Key Points
Question
Does administration of myo-inositol to premature infants for up to 10 weeks reduce the incidence of type 1 retinopathy of prematurity?
Findings
In this randomized clinical trial that included 638 premature infants younger than 28 weeks’ gestational age, treatment with myo-inositol for up to 10 weeks did not reduce the risk of type 1 retinopathy of prematurity or death compared with placebo (29% vs 21%, respectively).
Meaning
These findings do not support the use of myo-inositol among premature infants; however, the early termination of the trial limits definitive conclusions.
Abstract
Importance
Previous studies of myo-inositol in preterm infants with respiratory distress found reduced severity of retinopathy of prematurity (ROP) and less frequent ROP, death, and intraventricular hemorrhage. However, no large trials have tested its efficacy or safety.
Objective
To test the adverse events and efficacy of myo-inositol to reduce type 1 ROP among infants younger than 28 weeks’ gestational age.
Design, Setting, and Participants
Randomized clinical trial included 638 infants younger than 28 weeks’ gestational age enrolled from 18 neonatal intensive care centers throughout the United States from April 17, 2014, to September 4, 2015; final date of follow-up was February 12, 2016. The planned enrollment of 1760 participants would permit detection of an absolute reduction in death or type 1 ROP of 7% with 90% power. The trial was terminated early due to a statistically significantly higher mortality rate in the myo-inositol group.
Interventions
A 40-mg/kg dose of myo-inositol was given every 12 hours (initially intravenously, then enterally when feeding; n = 317) or placebo (n = 321) for up to 10 weeks.
Main Outcomes and Measures
Type 1 ROP or death before determination of ROP outcome was designated as unfavorable. The designated favorable outcome was survival without type 1 ROP.
Results
Among 638 infants (mean, 26 weeks’ gestational age; 50% male), 632 (99%) received the trial drug or placebo and 589 (92%) had a study outcome. Death or type 1 ROP occurred more often in the myo-inositol group vs the placebo group (29% vs 21%, respectively; adjusted risk difference, 7% [95% CI, 0%-13%]; adjusted relative risk, 1.41 [95% CI, 1.08-1.83], P = .01). All-cause death before 55 weeks’ postmenstrual age occurred in 18% of the myo-inositol group and in 11% of the placebo group (adjusted risk difference, 6% [95% CI, 0%-11%]; adjusted relative risk, 1.66 [95% CI, 1.14-2.43], P = .007). The most common serious adverse events up to 7 days of receiving the ending dose were necrotizing enterocolitis (6% for myo-inositol vs 4% for placebo), poor perfusion or hypotension (7% vs 4%, respectively), intraventricular hemorrhage (10% vs 9%), systemic infection (16% vs 11%), and respiratory distress (15% vs 13%).
Conclusions and Relevance
Among premature infants younger than 28 weeks’ gestational age, treatment with myo-inositol for up to 10 weeks did not reduce the risk of type 1 ROP or death vs placebo. These findings do not support the use of myo-inositol among premature infants; however, the early termination of the trial limits definitive conclusions.
This randomized clinical trial compares the effects of myo-inositol vs placebo on type 1 retinopathy of prematurity (ROP) among infants younger than 28 weeks’ gestational age.
Introduction
Retinopathy of prematurity (ROP) is a common morbidity among premature infants and a leading cause of childhood blindness worldwide.1,2 Investigations conducted from 1986 through 1992 of myo-inositol use as a surfactant component found that infants with respiratory distress syndrome who were treated with inositol had improved survival, lower rates of pneumothorax or intraventricular hemorrhage, and reduced rates of ROP.3,4,5,6,7
Furthermore, in 2000-2001, researchers comparing (1) human milk feedings (normally high in inositol) vs formulas that did not contain inositol and (2) inositol-supplemented formula vs non–inositol-supplemented formula found higher rates of ROP in the infants fed non–inositol-supplemented formula.8,9 Use of antenatal steroids and exogenous surfactant has since reduced respiratory morbidity from respiratory distress syndrome; however, ROP remains a serious sequela among survivors of extremely preterm birth.10,11 A 2012 Cochrane meta-analysis concluded that myo-inositol reduced preterm death, severe intraventricular hemorrhage, and ROP, which warranted the undertaking of a large multicenter randomized clinical trial.12
Retinal vessels first begin to vascularize the developing retina, growing in from the hyaloid artery at about 16 weeks’ gestational age. The retinal vessels generally reach the ora serrata (full vascularization) at 36 to 45 weeks’ postmenstrual age (PMA; defined as the sum of gestational age at birth plus No. of weeks postnatal), but this process is more prolonged if ROP develops.10,13 The stresses of preterm birth, most notably sustained high Pao2 levels that suppress the proangiogenic factors supporting growth, result in retinal ischemia as the retina becomes more metabolically active. As ischemia increases, excessive neovascularization follows. If mild, neovascularization may resolve spontaneously or it may progress to an uncontrolled level, leading to retinal detachment.10,13 Retinopathy of prematurity has been classified by various prognostic grades or types to assist ophthalmologists in selecting the best time to surgically intervene to prevent retinal detachments.10 Type 1 ROP meets the criteria for surgical intervention determined by serial retinal examinations. The purpose of this trial was to test the safety and efficacy of myo-inositol among infants younger than 28 weeks’ gestational age to reduce the rates of type 1 ROP.
Methods
This double-blind placebo-controlled randomized clinical trial was approved by the US Food and Drug Administration, and reviewed and approved by the institutional review boards at each recruiting institution and the Neonatal Research Network data coordinating center prior to enrollment. Infants were enrolled once written informed consent was obtained from a parent or guardian prior to 72 hours of age. The trial was conducted by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development Neonatal Research Network. The trial protocol appears in Supplement 1 and the statistical analysis plan appears in Supplement 2.
Population
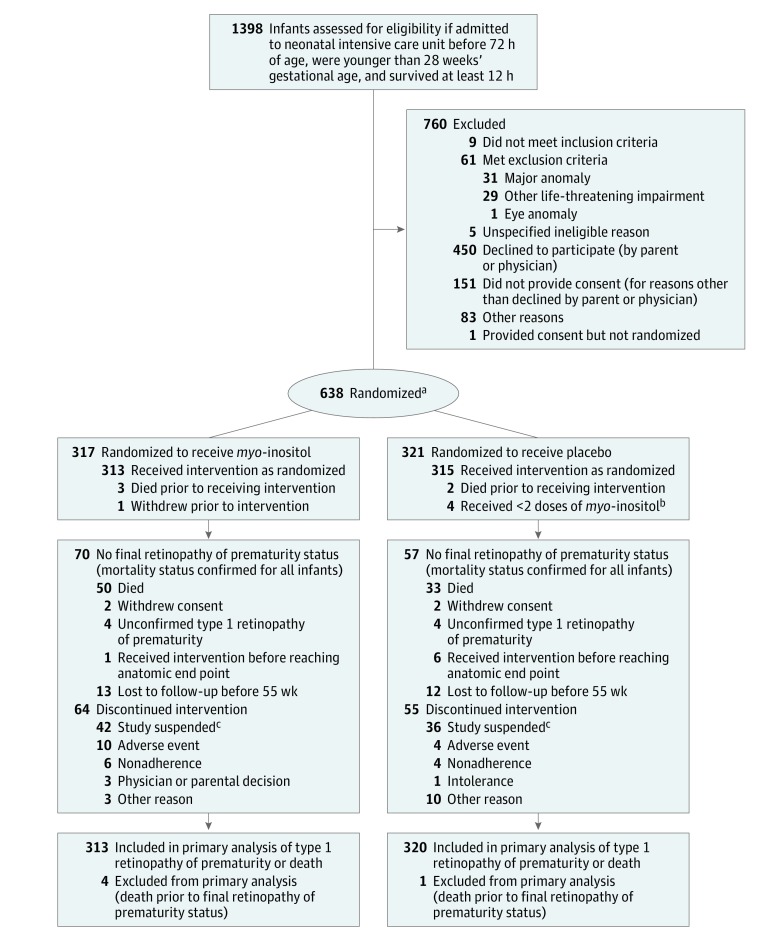
Infants born before 28 0/7 weeks of gestation, surviving for at least 12 hours, and admitted to 1 of the 18 Neonatal Research Network centers before 72 hours’ postnatal age were screened for eligibility (Figure). The centers included major universities throughout the United States.
Figure. Flow of Infants Through Study.
aOne ineligible infant (aged <12 hours) was randomized in error; however, this infant continued in the study.
bBecause these infants received only less than 2 of 100 possible doses, they remained in the placebo group for the safety analyses.
cAfter 20 months of study enrollment, the study was suspended because of a manufacturing issue. All study drug dosing was suspended at this time and was not restarted.
Exclusion criteria included any major congenital anomaly, any eye anomaly, or any moribund condition. Because type 1 ROP is known to vary among individuals from different racial/ethnic backgrounds, the mother’s self-reported race/ethnicity (selected from defined categories provided by the National Institutes of Health) was collected from electronic medical records.1,11
Randomization and Masking
Computer-generated and centrally administered randomization used a permuted block design with block sizes of 2 and 4. Participants were stratified within center and by subgroups of gestational age (<26 0/7 vs 26 0/7 to 27 6/7 weeks of gestation) and randomized in a 1:1 ratio. Infants from multiple births were randomized individually. With the exception of pharmacists, who prepared the daily unit doses of myo-inositol or placebo according to randomization assignment, all other clinical and research personnel and families were blind to group assignment.
Intervention
The active drug was an isotonic, sterile, pyrogen- and preservative-free aqueous solution of 5% myo-inositol (50 mg/mL) at neutral pH and was provided by Abbott Laboratories. After conducting pharmacokinetic and safety studies of myo-inositol in extremely preterm infants, a dose of 40 mg/kg every 12 hours was selected to achieve serum concentrations similar to those previously reported.14,15 A therapeutic duration of up to 10 weeks was chosen to sustain serum myo-inositol levels similar to those found in utero throughout the period of normal retinal vascular development and because of the reported benefits in the treatment of ROP and survival.12
The placebo was a solution of 5% glucose for intravenous infusion from pharmacy stock. Both solutions were clear, colorless, nonviscous, and slightly sweet if tasted. Doses were initially given intravenously, changed to enteral when the infant was receiving enteral feedings of 120 mL/kg/d, or changed sooner if intravenous fluids were discontinued. Treatment continued for up to 10 weeks, until hospital discharge, or until 34 weeks’ PMA.16 No changes to the protocol occurred during the trial.
Primary Outcome
The unfavorable primary outcome was type 1 ROP, which was defined as meeting the criteria for ophthalmological intervention to prevent retinal detachment, a more severe ROP type than ROP type 1 (eg, aggressive posterior ROP or rush disease), or death before the ROP outcome could be determined.10 The favorable primary outcome was survival with only milder ROP or no ROP. Infants were followed up as outpatients to determine the primary outcome up to a maximum of 55 weeks’ PMA.
The severity of ROP was determined with serial eye examinations by ophthalmologists highly experienced with ROP beginning at 31 weeks’ PMA.17,18 The lead ophthalmologist at each trial center had extensive experience in identifying and managing ROP, was certified in the diagnosis of ROP via the International Classification of Retinopathy of Prematurity, and ensured consistency among that center’s examiners.18,19 If an infant developed type 1 ROP in either eye, a second examination by an independent examiner or fundus photographs were required to confirm or document the findings prior to the surgical intervention.
For infants who missed follow-up visits, all available clinical ROP data were reviewed through an independent process to determine the ROP outcome of (1) most likely never had type 1 ROP or (2) most likely developed type 1 ROP. This adjudication was performed under the supervision of the data coordinating center.
The adjudicators, who were blind to trial group assignment and who did not review the data for any infant from the center at which he or she was employed, were ophthalmologists with extensive ROP experience. The adjudication process was performed to reduce missing data bias because incomplete follow-up is more common among participants with mild or no ROP than among those who develop type 1 ROP. A pocket guide was provided as a tool to assist research personnel with the recording of the ROP outcomes and for the scheduling of follow-up examinations (eFigure 1 in Supplement 3).
Secondary Outcomes
The preplanned secondary efficacy outcomes were occurrence of any type of ROP; self-resolving type 2 ROP or greater; and all-cause mortality up to 55 weeks’ PMA. All-cause mortality also is reported through the earliest of the following events: hospital transfer, hospital discharge, or 120 days of age.
Clinical diagnoses of bronchopulmonary dysplasia, bronchopulmonary dysplasia or death reported as caused by bronchopulmonary dysplasia, and severe intraventricular hemorrhage through hospital discharge, hospital transfer, death, or 120 days of age were secondary efficacy and safety outcomes and were recorded using uniform definitions established for the Neonatal Research Network protocols.20
Safety Outcomes
Additional clinical diagnoses were recorded as safety outcomes through hospital discharge, hospital transfer, death, or 120 days of age using Neonatal Research Network protocol definitions. For each death, the site principal investigator reviewed the infant’s course and verified the primary and contributing causes of death, taking into account discussion with the clinical team, autopsy results (if an autopsy was performed), and the results of any internal mortality review. Multiple causes of death could be listed per infant.
Adverse events were prospectively monitored and recorded according to organ system, severity, and possible relatedness to the trial drug. All adverse events (ie, any untoward medical occurrence in a patient temporally associated with the use of a drug in humans) either considered related to the drug or not related and occurring prior to 7 days after the end of dosing were collected with the exception of mild and expected events.
Prespecified Exploratory Outcomes
The components of the primary outcome (type 1 ROP and death before determination of ROP outcome) were prespecified exploratory outcomes along with weight, length, and head circumference measured through 7 days after the end of dosing. Cystic areas in the cerebral parenchyma were recorded through 28 days. Follow-up evaluations were scheduled for 22 to 26 months’ corrected age and are not reported herein.
Statistical Analyses
Based on an occurrence rate for the primary outcome of 30% in a large study,21 the estimated total enrollment was 1672 (836 per group) to achieve 90% power to detect an absolute reduction of 7% or greater in type 1 ROP or death prior to the determination of an ROP outcome (assuming a 2-tailed α level of .05 using a Mantel-Haenszel test).22 Inflating the sample size for an estimated lost to follow-up rate of 3% to 5%, and a single interim analysis for 1000 enrollees with final outcomes, the projected sample size was 1760. The analyses for all other secondary and safety outcomes were exploratory and not intended to be formal tests of hypotheses; therefore, no adjustments were made for multiplicity.
For the primary and secondary outcomes, the analyses were performed on an intention-to-treat basis and included adjudicated ROP outcomes according to the prespecified statistical analysis plan (Supplement 2). Individuals for whom adjudicated ROP outcomes could not be obtained were treated as missing completely at random and were excluded from the primary analysis. Poisson regression was used to estimate adjusted relative risks (RRs) and a generalized linear model with a binomial distribution and identity link was used to estimate the adjusted absolute risk differences (and associated 95% CIs) for myo-inositol vs placebo for the primary outcome and for all binary secondary outcomes.22,23,24 The analyses were adjusted for center when possible and gestational age strata. For the primary outcome, gestational age strata × sex interaction terms were explored to determine if the treatment effect differed by gestational age or sex. For continuous secondary outcomes, analysis of covariance models that controlled for strata defined by gestational age and center were used. Growth was assessed using repeated measure models that controlled for site and gestational age strata.
Causes of death (including due to infectious organisms) were summarized. Infectious organisms were explored post hoc in an attempt to understand the observed increase in mortality in the myo-inositol group that led to early trial termination. The post hoc analyses by drug lot used the same modeling approaches but compared lot groups instead of treatment groups.
All statistical analyses were performed using 2-sided tests with SAS software version 9.4 (SAS Institute Inc). A P value of less than .05 was considered statistically significant. No adjustments of the P value threshold were planned to account for the interim safety reviews of the trial data conducted by the data and safety monitoring committee.
Trial Termination
The trial protocol specified that the Neonatal Research Network data and safety monitoring committee would monitor safety and the overall trial performance at a preplanned frequency when approximately 25%, 50%, and 75% of the enrolled participants had completed trial therapy. The first preplanned safety review was scheduled to occur after 19 months of enrollment. At 18 months, trial enrollment and treatment were suspended because of a manufacturing issue.
Particulate matter was identified in lot 3 of the trial drug before it was released for use. Because of this, the stored quality control vials from lot 2 (then currently in use) were subjected to an additional examination. This examination revealed particulate matter had developed in 17 of the approximately 900 vials examined (1.9%). Quality control vials from lot 1 (no longer in use) had passed all scheduled inspections. The particulate matter was later identified as glass lamellae and is discussed below with the associated analyses.
Due to the timing of the trial suspension, a review of the safety data by lot was added to the first preplanned safety review of the accumulating data by the data and safety monitoring committee. The data and safety monitoring committee recommended the continued suspension of the trial while the remaining data were collected and a final recommendation was made approximately 4 months later that the trial be stopped early based on a statistically significant increase in all-cause deaths through 55 weeks’ PMA in the myo-inositol group, which was unrelated to the manufacturing issue.
The review of the lot data did not show any evidence of harm to neonates exposed to lot 2 of the trial drug compared with those exposed to lot 1. No further drug was given and full data collection was continued as planned for up to 55 weeks’ PMA.16
Results
Participants
From April 17, 2014, through September 4, 2015, 1398 infants were screened, 1323 met the eligibility criteria, and 638 (48% of 1323 eligible infants) were randomized. The final date of follow-up was February 12, 2016, and 589 infants (92%) had a study outcome. The major reason for not enrolling in the trial was lack of informed consent (601). Six randomized participants received no doses (4 in the myo-inositol group and 2 in the placebo group) because of death or trial withdrawal prior to the first dose.
Of the 317 infants randomized to receive myo-inositol, 313 received the trial drug. Forty-two did not complete treatment due to the suspension of the trial (34 of those received ≥10 days of dosing) and 297 reached a primary outcome.
In the placebo group, 319 of the 321 participants received the assigned treatment, 36 did not complete treatment due to trial suspension (32 with ≥10 days of dosing) and 297 reached a primary outcome. Participants who did not receive the full course of treatment were included in the analyses.
The baseline characteristics of infants in the myo-inositol group and in the placebo group were similar (Table 1). The mean gestational age was 26 weeks, the mean birthweight was 780 g, and 53% of enrollees were younger than 26 weeks’ gestational age.
Table 1. Baseline Characteristics of Infants.
| Characteristica |
Myo-inositol (n = 317)b |
Placebo (n = 321)b |
|---|---|---|
| Gestational age | ||
| Mean (SD), wk | 25.6 (1.4) | 25.7 (1.3) |
| <26 0/7 wk, No. (%) | 169 (53) | 170 (53) |
| Birth weight, mean (SD), g | 776 (195) | 785 (198) |
| Male sex, No. (%) | 158 (50) | 163 (51) |
| Racial/ethnic group, No. (%)c | ||
| Non-Hispanic white | 133 (44) | 132 (43) |
| Non-Hispanic black | 119 (39) | 113 (37) |
| Asian | 11 (4) | 6 (2) |
| Hispanic or Latino | 38 (13) | 45 (15) |
| Received antenatal steroids, No. (%) | 281 (89) | 283 (88) |
| Apgar score at 5 min, median (IQR)d | 7 (5-8) | 6 (4-8) |
| Early-onset sepsis (<72 h), No. (%) | 7 (2) | 9 (3) |
| Age at initiation of drug therapy, mean (SD), d | 2.8 (0.8) | 2.8 (0.8) |
Abbreviation: IQR, interquartile range.
These characteristics were chosen because they are believed to have an effect on neonatal morbidity and mortality outcomes in this extremely preterm population.
Includes all infants who were randomized and not the 313 in the myo-inositol group and the 320 in the placebo group who were included in the primary outcome analysis.
Self-reported by the family and selected from categories defined by the National Institutes of Health. There were missing data for 13 infants in the myo-inositol group and for 14 in the placebo group.
Summarizes the condition of the newborn after birth. Neonates are given a score of 0, 1, or 2 (best) for 5 items: color, tone, breathing, heart rate, and grimace resulting in a score from 0 to 10. There were missing data for 4 infants in the myo-inositol group and for 5 in the placebo group.
Primary Outcome
Adjudication of the ROP outcome was required for 39 infants (16 in the myo-inositol group and 23 in the placebo group). Death or type 1 ROP occurred more often in the myo-inositol group compared with the placebo group (29% vs 21%, respectively; adjusted risk difference, 7% [95% CI, 0% to 13%]; adjusted RR, 1.41 [95% CI, 1.08 to 1.83], P = .01; Table 2). For the prespecified exploratory analyses of the components of the primary outcome, death before determination of ROP occurred in 16% of the myo-inositol group vs 10% of the placebo group (adjusted risk difference, 4% [95% CI, −1% to 9%]; adjusted RR, 1.53 [95% CI, 1.03 to 2.25], P = .03).
Table 2. Primary Outcome and Exploratory Analysisa.
| No./Total No. (%) | Adjusted Risk Difference (95% CI), %b |
Adjusted RR (95% CI)c |
P Valued | ||
|---|---|---|---|---|---|
| Myo-inositol | Placebo | ||||
| Primary Outcome | |||||
| Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcomee,f | 91/313 (29) | 66/320 (21) | 7 (0 to 13) | 1.41 (1.08 to 1.83) | .01 |
| Prespecified Exploratory Analysisg | |||||
| Type 1 ROPe | 37/247 (15) | 28/264 (11) | 2 (−3 to 8) | 1.43 (0.91 to 2.24) | .12 |
| Death before determination of ROP outcome | 50/317 (16) | 33/321 (10) | 4 (−1 to 9) | 1.53 (1.03 to 2.25) | .03 |
| Type 1 ROP including adjudicated ROP outcomef | 41/263 (16) | 33/287 (11) | 3 (−3 to 8) | 1.38 (0.91 to 2.10) | .13 |
Abbreviations: ROP, retinopathy of prematurity; RR, relative risk.
Outcomes collected up to 55 weeks’ postmenstrual age.
A generalized linear model with a binomial distribution and identity link was used. The analyses were adjusted for center when possible and gestational age strata.
Poisson regression was used. The analyses were adjusted for center when possible and gestational age strata.
Tested based on a score test using the robust variance estimator of the treatment effect in the Poisson regression model.
Type 1 ROP defined as the level of severity meeting criteria for surgical intervention.10
The ROP outcome was assigned by adjudication for 16 infants in the myo-inositol group and 23 in the placebo group when the final ROP outcome was not available.
Included components of the primary outcome.
The rates for type 1 ROP only were not significantly different with or without the adjudicated ROP outcome. Using the adjudicated ROP outcome, 16% in the myo-inositol group and 11% in the placebo group developed type 1 ROP (adjusted risk difference, 3% [95% CI, −3% to 8%]; adjusted RR, 1.38 [95% CI, 0.91 to 2.10], P = .13). The overall treatment × sex interaction and treatment × gestational age strata interaction were not significant (P = .20 and P = .51, respectively).
Secondary Outcomes
The between-group differences for presence of any ROP outcome and the type 2 ROP or greater outcome were not significantly different. All-cause mortality up to 55 weeks’ PMA was 18% in the myo-inositol group and 11% in the placebo group (adjusted risk difference, 6% [95% CI, 0%-11%]; adjusted RR, 1.66 [95% CI, 1.14-2.43], P = .007; Table 3). The between-group differences for bronchopulmonary dysplasia and severe intraventricular hemorrhage were not significantly different (Table 3).
Table 3. Secondary Outcomes, Adverse Events, and Exploratory Outcomes.
| No./Total No. (%) | Adjusted Risk Difference (95% CI), %a |
Adjusted RR (95% CI)b |
P Valuec | ||
|---|---|---|---|---|---|
| Myo-inositol | Placebo | ||||
| Secondary Outcomes | |||||
| Any ROPd | 171/267 (64) | 183/286 (64) | 1 (−7 to 8) | 1.02 (0.91 to 1.14) | .75 |
| ≥Type 2 ROPd | 125/263 (48) | 142/285 (50) | −2 (−10 to 6) | 0.98 (0.83 to 1.14) | .76 |
| All-cause mortalityd | 56/317 (18) | 34/321 (11) | 6 (0 to 11) | 1.66 (1.14 to 2.43) | .007 |
| All-cause mortalitye | 50/317 (16) | 33/321 (10) | 4 (−1 to 9) | 1.53 (1.03 to 2.25) | .03 |
| Bronchopulmonary dysplasiae,f | 159/272 (58) | 165/288 (57) | 3 (−5 to 10) | 1.03 (0.91 to 1.16) | .66 |
| Bronchopulmonary dysplasia or death caused by it prior to 37 wke | 159/272 (58) | 165/288 (57) | 3 (−5 to 10) | 1.03 (0.91 to 1.16) | .66 |
| Severe intraventricular hemorrhage (grade 3 or 4)e | 51/311 (16) | 50/317 (16) | −1 (−6 to 4) | 1.04 (0.74 to 1.48) | .81 |
| Adverse Eventse | |||||
| Late-onset sepsis (>72 h of age) | 83/317 (26) | 64/321 (20) | 5 (−1 to 11) | 1.31 (0.99 to 1.73) | .06 |
| Suspected or proven necrotizing enterocolitis | 29/317 (9) | 30/321 (9) | −0 (−5 to 4) | 0.98 (0.60 to 1.59) | .93 |
| Surgical necrotizing enterocolitis | 14/317 (4) | 9/321 (3) | 2 (−1 to 4) | 1.57 (0.69 to 3.58) | .28 |
| Spontaneous gastrointestinal perforation without necrotizing enterocolitis | 19/317 (6) | 22/321 (7) | −1 (−4 to 2) | 0.87 (0.48 to 1.57) | .65 |
| Time receiving intervention, mean (95% CI), dg | |||||
| Ventilation through 28 d | 15.3 (14.1 to 16.5) |
15.4 (14.2 to 16.6) |
−0.07 (−1.62 to 1.47)h | .93 | |
| Oxygen through 28 d | 23.6 (22.7 to 24.5) |
23.9 (23.0 to 24.8) |
−0.26 (−1.42 to 0.90)h | .66 | |
| Parenteral nutrition | 29.6 (27.1 to 32.0) |
26.2 (23.7 to 28.7) |
3.33 (0.14 to 6.53)h | .04 | |
| Pulmonary hemorrhage | 27/317 (9) | 28/321 (9) | −1 (−5 to 3) | 0.97 (0.59 to 1.61) | .92 |
| Patent ductus arteriosus | 160/316 (51) | 165/321 (51) | −1 (−9 to 7) | 0.98 (0.85 to 1.14) | .83 |
| Requiring indomethacin | 64/316 (20) | 72/321 (22) | −2 (−8 to 4) | 0.90 (0.67 to 1.21) | .49 |
| Requiring surgery | 40/316 (13) | 43/321 (13) | −1 (−6 to 3) | 0.94 (0.63 to 1.40) | .76 |
| Seizure treatment ≥2 d | 9/317 (3) | 7/320 (2) | 1 (−2 to 3) | 1.29 (0.49 to 3.43) | .60 |
| Negative hearing screening result in either ear at hospital discharge | 35/211 (17) | 26/215 (12) | 5 (−2 to 11) | 1.38 (0.86 to 2.20) | .18 |
| Prespecified Exploratory Outcomee | |||||
| Cystic areas in the cerebral parenchyma measured through 28 d | 12/108 (11) | 8/97 (8) | 4 (−5 to 12) | 1.35 (0.59 to 3.12) | .47 |
Abbreviations: ROP, retinopathy of prematurity; RR, relative risk.
A generalized linear model with a binomial distribution and identity link was used. The analyses were adjusted for center when possible and gestational age strata.
Poisson regression was used. The analyses were adjusted for center when possible and gestational age strata.
For binary outcomes, tested based on a score test using the robust variance estimator of the treatment effect in the Poisson regression model. For continuous outcomes, tested based on analysis of covariance model.
Outcomes collected up to 55 weeks’ postmenstrual age.
Outcomes collected through the first event: death, hospital discharge, hospital transfer, or 120 days after birth.
Defined as requiring oxygen at 36 weeks’ postmenstrual age for oxygen saturation greater than 90%.
Analysis of covariance models were used that controlled for strata defined by gestational age and center.
Data expressed as difference in means (95% CI).
Adverse Events
The between-group differences for the majority of the clinical outcomes were not significantly different through hospital discharge, hospital transfer, death, or 120 days of age. However, the between-group difference for mean No. of days receiving parental nutrition was significant and there was a greater number of days reported on average in the myo-inositol group (29.6 vs 26.2 days in placebo group; between-group difference, 3.33 [95% CI, 0.14 to 6.53], P = .04). In both treatment groups, the most common cause of death before reaching the ROP outcome was respiratory distress syndrome (50% for myo-inositol vs 58% for placebo), followed by sepsis (40% vs 39%, respectively), and intracranial hemorrhage (14% vs 27%) (Table 4).
Table 4. Causes of Death Before Reaching Retinopathy of Prematurity (ROP) Outcome.
| No./Total No. (%)a | ||
|---|---|---|
| Myo-inositol | Placebo | |
| Death before reaching ROP outcome, No. (%) | 50/317 (16) | 33/321 (10) |
| Respiratory distress syndrome | 25/50 (50) | 19/33 (58) |
| Sepsis | 20/50 (40) | 13/33 (39) |
| Intracranial hemorrhage | 7/50 (14) | 9/33 (27) |
| Pulmonary hemorrhage | 5/50 (10) | 7/33 (21) |
| Necrotizing enterocolitis | 9/50 (18) | 5/33 (15) |
| Spontaneous gastrointestinal perforation | 5/50 (10) | 5/33 (15) |
| Immaturity | 4/50 (8) | 5/33 (15) |
| Bronchopulmonary dysplasia | 6/50 (12) | 2/33 (6) |
| Other infection | 6/50 (12) | 0 |
| Pulmonary hypoplasia | 0 | 3/33 (9) |
| Pneumonia | 2/50 (4) | 0 |
| Congenital malformation | 1/50 (2) | 1/33 (3) |
| Multiple organ failure | 0 | 1/33 (3) |
| Hepatic failure or cirrhosis | 0 | 1/33 (3) |
| Patent ductus arteriosus | 0 | 1/33 (3) |
| Any other cause of death | 11/50 (22) | 7/33 (21) |
There may have been more than 1 primary or contributing cause of death.
The most common serious adverse events up to 7 days of receiving the ending dose were necrotizing enterocolitis (6% for myo-inositol and 4% for placebo), spontaneous intestinal perforation without necrotizing enterocolitis (5% vs 6%, respectively), poor perfusion or hypotension (7% vs 4%), intraventricular hemorrhage (10% vs 9%), systemic infection (16% vs 11%), and respiratory distress (15% vs 13%).
Prespecified Exploratory Outcomes
There were no between-group differences for the prespecified exploratory outcomes of growth (weight gain, head circumference, and length) or the cystic areas in the cerebral parenchyma (Table 3 and eFigure 2 in Supplement 3).
Post hoc Analyses
Post hoc examination of deaths due to late-onset sepsis revealed the suspected causal organisms to be viral in 2% of deaths in the myo-inositol group vs 6% of deaths in the placebo group, gram negative in 8% vs 9% of deaths, respectively, gram positive in 20% vs 12% of deaths, and yeast in 4% vs 3% of deaths. Post hoc analyses of the primary outcome and the clinical outcomes of infants exposed only to lot 1 of myo-inositol compared with those exposed only to lot 2 of myo-inositol (particulates were found in 1.9% of stored lot 2 trial drug vials) showed no evidence of increased risk among infants exposed to lot 2 of myo-inositol (eTables 1 and 2 in Supplement 3).
Discussion
In this trial of supplemental myo-inositol to improve survival without type 1 ROP among extremely preterm infants, myo-inositol did not reduce type 1 ROP rates, and the trial was stopped early for an unexpected significant increase in mortality. The previous beneficial findings of myo-inositol were not observed in the current trial; however, there are several relevant differences between the current and former inositol studies.3,4,6 Antenatal steroids were not widely used, nor was surfactant available during the earlier trials. The prior studies treated infants for 3 to 10 days with myo-inositol during the acute phase of respiratory distress syndrome, whereas the present trial treated infants for up to 10 weeks to support retinal vascular development.
In the previous trials,3,4,6 infants were more mature at birth (mean, 27-29 weeks’ gestational age). One explanation could be that a benefit of myo-inositol on the rates of intraventricular hemorrhage, bronchopulmonary dysplasia, and ROP during the earlier studies may have resulted from the predicted beneficial effect of myo-inositol on surfactant function.3,4,5 Reducing the severity of respiratory distress syndrome could be expected to reduce these morbidities. Thus, a myo-inositol benefit on surfactant function in the current trial may have been outweighed by the beneficial effects of antenatal steroids, exogenous surfactant, and noninvasive ventilatory support in current use.
The dose of myo-inositol to produce serum concentrations was similar to those in the previous studies.5,6,15 However, the combination of longer treatment and the inclusion of infants with younger gestational ages may have resulted in the unexpected increase in mortality through as yet unknown mechanisms. In vitro data have shown that infection of macrophages by some intracellular bacteria is enhanced by their ability to use myo-inositol as an energy source.25
An additional issue for this trial was its suspension when particulates (later identified as glass lamellae) were found in the third lot of drug, which was never used. Glass particulates are a commonly cited reason for drug recalls. Delamination within glass vials is affected by both the glass manufacturing process and the chemical characteristics of the drug, particularly if acidic or caustic.26,27
The glass lamellae subsequently found in 1.9% of stored vials in lot 2 of the trial drug raised the question whether the observed harmful effect of myo-inositol could have been due to these particles. However, detailed analyses revealed that there were no differences in the outcomes for infants treated with myo-inositol between the 2 lots of the trial drug.
Limitations
This trial was terminated prematurely, thus limiting definitive conclusions. Because the trial did not enroll as many infants as the preplanned sample size, it was underpowered to make conclusions regarding the efficacy of myo-inositol. In addition, the trial was not formally powered to make a conclusive assessment regarding safety.
Conclusions
Among premature infants younger than 28 weeks’ gestational age, treatment with myo-inositol for up to 10 weeks did not reduce the risk of type 1 ROP or death vs placebo. These findings do not support the use of myo-inositol among premature infants; however, the early termination of the trial limits definitive conclusions.
Study protocol
Statistical analysis plan
eFigure 1. ROP endpoints: myo-inositol to reduce ROP
eFigure 2. Growth charts
eText. Evaluation of drug lot effects
eTable 1. Post-hoc analysis of primary and secondary efficacy outcomes for lot 1 use only vs lot 2 use only: on-treatment population
eTable 2. Post-hoc analysis of secondary efficacy and safety clinical outcomes for lot 1 use only vs lot 2 use only: on-treatment population
Data sharing statement
References
- 1.Palmer EA, Flynn JT, Hardy RJ, et al. ; Cryotherapy for Retinopathy of Prematurity Cooperative Group . Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98(11):1628-1640. doi: 10.1016/S0161-6420(91)32074-8 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77-82. doi: 10.1016/j.earlhumdev.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 3.Hallman M, Pohjavuori M, Bry K. Inositol supplementation in respiratory distress syndrome. Lung. 1990;168(suppl):877-882. doi: 10.1007/BF02718223 [DOI] [PubMed] [Google Scholar]
- 4.Hallman M, Järvenpää AL, Pohjavuori M. Respiratory distress syndrome and inositol supplementation in preterm infants. Arch Dis Child. 1986;61(11):1076-1083. doi: 10.1136/adc.61.11.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallman M, Arjomaa P, Hoppu K. Inositol supplementation in respiratory distress syndrome: relationship between serum concentration, renal excretion, and lung effluent phospholipids. J Pediatr. 1987;110(4):604-610. doi: 10.1016/S0022-3476(87)80561-9 [DOI] [PubMed] [Google Scholar]
- 6.Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M. Inositol supplementation in premature infants with respiratory distress syndrome. N Engl J Med. 1992;326(19):1233-1239. doi: 10.1056/NEJM199205073261901 [DOI] [PubMed] [Google Scholar]
- 7.Hallman M. Inositol during perinatal transition. NeoReviews. 2015;16(2):e84-e93. doi: 10.1542/neo.16-2-e84 [DOI] [Google Scholar]
- 8.Friedman CA, McVey J, Borne MJ, et al. Relationship between serum inositol concentration and development of retinopathy of prematurity: a prospective study. J Pediatr Ophthalmol Strabismus. 2000;37(2):79-86. [DOI] [PubMed] [Google Scholar]
- 9.Hylander MA, Strobino DM, Pezzullo JC, Dhanireddy R. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. J Perinatol. 2001;21(6):356-362. doi: 10.1038/sj.jp.7210548 [DOI] [PubMed] [Google Scholar]
- 10.Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684-1694. doi: 10.1001/archopht.121.12.1684 [DOI] [PubMed] [Google Scholar]
- 11.Kennedy KA, Wrage LA, Higgins RD, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Evaluating retinopathy of prematurity screening guidelines for 24- to 27-week gestational age infants. J Perinatol. 2014;34(4):311-318. doi: 10.1038/jp.2014.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howlett A, Ohlsson A, Plakkal N. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2012;(3):CD000366. [DOI] [PubMed] [Google Scholar]
- 13.Ogden T. Ocular embroyology and antomy In: Ryan S, ed. Basic Science and Rentinal Disease. Vol 1 2nd ed St Louis, MO: Mosby-Year Book Inc; 1994:10-12. [Google Scholar]
- 14.Phelps DL, Ward RM, Williams RL, et al. Pharmacokinetics and safety of a single intravenous dose of myo-inositol in preterm infants of 23-29 wk. Pediatr Res. 2013;74(6):721-729. doi: 10.1038/pr.2013.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phelps DL, Ward RM, Williams RL, et al. Safety and pharmacokinetics of multiple dose myo-inositol in preterm infants. Pediatr Res. 2016;80(2):209-217. doi: 10.1038/pr.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engle WA; American Academy of Pediatrics Committee on Fetus and Newborn . Age terminology during the perinatal period. Pediatrics. 2004;114(5):1362-1364. doi: 10.1542/peds.2004-1915 [DOI] [PubMed] [Google Scholar]
- 17.Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists . Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189-195. doi: 10.1542/peds.2012-2996 [DOI] [PubMed] [Google Scholar]
- 18.Reynolds JD, Dobson V, Quinn GE, et al. ; CRYO-ROP and LIGHT-ROP Cooperative Study Groups . Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol. 2002;120(11):1470-1476. doi: 10.1001/archopht.120.11.1470 [DOI] [PubMed] [Google Scholar]
- 19.An international classification of retinopathy of prematurity. Pediatrics. 1984;74(1):127-133. [PubMed] [Google Scholar]
- 20.Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. doi: 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlo WA, Finer NN, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362(21):1959-1969. doi: 10.1056/NEJMoa0911781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301-305. doi: 10.1093/aje/kwh221 [DOI] [PubMed] [Google Scholar]
- 23.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943. doi: 10.1093/aje/kwg074 [DOI] [PubMed] [Google Scholar]
- 24.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 25.Manske C, Schell U, Hilbi H. Metabolism of myo-inositol by Legionella pneumophila promotes infection of amoebae and macrophages. Appl Environ Microbiol. 2016;82(16):5000-5014. doi: 10.1128/AEM.01018-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guadagnino E, Zuccato D. Delamination propensity of pharmaceutical glass containers by accelerated testing with different extraction media. PDA J Pharm Sci Technol. 2012;66(2):116-125. doi: 10.5731/pdajpst.2012.00853 [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Lavalley V, Mangiagalli P, Wright JM, Bankston TE. Glass delamination: a comparison of the inner surface performance of vials and pre-filled syringes. AAPS PharmSciTech. 2014;15(6):1398-1409. doi: 10.1208/s12249-014-0167-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study protocol
Statistical analysis plan
eFigure 1. ROP endpoints: myo-inositol to reduce ROP
eFigure 2. Growth charts
eText. Evaluation of drug lot effects
eTable 1. Post-hoc analysis of primary and secondary efficacy outcomes for lot 1 use only vs lot 2 use only: on-treatment population
eTable 2. Post-hoc analysis of secondary efficacy and safety clinical outcomes for lot 1 use only vs lot 2 use only: on-treatment population
Data sharing statement