Key Points
Question
Can antiviral treatment (AVT) affect the incidences of microvascular invasion (MVI) and early tumor recurrence after hepatectomy for hepatitis B virus (HBV)–related hepatocellular carcinoma (HCC)?
Finding
In this cohort study of 2362 patients with HBV-related HCC who underwent hepatectomy, a preoperative HBV DNA level of 2000 IU/mL or greater was associated with an increased risk of MVI. Compared with patients who did not receive preoperative AVT, patients who received AVT more than 90 days preoperatively had decreased incidences of MVI and 1-year and 2-year recurrence and less chance to develop recurrences involving multiple hepatic segments.
Meaning
Preoperative AVT was associated with a decreased posthepatectomy early tumor recurrence in HBV-related HCC.
This cohort study examines the association of preoperative antiviral treatment with the incidences of microvascular invasion and posthepatectomy early tumor recurrence in adult patients with hepatitis B virus–related hepatocellular carcinoma.
Abstract
Importance
A reduced incidence of microvascular invasion (MVI) in hepatitis B virus (HBV)–related hepatocellular carcinoma (HCC) may be associated with a decreased risk of early tumor recurrence and better survival after partial hepatectomy.
Objective
To examine the association of preoperative antiviral treatment (AVT) with the incidences of MVI and posthepatectomy early tumor recurrence in HBV-related HCC.
Design, Setting, and Participants
Data on a cohort of 2362 patients who underwent R0 resection for HBV-related HCC between January 2008 and April 2010 at the Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China, were reviewed. The median (interquartile range) postoperative follow-up was 44.8 (22.8-59.3) months. Data were analyzed from June 2016 to October 2017.
Interventions
Preoperative AVT and partial hepatectomy.
Main Outcomes and Measures
Overall survival and time to recurrence after surgery were calculated and compared using the Kaplan-Meier method, log-rank test, and Cox regression analysis. Independent risk factors of MVI presence were assessed by logistic regression analysis.
Results
Among 2362 included patients, 1999 (84.6%) were men, and the median (interquartile range) age was 50.6 (43.1-57.3) years. A total of 2036 patients (86.2%) did not receive any preoperative AVT, while 326 (13.8%) received ongoing AVT more than 90 days before surgery. In the non-AVT group, compared with a preoperative HBV DNA level of less than 2000 IU/mL, a preoperative HBV DNA level of 2000 IU/mL or greater was associated with an increased risk of MVI (odds ratio [OR], 1.399; 95% CI, 1.151-1.701). Compared with the non-AVT group, patients receiving AVT had a lower incidence of MVI (38.7% [126 of 326] vs 48.6% [989 of 2036]; P = .001) and reduced risk of MVI (OR, 0.758; 95% CI, 0.575-0.998). A complete response to AVT was an independent protective factor of MVI (OR, 0.690; 95% CI, 0.500-0.952). Accordingly, preoperative AVT was associated with decreased 6-month, 1-year, and 2-year recurrences vs non-AVT (14.2%, 24.6%, and 38.5%, respectively, vs 23.4%, 37.1%, and 52.3%; P < .001); AVT was protective of early tumor recurrence (hazard ratio, 0.732; 95% CI, 0.605-0.886). In addition, patients in the non-AVT group were more likely to have multiple intrahepatic recurrences (49.1% [549 of 1119] vs 36.2% [54 of 149]; P = .003) and recurrences involving multiple hepatic segments compared with patients receiving AVT.
Conclusions and Relevance
A high preoperative HBV DNA level was an independent risk factor of MVI. Antiviral treatment administered more than 90 days before surgery was associated with reduced incidences of MVI and early tumor recurrence after partial hepatectomy for HBV-related HCC.
Introduction
Approximately 70% to 90% of hepatocellular carcinoma (HCC) cases are associated with hepatitis B virus (HBV) infection in the highly endemic Asia-Pacific region.1,2 Partial hepatectomy and liver transplantation are potential curative treatments for selected patients with HCC.3,4 Unfortunately, long-term surgical outcomes remain unsatisfactory because of high tumor recurrence rates.5,6
Early HCC recurrence, defined as recurrence within 2 years after partial hepatectomy, has been reported to account for more than 60% of all recurrent HCC events.7,8,9,10,11,12 Early recurrence has been associated with the presence of microvascular invasion (MVI),8,10,13,14 which is a histopathological feature of microscopic metastasis and has been identified in 15.0% to 57.1% of liver resection or transplantation specimens.15,16,17,18 In contrast, late recurrence commonly arises from a liver remnant with chronic inflammation caused mainly by viral infection and cirrhosis (de novo tumor formation).8,9 Accordingly, antiviral treatment (AVT) has been reported to reduce late recurrence by suppressing viral replication and inflammation of the liver microenvironment.8,9,19,20,21,22
Experimental studies in 200823 and 201024 strongly suggested a close association of HBV infection with MVI formation in HBV-related HCC, as HBV infection may augment the angiogenic processes by enhancing the expression of metastasis-associated protein 1. In addition, HBV infection may subvert the immune responses against tumor cells that escape from the primary tumor, thus leading to vascular metastases.25 Meanwhile, several clinical studies have demonstrated that patients with HBV-related HCC were more likely to develop MVI and microscopic metastasis than patients with HCC with other etiologies,26,27,28,29,30 and serum HBV levels might be associated with the extent of vascular invasion.31,32 These results suggested the potential association of preoperative AVT with MVI formation and early HCC recurrence. To date, clinical evidence to verify this hypothesis has been lacking. As such, the current study sought to examine the association of preoperative HBV DNA level as well as the use of preoperative AVT with the incidence of MVI and early tumor recurrence among patients with HBV-related HCC who underwent partial hepatectomy.
Methods
Study Population
The analytic population consisted of 3405 consecutive patients with HBV-related HCC31,33 who underwent R0 resection between January 2008 and April 2010 at the Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China. To clarify the association of HBV infection with long-term surgical outcomes, 853 patients were excluded because of presence of macroscopic vascular invasion (n = 312), preoperative anticancer therapy (n = 284), history of other malignancies (n = 64), concurrent hepatitis C virus antibody positivity (n = 52), perioperative mortality within 30 days of surgery (n = 32), and missing clinicopathological data (n = 109). Data on the remaining 2552 patients were analyzed (eFigure in the Supplement). The study was approved by the Institutional Ethics Committee of the Eastern Hepatobiliary Surgery Hospital. Written informed consent was obtained from all patients for their data to be used for the research.
Preoperative Investigations and Partial Hepatectomy
Routine preoperative investigations included liver function tests, hepatitis B and C antigens/antibodies, α-fetoprotein, abdominal ultrasonography, abdominal contrast-enhanced magnetic resonance imaging or computed tomography, and radiography of the chest. Quantitative analysis of serum HBV DNA level was carried out using polymerase chain reaction (ABI 7300 Real-Time PCR System; Applied Biosystems) with a linear range of quantification of 200 to 2 million IU/mL. The preoperative diagnosis of HCC was based on the criteria of the American Association for the Study of Liver Diseases.34
Partial hepatectomy was considered feasible when patients were in good general condition, all tumor nodules on imaging studies were technically resectable, and the liver remnant functional volume and reserve were assessed to be adequate. The type of hepatectomy was determined by the operating surgeon based on the patient’s general performance, tumor stage and location, cirrhosis, and the estimated volume of future liver remnant. An R0 resection was defined as complete removal of macroscopic nodules with a microscopically tumor-free resection margin.35 The Edmondson-Steiner classification36 was used to determine the degree of tumor differentiation. Satellite nodules were defined as tumors less than 2 cm in diameter and located less than 2 cm from the main tumor.37 Microvascular invasion was defined as the presence of tumor cells in a portal vein, hepatic vein, or large capsular vessel of the surrounding hepatic tissue lined by endothelium that was visible only on microscopy.38
Preoperative Antiviral Treatment and Patient Grouping
Preoperative AVT was defined as continuously using at least 1 type of AVT medication for more than 90 days before surgery, which was consistent with the previously reported definition.39,40 The selection criteria for AVT were mainly according to the contemporary guidelines for the management of patients with HBV infection.41,42,43 Antiviral agents used in this study are listed in eTable 1 in the Supplement.
Among these 2552 patients, 2036 (79.8%) did not receive any preoperative AVT; 326 (12.8%) met the preoperative AVT criteria, with a median (interquartile range) AVT duration of 23.6 (11.1-40.4) months before surgery. The remaining 190 patients also received AVT before surgery but failed to meet the preoperative AVT criteria. Of these, 13 underwent treatment before HCC diagnosis (8 and 5 received AVT less than 60 and 90 days, respectively), 30 had discontinuous treatment, and 147 received preoperative short-term AVT with a median (interquartile range) treatment duration of 19 (interquartile range, 15-22) days, mainly because of an HBV DNA level of 200 000 IU/mL or greater, which might increase the risks of postoperative hepatitis and hepatic decompensation44,45 (eFigure in the Supplement). Among all patients who were treated with AVT before surgery, 475 of 516 (92.1%) received nucleos(t)ide analogues (eTable 1 in the Supplement).
Follow-up and End Points
Patients were followed up once every 2 months in the first 2 years after discharge and then once every 3 to 6 months thereafter. The follow-up regimen, diagnosis, and treatment of HCC recurrence were similar to the management reported previously.35 In particular, patients who received preoperative AVT were routinely recommended to continue the treatment after surgery. For patients without any preoperative AVT, this treatment was recommended after surgery according to the indication proposed by the related consensus of the same period.41,42,43 In addition, a clear explanation on the need for any long-term medication and on the discontinuation of medication for any reason was given to all the patients who received AVT. On each follow-up visit, the use of medication was verified to assess drug resistance and adverse effects and to adjust medications.
The primary end points were overall survival (OS), which was measured from the date of surgery to the dates of patient death or last follow-up, and time to recurrence, which was calculated from the date of surgery to the date when tumor recurrence was diagnosed. Early and late recurrence were defined as recurrent disease developed within or beyond 2 years, respectively, after surgery.8,9,10,11 The secondary outcome was the presence of MVI on postoperative histopathology.
Statistical Analysis
Categorical variables were compared using the χ2 test or Fisher exact test. Continuous variables or variables with an abnormal distribution were compared using the unpaired t test or Mann-Whitney U test. Survival curves were calculated and compared using the Kaplan-Meier method and log-rank test. Cox regression analyses were used for univariate and multivariate analyses of OS and recurrence. Preoperative factors that might be associated with MVI presence were assessed by univariate logistic regression analysis, and the variables that were significant (P < .05) were subjected in the stepwise multivariate analysis. All analysis was performed using SPSS Statistics version 21.0 for Windows (IBM Corp). A 2-tailed P value less than .05 was considered to be statistically significant.
Results
Patient Characteristics
As shown in Table 1, among 2036 patients who did not receive any preoperative AVT, 1132 patients with a high viral level (≥2000 IU/mL) were more likely to be positive for hepatitis B e antigen (HBeAg) and had higher levels of alanine aminotransferase and aspartate aminotransferase, a prolonged prothrombin time, cirrhosis, and a surgical resection margin of less than 1 cm compared with 904 patients who had a low viral level (<2000 IU/mL). However, there was no difference in α-fetoprotein level, size and number of tumors, tumor encapsulation, satellite nodules, and tumor differentiation between low-titer vs high-titer patients. In contrast, patients with a high viral level were more likely to have MVI compared with patients who had a low viral level (51.9% [587 of 1132] vs 44.5% [402 of 904]; P = .001).
Table 1. Clinicopathological Characteristics in Patients Receiving Antiviral Treatment (AVT) and Those Not Receiving AVT.
| Variable | No. (%) | |||||
|---|---|---|---|---|---|---|
| Non-AVT Group | AVT Group | |||||
| Total (n = 2036) | Low Viral Level (n = 904) | High Viral Level (n = 1132) | P Valuea | Total (n = 326) | P Valueb | |
| Age, median (IQR), y | 50.6 (43.2-57.3) | 50.0 (43.0–57.5) | 51.2 (43.4-57.2) | .34 | 50.2 (42.3-57.2) | .93 |
| Male | 1717 (84.3) | 755 (83.5) | 962 (85.0) | .37 | 282 (86.5) | .31 |
| Diabetes | 196 (9.6) | 103 (11.4) | 93 (8.2) | .02 | 51 (15.6) | .001 |
| HBeAg positivity | 658 (32.3) | 180 (19.9) | 478 (42.2) | <.001 | 65 (19.9) | <.001 |
| HBeAb positivity | 1483 (72.8) | 740 (81.9) | 743 (65.6) | <.001 | 241 (73.9) | .68 |
| HBV DNA level ≥2000 IU/mL | 1132 (55.6) | 0 | 1132 (100) | <.001 | 74 (22.7) | <.001 |
| ALT level >44 U/L | 821 (40.3) | 286 (31.6) | 535 (47.3) | <.001 | 112 (34.4) | .04 |
| AST level >40 U/L | 879 (43.2) | 319 (35.3) | 560 (49.5) | <.001 | 106 (32.5) | <.001 |
| GGT level >64 U/L | 1017 (50.0) | 424 (46.9) | 593 (52.4) | .01 | 143 (43.9) | .04 |
| TBIL level >0.99 mg/dL | 516 (25.3) | 228 (25.2) | 288 (25.4) | .91 | 86 (26.4) | .69 |
| Albumin level <3.5 g/dL | 85 (4.2) | 26 (2.9) | 59 (5.2) | .009 | 12 (3.7) | .68 |
| PT >13 s | 328 (16.1) | 114 (12.6) | 214 (18.9) | <.001 | 78 (23.9) | .001 |
| Platelet count <100 ×103/μL | 384 (18.9) | 149 (16.5) | 235 (20.8) | .01 | 102 (31.3) | <.001 |
| Glucose level >126.13 mg/dL | 130 (6.4) | 70 (7.7) | 60 (5.3) | .03 | 26 (8.0) | .28 |
| Creatinine level >1.18 mg/dL | 23 (1.1) | 16 (1.8) | 7 (0.6) | .02 | 5 (1.5) | .58 |
| WBC count <4000/μL | 424 (20.8) | 168 (18.6) | 256 (22.6) | .03 | 87 (26.7) | .02 |
| Hemoglobin level, median (IQR), g/dL | 14.3 (13.2-15.3) | 14.3 (13.3-15.3) | 14.3 (13.2-15.2) | .33 | 14.4 (13.2-15.4) | .56 |
| α-Fetoprotein level >400 ng/mL | 741 (36.4) | 323 (35.7) | 418 (36.9) | .58 | 91 (27.9) | .003 |
| Blood loss >800 mL | 111 (5.5) | 56 (6.2) | 55 (4.9) | .19 | 12 (3.7) | .18 |
| Transfusion | 239 (11.7) | 108 (11.9) | 131 (11.6) | .79 | 26 (8.0) | .046 |
| Adjuvant TACE | 696 (34.2) | 292 (32.3) | 404 (35.7) | .11 | 113 (34.7) | .87 |
| Postoperative AVT | 522 (25.6) | 148 (16.4) | 374 (33.0) | <.001 | 259 (79.4) | <.001 |
| Surgical margin <1 cm | 1576 (77.4) | 675 (74.7) | 901 (79.6) | .008 | 248 (76.1) | .59 |
| Tumor diameter, median (IQR), cm | 4.6 (3.0-7.5) | 4.6 (3.0-7.6) | 4.6 (3.0-7.3) | .61 | 3.8 (2.5-5.6) | <.001 |
| Tumor >1 | 230 (11.3) | 92 (10.2) | 138 (12.2) | .15 | 34 (10.4) | .65 |
| Incomplete tumor encapsulation | 1078 (52.9) | 490 (54.2) | 588 (51.9) | .31 | 151 (46.3) | .03 |
| Microvascular invasion | 989 (48.6) | 402 (44.5) | 587 (51.9) | .001 | 126 (38.7) | .001 |
| Satellite nodules | 324 (15.9) | 134 (14.8) | 190 (16.8) | .23 | 54 (16.6) | .77 |
| Edmondson-Steiner grade III/IV36 | 1378 (67.7) | 616 (68.1) | 762 (67.3) | .69 | 209 (64.1) | .20 |
| Cirrhosis | 1036 (50.9) | 415 (45.9) | 621 (54.9) | <.001 | 217 (66.6) | <.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, λ-glutamyltransferase; HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; IQR, interquartile range; PT, prothrombin time; TACE, transarterial chemoembolization; TBIL, total bilirubin; WBC, white blood cell.
SI conversion factor: To convert ALT to microkatals per liter, multiply by 0.0167; AST to microkatals per liter, multiply by 0.0167; GGT to microkatals per liter, multiply by 0.0167; TBIL to micromoles per liter, multiply by 17.104; albumin to grams per liter, multiply by 10; platelet count to ×109/L, multiply by 1; glucose to millimoles per liter, multiply by 0.0555; creatinine to micromoles per liter, multiply by 88.4; WBC count to ×109/L, multiply by 0.001; hemoglobin to grams per liter, multiply by 10; α-fetoprotein to micrograms per liter, multiply by 1.
Comparison between patients not receiving AVT with a low (<2000 IU/mL) and a high (≥2000 IU/mL) viral level.
Comparison between patients not receiving AVT and patients receiving AVT.
Compared with 326 patients who met our preoperative AVT criteria, 2036 patients who did not receive AVT were more likely to be positive for HBeAg; had higher levels of alanine aminotransferase, aspartate aminotransferase, and α-fetoprotein; had larger tumor size; had incomplete tumor encapsulation; and received blood transfusion. Patients who received AVT had a lower platelet count level, prolonged prothrombin time, and cirrhosis. Tumor number, satellite nodules, surgical margin, and tumor differentiation were comparable between patients in the AVT and non-AVT groups. However, patients not receiving AVT had a higher incidence of MVI compared with patients receiving AVT (48.6% [989 of 2036] vs 38.7% [126 of 326]; P = .001) (Table 1).
Association of Preoperative HBV DNA Level With the Presence of MVI
To avoid any potential influence of preoperative AVT on the presence of MVI, an analysis was conducted on all 2036 patients not receiving AVT. Multivariate analysis demonstrated that a preoperative HBV DNA level of 2000 IU/mL or greater was an independent risk factor of MVI presence (odds ratio [OR], 1.399; 95% CI, 1.151-1.701), in addition to α-fetoprotein level greater than 400 ng/mL (to convert to micrograms per liter, multiply by 1), increasing tumor size, incomplete tumor encapsulation, presence of satellite nodules, grade III/IV of tumor differentiation, and cirrhosis (eTable 2 in the Supplement).
Association of Preoperative AVT With the Presence of MVI
Subsequent analyses were conducted on all patients receiving and not receiving AVT (n = 2362). Multivariate analysis demonstrated that in addition to a high α-fetoprotein level, large tumor size, incomplete tumor encapsulation, satellite nodules, grade III/IV differentiation, and cirrhosis, an HBV DNA level of 2000 IU/mL or greater was independently associated with an increased risk of MVI (OR, 1.409; 95% CI, 1.171-1.696). In contrast, AVT was associated with a decreased risk (OR, 0.758; 95% CI, 0.575-0.998) (Table 2). The differences in MVI incidence between the AVT and non-AVT groups were also identified in the subpopulations of patients stratified by a tumor diameter of 3 cm or less, 5 cm or less, and 10 cm or less (eTable 3 in the Supplement).
Table 2. Logistic Regression Analysis of Microvascular Invasion Presence in Patients Receiving Antiviral Treatment (AVT) and Those Not Receiving AVT (N = 2362).
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, y (continuous) | 0.989 (0.981-0.997) | .005 | NA | NA |
| Sex, male vs female | 1.176 (0.939-1.473) | .16 | NA | NA |
| Diabetes, yes vs no | 0.887 (0.680-1.156) | .37 | NA | NA |
| HBeAg, positive vs negative | 1.064 (0.893-1.267) | .49 | NA | NA |
| HBeAb, positive vs negative | 0.850 (0.709-1.020) | .08 | NA | NA |
| HBV DNA level, ≥2000 vs <2000 IU/mL | 1.462 (1.243-1.720) | <.001 | 1.409 (1.171-1.696) | <.001 |
| AVT, yes vs no | 0.667 (0.525-0.847) | .001 | 0.758 (0.575-0.998) | .049 |
| ALT level, >44 vs ≤44 U/L | 0.877 (0.743-1.035) | .12 | NA | NA |
| AST level, >40 vs ≤40 U/L | 1.360 (1.155-1.603) | <.001 | NA | NA |
| GGT level, >64 vs ≤64 U/L | 1.221 (1.039-1.436) | .02 | NA | NA |
| TBIL level, >0.99 vs ≤0.99 mg/dL | 1.007 (0.837-1.213) | .94 | NA | NA |
| Albumin level, <3.5 vs ≥3.5 g/dL | 0.777 (0.514-1.174) | .23 | NA | NA |
| PT, >13 vs ≤13 s | 1.004 (0.811-1.244) | .97 | NA | NA |
| Platelet count, <100 vs ≥100 ×103/μL | 0.851 (0.697-1.041) | .12 | NA | NA |
| Glucose level, >126.13 vs ≤126.13 mg/dL | 0.764 (0.550-1.063) | .11 | NA | NA |
| Creatinine level, >1.18 vs ≤1.18 mg/dL | 0.526 (0.237-1.167) | .11 | NA | NA |
| WBC count, <4000/μL vs ≥4000/μL | 0.867 (0.712-1.055) | .16 | NA | NA |
| Hemoglobin, g/L (continuous) | 1.000 (0.995-1.005) | <.99 | NA | NA |
| α-Fetoprotein level, >400 vs ≤400 ng/mL | 2.538 (2.133-3.019) | <.001 | 1.736 (1.431-2.106) | <.001 |
| Blood loss, >800 vs ≤800 mL | 1.509 (1.046-2.177) | .03 | NA | NA |
| Transfusion, yes vs no | 1.453 (1.124-1.879) | .004 | NA | NA |
| Surgical margin, <1 vs ≥1 cm | 1.049 (0.865-1.272) | .63 | NA | NA |
| Tumor diameter, cm (continuous) | 1.089 (1.064-1.114) | <.001 | 1.043 (1.015-1.072) | .002 |
| Tumor No., multiple vs single | 1.235 (0.956-1.596) | .11 | NA | NA |
| Tumor encapsulation, incomplete vs complete | 3.151 (2.661-3.730) | <.001 | 2.458 (2.048-2.951) | <.001 |
| Satellite nodules, presence vs absence | 2.672 (2.118-3.371) | <.001 | 1.832 (1.416-2.370) | <.001 |
| Edmondson-Steiner grade,36 III/IV vs I/II | 4.103 (3.391-4.963) | <.001 | 2.947 (2.388-3.637) | <.001 |
| Cirrhosis, yes vs no | 1.356 (1.152-1.595) | <.001 | 1.929 (1.590-2.339) | <.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, λ-glutamyltransferase; HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; NA, not applicable; OR, odds ratio; PT, prothrombin time; TBIL, total bilirubin; WBC, white blood cell.
SI conversion factor: To convert ALT to microkatals per liter, multiply by 0.0167; AST to microkatals per liter, multiply by 0.0167; GGT to microkatals per liter, multiply by 0.0167; TBIL to micromoles per liter, multiply by 17.104; albumin to grams per liter, multiply by 10; platelet count to ×109/L, multiply by 1; glucose to millimoles per liter, multiply by 0.0555; creatinine to micromoles per liter, multiply by 88.4; WBC count to ×109/L, multiply by 0.001; α-fetoprotein to micrograms per liter, multiply by 1.
Association of Preoperative AVT With the Presence of MVI Based on Virological Responses
Using the criteria on response to AVT,46 among 326 patients receiving AVT, 252 (77.3%) were complete responders to AVT, while the remaining 74 (22.7%) were incomplete responders. The baseline characteristics among complete responders and patients not receiving AVT with a low HBV DNA level were compared (eTable 4 in the Supplement); there were no significant differences in HBeAg positivity, HBV DNA level, alanine aminotransferase and γ-glutamyltransferase levels, surgical margin, tumor number, and satellite nodules between the 2 subgroups. However, the incidence of MVI in complete responders was lower than that in patients not receiving AVT with a low viral level (34.9% [88 of 252] vs 44.5% [402 of 904]; P = .007). Multivariate analysis demonstrated that a complete response to AVT (OR, 0.690; 95% CI, 0.500-0.952) was independently associated with MVI presence (eTable 5 in the Supplement).
Early Tumor Recurrence and Overall Survival Among AVT vs Non-AVT Groups
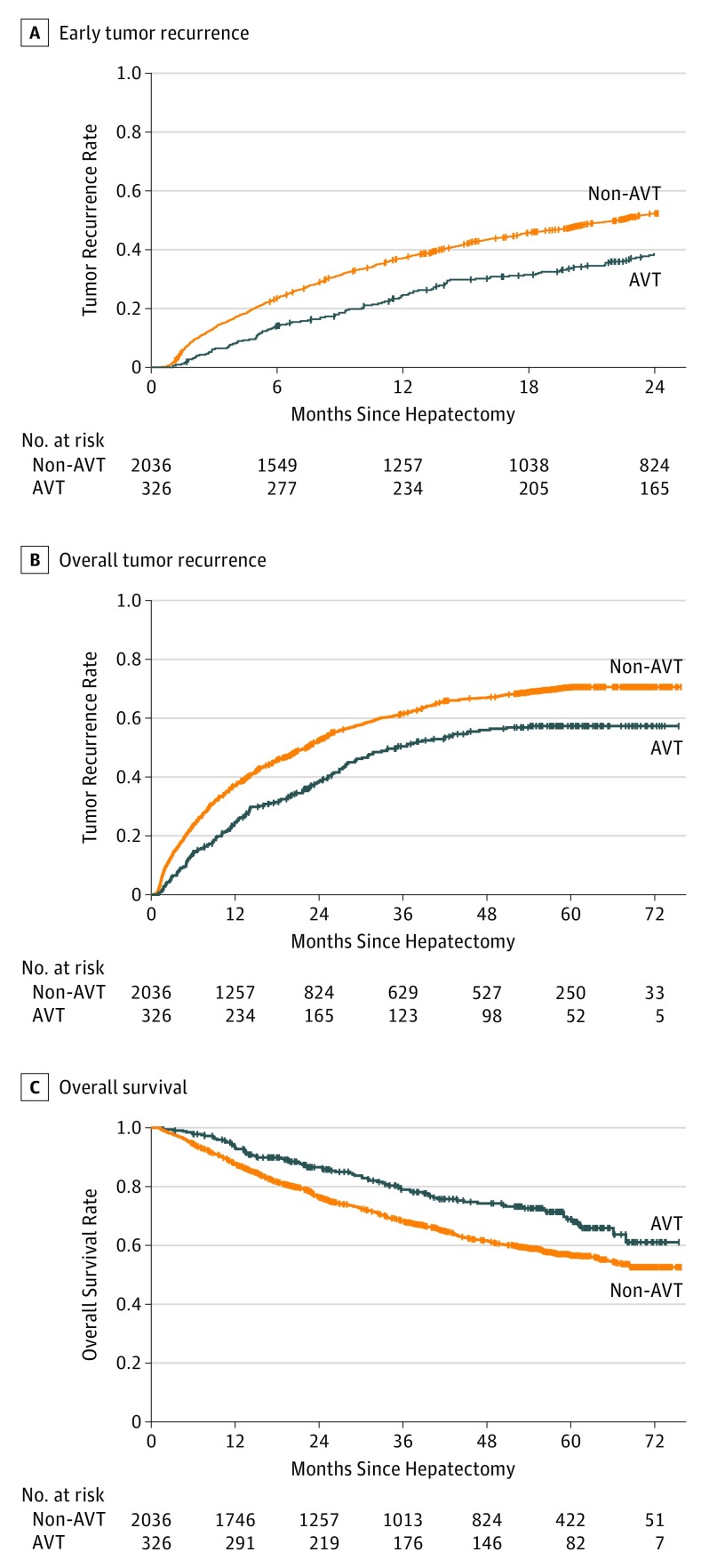
The median (interquartile range) follow-up was 44.8 (22.8-59.3) months. For patients receiving AVT, the 6-month, 1-year, and 2-year recurrence rates were 14.2%, 24.6%, and 38.5%, respectively; for patients not receiving AVT, recurrence rates were 23.4%, 37.1%, and 52.3% (P < .001) (Figure, A).
Figure. Early Tumor Recurrence and Overall Survival Between Patients Receiving Antiviral Treatment (AVT) and Not Receiving AVT After Hepatectomy for Hepatitis B Virus–Related Hepatocellular Carcinoma.
A, For patients receiving AVT, the 6-month, 1-year, and 2-year recurrence rates were 14.2%, 24.6%, and 38.5%, respectively; for patients not receiving AVT, recurrence rates were 23.4%, 37.1%, and 52.3% (P < .001). B, Overall 1-year, 3-year, and 5-year recurrence rates for patients receiving AVT were 24.7%, 50.7%, and 57.9%, respectively; for patients not receiving AVT, recurrence rates were 37.1%, 61.5%, and 70.8% (P < .001). C, Patients receiving AVT had better survival than patients not receiving AVT, with the corresponding 1-year, 3-year, and 5-year overall survival rate of 93.1%, 78.9%, and 69.1%, respectively, vs 87.6%, 68.4%, and 56.3% (P < .001).
The independent risk factors for early tumor recurrence were analyzed among all 2362 patients in the AVT and non-AVT groups, while the factors associated with late recurrence were assessed among the 989 patients who had a postoperative period of 2 years or more and did not have early recurrence. Multivariate analysis demonstrated that in addition to those factors associated with both early and late recurrences, presence of MVI (hazard ratio [HR], 1.458; 95% CI 1.287-1.652), preoperative AVT (HR, 0.732; 95% CI, 0.605-0.886), α-fetoprotein level greater than 400 ng/mL, surgical margin less than 1 cm, and grade III/IV differentiation were associated only with early recurrence (Table 3).
Table 3. Cox Regression Analysis of Early and Late Tumor Recurrences (N = 2362).
| Variable | Early Tumor Recurrence | Late Tumor Recurrence | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y (continuous) | 0.996 (0.990-1.002) | .17 | NA | NA | 1.005 (0.994-1.015) | .40 | NA | NA |
| Sex, male vs female | 1.280 (1.082-1.514) | .004 | NA | NA | 0.910 (0.696-1.189) | .49 | NA | NA |
| Diabetes, yes vs no | 0.925 (0.763-1.122) | .43 | NA | NA | 0.873 (0.612-1.244) | .45 | NA | NA |
| HBeAg, positive vs negative | 1.252 (1.108-1.413) | <.001 | 1.246 (1.101-1.409) | <.001 | 1.490 (1.190-1.866) | .001 | 1.497 (1.184-1.894) | .001 |
| HBeAb, positive vs negative | 0.858 (0.756-0.974) | .02 | NA | NA | 0.709 (0.563-0.893) | .003 | NA | NA |
| HBV DNA level, ≥2000 vs <2000 IU/mL | 1.212 (1.080-1.361) | .001 | NA | NA | 1.354 (1.093-1.678) | .006 | 1.266 (1.015-1.579) | .04 |
| AVT, yes vs no | 0.635 (0.526-0.767) | <.001 | 0.732 (0.605-0.886) | .001 | 0.818 (0.601-1.114) | .20 | NA | NA |
| ALT level, >44 vs ≤44 U/L | 1.309 (1.165-1.470) | <.001 | NA | NA | 1.210 (0.969-1.510) | .09 | NA | NA |
| AST level, >40 vs ≤40 U/L | 1.638 (1.459-1.838) | <.001 | 1.145 (1.004-1.307) | .04 | 1.318 (1.058-1.641) | .01 | NA | NA |
| GGT level, >64 vs ≤64 U/L | 1.618 (1.440-1.818) | <.001 | 1.201 (1.054-1.369) | .006 | 1.366 (1.102-1.693) | .004 | NA | NA |
| TBIL level, >0.99 vs ≤0.99 mg/dL | 1.063 (0.932-1.211) | .36 | NA | NA | 0.999 (0.778-1.283) | >.99 | NA | NA |
| Albumin level, <3.5 vs ≥3.5 g/dL | 1.352 (1.033-1.768) | .03 | NA | NA | 1.280 (0.749-2.186) | .37 | NA | NA |
| PT, >13 vs ≤13 s | 1.036 (0.891-1.206) | .65 | NA | NA | 1.229 (0.941-1.607) | .13 | NA | NA |
| Platelet count, <100 vs ≥100 ×103/μL | 0.968 (0.839-1.118) | .66 | NA | NA | 1.313 (1.027-1.680) | .03 | 1.353 (1.050-1.742) | .02 |
| Glucose level, >126.13 vs ≤126.13 mg/dL | 1.031 (0.819-1.298) | .79 | NA | NA | 0.973 (0.625-1.513) | .90 | NA | NA |
| Creatinine level, >1.18 vs ≤1.18 mg/dL | 0.689 (0.370-1.284) | .24 | NA | NA | 0.960 (0.358-2.574) | .94 | NA | NA |
| WBC count, <4000/μL vs ≥4000/μL | 0.883 (0.765-1.019) | .09 | NA | NA | 1.252 (0.983-1.593) | .07 | NA | NA |
| Hemoglobin, g/L (continuous) | 1.000 (0.997-1.004) | .92 | NA | NA | 0.995 (0.989-1.002) | .14 | NA | NA |
| α-Fetoprotein level, >400 vs ≤400 ng/mL | 1.619 (1.440-1.819) | <.001 | 1.266 (1.120-1.430) | <.001 | 0.880 (0.690-1.123) | .30 | NA | NA |
| Blood loss, >800 vs ≤800 mL | 1.832 (1.463-2.294) | <.001 | NA | NA | 1.747 (1.023-2.983) | .04 | NA | NA |
| Transfusion, yes vs no | 1.739 (1.477-2.046) | <.001 | NA | NA | 1.484 (1.020-2.159) | .04 | NA | NA |
| Adjuvant TACE, yes vs no | 1.098 (0.974-1.238) | .13 | NA | NA | 1.156 (0.924-1.447) | .20 | NA | NA |
| Postoperative AVT, yes vs no | 0.934 (0.825-1.057) | .28 | NA | NA | 0.767 (0.599-0.982) | .04 | 0.698 (0.543-0.898) | .005 |
| Surgical margin, <1 vs ≥1 cm | 1.744 (1.493-2.037) | <.001 | 1.350 (1.147-1.590) | <.001 | 1.247 (0.976-1.593) | .08 | NA | NA |
| Tumor diameter, cm (continuous) | 1.096 (1.081-1.111) | <.001 | 1.050 (1.033-1.067) | <.001 | 1.045 (1.013-1.078) | .006 | 1.045 (1.011-1.080) | .008 |
| Tumor No., multiple vs solitary | 1.400 (1.183-1.655) | <.001 | 1.250 (1.054-1.484) | .01 | 1.636 (1.192-2.247) | .002 | 1.572 (1.141-2.164) | .006 |
| Microvascular invasion, presence vs absence | 1.825 (1.625-2.051) | <.001 | 1.458 (1.287-1.652) | <.001 | 1.181 (0.950-1.470) | .14 | NA | NA |
| Tumor encapsulation, incomplete vs complete | 1.364 (1.214-1.532) | <.001 | NA | NA | 1.018 (0.822-1.262) | .87 | NA | NA |
| Satellite nodules, presence vs absence | 1.877 (1.632-2.160) | <.001 | 1.368 (1.181-1.584) | <.001 | 1.773 (1.299-2.420) | <.001 | 1.601 (1.161-2.206) | .004 |
| Edmondson-Steiner grade,36 III/IV vs I/II | 1.880 (1.644-2.151) | <.001 | 1.292 (1.116-1.496) | .001 | 1.123 (0.901-1.401) | .30 | NA | NA |
| Cirrhosis, yes vs no | 0.920 (0.820-1.033) | .16 | NA | NA | 1.040 (0.839-1.289) | .72 | NA | NA |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AVT, antiviral treatment; GGT, λ-glutamyltransferase; HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HR, hazard ratio; NA, not applicable; PT, prothrombin time; TACE, transarterial chemoembolization; TBIL, total bilirubin; WBC, white blood cell.
SI conversion factor: To convert ALT to microkatals per liter, multiply by 0.0167; AST to microkatals per liter, multiply by 0.0167; GGT to microkatals per liter, multiply by 0.0167; TBIL to micromoles per liter, multiply by 17.104; albumin to grams per liter, multiply by 10; platelet count to ×109/L, multiply by 1; glucose to millimoles per liter, multiply by 0.0555; creatinine to micromoles per liter, multiply by 88.4; WBC count to ×109/L, multiply by 0.001; α-fetoprotein to micrograms per liter, multiply by 1.
Overall 1-year, 3-year, and 5-year recurrence rates for patients receiving AVT were 24.7%, 50.7%, and 57.9%, respectively; for patients not receiving AVT, recurrence rates were 37.1%, 61.5%, and 70.8% (P < .001). Patients receiving AVT had better survival than patients not receiving AVT, with corresponding 1-year, 3-year, and 5-year OS of 93.1%, 78.9%, and 69.1%, respectively, vs 87.6%, 68.4%, and 56.3% (P < .001) (Figure, B and C). Multivariate analysis demonstrated that several factors were independently associated with OS and overall recurrence, including the presence of MVI (OS: HR, 1.518; 95% CI, 1.310-1.758; overall recurrence: HR, 1.392; 95% CI, 1.248-1.552), HBeAg positivity (OS: HR, 1.262; 95% CI, 1.092-1.460; overall recurrence: HR, 1.309; 95% CI, 1.174-1.459), and preoperative AVT (OS: HR, 0.752; 95% CI, 0.598-0.947; overall recurrence: HR, 0.764; 95% CI, 0.649-0.899) (eTable 6 in the Supplement).
Among 1115 patients with MVI, patients receiving AVT had decreased early and overall 1-year, 3-year, and 5-year recurrence rates and better 1-year, 3-year, and 5-year OS rates compared with patients not receiving AVT. Multivariate analysis identified that the use of preoperative AVT was an independent risk factor for both early tumor recurrence (HR, 0.717; 95% CI, 0.548-0.938) and overall recurrence (HR, 0.787; 95% CI, 0.622-0.996). Further stratified analyses showed significant differences in early and overall recurrence between the AVT vs non-AVT groups among all the patients who had an HCC of 10 cm or less (eTable 7 and 8 in the Supplement).
Patterns of Tumor Recurrence Among AVT vs Non-AVT Groups
There were 1329 and 167 patients who had recurrences in the non-AVT and AVT groups, respectively. Although patients not receiving AVT were more likely to develop extrahepatic metastasis than those receiving AVT (8.0% vs 4.2%), overall differences in the type of recurrence were not different between the groups (P = .17). However, patients not receiving AVT were more likely to have multiple intrahepatic recurrent nodules vs those receiving AVT (49.1% [549 of 1119] vs 36.2% [54 of 149]; P = .003). In addition, the sites of intrahepatic recurrence were also different between the non-AVT and AVT groups; recurrences adjacent to the surgical margin or involving multiple segments were more commonly seen in those not receiving AVT than those receiving AVT (Table 4).
Table 4. Patterns of Tumor Recurrence.
| Patterna | No. (%) | P Value | |
|---|---|---|---|
| Non-AVT (n = 1329) | AVT (n = 167) | ||
| Type of recurrence | .17 | ||
| Intrahepatic recurrence | 1119 (84.2) | 149 (89.2) | |
| Extrahepatic metastasis | 106 (8.0) | 7 (4.2) | |
| Synchronous intrahepatic and extrahepatic recurrences | 104 (7.8) | 11 (6.6) | |
| No. of intrahepatic recurrence | .003 | ||
| Single | 570 (50.9) | 95 (63.8) | |
| Multiple | 549 (49.1) | 54 (36.2) | |
| Site of intrahepatic recurrenceb | .006 | ||
| Local | 80 (7.1) | 5 (3.4) | |
| Adjacent segment | 298 (26.6) | 52 (34.9) | |
| Distal segment | 279 (24.9) | 47 (31.5) | |
| Multisegment | 462 (41.3) | 45 (30.2) | |
Abbreviation: AVT, antiviral treatment.
Observed at the first diagnosis of tumor recurrence.
Local recurrence is defined as any recurrence adjacent to the surgical margin within 2 cm, irrespective of additional recurrences in other parts of the liver remnant; adjacent segment recurrence refers to any recurrence in the adjacent segment or in the same segment after subsegmentectomy 2 cm away from the surgical margin; distal segment recurrence includes any recurrence not in the adjacent segment; and multisegment recurrences refers to the multiple recurrences involving adjacent and distal segments.
Outcomes Among Patients Who Underwent AVT Before Surgery But Did Not Meet the Preoperative AVT Criteria
Among 147 patients who received short-term AVT preoperatively, the incidence of MVI was 51.0% (75 of 147), which was higher than the incidence among 326 patients receiving AVT (38.7% [126 of 326]; P = .01) and similar to the incidence noted in patients not receiving AVT (48.6% [989 of 2036]; P = .57). Recurrences at 6 months, 1 year, and 2 years were 22.5%, 36.3%, and 53.9%, respectively, among these patients, which were higher than the recurrence noted among patients in the AVT group (P = .001) but not different from recurrence noted among the non-AVT group (P = .97).
Discussion
This study demonstrated that a high preoperative HBV DNA level was associated with an increased incidence of MVI in patients with HBV-related HCC. Patients who received consecutive AVT given more than 90 days preoperatively had a decreased incidence of MVI compared with patients who did not receive any preoperative AVT. In addition, preoperative AVT was significantly associated with a decrease in early HCC recurrence after partial hepatectomy.
In this study, the association of preoperative HBV DNA level with MVI presence was specifically assessed among patients not receiving AVT. For these patients, any association of preoperative AVT with MVI formation was absent. This result was also not affected by the presence of other risk factors of MVI, such as α-fetoprotein level, tumor stage, tumor encapsulation, and differentiation grade,17,31,47 as these factors were not different among those not receiving AVT with a high or a low HBV DNA level. The data demonstrated that patients with a high HBV DNA level had an increased incidence of MVI compared with patients who had a low viral level (51.9% vs 44.5%; P = .001). Furthermore, a high viral level was independently associated with an increased risk of MVI presence (OR, 1.399; 95% CI, 1.151-1.701).
There is a lack of evidence supporting an association of preoperative AVT with the presence of MVI and early tumor recurrence. This study demonstrated that patients receiving preoperative AVT had a decreased incidence of MVI compared with patients not receiving AVT (38.7% vs 48.6%; P = .001). Multivariate analysis demonstrated such AVT scheme was significantly associated with a decreased risk of MVI (OR, 0.758 95% CI, 0.575-0.998). In addition, the incidence of MVI was lower among complete responders to AVT compared with those not receiving AVT with a low HBV DNA level (34.9% vs 44.5%; P = .007). Complete response to AVT was a protective factor for the presence of MVI (OR, 0.690; 95% CI, 0.500-0.952).
Previous studies have reported that the importance of preoperative AVT is mainly to reduce postoperative HBV reactivation in patients with HBV-related HCC.33,44,45 Our data suggested that the association of preoperative AVT with reduced early tumor recurrence as the 6-month, 1-year, and 2-year recurrences, which are often associated with the presence of MVI, were significantly decreased in those receiving AVT vs those not receiving AVT. The presence of MVI was a risk factor for early tumor recurrence (HR, 1.458; 95% CI 1.287-1.652), while AVT was a protective factor (HR, 0.732; 95% CI, 0.605-0.886). In contrast, short-term AVT given before surgery (median [interquartile range] duration, 19 [15-22] days) failed to reduce MVI presence. Accordingly, early recurrence in patients undergoing short-term AVT was similar to that in patients not receiving AVT. Furthermore, the recurrence patterns in patients not receiving AVT were more closely associated with MVI, as these patients were more likely to have intrahepatic multiple recurrences, recurrent nodules involving multiple segments, and extrahepatic metastasis compared with those receiving AVT.
Limitations
Our study has several limitations. First, it is a single-institution study. Second, the indication of AVT in patients with HBV infection has been progressively expanded over the past decade,41,42,43,48,49 and the importance of AVT in patients with HBV-related HCC has been gradually recognized by surgeons and patients. As such, some patients did not receive AVT during the early period of this study even though they might meet the recent indications for AVT. Third, although short-term preoperative AVT decreased viral levels from 200 000 IU/mL or greater to 20 000 IU/mL or less in 136 of 147 patients (92.5%) before surgery, the incidences of MVI and early tumor recurrence in these patients had not been significantly reduced. Therefore, an optimal preoperative time window for AVT to affect MVI risk remains to be determined. In addition, this study did not provide evidence of the association of preoperative AVT with the tumor-immune microenvironment. Finally, in addition to HBV infection, the biological characteristics of HCC itself may affect the tumor invasiveness and recurrence after surgery.
Conclusions
In conclusion, a high preoperative HBV DNA level had a positive association with MVI formation. Antiviral treatment given more than 90 days before surgery was associated with decreased risks of MVI presence and early tumor recurrence for patients with HBV-related HCC after hepatectomy. Since MVI is also closely associated with tumor recurrence after liver transplantation for HCC,15 whether pretransplant AVT affects the incidence of MVI in viral-related HCC should be further investigated.
eFigure. Flowchart of the study.
eTable 1. Information on AVT medication.
eTable 2. Logistic regression analysis of MVI presence in non-AVT patients (n = 2036).
eTable 3. Incidence of MVI between AVT and non-AVT patients according to tumor size (n = 2362).
eTable 4. Clinicopathological characteristics between CRs to AVT and non-AVT patients with a low viral level (n = 1156).
eTable 5. Logistic regression analysis of MVI presence in CRs to AVT and non-AVT patients with a low viral level (n = 1156).
eTable 6. Cox regression analysis of OS and tumor recurrence in AVT and non-AVT patients (n = 2362).
eTable 7. Tumor recurrence and OS between AVT and non-AVT among patients with MVI (n = 1115).
eTable 8. Multivariate Cox regression analysis of tumor recurrence and OS between AVT and non-AVT among patients with MVI (n = 1115).
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):-. doi: 10.1016/S0140-6736(03)14964-1 [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Llovet JM. Hepatitis B virus and hepatocellular carcinoma. J Hepatol. 2003;39(suppl 1):S59-S63. doi: 10.1016/S0168-8278(03)00140-5 [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844-855. doi: 10.1136/gutjnl-2013-306627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura JT, Johnston FM, Tsai S, et al. Surgical resection versus ablation for hepatocellular carcinoma ≤ 3 cm: a population-based analysis. HPB (Oxford). 2015;17(10):896-901. doi: 10.1111/hpb.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232(1):10-24. doi: 10.1097/00000658-200007000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akamatsu N, Kokudo N. Liver transplantation for hepatocellular carcinoma from living-donor vs. deceased donor. Hepatobiliary Surg Nutr. 2016;5(5):422-428. doi: 10.21037/hbsn.2016.08.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89(3):500-507. doi: [DOI] [PubMed] [Google Scholar]
- 8.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200-207. doi: 10.1016/S0168-8278(02)00360-4 [DOI] [PubMed] [Google Scholar]
- 9.Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51(5):890-897. doi: 10.1016/j.jhep.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 10.Sohn W, Paik YH, Kim JM, et al. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol. 2014;21(7):2429-2435. doi: 10.1245/s10434-014-3621-x [DOI] [PubMed] [Google Scholar]
- 11.Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229-235. doi: 10.1097/01.sla.0000197706.21803.a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi GH, Kim DH, Kang CM, et al. Prognostic factors and optimal treatment strategy for intrahepatic nodular recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol. 2008;15(2):618-629. doi: 10.1245/s10434-007-9671-6 [DOI] [PubMed] [Google Scholar]
- 13.Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford). 2015;17(5):422-427. doi: 10.1111/hpb.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33(2):347-354. doi: 10.1111/jgh.13843 [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferro V, Llovet JM, Miceli R, et al. ; Metroticket Investigator Study Group . Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43. doi: 10.1016/S1470-2045(08)70284-5 [DOI] [PubMed] [Google Scholar]
- 16.Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108-113. doi: 10.1097/SLA.0b013e31821ad884 [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325-339. doi: 10.1245/s10434-012-2513-1 [DOI] [PubMed] [Google Scholar]
- 18.Kluger MD, Salceda JA, Laurent A, et al. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015;62(5):1131-1140. doi: 10.1016/j.jhep.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Lu JH, Zhai J, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol. 2012;38(8):683-691. doi: 10.1016/j.ejso.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 20.Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013;57(1):399-408. doi: 10.1002/hep.25937 [DOI] [PubMed] [Google Scholar]
- 21.Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58(1):98-107. doi: 10.1002/hep.26180 [DOI] [PubMed] [Google Scholar]
- 22.Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015;261(1):56-66. doi: 10.1097/SLA.0000000000000858 [DOI] [PubMed] [Google Scholar]
- 23.Ryu SH, Chung YH, Lee H, et al. Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology. 2008;47(3):929-936. doi: 10.1002/hep.22124 [DOI] [PubMed] [Google Scholar]
- 24.Bui-Nguyen TM, Pakala SB, Sirigiri RD, et al. NF-kappaB signaling mediates the induction of MTA1 by hepatitis B virus transactivator protein HBx. Oncogene. 2010;29(8):1179-1189. doi: 10.1038/onc.2009.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang P, Li QJ, Feng Y, et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22(3):291-303. doi: 10.1016/j.ccr.2012.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utsunomiya T, Shimada M, Kudo M, et al. ; Liver Cancer Study Group of Japan . A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg. 2015;261(3):513-520. doi: 10.1097/SLA.0000000000000821 [DOI] [PubMed] [Google Scholar]
- 27.Kaibori M, Ishizaki M, Matsui K, Kwon AH. Clinicopathologic characteristics of patients with non-B non-C hepatitis virus hepatocellular carcinoma after hepatectomy. Am J Surg. 2012;204(3):300-307. doi: 10.1016/j.amjsurg.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 28.Omichi K, Shindoh J, Yamamoto S, et al. Postoperative outcomes for patients with non-B non-C hepatocellular carcinoma: a subgroup analysis of patients with a history of hepatitis B infection. Ann Surg Oncol. 2015;22(suppl 3):S1034-S1040. doi: 10.1245/s10434-015-4845-0 [DOI] [PubMed] [Google Scholar]
- 29.Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35(4):858-867. doi: 10.1007/s00268-010-0928-z [DOI] [PubMed] [Google Scholar]
- 30.Sasaki K, Shindoh J, Nishioka Y, et al. Impact of viral etiology on postoperative de novo recurrence after hepatectomy for hepatocellular carcinoma in cirrhotic patients. J Gastrointest Surg. 2017;21(3):487-495. doi: 10.1007/s11605-016-3344-3 [DOI] [PubMed] [Google Scholar]
- 31.Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151(4):356-363. doi: 10.1001/jamasurg.2015.4257 [DOI] [PubMed] [Google Scholar]
- 32.Wei X, Li N, Li S, et al. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer. 2017;17(1):304-311. doi: 10.1186/s12885-017-3293-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dan JQ, Zhang YJ, Huang JT, et al. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2013;39(8):865-872. doi: 10.1016/j.ejso.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 34.Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208-1236. doi: 10.1002/hep.20933 [DOI] [PubMed] [Google Scholar]
- 35.Wang K, Liu J, Yan ZL, et al. Overexpression of aspartyl-(asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology. 2010;52(1):164-173. doi: 10.1002/hep.23650 [DOI] [PubMed] [Google Scholar]
- 36.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462-503. doi: [DOI] [PubMed] [Google Scholar]
- 37.Yang T, Lu JH, Lau WY, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: a propensity score matching analysis. J Hepatol. 2016;64(3):583-593. doi: 10.1016/j.jhep.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 38.Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850-855. doi: 10.1053/j.gastro.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906-1914. doi: 10.1001/2012.jama.11975 [DOI] [PubMed] [Google Scholar]
- 40.Lee TY, Lin JT, Zeng YS, Chen YJ, Wu MS, Wu CY. Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Hepatology. 2016;63(5):1517-1527. doi: 10.1002/hep.28266 [DOI] [PubMed] [Google Scholar]
- 41.Liaw YF, Leung N, Guan R, Lau GKK, Merican I; Asian-Pacific Consensus Working Parties on Hepatitis B . Asian-Pacific consensus statement on the management of chronic hepatitis B: an update. J Gastroenterol Hepatol. 2003;18(3):239-245. doi: 10.1046/j.1440-1746.2003.03037.x [DOI] [PubMed] [Google Scholar]
- 42.Society of Hepatology of Chinese Medical Association; Society of Infectious Diseases of Chinese Medical Association . The guideline of prevention and treatment for chronic hepatitis B. Chin J Integr Med. 2006;45(2):162-170. [Google Scholar]
- 43.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45(2):507-539. doi: 10.1002/hep.21513 [DOI] [PubMed] [Google Scholar]
- 44.Huang G, Lai EC, Lau WY, et al. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257(3):490-505. doi: 10.1097/SLA.0b013e318262b218 [DOI] [PubMed] [Google Scholar]
- 45.Jiang E, Shangguan AJ, Chen S, Tang L, Zhao S, Yu Z. The progress and prospects of routine prophylactic antiviral treatment in hepatitis B-related hepatocellular carcinoma. Cancer Lett. 2016;379(2):262-267. doi: 10.1016/j.canlet.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 46.Cho JY, Paik YH, Sohn W, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63(12):1943-1950. doi: 10.1136/gutjnl-2013-306409 [DOI] [PubMed] [Google Scholar]
- 47.Cucchetti A, Piscaglia F, Grigioni AD, et al. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52(6):880-888. doi: 10.1016/j.jhep.2009.12.037 [DOI] [PubMed] [Google Scholar]
- 48.Ye S; Expert Panel of Antiviral Therapy for Hepatocellular Carcinoma . Expert consensus on antiviral therapy to treat hepatitis B/C virus-related hepatocellular carcinoma [in Chinese]. Zhonghua Gan Zang Bing Za Zhi. 2014;22(5):321-326. [PubMed] [Google Scholar]
- 49.Hou JL, lai W; Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association . The guideline of prevention and treatment for chronic hepatitis B: a 2015 update [in Chinese]. Zhonghua Gan Zang Bing Za Zhi. 2015;23(12):888-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of the study.
eTable 1. Information on AVT medication.
eTable 2. Logistic regression analysis of MVI presence in non-AVT patients (n = 2036).
eTable 3. Incidence of MVI between AVT and non-AVT patients according to tumor size (n = 2362).
eTable 4. Clinicopathological characteristics between CRs to AVT and non-AVT patients with a low viral level (n = 1156).
eTable 5. Logistic regression analysis of MVI presence in CRs to AVT and non-AVT patients with a low viral level (n = 1156).
eTable 6. Cox regression analysis of OS and tumor recurrence in AVT and non-AVT patients (n = 2362).
eTable 7. Tumor recurrence and OS between AVT and non-AVT among patients with MVI (n = 1115).
eTable 8. Multivariate Cox regression analysis of tumor recurrence and OS between AVT and non-AVT among patients with MVI (n = 1115).