Key Points
Question
What is the association between aortic vascular inflammation by fludeoxyglucose F 18 (18F-FDG) positron emission tomography/computed tomography and coronary artery disease by coronary computed tomography angiography?
Findings
In this cross-sectional cohort study of 215 patients with psoriasis assessed with 18F-FDG positron emission tomography/computed tomography and coronary computed tomography angiography, aortic vascular inflammation was directly associated with quantitative burden of coronary artery disease, luminal stenosis severity within the coronary arteries, and the prevalence of high-risk coronary plaque beyond traditional cardiovascular risk factors.
Meaning
Aortic vascular inflammation by 18F-FDG positron emission tomography/computed tomography is associated with presence of coronary artery disease by coronary computed tomography angiography, suggesting that 18F-FDG PET/CT may provide a potential surrogate of early coronary artery disease.
Abstract
Importance
Inflammation is critical to atherosclerosis. Psoriasis, a chronic inflammatory disease associated with early cardiovascular events and increased aortic vascular inflammation (VI), provides a model to study the process of early atherogenesis. Fludeoxyglucose F 18 positron emission tomography/computed tomography (18F-FDG PET/CT) helps quantify aortic VI, and coronary computed tomography angiography provides coronary artery disease (CAD) assessment through evaluation of total plaque burden (TB) and noncalcified coronary plaque burden (NCB), luminal stenosis, and high-risk plaques (HRP). To our knowledge, association between aortic VI and broad CAD indices has not yet been assessed in a chronic inflammatory disease state. Such a study may provide information regarding the utility of aortic VI in capturing early CAD.
Objective
To assess the association between aortic VI and CAD indices, including TB, NCB, luminal stenosis, and HRP prevalence, in psoriasis.
Design, Setting, and Participants
In a cross-sectional cohort study at the National Institutes of Health, 215 consecutive patients with psoriasis were recruited from surrounding outpatient dermatology practices. All patients underwent 18F-FDG PET/CT for aortic VI assessment, and 190 of 215 patients underwent coronary computed tomography angiography to characterize CAD. The study was conducted between January 1, 2013, and May 31, 2017. Data were analyzed in March 2018.
Exposures
Aortic VI assessed by 18F-FDG PET/CT.
Main Outcomes and Measures
Primary outcome: TB and NCB. Secondary outcomes: luminal stenosis and HRP.
Results
Among 215 patients with psoriasis (mean [SD] age, 50.4 [12.6] years; 126 men [59%]), patients with increased aortic VI had increased TB (standardized β = 0.48; P < .001), and higher prevalence of luminal stenosis (OR, 3.63; 95% CI, 1.71-7.70; P = .001) and HRP (OR, 3.05; 95% CI, 1.42-6.47; P = .004). The aortic VI and TB association was primarily driven by NCB (β = 0.49; P < .001), whereas the aortic VI and HRP association was driven by low-attenuation plaque (OR, 5.63; 95% CI, 1.96-16.19; P = .001). All associations of aortic VI remained significant after adjustment for cardiovascular risk factors: aortic VI and TB (β = 0.23; P < .001), NCB (β = 0.24; P < .001), luminal stenosis (OR, 3.40; 95% CI, 1.40-8.24; P = .007), and HRP (OR, 2.72; 95% CI, 1.08-6.83; P = .03). No association was found between aortic VI and dense-calcified coronary plaque burden.
Conclusions and Relevance
Aortic VI is associated with broad CAD indices, suggesting that aortic VI may be a surrogate for early CAD. Larger prospective studies need to assess these associations longitudinally and examine treatment effects on these outcomes.
This study assesses the association between aortic vascular inflammation by fludeoxyglucose F 18 positron emission tomography/computed tomography and coronary artery disease indices by coronary computed tomography angiography.
Introduction
Inflammation is increasingly recognized as a driver of atherosclerosis.1 From early endothelial cell dysfunction to late-stage coronary plaque rupture, inflammatory mediators have a critical role in the atherosclerotic cascade.2 Psoriasis, a chronic inflammatory skin disorder, is associated with heightened cardiovascular risk, increased vascular inflammation (VI), and increased incidence of early cardiovascular events including myocardial infarction and stroke.3,4,5,6 Psoriasis being a systemic inflammatory state may therefore help understand the role of chronic inflammation in atherosclerosis in vivo.
In 2017 and 2018, findings from the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS),7,8 inflammation reduction was demonstrated to reduce cardiovascular events in direct proportion with the extent of high-sensitivity C-reactive protein reduction, despite null effects on lipids. While these findings highlight the role of residual inflammatory risk in atherosclerosis, emerging imaging techniques provide means to assess inflammation within the vasculature and effect on atherosclerotic plaque development and progression. Aortic vascular inflammation by fluorodeoxyglucose F 18 positron emission tomography computed tomography (18F-FDG PET/CT) has been used as a surrogate marker of cardiovascular risk and immune-mediated vascular disease during the last decade.9,10 Histologic studies corroborate that aortic VI by 18F-FDG PET/CT is associated with the activity of proinflammatory CD-68 macrophages within the arterial wall.11 Aortic VI is associated with hard cardiovascular events, improves cardiovascular risk prediction,12 and is also amenable to modulation, with known risk-modifying agents including statins, anti-inflammatory therapies, and therapeutic lifestyle changes.13,14,15,16 However, its context in treatment trials as a quantifiable primary end point is only now gaining momentum. Furthermore, how aortic VI is associated with vascular diseases in other territories, including the coronary arteries, is understudied. A prior study characterized the association between aortic VI and high-risk coronary plaque (HRP) morphology in patients with HIV17 and found that aortic VI was associated with coronary artery disease. To our knowledge, whether aortic VI is associated with broad coronary plaque characteristics has not been evaluated.
Coronary computed tomography angiography (CCTA) is an important imaging tool for the assessment of coronary plaque.18 Coronary computed tomography angiography provides qualitative assessment of coronary plaque composition and allows for the volumetric quantification of the burden of coronary artery disease (CAD)19,20,21 while sparing the risks of invasive assessment tools.22 Noncalcified coronary plaque burden (NCB) by CCTA has been shown to associate with thin cap fibroatheroma by histological evaluation23 and is elevated in psoriasis.21 Furthermore, noncalcified plaques are known to be the culprit lesion in most ST-segment elevation myocardial infarction.23,24,25 Total coronary plaque burden (TB) and NCB by CCTA are beneficial in the prediction of prospective cardiovascular outcomes.26,27 Additionally, CCTA also provides a reliable tool to characterize the lipid-rich, rupture-prone HRPs, which associate with an increased risk of prospective cardiovascular events.24 We have previously demonstrated that psoriasis is associated with a higher prevalence of such HRPs compared with age- and sex-matched healthy volunteers, to a similar extent as seen in hyperlipidemic patients 10 years older.21 Here, we used an ongoing cohort study of patients with psoriasis and leveraged multimodality imaging using 18F-FDG PET/CT and CCTA. Our goal was to analyze the association between aortic VI (Figure 1A) and CCTA-based quantitative and qualitative CAD indices including TB and NCB, luminal stenosis, and HRP characteristics (Figure 1B). We hypothesized that there would be a direct association between aortic VI and CAD indices.
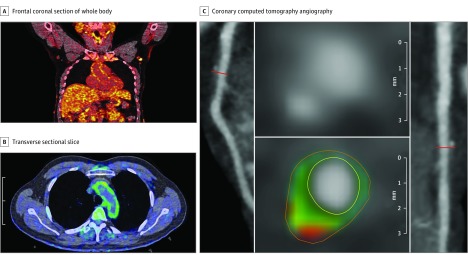
Figure 1. Aortic Vascular Inflammation by Fluorodeoxyglucose F 18 Positron Emission Tomography Computed Tomography (18F-FDG PET/CT) and Coronary Artery Disease Characterization by Coronary Computed Tomography Angiography.
A and B, Frontal coronal section of whole body 18F-FDG PET/CT scan (A) demonstrating increased uptake of FDG throughout the body in patients with psoriasis, with clearly increased uptake in the aortic wall. A transverse sectional slice from 18F-FDG PET/CT (B) demonstrates increased vascular inflammation in the aortic wall seen by higher intensity of FDG signal (green) in a patient with psoriasis. C, A panel of reconstructed images from the coronary computed tomography angiography demonstrates path of left anterior descending coronary artery (left), depicting noncalcified burden of the coronary artery and transverse section of left anterior descending coronary artery (right). The planar reconstruction (middle) reveals low-attenuation lipid-rich high-risk plaque (green and red).
Methods
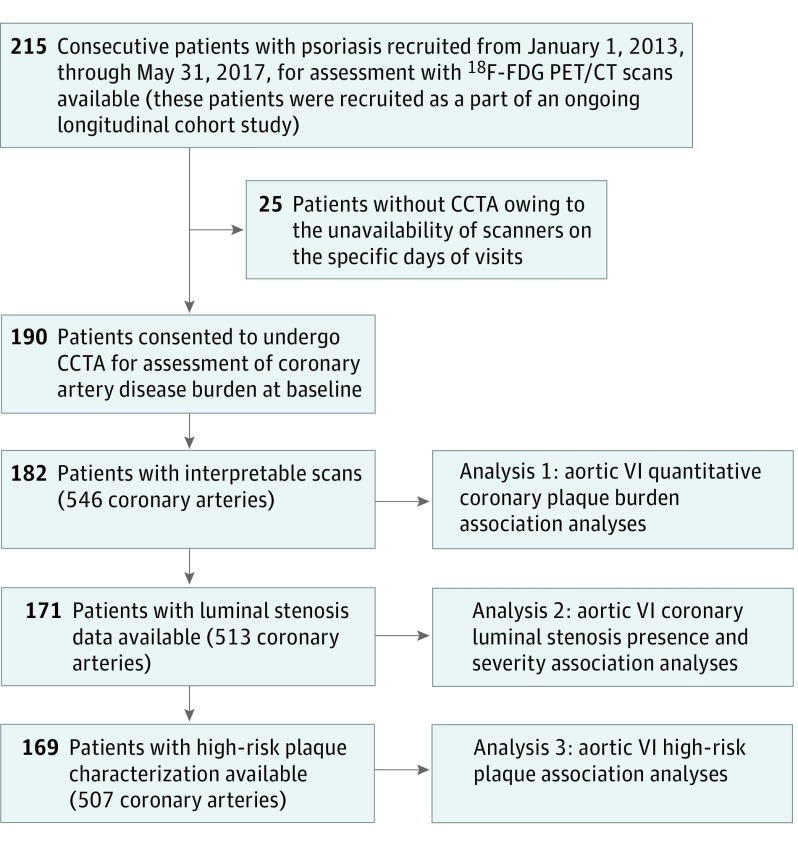
This study consisted of a cohort of consecutive patients with psoriasis (n = 215), all of whom were recruited between January 1, 2013, and May 31, 2017, and underwent 18F-FDG PET/CT. Of 215 patients, 25 patients did not undergo CCTA scans owing to the unavailability of scanners on their specific visit dates; thus, 190 patients had CCTA scans (Figure 2). Of 190 patients with CCTA, 182 scans (546 arteries) were interpretable and were included in the final analysis for quantitative plaque burdens. Furthermore, for analyses of luminal stenoses, scan reads were available for 171 patients (513 arteries), while HRP characterization was available in 169 patients (507 arteries). Coronary plaque burden was evaluated across 3 major epicardial coronary arteries using the dedicated software QAngio CT (Medis) by previously described methods.19 Semiautomated segmentation of each major coronary artery was performed. The need for manual adjustment was reviewed by assessing transverse reconstructed cross-sections at 0.5-mm increments. Coronary artery disease indices, such as TB and NCB volume indices, assessed as millimeters squared, were calculated by dividing total vessel plaque volume by total vessel length for standardization and were attenuated for luminal intensity for accuracy. Clinical reads were used for analysis of luminal stenosis. Quantitative stenosis grading recommended in Society of Cardiovascular Computed Tomography guidelines was used for categorization of stenosis severity.28 All coronary segments were analyzed for the presence of HRP, defined as a plaque with positive remodeling (PRP) (remodeling index ≥1.1) or presence of low-attenuation plaque (LAP) (<30 Hounsfield units). Low-attenuation plaque and PRP scores were calculated for each artery by calculating the number of LAPs or PRPs present in that artery. High-risk plaque score was calculated by addition of LAP and PRP scores. All scores were confirmed by 2 separate, trained, and blinded readers (intraclass correlation coefficient, 94% for intraexaminer and intraclass correlation coefficient, 93% for interexaminer analysis). The primary outcomes of our study were TB and NCB, whereas secondary outcomes were presence of luminal stenoses with its various severities, HRP assessed by CCTA, and various HRP scores. The main exposures were aortic VI by 18F-FDG PET/CT assessed as target-to-background ratio (TBR) and other cardiovascular risk factors. All participants provided written informed consent. The study protocols were approved by the institutional review board at the National Institutes of Health. All study protocols are in compliance with the Declaration of Helsinki.
Figure 2. Recruitment Scheme.
CCTA indicates coronary computed tomography angiography; 18F-FDG PET/CT, 18-fluorodeoxyglucose positron emission tomography computed tomography; VI, vascular inflammation.
Multivariable linear regressions were performed to examine the association between aortic VI and TB, NCB, and dense-calcified plaque. Standardized β values from these analyses were reported, which indicate number of SD change in the outcome variable (TB or NCB) per SD change in the exposure (aortic VI). Multivariable logistic regressions assessed the aortic VI–luminal stenosis and aortic VI–HRP associations, whereas ordered logistic regressions were used for studying the luminal stenosis severity–aortic VI as well as HRP score–aortic VI associations. Odds ratios (ORs) with 95% confidence intervals were reported for all logistic regression analyses. Detailed methods, including the comprehensive imaging protocols, image analyses protocols, covariate determinations, and statistical analyses, are provided in the eMethods in the Supplement. A 2-sided P value of less than .05 was considered statistically significant.
Results
Characteristics of the Study Group at Baseline
We summarize the characteristics of our study population in Table 1. The psoriasis cohort included 215 middle-aged patients (mean [SD] age, 50.4 [12.6] years), was male predominant (n = 126 [59%]), and had moderate skin disease severity by psoriasis area severity index score (median, 5.7; interqartile range [IQR], 3-10). Median psoriasis duration was 20 years. Patients with psoriasis had a mean (SD) body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) suggestive of overweight profiles (29.6 [6.2]) and a high prevalence of dyslipidemia (48%) despite near-normal lipid profiles, with mean (SD) total cholesterol of 183 (37.9) mg/dL (to convert to millimoles per liter, multiply by 0.0259) and low-density lipoprotein cholesterol of 102.2 (31.1) mg/dL. Sixty-nine of 215 patients with psoriasis (32%) were receiving lipid-lowering therapy at baseline. We observed increased insulin resistance in psoriasis (homeostatic model assessment insulin resistance, 2.8; IQR, 1.6-5.0), although the prevalence of diabetes mellitus was 11%. Moreover, patients with psoriasis were at low risk for cardiovascular disease by atherosclerotic cardiovascular disease 10-year risk (median, 2.2; IQR, 0.7-6.1). Aortic VI was high in patients with psoriasis at baseline (mean [SD] TBR, 1.70 [0.26]). The quantified TB was 1.14 (0.44) mm2, which was predominantly composed of NCB (mean [SD], 1.11 [0.46] mm2). A total of 82 patients (163 arteries) of 171 (513 arteries) evaluated had greater than 25% luminal stenosis. Finally, 57 patients of 169 evaluated had prevalent HRP in at least 1 major epicardial coronary artery.
Table 1. Characteristics of the Study Cohort Stratified by Severity of Aortic Vascular Inflammation: Baseline Data From 215 Patients.
| Parameter | Mean (SD) | P Value | ||
|---|---|---|---|---|
| Total (n = 215) | TBRa | |||
| <Median (n = 106) | ≥Median (n = 109) | |||
| Demographics and medical history | ||||
| Age, y | 50.4 (12.6) | 49.6 (13.5) | 51.2 (11.5) | .11 |
| Men, No. (%) | 126 (59) | 53 (50) | 73 (67) | .001 |
| White race/ethnicity, No. (%) | 168 (78) | 86 (81) | 82 (75) | .19 |
| Hypertension, No. (%) | 62 (29) | 25 (24) | 36 (33) | .13 |
| Hyperlipidemia, No. (%) | 102 (48) | 47 (44) | 54 (50) | .45 |
| Type 2 diabetes mellitus, No. (%) | 23 (11) | 8 (7) | 14 (14) | .20 |
| Current tobacco use, No. (%) | 20 (9) | 8 (7) | 11 (11) | .51 |
| Lipid treatment, No. (%) | 69 (32) | 33 (31) | 35 (32) | .88 |
| BMI | 29.6 (6.2) | 26.6 (3.9) | 32.7 (6.6) | <.001 |
| Clinical and laboratory values | ||||
| Systolic blood pressure, mm Hg | 122.6 (15.0) | 120.2 (13.0) | 125.2 (16.5) | .01 |
| Cholesterol, mg/dL | ||||
| Total | 183.0 (37.9) | 184.1 (41.2) | 181.8 (34.4) | .66 |
| High-density lipoprotein | 56.2 (17.9) | 62.5 (19.1) | 49.5 (13.7) | <.001 |
| Low-density lipoprotein | 102.2 (31.1) | 100.6 (33.5) | 103.9 (28.4) | .46 |
| Triglycerides, median (IQR), mg/dL | 101 (77-139) | 92 (74-121) | 113 (83-190) | <.001 |
| ASCVD 10-y risk, median (IQR) | 2.2 (0.7-6.1) | 1.5 (0.4-4.4) | 3.5 (1.6-7.9) | <.001 |
| HOMA-IR, median (IQR) | 2.8 (1.6-5.0) | 2.1 (1.4-3.2) | 4.1 (2.5-6.9) | <.001 |
| High-sensitivity C-reactive protein, median (IQR), mg/L | 2.0 (0.9-4.5) | 1.6 (0.7-3.5) | 2.6 (1.0-7.1) | .01 |
| Psoriasis characterization, median (IQR) | ||||
| Disease duration, y | 20 (10-30) | 19 (9-30) | 20 (10-30) | .41 |
| Psoriasis area severity index score | 5.7 (3-10) | 4.8 (2.8-8.0) | 6.4 (3.2-11.7) | .03 |
| Systemic or biologic treatment, No. (%) | 78 (37) | 36 (34) | 42 (39) | .49 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HOMA-IR, homeostasis model assessment of insulin resistance; IQR, interquartile range; TBR, target-to-background ratio.
SI conversion factor: To convert cholesterol levels to millimoles per liter, multiply by 0.0259; high-sensitivity C-reactive protein to nanomoles per liter, multiply by 9.524; triglycerides to millimoles per liter, multiply by 0.0113.
Median TBR = 1.66.
Association Between Aortic VI and CAD
We compared the patients with psoriasis with TBR at the median or higher (1.66) with those with TBR less than the median to assess whether coronary findings tracked alongside TBR. Patients with psoriasis with higher TBR (mean [SD], 1.89 [0.22]) had similar age compared with those with lower-than-median TBR (mean [SD], 1.51 [0.10]) but were predominantly composed of men (73 of 109 [67%] vs 53 of 106 [50%], P = .001) (Table 1). They also had a higher BMI (mean [SD], 32.7 [6.6] vs 26.6 [3.9]; P < .001), despite similar prevalence of diabetes, hyperlipidemia, and comparable traditional lipid profiles except high-density lipoprotein cholesterol, which was lower (mean [SD], 49.5 [13.7] mg/dL vs 62.5 [19.1] mg/dL; P < .001). Alhough both subgroups had low atherosclerotic cardiovascular disease 10-year risk, patients with higher TBR had increased atherosclerotic cardiovascular disease risk (3.5; IQR, 1.6-7.9 vs 1.5; IQR, 0.4-4.4; P < .001) compared with the other subgroup. Finally, despite similar psoriasis duration, patients with higher TBR had elevated insulin resistance and increased levels of high-sensitivity C-reactive protein concomitant with increased psoriasis severity.
On comparing the CCTA-based characteristics, quantitative TB was increased in patients with higher TBR (mean [SD], 1.33 [0.47] vs 0.97 [0.39]; P < .001), which was predominantly secondary to increased NCB (mean [SD], 1.30 [0.47] vs 0.93 [0.37]; P < .001) (Table 2). Dense-calcified burden did not differ between these groups (mean [SD], 0.04 [0.12] vs 0.04 [0.08]; P = .64). Concurrent with quantitative analyses, more patients with higher TBR had presence of luminal stenosis in at least 1 major epicardial coronary artery (49 of 82 [60%] vs 37 of 89 [37%]; P < .001) compared with patients with TBR lower than median. Number of arteries with luminal stenosis was also greater in this group (98 [40%] vs 65 [24.5%]; P < .001). Furthermore, severity of luminal stenosis was significantly worse in patients with higher TBR (P = .002). Finally, HRP prevalence was higher in those with higher TBR (42% vs 26%, P = .02). The distribution of HRP score differed significantly between 2 groups and was driven by the difference in LAP score, while PRP scores were essentially similar (Table 2). Similar analyses were performed to test whether the reverse was true for TBR, which demonstrated increased TBR in patients with luminal stenosis as well as in patients with presence of HRP compared with those without (eTables 1 and 2 in the Supplement).
Table 2. Baseline Coronary CTA-Based Characteristics of the Cohort Stratified by Median Value of Vascular Inflammation.
| Parameter | TBR, Mean (SD)a | P Valueb | |
|---|---|---|---|
| <Median (n = 106) | ≥Median (n = 109) | ||
| Aortic vascular inflammation | |||
| Aortic TBR | 1.51 (0.10) | 1.89 (0.22) | <.001 |
| Coronary CTA-based luminal stenoses, No. of patients/No. of arteries | 89 /267 | 82/246 | NA |
| Patients with luminal stenosis | 33 (37) | 49 (60) | <.001 |
| Arteries with luminal stenosis | 65 (24.5) | 98 (40) | <.001 |
| Severity of luminal stenosisc | |||
| Minimal/none | 202 (75.5) | 148 (60) | .002 |
| Mild | 59 (22) | 87 (35) | |
| Moderate | 4 (1.5) | 9 (4) | |
| Severe | 2 (1) | 2 (1) | |
| Coronary plaque burden, No. of patients/No. of arteries | 93/279 | 89/267 | NA |
| Burden, mm2 (×100) | |||
| Total | 0.97 (0.39) | 1.33 (0.47) | <.001 |
| Noncalcified | 0.93 (0.37) | 1.30 (0.47) | <.001 |
| Dense-calcified | 0.04 (0.08) | 0.04 (0.12) | .64 |
| High-risk plaque characterization, No. of patients/No. of arteries | 86/258 | 83/249 | NA |
| Presence of HRP, No. (%) | 22 (26) | 35 (42) | .02 |
| HRP score | |||
| 0 | 206 (80) | 184 (74) | .02 |
| 1 | 40 (15.5) | 48 (19) | |
| 2 | 11 (4) | 9 (4) | |
| ≥3 | 1 (0.5) | 8 (3) | |
| LAP score | |||
| 0 | 216 (83.5) | 203 (81.5) | .03 |
| 1 | 40 (15.5) | 33 (13.5) | |
| 2 | 2 (1) | 8 (3) | |
| ≥3 | 0 (0) | 5 (2) | |
| PRP score | |||
| 0 | 239 (92.5) | 226 (91) | .69 |
| 1 | 17 (6.5) | 18 (7) | |
| 2 | 1 (0.5) | 1 (0.5) | |
| ≥3 | 1 (0.5) | 4 (1.5) | |
Abbreivations: CTA, computed tomography angiography; HRP, high-risk plaque; IQR, interquartile range; LAP, low-attenuation plaque; NA, not applicable; PRP, positively remodeled plaque; TBR, target-to-background ratio.
Median TBR = 1.66.
P values were calculated by using t test or Mann-Whitney U test for continuous variables and Pearson χ2 test for categorical variables comparing various parameters between groups stratified by median value of TBR.
Luminal stenoses severities were categorized as minimal (0%-24%) or no stenosis, mild (25%-49%), moderate (50%-69%), and severe (≥70%). There were no vessels with complete occlusion.
Association of Aortic VI With Quantitative Total and Noncalcified Coronary Plaque Burden, Luminal Stenosis, and High-Risk Plaque Characteristics
Subsequent analyses showed that aortic VI was associated with both TB (β = 0.48; P < .001) and NCB (β = 0.49; P < .001) (Table 3). This association persisted after adjustment for age, sex, BMI, diabetes, hypertension, smoking, hyperlipidemia, lipid-lowering treatment with statins, high-sensitivity C-reactive protein, and psoriasis treatment with systemic/biologic agents (TB, β = 0.23; P < .001 and NCB, β = 0.24; P < .001) (eFigure 1 in the Supplement). No such association was observed for dense-calcified burden (eFigure 2 in the Supplement). For each 1-SD unit increase in aortic VI, we observed a 20% increase in NCB in unadjusted analyses and 10% higher NCB in fully adjusted analyses. Furthermore, aortic VI was associated with luminal stenosis in both unadjusted (OR, 3.63; 95% CI, 1.71-7.70; P = .001) and fully adjusted (OR, 3.40; 95% CI, 1.40-8.24; P = .007) analyses (eTable 3 in the Supplement). Analogous results were found for luminal stenosis severity (unadjusted OR, 4.03; 95% CI, 1.90-8.55; P < .001 and fully adjusted OR, 3.38; 95% CI, 1.42-8.07; P = .006). Per 1-SD increase in VI was associated with significantly increased odds of prevalent luminal stenosis (unadjusted, 1.38 and adjusted, 1.36) and severity of luminal stenosis (unadjusted, 1.42 and adjusted, 1.36). Finally, we also observed an association between aortic VI and HRP prevalence (OR, 3.05; 95% CI, 1.48-6.29; P = .002) that persisted beyond full adjustment (OR, 2.72; 95% CI, 1.08-6.83; P = .03) (eTable 4 in the Supplement). Similarly, aortic VI was also associated with HRP score (unadjusted OR, 3.03; 95% CI, 1.42-6.47; P = .004 and fully adjusted OR, 2.95; 95% CI, 1.21-7.18; P = .02), which was primarily driven by an association with LAP score (unadjusted OR, 5.63; 95% CI, 1.96-16.19; P = .001 and fully adjusted OR, 6.33; 95% CI, 1.88-21.28; P = .003), whereas PRP score did not associate with aortic VI (unadjusted OR, 1.51; 95% CI, 0.64-3.58; P = .70). Ultimately, per 1-SD increase in VI was associated with increased HRP prevalence at 1.34 times increased odds in unadjusted and 1.3 times increased odds in fully adjusted analyses.
Table 3. Association Between Coronary Computed Tomography Angiography–Based Quantitative Total, Noncalcified, and Dense-Calcified Coronary Plaque Burden and Vascular Inflammation.
| Model | Total Burden | Noncalcified Burden | Dense-Calcified Burden | |||
|---|---|---|---|---|---|---|
| Standardized β Coefficient | P Value | Standardized β Coefficient | P Value | Standardized β Coefficient | P Value | |
| Unadjusted | 0.48 | <.001 | 0.49 | <.001 | 0.03 | .43 |
| Adjusted for age, sex, BMI, diabetes, hypertension, hyperlipidemia, smoking, hsCRP, statins, and systemic/biologic psoriasis treatment | 0.23 | <.001 | 0.24 | <.001 | −0.05 | .24 |
Abbreviations: BMI, body mass index; hsCRP, high-sensitivity C-reactive protein.
Discussion
Major Findings
Our analysis of aortic VI by 18F-FDG PET/CT and CAD indices by CCTA demonstrates the following findings: (1) when stratified by high aortic VI, the extent of CAD is greater in those with elevated aortic VI, in line with greater quantitative total CAD burden, increased prevalence of luminal stenoses, more severe luminal stenosis, and a higher prevalence of HRP; (2) the increase in TB is predominantly driven by NCB, whereas dense-calcified burden does not differ by varying degrees of aortic VI; and (3) we found a strong association between aortic VI by 18F-FDG PET/CT and CAD indices derived from CCTA in unadjusted analyses and beyond adjustment for traditional cardiovascular risk factors. Our study adds to the growing body of literature demonstrating that aortic VI by 18F-FDG PET/CT may be a potential surrogate for early coronary disease.
Aortic VI by 18F-FDG PET/CT as a Marker of Atherosclerosis and CAD Evaluation by CCTA
Inflammation is critical to atherosclerosis.29 Psoriasis, a chronic inflammatory disease associated with increased systemic inflammation and aortic VI,5,6 greater burden of lipid-rich NCB,21 higher prevalence of HRP,21 and elevated risk of myocardial infarction and major adverse cardiovascular events,3,4 provides an opportunity to study the role of inflammation in early CAD. Within the last decade, aortic VI by 18F-FDG PET/CT has been validated as a surrogate marker of atherosclerosis and prospective cardiovascular risk.30 Aortic VI by 18F-FDG PET/CT is associated with prospective risk of cardiovascular events12 and modulates following cardiovascular risk-modifying therapies such as statins14,15 and therapeutic lifestyle changes.13 In the past, we have demonstrated that aortic VI is increased in psoriasis and is associated with psoriasis severity5,6 and novel nuclear magnetic resonance–derived markers of inflammation such as glycoprotein acetylation31 and is responsive to treatment with anti-inflammatory medications in patients with psoriasis.16
While 18F-FDG PET/CT provides a reliable tool to assess aortic VI, CCTA remains the ideal noninvasive modality for the comprehensive evaluation of CAD,22 including the assessment of coronary atherosclerotic plaque and its morphological features, beyond the coronary artery lumen.18 While CCTA-based analysis of luminal stenosis provides important prognostic information,32,33,34 quantitative volumetric coronary artery plaque assessment by CCTA has been shown to provide value in assessing future risk of major adverse cardiovascular events.20,27 Finally, imaging vulnerable coronary plaques with CCTA has also revealed significant prognostic implications in recent studies.18,24 Given the efficacy of multimodality imaging to detect different phases of atherosclerosis in multiple vascular beds, we used concurrent imaging with 18F-FDG PET/CT and CCTA in patients with psoriasis to understand the association between aortic VI and CAD indices.
Association Between Aortic VI by 18F-FDG PET/CT and CAD Indices by CCTA
In this study, we demonstrated an association between aortic VI and TB as well as between aortic VI and NCB, both of which persisted beyond adjustment for traditional risk factors, signifying that the presence of early vascular disease may in part be comparable across multiple major vascular beds. Interestingly, dense-calcified coronary plaque burden was not associated with aortic VI, which upheld the notion that aortic VI maybe a marker of early vascular disease.9 Furthermore, we demonstrated that aortic VI was directly associated with presence as well as severity of luminal stenosis in major epicardial coronary arteries, denoting aortic VI as a clinically reliable indicator of CAD. Finally, aortic VI was associated with HRP prevalence and HRP score in patients with psoriasis. This was principally driven by the association between aortic VI and LAP, whereas the association between aortic VI and PRP was statistically nonsignificant. While this is a biologically interesting finding, caution needs to be exercised in interpreting this result because the absolute number of PRP was small in our study. Taken together, these findings suggest that aortic VI may be a potential surrogate marker for the evaluation of early CAD. Finally, building on the prior literature, these associations suggest that the inflammatory processes captured by 18F-FDG PET/CT may be implicated in the development as well as progression of coronary atherosclerosis.35
Strengths and Limitations
Despite our significant findings, our study does have notable limitations. We lack hard cardiovascular outcomes. The small sample size, observational nature of our study, and cross-sectional analyses preclude any attempt at establishing causality and also make our analyses prone to unmeasured confounding. When imaging targets of small size, such as atherosclerotic plaques, the spatial resolution limitations of PET imaging create a close association between lesion size and the apparent lesion intensity.36,37 Thus, an interaction is possible between apparent plaque size measured by CT and standardized tracer activity of the aorta, considering that larger individuals will have larger coronary arteries and aortic wall sizes. To attenuate such an interaction, we included adjustment for BMI within our multivariable model and still observed significance in the association between CAD indices and aortic VI, albeit with attenuated strength. Another limitation of our study is that VI was assessed in the aorta and not in the coronary arteries. There are several technical hurdles in attempting to assess coronary inflammation by PET. Electrocardiogram and respiratory motion gating were not available for 18F-FDG PET/CT, thus precluding fusion of coronary PET imaging with CCTA; the lesion size expected for coronary plaques, with their low-level activity, is less than the resolution of PET to quantify regional background activity; and we did not have our patients consume a high-fat diet for suppression of intense myocardial activity that would be required for coronary VI quantification. We acknowledge that coronary VI imaging with PET/CT is feasible,35 especially with emerging tracers,38 but these techniques are still evolving. Finally, statin therapy reduces both aortic VI and CAD plaque burden.14,15,39,40 Although we adjusted for statin use in our regression models, we did not have data on duration of statin therapy in our patients.
Implications and Future Directions
Our findings build on previous evidence of an association between VI in the aortic arch by PET and LAP by CCTA in an inflammatory condition (eg, HIV).17 We confirm this association using VI from the entire aorta and also extend these findings by providing further evidence for an association between aortic VI and coronary plaque burden and composition, including the whole vessel NCB, HRP prevalence, and HRP subtypes. These findings are potentially generalizable to other inflammatory diseases but would need to be confirmed in noninflammatory states using larger generalizable cohorts in longitudinal studies. Additionally, future studies should use multimodality imaging, including CCTA concurrent with 18F-FDG PET/CT, to investigate the effect of reducing inflammation on atherosclerosis in various vascular beds.
Conclusions
Our findings demonstrate that aortic VI by 18F-FDG PET/CT is associated with multiple indices of coronary plaque extent and composition derived from CCTA. This provides evidence that aortic VI by 18F-FDG PET/CT may be a potential surrogate for assessment of early CAD captured by CCTA. Larger studies assessing these associations over longer periods of time are warranted. Concurrent use of multimodality imaging should assess whether targeted therapies to curb inflammation affect early vascular disease in the aorta and coronary arteries.
eMethods.
eResults.
eReferences.
eTable 1. Baseline Characteristics of the Study Cohort Stratified by Presence or Absence of Coronary Artery Stenoses
eTable 2. Baseline Characteristics of the Study Cohort Stratified by Presence or Absence of High-risk Plaque
eTable 3. Association Between Luminal Stenoses by Coronary Computed Tomography Angiography and Aortic Vascular Inflammation
eTable 4. Association Between Presence of High-Risk Plaque, Various High-risk Plaque Scores and Aortic Vascular Inflammation
eFigure 1. Aortic Vascular Inflammation by 18FDG PET/CT is Associated With Total and Noncalcified Coronary Plaque Burden
eFigure 2. Regression Plot Reveals No Association Between Aortic Vascular Inflammation and Dense-Calcified Coronary Plaque Burden
References
- 1.Hansson GK. Inflammation and atherosclerosis: the end of a controversy. Circulation. 2017;136(20):1875-1877. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204-212. [DOI] [PubMed] [Google Scholar]
- 3.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735-1741. [DOI] [PubMed] [Google Scholar]
- 4.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147(9):1031-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naik HB, Natarajan B, Stansky E, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group . Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319-328. [DOI] [PubMed] [Google Scholar]
- 9.Teague HL, Ahlman MA, Alavi A, et al. Unraveling vascular inflammation: from immunology to imaging. J Am Coll Cardiol. 2017;70(11):1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50(10):1611-1620. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa AL, Subramanian SS, Cury RC, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5(1):69-77. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa AL, Abdelbaky A, Truong QA, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6(12):1250-1259. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49(8):1277-1282. [DOI] [PubMed] [Google Scholar]
- 14.Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48(9):1825-1831. [DOI] [PubMed] [Google Scholar]
- 15.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909-917. [DOI] [PubMed] [Google Scholar]
- 16.Dey AK, Joshi AA, Chaturvedi A, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: a case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017;2(9):1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawakol A, Lo J, Zanni MV, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66(2):164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11(7):390-402. [DOI] [PubMed] [Google Scholar]
- 19.Salahuddin T, Natarajan B, Playford MP, et al. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur Heart J. 2015;36(39):2662-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwan AC, May HT, Cater G, et al. Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology. 2014;272(3):690-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136(3):263-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan AC, Cater G, Vargas J, Bluemke DA. Beyond coronary stenosis: coronary computed tomographic angiography for the assessment of atherosclerotic plaque burden. Curr Cardiovasc Imaging Rep. 2013;6(2):89-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narula J, Nakano M, Virmani R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61(10):1041-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66(4):337-346. [DOI] [PubMed] [Google Scholar]
- 25.Fleg JL, Stone GW, Fayad ZA, et al. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. 2012;5(9):941-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64(7):684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Versteylen MO, Kietselaer BL, Dagnelie PC, et al. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol. 2013;61(22):2296-2305. [DOI] [PubMed] [Google Scholar]
- 28.Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342-358. [DOI] [PubMed] [Google Scholar]
- 29.Harrington RA. Targeting inflammation in coronary artery disease. N Engl J Med. 2017;377(12):1197-1198. [DOI] [PubMed] [Google Scholar]
- 30.Rudd JH, Narula J, Strauss HW, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55(23):2527-2535. [DOI] [PubMed] [Google Scholar]
- 31.Joshi AA, Lerman JB, Aberra TM, et al. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res. 2016;119(11):1242-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724-1732. [DOI] [PubMed] [Google Scholar]
- 33.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324-2336. [DOI] [PubMed] [Google Scholar]
- 34.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161-1170. [DOI] [PubMed] [Google Scholar]
- 35.Singh P, Emami H, Subramanian S, et al. Coronary plaque morphology and the anti-inflammatory impact of atorvastatin: a multicenter 18f-fluorodeoxyglucose positron emission tomographic/computed tomographic study. Circ Cardiovasc Imaging. 2016;9(12):e004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huet P, Burg S, Le Guludec D, Hyafil F, Buvat I. Variability and uncertainty of 18F-FDG PET imaging protocols for assessing inflammation in atherosclerosis: suggestions for improvement. J Nucl Med. 2015;56(4):552-559. [DOI] [PubMed] [Google Scholar]
- 37.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S-150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarkin JM, Joshi FR, Evans NR, et al. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J Am Coll Cardiol. 2017;69(14):1774-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima JA, Desai MY, Steen H, Warren WP, Gautam S, Lai S. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation. 2004;110(16):2336-2341. [DOI] [PubMed] [Google Scholar]
- 40.Nissen SE, Nicholls SJ, Sipahi I, et al. ; ASTEROID Investigators . Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556-1565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eReferences.
eTable 1. Baseline Characteristics of the Study Cohort Stratified by Presence or Absence of Coronary Artery Stenoses
eTable 2. Baseline Characteristics of the Study Cohort Stratified by Presence or Absence of High-risk Plaque
eTable 3. Association Between Luminal Stenoses by Coronary Computed Tomography Angiography and Aortic Vascular Inflammation
eTable 4. Association Between Presence of High-Risk Plaque, Various High-risk Plaque Scores and Aortic Vascular Inflammation
eFigure 1. Aortic Vascular Inflammation by 18FDG PET/CT is Associated With Total and Noncalcified Coronary Plaque Burden
eFigure 2. Regression Plot Reveals No Association Between Aortic Vascular Inflammation and Dense-Calcified Coronary Plaque Burden