Key Points
Question
How do age, race/ethnicity, obesity, renal function, and atrial fibrillation influence natriuretic peptides (NP) in heart failure with preserved ejection fraction and does the prognostic significance of NPs vary in these clinically important subgroups?
Findings
In this secondary analysis of the Americas region of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT), 1057 participants had available NP concentrations that varied markedly across key subgroups and were consistently associated with excess cardiovascular risk.
Meaning
Natriuretic peptides represent important biomarkers of prognosis, even in populations with lower distributions of levels; single, absolute NP thresholds for inclusion in contemporary heart failure with preserved ejection fraction trials may lead to an underrepresentation of certain demographic and clinical subgroups.
Abstract
Importance
Contemporary clinical trials of heart failure with preserved ejection fraction (HFpEF) apply natriuretic peptide (NP) thresholds to identify patients who are more likely to have the disease of interest and to enrich the baseline risk of the enrolled cohort.
Objective
To determine whether age, race/ethnicity, obesity, renal function, and atrial fibrillation (AF) affect the levels of NPs in HFpEF and whether the prognostic significance of NPs varies in these clinically important subgroups.
Design, Setting, and Participants
This secondary analysis of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT) evaluated the distribution and prognostic significance of NPs across 6 subgroups comprising 1057 adult patients (60%) in the Americas region of TOPCAT with symptomatic heart failure (HF) and a left ventricular ejection fraction of 45% or more with available NPs at baseline.
Exposures
Natriuretic peptides were log-transformed and standardized (expressed per 1 SD, z score) and assessed in 6 subgroups: age (cutoff, 70 years), black race, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared; cutoff, 30 kg/m2), waist circumference (cutoff, 102 cm for men, 88 cm for women), estimated glomerular filtration rate (cutoff, 60 mL/min/1.73 m2), and a history of AF.
Main Outcomes and Measures
Time to composite cardiovascular death, hospitalization for HF, or aborted cardiac arrest at mean (SD) 2.4-year (1.5) follow-up.
Results
Of 1057 participants, the mean (SD) age was 72 (10) years, 183 (17.3%) were black, the mean (SD) BMI was 33.4 (8.6) kg/m2, the mean (SD) estimated glomerular filtration rate was 64.6 (21.8) mL/min/1.73 m2, and 472 (45%) had a history of AF. Median B-type NP (n = 698) and N-terminal pro-B-type NP concentrations (n = 359) were 257 (interquartile range, 149-443) ng/L and 959 (interquartile range, 554-2015) ng/L, respectively. Natriuretic peptide concentrations varied by up to 0.5 SD within the 6 subgroups, being higher in older patients with nonblack race, a lower BMI, a lower waist circumference, a lower estimated glomerular filtration rate, and a history of AF. Elevated NP levels (per 1-SD increase) were independently associated with an increased risk of the primary outcome (adjusted hazard ratio, 1.36; 95% CI, 1.22-1.54; P < .001) consistently across all investigated subgroups (interaction P > .05). In TOPCAT Americas (n = 1767), 791 (45%) were enrolled based on elevated NP levels as the qualifying criterion (as opposed to a history of HF hospitalization). This proportion was 31% (93 of 302), 34% (258 of 760), and 39% (443 of 1144) for black race, younger than 70 years, and a BMI of 30 kg/m2 or greater, respectively.
Conclusions and Relevance
Natriuretic peptides remain important biomarkers of prognosis in HFpEF, even in subgroups who tend to have lower NP levels. A single, absolute NP threshold for inclusion in contemporary HFpEF trials may lead to an underrepresentation of certain demographic and clinical subgroups.
Trial Registration
ClinicalTrials.gov Identifier: NCT00094302
This secondary analysis of the TOPCAT randomized clinical trial examines the association of age, race/ethnicity, obesity, renal function, and atrial fibrillation with levels of natriuretic peptides in heart failure with preserved ejection fraction in people in the Americas.
Introduction
Concentrations of circulating natriuretic peptides (NPs) are incorporated in diagnostic criteria for heart failure with preserved ejection fraction (HFpEF)1 and serve as important markers of cardiovascular prognosis.2 Contemporary clinical trials of HFpEF apply NP thresholds to identify patients who are more likely to have the disease of interest and to enrich baseline risk.3 However, NP levels are influenced by various clinical factors, including age, race/ethnicity, obesity, renal function, and the presence of atrial fibrillation (AF).4,5 It is uncertain whether the risk associated with a given NP level varies in these clinically important subgroups and whether heterogeneity in NP distributions alters the relative representation of these patients in contemporary HFpEF trials. As such, we examined the distribution and associated cardiovascular prognosis of baseline concentrations of NPs in subgroups of HFpEF that were defined by age, race/ethnicity, obesity, renal function, and AF status that were enrolled in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT).
Methods
TOPCAT was a global, phase-3, double-blind, placebo-controlled randomized clinical trial of spironolactone in HFpEF.6 Given significant regional heterogeneity,7 this analysis was restricted to the 1057 patients who were enrolled in the Americas region (United States, Canada, Brazil, and Argentina) with available NPs. Patients who were 50 years or older with symptomatic heart failure (HF) and a left ventricular ejection fraction of 45% or greater, well-controlled blood pressure, and a serum potassium of less than 5.0 mEq/L (to convert potassium to millimoles per liter, multiply by 1) were considered for enrollment. In addition, either HF hospitalization within 12 months or elevated NP concentration (B-type natriuretic peptide [BNP] ≥100 ng/L or N-terminal pro-BNP [NT-proBNP] ≥360 ng/L) within 60 days was required. The primary outcome for TOPCAT and for this analysis was time to composite cardiovascular death, hospitalization for HF, or aborted cardiac arrest.
Analyses were performed in all patients with available NPs, which were locally collected and processed as previously described.8 Before the analysis, we selected 6 key subgroups and relevant cutoffs a priori based on available clinical consensus documents9,10 and prior data in HFpEF2: age (cutoff, 70 years), black race, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) (cutoff, 30 kg/m2), waist circumference (cutoff, 102 cm for men; 88 cm for women), estimated glomerular filtration rate (eGFR) (cutoff, 60 mL/min/1.73 m2), and a history of AF. Consistent with a prior report,2 BNP and NT-proBNP levels were log-transformed and standardized (expressed per 1 SD; z score). Multivariable Cox proportional hazards models that accounted for age, sex, race/ethnicity, BMI, eGFR, history of AF, enrollment strata, and treatment randomization were used to assess the association between NP levels and the primary outcome. Interactions by key subgroups on the association between NPs and risk were assessed by linear regression. The incremental value of NPs in predicting the primary outcome was evaluated using the change in Harrell C statistics. Restricted cubic splines models with 3 knots were used to plot the flexible association between log-transformed, standardized NP as a continuous variable and the incidence of the primary end point for each of the key subgroups. All patients provided written informed consent, and the study was approved by institutional review boards or ethics committees at each participating institution. Statistical analyses were performed using Stata, version 14.1 (Stata Corp).
Results
There was minor variation across the key characteristics in those with (1057 [60%]) and without available NPs (710 [40%]) in TOPCAT Americas (eTable in the Supplement). As expected, patients with available NPs levels were more frequently enrolled in the NP strata (687 [65%]) than the hospitalization for HF strata (370 [35%]). In patients with available NP levels, the mean (SD) age was 72 (10) years, 514 (49%) were men, and 183 (17%) were black. The mean (SD) BMI was 33.4 (8.6) kg/m2, mean (SD) eGFR was 64.6 (21.8) mL/min/1.73 m2, and 472 (45%) had a history of AF (of whom 289 [61%] had AF on an inclusion electrocardiogram).
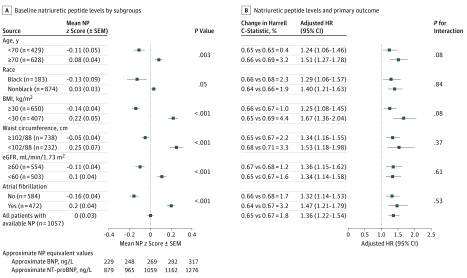
Overall, median BNP (n = 698) and NT-proBNP concentrations (n = 359) were 257 (interquartile range, 149-443) ng/L and 959 (interquartile range, 554-2015) ng/L, respectively (Table), and there were no differences in NP levels between the enrollment strata (eFigure 1 in the Supplement). The NP concentrations varied by up to 0.5 SDs within the 6 subgroups and were significantly higher in older patients with nonblack race, a lower BMI, a lower waist circumference, lower eGFR, and a history of AF (Figure 1). A similar variation was observed between patients with and without AF on a presenting electrocardiogram (z score [SD] 0.28 [0.90] vs −0.06 [1.03], respectively). In sensitivity analyses, similar qualitative differences across these subgroups were observed when analyzing concentrations of NPs in patients who were enrolled in the hospitalization stratum (n = 370; eFigure 2 in the Supplement) and in Russia and Georgia with available NPs (n = 366; eFigure 3 in the Supplement).
Table. Concentrations of BNP and NT-proBNP and Incidence Rate of the Primary Outcome in 6 Subgroups of the Americas Region of the TOPCAT Trial.
| Characteristic | Median (IQR) | Incidence Rate of the Primary Outcome per 100 Patient-Years (95% CI) | |
|---|---|---|---|
| BNP, ng/L | NT-proBNP, ng/L | ||
| Total population (N = 1057) | 257 (149-443) | 959 (554-2015) | 11.6 (10.3-13.0) |
| Age, y | |||
| <70 (n = 429) | 235 (136-427) | 937 (487-1813) | 12.7 (10.7-15.2) |
| ≥70 (n = 628) | 276 (165-461) | 962 (631-2027) | 10.9 (9.4-12.6) |
| Race | |||
| Black (n = 183) | 208 (128-454) | 1274 (599-2582) | 16.1 (12.5-20.6) |
| Nonblack (n = 874) | 268 (157-443) | 918 (554-1937) | 10.8 (9.5-12.3) |
| BMI, kg/m2 | |||
| ≥30 (n = 650) | 240 (143-402) | 885 (500-1611) | 12.5 (10.9-14.4) |
| <30 (n = 407) | 285 (170-518) | 1140 (665-2308) | 10.2 (8.4-12.3) |
| Waist circumference, cm | |||
| ≥102/88 (n = 738) | 252 (152-439) | 893 (502-1641) | 11.4 (9.9-13.0) |
| <102/88 (n = 232) | 282 (149-504) | 1229 (700-2720) | 10.8 (8.4-13.9) |
| eGFR, mL/min/1.73 m2 | |||
| ≥60 (n = 554) | 239 (146-414) | 882 (496-1720) | 8.9 (7.5-10.6) |
| <60 (n = 503) | 273 (162-484) | 1077 (672-2276) | 14.9 (12.8-17.3) |
| Atrial fibrillation | |||
| No (n = 584) | 234 (138-429) | 761 (475-1592) | 11.9 (10.2-13.8) |
| Yes (n = 472) | 293 (175-478) | 1275 (731-2276) | 11.3 (9.5-13.4) |
Abbreviations: AF, atrial fibrillation; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; IQR, interquartile range; NP, natriuretic peptide; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Figure 1. Baseline Natriuretic Peptides and Subsequent Cardiovascular Risk in 6 Subgroups of the Americas Region of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial.
Overall, 1057 patients had natriuretic peptides (NPs) available for analysis. Multivariate Cox regression models and risk reclassification models accounted for the following covariates: age, sex, race/ethnicity, body mass index (calculated as weight in kilograms divided by height in meters squared), estimated glomerular filtration rate (eGFR), history of atrial fibrillation (AF), enrollment strata, and treatment randomization. BNP indicates B-type natriuretic peptide; HR, hazard ratio; SEM, standard error of the mean.
Over a mean (SD) 2.4-year (1.5) follow-up, 300 primary outcome events occurred (incidence rate, 11.6; 95% CI, 10.3-13.0 per 100 patient-years) (Table). Elevated NP levels (per 1-SD increase in log-transformed, standardized NP) were independently associated with an increased risk of the primary outcome (adjusted hazard ratio, 1.36; 95% CI, 1.22-1.54; P < .001). The excess risk that was associated with NP levels was consistent in all the investigated subgroups (no significant interactions across subgroups). The incremental value of NP levels in predicting the primary outcome beyond the covariate set was modest (a 1.8% increase in C-statistic) and was comparable across investigated subgroups (Figure 1).
The incidence of the primary outcome at a given level of NP was higher in subgroups with left-shifted distributions of baseline NP concentrations (young, black, obese, higher eGFR, and no AF) compared with their respective counterparts (Figure 2). Overall, in TOPCAT Americas (n = 1767), 791 (45%) were enrolled based on elevated NP levels as the qualifying criterion (as opposed to a history of hospitalization for HF). This proportion was 31% (93 or 302), 34% (258 of 760), and 39% (443 of 1144) for black race, age younger than 70 years, and a BMI of 30 kg/m2 or greater, respectively.
Figure 2. Association Between Natriuretic Peptide (NP) Levels and Incidence of Primary Outcome in 6 Subgroups of the Americas Region of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial.
Restricted cubic splines models with 3 knots were constructed to display the association between log-transformed, standardized NP concentrations as a continuous variable and the incidence of the primary end point for each of the key subgroups. The dotted lines reflect the 95% confidence intervals. BNP indicates B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide. Body mass index is calculated as weight in kilograms divided by height in meters squared.
Discussion
The factors associated with variations in NP levels in the general population and in HF with reduced ejection fraction also appeared to modify NP levels in HFpEF.4,5,11 Key clinical subgroups (young, black, obese, better renal function, and no AF) have, on average, lower NP levels in HFpEF. Nevertheless, elevated NP concentrations are consistently associated with adverse cardiovascular outcomes, even in these populations with lower distributions of levels.
Applying a single NP threshold for trial entry may contribute to unbalanced selection and may result in an underrepresentation of certain subgroups. Natriuretic peptide thresholds are often used in trials as a risk enrichment strategy, but this approach may exclude patients in these subsets who truly have HFpEF and who experience high rates of cardiovascular events. For instance, most black patients (∼70%) in TOPCAT Americas were eligible for enrollment based on prior hospitalization for HF as opposed to elevated NP levels (∼30%), and enrolled black patients faced higher rates of the primary outcome compared with white patients across a broad range of NP levels. In addition, patients with lower NPs may potentially stand to benefit most from investigational therapies,2,12 perhaps due to improved responsiveness earlier in the natural history of HFpEF, which adds to the importance of identifying appropriate NP-based pathways to enroll these subgroups. Ongoing global advanced-phase pharmacological trials of HFpEF (NCT01920711, NCT03057951, and NCT02901184) are applying differential NP thresholds based on AF status, while select device trials (NCT03499236) are adjusting screening NP levels for BMI in determining trial eligibility. As high-quality data from global registries of HFpEF accrue, greater information regarding the typical distributions of NP levels across various clinical subgroups should be ascertained, and these identified factors should be considered in modifying NP-based trial entry criteria. Beyond this, markers are needed that are less sensitive to systematic variation across heterogeneous subsets for use in screening for therapeutic trials.
The mechanisms that drive NP distributions in HFpEF are varied. Natriuretic peptides are known to increase with age and with atrial arrhythmias, which are thought to be mediated by increased atrial and ventricular wall stress. Genetic polymorphisms that regulate NP levels may partially explain lower NP distributions in black patients.13,14 Obesity has been mechanistically linked with lower NP levels that are associated with increased adipocyte-mediated clearance, hyperinsulinemia, sex hormonal activity, and local epicardial adipose tissue-related effects.11,15 Because only a proportion of NPs are renally cleared, elevations in NP levels in renal impairment are likely multifactorial and are incompletely understood.
Limitations
The limitations in this exploratory analysis include restricting the study sample to patients with NP data available at baseline in a randomized clinical trial that used an NP threshold as one of the trial eligibility criteria. We evaluated NPs on a standardized scale given variation in circulating levels of BNP and NT-proBNP; however, the validity and prognostic utility of NPs appear independent of assay. Given the selected nature of this trial population, clear NP thresholds of risk for individual subgroups were not able to be established.
Conclusions
The burden of HFpEF is high in subgroups who tend to have lower NP levels, including black patients and patients with obesity. Natriuretic peptides remain important biomarkers of prognosis in HFpEF, even in these subgroups with lower distributions of levels. However, as the global population of HFpEF grows and becomes more heterogeneous, single, absolute NP thresholds for inclusion in contemporary HFpEF trials may bias against enrolling certain demographic and clinical subgroups.
eTable. Baseline characteristics of patients in TOPCAT Americas (n=1,767) in patients with and without available natriuretic peptide levels.
eFigure 1. Distribution of log-transformed, standardized natriuretic peptide (NP) levels by enrollment strata in TOPCAT Americas.
eFigure 2. Baseline natriuretic peptides in 6 subgroups of the hospitalization strata of TOPCAT Americas (n=370).
eFigure 3. Baseline natriuretic peptides in 5 subgroups of TOPCAT Russia and Georgia (n=366).
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 2.Anand IS, Claggett B, Liu J, et al. . Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail. 2017;5(4):241-252. doi: 10.1016/j.jchf.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 3.Solomon SD, Rizkala AR, Gong J, et al. . Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC Heart Fail. 2017;5(7):471-482. doi: 10.1016/j.jchf.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 4.Chow SL, Maisel AS, Anand I, et al. ; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research . Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135(22):e1054-e1091. doi: 10.1161/CIR.0000000000000490 [DOI] [PubMed] [Google Scholar]
- 5.Gupta DK, Claggett B, Wells Q, et al. . Racial differences in circulating natriuretic peptide levels: the atherosclerosis risk in communities study. J Am Heart Assoc. 2015;4(5):e001831. doi: 10.1161/JAHA.115.001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitt B, Pfeffer MA, Assmann SF, et al. ; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Claggett B, Assmann SF, et al. . Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34-42. doi: 10.1161/CIRCULATIONAHA.114.013255 [DOI] [PubMed] [Google Scholar]
- 8.Desai AS, Lewis EF, Li R, et al. . Rationale and design of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162(6):966-972.e910. [DOI] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 10.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825-830. doi: 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Larson MG, Levy D, et al. . Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109(5):594-600. doi: 10.1161/01.CIR.0000112582.16683.EA [DOI] [PubMed] [Google Scholar]
- 12.Anand IS, Rector TS, Cleland JG, et al. . Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4(5):569-577. doi: 10.1161/CIRCHEARTFAILURE.111.962654 [DOI] [PubMed] [Google Scholar]
- 13.Seidelmann SB, Vardeny O, Claggett B, et al. . An NPPB promoter polymorphism associated with elevated n-terminal pro-B-type natriuretic peptide and lower blood pressure, hypertension, and mortality. J Am Heart Assoc. 2017;6(4):e005257. doi: 10.1161/JAHA.116.005257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Levy D, et al. . Heritability and genetic linkage of plasma natriuretic peptide levels. Circulation. 2003;108(1):13-16. doi: 10.1161/01.CIR.0000081657.83724.A7 [DOI] [PubMed] [Google Scholar]
- 15.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71(20):2360-2372. doi: 10.1016/j.jacc.2018.03.509 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Baseline characteristics of patients in TOPCAT Americas (n=1,767) in patients with and without available natriuretic peptide levels.
eFigure 1. Distribution of log-transformed, standardized natriuretic peptide (NP) levels by enrollment strata in TOPCAT Americas.
eFigure 2. Baseline natriuretic peptides in 6 subgroups of the hospitalization strata of TOPCAT Americas (n=370).
eFigure 3. Baseline natriuretic peptides in 5 subgroups of TOPCAT Russia and Georgia (n=366).