Key Points
Question
For patients with severe obesity and type 2 diabetes, is there an association between bariatric surgery and incident macrovascular disease (defined as first occurrence of acute myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass grafting, ischemic stroke, hemorrhagic stroke, carotid stenting, or carotid endarterectomy)?
Findings
In this retrospective cohort study of patients with type 2 diabetes and severe obesity that included 5301 who underwent bariatric surgery and 14 934 control patients without surgery, bariatric surgery was associated with a significantly lower risk of macrovascular events at 5 years’ follow-up (2.1% vs 4.3% at 5 years; hazard ratio, 0.60).
Meaning
Bariatric surgery was associated with a lower risk of incident major macrovascular events.
Abstract
Importance
Macrovascular disease is a leading cause of morbidity and mortality for patients with type 2 diabetes, and medical management, including lifestyle changes, may not be successful at lowering risk.
Objective
To investigate the relationship between bariatric surgery and incident macrovascular (coronary artery disease and cerebrovascular diseases) events in patients with severe obesity and type 2 diabetes.
Design, Setting, and Participants
In this retrospective, matched cohort study, patients with severe obesity (body mass index ≥35) aged 19 to 79 years with diabetes who underwent bariatric surgery from 2005 to 2011 in 4 integrated health systems in the United States (n = 5301) were matched to 14 934 control patients on site, age, sex, body mass index, hemoglobin A1c, insulin use, observed diabetes duration, and prior health care utilization, with follow-up through September 2015.
Exposures
Bariatric procedures (76% Roux-en-Y gastric bypass, 17% sleeve gastrectomy, and 7% adjustable gastric banding) were compared with usual care for diabetes.
Main Outcomes and Measures
Multivariable-adjusted Cox regression analysis investigated time to incident macrovascular disease (defined as first occurrence of coronary artery disease [acute myocardial infarction, unstable angina, percutaneous coronary intervention, or coronary artery bypass grafting] or cerebrovascular events [ischemic stroke, hemorrhagic stroke, carotid stenting, or carotid endarterectomy]). Secondary outcomes included coronary artery disease and cerebrovascular outcomes separately.
Results
Among a combined 20 235 surgical and nonsurgical patients, the mean (SD) age was 50 (10) years; 76% of the surgical and 75% of the nonsurgical patients were female; and the baseline mean (SD) body mass index was 44.7 (6.9) and 43.8 (6.7) in the surgical and nonsurgical groups, respectively. At the end of the study period, there were 106 macrovascular events in surgical patients (including 37 cerebrovascular and 78 coronary artery events over a median of 4.7 years; interquartile range, 3.2-6.2 years) and 596 events in the matched control patients (including 227 cerebrovascular and 398 coronary artery events over a median of 4.6 years; interquartile range, 3.1-6.1 years). Bariatric surgery was associated with a lower composite incidence of macrovascular events at 5 years (2.1% in the surgical group vs 4.3% in the nonsurgical group; hazard ratio, 0.60 [95% CI, 0.42-0.86]), as well as a lower incidence of coronary artery disease (1.6% in the surgical group vs 2.8% in the nonsurgical group; hazard ratio, 0.64 [95% CI, 0.42-0.99]). The incidence of cerebrovascular disease was not significantly different between groups at 5 years (0.7% in the surgical group vs 1.7% in the nonsurgical group; hazard ratio, 0.69 [95% CI, 0.38-1.25]).
Conclusions and Relevance
In this observational study of patients with type 2 diabetes and severe obesity who underwent surgery, compared with those who did not undergo surgery, bariatric surgery was associated with a lower risk of macrovascular outcomes. The findings require confirmation in randomized clinical trials. Health care professionals should engage patients with severe obesity and type 2 diabetes in a shared decision making conversation about the potential role of bariatric surgery in the prevention of macrovascular events.
This cohort study investigates associations between bariatric surgery and incident macrovascular events (coronary artery and cerebrovascular diseases) in patients with type 2 diabetes.
Introduction
Macrovascular disease, including coronary artery disease and cerebrovascular disease, is one of the leading causes of morbidity and mortality for patients with type 2 diabetes. As a result, clinical guidelines for the management of type 2 diabetes emphasize lowering macrovascular disease risk factors by optimizing glycemic control, blood pressure, and serum lipid levels.1,2 This multifactorial approach has been shown to substantially reduce the incidence of macrovascular complications.2,3 However, most patients with type 2 diabetes do not achieve these recommended treatment goals,4 resulting in continued morbidity and costs.
There is evidence from randomized trials that weight loss interventions—including intensive lifestyle modification, pharmacotherapy, and bariatric surgery—can improve macrovascular disease risk factors in patients with type 2 diabetes. To our knowledge, clinical trials have not yet shown that intensive lifestyle intervention and pharmacotherapy for obesity can reduce macrovascular events.5,6,7,8,9 In comparison, patients who have bariatric surgery have better glycemic control and greater macrovascular risk factor reduction than those who get both intensive lifestyle and medical treatment combined.10,11,12
Several observational studies have provided evidence that bariatric surgery may reduce macrovascular complications of type 2 diabetes when compared with usual medical care,13 but limitations of these studies include the inability to study contemporary bariatric procedures because of small numbers of patients14 and unavailability of body mass index measurements for identifying a cohort of nonsurgical matches.15
To address these concerns, a study was conducted to test the hypothesis that patients undergoing contemporary bariatric procedures would experience a lower rate of macrovascular events than matched patients with severe obesity and type 2 diabetes who received usual care in 4 integrated health insurance and care delivery systems in the United States.
Methods
Settings
A retrospective observational matched cohort study was conducted of adults with type 2 diabetes who underwent bariatric surgery between 2005 and 2011 while enrolled in 1 of 4 US integrated health care systems from the Health Care Systems Research Network: Kaiser Permanente Washington in Washington state, HealthPartners in Minnesota, Kaiser Permanente Northern California, and Kaiser Permanente Southern California. All study procedures were reviewed in advance and approved by the institutional review board at each site and permitted to conduct the research without explicit consent from participants.
Data Sources
At each study site, standardized electronic medical records, insurance claims, and other data systems16 were used to extract information on enrollment; insurance coverage; demographics; blood pressure; height; weight; laboratory values; medications dispensed; deaths; outpatient, inpatient, and emergency department use; and diagnosis and procedure codes of all enrollees. Race/ethnicity data, which were collected as part of routine clinical care, were extracted from electronic medical records, where they had been entered by clinical staff based on patient self-report using fixed categories.
Surgical Participants
The bariatric surgery population included adults (aged 19-79 years) with severe obesity (body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] ≥35) and type 2 diabetes who had a primary (first observed) bariatric surgical procedure between January 1, 2005, and December 31, 2011. A combination of bariatric registries, medical record reviews, International Classification of Diseases, Ninth Revision (ICD-9) codes (43.89, 44.31, 44.38, 44.39, 44.68, 44.69, and 44.95), and Current Procedural Terminology (CPT) procedure codes (43633, 43644, 43645, 43659, 43770, 43775, 43842, 43843, 43844, 43845, 43846, and 43847) were used to identify bariatric procedures. Patients were classified as having type 2 diabetes if they met 1 of 2 criteria at the time of the procedure: (1) type 2 diabetes, defined as a hemoglobin A1c (HbA1c) level greater than or equal to 6.5% (48 mmol/mol) or fasting plasma glucose level greater than or equal to 126 mg/dL (to convert to mmol/L, multiply by 0.0555) at the most recent measurement within 2 years prior to surgery, or (2) medication-treated type 2 diabetes, defined as a current prescription for any oral or injectable diabetes medication at the time of bariatric surgery.
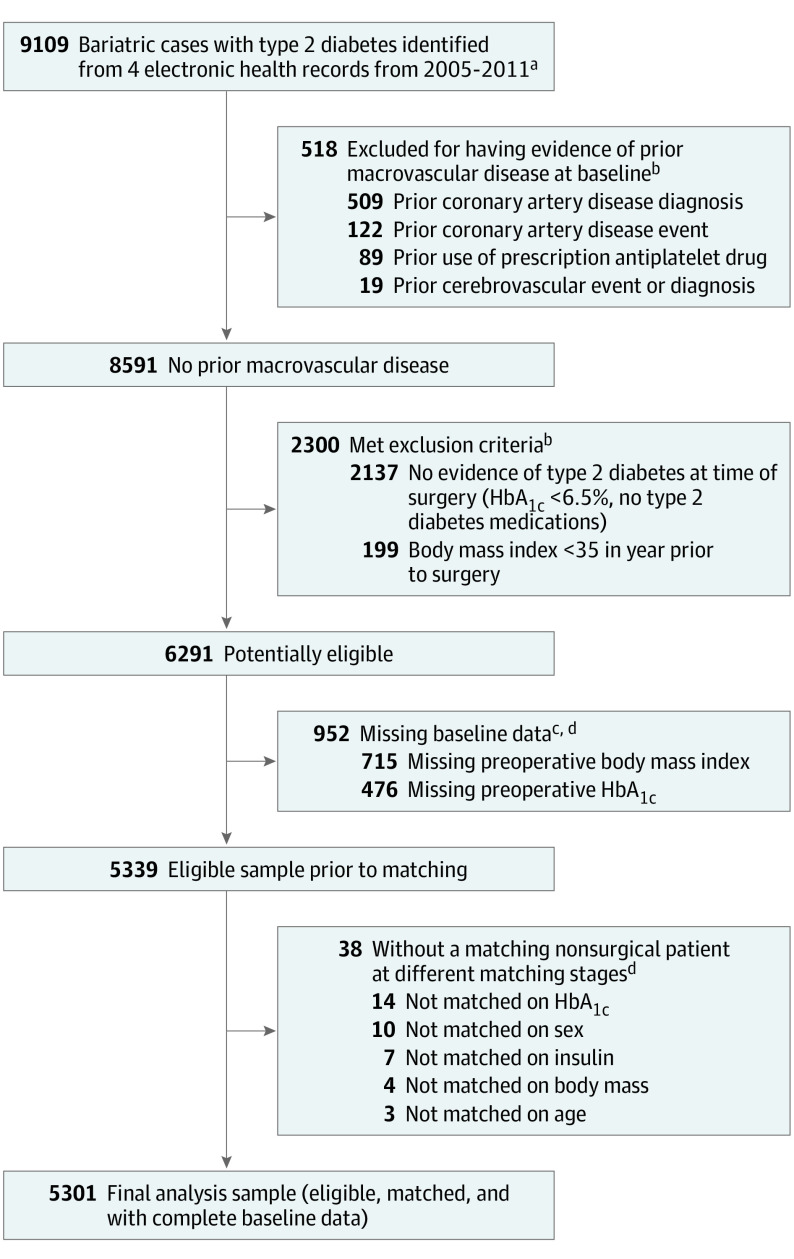
After population selection, the following exclusion criteria were applied based on information in the 2 years before the index date: (1) less than 1 full year of continuous enrollment, (2) a history of cancer (except nonmelanoma skin cancer), (3) pregnancy, (4) gestational diabetes when it was the sole diabetes diagnosis, (5) metformin as the sole indicator of possible type 2 diabetes (ie, no other type 2 diabetes laboratories or diagnoses), (6) pre-existing coronary artery disease or cerebrovascular disease (defined below) or the use of prescription antiplatelet medication, (7) maximum preoperative BMI less than 35, (8) missing preoperative BMI or HbA1c level in the 2 years before surgery, and (9) unable to match to at least 1 nonsurgical patient (Figure 1).
Figure 1. Flow Diagram for Identification of Eligible Patients Who Underwent Bariatric Surgery and Had Type 2 Diabetes Without a Preoperative History of Macrovascular Disease.
aAdults aged 19 to 79 years old who had a primary bariatric surgical procedure between January 1, 2005, and December 31, 2011. Bariatric procedures identified using a combination of bariatric registries, medical record reviews, International Classification of Diseases, Ninth Revision codes (43.89, 44.31, 44.38, 44.39, 44.68, 44.69, and 44.95), and Current Procedural Terminology procedure codes (43633, 43644, 43645, 43659, 43770, 43775, 43842, 43843, 43844, 43845, 43846, and 43847).
bPatients with the following were excluded: (1) less than 1 full year of continuous enrollment and drug coverage, (2) a history of gastrointestinal surgery for cancer, (3) gestational diabetes if it was the sole diabetes diagnosis, (4) individuals who experienced any time in pregnancy during the year prior to surgery, (5) any recorded use of prescription anticoagulants, and (6) metformin as the sole indicator of possible type 2 diabetes (no other type 2 diabetes medications, laboratories, or diagnoses). Patients may have more than 1 reason for exclusion.
cPatients may have more than 1 type of missing data (body mass index [calculated as weight in kilograms divided by height in meters squared], hemoglobin A1c level).
deTable 1 in the Supplement shows the characteristics of patients who were excluded due to missing data or inability to match compared with the final analytic sample.
Matched Nonsurgical Participants
For each patient with bariatric surgery, we identified up to 3 matched nonsurgical controls via a multistep process. First, among all patients with diabetes and BMI greater than or equal to 35 who did not undergo bariatric surgery during the study period (n = 320 345), we identified a pool of potential controls who were enrolled at the time of the surgery, satisfied the study inclusion/exclusion criteria, and matched the patient with bariatric surgery on the basis of study site, sex, age (±10 years), BMI (±5), HbA1c level (±absolute 1.5%), and insulin use. Second, for each control in the pool, we calculated their Mahalanobis distance (a measure of the distance that accounts for correlation between variables) from the bariatric patient on the basis of age, BMI, HbA1c level, diabetes duration, and the number of days of health care utilization in the 7 to 24 months prior to the date of surgery.17 Third, up to 3 controls were selected based on the shortest Mahalanobis distance. Throughout, nonsurgical patients could only be used as a control for 1 surgical patient (matching without replacement). Additional details on the process we used to establish the matched cohort are provided in the eAppendix in the Supplement.
Analyses
Outcome and Censoring Definitions
The a priori primary outcome measure was time to incident macrovascular disease (composite indicator of the first occurrence of a coronary artery disease or cerebrovascular event). Using methods from prior studies,17,18 coronary artery disease events included acute myocardial infarction, unstable angina, percutaneous coronary intervention, or coronary artery bypass grafting identified via ICD-9 diagnosis codes (410.x; 411.x as principal diagnosis or 411.x as secondary diagnosis with 414.x as principal diagnosis), ICD-9 procedure codes (36.01, 36.02, 36.03, 36.05, 36.06, 36.07, 36.10, 36.11, 36.12, 36.13, 36.14, 36.15, 36.16, 36.17, 36.19, 36.31, 36.32, 36.33, and 36.34), and CPT-4 procedure codes (92982, 92984, 92995, 92996, 92980, 92981, 33510, 33511, 33512, 33513, 33514, 33516, 33517, 33518, 33519, 33521, 33522, 33523, 33530, 33533, 33534, 33535, 33536, 93539, and 93540). Cerebrovascular events included ischemic stroke, hemorrhagic stroke, carotid stenting, or endarterectomy procedures identified via ICD-9 diagnosis codes (433.x1, 434.x1, 436.0, 430.x, and 431.x), ICD-9 procedure codes (38.12, 0.61, and 0.63), and CPT-4 procedure codes (37215, 37216, 0075T, 0076T, 35301, 37205, and 37206). Patients were censored at the first incidence of cancer (excluding nonmelanoma skin cancer), disenrollment, death, or study end (eTable 2 in the Supplement).
In secondary analyses, each of the coronary artery disease and cerebrovascular outcomes was considered separately. All-cause mortality was also examined as a post hoc exploratory outcome. As in prior studies,19 death information was drawn from a combination of electronic medical records, administrative databases, and state death indices.
Statistical Models
Cox proportional hazards regression models, fit via the usual partial likelihood, were used to investigate the association between bariatric surgery vs usual care (nonsurgical controls) and incident outcomes. Patients were followed from the index date (the date of bariatric surgery or, for nonsurgical patients, the date of surgery for the patient to whom they had been matched) until the first occurrence of either incident outcome or a censoring event. Based on preliminary analyses, the proportional hazards assumption was assessed to not hold for bariatric surgery vs usual care (P < .001 for an interaction with log-time in the Cox model). We therefore fit a flexible time-varying hazard ratio (HR) association as a function of time since surgery, using restricted cubic splines with knots at the fifth, 35th, 65th, and 95th percentiles of the observed follow-up time scale.20
Cox regression models were used for the primary and secondary outcomes and were adjusted for a priori–identified potential confounders: age, race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or non-Hispanic other), surgical year, BMI, smoking status (current, former, or never), duration of observed diabetes before surgery (defined as first observed diagnosis, laboratory value, or prescription indicating type 2 diabetes), insulin use, oral diabetes medication use, uncontrolled blood pressure (defined as either systolic ≥140 or diastolic ≥90 mm Hg at 2 consecutive measures on different days), use of angiotensin converting enzyme inhibitor, angiotensin receptor blocker medications, use of any other antihypertensive medication, insurance type (commercial, Medicare, Medicaid, or other), estimated glomerular filtration rate, low-density lipoprotein (LDL) cholesterol level greater than or equal to 100 mg/dL (to convert to mmol/L, multiply by 0.0259), serum triglyceride level greater than or equal to 150 mg/dL (to convert to mmol/L, multiply by 0.0113), use of cholesterol-lowering medication (statin or other), and history of peripheral arterial disease, hypertension, dyslipidemia, or microvascular disease (diabetic retinopathy, diabetic neuropathy, or diabetes with renal manifestations; end-stage renal disease; or dialysis) before surgery (defined based on ICD-9 and CPT-4 codes). Because of potential variation in care between health care systems, study site (HealthPartners, Kaiser Permanente Southern California, Kaiser Permanente Northern California, and Kaiser Permanente Washington) was adjusted for via stratification of the baseline hazard function. Race/ethnicity was included in the statistical models because it could confound the outcomes by either influencing a patient’s ability to receive bariatric surgery or by having a direct effect on the macrovascular outcomes by itself.
Missing data were encountered at baseline for race/ethnicity, smoking status, blood pressure, estimated glomerular filtration rate, LDL cholesterol, and triglyceride levels. We performed multiple imputation via chained equations, with unordered categorical variables and multinomial logistic regression for imputation of race/ethnicity and smoking status; logistic regression for elevated blood pressure, LDL cholesterol, and triglyceride levels; and linear regression for estimated glomerular filtration rate.21 Ten imputed data sets were generated, with the results combined using Rubin’s rules. Additional detail is provided in the eAppendix in the Supplement.
Sensitivity Analyses
Four sensitivity analyses were conducted to examine the effect of (1) not adjusting for potential confounders that were not included in the matching process (vs adjusting for those confounders in our main analysis), (2) using 1-to-1 and 10-to-1 matching, (3) incorporating all covariates into our Mahalanobis distance calculation (vs our original matching approach), and (4) an additional sensitivity analysis to assess the robustness of the 5-year results to unmeasured confounding using the E-value methodology of VanderWeele and Ding.22 This estimates what the relative risk would have to be for any unmeasured confounder to overcome the observed association of bariatric surgery with macrovascular disease in this study (see the eAppendix in the Supplement for details).22
The a priori level of statistical significance was set at a 2-sided P value of .05 for all analyses. Because the significance threshold for the secondary analyses was not adjusted for multiple comparisons, these should be interpreted as exploratory. Throughout, we used SAS version 9.4 (SAS Institute) for data manipulation, Stata version 15.1 (StataCorp) for multiple imputation and analysis, and R (R Foundation) for visualization.
Results
Participants
Table 1 presents descriptive statistics for the final analytic sample of 5301 surgical patients and 14 934 matched nonsurgical patients. Surgical patients were primarily middle-aged, female, had commercial insurance, and more than 40% were racial/ethnic minorities. Seventy-six percent of patients with bariatric surgery underwent Roux-en-Y (RYGB) from 2005 to 2011, 17% underwent sleeve gastrectomy (SG), and 7% underwent adjustable gastric band. Among all patients at the index date, 52% of patients had a BMI of 40 to 49.9, the mean HbA1c level was 7.2%, and 49% had type 2 diabetes for 5 years or more before baseline. Fewer surgical patients had been current smokers in the 2 years before surgery (9% vs 13%). More nonsurgical patients had a diagnosis of diabetic retinopathy, neuropathy, and renal manifestations, including end-stage renal disease or dialysis. Standardized differences indicate imbalance between surgical and matched nonsurgical patients on a number of variables, including insurance type, days of health care usage, use of other antihypertensive medications, and baseline prevalence of peripheral arterial disease.
Table 1. Baseline Characteristics of Patients With Type 2 Diabetes Who Underwent Bariatric Surgery and Matched Nonsurgical Patients, 2005-2011.
| Characteristic | No. (%)a | Standardized Differenceb | |
|---|---|---|---|
| Patients Who Underwent Bariatric Surgery | Matched Nonsurgical Patients | ||
| No. of patients | 5301 | 14 934 | |
| Procedure type | |||
| Roux-en-Y gastric bypass | 4036 (76) | ||
| Sleeve gastrectomy | 896 (17) | ||
| Adjustable gastric band | 369 (7) | ||
| Age, mean (SD), y | 49.5 (10.0) | 50.2 (10.1) | −7.0 |
| Age categories, y | |||
| 18-29 | 118 (2) | 341 (2) | −0.4 |
| 30-44 | 1531 (29) | 3950 (26) | 5.4 |
| 45-54 | 1857 (35) | 5154 (35) | 1.1 |
| 55-64 | 1517 (29) | 4519 (30) | −3.6 |
| 65-79 | 278 (5) | 970 (6) | −5.3 |
| Sex | |||
| Female | 4023 (76) | 11 180 (75) | 2.4 |
| Male | 1278 (24) | 3754 (25) | −2.4 |
| Race/ethnicity | |||
| Hispanic | 930 (18) | 2622 (18) | 0 |
| Non-Hispanic black | 812 (15) | 2521 (17) | −4.3 |
| Non-Hispanic white | 2534 (48) | 6314 (42) | 11.1 |
| Otherc | 400 (8) | 976 (7) | 3.9 |
| Unknown/missing | 625 (12) | 2501 (17) | −14.2 |
| Health care site | |||
| HealthPartners | 247 (5) | 691 (5) | 0.2 |
| Kaiser Permanente Northern California | 1290 (24) | 3834 (26) | −3.1 |
| Kaiser Permanente Southern California | 3381 (64) | 9293 (62) | 3.2 |
| Kaiser Permanente Washington | 383 (7) | 1116 (7) | −0.9 |
| Insurance type | |||
| Commercial | 4908 (93) | 12 825 (86) | 21.8 |
| Medicaid | 144 (3) | 553 (4) | −5.6 |
| Medicare | 172 (3) | 1268 (8) | −22.5 |
| Other | 77 (1) | 288 (2) | −3.7 |
| Year of surgery/index date | |||
| 2005 | 87 (2) | 239 (2) | 0.3 |
| 2006 | 304 (6) | 874 (6) | −0.5 |
| 2007 | 536 (10) | 1554 (10) | −1.0 |
| 2008 | 769 (15) | 2189 (15) | −0.4 |
| 2009 | 852 (16) | 2351 (16) | 0.9 |
| 2010 | 1163 (22) | 3219 (22) | 0.9 |
| 2011 | 1590 (30) | 4580 (30) | −0.4 |
| Total No. of days of health care use in 7-24 mo pre-index date, mean (SD) | 20.0 (12.7) | 16.2 (10.3) | 33.0 |
| BMI, mean (SD) | 44.7 (6.9) | 43.8 (6.7) | 13.2 |
| BMI categories | |||
| 35.0-39.9 | 1460 (28) | 4947 (33) | −12.2 |
| 40.0-49.9 | 2781 (52) | 7522 (50) | 4.2 |
| ≥50.0 | 1060 (20) | 2465 (17) | 9.0 |
| eGFR, mean (SD), mL/min/1.73 m2 | 90.5 (23.0) | 87.6 (26.6) | 11.7 |
| Hemoglobin A1c level, mean (SD), % | 7.17 (1.24) | 7.20 (1.22) | −2.4 |
| Observed duration of diabetes, mean (SD), y | 5.64 (4.06) | 5.64 (4.09) | 0 |
| 0-4 | 2704 (51) | 7752 (52) | −1.8 |
| ≥5 | 2597 (49) | 7182 (48) | 1.8 |
| Use of oral diabetes medication | 3639 (69) | 10172 (68) | 1.1 |
| Use of insulin | 1294 (24) | 3700 (25) | −0.8 |
| Peripheral arterial disease | 175 (3) | 946 (6) | −14.2 |
| Dyslipidemia | |||
| Triglyceride level ≥150 mg/dLd | 2483 (47) | 6880 (46) | 1.5 |
| Missing triglyceride leveld | 51 (1) | 545 (4) | −18.0 |
| Dyslipidemia diagnosise | 4405 (83) | 12 259 (82) | 2.7 |
| Use of a statind | 2893 (55) | 8365 (56) | −2.9 |
| Use of other lipid-lowering medicationsd | 337 (6) | 1028 (7) | −2.1 |
| LDL cholesterol level ≥100 mg/dLd | 855 (16) | 2439 (16) | −0.6 |
| Missing LDL cholesterol leveld | 3527 (67) | 9775 (65) | 2.3 |
| Hypertension | |||
| Uncontrolled hypertensiond,e,f | 422 (8) | 1632 (11) | −10.2 |
| Missing blood pressure measurementd | 23 (0) | 86 (1) | −2.0 |
| Hypertension diagnosise | 4080 (77) | 10775 (72) | 11.1 |
| Use of ACE inhibitors or ARBsd | 2992 (56) | 9205 (62) | −10.6 |
| Use of other antihypertensive medicationsd | 2421 (46) | 8499 (57) | −22.6 |
| Microvascular disease | |||
| Diabetes with renal manifestations, end-stage renal disease, or dialysise | 1256 (24) | 3923 (26) | −6.0 |
| Diabetic retinopathye | 642 (12) | 2147 (14) | −6.7 |
| Diabetic neuropathye | 1050 (20) | 3441 (23) | −7.9 |
| Smoking statuse | |||
| Current | 469 (9) | 1905 (13) | −12.6 |
| Former | 1701 (32) | 3901 (26) | 13.2 |
| Never | 3009 (57) | 8486 (57) | −0.1 |
| Missing | 122 (2) | 642 (4) | −11.2 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein.
SI conversion factors: To convert LDL cholesterol to mmol/L, multiply by 0.0259; and triglyceride level to mmol/L, multiply by 0.0113.
For categorical variables, counts and percentages are presented; for continuous variables, means (SDs) are presented.
The standardized difference is the difference between the 2 groups, divided by their average SD.
The “other” category includes patients who identified as Asian, Hawaiian/Pacific Islander, or Native American/Alaskan Native.
Values represent characteristics at the time of bariatric surgery for surgical patients (or equivalent index date for nonsurgical patients).
Values represent characteristics for the 2-year period prior to surgery.
Uncontrolled hypertension defined as either systolic blood pressure ≥140 or diastolic ≥90 mm Hg at 2 consecutive measures on different days.
The 1-, 3-, and 5-year retention rates in the analytic sample were similar for nonsurgical (93%, 79%, and 68%, respectively) and surgical (91%, 77%, and 67%, respectively) patients. Median follow-up durations were 4.7 years (interquartile range, 3.3-6.2) for surgical and 4.6 years (interquartile range, 3.1-6.1) for nonsurgical patients. Missing data were encountered for some characteristics at baseline, including race/ethnicity (12% in the surgical group vs 17% in the nonsurgical group), self-reported smoking status (2% vs 4%, respectively), blood pressure (0% vs 1%, respectively), triglyceride levels (1% vs 4%, respectively), and LDL cholesterol level (67% vs 65%, respectively).
Association of Bariatric Surgery vs Usual Care With Incident Macrovascular Disease
Figure 2 and Table 2 provide estimates of the cumulative probability of incident macrovascular disease over time after bariatric surgery and usual nonsurgical care (for matched controls), as well as the cumulative probability of each of the indicators of macrovascular disease (coronary artery disease and cerebrovascular events). The 1-, 3-, 5-, and 7-year rates of incident macrovascular disease were 0.5%, 1.1%, 2.1%, and 3.2%, respectively, after bariatric surgery, and 1.1%, 2.6%, 4.3%, and 6.2% for the matched controls. The cumulative number of macrovascular events at each of these time points is shown in eTable 3 in the Supplement. Rates of incident coronary artery disease and cerebrovascular events were also lower for patients with bariatric surgery than for matched nonsurgical patients (Figure 2B and C; Table 2).
Figure 2. Cumulative Incidence Rates at All Study Sites.
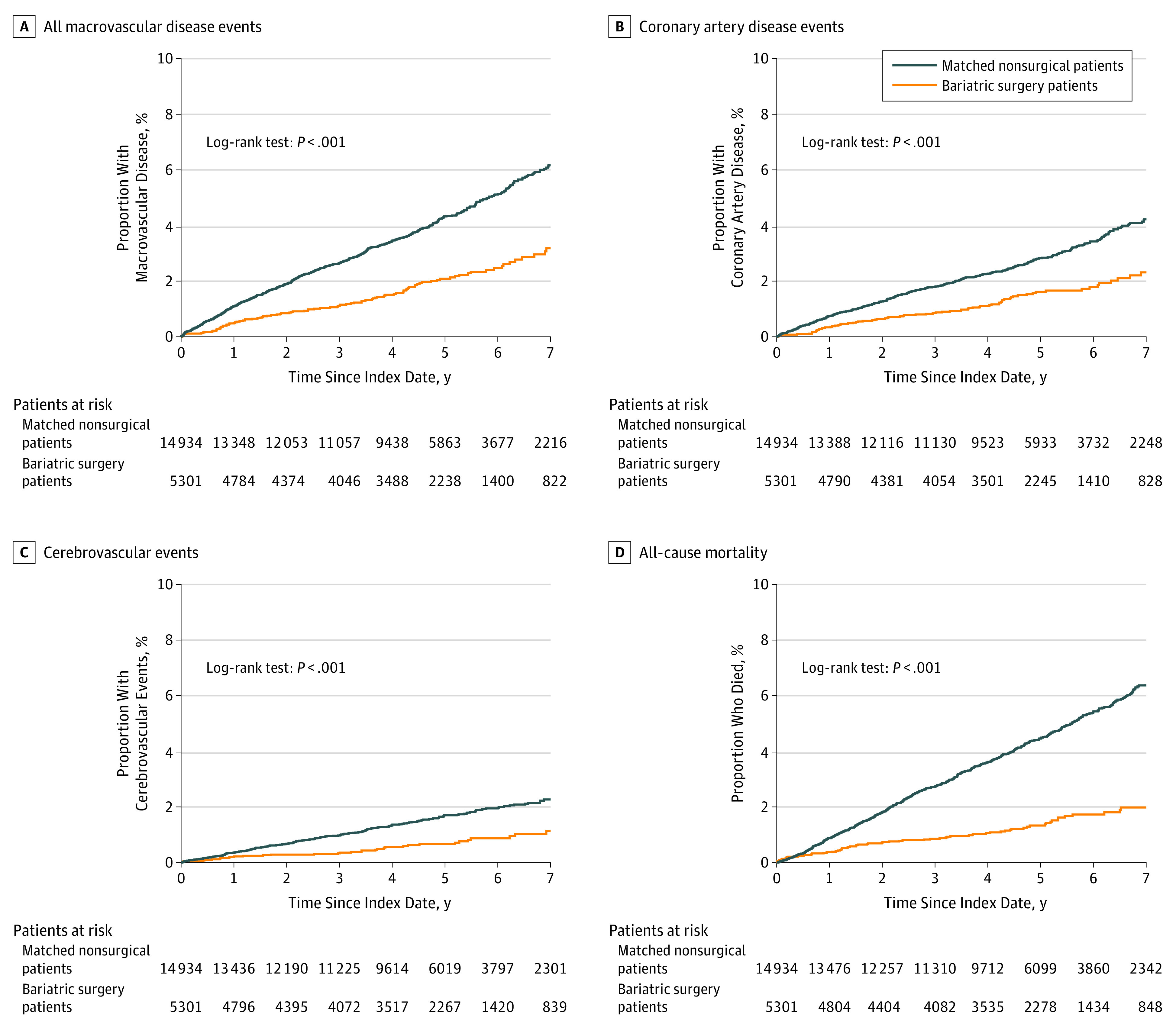
Kaplan-Meier estimates of the cumulative incidence of macrovascular disease and all-cause mortality following bariatric surgery vs matched nonsurgical patients. Separate estimates for coronary artery disease events (B) and cerebrovascular events (C) are shown, as well as a composite estimate for incident macrovascular disease due to either of these event classes (A) and all-cause mortality (D). The median follow-up time among surgical patients was 4.7 years (interquartile range, 3.2-6.2), and among matched nonsurgical patients was 4.6 years (interquartile range, 3.1-6.1).
Table 2. Cumulative Incidence Rates and 95% CIs for Macrovascular Disease Outcomes in Surgical Patients and Matched Nonsurgical Patientsa.
| Incidence Rate (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 y | 3 y | 5 y | 7 y | |||||
| Surgical Group | Nonsurgical Group | Surgical Group | Nonsurgical Group | Surgical Group | Nonsurgical Group | Surgical Group | Nonsurgical Group | |
| Composite Index of Incident Macrovascular Diseaseb | 0.5 (0.3-0.7) | 1.1 (0.9-1.3) | 1.1 (0.8-1.4) | 2.6 (2.4-2.9) | 2.1 (1.6-2.5) | 4.3 (3.9-4.7) | 3.2 (2.5-3.9) | 6.2 (5.6-6.7) |
| Incident cerebrovascular disease | 0.2 (0.1-0.3) | 0.4 (0.3-0.4) | 0.3 (0.2-0.5) | 1 (0.8-1.1) | 0.7 (0.4-0.9) | 1.7 (1.5-1.9) | 1.1 (0.7-1.6) | 2.3 (1.9-2.6) |
| Incident coronary artery disease | 0.3 (0.2-0.5) | 0.7 (0.6-0.9) | 0.9 (0.6-1.1) | 1.8 (1.6-2) | 1.6 (1.2-2) | 2.8 (2.5-3.1) | 2.3 (1.7-2.9) | 4.2 (3.7-4.7) |
| Death (post hoc analysis) | 0.4 (0.2-0.5) | 0.9 (0.7-1) | 0.9 (0.6-1.1) | 2.7 (2.4-3) | 1.3 (1-1.7) | 4.5 (4.1-4.8) | 2.0 (1.5-2.5) | 6.4 (5.8-7) |
Patients were matched on age, sex, body mass index, site, insulin use, hemoglobin A1c level, observed diabetes duration, and number of days of health care utilization in the 7 to 24 months prior to index date.
The Composite Index of Incident Macrovascular Disease indicates the first occurrence of cerebrovascular or coronary artery disease event.
Table 3 and Figure 3 show the results from the adjusted multivariable Cox models investigating the association between bariatric surgery vs usual care and incident macrovascular disease (see Table 4 for the fully adjusted model with all covariates). Because the effect of surgery was specified in the model as being time-varying using cubic splines, calculating the HRs shown in Table 3 required using all the components of Table 4 and specifying the time points at which the HRs were estimated. Table 3 shows the HRs estimated at 1, 3, 5, and 7 years and Figure 3 shows the HR estimation for all time points. In Table 4, the most appropriate interpretation of the surgery point estimate in row one is the HR comparing surgery vs no surgery at time zero (the index date). The point estimates for the surgery-by-spline interactions are uninterpretable because of the underlying basis function for the spline. The point estimates for other variables in the model should not be interpreted as unconfounded because this was a matched cohort with respect to surgery and the consideration of confounding was with respect to surgery only, not with respect to these other covariates.
Table 3. Results of Matched, Fully Adjusted Cox Proportional Hazards Model Comparing the Risk of Incident Macrovascular Disease Outcomes in Surgical Patients vs Matched Nonsurgical Patientsa.
| Years After Index Date, Hazard Ratio (95% CI) | ||||
|---|---|---|---|---|
| 1 | 3 | 5 | 7 | |
| Composite Index of Incident Macrovascular Diseaseb | 0.47 (0.34-0.65) | 0.52 (0.38-0.71) | 0.60 (0.42-0.86) | 0.58 (0.34-0.99) |
| Incident cerebrovascular disease | 0.39 (0.21-0.71) | 0.39 (0.22-0.68) | 0.69 (0.38-1.25) | 0.58 (0.25-1.36) |
| Incident coronary artery disease | 0.54 (0.37-0.78) | 0.63 (0.44-0.92) | 0.64 (0.42-0.99) | 0.56 (0.29-1.08) |
| Death (post hoc analysis)c | 0.38 (0.26-0.56) | 0.23 (0.15-0.35) | 0.33 (0.21-0.52) | 0.34 (0.15-0.74) |
At the end of the study period, there were 106 macrovascular events in the 5301 surgical patients (including 37 cerebrovascular and 78 coronary artery disease events over a median of 4.7 years [interquartile range, 3.2-6.2]) and 596 macrovascular events in the 14 934 matched nonsurgical patients (including 227 cerebrovascular and 398 coronary artery disease events over a median of 4.6 years [interquartile range, 3.1-6.1]). Patients were matched on age, sex, body mass index, site, insulin use, hemoglobin A1c level, observed diabetes duration, and number of days of health care utilization in the 7 to 24 months prior to index date. Models were adjusted for all matching variables plus race/ethnicity, insurance type, diabetes duration, oral diabetes medication use, hypertension diagnosis, baseline blood pressure, angiotensin converting enzyme inhibitor and angiotensin receptor blocker use, other antihypertensive medication use, dyslipidemia diagnosis, statin use, other lipid-lowering agent use, index year, estimated glomerular filtration rate, elevated low-density lipoprotein cholesterol level, elevated triglyceride level, peripheral artery disease, diabetic retinopathy, neuropathy, nephropathy, and smoking status.
The Composite Index of Incident Macrovascular Disease indicates the first occurrence of cerebrovascular or coronary artery disease event.
Death was specified as a post hoc analysis, and results should be viewed as exploratory.
Figure 3. Time-Varying Hazard Ratios Comparing Surgical and Matched Nonsurgical Patients.
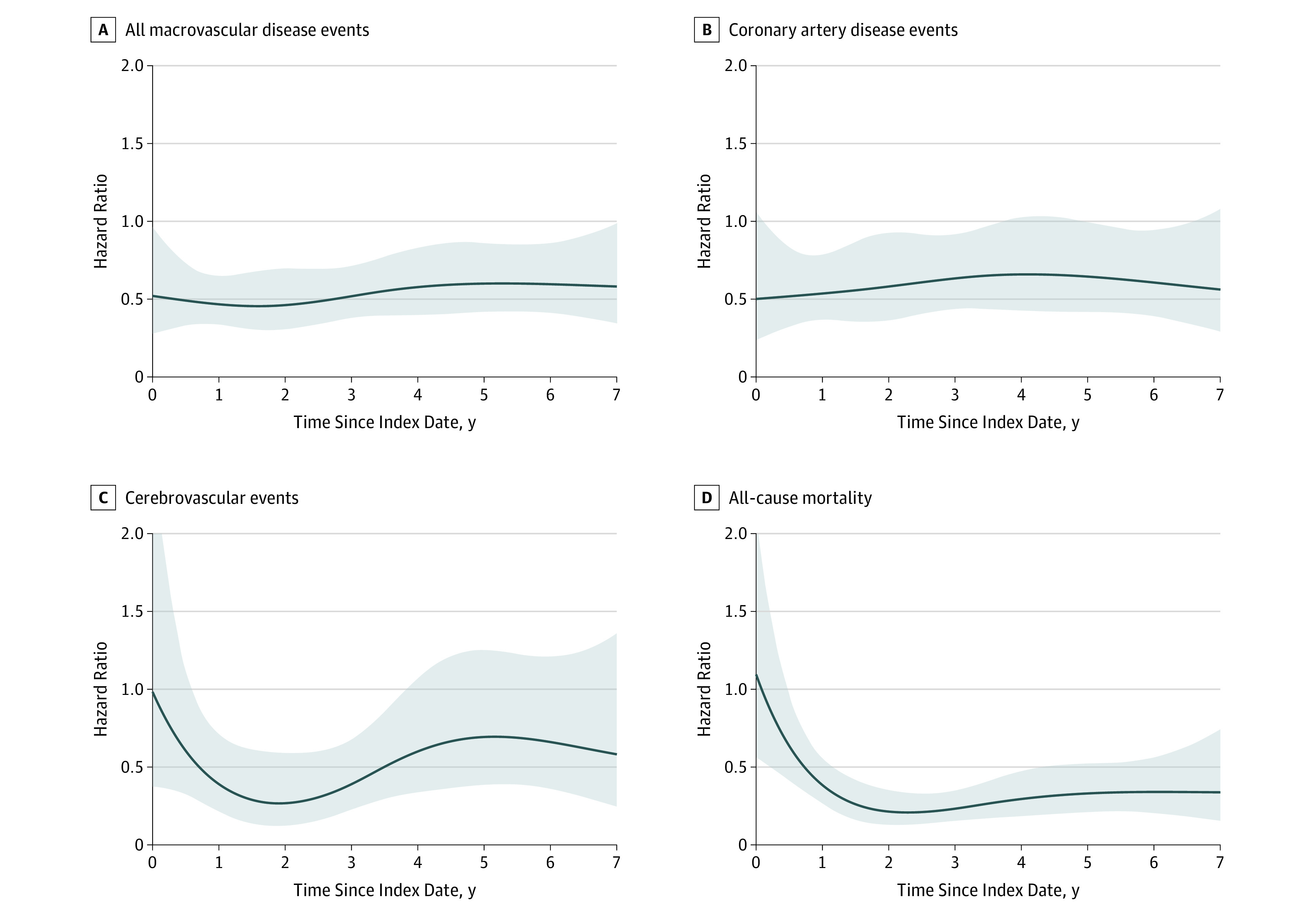
Time-varying hazard ratios comparing the risk of incident macrovascular disease and death following bariatric surgery vs matched nonsurgical patients. Separate estimates for coronary artery disease events (B) and cerebrovascular events (C) are shown, as well as a composite estimate for incident macrovascular disease due to either of these event classes (A) and all-cause mortality (D). The median follow-up time among surgical patients was 4.7 years (interquartile range, 3.2-6.2), and among matched nonsurgical patients was 4.6 years (interquartile range, 3.1-6.1). All analyses used the full cohort of patients who underwent surgery (n = 5301) and matched controls (n = 14 934). Shaded areas represent the 95% CIs.
Table 4. Fully Adjusted Cox Proportional Hazards Model of Time to Composite Indicator of Any Incident Macrovascular Disease.
| Hazard Ratio (95% CI) | ||||
|---|---|---|---|---|
| All Macrovascular Events | Coronary Artery Disease | Cerebrovascular Disease | Death | |
| Surgerya | 0.52 (0.28-0.97) |
0.50 (0.24-1.06) |
0.98 (0.37-2.58) |
1.09 (0.57-2.12) |
| Surgery by spline interactiona | ||||
| 1 | 1.00 (0.99-1.00) |
1.00 (0.99-1.00) |
1.00 (0.99-1.00) |
1.00 (1.00-1.00) |
| 2 | 1.00 (0.99-1.01) |
1.00 (0.99-1.01) |
1.01 (0.99-1.02) |
1.01 (1.00-1.02) |
| 3 | 0.99 (0.97-1.01) |
1.00 (0.97-1.02) |
0.97 (0.94-1.00) |
0.98 (0.95-0.99) |
| Age, y | ||||
| 18-29 | 0.81 (0.25-2.57) |
1.45 (0.45-4.7) |
NAb | 0.74 (0.23-2.35) |
| 30-44 | [Reference] | [Reference] | [Reference] | [Reference] |
| 45-54 | 1.58 (1.19-2.09) |
1.70 (1.2-2.41) |
1.36 (0.86-2.14) |
1.33 (1.00-1.78) |
| 55-64 | 2.18 (1.63-2.92) |
2.12 (1.48-3.03) |
2.20 (1.39-3.49) |
1.92 (1.44-2.57) |
| 65-79 | 2.10 (1.43-3.07) |
1.86 (1.15-2.99) |
2.19 (1.21-3.96) |
1.66 (1.16-2.39) |
| Sex | ||||
| Male | 1.30 (1.11-1.54) |
1.51 (1.24-1.84) |
0.99 (0.74-1.31) |
1.55 (1.32-1.82) |
| Female | [Reference] | [Reference] | [Reference] | [Reference] |
| Race/ethnicity | ||||
| Hispanic | 1.07 (0.85-1.34) |
0.89 (0.68-1.16) |
1.36 (0.93-2.01) |
0.79 (0.63-0.98) |
| Non-Hispanic black | 1.21 (0.96-1.51) |
0.86 (0.65-1.15) |
2.03 (1.47-2.82) |
0.91 (0.73-1.14) |
| Non-Hispanic white | [Reference] | [Reference] | [Reference] | [Reference] |
| Other | 1.17 (0.85-1.6) |
1.15 (0.79-1.68) |
1.42 (0.84-2.4) |
0.85 (0.59-1.21) |
| Insurance | ||||
| Commercial | [Reference] | [Reference] | [Reference] | [Reference] |
| Medicaid | 1.30 (0.86-1.96) |
1.25 (0.75-2.1) |
1.56 (0.83-2.91) |
1.70 (1.19-2.44) |
| Medicare | 1.14 (0.89-1.47) |
1.06 (0.78-1.45) |
1.32 (0.89-1.94) |
1.84 (1.49-2.27) |
| Other | 1.74 (1.12-2.71) |
1.82 (1.09-3.04) |
1.24 (0.5-3.04) |
0.67 (0.32-1.42) |
| Year of surgery | ||||
| 2005 | 2.63 (1.64-4.21) |
3.72 (2.18-6.35) |
0.78 (0.26-2.31) |
3.35 (1.74-6.43) |
| 2006 | 1.78 (1.27-2.5) |
2.12 (1.41-3.19) |
1.51 (0.86-2.63) |
2.50 (1.73-3.61) |
| 2007 | 1.37 (1.02-1.84) |
1.58 (1.1-2.27) |
1.17 (0.72-1.9) |
2.10 (1.57-2.81) |
| 2008 | 1.31 (1.01-1.7) |
1.42 (1.02-1.97) |
1.26 (0.83-1.91) |
1.57 (1.2-2.06) |
| 2009 | 1.34 (1.05-1.72) |
1.40 (1.03-1.91) |
1.27 (0.87-1.87) |
1.54 (1.2-1.97) |
| 2010 | 0.97 (0.75-1.24) |
1.07 (0.78-1.46) |
0.79 (0.52-1.18) |
1.24 (0.98-1.59) |
| 2011 | [Reference] | [Reference] | [Reference] | [Reference] |
| No. of days with any health care use in 7 to 24 mo before baseline |
1.01 (1.00-1.02) |
1.01 (1.00-1.02) |
1.01 (1.00-1.02) |
1.03 (1.02-1.03) |
| BMI category | ||||
| 34-39.9 | [Reference] | [Reference] | [Reference] | [Reference] |
| 40-49.9 | 0.97 (0.82-1.14) |
0.97 (0.79-1.18) |
0.98 (0.75-1.29) |
1.11 (0.92-1.32) |
| ≥50 | 0.71 (0.55-0.92) |
0.62 (0.45-0.85) |
0.81 (0.54-1.21) |
1.83 (1.47-2.28) |
| Estimated glomerular filtration rate | 0.99 (0.99-1) |
0.99 (0.98-0.99) |
1.00 (0.99-1.00) |
0.99 (0.98-0.99) |
| Hemoglobin A1c level at baseline | 1.07 (1-1.14) |
1.05 (0.97-1.13) |
1.15 (1.04-1.27) |
0.99 (0.92-1.06) |
| Diabetes duration, y | 1.01 (0.99-1.04) |
1.00 (0.98-1.03) |
1.03 (0.99-1.07) |
1.00 (0.98-1.03) |
| Oral hypoglycemic use | 0.69 (0.58-0.81) |
0.71 (0.57-0.87) |
0.69 (0.53-0.91) |
0.86 (0.73-1.02) |
| Insulin use | 1.11 (0.92-1.35) |
1.18 (0.94-1.48) |
1.04 (0.77-1.41) |
1.17 (0.97-1.41) |
| Dyslipidemia diagnosis | 1.88 (1.34-2.63) |
2.15 (1.38-3.36) |
1.62 (0.97-2.72) |
0.90 (0.66-1.22) |
| Statin use | 0.89 (0.75-1.06) |
0.85 (0.69-1.05) |
0.96 (0.72-1.28) |
0.90 (0.75-1.09) |
| Triglyceride level ≥150 mg/dL | 1.20 (1.02-1.41) |
1.41 (1.16-1.71) |
0.90 (0.69-1.17) |
0.95 (0.8-1.12) |
| Fibrate or niacin use | 0.91 (0.69-1.2) |
0.88 (0.64-1.21) |
1.00 (0.63-1.58) |
0.74 (0.55-0.99) |
| LDL cholesterol level >100 mg/dL | 1.08 (0.91-1.3) |
1.14 (0.82-1.58) |
0.96 (0.66-1.4) |
1.02 (0.78-1.34) |
| Blood pressure ≥140/90 mm Hg at baseline | 1.75 (1.45-2.11) |
1.93 (1.54-2.41) |
1.37 (0.99-1.88) |
1.19 (0.97-1.47) |
| Hypertension diagnosis | 1.09 (0.85-1.41) |
1.07 (0.78-1.45) |
1.02 (0.68-1.55) |
0.87 (0.66-1.14) |
| ACE inhibitor or ARB use | 0.81 (0.68-0.96) |
0.90 (0.73-1.12) |
0.69 (0.52-0.91) |
0.78 (0.66-0.93) |
| Use of other non-ACE/ARB antihypertensive medications |
1.39 (1.14-1.7) |
1.34 (1.05-1.71) |
1.60 (1.15-2.23) |
1.50 (1.2-1.86) |
| Diabetic retinopathy diagnosis | 1.32 (1.09-1.61) |
1.40 (1.11-1.78) |
1.12 (0.81-1.53) |
1.31 (1.07-1.6) |
| Renal disease diagnosis | 1.15 (0.94-1.41) |
1.01 (0.79-1.3) |
1.45 (1.07-1.98) |
1.60 (1.29-1.99) |
| Neuropathy diagnosis | 1.29 (1.08-1.54) |
1.33 (1.07-1.65) |
1.17 (0.87-1.57) |
1.21 (1.01-1.46) |
| Peripheral artery disease | 1.50 (1.19-1.87) |
1.41 (1.07-1.85) |
1.79 (1.26-2.55) |
1.64 (1.35-2.01) |
| Smoking | ||||
| Never | [Reference] | [Reference] | [Reference] | [Reference] |
| Current | 1.48 (1.17-1.87) |
1.65 (1.25-2.18) |
1.24 (0.81-1.89) |
1.91 (1.52-2.4) |
| Former | 1.27 (1.07-1.51) |
1.20 (0.98-1.47) |
1.36 (1.04-1.79) |
1.11 (0.93-1.32) |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LDL, low-density lipoprotein; NA, not applicable.
SI conversion factors: To convert LDL cholesterol to mmol/L, multiply by 0.0259; and triglyceride level to mmol/L, multiply by 0.0113.
The point estimates for the effect of surgery (row 1) on all outcomes are not easily interpretable because they arise from a spline-based model that includes 3 interactions with surgery (surgery by spline [1], surgery by spline [2], and surgery by spline [3]). Because the effect of surgery is specified as being time-varying through a cubic spline, calculating the hazard ratios in Table 3 requires using all components of Table 4 and specifying the time points at which the hazard ratios are to be estimated. In Table 3, we provide hazard ratios estimated at 1, 3, 5, and 7 years. In Figure 3, this hazard ratio estimation is presented at all time points. In Table 4, the most appropriate interpretation of the “surgery” point estimate in row 1 is the hazard ratio comparing surgery vs no surgery at time zero (the index date). The point estimates for the surgery by spline interactions are uninterpretable because of the underlying basis function for the spline. The point estimates for other variables in the model are hazard ratios, although these effects should not be interpreted as unconfounded because this is a matched cohort with respect of surgery and the consideration of confounding is with respect to surgery only, not with respect to these other covariates.
No cerebrovascular events were observed in the youngest age category.
As shown in Table 3, patients who underwent bariatric surgery had a significantly lower risk of incident macrovascular disease at 5 years when compared with matched nonsurgical patients who had not received surgery (2.1% in the surgical group vs 4.3% in the nonsurgical group; HR, 0.60 [95% CI, 0.42-0.86]), as well as a lower 5-year incidence of coronary artery disease (1.6% in the surgical group vs 2.8% in the nonsurgical group; HR, 0.64 [95% CI, 0.42-0.99]). The incidence of cerebrovascular disease was not significantly different between groups at the 5-year time point (0.7% in the surgical group vs 1.7% in the nonsurgical group; HR, 0.69 [95% CI, 0.38-1.25]).
Sensitivity Analyses
Results of the sensitivity analyses are provided in the eFigure in the Supplement. A matched, unadjusted analysis of our primary outcome was not significantly different than the fully adjusted model (eFigure, A in the Supplement). Primary outcome results using 3-to-1 matching were qualitatively similar to using 1-to-1 and 10-to-1 matching (eFigure, B in the Supplement). Primary outcome results were similar to results obtained when all covariates were included in the Mahalanobis distance calculation for matching (eFigure, C in the Supplement). The E-values (relative risk) for the point estimate and upper confidence bound for incident macrovascular disease at 5 years were 2.72 and 1.60, respectively (eAppendix in the Supplement).
Association of Bariatric Surgery vs Usual Care With Mortality (Post Hoc Exploratory Analyses)
The rates of mortality at 1, 3, 5, and 7 years were 0.4%, 0.9%, 1.3%, and 2.0%, respectively, for surgical and 0.9%, 2.7%, 4.5%, and 6.4% for nonsurgical patients (Table 2; Figure 2D). The cumulative number of deaths at each of these time points is shown in the eAppendix in the Supplement. The risk of all-cause mortality was significantly lower at 5 years (HR, 0.33 [95% CI, 0.21-0.52]) among patients who underwent bariatric surgery relative to the matched nonsurgical patients (Table 3 and Figure 3D).
Discussion
In this retrospective cohort study of patients with type 2 diabetes and severe obesity, bariatric surgery was associated with a lower risk of major macrovascular outcomes compared with usual medical care. These findings have strong biological plausibility and are consistent with other research. Randomized trials have demonstrated that bariatric procedures are more effective than the best-available intensive medical and lifestyle interventions in promoting weight loss, improving glycemic control and serum lipid levels, and reducing the need for medications used to control diabetes, hypertension, and dyslipidemia.10,11,12,23,24,25 Improvements in these parameters after bariatric surgery result in lower cardiovascular risk scores, as measured through validated tools such as the Framingham risk equation.26,27 The effect of bariatric surgery also appeared to be dose-dependent, where patients who underwent RYGB procedures (which is associated with greatest initial weight loss) had larger improvements in glycemic control and components of the metabolic syndrome than patients who underwent SG or underwent the adjustable gastric band procedure,28,29 although there may also be weight-independent effects of these procedures on glycemic control.13
A few other observational studies have also found an association between bariatric surgery and fewer macrovascular events when compared with usual care.13,14,15,30 The landmark Swedish Obese Subjects study prospectively recruited 2010 patients who underwent bariatric surgery and 2037 matched patients who received usual care and found that surgery was associated with an adjusted hazard ratio of 0.67 (95% CI, 0.54-0.83) for cardiovascular events (myocardial infarction or stroke).14 A subanalysis of 343 surgical and 260 usual care patients with type 2 diabetes in the Swedish Obese Subjects study found a similar association on macrovascular event rates (HR, 0.68 [95% CI, 0.54-0.85]; P = .001).14 These findings are also consistent with another recent single-center study of RYGB, which found fewer cardiovascular events in an RYGB cohort (HR, 0.58 [95% CI, 0.42-0.82]; P = .02) compared with matched controls.30
While accumulating data suggest a causal effect of intentional weight loss on lower cardiovascular events and premature mortality, randomized clinical trials to date have not confirmed these findings. The Look AHEAD study randomized 5145 patients with obesity and type 2 diabetes to intensive lifestyle intervention or usual care, but was stopped early because the intervention had no effect on the primary macrovascular disease end point (HR, 0.95 [95% CI, 0.83-1.09]; P = .51).31 The ongoing Alliance of Randomized Trials of Medicine vs Metabolic Surgery in T2DM (ARMMS-T2D) is an observational follow-up of 4 randomized trials comparing bariatric surgery vs intensive medical and lifestyle treatment, but, with approximately 300 randomized participants, that study may not be powered to assess macrovascular end points. Randomized clinical trials with sufficient power to assess these rare outcomes are unlikely to be conducted because such studies are challenging to conduct and prohibitively expensive.32
Limitations
The study has several limitations. First, the observational design precluded causal inference, and unmeasured confounding may have persisted despite model adjustment for all major cardiovascular risk factors. However, the sensitivity analysis using E-value methodology indicated that the observed 5-year HR of 0.60 for incident macrovascular disease could only be explained by an unmeasured confounder that was associated with both receipt of bariatric surgery and risk of macrovascular disease by a risk ratio of more than 2.72 above and beyond that of the confounders that were measured in this study (upper confidence bound, 1.60). Given that this risk ratio is much greater than any observed for known macrovascular disease risk factors examined in the current study, such as hypertension, diabetes, or hyperlipidemia, it is implausible that an unmeasured confounder exists that can overcome the effect of bariatric surgery observed in the current analysis study (Table 4).
Second, baseline health characteristics and outcomes were established using data collected during routine medical care and billing, which meant that some information was missing and some comorbid conditions could be misclassified (eg, ICD-9 diagnosis codes could be misapplied); however, major cardiac and cerebrovascular outcomes were more likely to be accurately captured claims for all diagnoses and procedures associated with emergency department and hospital admissions.
Third, cause-specific mortality was not examined because cause of death data were not extracted a priori.
Fourth, loss to follow-up could bias the result if patients who underwent bariatric surgery and left the integrated health care systems in this study had very different macrovascular outcomes than the nonsurgical patients who left these systems.
Fifth, the sample size was insufficient to compare the effectiveness of alternative bariatric procedures for these outcomes. There has been a shift toward increased use of the SG procedure in recent years in the United States, and although this study included 17% SG, it is unclear whether the benefits observed in a primarily RYGB population will be seen with SG.
Sixth, given sample size and statistical constraints related to the number of variables that could be accommodated in the matching process, we could not match on every available characteristic. This left some imbalances in other variables (Table 1; standardized differences >10%) that were not part of our matching algorithm. To further address confounding, all variables in Table 1 were adjusted for in our multivariable Cox models.
Conclusions
In this observational study of patients with type 2 diabetes and severe obesity who underwent surgery, compared with those who did not undergo surgery, bariatric surgery was associated with a lower risk of macrovascular outcomes. The findings require confirmation in randomized clinical trials. Health care professionals should engage patients with severe obesity and type 2 diabetes in a shared decision making conversation about the potential role of bariatric surgery in the prevention of macrovascular events.
eAppendix. Statistical Methods
eTable 1. Baseline Characteristics of Patients With Type 2 Diabetes Who Underwent Bariatric Surgery Who Were Included in the Study and Those That Were Excluded Due to Missing Data or Inability to Match to Nonsurgical Patients, 2005-2011
eTable 2. Reasons for Censoring for Surgical and Nonsurgical Populations
eTable 3. Cumulative Number of Incident Macrovascular Events and Deaths Among Bariatric Patients and Matched Nonsurgical Patients
eFigure. Sensitivity Analyses
References
- 1.American Diabetes Association . 9. Cardiovascular disease and risk management. Diabetes Care. 2017;40(suppl 1):S75-S87. doi: 10.2337/dc17-S012 [DOI] [PubMed] [Google Scholar]
- 2.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus: mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459-2502. doi: 10.1161/CIRCULATIONAHA.116.022194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383-393. doi: 10.1056/NEJMoa021778 [DOI] [PubMed] [Google Scholar]
- 4.Ji L, Hu D, Pan C, et al. ; CCMR Advisory Board; CCMR-3B STUDY Investigators . Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. 2013;126(10):925.e11-925.e22. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham JW, Wiviott SD. Modern obesity pharmacotherapy: weighing cardiovascular risk and benefit. Clin Cardiol. 2014;37(11):693-699. doi: 10.1002/clc.22304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston CA, Moreno JP, Foreyt JP. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. Curr Atheroscler Rep. 2014;16(12):457. doi: 10.1007/s11883-014-0457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arterburn DE, O’Connor PJ. A look ahead at the future of diabetes prevention and treatment. JAMA. 2012;308(23):2517-2518. doi: 10.1001/jama.2012.144749 [DOI] [PubMed] [Google Scholar]
- 8.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311(1):74-86. doi: 10.1001/jama.2013.281361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14(1):12-24. doi: 10.1038/nrendo.2017.122 [DOI] [PubMed] [Google Scholar]
- 10.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150(10):931-940. doi: 10.1001/jamasurg.2015.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59(5):945-953. doi: 10.1007/s00125-016-3903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery or intensive medical therapy for diabetes after 5 years. N Engl J Med. 2017;376(20):1997. [DOI] [PubMed] [Google Scholar]
- 13.Adams TD, Arterburn DE, Nathan DM, Eckel RH. Clinical outcomes of metabolic surgery: microvascular and macrovascular complications. Diabetes Care. 2016;39(6):912-923. doi: 10.2337/dc16-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297-2304. doi: 10.1001/jama.2014.5988 [DOI] [PubMed] [Google Scholar]
- 15.Johnson BL, Blackhurst DW, Latham BB, et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216(4):545-556. [DOI] [PubMed] [Google Scholar]
- 16.Ross TR, Ng D, Brown JS, et al. The HMO Research Network Virtual Data Warehouse: a public data model to support collaboration. EGEMS (Wash DC). 2014;2(1):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matlock DD, Groeneveld PW, Sidney S, et al. Geographic variation in cardiovascular procedure use among Medicare fee-for-service vs Medicare Advantage beneficiaries. JAMA. 2013;310(2):155-162. doi: 10.1001/jama.2013.7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87(1):93-100. doi: 10.1016/j.contraception.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 19.Arterburn D, Johnson ES, Butler MG, Fisher D, Bayliss EA. Predicting 90-day mortality after bariatric surgery: an independent, external validation of the OS-MRS prognostic risk score. Surg Obes Relat Dis. 2014;10(5):774-779. doi: 10.1016/j.soard.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE. Regression Modeling Strategies. Secaucus, NJ: Springer-Verlag New York, Inc; 2006. [Google Scholar]
- 21.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85-95. [Google Scholar]
- 22.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 23.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577-1585. doi: 10.1056/NEJMoa1200111 [DOI] [PubMed] [Google Scholar]
- 24.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240-2249. doi: 10.1001/jama.2013.5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arterburn D, Schauer DP, Wise RE, et al. Change in predicted 10-year cardiovascular risk following laparoscopic Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19(2):184-189. doi: 10.1007/s11695-008-9534-7 [DOI] [PubMed] [Google Scholar]
- 27.Torquati A, Wright K, Melvin W, Richards W. Effect of gastric bypass operation on Framingham and actual risk of cardiovascular events in class II to III obesity. J Am Coll Surg. 2007;204(5):776-782. [DOI] [PubMed] [Google Scholar]
- 28.Coleman KJ, Huang YC, Koebnick C, et al. Metabolic syndrome is less likely to resolve in Hispanics and non-Hispanic blacks after bariatric surgery. Ann Surg. 2014;259(2):279-285. doi: 10.1097/SLA.0000000000000258 [DOI] [PubMed] [Google Scholar]
- 29.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23(1):93-102. doi: 10.1007/s11695-012-0802-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benotti PN, Wood GC, Carey DJ, et al. Gastric bypass surgery produces a durable reduction in cardiovascular disease risk factors and reduces the long-term risks of congestive heart failure. J Am Heart Assoc. 2017;6(5):e005126. doi: 10.1161/JAHA.116.005126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing RR, Bolin P, Brancati FL, et al. ; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145-154. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courcoulas AP, Yanovski SZ, Bonds D, et al. Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg. 2014;149(12):1323-1329. doi: 10.1001/jamasurg.2014.2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Statistical Methods
eTable 1. Baseline Characteristics of Patients With Type 2 Diabetes Who Underwent Bariatric Surgery Who Were Included in the Study and Those That Were Excluded Due to Missing Data or Inability to Match to Nonsurgical Patients, 2005-2011
eTable 2. Reasons for Censoring for Surgical and Nonsurgical Populations
eTable 3. Cumulative Number of Incident Macrovascular Events and Deaths Among Bariatric Patients and Matched Nonsurgical Patients
eFigure. Sensitivity Analyses