This systematic review and meta-analysis compares long-term outcomes between the Ross procedure and mechanical aortic valve replacement in adults.
Key Points
Question
What is the optimal aortic valve substitute in young and middle-aged adults undergoing aortic valve replacement?
Findings
This meta-analysis included 3516 adults who underwent the Ross procedure and found a 46% lower incidence of all-cause mortality compared with patients undergoing mechanical aortic valve replacement, indicating a significant difference.
Meaning
In carefully selected young and middle-aged adults, the Ross procedure is associated with lower all-cause mortality compared with mechanical aortic valve replacement.
Abstract
Importance
The ideal aortic valve substitute in young and middle-aged adults remains unknown.
Objective
To compare long-term outcomes between the Ross procedure and mechanical aortic valve replacement in adults.
Data Sources
The Ovid versions of MEDLINE and EMBASE classic (January 1, 1967, to April 26, 2018; search performed on April 27, 2018) were screened for relevant studies using the following text word search in the title or abstract: (“Ross” OR “autograft”) AND (“aortic” OR “mechanical”).
Study Selection
All randomized clinical trials and observational studies comparing the Ross procedure to the use of mechanical prostheses in adults undergoing aortic valve replacement were included. Studies were included if they reported any of the prespecified primary or secondary outcomes. Studies were excluded if no clinical outcomes were reported or if data were published only as an abstract. Citations were screened in duplicate by 2 of the authors, and disagreements regarding inclusion were reconciled via consensus.
Data Extraction and Synthesis
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-analysis of Observational Studies in Epidemiology guidelines. Data were independently abstracted by 3 reviewers and pooled using a random-effects model.
Main Outcomes and Measures
The prespecified primary outcome was all-cause mortality.
Results
The search identified 2919 reports, of which 18 studies (3516 patients) met inclusion criteria, including 1 randomized clinical trial and 17 observational studies, with a median average follow-up of 5.8 (interquartile range, 3.4-9.2) years. Analysis of the primary outcome showed a 46% lower all-cause mortality in patients undergoing the Ross procedure compared with mechanical aortic valve replacement (incidence rate ratio [IRR], 0.54; 95% CI, 0.35-0.82; P = .004; I2 = 28%). The Ross procedure was also associated with lower rates of stroke (IRR, 0.26; 95% CI, 0.09-0.80; P = .02; I2 = 8%) and major bleeding (IRR, 0.17; 95% CI, 0.07-0.40; P < .001; I2 = 0%) but higher rates of reintervention (IRR, 1.76; 95% CI, 1.16-2.65; P = .007; I2 = 0%).
Conclusions and Relevance
Data from primarily observational studies suggest that the Ross procedure is associated with lower all-cause mortality compared with mechanical aortic valve replacement. These findings highlight the need for a large, prospective randomized clinical trial comparing long-term outcomes between these 2 interventions.
Introduction
Aortic valve replacement remains the only therapy that has been shown to improve the natural history in patients with severe symptomatic aortic valve disease.1,2 However, young and middle-aged adults with diseased aortic valves constitute a challenging population. Owing to a longer life expectancy, these patients present a higher cumulative lifetime risk of prosthesis-related complications. Because of their proven durability and ease of implantation, mechanical prostheses are the most frequently used option in this patient group.3 Recent evidence suggests better long-term survival in young and middle-aged adults who undergo aortic valve replacement with mechanical prostheses compared with biologic prostheses.4,5 However, mechanical valves are thrombogenic and require lifelong anticoagulation, exposing the patient to a continuous hazard of bleeding and thromboembolic events. Recent studies have reported excess mortality in young adults undergoing mechanical aortic valve replacement compared with the age- and sex-matched general population.6
Replacement of the aortic valve with a pulmonary autograft and placement of a homograft in the pulmonary position (Ross procedure) was first described by Donald Ross in 1967.7 In addition to alleviating the need for anticoagulation, this procedure remains the only aortic valve replacement operation that provides continued long-term viability of the aortic valve substitute, thus allowing adaptive remodeling and conferring a hemodynamic profile similar to that of the native aortic valve.8 After an initial wave of enthusiasm in the early 1990s, use of the Ross procedure has declined markedly over the last 2 decades owing to concerns over the perceived complexity of this operation, which is felt by many to increase surgical risk and lead to high rates of reintervention due to autograft failure.9,10 In addition, patients who undergo the Ross procedure are also at risk of late homograft failure, although this problem is increasingly managed using percutaneous approaches in the current era.11,12,13 Thus, there is equipoise regarding the ideal aortic valve substitute in young and middle-aged adults. Our aim was to compare early and late outcomes in adult patients who undergo the Ross procedure vs mechanical aortic valve replacement.
Methods
Search Strategy and Selection Criteria
We conducted a systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses and Meta-analysis of Observational Studies in Epidemiology guidelines (eTable 1 in Supplement 1).14,15 We systematically searched Ovid versions of MEDLINE (January 1, 1967, to April 26, 2018) and EMBASE classic (January 1, 1967, to April 26, 2018; search performed on April 27, 2018) for relevant studies using the following text word search in the title or abstract: (“Ross” OR “autograft”) AND (“aortic” OR “mechanical”). We also searched bibliographies of included studies. We imposed no language restrictions. We included all randomized clinical trials and observational studies comparing the Ross procedure to the use of mechanical prostheses in adults undergoing aortic valve replacement. Studies were included if they reported the primary outcome (ie, all-cause mortality) or any of the prespecified secondary clinical outcomes: operative mortality, perioperative complications, late complications (reoperation, stroke, major bleeding, endocarditis), echocardiographic outcomes at follow-up (mean aortic valve gradient, left ventricular ejection fraction), or quality of life. Studies were excluded if no clinical outcomes were reported or if data were published only as an abstract. Citations were screened in duplicate by 2 of the authors (A.M. and R.V.R.), and full text review, also in duplicate, was performed to determine eligibility when either screening reviewer felt a citation potentially met inclusion criteria. Disagreements regarding inclusion were reconciled via consensus. When there was overlap between 2 or more studies, we included only the report with the larger sample size, the longer duration of follow-up, and/or the most complete description of the data.
Data Analysis
Three reviewers (A.M., R.V.R., and J.O.F.) independently abstracted data including details of the publication (ie, study authors, enrollment period, year of publication, study design), inclusion/exclusion criteria, demographics of the enrolled patients, description of the interventions used, and outcome definitions and events. Risk of bias in randomized clinical trials (including blinding of participants, method of sequence generation and allocation concealment, intention-to-treat analysis, early trial stoppage for efficacy before planned enrollment completion, and loss to follow-up) and cohort studies (including retrospective vs prospective data collection, concurrent vs historical controls, and comparable baseline characteristics of cases and controls) were formally assessed using the Cochrane Collaboration’s Risk of Bias tool for randomized clinical trials and the Newcastle-Ottawa Scale for nonrandomized studies,16,17 respectively, with disagreements resolved by consensus.
All analyses were performed using Review Manager (RevMan version 5.2; Cochrane Collaboration) and the DerSimonian and Laird random-effects method, which incorporates between-trial heterogeneity.18 We assessed statistical heterogeneity among studies using I2, defined as the percentage of total variability across studies attributable to heterogeneity rather than chance, and used published guidelines for low (I2 = 25% to 49%), moderate (I2 = 50% to 74%), and high (I2 > 75%) heterogeneity.19 For perioperative outcomes, relative risks were used to pool binary outcomes. When necessary, we applied a continuity correction factor of 0.5 to allow inclusion of studies with no events in one of the comparator groups but excluded studies with no events in either comparator group.20 Weighted mean differences were used to pool continuous data. For skewed continuous data, means and SDs were estimated from medians and interquartile ranges or ranges as described by Wan et al.21 Imbalances in baseline characteristics were also assessed using standardized differences,22 including correction for small sample bias.23
For long-term outcomes with potentially different follow-up between groups, we pooled incidence rate ratios (IRRs) on the logarithmic scale using the generic inverse variance method. When hazard ratios (assumed to be equivalent to IRRs) were not provided, IRRs for each study were calculated in 1 of 2 ways: (1) using Kaplan-Meier survival curve estimates for each group and the log-rank survival curve P value to estimate the standard error of the logarithm-transformed IRR or otherwise (2) using absolute events divided by patient-years of follow-up (estimated as number of patients per group multiplied by group-specific mean duration of follow-up in years) when group-specific mean follow-up durations were provided, as previously described.24,25 Individual study and pooled summary results are reported with 95% CIs.
Study results were subgrouped by study type: randomized clinical trials vs propensity-score matched or risk-adjusted observational data vs unmatched/unadjusted observational data. In cases where observational studies reported both matched/risk-adjusted and unmatched/unadjusted data, the matched or risk-adjusted outcomes were preferentially used in calculating the overall pooled result, if available. Otherwise, the unmatched/unadjusted outcomes were used. This means that studies that report matched or risk-adjusted outcomes for some but not all outcomes appear in the matched/adjusted subgroup for some outcomes and the unmatched/unadjusted subgroup for other outcomes.
Results
Our search strategy identified 2919 citations, of which 69 were retrieved for more detailed assessment. Fifty-one studies were excluded following full text review. A total of 18 studies were included in the analysis: 1 randomized clinical trial (5.6%),26 10 matched/adjusted observational studies (55.6%),27,28,29,30,31,32,33,34,35,36 and 7 unmatched/unadjusted observational studies (38.9%) (eFigure 1 in Supplement 1).37,38,39,40,41,42,43
The selected studies included a total of 3516 patients, of whom 1552 (44.1%) underwent a Ross procedure, and 1964 (55.9%) underwent mechanical aortic valve replacement. Characteristics of the included studies are presented in the Table. Study size ranged from 11 to 692 patients. One study followed up patients only to hospital discharge. The median (interquartile range) average follow-up in the remaining studies was 5.8 (3.4 to 9.2) years (range, 11 months to 14.8 years). The single unblinded randomized clinical trial in this meta-analysis included a small number of patients (20 per group) with a short duration of follow-up (1 year in all patients). Risk of bias in this randomized clinical trial was rated as high owing to the absence of blinding and relatively stringent exclusion criteria (eTable 2 in Supplement 1). Risk of bias in the observational studies is summarized in the eTable 3 in Supplement 1. Five of 17 studies (29.4%) scored higher than 7 of 9 (denoting low risk of bias) on the Newcastle-Ottawa Scale for nonrandomized studies. Inadequate comparability of the 2 study groups (12 of 17 studies [70.6%]) and insufficient length of follow-up (<5 years in 7 of 17 studies [41.2%]) were the 2 most common sources of potential bias. Comparisons of baseline characteristics and intraoperative data between the groups are presented in eTable 4 in Supplement 1.
Table. Characteristics of Studies Included in the Meta-Analysis.
| Source | Country | No. of Centers | Design | Group | No. of Patients | Age, Mean (SD), y | Men, % | Follow-up, Mean (SD), y |
|---|---|---|---|---|---|---|---|---|
| Aicher et al,40 2011 | Germany | 1 | Retrospective observational; unadjusted | Ross | 39 | 40 (7) | 69 | 6.1 (2.6) |
| mAVR | 41 | 40 (7) | 86 | 6.5 (2.4) | ||||
| Akhyari et al,39 2009 | Germany | 1 | Retrospective observational; unadjusted | Ross | 18 | 38 (9) | 72 | 3.2 (1.8) |
| mAVR | 20 | 42 (7) | 75 | 4.2 (2.2) | ||||
| Andreas et al,32 2014 | Austria | 1 | Retrospective observational, adjusted | Ross | 159 | 35 (8) | 80 | 9.9 (6.0) |
| mAVR | 173 | 41 (7) | 75 | 7.9 (5.4) | ||||
| Basude et al,42 2014a | United Kingdom | 1 | Retrospective observational; unadjusted | Ross | 3 | 20 (14-32)b | 0 | 9 (9-16)b |
| mAVR | 8 | 25 (7-33)b | 0 | 13 (6-20)b | ||||
| Bouhout et al,35 2017 | Canada | 1 | Propensity score matching | Ross | 70 | 52 (45-59)c | 77 | Hospital stay |
| mAVR | 70 | 52 (45-59)c | 67 | Hospital stay | ||||
| Buratto et al,36 2018 | Australia | 1 | Propensity score matching | Ross | 275 | 43 (11) | 71 | 10 (7) |
| 31 | mAVR | 275 | 44 (11) | 73 | 10 (7) | |||
| Concha et al,30 2005 | Spain | 1 | Retrospective observational; adjusted | Ross | 63 | 35 (8) | 78 | 2.5 (1.6) |
| mAVR | 62 | 38 (7) | 76 | 2.5 (1.6) | ||||
| Doss et al,26 2005 | Germany | 1 | Randomized clinical trial | Ross | 20 | 49 (8) | 60 | 1 (0) |
| mAVR | 20 | 48 (7) | 55 | 1 (0) | ||||
| Heuvelman et al,41 2013a | Netherlands | 1 | Retrospective observational; unadjusted | Ross | 18 | 22 (7) | 0 | NR |
| mAVR | 9 | 31 (9) | 0 | NR | ||||
| Jaggers et al,27 1998 | United States | 1 | Retrospective observational; adjusted | Ross | 22 | 38 (11) | 77 | 0.9 (NR) |
| mAVR | 27 | 41 (11) | 63 | 2.5 (NR) | ||||
| Klieverik et al,37 2006 | Netherlands | 1 | Retrospective observational; unadjusted | Ross | 81 | 31 (9) | 63 | 7.7 (2.3) |
| mAVR | 204 | 45 (8) | 73 | 6.2 (3.2) | ||||
| Mazine et al,33 2016 | Canada | 1 | Propensity score matching | Ross | 208 | 37 (10) | 64 | 13.6 (5.8) |
| mAVR | 209 | 37 (11) | 63 | 14.8 (7.2) | ||||
| Mokhles et al,31 2011 | Germany/ Netherlands | 12 | Propensity score matching | Ross | 253 | 47 (9) | 76 | 5.1 (NR) |
| 1 | mAVR | 253 | 48 (11) | 73 | 6.3 (NR) | |||
| Nötzold et al,28 2001 | Germany | 1 | Retrospective observational; adjusted | Ross | 40 | 26 (6) | 73 | 2.2 (1.3) |
| mAVR | 40 | 27 (5) | 73 | 1.9 (0.7) | ||||
| Schmidtke et al,29 2001 | Germany | 1 | Retrospective observational; adjusted | Ross | 20 | 57 (9) | 70 | 1.9 (0.9) |
| mAVR | 40 | 58 (9) | 70 | 2.1 (1.1) | ||||
| Sharabiani et al,34 2016 | United Kingdom | 37 | Propensity score matching | Ross | 224 | NR | NR | NR |
| mAVR | 468 | |||||||
| Zacek et al,43 2016 | Czech Republic | 1 | Retrospective observational; unadjusted | Ross | 22 | 38 (12) | 83 | 1.75 (NR) |
| mAVR | 29 | 40 (7) | 69 | 1.75 (NR) | ||||
| Zsolt et al,38 2008 | Hungary | 1 | Retrospective observational; unadjusted | Ross | 17 | 26 (6) | 59 | 5.9 (2.3) |
| mAVR | 17 | 27 (5) | 88 | 4.8 (2.1) |
Abbreviations: mAVR, mechanical aortic valve replacement; NR, not reported.
These studies included only pregnant women.
Reported as median (range).
Reported as median (interquartile range).
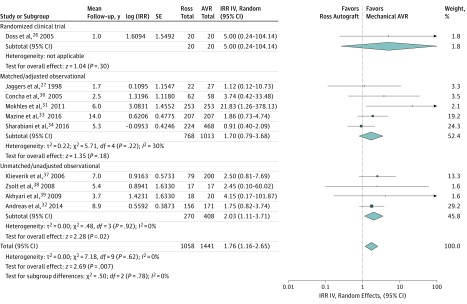
Compared with patients who underwent mechanical aortic valve replacement, patients in the Ross group had a statistically significant 46% reduction in the primary outcome of all-cause mortality (IRR, 0.54; 95% CI, 0.35-0.82; P = .004; I2 = 28%; 0.05%/y vs 0.10%/y) (Figure 1). Similarly, the incidence of valve- or cardiac-related mortality was significantly lower in the Ross group (IRR, 0.42; 95% CI, 0.18-0.97; P = .04; I2 = 34%; 0.04%/y vs 0.09%/y) (eFigure 2 in Supplement 1).
Figure 1. All-Cause Mortality.
Forest plot comparing all-cause mortality following the Ross procedure vs mechanical aortic valve replacement (AVR). Individual study and pooled incidence rate ratios (IRRs) are also presented separately for the randomized clinical trial, and subgroups of matched/adjusted observational studies, and unmatched/unadjusted observational studies. The pooled incidence rate ratios with 95% CIs were calculated using random-effects models. IV indicates inverse variance weighting; SE, standard error.
There was no difference in the rate of perioperative mortality between the groups (risk ratio [RR], 0.73; 95% CI, 0.37-1.44; P = .36; I2 = 0%; 17 of 2085 [0.8%] vs 25 of 1766 [1.4%]) (eFigure 3 in Supplement 1). Similarly, no significant differences were noted in the incidence of the following perioperative complications (eTable 5 in Supplement 1): acute myocardial infarction (RR, 1.96; 95% CI, 0.48-7.97; P = .35; I2 = 0%), stroke or transient ischemic attack (RR, 0.36; 95% CI, 0.10-1.32; P = .12; I2 = 0%), reoperation for bleeding (RR, 1.07; 95% CI, 0.66-1.75; P = .78; I2 = 14%), acute kidney injury (RR, 1.79; 95% CI, 0.30-10.49; P = .52; I2 = 40%), atrial fibrillation (RR, 1.39; 95% CI, 0.73-2.66; P = .32; I2 = 22%), and sternal wound infection (RR, 1.49; 95% CI, 0.26-8.48; P = .65; I2 = 0%). The Ross procedure was associated with lower rates of heart block requiring permanent pacemaker implantation (RR, 0.40; 95% CI, 0.17-0.94; P = .04; I2 = 0%).
Freedom from any operated valve reintervention was compared between groups. In the Ross group, this included any percutaneous or surgical reintervention on the pulmonary autograft and/or pulmonary homograft. We noted a significantly higher rate of reintervention in the Ross group (IRR, 1.76; 95% CI, 1.16-2.65; P = .007, I2 = 0%; 0.12%/y vs 0.06%/y) compared with mechanical aortic valve replacement (Figure 2).
Figure 2. Any Operated Valve Reintervention.
Forest plot comparing reintervention on any operated valve following the Ross procedure vs mechanical aortic valve replacement (AVR). In the Ross group, this includes any percutaneous or surgical reintervention on the pulmonary autograft and/or pulmonary homograft. Individual study and pooled incidence rate ratios (IRRs) are also presented separately for the randomized clinical trial, and the subgroups of matched/adjusted observational studies, and unmatched/unadjusted observational studies. The pooled incidence rate ratios with 95% CIs were calculated using random-effects models. Data from the study by Sharabiani et al34 were extracted from the graphs using a plot digitization software. IV indicates inverse variance weighting; SE, standard error.
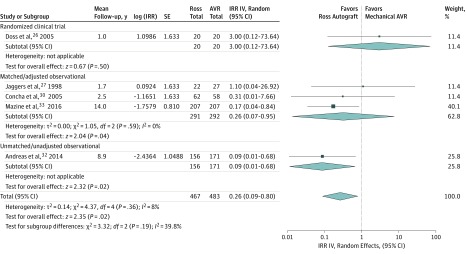
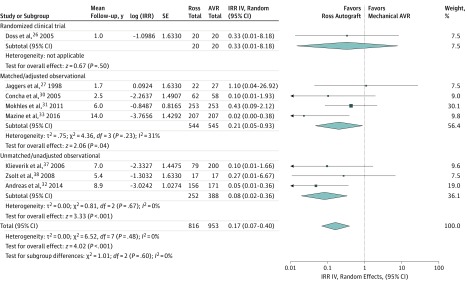
The Ross procedure was associated with significantly lower rates of stroke (IRR, 0.26; 95% CI, 0.09-0.80; P = .02; I2 = 8%; 0.04%/y vs 0.10%/y) (Figure 3) and major bleeding (IRR, 0.17; 95% CI, 0.07-0.40; P < .001; I2 = 0%; 0.01%/y vs 0.11%/y) (Figure 4) at follow-up, compared with mechanical aortic valve replacement. The incidence of endocarditis was low and not significantly different between the groups (P = .35) (eTable 5 in Supplement 1).
Figure 3. Stroke at Follow-up.
Forest plot comparing the incidence of stroke following the Ross procedure vs mechanical aortic valve replacement (AVR). Individual study and pooled incidence rate ratios (IRRs) are also presented separately for the randomized clinical trial, and subgroups of matched/adjusted observational studies, and unmatched/unadjusted observational studies. The pooled incidence rate ratios with 95% CIs were calculated using random-effects models. IV indicates inverse variance weighting; SE, standard error.
Figure 4. Major Bleeding at Follow-up.
Forest plot comparing the incidence of major bleeding following the Ross procedure vs mechanical aortic valve replacement (AVR). Individual study and pooled incidence rate ratios (IRRs) are also presented separately for the randomized clinical trial, and subgroups of matched/adjusted observational studies, and unmatched/unadjusted observational studies. The pooled incidence rate ratios with 95% CIs were calculated using random-effects models. IV indicates inverse variance weighting; SE, standard error.
Echocardiographic data were reported in 7 studies. At last follow-up (median average study follow-up, 2.4 years; range: hospital discharge, 5.4 years), patients in the Ross group had significantly lower mean transaortic valve gradients (mean difference, 9.8 mm Hg; 95% CI, 8.0-11.7; P < .001; I2 = 82%) (eFigure 4 in Supplement 1). There was high heterogeneity between studies regarding the magnitude but not the direction of this difference in mean gradients. A clinically and statistically insignificant improvement in left ventricular ejection fraction at follow-up (median average study follow-up, 2.2 years; range, 1.0-5.4 years) was also noted in the Ross group (mean difference, 3%; 95% CI, −2% to 8%; P = .20; I2 = 78%) (eTable 5 in Supplement 1).
Four studies compared quality of life following the Ross procedure vs mechanical aortic valve replacement using the 36-Item Short Form Survey. Our meta-analysis showed significantly higher scores (ie, improved quality of life) in the Ross group for the subcomponents of bodily pain (P < .001), social functioning (P = .03), and mental health (P = .002) (eFigures 5 and 6 in Supplement 1). All other subcomponents were not statistically different between the groups.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to compare the Ross procedure with mechanical aortic valve replacement in adults. This study included 3516 patients who underwent either the Ross procedure or mechanical aortic valve replacement. The main finding is that the Ross procedure is associated with improved freedom from all-cause mortality, largely driven by superior freedom from cardiac- and valve-related mortality. Secondary findings are that perioperative mortality and morbidity are not significantly different between the groups, with the exception of permanent pacemaker implantation, which is higher after mechanical aortic valve replacement. Furthermore, the Ross procedure is associated with lower rates of stroke and major bleeding at follow-up at the cost of a higher rate of reintervention. Quality of life and hemodynamics were enhanced after the Ross procedure. Finally, our systematic review highlighted the paucity of randomized data addressing this question.
The superior freedom from all-cause mortality in patients undergoing the Ross procedure may be explained by 2 main factors. First, in contrast to mechanical aortic valve replacement, the Ross procedure alleviates the need for lifelong anticoagulation through the avoidance of prosthetic valve material. This advantage is particularly significant in young and middle-aged adults who, because of their longer anticipated life expectancy and active lifestyle, present a higher cumulative lifetime risk of prosthesis-related complications. Second, because the pulmonary autograft is a living structure, it has the potential to reproduce the sophisticated functions of the normal aortic root. Thus, the enhanced survival observed in patients who have undergone the Ross procedure may reflect the beneficial effects of improved hemodynamics on left ventricular health, particularly in this young active patient population. These 2 hypotheses are supported by the observation of a significantly better freedom from cardiac- and valve-related mortality in the Ross group, as well as lower rates of stroke and bleeding, improved quality of life, and lower mean aortic gradients at follow-up. These findings are also in keeping with the results of several contemporary cohort studies with long-term follow-up that have demonstrated excellent survival well into the second postoperative decade after the Ross procedure.44,45,46,47,48,49,50 The majority of these studies have reported a survival that was similar to that of the age- and sex-matched general population. In contrast, large cohort studies have demonstrated that mechanical valves are associated with excess long-term mortality compared with the matched general population when implanted in young and middle-aged adults.4,6
While the superior survival observed in patients who have undergone Ross procedure is in large part attributable to the unique adaptive and hemodynamic characteristics of the living pulmonary autograft, there is no doubt that careful patient selection is equally important to achieve optimal outcomes with this operation. Although findings from this meta-analysis suggest better outcomes after a Ross procedure, this is not to say that, as a surgical approach, it is definitively superior to a mechanical aortic valve replacement. Instead, they should be seen as 2 treatment options providing very different outcomes, each offering unique opportunities to a given patient depending on their clinical profile and personal preferences. Achieving optimal outcomes therefore requires tailoring the appropriate surgical approach to the individual patient, based on shared decision making and consideration of patient values and preferences.51
From a technical standpoint, the ideal candidate for the Ross procedure is a young or middle-aged adult with aortic valve disease (ideally aortic stenosis), a small or nondilated aortic annulus (<25-27 mm), normal aortic dimensions, and a projected life expectancy of 10 to 15 years or longer. The operation is of particular benefit to patients with high levels of physical activity, those at risk of patient-prosthesis mismatch, and women contemplating pregnancy. In contrast, for patients presenting with preoperative aortic insufficiency, a dilated aortic annulus and/or aortic/pulmonary annular mismatch, a standard Ross procedure is associated with higher rates of autograft dilatation and need for reoperation.52 Nevertheless, a number of technical modifications and adjunct measures have been proposed to mitigate the risk of late failure, which are thought to improve the durability of the Ross procedure in these patients.53,54 However, the Ross procedure is contraindicated in patients with familial aortopathy and connective tissue disorder—owing to a prohibitive risk of autograft dilatation and failure—and certain autoimmune disorders (eg, lupus erythematosus, rheumatoid arthritis) as well as in the presence of any associated condition that may limit life expectancy (eg, radiation-induced mediastinal disease, end-stage renal disease).55
It is widely held that the Ross procedure is a technically complex operation and that this increased complexity may translate into greater operative risk. Nevertheless, our meta-analysis suggested equivalent rates of perioperative mortality and morbidity between the Ross procedure and mechanical aortic valve replacement. The only significant difference observed in early outcomes was a higher rate of permanent pacemaker implantation in the mechanical aortic valve replacement group. This difference may be explained by the potential impingement on the conduction system by the rigid sewing ring of mechanical prostheses, which is not present on a pulmonary autograft. Thus, our findings suggest that in dedicated centers, the long-term benefits of the Ross operation do not come at the cost of increased early risk. However, it should be mentioned that as with any complex procedure, outcomes of the Ross procedure are closely correlated with surgical volumes.56,57 It follows that this operation should not be carried out sporadically and that an adequate annual volume of root replacement and Ross procedures is required to achieve and maintain competence.
The higher rate of reintervention observed in the Ross group can be explained by the risk of late dilatation of the pulmonary autograft, as well as by the potential long-term failure of 2 valves rather than 1, which is considered by many to be the Achilles’ heel of the Ross procedure. Fortunately, homograft failure—and the ensuing right ventricular volume and/or pressure overload—is usually tolerated for a long time before requiring reintervention. Furthermore, in the current era, homograft failure is increasingly treated percutaneously using transcatheter valves, therefore obviating the need for open reinterventions.11,12,13
There is wide variability in the reported durability of the Ross operation, and certain early series have reported concerning rates of reintervention.10 However, proponents of the Ross procedure argue that the risk of reintervention may be largely mitigated by subtle technical refinements based on a thorough understanding of aortic and pulmonary root physiology and mechanisms of valve failure.58,59 Supporting this notion, several large recently published series have reported low rates of reintervention ranging from 0.5% to 1.0% per patient-year.44,45 These technical refinements and improved outcomes notwithstanding, a subset of patients who undergo the Ross procedure will invariably require reoperation, particularly after 15 to 20 years,46 and it is reasonable to assume this operation will always carry a risk of reintervention that is greater than that of mechanical valves. Our data suggest that this increased risk of reintervention is not enough to counterbalance the potential long-term benefits of the Ross procedure, resulting in an associated net survival advantage.
Strengths and Limitations
The strength of this study is the use of rigorous methodology, including a reproducible and comprehensive literature search, clearly defined inclusion criteria, duplicate citation review, data abstraction, and quality assessment of individual studies. Our systematic review highlights the limited number of published studies and enrolled patients per study. In contrast to the large number of case series describing medium- and long-term outcomes of the Ross procedure and mechanical aortic valve replacement, there is a paucity of comparative data and only a single small randomized clinical trial directly comparing the 2 approaches. Of note, risk of bias in this randomized clinical trial was rated as high, and while its results are presented separately in this meta-analysis, they should be interpreted cautiously in light of the small number of patients included (20 per group) and short duration of follow-up (1 year in all patients). Thus, the results of this meta-analysis are based almost exclusively on observational studies, most of which had baseline differences between the groups. Potential hidden confounders and known patient selection factors therefore undoubtedly contributed to the observed differences. Although we prioritized data from matched/adjusted studies that attempted to correct for these baseline differences, only an adequately powered randomized clinical trial would be able to definitively eliminate residual confounding inherent in nonrandomized studies. As a result, this meta-analysis is by no means prescriptive, nor is it intended to demonstrate the superiority of one approach over the other in absolute terms. Furthermore, the median average follow-up in the included studies was 5.8 years, which is insufficient to adequately address the long-term impact of structural valve deterioration following the Ross procedure, which predominantly manifests into the second decade after surgery. Of note, the 2 studies with an average follow-up longer than 10 years included in this meta-analysis demonstrated superior survival in the Ross group.33,36 Nonetheless, given the wide variability in published rates of reintervention following the Ross procedure,44,46,60,61 further comparative studies with long-term follow-up are required to conclusively determine whether some of the benefits of the Ross operation over mechanical aortic valve replacement (eg, living substitute, optimal hemodynamics, avoidance of anticoagulation) are offset by an increased risk of late structural valve deterioration and reintervention. Recent evidence suggests that the root replacement technique may provide superior long-term survival and freedom from reintervention compared with the subcoronary implantation technique.62 However, the effect of the technique used in patients who undergo the Ross procedure could not be assessed in this meta-analysis. In addition, the included studies did not report adequacy of anticoagulation (ie, adherence to treatment and time in therapeutic range) in the mechanical aortic valve replacement group, which could have an impact on rates of thrombosis and bleeding. Only 7 of 18 included studies specified the type of mechanical valve models used, and none stratified reported outcomes by type, making it impossible to assess differences in valve-related complications between different models of mechanical prostheses. The recent introduction of less thrombogenic mechanical valves requiring a lower target international normalized ratio (eg, On-X valve)63 may affect comparisons with the Ross procedure in the future. It is also unclear how closely outcomes within each study might have been associated with individual surgeons’ experience and level of comfort with each of the 2 techniques. Finally, the large number of statistical tests conducted in this analysis increases the risk of type I errors. As a result, outcomes with P values that are only slightly lower than the threshold of .05 should be considered as hypothesis-generating only because P values have not been adjusted for multiple testing.
Conclusions
In summary, data from this meta-analysis suggest long-term benefits of the Ross procedure in terms of association with better survival, freedom from thromboembolic and hemorrhagic complications, and quality of life. These findings are in stark contrast with clinical practice, where the use of the Ross procedure remains extremely limited and confined to only a handful of centers worldwide.9,64 The suboptimal outcomes associated with the use of prosthetic valves in young and middle-aged adults,4 as well as the accumulating evidence suggesting improved long-term outcomes with the Ross procedure,65 make it important for clinicians to discuss the pulmonary autograft when considering options for aortic valve replacement in young and middle-aged adults. Findings from this meta-analysis highlight the urgent need for a large, prospective randomized clinical trial comparing long-term outcomes between these 2 interventions.
eFigure 1. Study selection
eFigure 2. Cardiac- and valve-related mortality
eFigure 3. Perioperative mortality
eFigure 4. Mean aortic valve gradient at follow-up
eFigure 5. Quality of life (physical health)
eFigure 6. Quality of life (psychological health)
eTable 1. PRISMA checklist
eTable 2. Quality assessment of the included randomized controlled trial (Cochrane Collaboration Risk of Bias)
eTable 3. Quality assessment of included observational studies using the Newcastle-Ottawa Scale
eTable 4. Baseline patient characteristics and operative data
eTable 5. Summary of secondary outcomes
Data Sharing Statement
References
- 1.Bonow RO, Leon MB, Doshi D, Moat N. Management strategies and future challenges for aortic valve disease. Lancet. 2016;387(10025):1312-1323. doi: 10.1016/S0140-6736(16)00586-9 [DOI] [PubMed] [Google Scholar]
- 2.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373(9667):956-966. doi: 10.1016/S0140-6736(09)60211-7 [DOI] [PubMed] [Google Scholar]
- 3.Isaacs AJ, Shuhaiber J, Salemi A, Isom OW, Sedrakyan A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg. 2015;149(5):1262-1269. doi: 10.1016/j.jtcvs.2015.01.052 [DOI] [PubMed] [Google Scholar]
- 4.Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med. 2017;377(19):1847-1857. doi: 10.1056/NEJMoa1613792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaser N, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50-69 years. Eur Heart J. 2016;37(34):2658-2667. doi: 10.1093/eurheartj/ehv580 [DOI] [PubMed] [Google Scholar]
- 6.Bouhout I, Stevens LM, Mazine A, et al. Long-term outcomes after elective isolated mechanical aortic valve replacement in young adults. J Thorac Cardiovasc Surg. 2014;148(4):1341-1346. doi: 10.1016/j.jtcvs.2013.10.064 [DOI] [PubMed] [Google Scholar]
- 7.Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet. 1967;2(7523):956-958. doi: 10.1016/S0140-6736(67)90794-5 [DOI] [PubMed] [Google Scholar]
- 8.Rabkin-Aikawa E, Aikawa M, Farber M, et al. Clinical pulmonary autograft valves: pathologic evidence of adaptive remodeling in the aortic site. J Thorac Cardiovasc Surg. 2004;128(4):552-561. doi: 10.1016/j.jtcvs.2004.04.016 [DOI] [PubMed] [Google Scholar]
- 9.Reece TB, Welke KF, O’Brien S, Grau-Sepulveda MV, Grover FL, Gammie JS. Rethinking the ross procedure in adults. Ann Thorac Surg. 2014;97(1):175-181. doi: 10.1016/j.athoracsur.2013.07.036 [DOI] [PubMed] [Google Scholar]
- 10.Klieverik LM, Takkenberg JJ, Bekkers JA, Roos-Hesselink JW, Witsenburg M, Bogers AJ. The Ross operation: a Trojan horse? Eur Heart J. 2007;28(16):1993-2000. doi: 10.1093/eurheartj/ehl550 [DOI] [PubMed] [Google Scholar]
- 11.Alassas K, Mohty D, Clavel MA, et al. Transcatheter versus surgical valve replacement for a failed pulmonary homograft in the Ross population. J Thorac Cardiovasc Surg. 2018;155(4):1434-1444. doi: 10.1016/j.jtcvs.2017.10.141 [DOI] [PubMed] [Google Scholar]
- 12.Gillespie MJ, McElhinney DB, Kreutzer J, et al. Transcatheter pulmonary valve replacement for right ventricular outflow tract conduit dysfunction after the Ross procedure. Ann Thorac Surg. 2015;100(3):996-1002. doi: 10.1016/j.athoracsur.2015.04.108 [DOI] [PubMed] [Google Scholar]
- 13.Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter pulmonary valve replacement with the Edwards Sapien system: the Toronto experience. JACC Cardiovasc Interv. 2015;8(14):1819-1827. doi: 10.1016/j.jcin.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123-e130. [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. http://handbook-5-1.cochrane.org/. Accessed August 8, 2018.
- 17.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed November 15, 2017.
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5. doi: 10.1186/1471-2288-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol. 2008;8:32. doi: 10.1186/1471-2288-8-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815-2834. doi: [DOI] [PubMed] [Google Scholar]
- 25.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doss M, Wood JP, Martens S, Wimmer-Greinecker G, Moritz A. Do pulmonary autografts provide better outcomes than mechanical valves? a prospective randomized trial. Ann Thorac Surg. 2005;80(6):2194-2198. doi: 10.1016/j.athoracsur.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 27.Jaggers J, Harrison JK, Bashore TM, Davis RD, Glower DD, Ungerleider RM. The Ross procedure: shorter hospital stay, decreased morbidity, and cost effective. Ann Thorac Surg. 1998;65(6):1553-1557. doi: 10.1016/S0003-4975(98)00288-4 [DOI] [PubMed] [Google Scholar]
- 28.Nötzold A, Hüppe M, Schmidtke C, Blömer P, Uhlig T, Sievers HH. Quality of life in aortic valve replacement: pulmonary autografts versus mechanical prostheses. J Am Coll Cardiol. 2001;37(7):1963-1966. doi: 10.1016/S0735-1097(01)01267-0 [DOI] [PubMed] [Google Scholar]
- 29.Schmidtke C, Hüppe M, Berndt S, Nötzold A, Sievers HH. [Quality of life after aortic valve replacement: self-management or conventional anticoagulation therapy after mechanical valve replacement plus pulmonary autograft]. Z Kardiol. 2001;90(11):860-866. doi: 10.1007/s003920170084 [DOI] [PubMed] [Google Scholar]
- 30.Concha M, Aranda PJ, Casares J, et al. Prospective evaluation of aortic valve replacement in young adults and middle-aged patients: mechanical prosthesis versus pulmonary autograft. J Heart Valve Dis. 2005;14(1):40-46. [PubMed] [Google Scholar]
- 31.Mokhles MM, Körtke H, Stierle U, et al. Survival comparison of the Ross procedure and mechanical valve replacement with optimal self-management anticoagulation therapy: propensity-matched cohort study. Circulation. 2011;123(1):31-38. doi: 10.1161/CIRCULATIONAHA.110.947341 [DOI] [PubMed] [Google Scholar]
- 32.Andreas M, Wiedemann D, Seebacher G, et al. The Ross procedure offers excellent survival compared with mechanical aortic valve replacement in a real-world setting. Eur J Cardiothorac Surg. 2014;46(3):409-413. doi: 10.1093/ejcts/ezt663 [DOI] [PubMed] [Google Scholar]
- 33.Mazine A, David TE, Rao V, et al. Long-term outcomes of the Ross procedure versus mechanical aortic valve replacement: propensity-matched cohort study. Circulation. 2016;134(8):576-585. doi: 10.1161/CIRCULATIONAHA.116.022800 [DOI] [PubMed] [Google Scholar]
- 34.Sharabiani MT, Dorobantu DM, Mahani AS, et al. Aortic valve replacement and the Ross operation in children and young adults. J Am Coll Cardiol. 2016;67(24):2858-2870. doi: 10.1016/j.jacc.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 35.Bouhout I, Noly PE, Ghoneim A, et al. Is the Ross procedure a riskier operation? perioperative outcome comparison with mechanical aortic valve replacement in a propensity-matched cohort. Interact Cardiovasc Thorac Surg. 2017;24(1):41-47. doi: 10.1093/icvts/ivw325 [DOI] [PubMed] [Google Scholar]
- 36.Buratto E, Shi WY, Wynne R, et al. Improved survival after the Ross procedure compared with mechanical aortic valve replacement. J Am Coll Cardiol. 2018;71(12):1337-1344. doi: 10.1016/j.jacc.2018.01.048 [DOI] [PubMed] [Google Scholar]
- 37.Klieverik LM, Noorlander M, Takkenberg JJ, et al. Outcome after aortic valve replacement in young adults: is patient profile more important than prosthesis type? J Heart Valve Dis. 2006;15(4):479-487. [PubMed] [Google Scholar]
- 38.Zsolt N, Watterson KG. Ross procedure versus mechanical aortic valve replacement in young adults [in Hungarian]. Magy Seb. 2008;61(suppl):23-27. doi: 10.1556/MaSeb.61.2008.Suppl.7 [DOI] [PubMed] [Google Scholar]
- 39.Akhyari P, Bara C, Kofidis T, Khaladj N, Haverich A, Klima U. Aortic root and ascending aortic replacement. Int Heart J. 2009;50(1):47-57. doi: 10.1536/ihj.50.47 [DOI] [PubMed] [Google Scholar]
- 40.Aicher D, Holz A, Feldner S, Köllner V, Schäfers HJ. Quality of life after aortic valve surgery: replacement versus reconstruction. J Thorac Cardiovasc Surg. 2011;142(2):e19-e24. doi: 10.1016/j.jtcvs.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 41.Heuvelman HJ, Arabkhani B, Cornette JM, et al. Pregnancy outcomes in women with aortic valve substitutes. Am J Cardiol. 2013;111(3):382-387. doi: 10.1016/j.amjcard.2012.09.035 [DOI] [PubMed] [Google Scholar]
- 42.Basude S, Trinder J, Caputo M, Curtis SL. Pregnancy outcome and follow-up cardiac outcome in women with aortic valve replacement. Obstet Med. 2014;7(1):29-33. doi: 10.1177/1753495X13514382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zacek P, Holubec T, Vobornik M, et al. Quality of life after aortic valve repair is similar to Ross patients and superior to mechanical valve replacement: a cross-sectional study. BMC Cardiovasc Disord. 2016;16:63. doi: 10.1186/s12872-016-0236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David TE, David C, Woo A, Manlhiot C. The Ross procedure: outcomes at 20 years. J Thorac Cardiovasc Surg. 2014;147(1):85-93. doi: 10.1016/j.jtcvs.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 45.Sievers HH, Stierle U, Charitos EI, et al. A multicentre evaluation of the autograft procedure for young patients undergoing aortic valve replacement: update on the German Ross Registry. Eur J Cardiothorac Surg. 2016;49(1):212-218. doi: 10.1093/ejcts/ezv001 [DOI] [PubMed] [Google Scholar]
- 46.Martin E, Mohammadi S, Jacques F, et al. Clinical outcomes following the Ross procedure in adults: a 25-year longitudinal study. J Am Coll Cardiol. 2017;70(15):1890-1899. doi: 10.1016/j.jacc.2017.08.030 [DOI] [PubMed] [Google Scholar]
- 47.Mastrobuoni S, de Kerchove L, Solari S, et al. The Ross procedure in young adults: over 20 years of experience in our Institution. Eur J Cardiothorac Surg. 2016;49(2):507-512. doi: 10.1093/ejcts/ezv053 [DOI] [PubMed] [Google Scholar]
- 48.Skillington PD, Mokhles MM, Takkenberg JJ, et al. The Ross procedure using autologous support of the pulmonary autograft: techniques and late results. J Thorac Cardiovasc Surg. 2015;149(2)(suppl):S46-S52. doi: 10.1016/j.jtcvs.2014.08.068 [DOI] [PubMed] [Google Scholar]
- 49.da Costa FD, Takkenberg JJ, Fornazari D, et al. Long-term results of the Ross operation: an 18-year single institutional experience. Eur J Cardiothorac Surg. 2014;46(3):415-422. doi: 10.1093/ejcts/ezu013 [DOI] [PubMed] [Google Scholar]
- 50.Yacoub MH, Klieverik LM, Melina G, et al. An evaluation of the Ross operation in adults. J Heart Valve Dis. 2006;15(4):531-539. [PubMed] [Google Scholar]
- 51.Treasure T, King A, Hidalgo Lemp L, Golesworthy T, Pepper J, Takkenberg JJ. Developing a shared decision support framework for aortic root surgery in Marfan syndrome. Heart. 2018;104(6):480-486. doi: 10.1136/heartjnl-2017-311598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.David TE, Woo A, Armstrong S, Maganti M. When is the Ross operation a good option to treat aortic valve disease? J Thorac Cardiovasc Surg. 2010;139(1):68-73. doi: 10.1016/j.jtcvs.2009.09.053 [DOI] [PubMed] [Google Scholar]
- 53.Mazine A, Ghoneim A, El-Hamamsy I. The Ross procedure: how I teach it. Ann Thorac Surg. 2018;105(5):1294-1298. doi: 10.1016/j.athoracsur.2018.01.048 [DOI] [PubMed] [Google Scholar]
- 54.David TE. Aortic valve replacement with pulmonary autograft: subcoronary and aortic root inclusion techniques. Oper Tech Thorac Cardiovasc Surg. 2012;17(1):27-40. doi: 10.1053/j.optechstcvs.2011.09.002 [DOI] [Google Scholar]
- 55.Mazine A, El-Hamamsy I, Ouzounian M. The Ross procedure in adults: which patients, which disease? Curr Opin Cardiol. 2017;32(6):663-671. doi: 10.1097/HCO.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 56.Bashir M, Harky A, Fok M, et al. Acute type A aortic dissection in the United Kingdom: Surgeon volume-outcome relation. J Thorac Cardiovasc Surg. 2017;154(2):398-406.e1. doi: 10.1016/j.jtcvs.2017.02.015 [DOI] [PubMed] [Google Scholar]
- 57.Hughes GC, Zhao Y, Rankin JS, et al. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg. 2013;145(1):166-170. doi: 10.1016/j.jtcvs.2011.10.094 [DOI] [PubMed] [Google Scholar]
- 58.El-Hamamsy I, Poirier NC. What is the role of the Ross procedure in today’s armamentarium? Can J Cardiol. 2013;29(12):1569-1576. doi: 10.1016/j.cjca.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 59.Forcillo J, Cikirikcioglu M, Poirier N, El-Hamamsy I. The Ross procedure: total root technique. Multimed Man Cardiothorac Surg. 2014;2014:mmu018. doi: 10.1093/mmcts/mmu018 [DOI] [PubMed] [Google Scholar]
- 60.Mokhles MM, Rizopoulos D, Andrinopoulou ER, et al. Autograft and pulmonary allograft performance in the second post-operative decade after the Ross procedure: insights from the Rotterdam Prospective Cohort Study. Eur Heart J. 2012;33(17):2213-2224. doi: 10.1093/eurheartj/ehs173 [DOI] [PubMed] [Google Scholar]
- 61.Sievers HH, Stierle U, Petersen M, et al. Valve performance classification in 630 subcoronary Ross patients over 22 years. J Thorac Cardiovasc Surg. 2018;156(1):79-86.e2. doi: 10.1016/j.jtcvs.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 62.Berdajs DA, Muradbegovic M, Haselbach D, et al. Ross procedure: is the root replacement technique superior to the sub-coronary implantation technique? Long-term results. Eur J Cardiothorac Surg. 2014;46(6):944-951. doi: 10.1093/ejcts/ezu176 [DOI] [PubMed] [Google Scholar]
- 63.Puskas J, Gerdisch M, Nichols D, et al. ; PROACT Investigators . Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg. 2014;147(4):1202-1210. doi: 10.1016/j.jtcvs.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 64.Yacoub MH, El-Hamamsy I, Sievers HH, et al. Under-use of the Ross operation: a lost opportunity. Lancet. 2014;384(9943):559-560. doi: 10.1016/S0140-6736(14)61090-4 [DOI] [PubMed] [Google Scholar]
- 65.El-Hamamsy I, Eryigit Z, Stevens LM, et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376(9740):524-531. doi: 10.1016/S0140-6736(10)60828-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study selection
eFigure 2. Cardiac- and valve-related mortality
eFigure 3. Perioperative mortality
eFigure 4. Mean aortic valve gradient at follow-up
eFigure 5. Quality of life (physical health)
eFigure 6. Quality of life (psychological health)
eTable 1. PRISMA checklist
eTable 2. Quality assessment of the included randomized controlled trial (Cochrane Collaboration Risk of Bias)
eTable 3. Quality assessment of included observational studies using the Newcastle-Ottawa Scale
eTable 4. Baseline patient characteristics and operative data
eTable 5. Summary of secondary outcomes
Data Sharing Statement