Key Points
Question
In patients with heart failure and ejection fraction equal to or greater than 40%, is an interatrial shunt device (IASD) safe compared with a sham control treatment, and does it remain patent after 1 year?
Findings
In a randomized clinical trial, the IASD was found to be patent 12 months after randomization in all patients randomly assigned to IASD treatment. In addition, at 12 months of follow-up, there were no significant differences in major adverse cardiac, cerebral, and renal events, and there were no strokes in either participant group.
Meaning
Through 1 year of follow-up, IASD treatment appears to be safe in patients with heart failure and ejection fraction equal to or greater than 40%.
This randomized clinical trial evaluates the 1-year safety, patency, and clinical outcomes of a transcatheter interatrial shunt device for patients with heart failure, compared with patients treated with a sham control procedure.
Abstract
Importance
In patients with heart failure (HF) and left ventricular ejection fraction (LVEF) equal to or greater than 40%, a transcatheter interatrial shunt device (IASD; Corvia Medical) reduces exercise pulmonary capillary wedge pressure (PCWP) and is safe compared with sham control treatment at 1 month of follow-up. The longer-term safety and patency of the IASD has not yet been demonstrated in the setting of a randomized clinical trial (RCT).
Objective
To evaluate the 1-year safety and clinical outcomes of the IASD compared with a sham control treatment.
Design, Setting, and Participants
This phase 2, double-blind, 1-to-1 sham-controlled multicenter RCT of IASD implantation vs a sham procedure (femoral venous access and imaging of the interatrial septum without IASD) was conducted in 22 centers in the United States, Europe, and Australia on patients with New York Heart Association (NYHA) class III or ambulatory class IV HF, LVEF equal to or greater than 40%, exercise PCWP equal to or greater than 25 mm Hg, and PCWP-right atrial pressure gradient equal to or greater than 5 mm Hg.
Main Outcomes and Measures
Safety was assessed by major adverse cardiac, cerebrovascular, or renal events (MACCRE). Exploratory outcomes evaluated at 1 year were hospitalizations for HF, NYHA class, quality of life, a 6-minute walk test, and device patency.
Results
After 1 year, shunts were patent in all IASD-treated patients; MACCRE did not differ significantly in the IASD arm (2 of 21 [9.5%]) vs the control arm (5 of 22 [22.7%]; P = .41), and no strokes occurred. The yearly rate of hospitalizations for HF was 0.22 in the IASD arm and 0.63 in the control arm (P = .06). Median improvement in NYHA class was 1 class in the IASD arm (IQR, −1 to 0) vs 0 in the control arm (IQR, −1 to 0; P = .08). Quality of life and 6-minute walk test distance were similar in both groups. At 6 months, there was an increase in right ventricular size in the IASD arm (mean [SD], 7.9 [8.0] mL/m2) vs the control arm (−1.8 [9.6] mL/m2; P = .002), consistent with left-to-right shunting through the device; no further increase occurred in the IASD arm at 12 months.
Conclusions and Relevance
The REDUCE LAP-HF I phase 2, sham-controlled RCT confirms the longer-term patency of the IASD. Through 1 year of follow-up, IASD treatment appears safe, with no significant differences in MACCRE in patients receiving IASD compared with those who received sham control treatment.
Trial Registration
ClinicalTrials.gov identifier: NCT02600234
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF; defined by a left ventricular ejection fraction [LVEF] ≥50%)1,2 and HF with midrange ejection fraction (HFmrEF; defined by an LVEF between 40% and 49%)3,4 are common and associated with substantial morbidity and mortality. There is currently a lack of evidence-based treatments that can improve outcomes in these HF syndromes. Although diuretic therapy is useful for reducing symptoms and signs of congestion,4,5 it is associated with a number of untoward effects, including urinary frequency and urgency, electrolyte disturbances, kidney dysfunction, and orthostasis. A key feature of HFpEF is a steep increase in left atrial (LA) pressure during exercise, which is an important cause of exertional dyspnea in these patients.6,7,8 In addition, in the setting of HFpEF, the magnitude of rise in pulmonary capillary wedge pressure (PCWP) during exercise is directly associated with adverse outcomes.9 Thus, reducing LA pressure, particularly during exercise, could be an important therapy for improving both symptoms and outcomes in patients with HFpEF and HFmrEF.
We previously reported the early effects of a transcatheter Interatrial Shunt Device (IASD; Corvia Medical Inc) in patients with HFpEF or HFmrEF.10 The device is implanted in the interatrial septum using standard transseptal catheter techniques.11 The IASD implant creates an interatrial communication, which is intended to dynamically decompress LA pressure during exercise. We conducted a phase 2, sham-controlled randomized clinical trial (RCT) to evaluate the mechanistic effect of the IASD on invasively measured PCWP during exercise.11 As previously described,10 at 1 month after randomization, patients assigned to treatment with an IASD had a significantly greater reduction in PCWP during both passive leg raise maneuver and exercise (accounting for all stages of exercise). Mean PCWP at 20 W of exercise also decreased to a greater degree in the patients randomly assigned to IASD compared with those assigned to the sham control group. The IASD appeared safe compared with the sham control procedure periprocedurally and during short-term follow-up at 1 month. In this study, we report the 1-year results.
Methods
Study Design and Participants
The rationale and design of the REDUCE LAP-HF-I trial have been described previously11; the full trial protocol is available in Supplement 1. The primary objective of the REDUCE LAP-HF I clinical trial was to evaluate the mechanistic effect of implanting the IASD System II (Corvia Medical) in patients with HF who have EF equal to or greater than 40% and elevated LA pressure and who remained symptomatic despite optimal guideline-directed medical therapy. This was a multicenter, prospective, blinded RCT with a sham control group and 1-to-1 randomization. Patients were recruited between February 3, 2016, and November 23, 2016, at 22 centers in the United States, Belgium, France, Netherlands, the United Kingdom, and Australia. (eTable 1 in Supplement 2 lists all of the participating sites, principal investigators, and study coordinators for the trial.) eFigure 1 in Supplement 2 graphically displays the design of the REDUCE LAP-HF I trial.
The inclusion and exclusion criteria were designed to ensure that patients had symptomatic HF (defined as New York Heart Association [NYHA] class III or ambulatory class IV), elevated LA pressure with a pressure gradient between the LA and right atrium (RA), and no clinically important right heart dysfunction. Key inclusion criteria included documented chronic symptomatic HF and either (1) a prior hospitalization for HF or an acute care facility or emergency department intensification of diuretic therapy within the prior 12 months or (2) elevated B-type natriuretic peptide (BNP) or N-terminal pro-BNP within the past 6 months (with a BNP level greater than 70 pg/mL in normal sinus rhythm or 200 pg/mL in atrial fibrillation or an N-terminal pro-BNP level greater than 200 pg/mL in normal sinus rhythm or 600 pg/mL in atrial fibrillation); an EF of 40% or more; an age of 40 years or older; elevated LA pressure documented invasively by end-expiratory PCWP during supine bike exercise of 25 mm Hg or higher; and a PCWP–RA pressure (RAP) gradient of 5 mm Hg or more.
Key exclusion criteria included stage D HF; a cardiac index less than 2.0 L/min/m2; a history of stroke, transient ischemic attack, deep vein thrombosis, or pulmonary embolism within the past 6 months; hemodynamically significant valvular disease; hypertrophic or infiltrative cardiomyopathy; RV dysfunction (moderate or severe RV dysfunction, a tricuspid annular plane systolic excursion less than 1.4 cm, an RV size larger than the patient’s LV size, or an RV fractional area change less than 35%); a resting RAP gradient less than 14 mm Hg; or pulmonary vascular resistance greater than 4 Wood units. A full list of the inclusion and exclusion criteria are listed in the eAppendix in Supplement 2.
The study protocol was approved by the institutional review board or ethics committee at each of the 22 enrolling sites, and all enrolled patients provided written informed consent. A data safety monitoring committee oversaw the program and reviewed trial data for patient safety at regular intervals. A separate clinical events committee adjudicated all outcomes. All statistical analyses were performed independently by the Baim Institute for Clinical Research (Boston, Massachusetts).
Randomization and Blinding
Patients were eligible for randomization if they met all of the inclusion and exclusion criteria after undergoing the study qualification procedures, including noninvasive screening with echocardiography and supine bicycle exercise right heart catheterization. Immediately after qualifying, eligible patients were randomly assigned in a 1-to-1 ratio to the treatment or control groups. Randomization was performed via an eClinicalOS Interactive Web Response System (IBM Corporation). Patient blinding included sedation, earphones with music to preclude the patient from hearing discussions during the procedure, and blindfolding (or the use of opaque screens) to prevent the participant from viewing the imaging screens.
All patients assigned to either arm underwent sheath placement in the femoral vein. Patients randomly assigned to the control arm underwent intracardiac or transesophageal echocardiographic examination of the interatrial septum and LA appendage but no transseptal puncture. Patients randomly assigned to the treatment arm underwent a transseptal puncture and IASD System II implantation, as described previously.11,12,13 The IASD System II consists of a 1-piece self-expanding metal cage that has a double-disc design with an opening (barrel) in the center. The expanded external diameter of each disc is 19.4 mm. The inner diameter of the barrel in the center of the fully expanded implant is 8 mm, which is thought to be an optimal size.14
Participants and nonprocedural research staff were blinded to treatment assignment for 1 year after randomization. Each site was assigned blinded and unblinded staff members to facilitate unbiased patient assessments during follow-up. The physicians managing the patients clinically (including the treating cardiologist) and the research staff involved in conducting selected postrandomization evaluations, including those in the hemodynamic core laboratory, were blinded to study arm. At the 12-month visit, study participants and blinded study staff members at each site were asked to complete a questionnaire to determine their blinding status.
Efficacy and Safety End Points
An independent Clinical Events Committee adjudicated all major adverse cardiac, cerebrovascular, and renal events (MACCRE) and other outcome events. The 6-minute walk test (6MWT) was conducted by blinded study staff at each site. The echocardiography analyses were performed by an independent echocardiography core laboratory, as detailed previously.11,13,15
The primary efficacy end point of change in PCWP during exercise from baseline to 1 month, and the primary safety end point of periprocedural events and MACCRE at 1 month have been previously reported.10 Several efficacy and safety end points were prespecified for evaluation through 12 months after randomization and included (1) MACCRE, defined as the composite of cardiovascular death, embolic stroke, device-associated and/or procedure-associated adverse cardiac events, or new onset or worsening of kidney dysfunction (defined as a decrease in estimated glomerular filtration rate of more than 20 mL/min/1.73 m2); (2) HF hospitalization (which included intravenous diuretics at a health care facility); (3) change from baseline in loop diuretic dose, left heart structure or function (as recorded by echocardiography), NYHA class, quality of life (per the Kansas City Cardiomyopathy Questionnaire [KCCQ]16 and EuroQol 5-Dimension scale [EQ-5D]17), and 6MWT distance; and (4) assessment of shunt flow. Additional safety end points prespecified at 12 months included (1) the need for implant removal or occlusion of the implant; (2) major adverse cardiac events; (3) all-cause mortality and cardiovascular mortality; (4) newly acquired persistent or permanent atrial fibrillation or atrial flutter; (5) implant embolization and clinically significant device migration; (6) systemic embolic events; and (7) increase in RV size and decrease in RV function.
Statistical Analysis
All analyses for the 12-month efficacy and safety end points included here were based on an intention-to-treat (ITT) analytic data set of all randomized patients, except for 1 patient who withdrew from the trial at the index procedure, with documentation of missing data because of participant withdrawal from the study or because of deaths during follow-up. For outcomes that are associated with a change from baseline, the values at baseline and at the point of interest are presented. Dichotomous outcomes were compared between treatment arms using the Fisher exact test. Kaplan-Meier estimates of event rate were computed if there was a time-to-event component of the end point, and treatments were compared on such end points using the log-rank test. Treatment arms were compared using Poisson regression by their per patient-year rate of total HF-associated admissions or visits requiring intravenous diuretics. Changes in quality of life at 12 months, measured as changes in each of EQ-5D scale dimensions and KCCQ overall summary and clinical summary scores, were compared across treatment groups using analysis of covariance, adjusting for baseline quality of life. Additionally, a mixed-effect model repeated-measures analysis18 with baseline quality of life as a covariate was used to compare treatments simultaneously across all points through 12 months. The underlying within-patient covariance matrix was assumed to be unstructured. Change in NYHA class was compared across treatment groups using the Wilcoxon rank sum test.
All statistical tests were carried out at a 2-sided .05 level of significance, and all P values were 2-sided. Analyses were carried out using SAS version 9.4 (SAS Institute).
Results
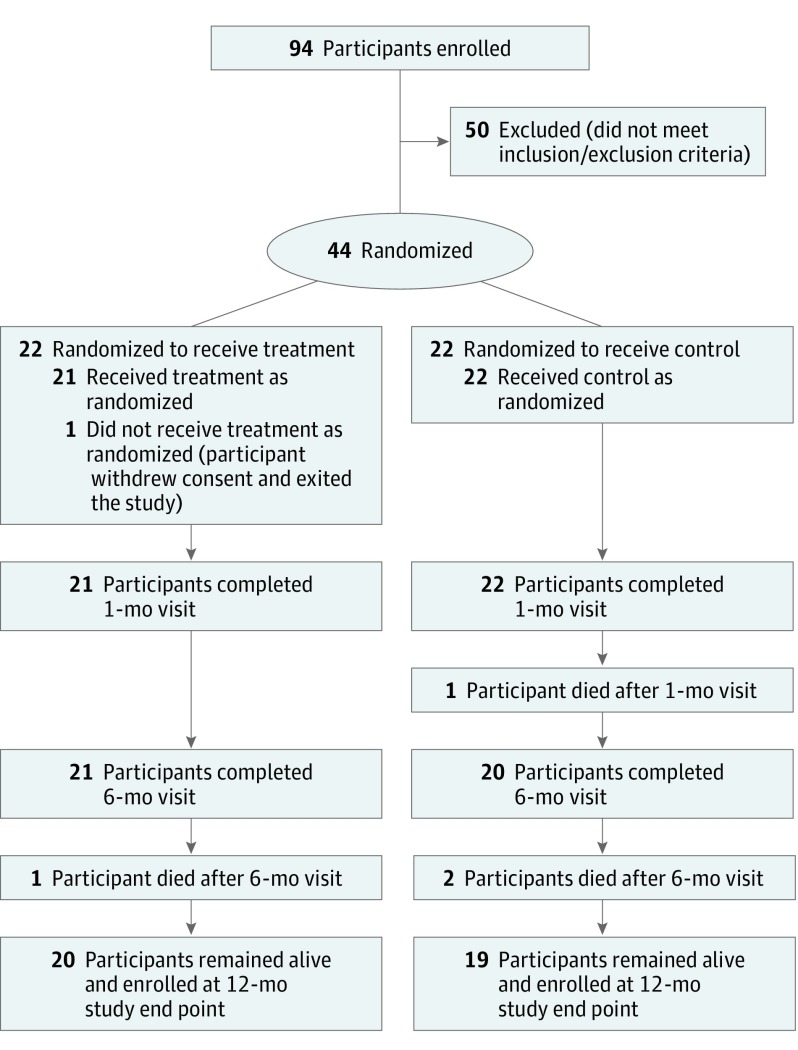
Figure 1 displays the study participant flow diagram up to 12 months, and Table 1 displays the baseline characteristics of the study participants in the 2 study arms (IASD vs sham-control groups) for all randomly assigned patients. There were no statistically significant differences between the study arms for any baseline characteristic, with the exception of race/ethnicity (in the IASD group: 0 African American patients, 19 white patients [86%], and 3 patients reporting other races/ethnicities [14%]; in the control group, 4 African American patients [18%], 18 white patients [82%], and 0 patients reporting other races/ethnicities; P = .03).
Figure 1. Study Participant Disposition Flowchart at 12 Months.
Table 1. Baseline Characteristics of All Patients by Treatment Group.
| Patient Characteristic | Patients, No. (%) | |
|---|---|---|
| IASD Group (n = 22) | Control Group (n = 22) | |
| Demographic factor | ||
| Age, median (IQR), y | 70.5 (67-75) | 71 (64-78) |
| Male | 14 (64) | 8 (36) |
| Race/ethnicity | ||
| Black or African American | 0 | 4 (18) |
| White | 19 (86) | 18 (82) |
| Other | 3 (14) | 0 |
| BMI, median (IQR) | 35.4 (33.4-39.1) |
35.2 (29.9-38.4) |
| Comorbidities/risk factors | ||
| Hypertension | 18 (82) | 20 (91) |
| Hyperlipidemia | 16 (73) | 16 (73) |
| Diabetes | 12 (55) | 12 (55) |
| Cardiopulmonary obstructive disease | 3 (14) | 7 (32) |
| Ischemic heart disease | 5 (23) | 5 (24)a |
| Prior myocardial infarction | 5 (23) | 4 (19)a |
| Prior coronary revascularization | 10 (48)a | 10 (46) |
| Atrial fibrillation | 12 (55) | 10 (45) |
| Atrial flutter | 1 (5) | 2 (9) |
| Stroke | 2 (9) | 3 (14)a |
| Transient ischemic attack | 3 (14) | 2 (9) |
| Peripheral arterial disease | 3 (14) | 2 (9) |
| Pulmonary embolism | 1 (5) | 1 (5) |
| Deep vein thrombosis | 3 (14) | 0a |
| Cardiac status | ||
| Left ventricular ejection fraction, site reported, median (IQR), % | 59.5 (56-65) | 60.0 (55-65) |
| New York Heart Association class | ||
| III | 22 (100) | 21 (96) |
| IV | 0 | 1 (5) |
| Loop diuretic dose (furosemide equivalents), median (IQR), mg | 40 (20-160) | 80 (40-160) |
| Hospitalization or emergency department/acute care visit for heart failure in the past 12 mo | 12 (55) | 16 (72.7) |
| Laboratory data, median (IQR) | ||
| Brain-type natriuretic peptide, pg/mLb | 89.6 (52.5-228.4) |
77 (51-120) |
| N-terminal pro–brain-type natriuretic peptide, pg/mLb | 298.5 (177-981.5) |
754 (236-1829) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 50 (33-60) | 50.5 (41-57) |
| Quality of life and exercise tolerance, median (IQR) | ||
| Kansas City Cardiomyopathy Questionnaire, median (IQR) | ||
| Overall summary score | 38.4 (29.2-55.7) | 40 (27.6-53.3) |
| Clinical summary score | 44.3 (34.9-54.2) | 44 (32.8-61.5) |
| EQ-5D scale score, median (IQR) | 0.7 (0.6-0.8) | 0.7 (0.4-0.8) |
| 6-min Walk test distance, median (IQR), m | 299.5 (231-334) | 242.5 (137-320) |
| Echocardiography, median (IQR)c | ||
| LV end-diastolic volume index, mL/m2 | 65.3 (53.1-75.2) |
57.3 (49.4-62.4) |
| LV end-systolic volume index, mL/m2 | 34.6 (26-42.2) | 28.3 (24.2-33.1) |
| LV ejection fraction, % | 47.5 (42.2-53) | 49.5 (45.4-53.3) |
| LA volume index, mL/m2 | 45.6 (34.9-52.8) |
36.5 (28.9-48.4) |
| Septal e′ velocity, cm/s | 6 (5-8) | 6 (5-7) |
| Lateral e′ velocity, cm/s | 8 (7-8) | 6 (5-8) |
| Average E/e′ ratio | 15.2 (10.2-17.5) |
15 (13-17.9) |
| RV end-diastolic volume index, mL/m2 | 19.3 (15.5-24.2) |
17.9 (14.6-24.8) |
| RV end-systolic volume index, mL/m2 | 7.5 (4.8-8.5) | 8.3 (4.6-11.1) |
| RV ejection fraction, % | 65.5 (57.5-73.3) | 65.1 (55.4-70) |
| Tricuspid annular plane systolic excursion, cm | 1.9 (1.7-2.3) | 2 (1.6-2.5) |
| RA volume index, mL/m2 | 29.7 (24-33.6) |
26.1 (18.5-33.7) |
| Estimated RA pressure, mm Hg | 3 (3-3) | 3 (3-3) |
| Invasive hemodynamicsc | ||
| RA pressure, mm Hg | 10.5 (9-12) | 8.5 (6-13) |
| Mean PA pressure, mm Hg | 28 (22-40) | 28 (23-33) |
| Cardiac output, L/min/m | 5.1 (4.3-6.2) | 4.6 (3.9-6.7) |
| Pulmonary vascular resistance, Wood units | 1.9 (1.2-3.1) | 1.2 (1-1.8) |
| Pulmonary capillary wedge pressure, mm Hg | ||
| With legs down | 19 (15-30) | 19 (17-25) |
| With legs up | 26 (22-33) | 22 (15-32) |
| Peak exercise | 38 (34-42) | 35 (33-45) |
| Pulmonary capillary wedge pressure–right atrial pressure gradient at rest | 9 (6-18) | 11 (7-14) |
| Workload-corrected, mm Hg/W/kg | 81.1 (69-97) |
83.5 (62.3-116.9) |
| Exercise duration, min | 7.5 (6-10) | 9.5 (5-11) |
| Peak exercise workload, W | 40 (20-60) | 40 (20-60) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; EQ-5D, EuroQol 5-dimension scale.
SI conversion factor: To convert picomoles per milliliter to nanograms per liter, multiply by 1.0.
Total patients included in this category were 21 (not 22).
Natriuretic peptide levels were measured locally at each site based on the available type of measurement (brain-type natriuretic peptide or N-terminal pro–brain-type natriuretic peptide). There were no statistically significant differences levels between groups; N-terminal pro–brain-type natriuretic peptide was numerically higher in the control group, and brain-type natriuretic peptide was numerically higher in the interatrial shunt device group. z Scores were calculated and the data was combined so that all patients in the study could be compared on the same scale (ie, via z scores for natriuretic peptide). When examined this way, the 2 treatment groups had identical median values (control group: median [IQR] z score, −0.37 [−0.48 to 0.01]; IASD group: −0.37 [−0.48 to 0.18]).
Measurements were made by independent core laboratories.
One patient withdrew from the study at the time of the index procedure after being randomly assigned because of an inability to access the right atrium from the femoral vein for insertion of the intracardiac echocardiography probe (because of a previously unrecognized inferior vena cava filter). The participant was unblinded immediately after the attempt and withdrew consent on learning that device placement was not feasible. Thus, the population used for 12-month analysis consisted of 21 patients in the IASD arm and 22 patients in the sham control arm.
As shown in Table 1, the patients enrolled in the REDUCE LAP-HF I trial were typical of other HFpEF studies: they were elderly (median [interquartile range (IQR)] age, 71 [66-76] years), obese (median [IQR] body mass index, 35 [31-39]), and had a high prevalence of multiple comorbidities (eg, hypertension in 38 of 44 participants [86%]). The patients had a quality of life (EQ-5D scale median [IQR] score: 0.7 [0.5-0.8]) that was worse than other contemporary HFpEF studies, such as the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial.19 Exercise tolerance was also quite low at baseline, as judged by a mean (SD) 6MWT distance of 257.4 (101.9) m across both groups. On echocardiography, LV ejection fraction was midrange or preserved in all participants (median [IQR], 60% [55%-65%]), mean LV and RV sizes were normal, and mean RV systolic function was normal (Table 1). Study participants had evidence of diastolic dysfunction, based on an enlarged LA and increased E/e′ ratio (a marker of LV filling pressures including early mitral filling velocity [E] and early diastolic mitral annular velocity [e′]; IASD group: median [IQR], 15.2 [10.2-17.5]; control group: 15 [13-17.9]). In addition, LA volume index (IASD group: median [IQR], 45.6 [34.9-52.8] mL/m2; control group: 36.5 [28.9-48.4] mL/m2) was larger than RA volume index (IASD group: median [IQR], 29.7 [24-33.6] mL/m2; control group: 26.1 [18.5-33.7] mL/m2), which is indicative of chronically increased LV filling pressures without considerable right-sided HF. Invasive hemodynamic testing revealed evidence of elevated PCWP with exercise (IASD group: PCWP with legs down: median [IQR], 19 [15-30] mm Hg; PCWP at peak exercise, 38 [34-42] mm Hg; control group: PCWP with legs down: 19 [17-25] mm Hg; PCWP at peak exercise, 35 [33-45] mm Hg) and PCWP pressure greater than RA pressure (median [IQR] gradient at rest: IASD group, 9 [6-18]; control group: 11 [7-14]), with no significant pulmonary arterial hypertension or RV failure.
Efficacy End Points
Through 1 year, there were no differences in MACCRE events among those assigned to the IASD arm (2 of 21 [9.5%]) compared with control group participants (5 of 22 [22.7%]; P = .41 by Fisher exact test; P = .20 by log-rank test). There was also no difference in all-cause mortality between the IASD group (1 of 21 [4.8%]) vs those in the control group (3 of 22 [13.6%]; P = .61), and no strokes in either group (eTable 2 in Supplement 2 and Figure 2A). There were no differences in HF-associated admissions or visits requiring intravenous diuretics among patients assigned to the IASD arm vs the control patients, and the rates of HF-related admissions or visits requiring intravenous diuretics were 0.22 (95% CI, 0.08-0.58) per patient-year for those assigned to the IASD arm compared with 0.63 in the control arm (95% CI, 0.33-1.21; P = .06; Table 2). Figure 2B displays the cumulative incidence of HF events over the first year of the trial. By the end of the year, the cumulative incidence of HF events requiring intravenous diuresis was 19% in the IASD arm vs 41% in the control arm (log-rank P = .08). Figure 2B also shows that there was a longer time from randomization to first HF event requiring intravenous diuresis in the IASD arm (mean [SD], 178 [47] days) vs the control arm (mean [SD], 70 [83] days).
Figure 2. Cumulative 12-Month Incidence of Major Adverse Cardiac, Cerebrovascular, and Renal Events and Heart Failure Events Requiring Intravenous Diuretic Treatment, Stratified by Treatment Group.
MACCRE indicates major adverse cardiac, cerebrovascular, and renal events.
Table 2. Key Secondary Outcome Measures at 12 Months.
| Measure | Median (Interquartile Range) | ||
|---|---|---|---|
| Participants With Interatrial Shunt Device (n = 21) | Control Participants (n = 22) | P Value | |
| Cardiovascular death | |||
| Available data, No. (%) [95% CI]a,b | 1 (4.8) [0.1-23.8] | 1 (4.5) [0.1-22.8] | >.99 |
| Kaplan-Meier cumulative incidence, % (95% CI)c | 4.8 (0.0-19.2) | 5.0 (0.0-17.6) | .99 |
| Total heart failure–associated admissions/visits, rate per patient-year (95% CI)d | 0.22 (0.08-0.58) | 0.63 (0.33-1.21) | .06 |
| Days alive and without hospitalization | 353 (339-363) | 340.5 (330-353) | .16 |
| Days alive without heart failure–associated hospitalization | 359 (351-365) | 351 (331-365) | .17 |
| Hospitalizations for a heart failure–associated event per patient, No. (%) | .09 | ||
| 0 | 18 (85.7) | 14 (63.6) | .13 |
| 1 | 1 (4.8) | 4 (18.2) | |
| 2 | 0 (0.0) | 1 (4.5) | |
| ≥3 | 2 (9.5) | 3 (13.6) | |
| Change from baseline values at 12 mo | |||
| Surviving participants, No. (%) | 20 (95) | 19 (86) | |
| New York Heart Asssociation class | −1 (−1 to 0) | 0 (−1 to 0) | .08 |
| 6-min Walk time distance | 16 (−57 to 30) | 13.6 (−10 to 72) | .31 |
| Kansas City Cardiomyopathy Questionnaire | |||
| Overall summary score | 10.5 (0.7 to 18.8) | 8.1 (−5.7 to 20.6) | .57e/.78f |
| Clinical summary score | 10.4 (−6.5 to 26.0) | 3.1 (−4.2 to 18.8) | .83e/.89f |
| EuroQol 5-dimension scale score | 0.0 (−0.2 to 0.1) | 0.0 (−0.1 to 0.2) | .81e/.25f |
Includes patients with at least 320 days of follow-up or a cardiovascular death within 365 days postprocedure; P values were calculated using the Fisher exact test.
The 95% CI for available data are based on the 2-sided exact CI of the percentage based on the binomial distribution for each treatment group.
Patients not experiencing cardiovascular death were censored at 12 months or last known follow-up examination; P values were calculated using the log-rank test.
Calculated as the total number of heart failure–associated admissions or visits requiring intravenous diuretics (including all admissions or visits to intensive care units, non–intensive care units, emergency departments, acute care facilities, and outpatient physician or nurse visits for heart failure in which the patient received diuretics) for all participants, divided by the total follow-up in years for all participants; Poisson regression was used to compare rates per patient-year.
The P value comparing treatment arms for changes at month 12 was computed using analysis of covariance adjusted for baseline value.
A mixed-effect repeated-measures model with baseline quality of life as a covariate was used to compare treatments simultaneously across all points through month 12; the underlying within-patient covariance matrix was assumed to be unstructured.
At 1 year, shunt patency was documented in all participants who received the IASD and were still alive. (eFigure 2 in Supplement 2 displays a transthoracic echocardiogram color Doppler example of left-to-right interatrial shunt flow in the subcostal view at 1 year.) Despite shunt patency and evidence of left-to-right interatrial shunt flow associated with IASD use, there was no evidence of greater increases in number of diuretic medications or total daily loop diuretic dose through 1 year in the IASD arm compared with the control group. There were also no significant changes in left heart structure or function at 6 and at 12 months (eFigure 3 in Supplement 2), and there was a nonsignificant difference in reduction in LA volume index in the IASD arm vs the control arm at 12 months (IASD group: mean [SD] reduction, −6.3 [10.7] mL/m2; control group: 1.5 [14.2] mL/m2; P = .08).
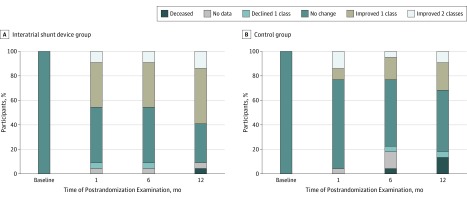
Figure 3 displays the change in NYHA class over time. There was a nonsignificant difference in improvement in NYHA class from baseline to 12 months in those assigned to the IASD group (median [IQR], −1 [−1 to 0]) compared with those in the control group (median [IQR], 0 [−1 to 0]; P = .08). There were no significant changes in other quality-of-life indicators (KCCQ or EQ-5D; Table 2). The statistical analysis of quality-of-life indicators using an mixed-effect model repeated-measures analysis instead of an analysis of covariance model showed similar findings.
Figure 3. Change in New York Heart Association Functional Class, Stratified by Study Arm.
Table 2 also shows that there were no significant differences in 6MWT distance between groups at 12 months. Although there were no statistically significant differences between the 2 groups in terms of clinical characteristics, the 6MWT distance was longer and the frequency of prior year HF hospitalizations was lower in the IASD-treated patients. Thus, we evaluated the association of these differences with MACCRE, HF hospitalization, 6MWT distance, and NYHA class. We found that adjustment for these baseline differences between groups had no association of treatment assignment with these outcomes (eTable 3 in Supplement 2).
Safety End Points
Implantation of the IASD generated the following observations. At 12 months, the rates of all-cause death were comparable between the 2 study arms (IASD group: 1 of 21 [4.8%]; control group: 3 of 22 [13.6%]; P = .61); rates were also comparable for cardiovascular death (IASD group: 1 of 21 [4.8%]; control group: 1 of 22 [4.6%]; P > .99; eTable 2 in Supplement 2). At 12 months, the rate of major adverse cardiovascular events was also comparable between the 2 study arms (eTable 2 in Supplement 2). None of the IASD-treated participants experienced device embolization, device occlusion, or device migration, and none required a second procedure for removal or occlusion of the device (eTable 2 in Supplement 2). Through 12 months of follow-up, there were also no strokes or transient ischemic attacks reported in the IASD arm. In addition, none of the IASD-treated participants developed persistent or permanent atrial fibrillation or atrial flutter through 12 months (eTable 2 in Supplement 2).
As shown in eFigure 3 in Supplement 2, at 6 months after randomization, there was a small increase in RV size in the IASD group (median [IQR], 9.1 [5.8-11.0] mL/m2) compared with the control group (median [IQR], −1.9 [−4.4 to 3.8] mL/m2; P = .002), which is consistent with left-to-right shunting through the device. There was no further increase in RV size in the IASD group at 12 months. In addition, the increase in RV size was not associated with a decrease in RV systolic function as assessed by RV ejection fraction or tricuspid annular plane systolic excursion at either 6 or 12 months. In patients in whom pulmonary artery systolic pressure was measurable by echocardiography, there was no change from baseline to 1 year (IASD group: median [IQR] change, −0.4 [−7.9 to 10.5] mm Hg; control group: −0.2 [−3.5 to 2.3] mm Hg; P = .82).
Blinding Status
At the 12-month visit, 18 patients in each group completed the blinding status questionnaire (1 withdrew consent, and 4 had died). Fifteen participants (83%) assigned to IASD and all 18 participants (100%) assigned to the control group were still blinded. Blinded study staff completed a total of 40 12-month blinding status questionnaires for the IASD-treated participants, and 57 questionnaires for the control participants. One blinded study coordinator was unblinded to IASD treatment in 1 of the participants, and 1 blinded research nurse was unblinded to the assignment of 2 control patients.
Discussion
The primary objective of the REDUCE LAP-HF I sham-controlled RCT was to evaluate the periprocedural safety and mechanistic effect of implanting the IASD System II in patients with HF who had an LVEF equal to or greater than 40% and elevated left-sided filling pressures, who remain symptomatic despite optimal guideline-directed medical therapy. As previously reported, at 1 month, exercise PCWP was significantly reduced in the IASD treatment group compared to the sham control.10 Here we documented the association of IASD use with clinical efficacy and safety end points at 1 year. The main findings of the 12-month analysis are that the IASD remained patent with left-to-right shunt flow in all patients and was safe compared with a sham control procedure. We also found that the IASD-treated patients had similar HF hospitalization rates, NYHA class, quality of life measures (via the KCCQ and EQ-5D), and exercise capacity as measured by the 6MWT distance.
Given the small study size (n = 44) and a primary objective to evaluate mechanistic efficacy (lowering of PCWP with exercise) and periprocedural safety through 1 month postrandomization, it is not surprising that the REDUCE LAP-HF I trial was underpowered to detect significant differences in 12-month end points. In addition, right heart catheterization and hemodynamic assessment were not repeated after 1 year.
However, it is reassuring that IASD placement appears safe up to 12 months. In particular, there were no strokes or transient ischemic attacks in the IASD-treated patients. In addition, there were no complications associated with the IASD (eg, device failure or migration) that would require removal of the device. Importantly, there was not a single adverse outcome that was more common in the IASD arm compared with the control arm. While the changes in NYHA class and rates of HF events requiring intravenous diuresis in the IASD-treated patients compared with the control participants are promising, clinical end points will need to be further evaluated in a larger, more adequately powered trial.
Some changes in cardiac structure and function are to be expected after implantation of an IASD. With shunting of blood from the overloaded LA to the lower-pressure RA, the RV must accommodate an increase in preload. Thus, as expected, there was a significant increase in RV size at 6 months, although this change appeared to plateau, with no significant further increase between 6 and 12 months. In addition, despite the rise in RV size in response to the IASD, there was no change in RV systolic function through the first year. The LA decreased in size in the participants in the IASD arm compared with those in the control arm, although this difference did not achieve statistical significance. A significant reduction in LA volume in response to the IASD, if corroborated in a larger trial, could be a promising finding, given that LA plays a central role in the pathogenesis of HFpEF20,21,22 and that reduction in LA volume over time may be associated with improved outcomes in this patient population.
The 12-month results of the REDUCE LAP-HF I trial shown here are consistent with our prior observations in an open-label, single arm study,13,15 which included 64 patients with LVEF equal to or greater than 40%, NYHA class II to IV symptoms, and elevated PCWP (≥15 mm Hg at rest or ≥25 mm Hg during supine bicycle exercise). The open-label study found that, 1 year after IASD implantation, there were sustained improvements in NYHA class, a small, stable reduction in LV end-diastolic volume index per echocardiography, and a small stable increase in RV end-diastolic volume index, all of which were highly statistically significant. Invasive hemodynamic studies performed in a subset of patients enrolled in the open-label trial demonstrated a sustained reduction in the workload-corrected exercise PCWP at 1 year after IASD implantation (P < .01). Survival at 1 year in this trial was 95%, and there was no evidence of device-associated complications. The results of the REDUCE LAP-HF I trial add to the prior open-label study findings, providing a gold-standard, sham-controlled RCT evidence of safety and device patency 1 year after IASD implantation. In addition, the improvement in NYHA class and the echocardiographic changes in response to the IASD were similar in the 2 studies, underscoring the consistency of these findings.
The finding that all IASD-treated patients had patent shunts with no obstruction to flow at 12 months is reassuring and an important difference between the Corvia IASD (which is valveless) and other interatrial shunt devices (which include a valve). Open-label studies of the V-wave device (V-Wave Medical), which is an hourglass-shaped interatrial shunt device that includes a valve, showed that by 12 months after implantation shunt occlusion occurred in 5 of 36 patients (14%), and 13 of 36 patients (36%) had shunt stenosis.23 Thus, at 1 year post-implantation, there was 50% device malfunction or failure in the V-wave device vs 0% in the open-label study and RCT of the Corvia IASD. The patency rate of the IASD demonstrates its durability.
Strengths
The major strength of the current study is the sham-controlled, blinded RCT design. In addition, patients were studied from multiple perspectives: physiologic (invasive hemodynamics and echocardiography), quality of life and functional limitation (KCCQ, EQ-5D, NYHA, and 6MWT), and clinical outcomes (MACCRE, HF hospitalization, and safety).
Limitations
The study is limited by its relatively small sample size and therefore it does not provide adequate power to definitively evaluate clinical benefit or safety. Although there were no statistically significant differences in clinical characteristics among groups, the control group had lower 6MWT distance and a higher frequency of HF hospitalization in the year before the study began. These imbalances did not affect the main 1-year results; however, these differences between treatment groups are also indicative of the limitation of a small sample size. An ongoing large-scale pivotal trial of the IASD in patients with HF who have EF equal to or greater than 40% is currently underway.
Conclusions
In patients with HF and EF equal to or greater than 40%, IASD implantation lowered exercise PCWP at 1 month and was found to be safe at 1 year compared with a sham procedure. In addition, device patency was confirmed at 12 months in all participants randomized to the IASD. Although, as anticipated, RV volumes increased slightly in the IASD-treated patients, no adverse safety signal was observed. These results are encouraging, but they require a larger study for further clinical evaluation. A larger-scale, blinded, sham-controlled, pivotal RCT is currently underway to determine the clinical efficacy of the IASD in HF and EF equal to or greater than 40%.
Trial Protocol.
eAppendix. Inclusion/Exclusion Criteria
eTable 1. Recruiting Sites, Investigators, and Clinical Research Coordinators
eTable 2. Combined In- and Out-of-Hospital Events Through 12 Months
eTable 3. One-year Outcomes Adjusted for Baseline 6-Minute Walk Test Distance and Prior Year Heart Failure Hospitalization
eFigure 1. Study Design
eFigure 2. Example of Left-to-Right Shunting Through a Patent IASD at 12 Months in a REDUCE LAP-HF I Study Participant
eFigure 3. Bar Graphs Displaying Baseline, 6-Month, and 12-Month Echocardiographic Parameters of Cardiac Structure and Function, Stratified by Treatment Group.
Data Sharing Statement.
References
- 1.Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375(19):1868-1877. doi: 10.1056/NEJMcp1511175 [DOI] [PubMed] [Google Scholar]
- 2.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73-90. doi: 10.1161/CIRCULATIONAHA.116.021884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam CS, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40-50%). Eur J Heart Fail. 2014;16(10):1049-1055. doi: 10.1002/ejhf.159 [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed). 2016;69(12):1167. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11(9):507-515. doi: 10.1038/nrcardio.2014.83 [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670-679. doi: 10.1093/eurheartj/ehq426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zile MR, Bennett TD, El Hajj S, et al. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail. 2017;10(1):e003594. doi: 10.1161/CIRCHEARTFAILURE.116.003594 [DOI] [PubMed] [Google Scholar]
- 9.Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35(44):3103-3112. doi: 10.1093/eurheartj/ehu315 [DOI] [PubMed] [Google Scholar]
- 10.Feldman T, Mauri L, Kahwash R, et al. ; REDUCE LAP-HF I Investigators and Study Coordinators . Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [reduce elevated left atrial pressure in patients with heart failure]): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137(4):364-375. doi: 10.1161/CIRCULATIONAHA.117.032094 [DOI] [PubMed] [Google Scholar]
- 11.Feldman T, Komtebedde J, Burkhoff D, et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE elevated left atrial pressure in heart failure (REDUCE LAP-HF I). Circ Heart Fail. 2016;9(7):e003025. doi: 10.1161/CIRCHEARTFAILURE.116.003025 [DOI] [PubMed] [Google Scholar]
- 12.Hasenfuss G, Gustafsson F, Kaye D, et al. ; Reduce LAP-HF Trial Investigators . Rationale and design of the reduce elevated left atrial pressure in patients with heart failure (Reduce LAP-HF) Trial. J Card Fail. 2015;21(7):594-600. doi: 10.1016/j.cardfail.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuß G, Hayward C, Burkhoff D, et al. ; REDUCE LAP-HF study investigators . A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387(10025):1298-1304. doi: 10.1016/S0140-6736(16)00704-2 [DOI] [PubMed] [Google Scholar]
- 14.Kaye D, Shah SJ, Borlaug BA, et al. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20(3):212-221. doi: 10.1016/j.cardfail.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 15.Kaye DM, Hasenfuß G, Neuzil P, et al. One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9(12):e003662. doi: 10.1161/CIRCHEARTFAILURE.116.003662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph SM, Novak E, Arnold SV, et al. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6(6):1139-1146. doi: 10.1161/CIRCHEARTFAILURE.113.000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui O, Hung HM, O’Neill R. MMRM vs. LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. J Biopharm Stat. 2009;19(2):227-246. doi: 10.1080/10543400802609797 [DOI] [PubMed] [Google Scholar]
- 19.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6(2):184-192. doi: 10.1161/CIRCHEARTFAILURE.112.972794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed BH, Daruwalla V, Cheng JY, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9(3):e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freed BH, Shah SJ. Stepping out of the left ventricle’s shadow: time to focus on the left atrium in heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2017;10(4):e006267. doi: 10.1161/CIRCIMAGING.117.006267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail. 2014;7(6):1042-1049. doi: 10.1161/CIRCHEARTFAILURE.114.001276 [DOI] [PubMed] [Google Scholar]
- 23.Rodés-Cabau J. Interatrial shunting for treating heart failure: early and late results of the first-in-human experience with the V-wave interatrial shunt system. http://www.crtonline.org/Assets/a840443d-4ef1-4917-a928-bf4c35fb6722/636564421327500000/v-wave-acc-21-02-2018-jrc-v2-pdf. Published March 2018. Accessed August 8, 2018. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eAppendix. Inclusion/Exclusion Criteria
eTable 1. Recruiting Sites, Investigators, and Clinical Research Coordinators
eTable 2. Combined In- and Out-of-Hospital Events Through 12 Months
eTable 3. One-year Outcomes Adjusted for Baseline 6-Minute Walk Test Distance and Prior Year Heart Failure Hospitalization
eFigure 1. Study Design
eFigure 2. Example of Left-to-Right Shunting Through a Patent IASD at 12 Months in a REDUCE LAP-HF I Study Participant
eFigure 3. Bar Graphs Displaying Baseline, 6-Month, and 12-Month Echocardiographic Parameters of Cardiac Structure and Function, Stratified by Treatment Group.
Data Sharing Statement.