Key Points
Question
Are psychoticlike experiences in adolescents associated with altered prefrontal and striatal activation during reward processing?
Findings
In this cohort study of 298 adolescents, those with an elevated rate of psychoticlike experiences at age 14 years demonstrated reduced activation in prefrontal and limbic cortical areas during reward processing compared with adolescents with no psychoticlike experiences. However, by age 19 years, the group with the elevated rate of psychoticlike experiences showed differentially increased activation of the right prefrontal cortex and reduced activation of dorsal striatum.
Meaning
Adolescents with an elevated rate of psychoticlike experiences show differential activation in frontostriatal brain areas engaged during reward processing compared with control adolescents; given the nonclinical nature of the sample, the increase in prefrontal cortical activation from early to late adolescence may reflect a compensatory cognitive mechanism in the presence of abnormal striatal reward processing to contextualize these abnormal experiences.
Abstract
Importance
Psychoticlike experiences (PLEs) are subclinical manifestations of psychotic symptoms and may reflect an increased vulnerability to psychotic disorders. Contemporary models of psychosis propose that dysfunctional reward processing is involved in the cause of these clinical illnesses.
Objective
To examine the neuroimaging profile of healthy adolescents at 14 and 19 years old points with PLEs, using a reward task.
Design, Setting, and Participants
A community-based cohort study, using both a cross-sectional and longitudinal design, was conducted in academic centers in London, Nottingham, United Kingdom, and Dublin, Ireland; Paris, France; and Berlin, Hamburg, Mannheim, and Dresden, Germany. A group of 1434 healthy adolescent volunteers was evaluated, and 2 subgroups were assessed at ages 14 and 19 years. Those who scored as either high or low PLE (based on the upper and lower deciles) on the Community Assessment of Psychic Experiences Questionnaire (CAPE-42) at age 19 years were included in the analysis. The study was conducted from January 1, 2016, to January 1, 2017.
Main Outcomes and Measures
Participants were assessed at age 14 and 19 year points using functional magnetic resonance imaging while performing a monetary incentive delay reward task. A first-level model focused on 2 predefined contrasts of anticipation and feedback of a win. The second-level analysis examined activation within the reward network using an a priori–defined region of interest approach. The main effects of group, time, and their interaction on brain activation were examined.
Results
Of the 1434 adolescents, 2 groups (n = 149 each) (high PLEs, n = 149, 50 [33.6%] male; low PLEs, n = 149, 84 [56.4%] male) were compared at ages 14 and 19 years. Two regions within the left and right middle frontal gyri showed a main effect of time on brain activation (F1, 93 = 5.559; P = .02; F1, 93 = 5.009; P = .03, respectively); there was no main effect of group. One region within the right middle frontal gyrus demonstrated a significant time × group interaction (F1, 93 = 7.448; P = .01).
Conclusion and Relevance
The findings are consistent with evidence implicating alterations in prefrontal and striatal function during reward processing in the etiology of psychosis. Given the nature of this nonclinical sample this may reflect a combination of aberrant salience yielding abnormal experiences and a compensatory cognitive control mechanism necessary to contextualize them.
This cohort study examines the rate of psychoticlike experiences in adolescents.
Introduction
Psychoticlike experiences (PLEs) describe transitory symptoms that, if they persist, lead to clinically relevant symptoms with functional impairment.1 This contemporary view proposes that psychotic symptoms are not “all-or-nothing” pathologic phenomena, but rather fall within the spectrum of normal experiences, conceptualized as the continuum model of psychosis.2 This view is supported by the high prevalence rate of delusional or hallucinatory experiences in the general population (10% and 30%), which is substantially higher than the prevalence rates of psychotic disorders.3 Late adolescence is a critical neurodevelopmental period in psychosis, with the classic age at onset being in early adulthood. Approximately 20% to 35% of individuals aged 12 to 35 years who meet clinical criteria for a prodromal risk syndrome (ie, the Comprehensive Assessment of the At-Risk Mental State structured questionnaire administered by clinicians) convert to psychosis within 2 years.4 The presence of psychotic symptoms at age 11 years and cannabis use by the age of 15 years were the 2 strongest predictors of psychosis outcomes at age 26 years.5 The most widely used community assessment of PLEs is the Community Assessment of Psychic Experiences Questionnaire (CAPE).6
Psychoticlike experiences offer a useful, nonclinical phenotype to study psychotic disorders7 with the advantages of a lack of exposure to the illness and antipsychotic medication, a critical phase of neurodevelopment for psychosis in drug-naive individuals. The aberrant salience model of psychosis suggests that positive psychotic symptoms occur when dysregulated dopamine firing in the mesocorticolimbic system gives rise to attribution of significance to irrelevant perceptual stimuli.8 Patients with first-episode psychosis exhibit abnormal physiologic responses associated with reward prediction error in the dopaminergic midbrain, striatum, and limbic system and subtle abnormalities in discriminating between motivationally salient and more neutral stimuli.9
A small functional magnetic resonance imaging (fMRI) study of individuals with PLEs (n = 27) at age 14 years using a reward task demonstrated increased amygdala/hippocampal activation in face perception tasks and reduced right dorsolateral prefrontal activation in response inhibition tasks.10 An earlier study suggested that PLEs arise as a consequence of decreased cognitive control,11 a view that is supported by a more recent systematic review of schizophrenia.12 In summary, the presence of psychosis symptoms is associated with decreases in striatal activation during reward tasks and a reduction in prefrontal activation in areas implicated in cognitive control. However, the frequency and intensity of psychotic symptoms differ from those in the general population sample through ultra-high-risk (UHR) states to schizophrenia and the latter is further differentiated by the presence of functional deterioration.13 If the cognitive control hypothesis is correct, one might anticipate dysregulation in subcortical reward processing in healthy adolescents that is compensated for by intact cognitive control mechanisms.
A combination of the aberrant salience and cognitive control hypotheses suggests that the presence of aberrant salience may generate PLEs, but these experiences are contextualized appropriately (ie, with no significant functional consequences) if cognitive control mechanisms are operating efficiently. Failure of cognitive control mechanisms would manifest as clinically relevant symptoms with consequent functional deterioration evidenced as psychotic illness.
The aim of the study was to determine whether PLEs in adolescence are associated with altered prefrontal and striatal activation during reward processing. We used the IMAGEN database14 sample of 1434 healthy adolescents to examine the association between elevated CAPE-42 scores and striatal and prefrontal activation associated with salience and cognitive control, respectively. Two hypotheses were evaluated: the presence of high PLEs will be associated with decreased activation of the prefrontal cortex and striatum during reward processing15,16 and the pattern of activation in the prefrontal cortex and striatum will vary between high and low PLE groups over time between early and late adolescence, consistently with reports in the literature on patients with psychosis.
Methods
Participants and Settings
Neuroimaging and clinical data of healthy adolescents were obtained from the IMAGEN database.14 The IMAGEN study received ethical approval by the ethics research committees of the academic centers at which the study was conducted (London, Nottingham, United Kingdom, and Dublin, Ireland; Paris, France; and Berlin, Hamburg, Mannheim, and Dresden, Germany). All adult participants provided written consent; minors provided oral consent and written consent was obtained by their parents or legal guardians. The present study was conducted from January 1, 2016, to January 1, 2017, from deidentified data. Access to IMAGEN database for the conduction of the present study did not require a separate informed consent; a waiver was applied owing to anonymized data. We used data from age 14 and 19 year points. A total of 1434 adolescents was initially selected based on quality controls and completeness of their behavioral and neuroimaging data sets. Two subgroups were assessed at ages 14 and 19 years. Those who scored as either high or low PLE (based on the upper and lower deciles) on the CAPE 42 items instrument (CAPE-42) at age 19 years were included in the analysis. The epidemiologic features of our sample are described in Table 1.
Table 1. Participant Characteristics and CAPE-42 Score Stratification.
| Characteristic | CAPE-42 Total Score Stratification | |||||
|---|---|---|---|---|---|---|
| High (n = 149)a |
Low (n = 149)b |
|||||
| Mean | SE | SD | Mean | SE | SD | |
| Male, No. (%) | 50 (33.6) | 84 (56.4) | ||||
| Right handed, No. (%) | 128 (85.9) | 123 (82.6) | ||||
| Age, y | ||||||
| Baseline | 14.47 | 0.03 | 0.39 | 14.43 | 0.03 | 0.38 |
| Follow-up | 19.02 | 0.06 | 0.76 | 18.98 | 0.06 | 0.74 |
| WISC baseline score c | ||||||
| Verbal | 111.15 | 1.32 | 15.66 | 106.72 | 1.27 | 15.24 |
| Performance | 108.49 | 1.33 | 15.79 | 105.2 | 1.20 | 14.42 |
| ADRS total score, follow-upd | 15.89 | 0.24 | 2.96 | 19.7 | 0.06 | 0.72 |
| AUDIT total score, follow-upe | 7.5 | 0.44 | 5.34 | 5.26 | 0.32 | 3.87 |
| DAST total score, follow-upf | 1.59 | 0.21 | 2.52 | 0.54 | 0.08 | 1.02 |
| CAPE-42 score, follow-upg | ||||||
| Total | 111.64 | 1.74 | 21.26 | 9.54 | 0.39 | 4.75 |
| Positive symptoms | 33.09 | 1.27 | 15.48 | 3.23 | 0.24 | 2.98 |
| Bizarre delusions | 13.17 | 0.97 | 11.82 | 0.37 | 0.09 | 1.09 |
| Social delusions | 19.91 | 0.54 | 6.57 | 2.87 | 0.21 | 2.61 |
| Negative symptoms | 46.37 | 0.93 | 11.37 | 2.22 | 0.21 | 2.61 |
| Depressive symptoms | 32.18 | 0.61 | 7.49 | 4.09 | 0.21 | 2.54 |
Abbreviations: ADRS, Adolescent Depression Rating Scale; AUDIT, Alcohol Use Disorders Identification Test; CAPE-42, Community Assessment of Psychic Experiences Questionnaire, 42 items instrument; DAST, Drug Abuse Screening Test for Cannabis; WISC, Wechsler Intelligence Scale for Children.
Scorers in upper 10% of CAPE-42 total score.
Scorers in lower 10% of CAPE-42 total score.
The average score is 100; higher scores indicate higher than average intelligence and lower scores indicate lower levels of intelligence.37
Scores range from 0 to 60; higher scores indicate higher levels of adolescent depression.38
Scores range from 0 to 40; higher scores indicate greater levels of alcohol abuse.39
Scores range from 0 to 10; higher scores indicate greater levels of cannabis abuse.40
Scores range from 0 to 294; higher scores indicate elevated presence of prodromal psychotic symptoms.6
Measures
The CAPE-42 questionnaire6 was used as a measure of PLEs in our adolescent population at age 19 years. In its extended version CAPE-42 includes 42 items that are grouped in 3 dimensions: positive, negative, and depressive. Each item is scored for frequency and severity in a scale from 0 to 7; total scores range from 0 to 294. Higher scores indicate an elevated presence of prodromal psychotic symptoms.
The Cambridge Neuropsychological Test Automated Battery (CANTAB) includes highly sensitive, precise, and objective measures of cognitive function.17 Our study focused on the Affective Go-NoGo Task (AGN), providing an assessment of the information processing biases for positive and negative stimuli; this task was chosen as a proxy for “hot” cognition, as related to the inhibitory/affective function of frontal and limbic areas of the brain.
The adapted monetary incentive delay (MID) task is a widely used assessment of rewarded learning. It measures participant reactions to a brief visual target. The MID task includes reward anticipation and receipt of feedback of win or no-win outcomes.
Stratification of the Sample
We defined 2 subgroups with high or low PLEs, based on total CAPE-42 scores. Because the CAPE-42 total scores did not follow a normal distribution, we selected participants with high and low scores using the upper and lower deciles. This distinction resulted in 149 adolescents in the high PLEs group and 149 in the low PLEs group. The high group CAPE-42 total score ranged from 91 to 182, corresponding to an itemized score range of 2.17 to 4.33. Cutoff levels in the area of 2.0 per CAPE-42 item provide adequate positive predictive value for transition to psychosis18; the level formed the lower bound of the high PLEs group. The 2 groups were matched for handedness, age, and IQ.
fMRI Acquisition and Analysis
The scanning parameters and sequence protocol were chosen to be uniform for all sites and scanners. A full description of the scanning protocols, cross-site standardization and quality checks, and preprocessing of resulting data are provided elsewhere.19
First-Level Analysis
Two within-participant contrasts reflecting core reward processing, as previously described,20,21 were selected for investigation. The fMRIs were conducted at ages 14 and 19 years, using SPM12 (http://www.fil.ion.ucl.ac.uk/spm; Wellcome Trust Centre for Neuroimaging): anticipation of large win – anticipation of no win and feedback of large win – feedback of no win.
Second-Level Analysis
Whole-brain analysis focused only on high and low CAPE-42 scorers and task-related regions of interest for the 2 first-level contrasts were collapsed across the high and low groups. This approach provides an unbiased estimate of the activation, as the group average positive/negative is orthogonal to high greater than low or low greater than high and is also supported by literature on functional localizers.22
In a factorial analysis, the main effect of time, group, and the interaction of time × group on brain activation levels was examined by employing a mixed-model 2-way analysis of variance, with group as fixed and subject as random effects. Any main effects or interactions were further examined by post hoc paired, independent, 2-tailed t tests, with P < .05 as the statistically significant threshold. Additional exploratory analysis was also reported, when statistically significant.
Results
Two groups of adolescents (high PLEs, n = 149; 50 [33.6%] males and low PLEs, n = 149; 84 [56.4%) males]) were compared at ages 14 and 19 years. The 2 groups were matched for handedness, age, and IQ.
Results of fMRI Analysis
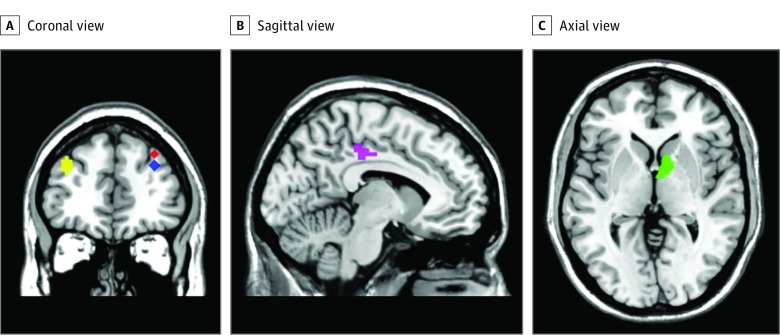
Five regions of interest (ROIs) based on previous studies of reward processing in psychosis and UHR for psychosis15,16 and located in the prefrontal cortices, the limbic areas, and the striatum, were selected for factorial analysis. The ROIs (Montreal Neurological Institute space coordinates) were right caudate head (9, 8, 1), right middle frontal gyrus (33, 41, 40), right middle frontal gyrus (33, 44, 31), left middle frontal gyrus (−36, 47, 31), and left cingulate gyrus (−12, −28, 40) (Figure 1 and eFigure 1, eFigure 2, and eFigure 3 in the Supplement provide a visual representation of the ROIs and eTable 1 in the Supplement reports the results of the functional regions of interest brain analysis).
Figure 1. Regions of Interest Showing Differences in Brain Activation Between Low (L) and High (H) Psychoticlike Experiences Groups.
Coronal (A), sagittal (B), and axial (C) views. At age 14 years: right middle frontal gyrus (33, 41, 40 [red]) (L>H [low group has shown greater brain activation than high group]); right middle frontal gyrus (33, 44, 31 [blue]) (L>H); left cingulate gyrus (−12, −28, 40 [purple]) (L>H); and left middle frontal gyrus (L>H) (−36, 47, 31 [yellow]). At age 19 years: (9, 8, 1 [green]) right caudate head (L>H).
Right Caudate Head (9, 8, 1)
No effects of group × time, group, or time were found. Additional exploratory analysis showed higher brain activation of the low PLEs group compared with the high PLEs group at age 19 years during anticipation (t = −2.846; P = .01) (eTable 2 and eTable 3 in the Supplement).
Right Middle Frontal Gyrus (33, 41, 40)
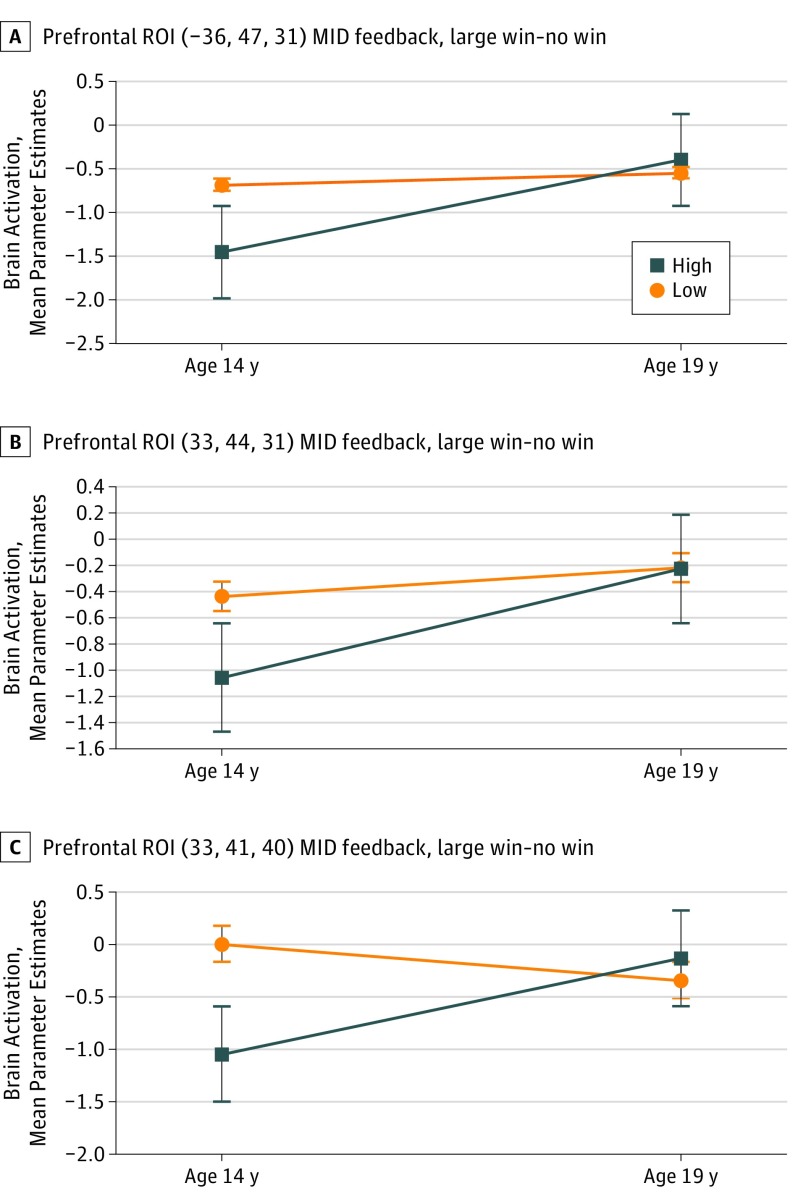
There was an interaction effect of group × time (F1, 93 = 7.448; P = .01), showing significant increase in the high PLEs group from ages 14 to 19 years (t = −3.18; P = .003) with no change in the low PLEs group. Additional exploratory analysis exhibited higher brain activation of the low PLEs group compared with the high PLEs group at age 14 years during feedback (t = −5.069; P < .001) (Figure 2, Table 2, and eTable 3 and eTable 4 in the Supplement).
Figure 2. Mean Brain Activation Parameter Estimates at Ages 14 and 19 Years for Functional Regions of Interest.
Statistically significant changes at P = .05 level for the high psychoticlike experiences group only; SE bars are displayed. MID indicates monetary incentive delay.
Table 2. Mean Brain Activation Contrast Parameter Estimates Factorial Analysis.
| Characteristic | Type III Sum of Squares | df | Mean Square | F Value | P Value | r Valuea |
|---|---|---|---|---|---|---|
| Frontal ROI (33, 41, 40) brain activation | ||||||
| Time | 7.632 | 1 | 7.632 | 1.527 | .22b | 0.127 |
| Group | 4.108 | 1 | 4.108 | 3.598 | .06b | 0.193 |
| Time × group | 37.236 | 1 | 37.236 | 7.448 | .008 | 0.272 |
| Error (time) | 464.966 | 93 | 5.000 | NA | NA | NA |
| Error (group) | 106.187 | 93 | 1.142 | NA | NA | NA |
| Frontal ROI (33, 44, 31) brain activation | ||||||
| Time | 25.604 | 1 | 25.604 | 5.009 | .03 | 0.226 |
| Group | 2.323 | 1 | 2.323 | 1.821 | .18b | 0.139 |
| Time × group | 8.606 | 1 | 8.606 | 1.684 | .20b | 0.133 |
| Error (time) | 475.398 | 93 | 5.112 | NA | NA | NA |
| Error (group) | 118.659 | 93 | 1.276 | NA | NA | NA |
| Frontal ROI (−36, 47, 31) brain activation | ||||||
| Time | 33.354 | 1 | 33.354 | 5.559 | .02 | 0.237 |
| Group | 2.24 | 1 | 2.24 | 1.77 | .19b | 0.137 |
| Time × group | 19.871 | 1 | 19.871 | 3.312 | .07b | 0.185 |
| Error (time) | 558.029 | 93 | 6 | NA | NA | NA |
| Error (group) | 117.68 | 93 | 1.265 | NA | NA | NA |
Abbreviations: ROI, region of interest; NA, not applicable.
Pearson correlation coefficient.
Not statistically significant at a P = .05 level.
Right Middle Frontal Gyrus (33, 44, 31)
There was a main effect of time (F1, 93 = 5.009; P = .03), driven by an increase in brain activation from ages 14 to 19 years, significant only for the high PLEs group (t = −2.902, P = .01). Additional exploratory analysis exhibited higher brain activation of the low PLEs group compared with the high PLEs group at age 14 years during feedback (t = −3.029; P = .003) (Figure 2, Table 2, and eTable 3 and eTable 4 in the Supplement).
Left Middle Frontal Gyrus (−36, 47, 31)
There was a main effect of time (F1, 93 = 5.559; P = .02) that was driven by an increase in brain activation from ages 14 to 19 years, but was significant only for the high PLEs group (t = −2.851; P = .01). Additional exploratory analysis exhibited higher brain activation of the low PLEs group compared with the high PLEs group at age 14 years during feedback (t = −2.818; P = .01) (Figure 2, Table 2, and eTable 3 and eTable 4 in the Supplement).
Left Cingulate Gyrus (−12, −28, 40)
No effects of group × time, group, or time were reported. Additional exploratory analysis exhibited higher brain activation of the low PLEs group compared with the high PLEs group at age 14 years during feedback (t = −2.82; P = .01) (eTable 2 and eTable 3 in the Supplement).
Results CANTAB Measures Analysis
There was a significant effect of time on AGN total omissions for both positive (F1, 159 = 58.778; P < .001) and negative (F1, 159 = 50.236; P < .001) stimuli; however, there was no significant main effect of group and no interaction (Table 3). Post hoc analyses demonstrated the AGN total omissions for both positive and negative stimuli showed a decrease from ages 14 to 19 years across the high PLEs group (AGN positive: t = 4.529; P < .001; AGN negative: t = 3.745; P < .001) and the low PLEs group (AGN positive: t = 6.352; P < .001; AGN negative: t = 6.385; P < .001). The high PLEs group scored lower on AGN total omissions for both positive and negative stimuli at age 14 years (Table 3 and eTable 5, eTable 6, and eFigure 4 in the Supplement).
Table 3. CANTAB Measures Factorial Analysisa.
| CANTAB Variableb | Factors | Type III Sum of Squares | df, dfR | Mean Square | F Value | P Value | r Valuec |
|---|---|---|---|---|---|---|---|
| AGN total omissions negative | Time | 3006.213 | 1, 159 | 3006.213 | 50.236 | <.001 | 0.490 |
| Group | 73.817 | 1, 159 | 73.817 | 2.498 | .12d | 0.124 | |
| Time × group | 144.599 | 1, 159 | 144.599 | 2.416 | .12d | 0.122 | |
| AGN total omissions positive | Time | 3423.720 | 1, 159 | 3423.720 | 58.778 | <.001 | 0.520 |
| Group | 77.094 | 1, 159 | 77.094 | 2.919 | .09d | 0.134 | |
| Time × group | 77.335 | 1, 159 | 77.335 | 1.328 | .25d | 0.091 |
Abbreviations: AGN, Affective Go-NoGo Task; CANTAB, Cambridge Neuropsychological Test Automated Battery; dfR, df(error).
Mixed-model, 2-way analysis of variance factorial analysis.
Total number of missed responses to targets in the blocks specified by the value of target type (negative, positive).
Pearson correlation coefficient.
Not statistically significant at a P = .05 level.
Discussion
Adolescents with high levels of PLEs at age 19 years demonstrated a novel significant increase in right frontal activation during a reward processing task between the ages of 14 and 19 years. They also exhibited decreased activation of the head of caudate during reward processing at 19 years, which is in line with earlier findings.23 Previous studies in early adolescent high-risk populations have found decreased frontal brain activation compared with controls16; this is in line with our findings of lower frontal activation in our high PLEs group at age 14 years.
The contemporary view suggests that abnormal perceptions, such as PLEs, may be a relatively common occurrence and, in most cases, they are appropriately contextualized—a function of cognitive control—and no functional alterations occur. The pattern of decreased striatal activation and increased prefrontal activation supports the suggestion that the evolution of clinically relevant psychotic symptoms requires not only a deficit in striatal reward processing, but also a putative failure of compensatory frontal executive processes.24,25 Thus, this increase in right frontal activation in PLEs may serve as a proxy of executive function—a compensatory mechanism to ensure that any unusual experiences secondary to reward processing deficits do not become incorrectly represented given the current context. The lack of any neuropsychological performance differences in executive functioning between the low and high PLE adolescent groups also lends additional support to this view. However, other possibilities are that this pattern of change in processing reward engages prefrontal cortex and striatum in a different way to generate PLEs in these individuals or that the differences reflect the allocation of potential resources in a proactive, healthy way.
In a study using an MID task, anticipation of reward loss-avoidance elicited significant activation of the ventral striatum in both controls and active participants with UHR for psychosis, with a tendency for less activation in the UHR group.15 Individuals with UHR have been shown to be more likely to attribute motivational salience to irrelevant stimulus features, and this bias (and ventral striatum responses) were correlated with delusionlike symptoms26; ventral striatum/pallidum connectivity to the midbrain is also altered in people with UHR for psychosis.27 Functional MRI studies with UHR individuals have shown ventral striatum hypoactivation and frontal hyperactivation during reward anticipation.15,28
Early hypoactivation in frontal areas might represent a trait in the development of psychosis, as it is commonly reported across all phases of the psychosis continuum, from prodromal to schizophrenia. Hypofrontality29 and functional dysconnectivity of frontostriatal circuitry30 may represent a risk phenotype for psychosis. A meta-analysis of neurofunctional correlates of vulnerability to psychosis revealed hypoactivation of dorsolateral prefrontal cortex and ventrolateral prefrontal cortex as the most common finding in UHR and first-episode psychosis populations.31 Even in healthy individuals, aberrant frontostriatal prediction error signals correlate with delusionlike beliefs.32 Our longitudinal studies confirmed the involvement of 3 frontal areas, showing hypoactivation in the high PLEs group at age 14 years, which also manifested higher levels of activation at 19 years. This change might represent the outcome of a corrective mechanism, targeting brain areas with an activation deficit at an early phase.
The dopamine hypothesis of schizophrenia specified subcortical hyperdopaminergia combined with prefrontal hypodopaminergia as the cardinal features of the neuropathologic cause of schizophrenia; this hypothesis has been modified by inclusion of a presynaptic striatal dopamine dysregulation conceptualized as the final common pathway responsible for the misappraisal of stimuli characteristic of emergent psychosis.33 In our study, we showed that aberrant activation in this neural circuit (frontal areas and caudate head) is present at ages 14 and 19 years in individuals with a prodromal psychotic phenotype.
Ethologic observations have traditionally distinguished between appetitive (anticipation or reward, motivational phase) and consummatory (outcome/feedback of reward, hedonic response) stages of reward processing involving ventral striatum and ventromedial frontal cortex.34,35 In our studies, 3 ROIs that resulted from the feedback contrast were located in left and right middle frontal gyri; 1 ROI that resulted from the anticipation contrast was located in dorsal striatum. A recent, small fMRI study from the IMAGEN sample10 showed increased activation in the right anterior/middle cingulate gyrus and decreased activation in the left fusiform gyrus in the MID in individuals with PLEs. However, the authors of that study used a smaller sample, assessed PLEs at age 14 years, and used a less-extended assessment tool focusing on perceptual abnormalities and delusional thoughts.
The neuropsychological assessment showed that the low PLEs group manifested higher omission errors compared with the high PLEs group at age 14 years, suggestive of reduced inhibitory control, despite the cross-sectional neuroimaging finding of increased frontal brain activation. However, a decrease in AGN scores from ages 14 to 19 years, which corresponds to an improvement in affective/inhibitory control, was observed in both the high and low PLEs groups and was associated with the longitudinal neuroimaging finding of an increase in brain activation at frontal ROIs from ages 14 to 19 years, which was significant only for the high PLEs group. This finding lends support to the proposal that there were no significant differences in explicit cognitive control between the high and low PLEs groups compared with an implicit compensatory cognitive control mechanism that was seen only in the high PLEs group.
Limitations
There are some limitations in our study; we selected high and low decile scorers on CAPE-42 to identify the 2 most polarized groups that would best represent the relative high and low risk for psychosis phenotypes; however, CAPE-42 scores were moderate in our sample. Because our stratification was based on CAPE-42 scores at age 19 years and we did not have available scores at age 14 years or intermediate ages, it was not possible to track the evolution of PLEs between the 2 points. We have viewed the high PLEs phenotype as a proxy for the prodrome for psychosis; however, PLEs can have multiple clinical outcomes, thus leading to a variety of psychopathologic conditions other than psychosis. The lack of transition to psychosis data limits the use of our high PLEs group as a measure for the UHR population. There was a greater representation of the male sex in our low vs high PLEs groups, despite a male preponderance being a common epidemiologic trend in this clinical field.36
Conclusions
Adolescents with high levels of PLEs performing a reward processing task demonstrate decreased activation of frontal and limbic areas at age 14 years and a differential increase in right middle frontal activation from ages 14 to 19 years. Given the nonclinical nature of this sample, this finding could represent a compensatory developmental change that permits cognitive control mechanisms to contextualize the PLEs sufficiently to preclude their evolution to clinical intensity. This finding lends support to a 2-stage process in the emergence of psychotic symptoms, with failure of cognitive control as the key event in transition to functionally relevant symptoms. The ongoing follow-up of this sample will permit evaluation of this change as a useful brain biomarker for the psychosis or other broader psychiatric phenotypes.
eFigure 1. MID Study, ROIs selected for analysis, Illustration (Coronal View)
eFigure 2. MID Study, ROIs selected for analysis, Illustration (Sagittal View)
eFigure 3. MID Study, ROIs selected for analysis, Illustration (Axial View)
eTable 1. MID Study, fROI Brain Analysis
eTable 2. MID Study, fROIs Factorial Analysis, Mixed Model 2-Way ANOVA
eTable 3. MID Study, Exploratory Cross-Sectional Analysis, Independent T-Tests
eTable 4. MID Study, Exploratory Longitudinal Analysis, Paired T-Tests
eTable 5. CANTAB Measures Exploratory Cross-Sectional Analysis, Independent T-Tests
eTable 6. CANTAB Measures Exploratory Longitudinal Analysis, Paired T-Tests
eFigure 4. CANTAB Measures: Mean AGN Total Omissions Positive and Negative Stimuli Scores at age 14 and 19
References
- 1.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195. doi: 10.1017/S0033291708003814 [DOI] [PubMed] [Google Scholar]
- 2.Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385. doi: 10.2174/138161212799316136 [DOI] [PubMed] [Google Scholar]
- 3.Verdoux H, van Os J. Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophr Res. 2002;54(1-2):59-65. doi: 10.1016/S0920-9964(01)00352-8 [DOI] [PubMed] [Google Scholar]
- 4.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107-120. doi: 10.1001/jamapsychiatry.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325(7374):1212-1213. doi: 10.1136/bmj.325.7374.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Os J, Verdoux H, Hanssen M CAPE42. http://cape42.homestead.com/files/CAPE-42.htm. Accessed January 1, 2018.
- 7.Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41(1):1-6. doi: 10.1017/S0033291710001005 [DOI] [PubMed] [Google Scholar]
- 8.Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis—linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79(1):59-68. doi: 10.1016/j.schres.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 9.Murray GK, Corlett PR, Clark L, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13(3):239–,267-276.. doi: 10.1038/sj.mp.4002157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourque J, Spechler PA, Potvin S, et al. ; IMAGEN Consortium . functional neuroimaging predictors of self-reported psychotic symptoms in adolescents. Am J Psychiatry. 2017;174(6):566-575. doi: 10.1176/appi.ajp.2017.16080897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modinos G, Ormel J, Aleman A. Altered activation and functional connectivity of neural systems supporting cognitive control of emotion in psychosis proneness. Schizophr Res. 2010;118(1-3):88-97. doi: 10.1016/j.schres.2010.01.030 [DOI] [PubMed] [Google Scholar]
- 12.Tseng HH, Bossong MG, Modinos G, Chen KM, McGuire P, Allen P. A systematic review of multisensory cognitive-affective integration in schizophrenia. Neurosci Biobehav Rev. 2015;55:444-452. doi: 10.1016/j.neubiorev.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 13.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971. doi: 10.1080/j.1440-1614.2005.01714.x [DOI] [PubMed] [Google Scholar]
- 14.Welcome to the IMAGEN Study. https://imagen-europe.com/. Accessed January 1, 2018.
- 15.Juckel G, Friedel E, Koslowski M, et al. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66(1):50-56. doi: 10.1159/000337130 [DOI] [PubMed] [Google Scholar]
- 16.Jacobson S, Kelleher I, Harley M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11-13 year old schoolchildren. Neuroimage. 2010;49(2):1875-1885. doi: 10.1016/j.neuroimage.2009.09.015 [DOI] [PubMed] [Google Scholar]
- 17.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85(7):399-402. [PMC free article] [PubMed] [Google Scholar]
- 18.Mossaheb N, Becker J, Schaefer MR, et al. The Community Assessment of Psychic Experience (CAPE) questionnaire as a screening-instrument in the detection of individuals at ultra-high risk for psychosis. Schizophr Res. 2012;141(2-3):210-214. doi: 10.1016/j.schres.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 19.Schumann G, Loth E, Banaschewski T, et al. ; IMAGEN Consortium . The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15(12):1128-1139. doi: 10.1038/mp.2010.4 [DOI] [PubMed] [Google Scholar]
- 20.Whelan R, Watts R, Orr CA, et al. ; IMAGEN Consortium . Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185-189. doi: 10.1038/nature13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stringaris A, Vidal-Ribas Belil P, Artiges E, et al. ; IMAGEN Consortium . The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry. 2015;172(12):1215-1223. doi: 10.1176/appi.ajp.2015.14101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN. A critique of functional localisers. Neuroimage. 2006;30(4):1077-1087. doi: 10.1016/j.neuroimage.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 23.Radua J, Schmidt A, Borgwardt S, et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72(12):1243-1251. doi: 10.1001/jamapsychiatry.2015.2196 [DOI] [PubMed] [Google Scholar]
- 24.Vanes LD, Mouchlianitis E, Collier T, Averbeck BB, Shergill SS. Differential neural reward mechanisms in treatment-responsive and treatment-resistant schizophrenia. Psychol Med. 2018;1-10. doi: 10.1017/S0033291718000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe P, Krivoy A, Porffy L, Henriksdottir E, Eromona W, Shergill SS. When the drugs don’t work: treatment-resistant schizophrenia, serotonin and serendipity. Ther Adv Psychopharmacol. 2018;8(1):63-70. doi: 10.1177/2045125317737003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2013;39(6):1328-1336. doi: 10.1093/schbul/sbs147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winton-Brown T, Schmidt A, Roiser JP, et al. Altered activation and connectivity in a hippocampal-basal ganglia-midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl Psychiatry. 2017;7(10):e1245. doi: 10.1038/tp.2017.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wotruba D, Heekeren K, Michels L, et al. Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Front Behav Neurosci. 2014;8:382. doi: 10.3389/fnbeh.2014.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110(4):243-256. doi: 10.1111/j.1600-0447.2004.00376.x [DOI] [PubMed] [Google Scholar]
- 30.Fornito A, Harrison BJ, Goodby E, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70(11):1143-1151. doi: 10.1001/jamapsychiatry.2013.1976 [DOI] [PubMed] [Google Scholar]
- 31.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31(4):465-484. doi: 10.1016/j.neubiorev.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 32.Corlett PR, Fletcher PC. The neurobiology of schizotypy: fronto-striatal prediction error signal correlates with delusion-like beliefs in healthy people. Neuropsychologia. 2012;50(14):3612-3620. doi: 10.1016/j.neuropsychologia.2012.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549-562. doi: 10.1093/schbul/sbp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683-3687. doi: 10.1097/00001756-200112040-00016 [DOI] [PubMed] [Google Scholar]
- 36.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60(6):565-571. doi: 10.1001/archpsyc.60.6.565 [DOI] [PubMed] [Google Scholar]
- 37.Wechsler D. Intelligence scale for children. New York: Psychological Corp; 1949. 1992. [Google Scholar]
- 38.Revah-Levy A, Birmaher B, Gasquet I, Falissard B. The Adolescent Depression Rating Scale (ADRS): a validation study. BMC Psychiatry. 2007;7(1):2. doi: 10.1186/1471-244X-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423-432. doi: 10.15288/jsa.1995.56.423 [DOI] [PubMed] [Google Scholar]
- 40.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363-371. doi: 10.1016/0306-4603(82)90005-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. MID Study, ROIs selected for analysis, Illustration (Coronal View)
eFigure 2. MID Study, ROIs selected for analysis, Illustration (Sagittal View)
eFigure 3. MID Study, ROIs selected for analysis, Illustration (Axial View)
eTable 1. MID Study, fROI Brain Analysis
eTable 2. MID Study, fROIs Factorial Analysis, Mixed Model 2-Way ANOVA
eTable 3. MID Study, Exploratory Cross-Sectional Analysis, Independent T-Tests
eTable 4. MID Study, Exploratory Longitudinal Analysis, Paired T-Tests
eTable 5. CANTAB Measures Exploratory Cross-Sectional Analysis, Independent T-Tests
eTable 6. CANTAB Measures Exploratory Longitudinal Analysis, Paired T-Tests
eFigure 4. CANTAB Measures: Mean AGN Total Omissions Positive and Negative Stimuli Scores at age 14 and 19