Key Points
Question
What are the trajectories of the established cardiometabolic risk factors in the 14 years before incidence of dementia?
Findings
In this nested case-control study of 3925 participants, both body mass index and blood pressure trajectories of future dementia cases deviated from those of dementia-free controls, leading to lower levels at the approach of diagnosis, whereas trajectories of blood lipid levels remained roughly similar between incident dementia cases and matched controls. Elevated glycemia was the only cardiometabolic risk factor with constantly higher values among dementia cases up to 14 years before diagnosis.
Meaning
Appropriate control of elevated glycemia levels and screening of both low blood pressure and weight loss may be key components of cardiovascular health management for the primary and secondary prevention of dementia in older persons.
Abstract
Importance
Cardiometabolic risk factors have been associated with an increased risk of dementia; yet, the optimal targets and time window for the management of cardiometabolic health to prevent dementia remain unknown.
Objectives
To model concurrently and compare the trajectories of cardiometabolic risk factors up to 14 years preceding diagnosis in individuals with dementia and matched controls free of dementia.
Design, Setting, and Participants
A case-control study nested within the Three-City study, a French population-based cohort of older persons (≥65 years), included 6 home visits with neuropsychological testing between 1999 and 2014. Data analysis was performed in September 2017. A total of 785 incident dementia cases and 3140 controls matched by sex, age, educational level, and cohort center at the time of diagnosis were evaluated.
Exposures
Repeated measures of body mass index (BMI) and systolic (SBP) and diastolic (DBP) blood pressure, high-density lipoprotein (HDL-C) and low-density lipoprotein cholesterol (LDL-C), triglycerides, and glycemia levels between 1999 and 2014.
Main Outcomes and Measures
Incidence of dementia based on systematic detection and validated diagnosis.
Results
A total of 785 cases and 3140 controls (2530 [65%] women; mean [SD] age, 76 [5] years) were included in the study. Cases presented a faster decline in BMI, slower increase of SBP and constantly lower DBP. Mean values (95% CI) 14 years before diagnosis (−14 years) and at diagnosis (year 0) for the most common profile were BMI, 26.1 (25.6-26.5) and 24.8 (24.5-25.1) for a case, and 25.7 (25.4-26.1) and 25.3 (25.0-25.5) for a control; for SBP, 135.2 (131.8-138.7) and 142.1 (140.3-143.9) mm Hg for a case, and 135.8 (132.9-138.6) and 144.9 (143.7-146.1) mm Hg for a control; for DBP, 76.5 (74.7-78.5) and 74.0 (73.1-74.9) mm Hg for a case, and 76.7 (75.1-78.3) and 75.0 (74.2-75.7) mm Hg for a control. In contrast, glycemia was higher among cases (mean fasting glucose values [95% CI] at −14 years and year 0: 89.4 [86.9-92.1] and 96.4 [93.7-99.3] mg/dL for a case, and 87.1 [85.1-89.2] and 95.3 [93.5-97.1] mg/dL for a control), with a significant case-control difference from −1.6 to −14 years prior to diagnosis. There were no significant case-control differences in trajectories of blood lipid levels (mean values [95% CI] at −14 years and year 0: for HDL-C, 70.6 [67.6-73.9] and 61.3 [58.9-63.8] mg/dL for a case, and 70.4 [67.5-73.3] and 62.3 [60.2-64.3] mg/dL for a control; for LDL-C: 147.2 [140.5-154.5] and 141.6 [136.6-146.7] mg/dL for a case, and 144.3 [138.7-150.4] and 141.2 [137.5-145.2] mg/dL for a control; for triglycerides: 115.5 [103.6-149.1] and 112.6 [104.8-120.9] mg/dL for a case, and 112.5 [103.8-144.4] and 109.7 [105.0-114.8] mg/dL for a control).
Conclusions and Relevance
In this large cohort of older persons, BMI declined in prodromal dementia, possibly reflecting early preclinical changes. Lower BP prior to dementia may reflect both a consequence and a contributing factor for the disease, whereas higher blood glucose levels may constitute a risk factor for dementia in the older age range. Overall, these findings suggest that elevated glycemia, low BP, and weight loss may be primary targets for the management of cardiometabolic health for primary and secondary prevention of dementia in the older age range.
This case-control study compares trajectories of dementia cardiometabolic risk factors in individuals older than 65 years who develop vs those who do not develop dementia.
Introduction
Appropriate control of cardiometabolic risk factors could be a primary strategy to reduce the incidence of dementia and its most common form, Alzheimer disease (AD).1,2,3,4 Epidemiologic studies found associations between overweight/obesity, hypertension, hypercholesterolemia, and diabetes in midlife and increased risk of dementia,3,5,6,7,8,9,10,11,12 although some inconsistent findings were also reported.13,14 When measured in late life, cardiometabolic risk factors have been inconsistently associated with dementia, with both increased and decreased risks reported in epidemiologic research15,16,17,18,19,20; notable exceptions are high glucose levels and diabetes, which have been consistently associated with a greater cognitive decline and a higher risk of dementia.14,21,22,23
Limited research has examined trajectories of risk factors in the long period preceding dementia diagnosis. A few longitudinal studies have reported a decline in blood pressure (BP) and total cholesterol levels several years prior to dementia.9,18,19 Likewise, a decline in both body mass index (BMI) and physical activity a few years before dementia diagnosis was reported in the Cardiovascular Risk Factors, Aging and Dementia5 and Whitehall II24,25 studies. To our knowledge, no large cohort study has concurrently examined the trajectories of all main cardiovascular risk factors in the years preceding dementia diagnosis, and the optimal targets and time windows in older ages for the management of cardiometabolic health for dementia prevention have remained unclear.
The present study aimed to concurrently describe trajectories of major cardiometabolic risk factors up to 14 years preceding dementia diagnosis in a large, population-based cohort: the Three-City (3C) study. We used a case-control approach to contrast trajectories between individuals who developed dementia and dementia-free controls and identify time lags when their trajectories significantly differed. Based on previous studies on trajectories of cognition26 and depressive symptoms27 that showed acceleration of impairments in prodromal dementia, we established a priori that a stronger change of risk factors among dementia cases at the approach of diagnosis might reflect reverse causation. By contrast, different, yet parallel, evolutions between cases and controls may reflect causal association. For BP, the biological rationale was that both high values (mediated by cerebral vascular disease) and low values (mediated by hypoperfusion) may cause brain injury28; thus, we set that a lower trajectory among cases may indicate either reverse causation or causal association.
Methods
Population
The 3C study is an ongoing, prospective cohort that started in 1999 including 9294 noninstitutionalized participants aged 65 years or older from 3 French cities (Bordeaux, 2104; Dijon, 4931; and Montpellier, 2259).29 At baseline (hereafter labeled T0) and at follow-up visits attended every 2 to 3 years until 2014, trained psychologists conducted an in-person interview including assessment of sociodemographic, lifestyle, and medical information (including recording of current medication use30), neuropsychological testing, and anthropometric and BP measurements. In Bordeaux and Montpellier, 6 follow-up visits were carried out 2, 4, 7, 10, 12, and 14 years after inclusion (hereafter labeled T2, T4, T7, T10, T12, and T14 respectively). In Dijon, 5 follow-up visits were conducted at T2, T4, T7, T10, and T12. Moreover, a blood sample was collected on 3 occasions in Bordeaux (T0, T4, T10) and 2 occasions in Montpellier (T0, T10) and Dijon (T0, T4). All participants provided written consent, and the study protocol was approved by the ethics committee of the Kremlin-Bicêtre University-Hospital, Paris, France. Participants do not receive financial compensation.
Diagnosis of Dementia
At each visit, diagnosis of dementia was based on a 2-step procedure.29 Neurologists examined participants with suspected dementia based on their neuropsychological performances. Diagnosis and cause of dementia were then reviewed and validated by an independent expert committee of neurologists and geriatricians with expertise in dementia following the criteria of the DSM-IV. Alzheimer disease cases were classified as possible or probable using the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association criteria.31
Nested Case-Control Sample
Among the initial 9294 potential participants evaluated at baseline, we excluded 651 individuals for whom no blood sample was available (n = 576) or who were not fasting (n = 75), 173 prevalent cases of dementia, and 551 persons with at least 1 missing value among the cardiometabolic measures of interest. Moreover, we excluded 576 individuals without at least 1 follow-up visit after inclusion and 3 with a missing educational level. To avoid a large incertitude on the date of dementia onset, we also excluded 36 incident cases for whom no negative diagnosis was established in the last 2 visits preceding the positive diagnosis. Among the 7304 remaining individuals, 841 developed dementia during the follow-up period.
Each dementia case was matched to 4 controls at the diagnosis visit. To ensure independence of control samples across every risk set,32 we used random sampling with replacement between visits (and without replacement within a same follow-up visit). To be included in the control sample, individuals had to be (1) both examined and free of dementia at the matching visit, (2) from the same cohort center as the case, and (3) of similar sex, age (±3 years), and educational level (coded in 3 classes: less than high school, high school, and more than high school). Among the 841 incident cases identified in the initial study sample, 785 (93.3%) were successfully matched to 4 controls, leading to a nested case-control study sample of 3925 individuals.
Ascertainment of Cardiometabolic Risk Factors
We investigated the trajectories of cardiometabolic variables through repeated measures of BMI, systolic BP (SBP), diastolic BP (DPB), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, and blood glucose levels. The BMI was computed as weight in kilograms divided by height in meters squared, using height measured (or self-reported for 25.4% of cases and 26.3% of controls) at baseline and weight measured (or self-reported for 43.7% of cases and 44.2% of controls) at T0 and T2 and self-reported thereafter. Blood pressure was measured twice on the right arm with the participant in a sitting position.29 We used the mean of the 2 measures or the single measure for 2.0% over all repeated measures when 1 measure was missing. Fasting total cholesterol, HDL-C, triglycerides, and blood glucose levels were measured using standard enzymatic methods. The LDL-C level was computed according to the Friedwald equation.33
Statistical Analysis
Mean trajectories of each cardiometabolic risk factor were estimated using latent-process mixed models (ie, mixed models that handle non-Gaussian longitudinal markers as reported in the eMethods in the Supplement).34 We used retrospective time since the matching visit as the timescale (in years); thus, for each quintet (1 case, 4 matched controls), year 0 was the diagnosis visit of the case.
Models included a quadratic function of retrospective time and were adjusted for case-control status, matching variables (age, sex, educational level, and cohort center), and their interactions with both time and time squared. The within-participant correlation was captured by correlated random intercept and slopes on time and time squared. For BP, models included 2 additional indicators to control for potential white-coat effect (eMethods and eFigure 1 in the Supplement).
We tested the differences in trajectories between cases and controls using Wald tests. Two comparisons were made. First, we compared risk factor trajectories during the entire time through a global evaluation of group-by-time interactions. Second, we compared mean predicted risk factor values at different time points. For the latter measures, we primarily used the nominal 5% significance level and secondarily used a corrected threshold to account for the multiple testing (eMethods in the Supplement). Significance was determined using 2-tailed, unpaired testing. The primary significance level considered overall was 5%; the thresholds then corrected for test multiplicity were 1.9% for BMI, 1.3% for BP, and 1.2% for blood lipid and glucose levels.
We used SAS, version 9.4 (SAS Institute Inc) and macro %match35 for the selection of controls, and R, version 3.3.1 (R Foundation) and lcmm function of lcmm package, version 1.7.836 for latent process mixed models. eAppendix in the Supplement provides the R code to reproduce the analyses. Data analysis was performed in September 2017.
Supplementary Analyses
Several additional analyses were performed. We investigated trajectories of each cardiometabolic risk factor among 2 dementia subtypes: possible or probable AD and mixed dementia or vascular dementia (VD). For biological factors, we also explored whether differences in trajectories between cases and controls were modified by the total number of medications at baseline and by factor-specific medication use during follow-up (eMethods and eTable 1 in the Supplement). Finally, we conducted sensitivity analyses to evaluate (1) the association between diabetes status and glycemia trajectories (excluding participants with diabetes during the study period) and (2) the robustness of our findings to statistical assumptions (eMethods in the Supplement).
Results
The nested case-control sample was composed of 785 incident cases of dementia (including 537 [68.4%] AD and 162 [20.6%] VD) and 3140 controls (2530 [65%] women; mean [SD] age, 76 [5] years). The mean (SD) duration of follow-up until the matching visit was 7.8 (3.8) years. At baseline, incident cases were more often carriers of the ε4 allele of the APOE gene, had slightly lower Mini-Mental State Examination scores, and had similar smoking status compared with controls (Table). Moreover, cardiometabolic risk factors and medication use at baseline were similar between the groups except that cases included more participants with diabetes (glucose levels ≥125 mg/dL [to convert to millimoles per liter, multiply by 0.0555] or treatment for diabetes).
Table. Baseline Characteristics of Incident Dementia Cases and Matched Controls.
| Characteristic | Cases (n = 785) | Controls (n = 3140) |
|---|---|---|
| Age, mean (SD), ya | 75.9 (4.9) | 75.8 (4.8) |
| Women, No. (%)a | 506 (64.5) | 2024 (64.5) |
| Highest educational level, No. (%)a | ||
| <High school | 328 (41.8) | 1312 (41.8) |
| High school | 189 (24.1) | 756 (24.1) |
| >High school | 268 (34.1) | 1072 (34.1) |
| Study center, No. (%)a | ||
| Bordeaux | 255 (32.5) | 1020 (32.5) |
| Dijon | 362 (46.1) | 1448 (46.1) |
| Montpellier | 168 (21.4) | 672 (21.4) |
| APOE ε4 carrier, ≥1 allele, No. (%)b | 216 (27.7) | 525 (16.8) |
| MMSE score, mean (SD)b | 26.6 (2.1) | 27.5 (1.8) |
| Smoking, No. (%) | ||
| Never | 511 (65.1) | 2054 (65.4) |
| Former | 244 (31.1) | 949 (30.2) |
| Current | 30 (3.8) | 137 (4.4) |
| BMI, mean (SD) | 25.6 (4.0) | 25.6 (3.9) |
| Arterial BP, mean (SD), mm Hgc | ||
| Systolic | 145.8 (20.7) | 146.8 (21.0) |
| Diastolic | 80.8 (11.0) | 81.8 (10.8) |
| Fasting plasma lipid levels, mg/dL | ||
| HDL-C, mean (SD) | 61.8 (11.6) | 61.8 (15.4) |
| LDL-C, mean (SD) | 142.9 (34.8) | 139.0 (34.8) |
| Triglycerides, mean (SD) | 115.0 (53.1) | 106.2 (53.1) |
| Triglycerides, median (IQR) | 97.4 (79.7-141.6) | 97.4 (79.7-132.7) |
| Fasting plasma glucose, mg/dL | ||
| Mean (SD) | 95.5 (21.6) | 91.9 (18.0) |
| Median (IQR) | 90.1 (82.9-99.1) | 88.3 (82.9-95.5) |
| Total No. of medications ≥4, No. (%) | 519 (66.1) | 1825 (58.1) |
| Antihypertensive medication, No. (%) | 426 (54.3) | 1650 (52.6) |
| Hypertension, No. (%)d | 622 (79.2) | 2513 (80.0) |
| Lipid-lowering medication, No. (%) | 238 (30.3) | 932 (29.7) |
| Antidiabetic medication, No. (%) | 82 (10.4) | 161 (5.1) |
| Diabetes, No. (%)e | 100 (12.7) | 211 (6.7) |
Abbreviations: APOE ε4, ε4 allele of the apolipoprotein E gene; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; DBP, diastolic BP; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; MMSE, Mini-Mental State Examination; SBP, systolic BP.
SI conversion factors: To convert HDL-C and LDL-C to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, 0.0113; and glucose to millimoles per liter, 0.0555.
Matching variables (with age ±3 years).
Summary measures computed among nonmissing values (data were missing for 0.5% of cases and 0.2% of controls for MMSE and for 0.5% of both groups for APOE).
Mean of the 2 measures of BP (4.6% of cases and 2.6% of controls had a single BP value at inclusion and were not included in this table).
SBP of 140 mm Hg or greater, DBP of 90 mm Hg or greater, or use of antihypertensive medication.
Glucose level of 125 mg/dL or greater or use of antidiabetic medication.
Trajectories of Cardiometabolic Risk Factors Preceding Dementia
For each cardiometabolic risk factor, trajectories were represented retrospectively from the matching visit among cases and controls for the most common profile of covariates (Figure 1, Figure 2, Figure 3, and Figure 4; see eFigure 2 in the Supplement for trajectories from a nonadjusted model and eFigure 3 in the Supplement for observed mean cardiometabolic values).
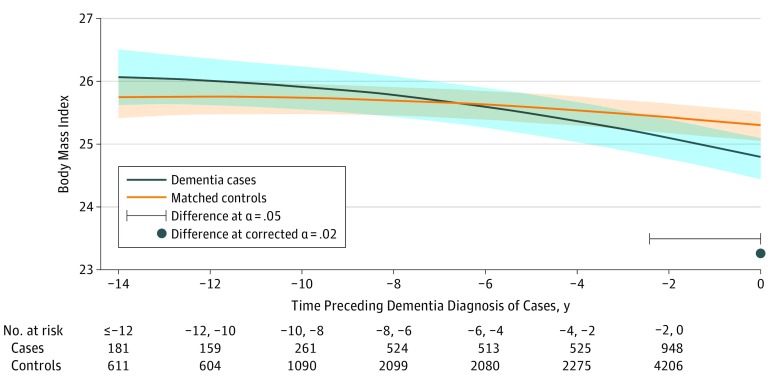
Figure 1. Predicted Mean Trajectories of Body Mass Index up to 14 Years Before Dementia Diagnosis Among Dementia Cases (n = 785) and Matched Controls (n = 3140).
Mean trajectories (solid lines) with 95% pointwise CIs (indicated with shading) were predicted by a latent process linear mixed model in the retrospective time since the matching visit. The model included a quadratic function of time (t, t2); case-control status, matching variables (ie, sex, age, educational level, and cohort center), and their interactions with the quadratic function of time; and correlated random effects on the intercept, time and time2. Body mass index (calculated as weight in kilograms divided by height in meters squared) observations were normalized by I-splines with 2 internal knots. Trajectories were plotted for the most common profile of the study sample: a woman from an average study center, aged 76 years at inclusion and with educational level lower than high school. Note that the choice of the profile affects only the level of the trajectories; it does not affect the differences between cases and controls or test significance.
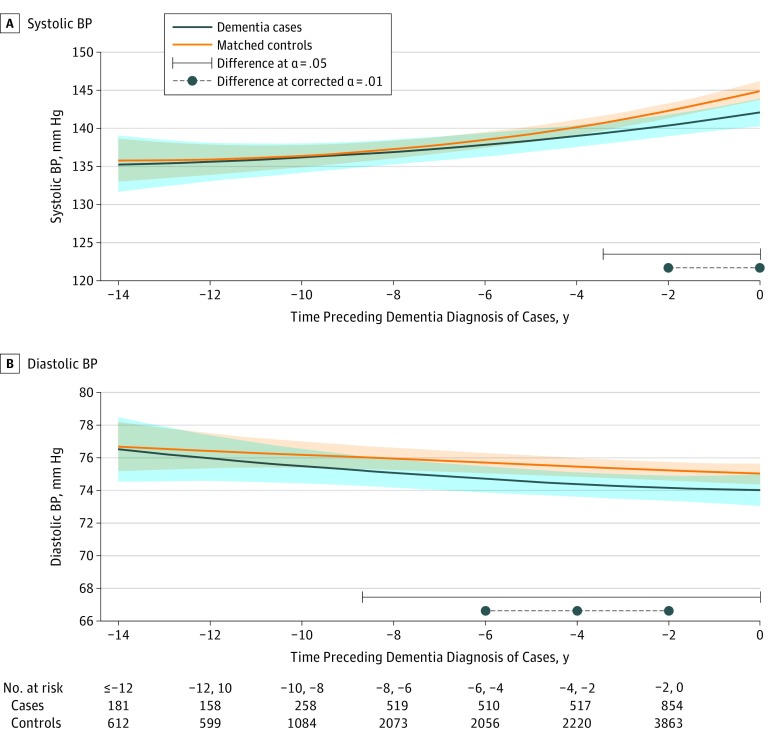
Figure 2. Predicted Mean Trajectories of Systolic and Diastolic Blood Pressure (BP) up to 14 Years Before Dementia Diagnosis Among Cases (n = 785) and Matched Controls (n = 3140).
Mean trajectories (solid lines) of systolic BP (A) and diastolic BP (B) with 95% pointwise CIs (indicated with shading) were predicted by a latent process linear mixed model in the retrospective time since the matching visit. The models included a quadratic function of time (t, t2); case-control status, matching parameters (ie, sex, age, educational level, and cohort center), and their interactions with the quadratic function of time; 2 binary indicators for white-coat effect (value measured at baseline vs later, and value based on mean of 2 measures vs 1 measure); and correlated random effects on the intercept, time, and time2. Blood pressure observations were normalized by I-splines with 2 internal knots. Trajectories were plotted for the most common profile of the study sample: a woman from an average study center, aged 76 years at inclusion, and with educational level lower than high school. Note that the choice of the profile affects only the level of the trajectories; it does not affect the differences between cases and controls or test significance.
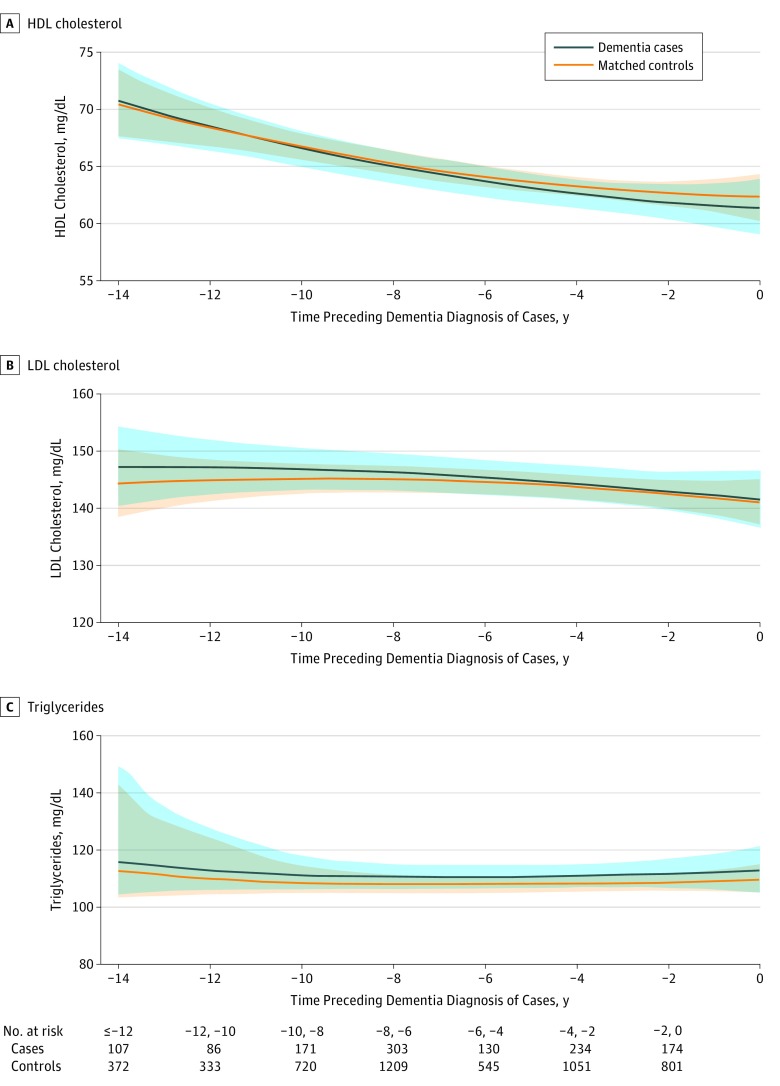
Figure 3. Predicted Mean Trajectories of High-Density Lipoprotein (HDL) Cholesterol, Low-Density Lipoprotein (LDL) Cholesterol, and Triglyceride Levels up to 14 Years Before Dementia Diagnosis Among Cases (n = 785) and Matched Controls (n = 3140).
Mean trajectories (solid lines) trajectories of HDL cholesterol (A), LDL cholesterol (B), and triglyceride (C) levels with 95% pointwise CIs (indicated with shading) were predicted by a latent process linear mixed model in the retrospective time since the matching visit. The models included a quadratic function of time (t, t2); case-control status, matching variables (ie, sex, age, educational level, and cohort center), and their interactions with the quadratic function of time; and correlated random effects on the intercept, time and time2. Plasma lipid level observations were normalized by I-splines with 3 internal knots. Trajectories were plotted for the most common profile of the study sample: a woman from an average study center, aged 76 years at inclusion, and with educational level lower than high school. Note that the choice of the profile affects only the level of the trajectories; it does not affect the differences between cases and controls or test significance. To convert HDL and LDL cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, 0.0113.
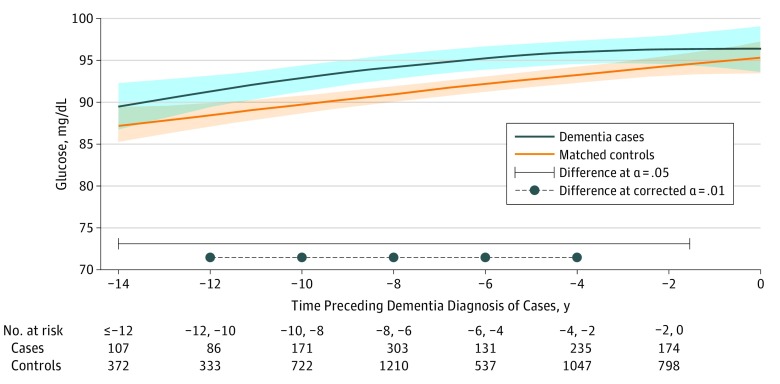
Figure 4. Predicted Mean Trajectories of Glucose Levels up to 14 Years Before Dementia Diagnosis Among Cases (n = 785) and Matched Controls (n = 3140).
Mean trajectories (solid lines) with 95% pointwise CIs (indicated with shading) were predicted by a latent process linear mixed model in the retrospective time since the matching visit. The model included a quadratic function of time (t, t2); case-control status, matching variables (ie, sex, age, educational level, and cohort center), and their interactions with the quadratic function of time; and correlated random effects on the intercept, time, and time2. Glucose level observations were normalized by I-splines with 3 internal knots. Trajectories were plotted for the most common profile of the study sample: a woman from an average study center, aged 76 years at inclusion, and with educational level lower than high school. Note that the choice of the profile affects only the level of the trajectories; it does not affect the differences between cases and controls or test significance. To convert glucose to millimoles per liter, multiply by 0.0555.
Mean BMI declined significantly over time for both cases and controls (Figure 1; P < .001 for time and time2 parameters; mean predicted values for the reference profile represented in the figure were 14 years before diagnosis [−14 years] and at diagnosis [year 0]: 26.1 [95% CI, 25.6-26.5] and 24.8 [95% CI, 24.5-25.1] for a case and 25.7 [95% CI, 25.4-26.1] and 25.3 [95% CI, 25.0-25.5] for a control). Nonetheless, BMI decline was more pronounced among cases (P < .001 for group-by-time interaction). Starting slightly above the predicted level of controls 14 years preceding the matching visit, the BMI of cases dropped below that of the controls about 7 years before diagnosis, with significantly different values 2.4 years before the matching visit (BMI, 25.1 for cases vs 25.5 for controls; P = .05).
The SBP increased significantly over time in both groups (Figure 2A; P < .001 for time and time2 parameters; mean values at −14 years and year 0: 135.2 mm Hg [95% CI, 131.8 mm Hg-138.7 mm Hg] and 142.1 mm Hg [95% CI, 140.3 mm Hg-143.9 mm Hg] for a case and 135.8 mm Hg [95% CI, 132.9 mm Hg-138.6 mm Hg] and 144.9 mm Hg [95% CI, 143.7 mm Hg-146.1 mm Hg] for a control). There was also a significant group-by-time interaction (P = .049 for group-by-time interaction): cases had a slower increase than controls, with a significant divergence 3.4 years prior to diagnosis. In contrast to SBP, trajectories of DBP decreased for both groups (Figure 2B; P = .02 for time and time2 parameters; mean values [95% CI] at −14 years and year 0: 76.5 mm Hg [74.7 mm Hg-78.5 mm Hg] and 74.0 mm Hg [73.1 mm Hg-74.9 mm Hg] for a case and 76.7 mm Hg [75.1 mm Hg-78.3 mm Hg] and 75.0 mm Hg [74.2 mm Hg-75.7 mm Hg] for a control). Although the shape of DBP decline did not differ significantly overall between groups (P = .58 for group-by-time interaction), a significant difference between cases and controls was found up to 8.7 years before diagnosis.
We observed a decreasing trend of HDL-C levels for both cases and controls (Figure 3A; P < .001 for time and time2 parameters; mean predicted values at −14 years and year 0: 70.6 mg/dL [95% CI, 67.6 mg/dL-73.9 mg/dL] and 61.3 mg/dL [95% CI, 58.9 mg/dL-63.8 mg/dL] for a case; 70.4 mg/dL [95% CI, 67.5 mg/dL-73.3 mg/dL] and 62.3 mg/dL [95% CI, 60.2 mg/dL-64.3 mg/dL] for a control), while LDL-C and triglycerides levels remained stable in both groups (Figure 3B and C; P ≥ 0.15 for time and time2 parameters; mean predicted values at −14 years and year 0: for LDL-C, 147.2 mg/dL [95% CI, 140.5 mg/dL-154.5 mg/dL] and 141.6 mg/dL [95% CI, 136.6 mg/dL-146.7 mg/dL] for a case and 144.3 mg/dL [95% CI, 138.7 mg/dL-150.4 mg/dL] and 141.2 mg/dL [95% CI, 137.5 mg/dL-145.2 mg/dL] for a control; for triglycerides, 115.5 mg/dL [95% CI, 103.6 mg/dL-149.1 mg/dL] and 112.6 mg/dL [95% CI, 104.8 mg/dL-120.9 mg/dL] for a case and 112.5 mg/dL [95% CI, 103.8 mg/dL-144.4 mg/dL] and 109.7 mg/dL [95% CI, 105.0 mg/dL-114.8 mg/dL] for a control). We found no significant difference in mean levels and in trajectories between cases and controls for any plasma lipid of interest (all P ≥ .57 for group-by-time interaction).
Finally, glucose levels increased significantly in both groups in the reference profile (Figure 4; P < .001 for time and time2 parameters; mean predicted values at −14 years and year 0: 89.4 mg/dL [95% CI, 86.9 mg/dL-92.1 mg/dL] and 96.4 mg/dL [95% CI, 93.7 mg/dL-99.3 mg/dL] for a case and 87.1 mg/dL [95% CI, 85.1 mg/dL-89.2 mg/dL] and 95.3 mg/dL [95% CI, 93.5 mg/dL-97.1 mg/dL] for a control), with no significant difference in the overall change between the 2 groups (P = .29 for group-by-time interaction). However, compared with controls, predicted glucose levels were constantly higher among cases during the study period, with significant differences in a large window spanning from 1.6 years to 14.0 years before the matching visit. The global evaluation of group-by-time interactions and time-specific tests of difference in trajectories between groups are presented in eTable 2 in the Supplement.
Supplementary Analyses
In the sub–case-control samples of AD and VD, cardiometabolic trajectories were generally similar to those found in the all-cause dementia sample (eFigure 4 in the Supplement), although most differences did not reach statistical significance (except for blood glucose levels). When considering medication use, neither polymedication at baseline nor factor-specific medication during follow-up were differently associated with trajectories of biological factors (all P values for global interaction tests ≥.07), suggesting that medication had little association with the differential trajectories between cases and controls. When excluding participants with diabetes, the shapes of the trajectory in both groups were roughly the same as those found in the main analysis (eFigure 5 in the Supplement), suggesting that glucose levels were higher among dementia cases than controls, even in the nondiabetic population. Results also remained virtually unchanged when we log-transformed triglyceride and glucose levels (eFigure 6 in the Supplement) or re-estimated the models by allowing more flexible trajectories over time with natural cubic splines (eFigure 7 in the Supplement).
Discussion
In this large prospective cohort spanning 14.0 years in older-aged participants, we found (1) a general decline of BMI, DBP, and HDL-C levels with aging, (2) an increase of SBP and blood glucose levels, and (3) a stable evolution of non-HDL-C lipid levels. Moreover, evolution of several risk factors diverged in prodromal dementia. Compared with controls, dementia cases had a steeper decline of BMI up to 7 years before diagnosis. Cases also had a significantly slower SBP increase, consistently lower DBP values, and higher glucose levels. Finally, blood lipid trajectories did not significantly differ. Thus, overall in prodromal dementia compared with natural aging, BMI and BP decreased and glucose was the only risk factor with constantly higher levels. These trajectories were based on observational findings, and any conclusion regarding causal associations should be made with caution. However, there is a biological rationale supporting the interpretation of BMI and BP trajectories in prodromal dementia toward reverse causation because of incipient evolution of neuropathologic changes and subtle cognitive impairment (trajectories of cognition and depressive symptoms show progressive prediagnosis acceleration in eFigure 8 in the Supplement). Yet, because low BP may cause hypoperfusion and lead to brain damage, the lower BP in prodromal dementia may also reflect a causal association. Finally, these findings suggest that elevated glucose levels may be a risk factor for dementia in the older age range.
The decline of BMI and HDL-C found with general aging in this cohort and in previous studies37,38,39,40,41,42,43 may reflect weight loss and malnutrition associated with the loss of muscle mass, appetite, and olfactory function in older persons. Likewise, the increase of SBP and decrease of DBP may be a consequence of age-related development of large-artery stiffness and atherosclerosis.44 Aging also affects glucose homeostasis,45 leading to glucose intolerance, insulin resistance, and consequent elevation of blood glucose levels as observed in our study and others.39,43
Beyond general age-related biological changes, our longitudinal approach revealed specific risk factor evolutions in prodromal dementia. A decrease in both BMI and BP in the years preceding dementia has been found in previous cohorts,24,46,47,48,49,50,51,52,53 although some inconsistent results were also reported.51,52,53,54,55 Alterations of food behavior and appetite causing malnutrition and weight loss may occur years before dementia diagnosis as a result of a more acute loss of olfactory function and taste than in general aging,56,57 depression,26 and early cognitive impairment.58 Malnutrition may also lower plasma lipid levels, and studies reported a decline of cholesterol levels in persons who developed dementia,54,55 although this result was not consistently found in all studies,59 including ours. As with BMI, the drop in BP in prodromal dementia, specifically DBP in our study, may be explained by systemic effects of underlying disease. In AD, early neurodegeneration may disturb brain processes involved in BP regulation and eventually decrease arterial pressure.60,61 Concurrently, atherosclerosis, fluctuations of BP, and hypotension with aging may induce cerebral hypoperfusion, ischemia, and hypoxia, thereby promoting neurodegeneration and eventually cognitive decline and dementia.44 Thus, overall lower BP in prodromal dementia may reflect both underlying disease and true causal association. In our cohort, blood glucose level was the only cardiometabolic factor with consistently higher mean levels among dementia cases compared with controls up to 14.0 years prior to diagnosis, even among participants who remained free of diabetes during the study. An increased risk of dementia associated with higher blood glucose levels, even in the normoglycemic range, over the preceding 5 years was previously demonstrated.21 Elevated glucose levels could damage the brain through multiple mechanisms, including insulin resistance, cerebral small vessel disease, and brain accumulation of amyloid.62
Strengths and Limitations
This study has several strengths. Trajectories were based on a large, community-based prospective study over a long follow-up, with longitudinal assessment of cardiometabolic health based mostly on objective measures, standardized clinical assessment of dementia, and a consensus-based clinical diagnosis. We applied a rigorous methodologic approach using incidence density case-control sampling that ensured formal statistical testing of differences between groups, coupled with a flexible statistical model capable of capturing nonlinear trajectories and handling non-Gaussian markers. Finally, by concurrently exploring trajectories of main cardiometabolic factors, our study helps to disentangle the risk factors for dementia (ie, elevated glucose levels, low BP) from risk factors potentially reflecting early preclinical disease (eg, decreased BMI).
Our study also has limitations. The 3C study includes French participants–mostly urban–and our findings may not apply to populations from different sociogeographic origins. Furthermore, although based on a long follow-up period, we did not cover early windows of exposures (eg, since midlife); thus, our results may not generalize to prevention in early adulthood. Moreover, cardiometabolic health factors were ascertained through a limited and somehow imperfect list of longitudinal risk factors. For instance, the waist to hip ratio may be a more sensitive indicator of cardiovascular disease risk than BMI.63 However, both BMI and waist to hip ratio demonstrated similar associations with risk of cardiovascular diseases in a previous study, suggesting that BMI appropriately captures risk for adverse health.64 Finally, the number of repeated measures for biological variables was small. Although using retrospective time since diagnosis as a timescale enabled biological data to be continuously observed over the study period, differences remote from diagnosis should be interpreted with caution because they are based on limited data.
Conclusions
Management of cardiometabolic health may be a key component of dementia prevention. Yet, with metabolic changes owing to both aging and potential underlying chronic diseases, including dementia, studies in older persons have been discordant, and there is no clear consensus on the strategy to use for their management in late adulthood for the purpose of delaying or preventing dementia onset. Most previous studies have examined risk factors individually and did not formally model trajectories over a long time prior to dementia. By modeling concurrently the trajectories of main cardiometabolic risk factors in prodromal dementia in a large prospective cohort, we provide evidence that BMI declines several years before dementia diagnosis and might indicate preclinical disease, whereas BP, specifically DBP, is consistently lower among future dementia cases, which may reflect both underlying disease and a causal association between low BP and dementia. Finally, elevated glucose levels over the course of older adulthood were higher among those who eventually developed dementia and may thus represent a risk factor for dementia.
Whether confirmed and extended to other populations, these findings emphasizing blood glucose control, low BP, and weight loss as key components of cardiovascular health management for primary and secondary prevention of dementia in older persons may have important implications for preventive care practice in geriatric populations.
eMethods. Detailed Methodology
eTable 1. Consumption of at Least One Cardiometabolic Treatment Medication Between Baseline and the Matching Visit of Incident Dementia Cases and Matched Controls
eFigure 1. Means and 95% Confidence Intervals of the Observed Measures of Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) in the Retrospective Time Since the Diagnosis of Dementia According to Measurement Occasion and the Total Number of Measures at Any Visit
eAppendix. R Code for the Analyses of Body Mass Index
eFigure 2. Trajectories of BMI, SBP, DBP, Glucose, HDL and LDL Cholesterol, and Triglycerides From a Non-Adjusted Model for Confounding Factors Among Incident Cases of Dementia
eFigure 3. Means and 95% Confidence Intervals of the Observed Repeated Measures of BMI, SBP, DBP, HDL and LDL Cholesterol, Triglycerides and Glucose in the Retrospective Time Since the Diagnosis of Dementia Visit
eTable 2. Table Summarizing the Statistical Tests of Difference Between Cases and Controls Trajectories Over the Entire Time Period and at Specific Times
eFigure 4. Trajectories of BMI, SBP, DBP, Glucose, HDL, LDL Cholesterol and Triglycerides Among Alzheimer Disease Subsample and Vascular Dementia Subsample
eFigure 5. Trajectories of Glucose Among Incident Cases of Dementia (n = 650) and Matched Controls (n = 2794) in Participants Without Diabetes
eFigure 6. Distributions of Repeated Measures (Pooled Over Study Visits) of Body Mass Index, Systolic and Diastolic Blood Pressure, HDL and LDL Cholesterol, Triglycerides, Log(Triglycerides), Glucose, and Log(Glucose) in the Nested Cases-Control Sample
eFigure 7. Trajectories of BMI, SBP, DBP, Glucose, HDL, LDL Cholesterols and Triglycerides Among Incident Cases of Dementia and Matched Controls
eFigure 8. Trajectories of Global Cognition and Depressive Symptomatology Among Incident Cases of Dementia and Matched Controls
References
- 1.Breteler MM. Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging. 2000;21(2):153-160. doi: 10.1016/S0197-4580(99)00110-4 [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260(3):211-223. doi: 10.1111/j.1365-2796.2006.01687.x [DOI] [PubMed] [Google Scholar]
- 3.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 4.Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15(5):455-532. doi: 10.1016/S1474-4422(16)00062-4 [DOI] [PubMed] [Google Scholar]
- 5.Tolppanen AM, Ngandu T, Kåreholt I, et al. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38(1):201-209. doi: 10.3233/JAD-130698 [DOI] [PubMed] [Google Scholar]
- 6.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447-1451. doi: 10.1136/bmj.322.7300.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anstey KJ, Ashby-Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis. 2017;56(1):215-228. doi: 10.3233/JAD-160826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75-80. doi: 10.1159/000231980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuligenga RH, Dugravot A, Tabák AG, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol. 2014;2(3):228-235. doi: 10.1016/S2213-8587(13)70192-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2000;21(1):49-55. doi: 10.1016/S0197-4580(00)00096-8 [DOI] [PubMed] [Google Scholar]
- 12.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277-281. doi: 10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- 13.Qizilbash N, Gregson J, Johnson ME, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(6):431-436. doi: 10.1016/S2213-8587(15)00033-9 [DOI] [PubMed] [Google Scholar]
- 14.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246-1254. doi: 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: Cardiovascular Health Study. Arch Neurol. 2009;66(3):336-342. doi: 10.1001/archneurol.2008.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426-e437. doi: 10.1111/j.1467-789X.2010.00825.x [DOI] [PubMed] [Google Scholar]
- 17.Gottesman RF, Schneider ALC, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443-1450. doi: 10.1001/jama.2017.3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen Project: a 6-year follow-up study. Arch Neurol. 2003;60(2):223-228. doi: 10.1001/archneur.60.2.223 [DOI] [PubMed] [Google Scholar]
- 19.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141-1145. doi: 10.1016/S0140-6736(96)90608-X [DOI] [PubMed] [Google Scholar]
- 20.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163(13):1524-1528. doi: 10.1001/archinte.163.13.1524 [DOI] [PubMed] [Google Scholar]
- 21.Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369(6):540-548. doi: 10.1056/NEJMoa1215740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58(1):71-77. doi: 10.2337/db08-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484-491. doi: 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- 24.Singh-Manoux A, Dugravot A, Shipley M, et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement. 2018;14(2):178-186. doi: 10.1016/j.jalz.2017.06.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabia S, Dugravot A, Dartigues JF, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ. 2017;357:j2709. doi: 10.1136/bmj.j2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64(5):492-498. doi: 10.1002/ana.21509 [DOI] [PubMed] [Google Scholar]
- 27.Singh-Manoux A, Dugravot A, Fournier A, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry. 2017;74(7):712-718. doi: 10.1001/jamapsychiatry.2017.0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22(5):646-659. doi: 10.1097/EDE.0b013e31822708b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.3C Study Group Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22(6):316-325. doi: 10.1159/000072920 [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization Guidelines for ATC Classification and DDD Assignment. Oslo, Norway: Norwegian Institute of Public Health; 2002. [Google Scholar]
- 31.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 32.Robins JM, Gail MH, Lubin JH. More on “biased selection of controls for case-control analyses of cohort studies.” Biometrics. 1986;42(2):293-299. doi: 10.2307/2531050 [DOI] [PubMed] [Google Scholar]
- 33.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 34.Proust-Lima C, Amieva H, Jacqmin-Gadda H. Analysis of multivariate mixed longitudinal data: a flexible latent process approach. Br J Math Stat Psychol. 2013;66(3):470-487. [DOI] [PubMed] [Google Scholar]
- 35.Bergstralh E, Kosanke J. Computerized matching of controls In: Technical Report Series, Number 56. Rochester, MN: Mayo Clinic Department of Health Sciences Research; 1995. [Google Scholar]
- 36.Proust-Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw. 2017;78(2):1-56. doi: 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 37.Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women: the Rancho Bernardo Study 1984-1994. Circulation. 1997;96(1):37-43. doi: 10.1161/01.CIR.96.1.37 [DOI] [PubMed] [Google Scholar]
- 38.Wilson PW, Anderson KM, Harris T, Kannel WB, Castelli WP. Determinants of change in total cholesterol and HDL-C with age: the Framingham Study. J Gerontol. 1994;49(6):M252-M257. doi: 10.1093/geronj/49.6.M252 [DOI] [PubMed] [Google Scholar]
- 39.Loh TP, Ma S, Heng D, Khoo CM. Age-related changes in the cardiometabolic profiles in Singapore resident adult population: findings from the National Health Survey 2010. PLoS One. 2016;11(8):e0162102. doi: 10.1371/journal.pone.0162102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franklin SS, Gustin W IV, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96(1):308-315. doi: 10.1161/01.CIR.96.1.308 [DOI] [PubMed] [Google Scholar]
- 41.van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32(2):454-460. doi: 10.1161/01.STR.32.2.454 [DOI] [PubMed] [Google Scholar]
- 42.Bots ML, Witteman JC, Hofman A, de Jong PT, Grobbee DE. Low diastolic blood pressure and atherosclerosis in elderly subjects: the Rotterdam Study. Arch Intern Med. 1996;156(8):843-848. doi: 10.1001/archinte.1996.00440080029004 [DOI] [PubMed] [Google Scholar]
- 43.Menke A, Rust KF, Savage PJ, Cowie CC. Hemoglobin A1c, fasting plasma glucose, and 2-hour plasma glucose distributions in US population subgroups: NHANES 2005-2010. Ann Epidemiol. 2014;24(2):83-89. doi: 10.1016/j.annepidem.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487-499. doi: 10.1016/S1474-4422(05)70141-1 [DOI] [PubMed] [Google Scholar]
- 45.Szoke E, Shrayyef MZ, Messing S, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31(3):539-543. doi: 10.2337/dc07-1443 [DOI] [PubMed] [Google Scholar]
- 46.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63(9):1312-1317. doi: 10.1001/archneur.63.9.1312 [DOI] [PubMed] [Google Scholar]
- 47.Stewart R, Masaki K, Xue Q-L, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55-60. doi: 10.1001/archneur.62.1.55 [DOI] [PubMed] [Google Scholar]
- 48.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739-746. doi: 10.1212/01.wnl.0000267661.65586.33 [DOI] [PubMed] [Google Scholar]
- 49.Ogunniyi A, Gao S, Unverzagt FW, et al. Weight loss and incident dementia in elderly Yoruba Nigerians: a 10-year follow-up study. Int Psychogeriatr. 2011;23(3):387-394. doi: 10.1017/S1041610210001390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44(10):1147-1152. doi: 10.1111/j.1532-5415.1996.tb01362.x [DOI] [PubMed] [Google Scholar]
- 51.McGrath ER, Beiser AS, DeCarli C, et al. Blood pressure from mid- to late life and risk of incident dementia. Neurology. 2017;89(24):2447-2454. doi: 10.1212/WNL.0000000000004741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joas E, Bäckman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59(4):796-801. doi: 10.1161/HYPERTENSIONAHA.111.182204 [DOI] [PubMed] [Google Scholar]
- 53.Stewart R, Xue Q-L, Masaki K, et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension. 2009;54(2):233-240. doi: 10.1161/HYPERTENSIONAHA.109.128744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart R, White LR, Xue Q-L, Launer LJ. Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2007;64(1):103-107. doi: 10.1001/archneur.64.1.103 [DOI] [PubMed] [Google Scholar]
- 55.Mielke MM, Zandi PP, Shao H, et al. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology. 2010;75(21):1888-1895. doi: 10.1212/WNL.0b013e3181feb2bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78(1):30-35. doi: 10.1136/jnnp.2006.099721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci. 2009;1170:730-735. doi: 10.1111/j.1749-6632.2009.04013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pérès K, Helmer C, Amieva H, et al. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. J Am Geriatr Soc. 2008;56(1):37-44. doi: 10.1111/j.1532-5415.2007.01499.x [DOI] [PubMed] [Google Scholar]
- 59.Tan ZS, Seshadri S, Beiser A, et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med. 2003;163(9):1053-1057. doi: 10.1001/archinte.163.9.1053 [DOI] [PubMed] [Google Scholar]
- 60.Burke WJ, Coronado PG, Schmitt CA, Gillespie KM, Chung HD. Blood pressure regulation in Alzheimer’s disease. J Auton Nerv Syst. 1994;48(1):65-71. doi: 10.1016/0165-1838(94)90160-0 [DOI] [PubMed] [Google Scholar]
- 61.Tzourio C, Laurent S, Debette S. Is hypertension associated with an accelerated aging of the brain? Hypertension. 2014;63(5):894-903. doi: 10.1161/HYPERTENSIONAHA.113.00147 [DOI] [PubMed] [Google Scholar]
- 62.Strachan MWJ. R D Lawrence Lecture 2010: the brain as a target organ in type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med. 2011;28(2):141-147. doi: 10.1111/j.1464-5491.2010.03199.x [DOI] [PubMed] [Google Scholar]
- 63.Gustafson D. Adiposity indices and dementia. Lancet Neurol. 2006;5(8):713-720. doi: 10.1016/S1474-4422(06)70526-9 [DOI] [PubMed] [Google Scholar]
- 64.Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol. 2008;52(8):605-615. doi: 10.1016/j.jacc.2008.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methodology
eTable 1. Consumption of at Least One Cardiometabolic Treatment Medication Between Baseline and the Matching Visit of Incident Dementia Cases and Matched Controls
eFigure 1. Means and 95% Confidence Intervals of the Observed Measures of Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) in the Retrospective Time Since the Diagnosis of Dementia According to Measurement Occasion and the Total Number of Measures at Any Visit
eAppendix. R Code for the Analyses of Body Mass Index
eFigure 2. Trajectories of BMI, SBP, DBP, Glucose, HDL and LDL Cholesterol, and Triglycerides From a Non-Adjusted Model for Confounding Factors Among Incident Cases of Dementia
eFigure 3. Means and 95% Confidence Intervals of the Observed Repeated Measures of BMI, SBP, DBP, HDL and LDL Cholesterol, Triglycerides and Glucose in the Retrospective Time Since the Diagnosis of Dementia Visit
eTable 2. Table Summarizing the Statistical Tests of Difference Between Cases and Controls Trajectories Over the Entire Time Period and at Specific Times
eFigure 4. Trajectories of BMI, SBP, DBP, Glucose, HDL, LDL Cholesterol and Triglycerides Among Alzheimer Disease Subsample and Vascular Dementia Subsample
eFigure 5. Trajectories of Glucose Among Incident Cases of Dementia (n = 650) and Matched Controls (n = 2794) in Participants Without Diabetes
eFigure 6. Distributions of Repeated Measures (Pooled Over Study Visits) of Body Mass Index, Systolic and Diastolic Blood Pressure, HDL and LDL Cholesterol, Triglycerides, Log(Triglycerides), Glucose, and Log(Glucose) in the Nested Cases-Control Sample
eFigure 7. Trajectories of BMI, SBP, DBP, Glucose, HDL, LDL Cholesterols and Triglycerides Among Incident Cases of Dementia and Matched Controls
eFigure 8. Trajectories of Global Cognition and Depressive Symptomatology Among Incident Cases of Dementia and Matched Controls