Abstract
Importance
Spontaneous pneumothorax is a common disease known to have an unusual epidemiological profile, but there are limited contemporary population-based data.
Objective
To estimate the incidence of hospital admissions for spontaneous pneumothorax, its recurrence and trends over time using large, longstanding hospitalization data sets in England.
Design, Setting, and Participants
A population-based epidemiological study was conducted using an English national data set and an English regional data set, each spanning 1968 to 2016, and including 170 929 hospital admission records of patients 15 years and older. Final date of the study period was December 31, 2016.
Exposures
Calendar year (for incidence) and readmission to hospital for spontaneous pneumothorax (for recurrence).
Main Outcomes and Measures
Primary outcomes were rates of hospital admissions for spontaneous pneumothorax and recurrence, defined as a subsequent hospital readmission with spontaneous pneumothorax. Record-linkage was used to identify multiple admissions per person and comorbidity. Risk factors for recurrence over 5 years of follow-up were assessed using cumulative time-to-failure analysis and Cox proportional hazards regression.
Results
From 1968 to 2016, there were 170 929 hospital admissions for spontaneous pneumothorax (median age, 44 years [IQR, 26-88]; 73.0% male). In 2016, there were 14.1 spontaneous pneumothorax admissions per 100 000 population 15 years and older (95% CI, 13.7-14.4), a significant increase compared with earlier years, up from 9.1 (95% CI, 8.1-10.1) in 1968. The population-based rate per 100 000 population 15 years and older was higher for males (20.8 [95% CI, 20.2-21.4]) than for females (7.6 [95% CI, 7.2-7.9]). Of patients with spontaneous pneumothorax, 60.8% (95% CI, 59.5%-62.0%) had chronic lung disease. Record-linkage analysis demonstrated that the overall increase in admissions over time could be due in part to an increase in repeat admissions, but there were also significant increases in the annual rate of first-known spontaneous pneumothorax admissions in some population subgroups, for example in women 65 years and older (annual percentage change from 1968 to 2016, 4.08 [95% CI, 3.33-4.82], P < .001). The probability of recurrence within 5 years was similar by sex (25.5% [95% CI, 25.1%-25.9%] for males vs 26.0% [95% CI, 25.3%-26.7%] for females), but there was variation by age group and presence of chronic lung disease. For example, the probability of readmission within 5 years among males aged 15 to 34 years with chronic lung disease was 39.2% (95% CI, 37.7%-40.7%) compared with 19.6% (95% CI, 18.2%-21.1%) in men 65 years and older without chronic lung disease.
Conclusions and Relevance
This study provides contemporary information regarding the trends in incidence and recurrence of inpatient-treated spontaneous pneumothorax.
This population epidemiological study uses English national hospital data sets to characterize trends in the incidence and recurrence of hospital admissions for spontaneous pneumothorax to identify risk factors for recurrence.
Key Points
Question
What are the rates of incidence and recurrence of spontaneous pneumothorax, and how have population-based trends changed over time?
Findings
In this analysis of 170 929 hospital admissions in England for spontaneous pneumothorax between 1968 and 2016, the annual hospital admission rate increased from 9.1 to 14.1 per 100 000 population, with differences by sex and by age.
Meaning
This study provides contemporary information regarding trends in hospitalization for spontaneous pneumothorax.
Introduction
Spontaneous pneumothorax is a common pathology. Primary spontaneous pneumothorax conventionally refers to patients with no underlying lung disease, whereas those with established lung pathology are classified as secondary spontaneous pneumothorax. Previous estimates of the incidence of spontaneous pneumothorax are based on studies1,2 (which were often small, many years old, and from single centers) and 2 national databases (each covering 4 years [1991-19943 and 2008-20114]). These have reported an overall incidence per 100 000 population of spontaneous pneumothorax of 17 to 24 per annum for men and 1 to 6 per annum for women1,2,3,4 (eTable 1 in the Supplement).
Recurrence estimates for spontaneous pneumothorax are based upon mostly retrospective case series data, which report a recurrence risk anywhere between 21% and 54% within 1 to 2 years.5,6,7,8,9,10,11
The aim of this study was to determine the contemporary and historical incidence of inpatient-treated spontaneous pneumothorax in England, and its recurrence, using longstanding data sets of hospital records.
Methods
Ethical approval for a multipurpose program of work on the linked and unlinked data sets (described below) was granted by the Central and South Bristol Multi-Centre Research Ethics Committee. The committee determined that individual patient consent was not required on the basis that this was a study without patient recruitment using routine administrative data in which the records contained no personally identifiable information or were pseudonymized through encryption of personal identifiers.
Data Sources
The English national data set comprised records from the Hospital In-Patient Enquiry (HIPE) 1968-1985 (not linkable, meaning that personally identifiable information was not collected, thus preventing the identification of multiple admissions per person during this period) and Hospital Episode Statistics (HES) 1990-2016 (linked from 1999). HIPE was a random 1 in 10 sample of all hospital discharge records in England, which were collected as statistical abstracts by all English hospitals and collated nationally by the national Office of Population Censuses and Surveys from 1968 to 1985. This sample was intended to be representative of National Health Service (NHS) hospital care throughout England. Hospital statistics were not collected nationally between 1986 and 1989. Since 1990, statistical abstracts of every day case and inpatient admission from every NHS hospital in England have been collected by NHS Digital (formerly the national Health and Social Care Information Centre) in the form of HES, covering the 52 million population. HES only became linkable from 1999 onwards. Using encrypted personal identifiers supplied by NHS Digital, linkage of HES from 1999 to 2016 was conducted by the Unit of Health-Care Epidemiology, University of Oxford. Details of the record-linkage methods are reported elsewhere.12
The English national data were compared with regional data from the Oxford Record Linkage Study (ORLS) 1968-2016 (linked throughout, also by the Unit of Health-Care Epidemiology using methods reported elsewhere12). The ORLS data set comprises statistical abstracts of all NHS day case and inpatient admissions occurring within what was formerly the Oxford NHS regional health authority area.
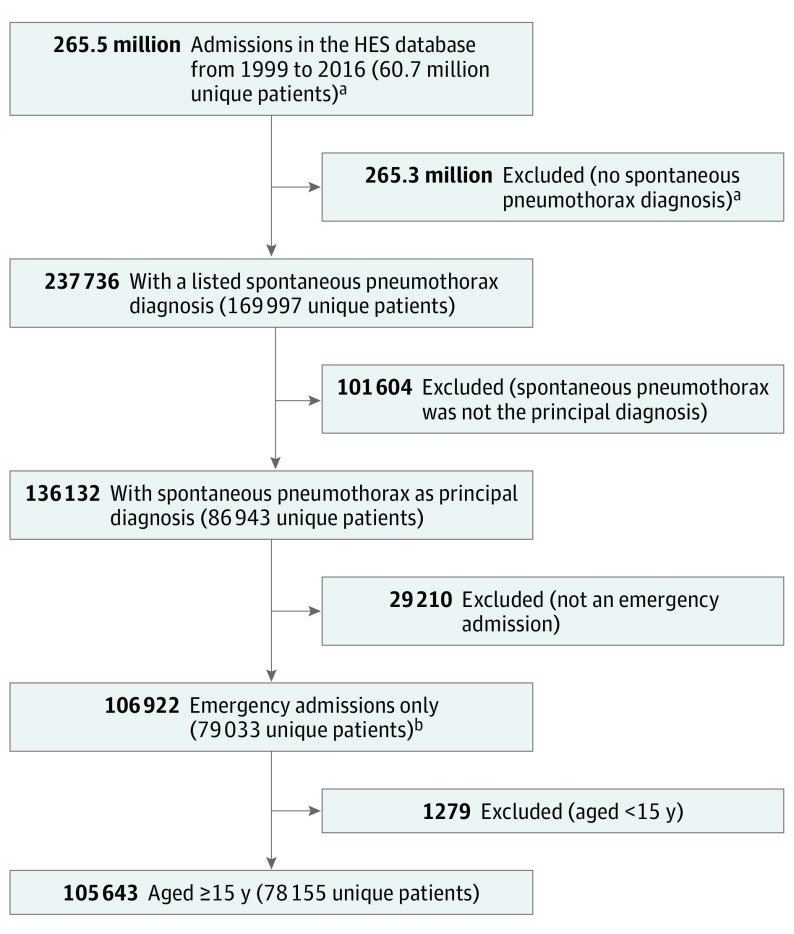
In all data sets, each discharge record contains a field for the principal reason for the admission (the primary diagnosis), along with other secondary or subsidiary diagnoses, coded using the International Classification of Diseases (ICD). The ICD codes used to identify spontaneous pneumothorax from the discharge records were ICD10 J93 (1995-current), ICD9 512 (1979-1994), and ICD8 512 (1968-1978). Traumatic spontaneous pneumothoraxes were excluded by removing records containing a concurrent diagnosis of fractures of ribs or vertebrae (ICD10 S22), traumatic hemothorax (ICD10 S27) or fracture of upper body (ICD10 S42). Spontaneous pneumothorax admissions were divided according to whether the spontaneous pneumothorax was recorded as the primary or nonprimary diagnosis, and according to whether the episode was an emergency or nonemergency admission. All data analysis was based on emergency admissions and where spontaneous pneumothorax was the primary diagnosis on the hospital record (Figure 1). The ICD does not distinguish between primary and secondary spontaneous pneumothorax. Therefore, an admission was regarded as secondary spontaneous pneumothorax if the patient had a diagnosis code indicating chronic lung disease (defined as any 1 of the 73 ICD codes listed in eTable 2 in the Supplement) coded either on the same spontaneous pneumothorax record or, using record linkage, on any known previous hospital record or any record within the next 12 months. Primary spontaneous pneumothorax was defined as any spontaneous pneumothorax admission that was not secondary. The variables used in these analyses (age, sex, date of admission, method of admission, recorded diagnoses) are all fundamental to the reporting of routine hospital statistics in England; therefore, missing data for these variables was extremely low. The variable with the most amount of missing data was admission method, which was missing on only 0.05% of all screened spontaneous pneumothorax records.
Figure 1. Flow of Pneumothorax Admissions from the English Hospital Episode Statistics (HES) From 1999 Through 2016 for Inclusion in the Analysis.
Derivations for other data sets not shown.
aRounded values.
bEmergency admissions defined in the HES Data Dictionary—Admitted Patient Care as those for which the admission is “unpredictable and at short notice because of clinical need” and for which admission method was coded 21, 22, 23, 24, 25, 2A, 2B, 2C, 2D or 28.
Incidence
The data were split by calendar year, age, sex, and presumed spontaneous pneumothorax type (primary vs secondary). Historical hospitalization rates per 100 000 population 15 years and older (hereafter referred to as population) were calculated for each year in England and ORLS from 1968 through 2016. Age-specific population counts for England and the ORLS region in each calendar year were obtained from the Office for National Statistics. Age-specific rates were calculated in 5-year age groups. Rates for broader age groups (15-34, 35-49, 50-64, ≥65 years) and all-ages combined were directly standardized to the European Standard Population 1976. The 2015 rates (rather than the 2016 rates) were considered to be the most reliable contemporary estimates when distinguishing between primary and secondary spontaneous pneumothorax because these incorporated the longest patient history of prior-recorded comorbidity, while also allowing for inspection of records occurring up to 12 months subsequently. Analyses using record-linkage were undertaken to identify multiple spontaneous pneumothorax records per person. Record-linkage enabled distinction between “admission-based” annual rates (for which every day case or inpatient record for spontaneous pneumothorax was counted, regardless of how many spontaneous pneumothorax records each individual had) and “person-based” annual rates (for which only 1 spontaneous pneumothorax record per individual was counted per year). Annual admission-based rates for spontaneous pneumothorax (which include both hospital transfers and readmissions for the same disease event) measure the burden of the disease on hospital services over time. Annual person-based rates indicate whether at least 1 spontaneous pneumothorax is affecting more or fewer individuals in any 1 year compared with another, and, therefore, measures of the burden of disease in the population. Within the ORLS, the data were linkable throughout the period of coverage, which enabled the calculation of person-based rates from 1968 to 2016. The admissions data for 2015 was split by age, sex, and known comorbid respiratory disease. Differences in the annual 2015 rates of spontaneous pneumothorax between these groups were assessed using z tests for independent proportions (or single proportions, where appropriate) with a statistical significance threshold of a 2-sided P value less than .05.
Where visual inspection of the annual rates indicated an upward or downward trend, annual percentage change (APC) was calculated with associated 95% CIs by fitting a regression model to the logarithms of the annual rates assuming a constant rate of change, with standard errors weighted to account for heteroscedasticity across the years. Where the null hypothesis was 0 APC, statistical significance was regarded as a 2-sided P value less than .05.
Recurrence
The record-linked data set in England (1999-2016) was used to determine the cumulative probability of inpatient-treated spontaneous pneumothorax recurrence within 7 days, 30 days, 90 days, 1 year, and 5 years by age and sex. Recurrence was defined as a second admission for which spontaneous pneumothorax was again recorded as the principal diagnosis and the patient was admitted as an emergency. Recurrence probabilities were calculated using a cumulative time-to-failure analysis, censoring individuals for death using mortality records obtained from the Office for National Statistics. Entry date was defined for each individual as the date of discharge on their first known spontaneous pneumothorax record that did not indicate an interhospital transfer on discharge. “Failure date” was defined as the date of admission for the earliest known recurrence event. It was considered that a subsequent spontaneous pneumothorax admission might sometimes represent an ongoing pneumothorax, rather than a true new spontaneous pneumothorax. As such, sensitivity analyses were conducted excluding spontaneous pneumothorax readmissions that occurred (1) within 7 days and (2) within 30 days after the index spontaneous pneumothorax admission.
A multivariable Cox proportional hazards model was developed to assess the independent associations of sex, age group, spontaneous pneumothorax type, and calendar period of first spontaneous pneumothorax admission on the risk of a second emergency admission for spontaneous pneumothorax within specified follow-up time intervals: less than 30 days, 30 to 89 days, 90 days to 1 year, and 1 to 5 years. Thus, the hazard ratios (HRs) were allowed to vary between periods because each period was assessed separately. The assumption of proportionality within each period was checked visually using Nelson-Aalen plots. Statistical significance of the HRs was regarded as a 2-sided P value less than .05.
A potential source of bias was an ascertainment bias in cases in which the only documented chronic lung disease came from the recurrent spontaneous pneumothorax record. A sensitivity analysis was therefore undertaken removing these cases. All analyses were performed using Stata (StataCorp), version 14.1.
Results
From 1968 to 2016, there were 170 929 hospital admissions for spontaneous pneumothorax (median age, 44 years [interquartile range, 26-88]; 73.0% male). Based on the most contemporary linked national data set in England, the proportion of patients with spontaneous pneumothorax who had chronic lung disease (ie, those with a secondary spontaneous pneumothorax) was 60.8% (95% CI, 59.5%-62.0%). During the periods of HIPE (1968-1985, 10% national sample) and unlinked HES (1990-1998), there were 7106 and 47 795 eligible spontaneous pneumothorax admissions, respectively. During the period of record-linked HES (1999-2016), when multiple admissions per individual could be identified, there were 105 643 eligible spontaneous pneumothorax admissions pertaining to 78 155 patients. Of these admissions, 17 650 (16.7%) resulted in transfer to another hospital and 21 687 (20.5%) were nontransfer readmissions that were deemed recurrent spontaneous pneumothorax events. Figure 1 demonstrates for the record-linked HES period, 1999-2016, how these eligible patients and their records were derived from a wider pool of 169 997 patients with spontaneous pneumothorax. In the ORLS region period, 1968-2016, there were 10 385 eligible spontaneous pneumothorax admissions pertaining to 7837 patients.
Annual Incidence
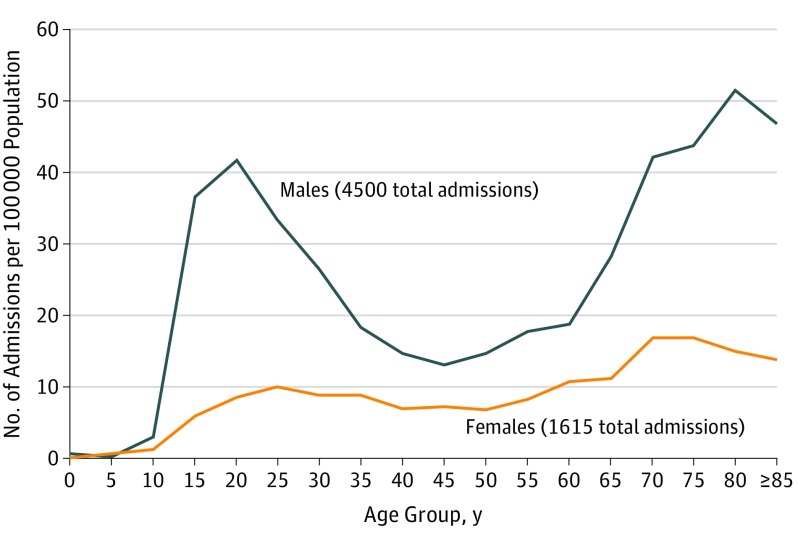
In 2016, there were 6372 eligible spontaneous pneumothorax admissions in England pertaining to 5245 patients 15 years and older in a corresponding total population of 45 340 600 individuals, giving an admission-based rate of 14.1 per 100 000 population (95% CI, 13.7-14.4) and a person-based rate of 11.6 per 100 000 population (95% CI, 11.3-11.9). This represented a significant increase compared with 1968 when the hospital admission rate was 9.1 per 100 000 population (95% CI, 8.1-10.1). Spontaneous pneumothorax admissions were more common among males than females (Figure 2): the admission-based rate per 100 000 population for males in 2016 was 20.8 (95% CI, 20.2-21.4) and for females was 7.6 (95% CI, 7.2-7.9); male:female ratio, 2.7. There was a bimodal age distribution with a first peak at age 15 to 34 years and an increasing incidence beyond 60 years in both males and females (Figure 2).
Figure 2. Spontaneous Pneumothorax Admissions by Age-Specific Population in England, 2015.
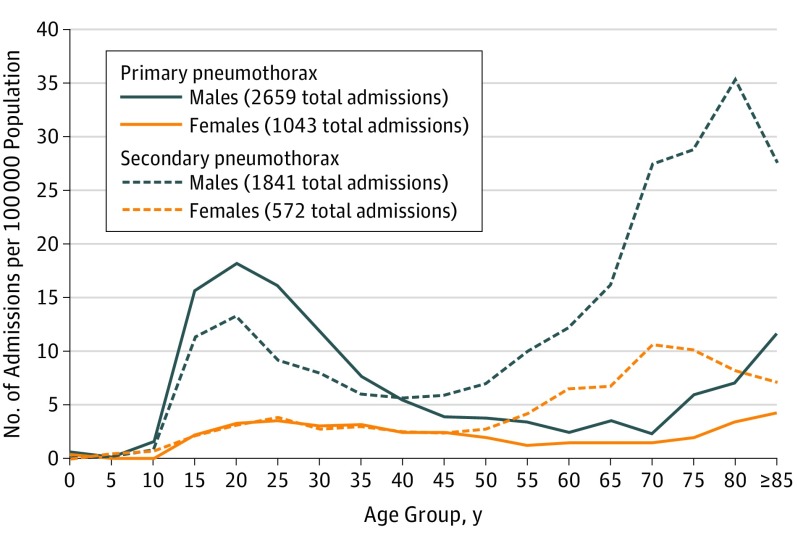
The majority of admissions were of patients with chronic lung disease (59.4% in males and 64.5% in females), but there was significant variation by age group in the proportion of spontaneous pneumothorax events that were identified as secondary (Table 1). Among those aged 15 to 34 years, primary spontaneous pneumothorax was significantly more common than secondary spontaneous pneumothorax in males (admission rates per 100 000 population: 15.5 [95% CI, 14.6-16.4] for primary spontaneous pneumothorax and 10.4 [95% CI, 9.7-11.2] for secondary spontaneous pneumothorax; z test for single proportion, P < .001), but not in females (3.1 [95% CI, 2.7-3.5] for primary spontaneous pneumothorax and 3.0 [95% CI, 2.6-3.4] for secondary spontaneous pneumothorax; z test for single proportion, P = .81). Among men and women aged 35 to 49 years, neither spontaneous pneumothorax type was significantly more common than the other (admission rates per 100 000 population for both sexes combined: primary spontaneous pneumothorax, 4.1 [95% CI, 3.7-4.5]; secondary spontaneous pneumothorax, 4.2 [95% CI, 3.9-4.6]; P = .69). Secondary spontaneous pneumothorax was significantly more common than primary spontaneous pneumothorax in both men and women 50 years and older (admission rates per 100 000 population for both sexes combined: primary spontaneous pneumothorax, 2.9 [95% CI, 2.7-3.2]; secondary spontaneous pneumothorax, 11.4 [95% CI, 10.9-11.8]; P < .001). Figure 3 shows the full age-sex distribution of the population-based rates for 2015 of primary spontaneous pneumothorax and secondary spontaneous pneumothorax separately. Figure 3 illustrates 2 peaks in the age-specific rates of primary spontaneous pneumothorax, the first between ages 15 to 34 years, the second after age 80 years. Further investigation of the latter age group revealed that, although respiratory comorbidities were not identified, other comorbidities were very common: 83% of these patients also had a diagnosis of cardiovascular disease (ICD10 I00-I99) coded at some point on their hospitalization records. Figure 3 also illustrates that the age-specific rates of secondary spontaneous pneumothorax became higher after age 50 years. The chronic lung diseases most commonly recorded in patients with secondary spontaneous pneumothorax in every age group were chronic obstructive pulmonary disease, asthma, or both (Table 2). Diagnoses of interstitial lung disease and malignancy were significantly more common in those 65 years and older than in the younger age groups combined (z test for independent proportions, P < .001).
Table 1. Spontaneous Pneumothorax Inpatient Admissions in England in 2015, and Rates Per 100 000 Population 15 Years and Older, by Age Group and Sex.
| Overall Spontaneous Pneumothorax | Primary Spontaneous Pneumothorax | Secondary Spontaneous Pneumothorax | Population in 2015 | ||||
| No. of Admissions | Rate (95% CI) | No. of Admissions | Rate (95% CI) | No. of Admissions | Rate (95% CI) | ||
| Male, by Age Group, y | |||||||
| 15-34 | 1872 | 25.9 (24.7-27.1) | 1118 | 15.5 (14.6-16.4) | 754 | 10.4 (9.7-11.2) | 7 228 300 |
| 35-49 | 625 | 11.5 (10.6-12.4) | 305 | 5.6 (5.0-6.3) | 320 | 5.9 (5.2-6.5) | 5 457 500 |
| 50-64 | 629 | 12.8 (11.8-13.8) | 161 | 3.3 (2.8-3.8) | 468 | 9.5 (8.7-10.4) | 4 927 600 |
| ≥65 | 1321 | 30.0 (28.4-31.7) | 220 | 5.0 (4.4-5.7) | 1101 | 25.0 (23.6-26.5) | 4 401 100 |
| Overall | 4447 | 20.2 (19.6-20.8) | 1804 | 8.2 (7.8-8.6) | 2643 | 12.0 (11.6-12.5) | 22 014 500 |
| Female, by Age Group, y | |||||||
| 15-34 | 427 | 6.0 (5.5-6.6) | 216 | 3.1 (2.7-3.5) | 211 | 3.0 (2.6-3.4) | 7 063 400 |
| 35-49 | 293 | 5.3 (4.7-5.9) | 148 | 2.7 (2.3-3.1) | 145 | 2.6 (2.2-3.1) | 5 539 200 |
| 50-64 | 298 | 5.9 (5.2-6.6) | 79 | 1.6 (1.2-1.9) | 219 | 4.3 (3.8-4.9) | 5 066 000 |
| ≥65 | 572 | 10.8 (9.9-11.7) | 121 | 2.3 (1.9-2.7) | 451 | 8.5 (7.7-9.3) | 5 310 200 |
| Overall | 1590 | 6.9 (6.6-7.3) | 564 | 2.5 (2.3-2.7) | 1026 | 4.5 (4.2-4.7) | 22 978 800 |
Figure 3. Primary and Secondary Spontaneous Pneumothorax Admissions by Age-Specific Population And Sex in England, 2015.
Table 2. Percentage Distribution of Comorbidities Among Secondary Spontaneous Pneumothorax Admissions, England 2015.
| Secondary Pneumothorax Admissions (% of Total)a | Distribution of Respiratory Comorbidities, %b | |||||||
|---|---|---|---|---|---|---|---|---|
| COPD/ Emphysema | Asthma | Interstitial Lung Disease | Malignancy | Tuberculosis | Sarcoidosis | Cystic Fibrosis | ||
| Male, by Age Group, y | ||||||||
| 15-34 | 754 (40.3) | 71.9 | 41.2 | 6.6 | 0.1 | 0.8 | 0.8 | 1.2 |
| 35-49 | 320 (51.2) | 74.1 | 40.6 | 10.0 | 3.1 | 0.6 | 2.2 | 0.9 |
| 50-64 | 468 (74.4) | 84.2 | 31.4 | 10.7 | 13.0 | 2.6 | 0.9 | 0.0 |
| ≥65 | 1101 (83.3) | 87.6 | 28.2 | 22.2 | 16.3 | 0.6 | 0.6 | 0.2 |
| Overall | 2643 (59.4) | 80.9 | 34.0 | 14.2 | 9.5 | 1.0 | 0.9 | 0.5 |
| Female, by Age Group, y | ||||||||
| 15-34 | 211 (49.4) | 53.6 | 55.0 | 6.6 | 1.9 | 2.8 | 0.0 | 0.9 |
| 35-49 | 145 (49.5) | 57.2 | 51.0 | 6.9 | 12.4 | 0.0 | 0.0 | 0.0 |
| 50-64 | 219 (73.5) | 84.5 | 35.6 | 9.6 | 17.8 | 0.0 | 0.5 | 0.0 |
| ≥65 | 451 (78.8) | 79.4 | 38.6 | 18.6 | 24.8 | 1.8 | 1.3 | 0.0 |
| Overall | 1026 (64.5) | 72.0 | 43.1 | 12.6 | 16.9 | 1.4 | 0.7 | 0.2 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
An admission was regarded as secondary spontaneous pneumothorax if the patient had a diagnosis code indicating chronic lung disease (defined as any 1 of the 73 International Classification of Diseases codes listed in eTable 2 in the Supplement) coded either on the same pneumothorax record or, using record linkage, on any known previous hospital record or any record within the next 12 mo.
Patients may have >1 respiratory comorbidity.
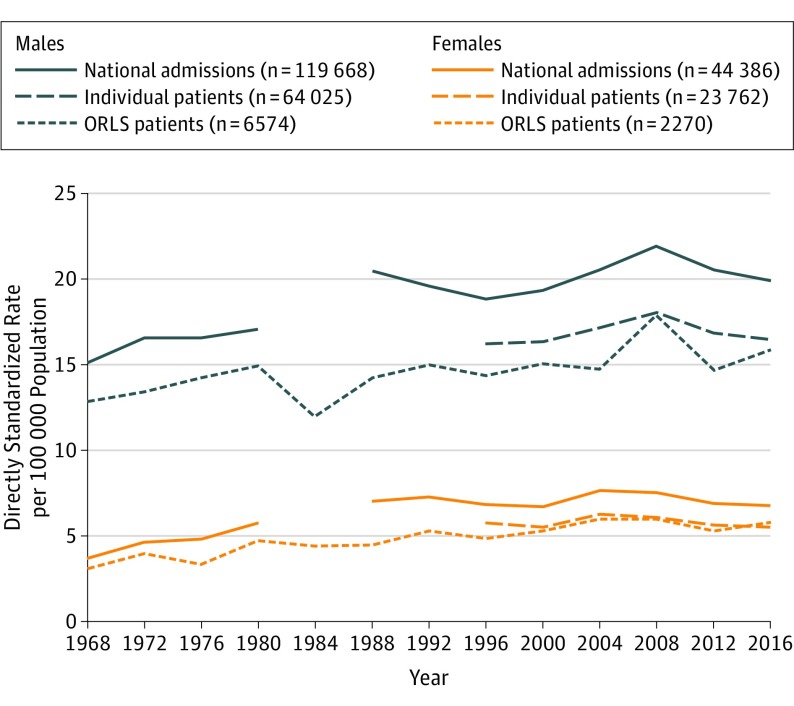
From 1968 to 2016, age-standardized, admission-based rates in England (Figure 4) increased significantly both for males (APC, 0.51 [95% CI, 0.29 to 0.73]; P < .001) and females (APC, 0.56 [95% CI, 0.23 to 0.89]; P < .001). Analysis of the record-linked ORLS data from 1968 to 2016, for which multiple admissions per person were excluded, demonstrated a small but significant increase in the annual rate of unique people admitted for spontaneous pneumothorax each year both in females (APC, 1.12 [95% CI, 0.79 to 1.45]; P < .001) and in males (APC, 0.44 [95% CI, 0.22 to 0.66]; P < .001) (Figure 4). eFigure 1 in the Supplement illustrates the change in age profile over the decades based on person-based rates in ORLS. In women, spontaneous pneumothorax became significantly more common since 1968 among age groups 50 to 64 years (APC, 0.93 [95% CI, 0.04 to 1.83]; P = .04) and 65 years and older (APC, 4.08 [95% CI, 3.33 to 4.82]; P < .001). In men, significant increases since 1968 were confined to ages 65 years and older (APC, 1.10 [95% CI, 0.66 to 1.54]; P < .001) (eFigure 1 in the Supplement). Analysis by age group of the shorter timeframe but higher-powered, linked national data from 1999-2016 (eFigure 2 in the Supplement) demonstrated that in more recent years, person-based rates increased significantly since 1999 for women aged 65 years and older (APC, 2.10 [95% CI, 1.58 to 2.62]; P < .001), but decreased significantly in females aged 15 to 34 years (APC, −1.98 [95% CI, −2.91 to −1.04]; P < .001). Among males, person-based rates did not change significantly in any broad age group since 1999 (eFigure 2 in the Supplement).
Figure 4. Age-Standardized Hospitalization Rates for Spontaneous Pneumothorax in England (National Data; Record-Linked From 1999 only) and ORLS (Regional Data; Record-Linked From 1968) by Sex.
ORLS indicates Oxford Record Linkage Study.
Data gaps were due to hospital statistics not being collected nationally between 1986 and 1989. England (People) starts at 1999 because record-linked analysis was only possible from 1999 onwards
Recurrence
Analysis of linked national data from 1999-2016 (Table 3) demonstrated the cumulative probability of inpatient-treated spontaneous pneumothorax recurrence within 7 days, 30 days, 90 days, 1 year, and 5 years, by age, sex and spontaneous pneumothorax type. The cumulative probability of recurrence within 1 year was 18.8% (95% CI, 18.4%-19.1%) for males and 19.5% (95% CI, 18.9%-20.1%) for females, and within 5 years was 25.5% (95% CI, 25.1%-25.9%) for males and 26.0% (95% CI, 25.3%-26.7%) for females. This probability of recurrence ranged between 10% to 33% at 1 year, and 13% to 39% at 5 years, depending on age group and presence of chronic lung disease. Those with the highest cumulative probability of recurrence over 5 years were males aged 15 to 34 years with chronic lung disease (39.2% [95% CI 37.7%-40.7%]) (Table 3).
Table 3. Cumulative Probability of Spontaneous Pneumothorax Recurrence Within 7 Days, 30 Days, 90 Days, 1 Year, and 5 Years, England.
| Age Group, y | Patients with index pneumothorax, No. | Time Interval for Spontaneous Pneumothorax Recurrence, Probability (95% CI), % | Patients With 5 Years Follow-Up, No. (% of Total) a | |||||
|---|---|---|---|---|---|---|---|---|
| <7 db | <30 d | <90 d | <1 y | <5 y | ||||
| Male | ||||||||
| Primary spontaneous pneumothorax | ||||||||
| 15-34 | 17562 | 4.0 (3.7-4.3) | 7.4 (7.0-7.8) | 9.7 (9.3-10.1) | 13.7 (13.2-14.2) | 22.0 (21.3-22.6) | 13 327 (75.9) | |
| 35-49 | 5108 | 4.2 (3.7-4.8) | 7.5 (6.8-8.2) | 9.2 (8.5-10.0) | 15.9 (15.1-16.8) | 17.3 (16.3-18.4) | 3990 (78.1) | |
| 50-64 | 2983 | 4.7 (4-5.5.0) | 8.8 (7.8-9.9) | 11.3 (10.2-12.5) | 13.7 (12.5-15.0) | 18.7 (17.3-20.2) | 2335 (78.3) | |
| ≥65 | 3589 | 3.7 (3.1-4.4) | 8.0 (7.2-8.9) | 11.3 (10.3-12.4) | 14.2 (13.1-15.4) | 19.6 (18.2-21.1) | 2735 (76.2) | |
| Overall | 29242 | 4.1 (3.8-4.3) | 7.6 (7.3-7.9) | 10.0 (9.6-10.3) | 13.4 (13.0-13.8) | 20.6 (20.1-21.1) | 22 387 (76.6) | |
| Secondary spontaneous pneumothorax | ||||||||
| 15-34 | 4543 | 7.0 (6.3-7.7) | 15.9 (14.9-17.0) | 22.5 (21.3-23.8) | 32.9 (31.5-34.3) | 39.2 (37.7-40.7) | 2881 (63.4) | |
| 35-49 | 2018 | 7.5 (6.4-8.7) | 14.8 (13.4-16.5) | 20.9 (19.2-22.8) | 27.5 (25.6-29.6) | 32.9 (30.8-35.1) | 1251 (62.0) | |
| 50-64 | 3823 | 7.8 (7.0-8.7) | 15.7 (14.5-16.9) | 21.7 (20.5-23.1) | 27.8 (26.3-29.3) | 33.8 (32.2-35.5) | 2599 (68.0) | |
| ≥65 | 8856 | 5.8 (5.4-6.3) | 12.6 (11.9-13.3) | 17.7 (16.9-18.6) | 24.1 (23.2-25.1) | 29.8 (28.6-31.0) | 5721 (64.6) | |
| Overall | 19240 | 6.7 (6.3-7.0) | 14.2 (13.7-14.7) | 20.0 (19.5-20.6) | 27.4 (26.8-28.1) | 33.4 (32.6-34.1) | 12 452 (64.7) | |
| Female | ||||||||
| Primary spontaneous pneumothorax | ||||||||
| 15-34 | 4223 | 4.7 (4.1-5.4) | 9.3 (8.5-10.2) | 12.4 (11.5-13.5) | 20.9 (0.6-15.6) | 25.1 (23.8-26.5) | 3396 (80.4) | |
| 35-49 | 2380 | 5.6 (4.7-6.6) | 9.8 (8.7-11.1) | 12.1 (10.8-13.5) | 16.6 (15.1-18.1) | 26.9 (25.4-28.4) | 1867 (78.4) | |
| 50-64 | 1216 | 4.7 (3.7-6.1) | 9.2 (7.7-11.0) | 10.9 (9.2-12.8) | 18.7 (17.3-20.2) | 22.6 (20.9-24.4) | 935 (76.9) | |
| ≥65 | 1637 | 2.4 (1.8-3.3) | 5.3 (4.3-6.5) | 7.6 (6.4-9.0) | 9.5 (8.2-11.1) | 13.3 (11.6-15.3) | 1189 (72.6) | |
| Overall | 9456 | 4.5 (4.1-5.0) | 8.7 (8.2-9.3) | 11.3 (10.7-12) | 14.9 (14.2-15.7) | 21.6 (20.7-22.4) | 7387 (78.1) | |
| Secondary spontaneous pneumothorax | ||||||||
| 15-34 | 1552 | 6.2 (5.1-7.6) | 16.1 (14.4-18.1) | 24.1 (22.0-26.3) | 32.3 (30.0-34.7) | 38.9 (36.4-41.5) | 1069 (68.9) | |
| 35-49 | 1303 | 7.7 (6.3-9.2) | 15.0 (13.2-17.1) | 21.1 (19.0-23.4) | 28.7 (26.2-31.3) | 35.1 (32.4-37.9) | 894 (68.6) | |
| 50-64 | 1815 | 6.2 (5.1-7.4) | 12.5 (11.0-14.1) | 17.7 (16.0-19.6) | 23.2 (21.3-25.3) | 29.3 (27.0-31.7) | 1173 (64.6) | |
| ≥65 | 3676 | 4.4 (3.8-5.1) | 10.1 (9.2-11.1) | 15.4 (14.3-16.7) | 21.1 (19.7-22.5) | 27.3 (25.5-29.2) | 2282 (62.1) | |
| Overall | 8346 | 5.6 (5.1-6.1) | 12.5 (11.8-13.3) | 18.5 (17.6-19.3) | 24.9 (24.0-25.9) | 31.3 (30.2-32.5) | 5418 (64.9) | |
Patients whose index pneumothorax record occurred >5 y before the study end date and who did not die during follow-up.
The 7-d recurrence may include subsequent readmissions in patients with incomplete resolution of the index pneumothorax (rather than a true new recurrent event), but it is included to illustrate the probability of readmission across all time points.
The Cox proportional hazards model (Table 4) quantifies the independent association of each patient characteristic with recurrence risk at each stage of follow-up. Visual inspection of the Nelson-Aalen plots demonstrated that the assumption of proportionality within each period was met. There was a significant interaction between secondary spontaneous pneumothorax and age (hence adjusted hazard ratios [aHRs] displayed by age group). The model demonstrated that, after adjustment for sex and calendar period of index admission, the presence of chronic lung disease was significantly associated with a higher probability of recurrence at nearly every stage of follow-up. In every age group, a chronic lung condition was significantly associated with a higher probability of recurrence within 1 year (P < .001) (Table 4). The association was greatest in younger patients: for example, in the 15 to 34 years age group, the aHRs (comparing secondary with primary spontaneous pneumothorax) were 2.10 (95% CI, 1.94-2.28) within 0 to 29 days, 3.23 (95% CI, 2.83-3.67) from 30 to 89 days, and 3.00 (95% CI, 2.69-3.34) for 90 days to 1 year.
Table 4. Adjusted Hazard Ratios (aHRs) for First Spontaneous Pneumothorax Recurrence by Sex, Spontaneous Pneumothorax Type, Age Group, and Calendar Period, Split by Time Since Index Pneumothorax Eventa.
| Time Since Index Spontaneous Pneumothorax Event, aHR (95% CI) | ||||
|---|---|---|---|---|
| <30 d | 30-89 d | 90 d-1 y | 1-5 y | |
| Female:male ratio | 0.98 (0.93-1.03) | 1.06 (0.97-1.15) | 0.98 (0.90-1.06) | 1.02 (0.94-1.10) |
| Primary:secondary spontaneous pneumothorax ratio | ||||
| Age group, y | ||||
| 15-34 | 2.10 (1.94-2.28) | 3.23 (2.83-3.67) | 3.00 (2.69-3.34) | 0.99 (0.88-1.12) |
| 35-49 | 1.86 (1.65-2.09) | 3.49 (2.81-4.34) | 2.82 (2.33-3.41) | 1.18 (0.98-1.44) |
| 50-64 | 1.67 (1.47-1.88) | 2.78 (2.21-3.50) | 3.28 (2.57-4.19) | 1.54 (1.26-1.90) |
| ≥65 | 1.67 (1.49-1.88) | 1.88 (1.57-2.26) | 2.66 (2.18-3.25) | 1.31 (1.08-1.59) |
| Age group:≥65 y group ratiob | ||||
| Primary spontaneous pneumothorax | ||||
| 15-34 | 1.09 (0.97-1.22) | 0.83 (0.69-0.99) | 1.57 (1.29-1.90) | 1.79 (1.52-2.11) |
| 35-49 | 1.16 (1.02-1.32) | 0.65 (0.52-0.82) | 1.13 (0.90-1.42) | 1.24 (1.03-1.50) |
| 50-64 | 1.26 (1.09-1.46) | 0.78 (0.60-1.01) | 0.81 (0.61-1.07) | 0.97 (0.78-1.21) |
| Secondary spontaneous pneumothorax | ||||
| 15-34 | 1.37 (1.26-1.49) | 1.42 (1.25-1.61) | 1.76 (1.57-1.98) | 1.35 (1.15-1.58) |
| 35-49 | 1.29 (1.16-1.43) | 1.21 (1.03-1.42) | 1.20 (1.02-1.40) | 1.12 (0.92-1.37) |
| 50-64 | 1.26 (1.15-1.37) | 1.16 (1.01-1.33) | 1.00 (0.86-1.15) | 1.14 (0.95-1.36) |
| 2008-2016:1999-2007 ratio | 1.08 (1.03-1.13) | 0.95 (0.88-1.03) | 0.92 (0.85-0.99) | 0.99 (0.93-1.07) |
The Cox proportional hazards model contained the following terms: sex, pneumothorax type, age group, pneumothorax type × age group (interaction), calendar period.
The reference category for all age groups is ≥65 years.
Younger age was associated with a significantly higher risk of recurrence independently of sex, spontaneous pneumothorax type, and calendar period of index admission (Table 4). Individuals who had their first (index) spontaneous pneumothorax admission after 2008 were more likely to have a recurrent event within the first 30 days of follow-up than those who had their first admission before 2008. However, in this model, effect size was small (aHR, 1.08 [95% CI, 1.03-1.13]) and the risk of recurrence beyond 30 days did not appear to vary by calendar period. Sex was not associated with increased risk of recurrence at any period of follow-up (Table 4).
A potential source of bias was an ascertainment bias for cases in which the only documented chronic lung disease came from the second spontaneous pneumothorax record. A sensitivity analysis was therefore undertaken removing these cases. This led to an attenuation of the apparent association between chronic lung disease and recurrence risk, but the margin of change was small and the pattern of significant associations in younger people with secondary spontaneous pneumothorax persisted; for example, the overall HR of recurrence within 5 years of follow-up, comparing secondary with primary spontaneous pneumothorax in patients aged 15 to 34 years, dropped from 2.0 (95% CI, 1.9-2.1) in the main analysis to 1.8 (95% CI, 1.7-1.9) in the sensitivity analysis.
The exclusion of readmissions that occurred within 7 or 30 days led to a reduction in overall cumulative recurrence at 1 and 5 years; for example, in males, cumulative recurrence at 5 years dropped from 20.6% (95% CI, 20.1%-21.1%) to 17.4% (95% CI, 16.9%-17.8%) when subsequent spontaneous pneumothorax diagnoses within 7 days were excluded, and to 14.2 (95% CI, 13.8-14.6) when diagnoses within 30 days were excluded (eTables 3A and 3B in the Supplement).
Discussion
Among a cohort of patients in England, age-standardized hospital admission rates for spontaneous pneumothorax increased over the period 1968-2016 for both males and females overall. This was, in part, attributable to an increase in multiple admissions in the same individuals. Chronic lung disease was associated with a significantly increased probability of spontaneous pneumothorax recurrence across all age groups. Sex was not significantly associated with spontaneous pneumothorax recurrence despite higher overall incidence of the disease in males.
The measures of admission-based rates (ie, in which multiple admissions for spontaneous pneumothorax per person per year were included in the counts) are useful because these are the commonest available measures in hospital admission statistics in many countries, against which these data can be compared, and provide information on the hospital “burden of care” for spontaneous pneumothorax. Data from the record-linked data sets suggest that the annual person-based rates (ie, in which only 1 spontaneous pneumothorax admission per person per year were included in the counts) have remained steady overall, but these are also significantly higher in recent years than they were in previous decades for women older than 50 years and men older than 65 years. The regional ORLS record-linked data set from 1968 demonstrates that the increase in spontaneous pneumothorax admissions seen nationally held true in the ORLS region even after admission counts were restricted to 1 per person per year. From this it may be deduced that the rate increase seen nationally was not solely due to an increase in multiple admissions per year in the same individuals. Females aged 15 to 34 years were the only subgroup to experience a significant decrease in person-based rates in recent years (since 1999). The contemporary admission-based rates and sex ratios are consistent with previous smaller epidemiological studies from the United Kingdom (male:female ratio, 2.9)3 and France (male:female ratio, 3.3).4
The bimodal nature of the age distribution for spontaneous pneumothorax has again been demonstrated in this data set. Although described previously4,5,13 this data shows on a large scale that the first peak (age 15-34 years) predominantly comprises those with no known lung disease (primary) with the latter peak mainly due to those with secondary spontaneous pneumothorax. That the ratio of primary:secondary spontaneous pneumothorax was higher in those older than 80 years than in the less elderly (Figure 3) could potentially be attributed to a survivor effect, as those with chronic lung disease are known to die younger,14 or may be explained by chronic respiratory conditions being less-reliably recorded in the very elderly for whom there is often multimorbidity, potentially leading to greater misclassification.
Previous data on recurrence risk for spontaneous pneumothorax are poorly defined for specific age groups, by sex and spontaneous pneumothorax type. Numerous small and mostly retrospective case series report a recurrence risk (ie, number of recurrences divided by the total population with spontaneous pneumothorax) from 21% to 54% at 1 and 2 years.5,6,7,8,9,10,11 Data from control or noninterventional cohorts of randomized controlled trials show a range of risk of recurrence from 21% to 49%.15,16,17,18,19,20
This population-based data, using cumulative failure analysis with longer follow-up and much larger numbers of cases than previous studies, found no difference by sex. This was unexpected given the marked difference in the respective rates of first occurrence per 100 000 sex-specific population. For both males and females, around a quarter of patients with spontaneous pneumothorax may experience an inpatient-treated recurrence within 5 years, but around three-quarters of these will occur within the first year. However, the probability of recurrence varies markedly by age group and is increased significantly by the presence of chronic lung disease.
Previous small and retrospective studies of spontaneous pneumothorax have suggested a higher rate of recurrence in patients with preexisting lung disease compared with those without.8,21 This study confirms the increased risk, and, for the first time, quantifies the varying risks of recurrence among secondary spontaneous pneumothorax patients across age groups. The relatively high probability of recurrence in younger patients with chronic lung disease may be a marker of disease severity of differing etiology. For example, cystic fibrosis is known to be a risk factor for recurrent spontaneous pneumothorax, which will affect younger populations.22 Longitudinal studies of spontaneous pneumothorax recurrence by comorbid disease type would help further quantify these relationships.
Analysis of recurrence by calendar year indicates that recurrence within the first 30 days has become more common in recent years, even after adjustment for age, sex, and spontaneous pneumothorax type. The potential statistical noise of increased multiple admissions for the same spontaneous pneumothorax event was minimized by excluding hospital transfers, by defining recurrence as a subsequent admission in which spontaneous pneumothorax was recorded as the principal diagnosis, and by restricting to emergency admissions only. The increased risk of recurrence within 30 days in more recent years may be a real effect, reflecting a change in clinical practice. For example, if physicians are treating an increasing proportion of patients conservatively, or with needle aspiration with shorter hospital stay (rather than chest drain and prolonged admission), this might lead to a greater recurrence rate in the first 30 days.
Current UK guidelines suggest that all patients older than 50 years who smoke should be treated as a secondary spontaneous pneumothorax.23 Although this cutoff was relatively arbitrarily chosen, data presented in this study suggest that this age limit coincides with the nadir between the 2 bimodal peaks, and captures the majority of patients with secondary spontaneous pneumothorax. However, this data shows that there is overlap of the age ranges for primary and secondary spontaneous pneumothorax, and there is an additional peak (albeit lower) of secondary spontaneous pneumothorax in patients younger than 50 years. The risk of recurrence appears highest among this group, and it is therefore important to consider the evidence of underlying lung disease in younger populations when counselling on risk of recurrence. Although the etiology of primary spontaneous pneumothorax still remains largely unknown, as imaging and diagnostic techniques improve, there is increasing evidence of underlying lung abnormalities (ie, blebs and emphysema-like change) in patients traditionally labeled as having primary spontaneous pneumothorax.24 The most common respiratory comorbidity among younger patients was chronic obstructive pulmonary disease or emphysema, which could represent identification of these abnormalities, thereby coded on their pneumothorax episode. In the future, the clinical distinction between primary and secondary spontaneous pneumothorax may become less important in favor of risk-stratifying all patients based on specific comorbidities, likely outcomes and potential management options. Further epidemiological and prospective clinical studies are required to convert these findings into clinical practice.
This analysis of all hospitalizations for spontaneous pneumothorax in England represents the largest study in the published literature to our knowledge, with national estimates of incidence and recurrence by age group, sex, and presence or absence of chronic lung disease, set in a historical context. This study compared 2 long-standing data sets, including an English national population of 52 million. The record-linked ORLS data set (1968-2016) and the record-linked era of the national data set (1999-2016) allow follow-up of individual patients to assess recurrence and its relationship to admission-based and person-based incidence.
Limitations
This study has several limitations. First, the data only provide information on patients admitted to the hospital. Patients who may be managed in the community or conservatively without admission and patients admitted to hospitals outside the study region are not included. In the recurrence analysis, this limitation applies both to the index spontaneous pneumothorax (ie, first-known spontaneous pneumothorax hospital record, which may not necessarily represent the patient’s first-ever spontaneous pneumothorax), as well as to the first-known recurrence. Second, migration data was not available. This data may therefore underestimate the true incidence and recurrence of spontaneous pneumothorax.
Third, because only the years 1999 to 2016 were linked in the English national dataset (as explained in the Methods section), it was not possible to determine the exact number of unique individuals to whom the admissions for spontaneous pneumothorax that occurred prior to 1999 pertain. Fourth, early recurrence events (which were excluded in sensitivity analyses) could potentially represent readmissions related to an ongoing pneumothorax, rather than a new episode. However, although continuation of a first episode requiring readmission may account for some proportion of readmissions in the main analysis, that proportion is likely to be relatively low for 3 main reasons: (1) a patient with spontaneous pneumothorax is unlikely to be discharged from the hospital after a first spontaneous pneumothorax without stabilization, (2) the threshold for any emergency (re)admission to the hospital is relatively high and would signify a new event from the perspectives of both the patient and the hospital, and (3) the principal diagnosis on the readmission record was not some complication from spontaneous pneumothorax but spontaneous pneumothorax itself.
Fifth, the analysis is reliant on the accuracy of the coding for each hospital episode; and spontaneous pneumothorax is covered by relatively few ICD codes. Other diagnoses listed alongside spontaneous pneumothorax may not always be reliably recorded, making it harder to distinguish particular spontaneous pneumothorax subtypes. For example, a proportion of spontaneous pneumothorax events in women of child-bearing age captured in these data sets could be catamenial pneumothorax (due to thoracic endometriosis), an underreported phenomenon25,26 for which there is no specific ICD10 code. Although the primary diagnosis (ie, the principal reason for admission to the hospital) has been well recorded over time, the reliability and consistency of recording secondary or subsidiary diagnosis codes is less clear. The benefit of record linkage is that secondary spontaneous pneumothorax could be identified from chronic lung disease coded on either the spontaneous pneumothorax record, or any known previous hospital record, or up to 12 months afterwards. This method was chosen to capture patients with secondary spontaneous pneumothorax whose coding may have been incomplete on their index spontaneous pneumothorax episode, but who did in fact have an underlying lung disease. The HES database does not contain patient identifiable data and is not linked to other data sets. Therefore, direct validation of the accuracy of comorbidity coding is not possible. Furthermore, indirect validation would be problematic, especially for the early years, because there is no (current or historical) population-based registry or gold-standard against which to compare. Spontaneous pneumothorax recurrence risk may be related to smoking and body mass index. HES data sets do not include these data to allow assessment in this model.
Conclusions
This study provides contemporary information regarding the trends in inpatient-treated incidence and recurrence of spontaneous pneumothorax.
eFigure 1. Person-Based Hospitalization Rates for Pneumothorax per 100 000 Population by Age Group in ORLS, 1968-2016
eFigure 2. Person-Based Hospitalization Rates for Pneumothorax per 100 000 Population by Age Group in England, 1999-2016
eTable 1. Summary of Previous Epidemiological Studies of Spontaneous Pneumothorax
eTable 2. List of ICD-10 Codes for Chronic Lung Disease Used to Define Secondary Spontaneous Pneumothorax
eTable 3A. Sensitivity Analysis: Probability of Spontaneous Pneumothorax Recurrence Within 30 Days, 90 Days, 1 Year and 5 Years—Excluding Readmission <7 Days
eTable 3B. Sensitivity Analysis: Probability of Spontaneous Pneumothorax Recurrence Within 30 Days, 90 Days, 1 Year and 5 Years—Excluding Readmission <30 Days
References
- 1.Melton LJ III, Hepper NGG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120(6):1379-1382. [DOI] [PubMed] [Google Scholar]
- 2.Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest. 1987;92(6):1009-1012. doi: 10.1378/chest.92.6.1009 [DOI] [PubMed] [Google Scholar]
- 3.Gupta D, Hansell A, Nichols T, Duong T, Ayres JG, Strachan D. Epidemiology of pneumothorax in England. Thorax. 2000;55(8):666-671. doi: 10.1136/thorax.55.8.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobbio A, Dechartres A, Bouam S, et al. Epidemiology of spontaneous pneumothorax: gender-related differences. Thorax. 2015;70(7):653-658. doi: 10.1136/thoraxjnl-2014-206577 [DOI] [PubMed] [Google Scholar]
- 5.Ruckley CV, McCormack RJM. The management of spontaneous pneumothorax. Thorax. 1966;21(2):139-144. doi: 10.1136/thx.21.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seremetis MG. The management of spontaneous pneumothorax. Chest. 1970;57(1):65-68. doi: 10.1378/chest.57.1.65 [DOI] [PubMed] [Google Scholar]
- 7.O’Rourke JP, Yee ES. Civilian spontaneous pneumothorax: treatment options and long-term results. Chest. 1989;96(6):1302-1306. doi: 10.1378/chest.96.6.1302 [DOI] [PubMed] [Google Scholar]
- 8.Lippert HL, Lund O, Blegvad S, Larsen HV. Independent risk factors for cumulative recurrence rate after first spontaneous pneumothorax. Eur Respir J. 1991;4(3):324-331. [PubMed] [Google Scholar]
- 9.Alfageme I, Moreno L, Huertas C, Vargas A, Hernandez J, Beiztegui A. Spontaneous pneumothorax: long-term results with tetracycline pleurodesis. Chest. 1994;106(2):347-350. doi: 10.1378/chest.106.2.347 [DOI] [PubMed] [Google Scholar]
- 10.Sadikot RT, Greene T, Meadows K, Arnold AG. Recurrence of primary spontaneous pneumothorax. Thorax. 1997;52(9):805-809. doi: 10.1136/thx.52.9.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olesen WH, Lindahl-Jacobsen R, Katballe N, et al. Recurrent Primary Spontaneous Pneumothorax is Common Following Chest Tube and Conservative Treatment. World J Surg. 2016;40(9):2163-2170. doi: 10.1007/s00268-016-3508-z [DOI] [PubMed] [Google Scholar]
- 12.Holland J, Hall N, Yeates DG, Goldacre M. Trends in hospital admission rates for anorexia nervosa in Oxford (1968-2011) and England (1990-2011): database studies. J R Soc Med. 2016;109(2):59-66. doi: 10.1177/0141076815617651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Primrose WR. Spontaneous pneumothorax: a retrospective review of aetiology, pathogenesis and management. Scott Med J. 1984;29(1):15-20. doi: 10.1177/003693308402900105 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. World Health Statistics 2011. http://www.who.int/whosis/whostat/2011/en/. Accessed July 5, 2017.
- 15.Light RW, O’Hara VS, Moritz TE, et al. Intrapleural tetracycline for the prevention of recurrent spontaneous pneumothorax: results of a Department of Veterans Affairs cooperative study. JAMA. 1990;264(17):2224-2230. doi: 10.1001/jama.1990.03450170072025 [DOI] [PubMed] [Google Scholar]
- 16.Harvey J, Prescott RJ; British Thoracic Society Research Committee . Simple aspiration versus intercostal tube drainage for spontaneous pneumothorax in patients with normal lungs. BMJ. 1994;309(6965):1338-1339. doi: 10.1136/bmj.309.6965.1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrivet P, Djedaini K, Teboul JL, Brochard L, Dreyfuss D. Spontaneous pneumothorax. Comparison of thoracic drainage vs immediate or delayed needle aspiration. Chest. 1995;108(2):335-339. doi: 10.1378/chest.108.2.335 [DOI] [PubMed] [Google Scholar]
- 18.Noppen M, Alexander P, Driesen P, Slabbynck H, Verstraeten A. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. Am J Respir Crit Care Med. 2002;165(9):1240-1244. doi: 10.1164/rccm.200111-078OC [DOI] [PubMed] [Google Scholar]
- 19.Ayed AK, Chandrasekaran C, Sukumar M. Aspiration versus tube drainage in primary spontaneous pneumothorax: a randomised study. Eur Respir J. 2006;27(3):477-482. doi: 10.1183/09031936.06.00091505 [DOI] [PubMed] [Google Scholar]
- 20.Chen JS, Chan WK, Tsai KT, et al. Simple aspiration and drainage and intrapleural minocycline pleurodesis versus simple aspiration and drainage for the initial treatment of primary spontaneous pneumothorax: an open-label, parallel-group, prospective, randomised, controlled trial. Lancet. 2013;381(9874):1277-1282. doi: 10.1016/S0140-6736(12)62170-9 [DOI] [PubMed] [Google Scholar]
- 21.Sousa C, Neves J, Sa N, Goncalves F, Oliveira J, Reis E. Spontaneous pneumothorax: a 5-year experience. J Clin Med Res. 2011;3(3):111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest. 2005;128(2):720-728. doi: 10.1378/chest.128.2.720 [DOI] [PubMed] [Google Scholar]
- 23.MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group . Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii18-ii31. doi: 10.1136/thx.2010.136986 [DOI] [PubMed] [Google Scholar]
- 24.Bintcliffe OJ, Hallifax RJ, Edey A, et al. Spontaneous pneumothorax: time to rethink management? Lancet Respir Med. 2015;3(7):578-588. doi: 10.1016/S2213-2600(15)00220-9 [DOI] [PubMed] [Google Scholar]
- 25.Visouli AN, Zarogoulidis K, Kougioumtzi I, et al. Catamenial pneumothorax. J Thorac Dis. 2014;6(suppl 4):S448-S460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legras A, Mansuet-Lupo A, Rousset-Jablonski C, et al. Pneumothorax in women of child-bearing age: an update classification based on clinical and pathologic findings. Chest. 2014;145(2):354-360. doi: 10.1378/chest.13-1284 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Person-Based Hospitalization Rates for Pneumothorax per 100 000 Population by Age Group in ORLS, 1968-2016
eFigure 2. Person-Based Hospitalization Rates for Pneumothorax per 100 000 Population by Age Group in England, 1999-2016
eTable 1. Summary of Previous Epidemiological Studies of Spontaneous Pneumothorax
eTable 2. List of ICD-10 Codes for Chronic Lung Disease Used to Define Secondary Spontaneous Pneumothorax
eTable 3A. Sensitivity Analysis: Probability of Spontaneous Pneumothorax Recurrence Within 30 Days, 90 Days, 1 Year and 5 Years—Excluding Readmission <7 Days
eTable 3B. Sensitivity Analysis: Probability of Spontaneous Pneumothorax Recurrence Within 30 Days, 90 Days, 1 Year and 5 Years—Excluding Readmission <30 Days