Key Points
Questions
Can the Dizziness Catastrophizing Scale reliably and validly assess catastrophic thinking in patients with dizziness, and is dizziness catastrophizing associated with dizziness-related disability over and above related constructs, such as negative affectivity (eg, symptoms of anxiety and depression)?
Findings
In this medical record review of 457 adults with a balance disorder, the Dizziness Catastrophizing Scale demonstrated strong psychometric properties and was independently associated with dizziness-related disability.
Meaning
Dizziness catastrophizing requires greater attention and treatment in clinical settings because of its association with dizziness-related disability.
This medical record review validates a measure of dizziness catastrophizing and assesses its association with dizziness-related disability compared with other negative affect constructs (eg, anxiety and depression) in adults with a balance disorder.
Abstract
Importance
Catastrophizing is a maladaptive thought process that involves irrational fear and worry about anticipated or actual symptoms. Although clinically relevant, the role of catastrophizing in patients with chronic dizziness or imbalance has not yet been explored to our knowledge.
Objectives
To validate a measure of dizziness catastrophizing and to assess its association with dizziness-related disability compared with other negative affect constructs (eg, anxiety and depression).
Design, Setting, and Participants
For this retrospective medical record review, the Dizziness Catastrophizing Scale (DCS), a dizziness-specific catastrophizing assessment tool, was adapted from the previously validated Pain Catastrophizing Scale. Psychometric evaluation of the DCS was performed. In addition, the associations of dizziness catastrophizing and positive and negative affectivity with dizziness-related disability were assessed using structural equation modeling and regression analyses. Data were collected using a retrospective medical record review from April 27, 2010, to June 25, 2014. The dates of analysis were June 3 to August 15, 2017. The setting was the Multidisciplinary Neurotology Clinic at the Toronto General Hospital (Toronto, Ontario, Canada). Participants were 457 adult outpatients with dizziness or imbalance who were referred to the clinic.
Main Outcomes and Measures
Psychometric properties of the DCS and its association with dizziness-related disability, as measured with the Dizziness Handicap Inventory.
Results
Among 457 patients (mean [SD] age, 53.4 [15.4] years; 154 [33.7%] male), the DCS demonstrated good convergent (r = 0.78, P < .001) and discriminant validity (r = −0.40, P < .001) with the negative and positive affectivity, respectively; internal consistency (α = .95); and test-retest reliability (intraclass correlation coefficient, 0.92; P < .001 at the 95% CI). An exploratory dimension reduction analysis revealed a single latent component of the DCS. The results of the structural equation modeling and regression analyses revealed that dizziness catastrophizing, although associated with negative affectivity (eg, symptoms of anxiety and depression), was independently associated with dizziness-related disability (standardized β = 0.378; P < .001). Furthermore, a strong association was found between catastrophizing and dizziness-related disability across different dizziness-related diagnoses (r ≥ 0.6; P < .001).
Conclusions and Relevance
In this study, the DCS was a valid and reliable measure for evaluating catastrophic thinking in patients with dizziness, which was independently associated with dizziness-related disability. Future studies should investigate the influence of alleviating symptoms of catastrophizing on functional outcomes in patients with dizziness or imbalance, the results of which will help guide novel approaches to the clinical care of patients with chronic dizziness.
Introduction
Dizziness or vertigo, one of the most common symptoms in medicine, affects 20% to 30% of patients in the general population and is associated with reduced quality of life.1,2,3 Patients with chronic dizziness frequently have catastrophic thinking, which observationally appears to contribute to symptom severity and negative treatment outcomes. While pain catastrophizing is a well-known phenomenon,4 the literature on dizziness catastrophizing is limited. Many patients with chronic dizziness and imbalance exhibit a mismatch between the extent of an identified organic lesion and the degree of subjective symptom severity, which may be associated with psychological factors, including catastrophic thinking.5,6
Catastrophizing is a maladaptive thought process that involves irrational fear and worry about anticipated or actual symptoms, and it is often exhibited by patients with anxiety and depressive disorders.7 Several early studies4,8,9,10,11 on catastrophizing indicate that catastrophizers often experience high levels of physical and emotional distress. More recently, empirical evidence has accrued to suggest that pain catastrophizing contributes to overall clinical burden in patients with chronic pain, including increased symptom severity, pain-related disability, comorbidity, and changes in social support.7,12,13 Clinical observations suggest that catastrophizing may similarly contribute to negative clinical outcomes in adults with chronic dizziness.
Anxiety and depression are often comorbid with chronic dizziness14,15 and contribute to patient distress.3,16 Anxiety arousal and depression may trigger or contribute to catastrophic thinking in patients with dizziness.17,18 While there are no studies specific to dizziness catastrophizing, research on pain catastrophizing provides conflicting evidence about the degree to which catastrophizing overlaps with negative affect constructs (eg, anxiety and depression).4,19 The results of some studies20,21 on pain suggest a strong overlap between catastrophizing and negative affectivity, whereas the findings of other studies4,22 suggest that, although associated with anxiety and depression, pain catastrophizing is a unique contributor to the subjective experience of pain intensity.
We are not aware of any studies that have specifically evaluated the role of catastrophizing in patients with dizziness. A validated measure of dizziness catastrophizing is needed to facilitate our understanding of its association with negative clinical outcomes in patients with dizziness. Therefore, the aims of this study were to (1) psychometrically test a novel measure of dizziness catastrophizing in a sample of outpatients with dizziness due to a wide range of clinical diagnoses and (2) assess the measure’s distinctness from negative affectivity (eg, symptoms of panic, anxiety, and depression) in relation to dizziness-related disability using structural equation modeling (SEM). Based on our clinical observations and the literature on pain catastrophizing, we hypothesized that the concept of dizziness catastrophizing would be associated with negative affectivity and would also be independently correlated with dizziness-related disability.
Methods
Ethics Approval
This study was approved by the University Health Network Research Ethics Board. Informed consent requirements were waived because the study used a retrospective medical record review design.
Scale Development and Validation
The Dizziness Catastrophizing Scale (DCS) is a 13-item self-report measure of catastrophic thinking associated with dizziness at the time of the assessment (eFigure in the Supplement). The items and scoring of the DCS were adapted from the previously validated Pain Catastrophizing Scale (PCS) by replacing the term pain with dizziness.4 The present work was done with the support of the developer of the PCS.
All items are scored using a 5-point Likert-type scale ranging from “not at all” to “all the time.” Like the PCS, the DCS requires approximately a grade 6 reading level, as measured by the Flesch-Kincaid grade level formula,23 and takes less than 5 minutes to complete.
The DCS was completed by 457 adult outpatients (mean [SD] age, 53.4 [15.4] years; 154 [33.7%] male) with dizziness or imbalance at the Multidisciplinary Neurotology Clinic at the Toronto General Hospital, University Health Network (Toronto, Ontario, Canada). The DCS scores and additional clinical information were extracted as part of a retrospective medical record review. Baseline assessments and follow-up visits of patient medical records were selected from April 27, 2010, to June 25, 2014. The dates of analysis were June 3 to August 15, 2017. Patients were categorized into 11 dizziness-related diagnoses, including benign paroxysmal positional vertigo, Ménière’s disease, and central vestibular lesions. Demographic information and scores on clinical assessments, which included the DCS, Dizziness Handicap Inventory (DHI), and Positive and Negative Affect Schedule (PANAS), were obtained from patients’ medical records. The PANAS is a valid and reliable self-report measure of positive and negative emotional states in nonclinical (eg, nonpsychiatric) adult samples.24,25 The DHI is a psychometrically validated scale of self-perceived disability due to dizziness in patients with vestibular dysfunction.26
Statistical Analysis
Statistical analyses were performed using a software program (SPSS, version 24.0; IBM Corp). Means, SDs, and frequencies were calculated for the demographic and clinical variables as appropriate. Pearson product moment correlations (r) were performed between these variables (ie, age, duration of symptoms, the DHI, and the PANAS) and the DCS scores, with correlation coefficients from 0.20 to 0.39 defined as weak, 0.40 to 0.59 as moderate, and greater than 0.60 as strong. Mann-Whitney tests were used to compare the DCS and DHI scores among men and women. Analysis of variance was performed to assess differences in the DCS and DHI scores among the 11 dizziness-related diagnostic categories. Subsequent post hoc Tukey tests were performed if group differences were found. Statistical significance was set at P ≤ .05 (2-tailed).
Scale Validity and Scale Reliability
Convergent and discriminant validity were evaluated using Pearson product moment correlations between the DCS and the PANAS positive and negative affect subscales. For scale reliability, single item scores of the DCS were available for 144 patients. Internal consistency of the DCS was assessed using Cronbach α and corrected item-total correlation. Test-retest reliability for the DCS was assessed at 2 months after the initial visit. Reliability analyses were conducted using 2-way mixed intraclass correlation coefficients for absolute agreement set at a 95% CI.27
Exploratory Factor Analysis
To evaluate the factor structure of the 13-item DCS, we initially conducted a parallel analysis to identify the number of components to extract for a subsequent exploratory factor analysis.28 The number of components to extract is assessed by comparing eigenvalues from the original data with 95th percentile or higher eigenvalues generated from 1000 random permutations of the same data set.28,29 The number of eigenvalues from the original data that exceed the randomly generated eigenvalues represents the number of components to extract.28,29 Next, to perform a confirmatory factor analysis, we randomly divided the data into 2 equal subsets. The first subset was used to conduct exploratory factor analysis, and the second subset was used to perform a confirmatory factor analysis.
The exploratory and confirmatory factor analyses were performed with a software program (Mplus, version 6.12; Muthén & Muthén) using weighted least squares mean and variance-adjusted estimation. Cutoff levels for the following indexes were used to assess model fit of the exploratory factor analysis: χ2 test of model fit significance greater than .05, root-mean-square error of approximation (RMSEA) less than 0.08, Comparative Fit Index (CFI) of 0.95 or higher, Tucker-Lewis Index (TLI) of 0.96 or higher, standardized root-mean-square residual (SRMR) of 0.08 or lower, and weighted root-mean-square residual (WRMR) of 0.90 or lower.30,31
Structural Equation Modeling
Structural equation modeling was used to explore the associations of dizziness catastrophizing and positive and negative affectivity with dizziness-related disability. It was conducted using the full information maximum likelihood method in SPSS (Amos 21.0), which considers missing data and estimates the mean and intercept.32 This analysis included 457 patients to investigate if dizziness-related disability, as measured with the DHI, is predicted by measures of dizziness catastrophizing (evaluated with the DCS) and positive and negative affect (evaluated with the PANAS). Complete data were available for 352 of the 457 participants.
Several model fit indexes were used to evaluate the extent to which the model represented the data.33 As there were zero df, our proposed models were saturated, which means that all of the variables for estimating model fit were perfect; therefore, all possible associations within the models were accounted for.
Regression Analysis
A hierarchical multiple regression analysis was performed with the DHI as the dependent variable. The predictor variables were entered into the model in the order of the DCS in block 1 and the PANAS positive and PANAS negative in block 2.
Results
Demographic and Clinical Characteristics
The clinical and demographic characteristics of the study participants whose data contributed to the validity and reliability assessments of the DCS are listed in Table 1 and Table 2. The participants generally had a moderate level of catastrophic thinking associated with their dizziness (DCS score mean [SD], 24.5 [14.6]; range, 0-52). No significant associations were found between the DCS and age (r = 0.04, P = .38), sex (r = 0.04, P = .44), or duration of symptoms (r = −0.02, P = .68), whereas a moderate positive correlation was found between dizziness-related disability and duration of symptoms (r = 0.13, P = .03) (Table 1). Women had higher dizziness-related disability than men (z = −2.97, P = .003), with the mean (SD) DHI scores being 46.0 (25.1) in men and 53.8 (24.7) in women. A positive correlation was also found between the DCS and DHI scores (r = 0.67, P < .001), which remained significant after Bonferroni correction.
Table 1. Demographic and Clinical Characteristics and Their Correlations With the DCS, DHI, PANAS Positive, and PANAS Negative Mean Total Scores Among 457 Adults With a Balance Disorder.
| Variable | Mean (SD) | r | |
|---|---|---|---|
| DCS | DHI | ||
| No. | 457 | ||
| Age, y | 53.4 (15.4) | 0.04 | 0.04 |
| % Male | 33.7 | 0.04 | 0.15a |
| Duration of symptoms, mo | 64.1 (73.6) | −0.02 | 0.13b |
| Clinical scale total scores | |||
| DCS | 24.5 (14.6) | NA | 0.67a |
| DHI | 51.1 (25.1) | 0.67a | NA |
| PANAS positive | 30.2 (8.8) | −0.40a | −0.44a |
| PANAS negative | 21.7 (9.0) | 0.78a | 0.66a |
Abbreviations: DCS, Dizziness Catastrophizing Scale; DHI, Dizziness Handicap Inventory; NA, not applicable; PANAS, Positive and Negative Affect Schedule.
P ≤ .001.
P ≤ .05.
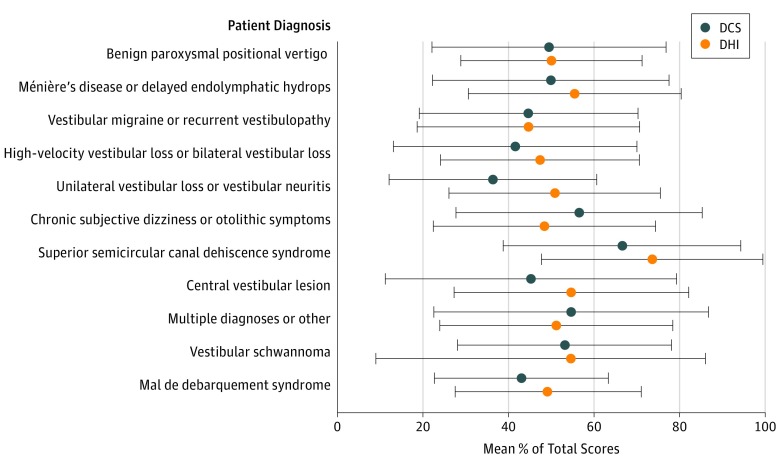
Table 2. Association Between the DCS and DHI Mean Total Scores by Diagnostic Categories Among 438 Patients.
| Patient Diagnosis | No. (%) | Mean (SD) | r | |
|---|---|---|---|---|
| DCS | DHI | |||
| Benign paroxysmal positional vertigo | 64 (14.6) | 25.7 (14.4) | 50.1 (21.0) | 0.63a |
| Ménière’s disease or delayed endolymphatic hydrops | 79 (18.0) | 25.9 (14.4) | 55.4 (24.9) | 0.67a |
| Vestibular migraine or recurrent vestibulopathy | 97 (22.1) | 23.2 (13.4) | 44.6 (26.0) | 0.65a |
| High-velocity vestibular loss or bilateral vestibular loss | 42 (9.6) | 21.6 (14.8) | 47.4 (23.2) | 0.53a |
| Unilateral vestibular loss or vestibular neuritisb | 39 (8.9) | 18.8 (12.7) | 50.7 (24.8) | 0.55a |
| Chronic subjective dizziness or otolithic symptomsb | 50 (11.4) | 29.3 (15.1) | 48.5 (26.0) | 0.84a |
| Superior semicircular canal dehiscence syndrome | 8 (1.8) | 34.6 (14.4) | 73.5 (26.0) | 0.74c |
| Central vestibular lesion | 34 (7.8) | 23.5 (17.7) | 56.8 (27.3) | 0.81a |
| Multiple diagnoses or other | 13 (3.0) | 28.4 (16.8) | 51.3 (27.2) | 0.61c |
| Vestibular schwannoma | 5 (1.1) | 27.6 (13.1) | 54.8 (31.3) | 0.99a |
| Mal de debarquement syndrome | 7 (1.6) | 22.4 (10.6) | 49.1 (22.6) | 0.19 |
Abbreviations: DCS, Dizziness Catastrophizing Scale; DHI, Dizziness Handicap Inventory.
P ≤ .001.
Post hoc Tukey test mean significant differences in the DCS scores between these 2 diagnoses.
P ≤ .05.
Moderate to strong associations were found between catastrophizing and dizziness-related disability across diagnostic classifications (Table 2). Diagnostic group differences were found for the DCS scores (F10,427 = 2.064, P = .03) but not for the DHI scores (F10,386 = 1.591, P = .11) (Figure 1 and Table 2). Post hoc Tukey tests demonstrated a single group difference among the 11 diagnostic categories. Patients with chronic subjective dizziness or otolithic symptoms had higher DCS scores than patients with unilateral vestibular loss or vestibular neuritis symptoms (mean [SD], 29.3 [15.1] vs 18.8 [12.7]) (mean difference, 10.5; 95% CI, 0.2-20.8; P = .04).
Figure 1. DCS and DHI Mean Percentage of Total Scores by Diagnostic Category.
DCS indicates Dizziness Catastrophizing Scale (score range, 0-52); DHI, Dizziness Handicap Inventory (score range, 0-100). Error bars represent ±1 SD.
Convergent and Discriminant Validity
The DCS was strongly correlated with the PANAS negative affect score (r = 0.78, P < .001) and was modestly to moderately negatively correlated with the PANAS positive affect score (r = −0.40, P < .001). These findings suggested good convergent and divergent validity, respectively (Table 1).
Scale Reliability
The DCS demonstrated strong internal consistency (α = .95). The factor loadings for the single latent component and corrected item-total correlations for each DCS item are listed in the eTable in the Supplement.
Follow-up visit data to assess test-retest reliability were available for 35 patients. The intraclass correlation coefficient for the average 2-month (ie, mean [SD], 66.7 [20.5] days; range, 7-91 days) test-retest reliability for the DCS total score was 0.92 (P < .001 at the 95% CI).
Exploratory Factor Analysis
One component emerged from the parallel analysis with an eigenvalue that was greater than randomly generated eigenvalues. The first 3 eigenvalues generated from actual data were 8.39, 0.87, and 0.71, and the first three 95th percentile eigenvalues generated from random data were 1.65, 1.49, and 1.37; therefore, the retention of 1 factor was suggested. The subsequent exploratory analysis indicated a satisfactory fit for the 1-factor model (χ2 test of model fit significance >.05, RMSEA of 0.131, CFI of 0.975, TLI of 0.970, and SRMR of 0.055). The single component extracted for the exploratory factor analysis explained 61.6% of the variance in the DCS. The factor loadings for the individual items were all 0.726 or higher (eTable in the Supplement).
For the confirmatory factor analysis, the data were divided into 2 equal subsets. One component emerged from the parallel analysis of the first data subset with an eigenvalue that was greater than the randomly generated eigenvalues. The first 3 eigenvalues generated from actual data were 8.57, 1.02, and 0.64, and the first three 95th percentile eigenvalues generated from random data were 1.98, 1.70, and 1.52; therefore, the retention of 1 factor was suggested. In the first data subset, satisfactory indexes were found for a 1-factor model (χ2 test of model fit significance <.05, RMSEA of 0.105, CFI of 0.986, TLI of 0.984, and SRMR of 0.060), and the factor loadings for the individual items were all 0.754 or greater. The single component extracted for the principal component analysis explained 72.2% of the variance in the DCS. The subsequent confirmatory factor analysis using the second data subset also suggested a satisfactory fit for a 1-factor model (χ2 test of model fit significance <.05, RMSEA of 0.164, CFI of 0.967, TLI of 0.960, and WRMR of 1.059).
Structural Equation Modeling
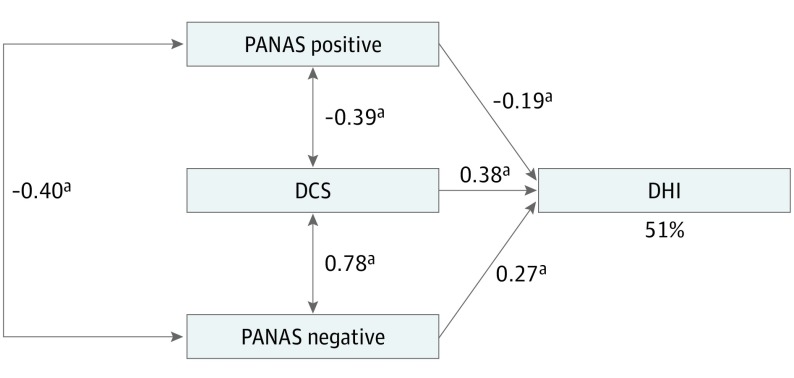
Figure 2 shows the model representing the hypothesis that dizziness-related disability (the DHI) is predicted by affect (the PANAS positive and negative) and catastrophizing (the DCS). The paths from positive affect (standardized β, −0.187), negative affect (standardized β, 0.275), and catastrophizing (standardized β, 0.378) to dizziness-related disability were statistically significant (P < .001 for all).
Figure 2. Saturated Path Model of the Interrelationships Between the DCS, PANAS Positive, and PANAS Negative and Their Contribution to the Variance of the DHI.
Rectangles represent observed variables. Standardized regression coefficients (β) are presented beside single-headed arrows. Correlation coefficients between predictor variables are presented beside double-headed arrows. The variance (R2) of the DHI is represented by a percentage value. Goodness-of-fit indexes are χ20 = 0.000, P = NA, and χ2 / df = NA. Comparative Fit Index is 1, Tucker-Lewis Index is NA, and root-mean-square of approximation is NA. DCS indicates Dizziness Catastrophizing Scale; DHI, Dizziness Handicap Inventory; NA, not applicable; and PANAS, Positive and Negative Affect Schedule. aP < .001.
Regression Analysis
Table 3 summarizes the hierarchical regression for dizziness-related disability (ie, the DHI). The DCS was independently associated with the DHI and remained associated after positive and negative affectivity (ie, the PANAS positive and negative subscale scores) was entered into the model. Dizziness catastrophizing (ie, the DCS) independently accounted for 47.1% of the variance in dizziness-related disability. All variables accounted for 52.9% of the variance in dizziness-related disability.
Table 3. Regression Analysis for the DHI,a Including the DCS, PANAS Positive, and PANAS Negative as Associated Variables.
| Predictor | R2 | F Change | P Value | B | β (95% CI) | Semipartial Correlation | P Value |
|---|---|---|---|---|---|---|---|
| Model 1 | 0.471 | 311.366 | <.001 | NA | NA | NA | NA |
| DCS | NA | NA | NA | 1.145 | 0.686 (1.017 to 1.272) | 0.686 | <.001 |
| Model 2 | 0.529 | 21.338 | <.001 | NA | NA | NA | NA |
| DCS | NA | NA | NA | 0.689 | 0.413 (0.491 to 0.886) | 0.252 | <.001 |
| PANAS positive | NA | NA | NA | −0.469 | −0.167 (−0.694 to −0.245) | −0.151 | <.001 |
| PANAS negative | NA | NA | NA | 0.719 | 0.263 (0.394 to 1.043) | 0.160 | <.001 |
Abbreviations: B, unstandardized β coefficient; DCS, Dizziness Catastrophizing Scale; DHI, Dizziness Handicap Inventory; NA, not applicable; PANAS, Positive and Negative Affect Schedule.
The DHI is the dependent variable in this analysis.
Discussion
To our knowledge, this is the first study to investigate the role of catastrophizing in patients with dizziness. Our results suggest that catastrophizing is a strong independent predictor of dizziness-related disability across diagnoses (Figures 1 and 2). We discovered this through the psychometric evaluation of the DCS, a novel measure of catastrophizing in dizziness, which was adapted from the well-established PCS.4
Psychometrically, the DCS demonstrated good convergent and discriminant validity, as well as reliability. While the DCS total score was convergent with the PANAS negative affect subscale, the DCS total score was only moderately negatively correlated with the PANAS positive affect subscale. The results of our exploratory factor analysis suggest a single-factor solution for the DCS, which differs from the 3-factor solution of the PCS.4 Additional tests of the factor structure of the DCS in larger samples are required.
The results of our path and regression analyses revealed that catastrophizing (ie, higher DCS scores), although associated with negative affectivity (ie, the PANAS negative affect subscale), was independently correlated with dizziness-related disability, as assessed with the DHI, the criterion standard measure for quantifying impairment attributable to dizziness (Figure 2 and Table 3). Our results are in line with previous findings4,34,35,36 suggesting that catastrophizing is a related but unique construct from depression and other manifestations of anxiety and emotional distress. In another study,37 although weak to moderate correlations were found between catastrophizing and depression, some patients with musculoskeletal pain had either catastrophic thinking or negative affect associated with their condition but not both. In the present study, the strong association found between catastrophizing and dizziness-related disability was present across diagnostic classifications, including diagnoses traditionally identified as organic (eg, benign paroxysmal positional vertigo and central vestibular lesion) vs nonorganic (eg, chronic subjective dizziness or otolithic symptoms). This suggests that, similar to pain,7,12 patients’ subjective experience of their dizziness is an important contributor to dizziness-related disability regardless of diagnosis. Moreover, these results support a holistic approach to the care of patients with dizziness, which includes the treatment of the emotional and psychiatric factors contributing to disability.
The strong test-retest reliability of the DCS (DCS total score of 0.92, P < .001) at 2-month follow-up has significant clinical implications. This result points to the persistence of catastrophizing in patients with dizziness if left untreated. Alternatively, it may also appear that the DCS is not sensitive to changes over time. However, evidence from the pain catastrophizing literature suggests that catastrophizing has a temporal stability in the absence of intervention4 but can be significantly reduced by interventions that facilitate the use of healthier coping strategies (eg, psychiatric and rehabilitation support).38,39,40 Although we are not aware of any studies specific to the treatment of catastrophizing in chronic dizziness, a substantial amount of literature supports the treatment of comorbid mood or anxiety symptoms to improve clinical outcomes in patients with long-term dizziness.41,42,43 Moreover, there is also evidence that pharmacological treatments originally designed for the treatment of depression and anxiety improve chronic dizziness even in individuals who do not have symptoms of anxiety or depression.44,45
Limitations
Our study has a few limitations. First, some data were missing because of measures being only partially completed by patients, which may have influenced the results of our SEM. However, the large sample that remained with complete data (n = 352) and the use of an SEM method (ie, full information maximum likelihood) to account for missing data were likely sufficient to overcome this issue. Future studies adopting a prospective design are recommended to minimize the likelihood of missing data that can occur with retrospective medical record reviews. Second, responses to self-report English-language questionnaires may have been influenced by difficulties experienced among those who were not native English speakers or patients with advanced disabilities. Third, our study sample may not be representative of the general population with dizziness. Because many of the patients assessed at the Multidisciplinary Neurotology Clinic are referrals from other specialists, these individuals likely reflect more complex cases than those seen in primary care settings.
Conclusions
The results of this study support the validity and reliability of the DCS as a measure of catastrophic thinking in dizziness, which is associated with dizziness-related disability independent of positive or negative affectivity (eg, symptoms of anxiety and depression). Our findings suggest that the evaluation of dizziness catastrophizing in clinical settings is essential because of its unique contribution to the degree of dizziness-related disability regardless of diagnosis (eg, organic vs nonorganic). Future studies should consider exploring the association of treatment specific for catastrophizing with clinical outcomes in patients with chronic dizziness.
eFigure. Dizziness Catastrophizing Scale
eTable. Dizziness Catastrophizing Scale (DCS) Factor Loadings and Internal Reliability
References
- 1.Lee HJ, Choi-Kwon S. Quality of life and the related factors in patients with dizziness [in Korean]. J Korean Acad Nurs. 2009;39(5):751-758. doi: 10.4040/jkan.2009.39.5.751 [DOI] [PubMed] [Google Scholar]
- 2.Weidt S, Bruehl AB, Straumann D, Hegemann SC, Krautstrunk G, Rufer M. Health-related quality of life and emotional distress in patients with dizziness: a cross-sectional approach to disentangle their relationship. BMC Health Serv Res. 2014;14:317. doi: 10.1186/1472-6963-14-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karatas M. Central vertigo and dizziness: epidemiology, differential diagnosis, and common causes. Neurologist. 2008;14(6):355-364. doi: 10.1097/NRL.0b013e31817533a3 [DOI] [PubMed] [Google Scholar]
- 4.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 5.Carleton RN, Kachur SS, Abrams MP, Asmundson GJ. Waddell’s symptoms as indicators of psychological distress, perceived disability, and treatment outcome. J Occup Rehabil. 2009;19(1):41-48. doi: 10.1007/s10926-009-9165-4 [DOI] [PubMed] [Google Scholar]
- 6.Chitsaz A, Khourvash F, Tolou-Ghamari Z, Gholamrezaei A, Noormohamadi A. Types of dizziness and its relationship with psychological symptoms in patients with chronic dizziness. Am J Exp Clin Res. 2016;3(1):141-145. [Google Scholar]
- 7.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9(5):745-758. doi: 10.1586/ern.09.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaves JF, Brown JM. Spontaneous cognitive strategies for the control of clinical pain and stress. J Behav Med. 1987;10(3):263-276. doi: 10.1007/BF00846540 [DOI] [PubMed] [Google Scholar]
- 9.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33-44. doi: 10.1016/0304-3959(83)90125-2 [DOI] [PubMed] [Google Scholar]
- 10.Spanos NP, Radtke-Bodorik HL, Ferguson JD, Jones B. The effects of hypnotic susceptibility, suggestions for analgesia, and the utilization of cognitive strategies on the reduction of pain. J Abnorm Psychol. 1979;88(3):282-292. doi: 10.1037/0021-843X.88.3.282 [DOI] [PubMed] [Google Scholar]
- 11.Turner JA, Clancy S. Strategies for coping with chronic low back pain: relationship to pain and disability. Pain. 1986;24(3):355-364. doi: 10.1016/0304-3959(86)90121-1 [DOI] [PubMed] [Google Scholar]
- 12.Farin E. The reciprocal effect of pain catastrophizing and satisfaction with participation in the multidisciplinary treatment of patients with chronic back pain. Health Qual Life Outcomes. 2015;13:163. doi: 10.1186/s12955-015-0359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristiansen FL, Olesen AE, Brock C, et al. The role of pain catastrophizing in experimental pain perception. Pain Pract. 2014;14(3):E136-E145. doi: 10.1111/papr.12150 [DOI] [PubMed] [Google Scholar]
- 14.Peluso ET, Quintana MI, Ganança FF. Anxiety and depressive disorders in elderly with chronic dizziness of vestibular origin. Braz J Otorhinolaryngol. 2016;82(2):209-214. doi: 10.1016/j.bjorl.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckhardt-Henn A, Breuer P, Thomalske C, Hoffmann SO, Hopf HC. Anxiety disorders and other psychiatric subgroups in patients complaining of dizziness. J Anxiety Disord. 2003;17(4):369-388. doi: 10.1016/S0887-6185(02)00226-8 [DOI] [PubMed] [Google Scholar]
- 16.Staab JP, Rohe DE, Eggers SD, Shepard NT. Anxious, introverted personality traits in patients with chronic subjective dizziness. J Psychosom Res. 2014;76(1):80-83. doi: 10.1016/j.jpsychores.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 17.Tschan R, Best C, Wiltink J, Beutel ME, Dieterich M, Eckhardt-Henn A. Persistence of symptoms in primary somatoform vertigo and dizziness: a disorder “lost” in health care? J Nerv Ment Dis. 2013;201(4):328-333. doi: 10.1097/NMD.0b013e318288e2ad [DOI] [PubMed] [Google Scholar]
- 18.Lefebvre MF. Cognitive distortion and cognitive errors in depressed psychiatric and low back pain patients. J Consult Clin Psychol. 1981;49(4):517-525. doi: 10.1037/0022-006X.49.4.517 [DOI] [PubMed] [Google Scholar]
- 19.Leung L. Pain catastrophizing: an updated review. Indian J Psychol Med. 2012;34(3):204-217. doi: 10.4103/0253-7176.106012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mounce C, Keogh E, Eccleston C. A principal components analysis of negative affect–related constructs relevant to pain: evidence for a three component structure. J Pain. 2010;11(8):710-717. doi: 10.1016/j.jpain.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 21.Hirsh AT, George SZ, Riley JL III, Robinson ME. An evaluation of the measurement of pain catastrophizing by the Coping Strategies Questionnaire. Eur J Pain. 2007;11(1):75-81. doi: 10.1016/j.ejpain.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 22.Geisser ME, Robinson ME, Keefe FJ, Weiner ML. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain. 1994;59(1):79-83. doi: 10.1016/0304-3959(94)90050-7 [DOI] [PubMed] [Google Scholar]
- 23.Kincaid JP, Fishburne RP Jr, Rogers RL, Chissom BS. Derivation of New Readability Formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy Enlisted Personnel. Millington, TN: Institute for Simulation and Training; 1975. Paper 56. doi: 10.21236/ADA006655 [DOI] [Google Scholar]
- 24.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063-1070. doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 25.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43(Pt 3):245-265. doi: 10.1348/0144665031752934 [DOI] [PubMed] [Google Scholar]
- 26.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424-427. doi: 10.1001/archotol.1990.01870040046011 [DOI] [PubMed] [Google Scholar]
- 27.Fleiss JL. The Design and Analysis of Clinical Experiments. New York, NY: Wiley; 1986. [Google Scholar]
- 28.O’Connor BP. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer’s MAP test. Behav Res Methods Instrum Comput. 2000;32(3):396-402. doi: 10.3758/BF03200807 [DOI] [PubMed] [Google Scholar]
- 29.Quilty LC, Ayearst L, Chmielewski M, Pollock BG, Bagby RM. The psychometric properties of the personality inventory for DSM-5 in an APA DSM-5 field trial sample. Assessment. 2013;20(3):362-369. doi: 10.1177/1073191113486183 [DOI] [PubMed] [Google Scholar]
- 30.Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting structural equation modeling and confirmatory factor analysis results: a review. J Educ Res. 2006;99(6):323-338. doi: 10.3200/JOER.99.6.323-338 [DOI] [Google Scholar]
- 31.Brown TA. Confirmatory Factor Analysis for Applied Research. New York, NY: The Guilford Press; 2006. [Google Scholar]
- 32.Allison PD. Missing data techniques for structural equation modeling. J Abnorm Psychol. 2003;112(4):545-557. doi: 10.1037/0021-843X.112.4.545 [DOI] [PubMed] [Google Scholar]
- 33.Ullman JB, Bentler PM. Structural equation modeling In: Weiner IB, ed. Handbook of Psychology. 2nd ed Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 34.Tran ST, Jastrowski Mano KE, Hainsworth KR, et al. Distinct influences of anxiety and pain catastrophizing on functional outcomes in children and adolescents with chronic pain. J Pediatr Psychol. 2015;40(8):744-755. doi: 10.1093/jpepsy/jsv029 [DOI] [PubMed] [Google Scholar]
- 35.Makino S, Jensen MP, Arimura T, et al. Alexithymia and chronic pain: the role of negative affectivity. Clin J Pain. 2013;29(4):354-361. doi: 10.1097/AJP.0b013e3182579c63 [DOI] [PubMed] [Google Scholar]
- 36.Jensen MP, Turner JA, Romano JM. Changes after multidisciplinary pain treatment in patient pain beliefs and coping are associated with concurrent changes in patient functioning. Pain. 2007;131(1-2):38-47. doi: 10.1016/j.pain.2006.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linton SJ, Nicholas MK, MacDonald S, et al. The role of depression and catastrophizing in musculoskeletal pain. Eur J Pain. 2011;15(4):416-422. doi: 10.1016/j.ejpain.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 38.Lerman SF, Finan PH, Smith MT, Haythornthwaite JA. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain. 2017;158(11):2189-2195. doi: 10.1097/j.pain.0000000000001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spanos NP, Brown JM, Jones B, Horner D. Cognitive activity and suggestions for analgesia in the reduction of reported pain. J Abnorm Psychol. 1981;90(6):554-561. doi: 10.1037/0021-843X.90.6.554 [DOI] [PubMed] [Google Scholar]
- 40.Thorn BE, Boothby JL, Sullivan MJL. Targeted treatment of catastrophizing for the management of chronic pain. Cognit Behav Pract. 2002;9(2):127-138. doi: 10.1016/S1077-7229(02)80006-2 [DOI] [Google Scholar]
- 41.Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am. 2009;42(1):71-77, ix. doi: 10.1016/j.otc.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 42.Ballas CA, Staab JP. Medically unexplained physical symptoms: toward an alternative paradigm for diagnosis and treatment. CNS Spectr. 2003;8(12)(suppl 3):20-26. doi: 10.1017/S1092852900008245 [DOI] [PubMed] [Google Scholar]
- 43.Staab JP. Chronic dizziness: the interface between psychiatry and neuro-otology. Curr Opin Neurol. 2006;19(1):41-48. doi: 10.1097/01.wco.0000198102.95294.1f [DOI] [PubMed] [Google Scholar]
- 44.Staab JP, Ruckenstein MJ, Amsterdam JD. A prospective trial of sertraline for chronic subjective dizziness. Laryngoscope. 2004;114(9):1637-1641. doi: 10.1097/00005537-200409000-00025 [DOI] [PubMed] [Google Scholar]
- 45.Staab JP, Ruckenstein MJ, Solomon D, Shepard NT. Serotonin reuptake inhibitors for dizziness with psychiatric symptoms. Arch Otolaryngol Head Neck Surg. 2002;128(5):554-560. doi: 10.1001/archotol.128.5.554 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Dizziness Catastrophizing Scale
eTable. Dizziness Catastrophizing Scale (DCS) Factor Loadings and Internal Reliability