This randomized clinical trial investigates if peer comparison letters targeting high-volume primary care prescribers of quetiapine meaningfully reduce their prescribing and examines quetiapine receipt by patients with low-value or guideline-concordant indications for therapy.
Key Points
Question
Can behavioral nudges reduce inappropriate prescribing of antipsychotic agents and raise clinical quality for older and disabled patients, who often receive these drugs?
Findings
In this randomized clinical trial, a peer comparison letter randomized across the 5055 highest Medicare prescribers of the antipsychotic quetiapine fumarate reduced prescribing for at least 2 years. Effects were larger than those observed in existing large-scale behavioral interventions, potentially because of the content of the peer comparison letter, which mentioned the potential for a review of prescribing activity.
Meaning
Behavioral nudge interventions can raise the quality of prescribing, but research is still needed on how to most precisely target unsafe prescribing behavior.
Abstract
Importance
Antipsychotic agents, such as quetiapine fumarate, are frequently overprescribed for indications not supported by clinical evidence, potentially causing harm.
Objective
To investigate if peer comparison letters targeting high-volume primary care prescribers of quetiapine meaningfully reduce their prescribing.
Design, Setting, and Participants
Randomized clinical trial (intent to treat) conducted from 2015 to 2017 of prescribers and their patients nationwide in the Medicare program. The trial targeted the 5055 highest-volume primary care prescribers of quetiapine in 2013 and 2014 (approximately 5% of all primary care prescribers of quetiapine).
Interventions
Prescribers were randomized (1:1 ratio) to receive a placebo letter or 3 peer comparison letters stating that their quetiapine prescribing was high relative to their peers and was under review by Medicare.
Main Outcomes and Measures
The primary outcome was the total quetiapine days supplied by prescribers from the intervention start to 9 months. Secondary outcomes included quetiapine receipt from all prescribers by baseline patients, quetiapine receipt by patients with low-value or guideline-concordant indications for therapy, mortality, and hospital use. In exploratory analyses, the study followed outcomes to 2 years.
Results
Of the 5055 prescribers, 231 (4.6%) were general practitioners, 2428 (48.0%) were in family medicine, and 2396 (47.4%) were in internal medicine; 4155 (82.2%) were male. All were included in the analyses. Over 9 months, the treatment arm supplied 11.1% fewer quetiapine days per prescriber vs the control arm (2456 vs 2864 days; percentage difference, 11.1% fewer days; 95% CI, −13.1% to −9.2% days; P < .001; adjusted difference, −319 days; 95% CI, −374 to −263 days; P < .001), which persisted through 2 years (15.6% fewer days; 95% CI, −18.1% to −13.0%; P < .001). At the patient level, individuals in the treatment arm received 3.9% (95% CI, −5.0% to −2.9%; P < .001) fewer days of quetiapine from all prescribers over 9 months, with a larger decrease among patients with low-value vs guideline-concordant indications (−5.9% [95% CI, −8.0% to −3.9%] vs −2.4% [95% CI, −4.0% to −0.9%], P = .01 for test that effects were equal for both patient groups). There was no evidence of substitution to other antipsychotics, and 9-month mortality and hospital use were similar between the treatment vs control arms.
Conclusions and Relevance
Peer comparison letters caused substantial and durable reductions in quetiapine prescribing, with no evidence of negative effects on patients.
Trial Registration
ClinicalTrials.gov identifier: NCT02467933
Introduction
Every year, millions of older adults are prescribed atypical antipsychotic agents for off-label use beyond the indications approved by the US Food and Drug Administration (FDA), which are limited to schizophrenia, bipolar disorder, and some cases of depression.1 Off-label prescribing to older adults for other indications, such as behavioral symptoms in dementia, anxiety, and insomnia, has continued2,3,4 despite a large body of evidence that the use of atypical antipsychotics is associated with significant harm in these populations.5,6,7,8,9 These harms include a host of adverse outcomes, such as increased risk of death, cognitive decline, extrapyramidal symptoms, and sedation.7,10,11,12
This evidence has contributed to a broad consensus among psychiatric experts that excessive off-label use of antipsychotic medications in older adults, particularly those with dementia, is a serious problem. Multiple Choosing Wisely recommendations from the American Psychiatric Association target off-label use of antipsychotics.13 The FDA has warned against the use of antipsychotics for the treatment of elderly individuals with dementia.14 The American Geriatrics Society recommends that these drugs be used only when other interventions have failed and the patient threatens self-harm or harm to others.15
Quetiapine fumarate is an atypical antipsychotic that is prescribed at a particularly high frequency for off-label use. In the United States, 2.8 million patients fill a prescription for quetiapine annually,16 but as much as 75% of quetiapine prescribing lacks a basis in clinical evidence, making it an attractive target for interventions to reduce off-label prescribing.17
The widespread off-label use of antipsychotics in spite of clear guidelines has attracted the attention of the Centers for Medicare & Medicaid Services (CMS) and federal oversight agencies.2,18 However, there is a gap between the need to curb antipsychotic overprescribing and the evidence base of effective interventions to change prescriber behavior. One existing approach focuses on changing health care professionals’ beliefs about the clinical benefits of prescribing; this intensive education can raise the quality of psychiatric medication prescribing.19,20 Another set of techniques based on behavioral economics involves harnessing peer comparison messaging to nudge physicians to change behavior without financial incentives.21,22,23,24,25,26 Yet, there is limited evidence on bringing health care professional education or behavioral nudges to a national scale. To our knowledge, no large-scale randomized behavioral interventions have targeted antipsychotic prescribing.
We performed a randomized clinical trial (intent to treat) of peer comparison letters to high quetiapine-prescribing primary care physicians with the goal of reducing excessive prescribing to Medicare program beneficiaries. Because peer comparison letters are inexpensive and easily scaled, they could be a powerful approach to improve the safety of antipsychotic prescribing.
Methods
Study Design and Participants
This study used a placebo-controlled, parallel-group design with balanced randomization (1:1 ratio) to the control arm (placebo letter) and treatment arm (peer comparison letter). The study was overseen by an interdisciplinary team at CMS and the US Office of Evaluation Sciences (Washington, DC), as well as institutional review boards at Columbia University (New York, New York), Harvard University (Boston, Massachusetts), and the Massachusetts Institute of Technology (Cambridge). The institutional review boards each waived informed consent for prescribers. The trial protocol can be found in Supplement 1.
Study participants were primary care practitioners (PCPs) or prescribers chosen by a CMS analysis of quetiapine prescribing in Medicare Part D (prescription drug coverage) in 2013 and 2014. We chose PCPs (prescribers with a specialty of general practice, family medicine, or internal medicine) because the lack of psychiatric specialization suggested less formal training in prescribing of antipsychotics. We defined quetiapine prescriptions as prescriptions for branded Seroquel (AstraZeneca Pharmaceuticals LP), Seroquel XR (AstraZeneca Pharmaceuticals LP), or generic quetiapine.
Power calculations indicated that a sample of 5000 would have 80% statistical power to detect an intervention effect of 1.5% to 1.7% on overall prescribing at the 5% significance level. Study participants were identified from the pool of PCPs with at least 10 quetiapine prescriptions in 2013 and 2014 who prescribed significantly more quetiapine than other such prescribers in their state. The PCPs were classified as high prescribers if their prescribing was at or above the 75th percentile plus a multiplier factor of the interquartile range vs other PCPs in the same state (a modified Tukey outlier method27) on 2 measures of quetiapine prescribing. These measures were (1) the number of quetiapine prescription fills supplied and (2) the total days of quetiapine supplied regardless of the number of patients (Supplement 1). A multiplier factor of 0.25 identified the 5055 highest-volume primary care prescribers (approximately 5% of all PCP prescribers of quetiapine) exceeding the outlier threshold for both measures in 2013 and 2014, which met our power calculations and became the study sample.
Intervention
The intervention was a mailed peer comparison letter using social norms from the Center for Program Integrity (Baltimore, Maryland) within CMS on PCPs’ quetiapine-prescribing behavior.23 Its message and format drew on insights from previous randomized evaluations of letter interventions.25,28,29 The letter (Supplement 1) indicated that the prescriber’s quetiapine prescribing was under review by CMS and was extremely high relative to the within-state peers. The text of the letter discussed that high quetiapine prescribing could be appropriate but was concerning for medically unjustified use. The letter encouraged PCPs to review their prescribing patterns and explained that PCPs could expect to receive future communications from CMS. The placebo intervention was a letter and pamphlet discussing an unrelated Medicare enrollment regulation, sent to allow CMS to observe whether letters were returned to sender in the full sample.
Placebo and intervention letters were mailed in April 2015. Drawing on literature30 that found that effects of letters grow when they are sent repeatedly, 2 follow-up intervention letters with more recent prescribing data were sent in August and October 2015 to treatment arm prescribers. An additional notice was sent to the control arm in June 2015 clarifying the enrollment process and the regulation.
The trial ended after the second follow-up letter on the request by CMS that the study team report the effect of the intervention. The prespecified analysis plan was finalized in March 2016, and researchers were then unmasked to the postintervention data.
Randomization
Prescribers were allocated by the first study author (A.S.) to control and treatment arms. A random sequence of numbers and a prespecified rerandomization procedure were used (Supplement 1).
Data Sources
We analyzed prescribers and patients using 100% Medicare claims data from 2013 to 2017, enrollment data from 2015 to 2017, and risk-adjustment data from 2013 and 2014. Data were analyzed using statistical software (Stata/MP, version 13; StataCorp LP).
Prescriber-Level and Patient-Level Outcomes
The primary outcome was measured at the prescriber level and was prespecified as the cumulative total number of quetiapine days supplied by PCPs in the 9 months after the intervention start (the initial mailing of letters). This outcome measure counts the number of quetiapine fills at pharmacies paid by Medicare Part D that were attributed to the targeted prescriber, quantified using the total number of days of quetiapine in the prescription fills. We chose the total number of days of quetiapine to integrate both changes in prescribing to continuing patients and initiations to new patients. As an exploratory outcome, we also assessed total number of days of quetiapine over an extended duration of 2 years.
We prespecified several additional secondary outcomes at the prescriber level and the patient level; we highlight several herein and provide the full set in Supplement 1. At the prescriber level, we also examined new quetiapine starts by PCPs, defined as all quetiapine days supplied to patients who had not received quetiapine from the study PCP during the last year. We also examined possible substitution toward similar atypical antipsychotic agents, the same drug class as quetiapine, as well as other psychiatric medications.
For patient-level outcomes, we defined a baseline cohort of patients as those receiving quetiapine from any study prescriber in the year before the intervention (Table 1 and Supplement 1). For this cohort, we examined the number of quetiapine fills over 9 months and 2 years, measured in days of quetiapine from all prescribers, divided into the following 3 mutually exclusive sources: the patient’s baseline study prescriber, other nonpsychiatric prescribers, and other psychiatric prescribers. We further examined health care use after the intervention, including inpatient admissions, emergency department visits, and psychiatrist outpatient visits, all cumulative to 9 months.
Table 1. Characteristics of Study Participants at Baselinea.
| Variable | Control | Treatment |
|---|---|---|
| Characteristics of Prescribers | (n = 2528) | (n = 2527) |
| Quetiapine days supplied in 9-mo baseline period, mean (SD) | ||
| To all patients | 2960 (2669) | 2872 (2401) |
| To new patients | 229 (260) | 225 (243) |
| To low-value patients | 846 (1307) | 794 (1250) |
| To guideline-concordant patients | 786 (924) | 769 (798) |
| Prescriber enrolled to bill Original Medicare, No. (%)b | 1745 (69.0) | 1784 (70.6) |
| Female sex, No. (%) | 447 (17.7) | 453 (17.9) |
| Specialty, No. (%) | ||
| General practitioner | 104 (4.1) | 127 (5.0) |
| Family medicine | 1186 (46.9) | 1242 (49.1) |
| Internal medicine | 1238 (49.0) | 1158 (45.8) |
| Characteristics of Baseline Patients | (n = 45 589) | (n = 43 911) |
| No. of patients by patient group | ||
| Low value | 12 105 | 11 385 |
| Guideline concordant | 13 050 | 12 630 |
| Quetiapine days received in 9-mo baseline period, mean (SD) | 193 (118) | 192 (117) |
| Quetiapine days received by patient group, mean (SD)c | ||
| Low value, 26.2% of 89 500 patients | 191 (116) | 189 (116) |
| Guideline concordant, 28.7% of 89 500 patients | 202 (118) | 203 (115) |
| Age, mean (SD), y | 70.4 (16.2) | 70.3 (16.2) |
| Nonwhite race/ethnicity, No. (%) | 13 415 (29.4) | 13 200 (30.1) |
| Female sex, No. (%) | 29 144 (63.9) | 27 963 (63.7) |
| Dementia or Alzheimer disease, No. (%) | 20 790 (45.6) | 19 558 (44.5) |
| Major psychiatric illness, No. (%) | 21 735 (47.7) | 20 803 (47.4) |
| Institutionalized in a long-term care facility, No. (%) | 7178 (15.7) | 6468 (14.7) |
| Qualifies for Medicare by disability, No. (%) | 17 028 (37.4) | 16 315 (37.2) |
| Dual Medicare-Medicaid eligible, No. (%) | 27 222 (59.7) | 26 158 (59.6) |
No. (%) is the number of observations (percentage of observations). The mean (SD) of days supplied or received refer to quetiapine fills in the baseline period, the 9 months before the intervention began. The only significant difference in control vs treatment baseline characteristics was in prescriber specialty (P = .04). The sample was the 5055 study prescribers (prescriber rows) and 89 500 patients (patient rows).
Original Medicare is the government health care payer in Medicare and is also called fee-for-service Medicare.
The low-value and guideline-concordant patient shares do not sum to 100% because they exclude patients who carried both low-value and guideline-concordant diagnoses (18.8% [16 858 of 89 500] of baseline patients), neither a low-value nor a guideline-concordant diagnosis (24.0% [21 521 of 89 500] of patients), or no diagnosis data in 2013 and 2014 (2.2% [1951 of 89 500] of patients).
Across several outcomes, we also assessed the effect of the intervention based on the likely indication for quetiapine prescribing. We defined the following 2 cohorts of patients: (1) those whose indications likely fell under the FDA’s quetiapine black box warning (low-value prescribing) and (2) those with FDA-approved indications (guideline-concordant prescribing),14,31 which also aligns with existing clinical guidelines.15 Using preintervention diagnoses in 2013 and 2014, quetiapine prescribing for patients with schizophrenia, bipolar disorder, or major depression without dementia or Alzheimer disease was deemed guideline concordant, whereas quetiapine prescribing for patients with dementia or Alzheimer disease but none of the major psychiatric illnesses above was considered low value (eTable 1 in Supplement 2). Patients in the low-value and guideline-concordant groups comprised 23 490 of 89 500 (26.2%) and 25 680 of 89 500 (28.7%) of the total baseline patient cohort, respectively (Table 1 and eTable 2 in Supplement 2). The residual group was composed of patients with no history of either category of diagnoses or with a history of diagnoses in both categories (Supplement 1); exploratory analyses of this group showed effects similar to the overall effects.
Statistical Analysis
We used multivariable linear regression models to evaluate the effect of the intervention. To increase the statistical power of our analyses, we prespecified multivariable adjustment for the level of the outcome before the start of the intervention and for several additional characteristics (Supplement 1).32,33 We used robust variance techniques in all statistical models, and patient-level analyses accounted for intraprescriber correlation with clustering at the prescriber level. Two-sided hypothesis tests with P < .05 were considered significant. To facilitate comparisons of outcomes with different levels, in some analyses we estimated a percentage effect by dividing the absolute effect (eg, absolute difference in quetiapine days supplied) and 95% CI by the control arm mean outcome.
Results
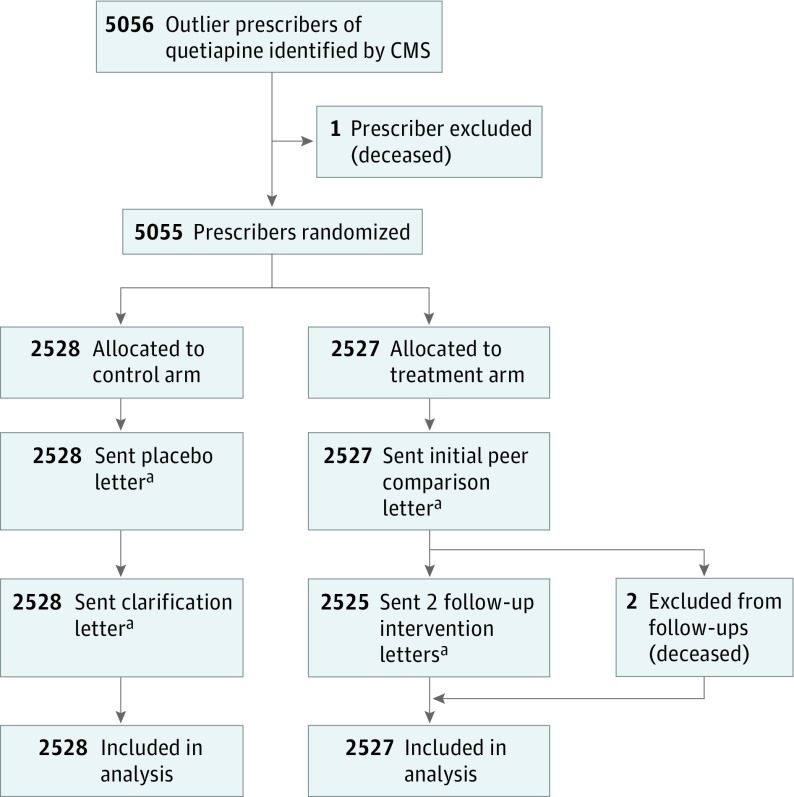
Of the 5055 study prescribers, 2528 prescribers were allocated to the control arm (placebo letter), and 2527 prescribers were allocated to the treatment arm (peer comparison letter). Two prescribers were not sent follow-up letters because they had died. Of the 5055 prescribers, 231 (4.6%) were general practitioners, 2428 (48.0%) were in family medicine, and 2396 (47.4%) were in internal medicine; 900 (17.8%) were female. All 5055 prescribers were included in analyses (Figure 1). The baseline patient cohort contained 89 500 patients, 45 589 aligned to the control arm and 43 911 aligned to the treatment arm (Table 1 and eFigure 1 in Supplement 2).
Figure 1. CONSORT Flow Diagram of Prescribers in the Study.
CMS indicates Centers for Medicare & Medicaid Services; CONSORT, Consolidated Standards of Reporting Trials.
aReproductions of letters can be found in Supplement 1.
The average prescriber in the study was responsible for supplying 2916 days (97 months) of quetiapine during the 9 months before the intervention (or about 3 months of quetiapine per week). On average, 820 (28.1%) of these days were to patients for likely low-value indications, and 778 (26.7%) were to patients for likely guideline-concordant indications. The average baseline patient received 193 days (6 months) of quetiapine during the 9-month preintervention period.
Prescriber-Level Outcomes
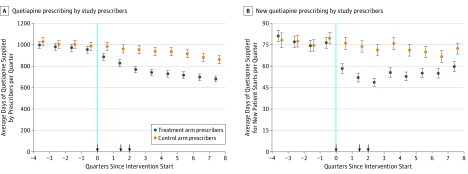
During the 9-month postintervention period, the average treatment arm prescriber supplied 2456 days (82 months) of quetiapine vs 2864 days (96 months) in the control arm, an adjusted difference of −319 days (95% CI, −374 to −263 days) per prescriber or an 11.1% (95% CI, −13.1% to −9.2%; P < .001) decrease vs control (Table 2 and Figure 2A). Extending the postintervention period to 2 years, the cumulative effect was a 15.6% (95% CI, −18.1% to −13.0%; P < .001) relative decrease vs control. The intervention was also associated with a significant decrease of 27.1% (95% CI, −31.1% to −23.1%; P < .001) relative to control in the volume of new quetiapine prescriptions over 9 months, which persisted cumulative to 2 years (−24.3% relative decrease; 95% CI, −28.0% to −20.6%; P < .001) (Table 2 and Figure 2B).
Table 2. Effect of the Intervention on Primary and Key Secondary Outcomesa.
| Variable | No.b | Control Mean | Treatment Mean | Difference (95% CI) | P Value | Adjusted Difference (95% CI)c | Percentage Difference (95% CI)d | P Value |
|---|---|---|---|---|---|---|---|---|
| Cumulative Total Quetiapine Days Over 9 mo | ||||||||
| Per prescriber: quetiapine days supplied | ||||||||
| To all patients | 5055 | 2864 | 2456 | −408 (−548 to −268) | <.001 | −319 (−374 to −263) | −11.1 (−13.1 to −9.2) | <.001 |
| To new patients | 5055 | 219 | 157 | −62 (−74 to −49) | <.001 | −59 (−68 to −50) | −27.1 (−31.1 to −23.1) | <.001 |
| To low-value patients | 5055 | 753 | 619 | −134 (−196 to −71) | <.001 | −91 (−115 to −67) | −12.1 (−15.3 to −8.9) | <.001 |
| To guideline-concordant patients | 5055 | 753 | 665 | −88 (−135 to −42) | <.001 | −74 (−95 to −53) | −9.8 (−12.6 to −7.1) | <.001 |
| P valuee | .24 | .26 | .25 | |||||
| Per baseline patient: quetiapine days received | ||||||||
| All patients | 89 500 | 169.7 | 162.9 | −6.8 (−10.3 to −3.2) | <.001 | −6.7 (−8.5 to −4.9) | −3.9 (−5.0 to −2.9) | <.001 |
| Low-value patients | 23 490 | 158.7 | 147.9 | −10.9 (−15.0 to −6.7) | <.001 | −9.4 (−12.6 to −6.2) | −5.9 (−8.0 to −3.9) | <.001 |
| Guideline-concordant patients | 25 680 | 182.1 | 177.9 | −4.3 (−9.4 to 0.9) | .10 | −4.5 (−7.2 to −1.7) | −2.4 (−4.0 to −0.9) | .002 |
| P valuee | .04 | .02 | .01 | |||||
| Cumulative Total Quetiapine Days Over 2 yf | ||||||||
| Per prescriber: quetiapine days supplied | ||||||||
| To all patients | 5055 | 7436 | 6052 | −1384 (−1752 to −1015) | <.001 | −1157 (−1343 to −970) | −15.6 (−18.1 to −13.0) | <.001 |
| To new patients | 5055 | 578 | 438 | −140 (−173 to −108) | <.001 | −141 (−162 to −119) | −24.3 (−28.0 to −20.6) | <.001 |
| To low-value patients | 5055 | 1801 | 1401 | −400 (−549 to −251) | <.001 | −306 (−379 to −233) | −17.0 (−21.0 to −13.0) | <.001 |
| To guideline-concordant patients | 5055 | 1922 | 1619 | −303 (−418 to −187) | <.001 | −264 (−327 to −201) | −13.7 (−17.0 to −10.5) | <.001 |
| P valuee | .29 | .34 | .17 | |||||
| Per baseline patient: quetiapine days received | ||||||||
| All patients | 89 500 | 385.7 | 364.9 | −20.8 (−29.2 to −12.3) | <.001 | −21.5 (−26.4 to −16.6) | −5.6 (−6.8 to −4.3) | <.001 |
| Low-value patients | 23 490 | 327.1 | 298.5 | −28.5 (−38.0 to −19.0) | <.001 | −25.8 (−33.9 to −17.8) | −7.9 (−10.4 to −5.4) | <.001 |
| Guideline-concordant patients | 25 680 | 442.1 | 424.9 | −17.2 (−30.3 to −4.2) | .01 | −17.6 (−25.1 to −10.1) | −4.0 (−5.7 to −2.3) | <.001 |
| P valuee | .15 | .13 | .01 | |||||
All outcomes count quetiapine days supplied or received cumulative to 9 months or cumulative to 2 years (as specified), beginning at the start of the intervention.
Indicates the number of study prescribers (prescriber rows) or the number of baseline patients (patient rows) included in the estimates.
Adjusts for baseline supply or receipt and other characteristics to raise statistical power (see the Statistical Analysis subsection of the Methods section and the Regression Control Variables subsection of the Statistical Approach section of Supplement 1 for more details).
Reports adjusted difference (95% CI) divided by the control mean.
Test that the low-value effect and guideline-concordant effect were equal.
Exploratory extension of outcome duration.
Figure 2. Quarterly Average Quetiapine Prescribing in Control and Treatment Arms.
A, Counts all days supplied by the prescribers. B, Counts only days supplied for new patient starts. Each point represents the average number of quetiapine days supplied in each quarter per prescriber relative to the intervention start date. Error bars indicate 95% CIs. Arrowheads denote when letters were sent to prescribers.
At the prescriber level, the intervention reduced quetiapine prescribing to both low-value and guideline-concordant patients (Table 2 and eFigure 2A in Supplement 2). There was a smaller decrease in prescribing to guideline-concordant patients, although the effect was not statistically different compared with the decrease for low-value patients (P = .25 for test that effects were equal over 9 months and P = .17 cumulative to 2 years).
Patient-Level Outcomes
We also examined quetiapine prescribing at the patient level (ie, how the intervention affected the average baseline patient’s receipt of quetiapine from all prescribers over the outcome period). The intervention was associated with a reduction of 6.7 days of quetiapine (95% CI, −8.5 to −4.9 quetiapine days; P < .001) received per patient over 9 months or a 3.9% (95% CI, −5.0% to −2.9%; P < .001) relative decrease (Table 2). The cumulative effect at 2 years grew to a 5.6% relative decrease (95% CI, −6.8% to −4.3%; P < .001).
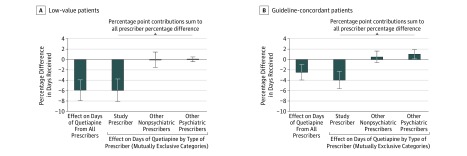
There was a significantly smaller reduction in the receipt of quetiapine for guideline-concordant patients than for low-value patients (P = .01 for difference in percentage reduction between the 2 patient groups both over 9 months and cumulative to 2 years; tests compare effects for low-value patients in this paragraph with effects for guideline-concordant patients in the following paragraph) (Table 2 and eFigure 2B in Supplement 2). For low-value patients, the intervention was associated with a 5.9% (95% CI, −8.0% to −3.9%; P < .001) reduction in quetiapine receipt over 9 months and with a larger 7.9% (95% CI, −10.4% to −5.4%; P < .001) decrease cumulative to 2 years. An exploratory analysis showed that the entirety of this effect came from the study prescribers, with no compounding or offsetting change from other (nonbaseline) prescribers (Figure 3A).
Figure 3. Cumulative Effect on the Receipt of Quetiapine by Low-Value and Guideline-Concordant Patients Over 9 Months.
In each panel, the left-most bar shows the percentage difference in quetiapine days between control and treatment patients from all prescribers in the 9 months after the start of the intervention. The next 3 bars display percentage point contributions to the percentage difference of the following 3 mutually exclusive categories: the patient’s study prescriber, other nonpsychiatric prescribers, and other psychiatric prescribers. The contributions of these 3 categories sum to the all prescriber percentage difference. Each bar reports an adjusted percentage difference (difference between control and treatment means, divided by the control mean; difference adjusted for baseline receipt and other characteristics described in the Statistical Analysis subsection of the Methods section and the Regression Control Variables subsection of the Statistical Approach section of Supplement 1). Error bars indicate 95% CIs. See eTable 3 in Supplement 2 for coefficients.
For guideline-concordant patients, there was a relative reduction of 2.4% (95% CI, −4.0% to −0.9%; P = .002) over 9 months and 4.0% (95% CI, −5.7% to −2.3%; P < .001) over 2 years in quetiapine receipt (Table 2). In exploratory analyses, we found that 39.8% of the reduction for guideline-concordant patients from study physicians was offset by shifting prescriptions to other prescribers (Figure 3B and eTable 3 in Supplement 2). Most of the offset was because of an increase in quetiapine receipt from other (nonbaseline) physicians with psychiatric specialization (the remainder came from other prescribers, including study prescribers from whom the patient did not previously receive quetiapine and nonpsychiatric prescribers outside of the study).
To test for effects on the total cessation of quetiapine, we considered whether patients received any quetiapine in each quarter in an exploratory analysis (eFigure 3 in Supplement 2). Percentage effects on the total cessation were roughly twice as large for low-value patients as for guideline-concordant patients.
There was no statistically significant effect of the intervention on PCPs prescribing or patients receiving other antipsychotics, antianxiety drugs, sleep aids, and antidepressants (eTable 4 and eTable 5 in Supplement 2). We studied the receipt of all antipsychotics for the low-value and guideline-concordant patient groups in an exploratory analysis (eTable 6 in Supplement 2). While both patient groups experienced increases in the receipt of other antipsychotics, the magnitudes were small, leaving the qualitative effect of the intervention on the total receipt unchanged.
There was no significant change in mortality, inpatient admissions, emergency department visits, or psychiatrist outpatient visits for baseline patients during the 9-month outcome period. Exploratory analyses of the patient groups detected only a reduction in emergency department visits for guideline-concordant patients (eTable 7 and eFigure 4 in Supplement 2).
Discussion
In this randomized clinical trial, we found that peer comparison letters targeting the 5055 highest quetiapine-prescribing PCPs nationwide in the Medicare program led to statistically significant, persistent decreases in quetiapine prescribing. The decrease was pronounced for new quetiapine prescribing, suggesting a particular effect on physicians’ decision making about whether to initiate quetiapine treatment. The intervention was associated with reductions in prescribing to both low-value and guideline-concordant patients at the prescriber level; however, at the patient level, low-value patients had a significantly greater decline in quetiapine receipt. We detected no adverse effects of the letters on baseline patients according to mortality data and health care use. These results provide encouraging evidence that high prescribers of antipsychotics can decrease quetiapine prescribing, without adverse clinical consequences, in response to a letter highlighting their overall high rates of prescribing.
Compared with existing work on prescribing quality, this study provides a unique example of a large-scale intervention yielding clinically meaningful, persistent effects. For example, a recent antibiotic prescribing nudge targeting general practitioners throughout England reduced inappropriate prescribing by 3.3%.24 Effects in the present study were smaller than those of other promising behavioral interventions on prescribing that targeted a more limited number of health care professionals (eg, where a peer comparison message reduced inappropriate antibiotic prescribing by 22% and effects endured after the intervention23), although those interventions involved more complex changes, such as modifying electronic health record systems.22,23,34
The findings herein also contrast with the null effect of a similar intervention performed by several members of our study team targeting high prescribers of controlled substances, including opioids.25 The present study incorporated lessons from that work that could have contributed to the more substantial effect we observed here. First, our study targeted a wider range of high prescribers (approximately 5% of quetiapine-prescribing PCPs) vs the top 0.3% of all schedule II controlled substance prescribers in the previous study. Second, the letters in the present study had stronger wording regarding the possibility that prescribing was inappropriate and could be reviewed, which may have led physicians to take them more seriously. This finding can guide future evaluations of randomized letters with a variety of framings to find optimally effective approaches to communication.
In many domains we did not observe evidence consistent with significant unintended consequences from the present intervention, such as substitution away from quetiapine toward another antipsychotic agent. We observed reductions in the receipt of quetiapine among guideline-concordant patients, which could represent negative effects from PCPs cutting quetiapine use indiscriminately, even for patients who may need it. If this represented a harmful change for patients, we may have expected to see higher rates of adverse outcomes in the guideline-concordant patient group as prescribing rates decreased. However, if anything, guideline-concordant patients experienced lower rates of hospital encounters after the intervention. Although there are negative outcomes beyond these that we may not have observed, these results suggest that PCPs may be able to target guideline-concordant patients for whom stopping quetiapine treatment may be clinically justifiable while maintaining access for patients who experience clinical benefits (by continuing to prescribe to these patients or by shifting them to psychiatrists). In future interventions, it will be important to specifically target low-value care (eg, by selecting physicians not only by their high overall prescribing but also by their high rates of low-value prescribing).
Limitations
This study has several limitations. First, our analysis included only prescribing covered by Medicare Part D. The letters may have encouraged physicians to reevaluate their prescribing to patients with private insurance, Medicaid, or no insurance coverage. This spillover effect could amplify or dampen the magnitude of our findings, depending on the nature of the spillovers. Second, another limitation concerns the external validity of the study if it was scaled or repeated in a different population. The effectiveness of the letters may have come from their novelty, and the magnitude of effects may decline if letters are used frequently or across multiple settings (eg, antibiotics, opioids, and benzodiazepines) similar to the well-documented phenomenon of alert fatigue.35 Letters sent to other populations, such as prescribers who were not high-volume outliers, could have different effects. Third, we classify low-value and guideline-concordant prescribing using administrative data, which may have measurement error. Validation studies would enable future interventions to use these data more confidently. Fourth, our outcomes did not measure quality of life or mental health directly, which may have been the most likely domains for detecting a negative effect if the intervention caused harm.
Fifth, because of limitations in data access, we could not estimate effects for patients who were classified as neither low value nor guideline concordant. Imputed effects for this patient group were similar to the overall effects, but we did not report them because it was not possible to impute 95% CIs. We also were not able to assess the characteristics of the psychiatric (and nonpsychiatric) care providers who offset reductions in quetiapine prescribing by study PCPs.
Conclusions
We found that a low-cost series of peer comparison letters targeting PCPs who were high prescribers of quetiapine in the Medicare program resulted in large, sustained decreases in prescribing. We observed greater decreases in likely low-value, off-label prescribing than in potentially guideline-concordant prescribing, with little evidence that prescribers simply switched patients to other similar drugs and with no detected negative effects on patients. With increasing awareness of the dangers of inappropriate prescribing, this study provides evidence that peer comparison letters targeted at high-risk medications could effectively and efficiently create durable improvements in prescribing patterns.
Trial Protocol
eTable 1. Classification Rules for Low-Value and Guideline-Concordant Patients
eTable 2. Summary Statistics About Patients
eTable 3. Effect of Intervention on Source of Quetiapine by Guideline Conformity
eTable 4. Effect of Intervention on Prescribing of Other Psychiatric Drugs
eTable 5. Effect of Intervention on Patient Receipt of Other Psychiatric Drugs
eTable 6. Effect of Intervention on Patient Receipt of Antipsychotics by Guideline Conformity
eTable 7. Effect of Intervention on Mortality and Health Care Utilization
eFigure 1. CONSORT Flow Diagram of Baseline Patients in Study
eFigure 2. Quarterly Effect of Intervention on Prescribers and Patients, by Guideline Conformity
eFigure 3. Quarterly Effect of Intervention on Any Receipt of Quetiapine
eFigure 4. Cumulative Effect on Health Care Utilization for All, Low-Value, and Guideline-Concordant Patients Over 9 Months
References
- 1.Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184. doi: 10.1002/pds.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Government Accountability Office (GAO) Antipsychotic Drug Use: HHS Has Initiatives to Reduce Use Among Older Adults in Nursing Homes, but Should Expand Efforts to Other Settings. Washington, DC: GAO; 2015. [Google Scholar]
- 3.Semla TP, Lee A, Gurrera R, et al. . Off-label prescribing of second-generation antipsychotics to elderly veterans with posttraumatic stress disorder and dementia. J Am Geriatr Soc. 2017;65(8):1789-1795. doi: 10.1111/jgs.14897 [DOI] [PubMed] [Google Scholar]
- 4.Driessen J, Baik SH, Zhang Y. Trends in off-label use of second-generation antipsychotics in the Medicare population from 2006 to 2012. Psychiatr Serv. 2016;67(8):898-903. doi: 10.1176/appi.ps.201500316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang YJ, Dixon SN, Reiss JP, et al. . Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med. 2014;161(4):242-248. doi: 10.7326/M13-2796 [DOI] [PubMed] [Google Scholar]
- 6.Maust DT, Kim HM, Seyfried LS, et al. . Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445. doi: 10.1001/jamapsychiatry.2014.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maher AR, Maglione M, Bagley S, et al. . Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306(12):1359-1369. doi: 10.1001/jama.2011.1360 [DOI] [PubMed] [Google Scholar]
- 8.El-Saifi N, Moyle W, Jones C, Tuffaha H. Quetiapine safety in older adults: a systematic literature review. J Clin Pharm Ther. 2016;41(1):7-18. doi: 10.1111/jcpt.12357 [DOI] [PubMed] [Google Scholar]
- 9.Maglione M, Maher AR, Hu J, et al. . Off-Label Use of Atypical Antipsychotics: An Update. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK66081/. Published September 2011. Accessed June 13, 2018. [PubMed] [Google Scholar]
- 10.Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: managing safety concerns. Am J Psychiatry. 2012;169(9):900-906. doi: 10.1176/appi.ajp.2012.12030342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943. doi: 10.1001/jama.294.15.1934 [DOI] [PubMed] [Google Scholar]
- 12.Ballard C, Margallo-Lana M, Juszczak E, et al. . Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ. 2005;330(7496):874. doi: 10.1136/bmj.38369.459988.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychiatric Association: Choosing Wisely. Five things physicians and patients should question. http://www.choosingwisely.org/societies/american-psychiatric-association/. Released September 20, 2013; recommendation 5 updated August 21, 2014; recommendation 3 updated April 22, 2015. Accessed September 26, 2017.
- 14.Seroquel (quetiapine fumarate). Highlights of prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020639s064lbl.pdf. Published June 2016. Accessed June 13, 2018.
- 15.American Geriatrics Society 2015 Beers Criteria Update Expert Panel American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 16.Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, US Food and Drug Administration Pediatric postmarketing pharmacovigilance and drug utilization review: Seroquel and Seroquel XR. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM494485.pdf. Published February 19, 2016. Accessed June 13, 2018.
- 17.Walton SM, Schumock GT, Lee KV, Alexander GC, Meltzer D, Stafford RS Developing Evidence-Based Research Priorities for Off-Label Drug Use. Rockville, MD: Agency for Healthcare Research and Quality. Effective Health Care Research Report 12. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/off-label-use-research-priorities_research.pdf. Published May 2009. Accessed June 13, 2018.
- 18.Mitka M. CMS seeks to reduce antipsychotic use in nursing home residents with dementia. JAMA. 2012;308(2):119–, 121.. doi: 10.1001/jama.2012.7422 [DOI] [PubMed] [Google Scholar]
- 19.Avorn J, Soumerai SB, Everitt DE, et al. . A randomized trial of a program to reduce the use of psychoactive drugs in nursing homes. N Engl J Med. 1992;327(3):168-173. doi: 10.1056/NEJM199207163270306 [DOI] [PubMed] [Google Scholar]
- 20.Thompson A, Sullivan SA, Barley M, et al. . The DEBIT trial: an intervention to reduce antipsychotic polypharmacy prescribing in adult psychiatry wards: a cluster randomized controlled trial. Psychol Med. 2008;38(5):705-715. doi: 10.1017/S003329170700147X [DOI] [PubMed] [Google Scholar]
- 21.Arditi C, Rège-Walther M, Wyatt JC, Durieux P, Burnand B. Computer-generated reminders delivered on paper to healthcare professionals; effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2012;12:CD001175. [DOI] [PubMed] [Google Scholar]
- 22.Meeker D, Knight TK, Friedberg MW, et al. . Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med. 2014;174(3):425-431. doi: 10.1001/jamainternmed.2013.14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meeker D, Linder JA, Fox CR, et al. . Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi: 10.1001/jama.2016.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallsworth M, Chadborn T, Sallis A, et al. . Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet. 2016;387(10029):1743-1752. doi: 10.1016/S0140-6736(16)00215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacarny A, Yokum D, Finkelstein A, Agrawal S. Medicare letters to curb overprescribing of controlled substances had no detectable effect on providers. Health Aff (Millwood). 2016;35(3):471-479. doi: 10.1377/hlthaff.2015.1025 [DOI] [PubMed] [Google Scholar]
- 26.Ivers N, Jamtvedt G, Flottorp S, et al. . Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tukey JW. Exploratory Data Analysis. Indianapolis, IN: Addison-Wesley Publishing Co; 1977. [Google Scholar]
- 28.Castro L, Scartascini C. Tax compliance and enforcement in the Pampas: evidence from a field experiment. J Econ Behav Organ. 2015;116:65-82. doi: 10.1016/j.jebo.2015.04.002 [DOI] [Google Scholar]
- 29.Fellner G, Sausgruber R, Traxler C. Testing enforcement strategies in the field: threat, moral appeal and social information. J Eur Econ Assoc. 2013;11(3):634-660. doi: 10.1111/jeea.12013 [DOI] [Google Scholar]
- 30.Allcott H, Rogers T. The Short-Run and Long-Run Effects of Behavioral Interventions: Experimental Evidence From Energy Conservation. Cambridge, MA: National Bureau of Economic Research; 2012. http://www.nber.org/papers/w18492.pdf. Published October 2012. Accessed August 25, 2016. doi: 10.3386/w18492 [DOI] [Google Scholar]
- 31.Seroquel XR (quetiapine fumarate). Highlights of prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022047s038lbl.pdf. Published June 2016. Accessed June 13, 2018.
- 32.Cox DR, McCullagh P. Some aspects of analysis of covariance. Biometrics. 1982;38(3):541-561. doi: 10.2307/2530040 [DOI] [PubMed] [Google Scholar]
- 33.Athey S, Imbens GW. The econometrics of randomized experiments In: Banerjee AV, Duflo E, eds. Handbook of Economic Field Experiments. Vol 1. New York, NY: Elsevier; 2017:73-140. doi: 10.1016/bs.hefe.2016.10.003 [DOI] [Google Scholar]
- 34.Linder JA, Meeker D, Fox CR, et al. . Effects of behavioral interventions on inappropriate antibiotic prescribing in primary care 12 months after stopping interventions. JAMA. 2017;318(14):1391-1392. doi: 10.1001/jama.2017.11152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agency for Healthcare Research and Quality Alert fatigue. https://psnet.ahrq.gov/primers/primer/28/alert-fatigue. Last updated June 2017. Accessed June 13, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Classification Rules for Low-Value and Guideline-Concordant Patients
eTable 2. Summary Statistics About Patients
eTable 3. Effect of Intervention on Source of Quetiapine by Guideline Conformity
eTable 4. Effect of Intervention on Prescribing of Other Psychiatric Drugs
eTable 5. Effect of Intervention on Patient Receipt of Other Psychiatric Drugs
eTable 6. Effect of Intervention on Patient Receipt of Antipsychotics by Guideline Conformity
eTable 7. Effect of Intervention on Mortality and Health Care Utilization
eFigure 1. CONSORT Flow Diagram of Baseline Patients in Study
eFigure 2. Quarterly Effect of Intervention on Prescribers and Patients, by Guideline Conformity
eFigure 3. Quarterly Effect of Intervention on Any Receipt of Quetiapine
eFigure 4. Cumulative Effect on Health Care Utilization for All, Low-Value, and Guideline-Concordant Patients Over 9 Months