Key Points
Question
Can a specific mutation in myeloid differentiation primary response gene (MYD88 L265P) be detected by a sensitive droplet digital polymerase chain reaction (ddPCR) in minimally invasive aqueous humor aspirates of patients with vitreoretinal lymphoma (VRL)?
Findings
In this cohort study of 63 patients (23 with VRL and 40 with uveitis), 74% of patients with VRL harbored the MYD88 L265P mutation. This mutation was detectable by ddPCR in both vitreous fluid (sensitivity, 75%) and aqueous humor (sensitivity, 67%), showing a high concordance rate (89%) in 12 paired vitreous fluid–aqueous humor samples.
Meaning
These findings suggest MYD88 L265P detection by ddPCR is feasible in small volumes of accessible aqueous humor from patients with VRL.
This cohort study assesses whether droplet digital polymerase chain reaction testing could accurately detect MYD88 L265P mutation in the aqueous humor and vitreous fluid of patients with vitreoretinal lymphoma.
Abstract
Importance
The diagnostic workup of patients suspected of having vitreoretinal lymphoma (VRL) is primarily based on vitreous fluid analysis, including the recently emerging myeloid differentiation primary response gene 88 (MYD88) mutation analysis. Aqueous humor paracentesis is a relatively less invasive and safer procedure than taking vitreous fluid specimens, and aqueous humor–based MYD88 mutation analysis would provide an additional liquid biopsy tool to diagnose and monitor patients with VRL.
Objective
To investigate whether the detection of MYD88 L265P by highly sensitive droplet digital polymerase chain reaction (ddPCR) is feasible in the vitreous fluid and aqueous humor of patients with VRL.
Design, Setting, and Participants
This cohort study includes aqueous humor and vitreous fluid samples from patients with VRL who were treated at the University Medical Center Utrecht, in Utrecht, the Netherlands, from August 2005 to August 2017. Ocular fluids were randomized and masked before MYD88 L265P analysis, which was performed using an in-house validated ddPCR platform. Patients with uveitis were included as a comparison group.
Main Outcomes and Measures
The presence of MYD88 L265P mutation detected by ddPCR in AH and VF.
Results
The study included 96 samples from 63 individuals, including 23 patients with VRL (of whom 10 were female and 13 male, with a mean [SD] age of 72 [7.3] years) and 40 individuals with uveitis (of whom 23 were female and 17 male, with a mean [SD] age of 58 [20.9] years). In 17 of 23 patients with VRL (74%), MYD88 L265P was detected; it was not detected in any of the patients with uveitis. It was detectable in both vitreous fluid and aqueous humor samples. In the paired samples, the mutation was detected in 8 of 9 aqueous humor samples (89%) of the MYD88 L265P–positive vitreous fluid samples. In vitreous fluid, the MYD88 ddPCR test showed a sensitivity of 75% (95% CI, 50%-92%) and a positive predictive value of 100%; in aqueous humor, sensitivity was 67% (95% CI, 42%-92%), and positive predictive value was 100%. Specificity was 100% in both fluids. After treatment, the mutation was no longer detectable in any ocular fluids.
Conclusions and Relevance
The high concordance between aqueous humor and vitreous fluid samples suggests that use of the easily accessible aqueous humor is nearly as informative as vitreous fluid in the identification of key somatic mutations in patients with VRL. This approach may provide an additional minimally invasive tool for accurate diagnosis, detection of recurrence, and monitoring of treatment.
Introduction
Vitreoretinal lymphoma (VRL) is a rare but potentially fatal eye condition for which early diagnosis is crucial to ensuring appropriate treatment and improving the unfavorable prognosis. However, the notoriously insidious course of this disease, as well as its masquerading presentation (eg, as intraocular inflammation), often hamper accurate diagnosis.1 A unique hallmark of VRL is the presence in approximately 75% of cases of an oncogenic hotspot mutation in myeloid differentiation primary response gene 88 (MYD88), which changes the amino acid leucine to proline at amino acid position 265 (L265P).2 Since MYD88 L265P is an oncogenic mutation not present in nonneoplastic proliferations,2,3 detection of this tumor-specific mutation provides definite evidence of a malignant neoplasm (ie, VRL). In other words, patients who are clinically suspected of having VRL and who present with MYD88 L265P in ocular tissues can be definitively diagnosed with VRL and receive prompt chemotherapy treatment.2 In principle, the diagnosis of VRL is established by the detection of malignant cells in ocular fluids (routinely in vitreous fluid [VF]) by cytomorphologic evaluation, supported by the use of flow cytometry or immunoglobulin gene rearrangement analysis to expose clonal populations.4,5,6 Unfortunately, the presence of clonal cell populations is not limited to VRL, and more importantly, cytomorphologic evaluation often fails to detect lymphoma cells because of low cellularity or cell lysis during VF biopsies.7,8,9
Although a vitreous tap or diagnostic vitrectomy is a relatively safe procedure, it can be associated with serious complications, such as vitreoretinal traction or hemorrhage, retinal detachment, and cataract formation in a later stage.10 Aqueous humor (AH) sampling is a more safe and simple procedure with a lower risk of adverse reactions.11,12 This makes AH paracentesis less invasive than collection of VF and thus provides a highly attractive medium for (repeated) sampling to diagnose or monitor patients with VRL.
However, sample volumes of AH are usually even smaller than VF and reliable molecular diagnostics are highly challenging for conventional methods because of low DNA yield. Therefore, mutation analysis in AH demands a highly sensitive detection strategy, suitable for low-volume and/or low DNA content. Droplet digital polymerase chain reaction (ddPCR) is such a technique, because it is not restricted by DNA input.13 This approach has already been successfully applied for mutation analysis in similar low-cellularity liquid biopsies, such as cerebrospinal fluid.14
Using this innovative technique, we tested the feasibility of MYD88 L265P detection in AH and VF samples from patients with VRL or uveitis. In addition, we assessed the added value of MYD88 L265P detection compared with routine biomarkers used in the current diagnostic workup of VRL.
Methods
Sample Selection and Case Characteristics
This study was conducted in accordance with the Helsinki Declaration and was approved by the institutional review board of the University Medical Center Utrecht, in Utrecht, the Netherlands. Use of anonymous or deidentified leftover diagnostic material for scientific purposes is part of the standard treatment agreement with patients, and therefore ethics approval and informed consent procedure are not required according to Dutch legislation (the Medical Research Involving Human Subjects Act).
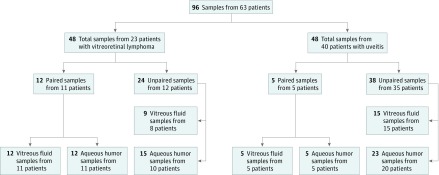
For this study, AH and VF samples collected between August 2005 and August 2017 were taken for routine diagnostic workups; 12.5 μL of each of these was used for ddPCR analysis. Twelve paired AH and VF samples from 11 patients with VRL and 5 paired AH and VF samples from 5 patients with uveitis were simultaneously collected during diagnostic vitrectomy, except for 1 pair, which was the remainder of diagnostic AH and VF samples collected during a diagnostic workup period that lasted 5 months. No treatment was started during this period.
Unpaired samples included those samples of which only VF or AH was available for analysis. Several such samples came from the same patients but were not considered paired samples. Nine VF samples were collected from 8 patients with VRL, and 15 AH samples were collected from 10 patients with VRL; of the total group of 12 patients, 6 patients contributed both VF and AH samples, of which VF and AH were collected from the same eye in 5 patients (eTable 1 in the Supplement). Twenty-three AH samples were collected from 20 patients with uveitis, and 15 samples of VF were collected from 15 patients with uveitis; these groups were nonoverlapping.
Diagnosis of VRL was confirmed for all patients with VRL during the course of the disease. This was based on cytomorphology or immunocytochemistry of vitreous cells, histopathological investigation of retinal biopsies, or proven central nervous system lymphoma in patients with ocular findings suspicious of lymphoma. None of the patients had received any treatment for VRL (eg, intravitreal chemotherapy, systemic chemotherapy, or local irradiation) at the time of sample collection. Available clinical parameters including cytomorphology and flow cytometry were collected for comparison. We calculated sensitivity, specificity, and positive predictive value of these clinical parameters and MYD88 ddPCR using SPSS version 21.0 (IBM Corporation). The 95% confidence intervals of the observed performance statistics were estimated using bootstrap sampling (n = 1000).
All samples from patients with uveitis were the remainders of samples taken for diagnostic purposes. All uveitis entities were analyzed by ddPCR and Goldmann-Witmer coefficient, as described previously,15 and classified according to the Standardization of Uveitis Nomenclature Working Group.16 Specific etiologies of uveitis cases are outlined in eTable 2 in the Supplement. All ocular samples were immediately stored at −80°C for further analysis.
In addition, we had the opportunity to compare the presence of MYD88 mutation in paired AH and VF samples of 5 patients with VRL in which intravitreal, systemic, or methotrexate-based treatment was started during the period of sample collection. We also observed this in 4 AH samples of 1 patient with VRL.
DNA Isolation
AH and Undiluted VF Samples: DNA Isolation of Total Nucleic Acids
All ocular fluid samples were isolated using the MagNa Pure 96 DNA and Viral NA Large Volume Kit (Roche Diagnostics), according to the manufacturer’s instructions. A total of 12.5 μL of ocular fluid was eluted in 100 μL (with a dilution of 1:8). After isolation, DNA concentrations were measured by Qubit (ThermoFischer Scientific).
Diluted VF Samples: DNA Isolation of Cell Pellet (Cellular DNA) and Supernatant (Cell-Free DNA)
We had the opportunity to test MYD88 L265P ddPCR in 3 available samples of diluted vitreous aspiration fluid that had been obtained during diagnostic vitrectomy; these revealed cytomorphological detection of tumor cells in the diluted vitreous humor. An undiluted and diluted mixture of VF and infusion fluid were available from 1 patient, while in the other patients only diluted material was available for molecular analysis (eFigure 1 in the Supplement). These VF samples were centrifuged for 10 minutes at 3000 rpm and cell pellets were separated from the supernatant. The limited supernatant from the undiluted VF was discarded, while 10 mL of supernatant of the diluted VF (total volume, >100 mL) was used for cell-free DNA isolation. Cell-free DNA isolation of the supernatant was performed with the Quick-cfDNA Serum and Plasma Kit (Zymo Research). Purified DNA was eluted in 35 μL.
The isolation of DNA from cell pellets of the diluted and undiluted VF samples was achieved by direct lysis in 30 μL of 50mM TRIS/hydrochloric acid buffer and 10 μL of proteinase K (10 mg/mL), after which the mixture is heated for 1 hour at 56°C, cooked for 10 minutes, and centrifuged for 2 minutes at 8000 rpm.14
Droplet Digital PCR for MYD88 L265P Detection
Polymerase chain reaction tests were performed with QX200 Droplet Digital PCR (Bio-Rad Laboratories Inc) according to the manufacturer’s instructions on randomized and masked samples, and the data were analyzed by QuantaSoft Software (version 1.7.4.0917; Bio-Rad Laboratories Inc), as described elsewhere.14 Ocular fluid samples were measured in duplo with a sample input of 4 and 8 μL of the DNA eluate, including positive and negative controls (MilliQ; Millipore Corporation). A validated cutoff threshold for detection was maintained at 2 mutant droplets.14
Flow Cytometry
Flow cytometry was performed on VF samples according to standard diagnostic workflow procedures. All VF samples were collected in fetal calf serum and washed in phosphate-buffered saline, after which cells were stained with CD3, CD4, CD5, CD8, CD10, CD14, CD19, CD20, CD45, κ, and λ in phosphate-buffered saline, supplemented with human serum albumin and azide. All monoclonal antibodies were from Beckman Dickinson except for CD45 (Life Technologies). After staining and incubation with lysing solution, cells were washed and analyzed in phosphate-buffered saline or hepatocyte-specific antigen on a BD FACSCanto cytometer (Beckman Dickinson) using BD FACSDiva software version 8 (Beckman Dickinson).
Results
For this study, a total of 41 VF and 55 AH samples from 23 patients with VRL and 40 patients with uveitis were included (Figure). Clinical and demographic details of patients investigated in this study are depicted in Table 1. Briefly, the study included 10 female patients and 13 male patients with VRL (mean [SD] age, 72 [7.3] years) and 23 female patients and 17 male patients with uveitis (mean [SD] age of 58 [20.9] years). Ophthalmological findings and demographics of the 23 patients with VRL are outlined in eTable 3 in the Supplement.
Figure. Flow Diagram for Sample Selection and Inclusion.
Table 1. Descriptive Characteristics of Patients With Vitreoretinal Lymphoma (VRL) and Uveitis.
| Characteristics | Patients With VRL | Patients With Uveitis |
|---|---|---|
| Total, No. (%) | 23 (37) | 40 (63) |
| Samples, No. (patients, No.) | ||
| Vitreous fluida | 21 (19) | 20 (20) |
| Aqueous humorb | 27 (21) | 28 (25) |
| Paired pretreatment samplesc | 12 (11) | 5 (5) |
| Age, y | ||
| Mean | 72 | 58 |
| Median (range) | 73 (56-83) | 61 (21-89) |
| Sex, No. (%) | ||
| Male | 13 (45) | 17 (42) |
| Female | 10 (30) | 23 (58) |
This total includes 12 samples from 11 patients with VRL (from the paired sample group) and 9 samples from 8 patients with VRL (from the unpaired sample group), as well as 5 samples from 5 patients with uveitis (from the paired sample group) and 15 samples from 15 patients with uveitis (from the unpaired sample group).
This total includes 12 samples from 11 patients with VRL (from the paired sample group) and 15 samples from 10 patients with VRL (from the unpaired sample group), as well as 5 samples from 5 patients with uveitis (from the paired sample group) and 23 samples from 20 patients with uveitis (from the unpaired sample group).
Included aqueous humor and vitreous fluid samples collected within a 5-month span.
MYD88 L265P Detection in AH Samples of Patients With VRL
Sixteen of 21 VF biopsies (76%) and 11 of 27 AH samples (41%) were positive for MYD88 L265P. The MYD88 L265P hotspot mutation was detected in 17 of 23 patients with VRL (74%) and none of the 40 patients with uveitis (sensitivity, 73.9% [95% CI, 57%-91%]; specificity, 100%; Table 2). No discrepancy in test outcome for either AH or VF samples was observed in cases with multiple samples available for analysis.
Table 2. MYD88 L265P Analysis ddPCR in Vitreous Fluid and Aqueous Humor of Patients With Vitreoretinal Lymphoma or Uveitis.
| Result | Samples, No. (%) | |
|---|---|---|
| Patients With Vitreoretinal Lymphoma | Patients With Uveitis | |
| Vitreous fluid | ||
| Patients | 19 (49) | 20 (51) |
| Total samples | 21 (51) | 20 (49) |
| Positive for MYD88 L265P | 16 (76) | 0 |
| Negative for MYD88 L265P | 5 (24) | 20 (100) |
| Aqueous humor | ||
| Patients | 21 (46) | 25 (54) |
| Total samples | 27 (49) | 28 (51) |
| Positive for MYD88 L265P | 11 (41) | 0 |
| Negative for MYD88 L265P | 16 (59) | 28 (100) |
Abbreviations: ddPCR, droplet digital polymerase chain reaction; MYD88, myeloid differentiation primary response gene 88.
To determine the feasibility of MYD88 L265P detection in AH samples, we compared the available paired VF and AH samples (12 samples from 11 patients with VRL and 5 samples from 5 patients with uveitis) for concordance of mutation detection (Table 3). We detected MYD88 L265P in 9 of 12 VF samples (75%) of patients with VRL, and none of the 5 patients with uveitis, which closely reflected the distribution within the entire study population. Of the 9 samples from patients with VRL who were positive for MYD88 L265P, ddPCR was able to detect the mutation in nearly all paired AH samples (8 of 9 [89%]). The only discrepant MYD88 L265P–negative AH sample harbored very little DNA; only 6 wild-type droplets were detected by ddPCR. In this paired analysis, MYD88 L265P detection showed a sensitivity of 75% (95% CI, 50%-92%) and positive predictive value of 100% in VF samples, vs a sensitivity of 67% (95% CI, 42%-92%) and a positive predictive value of 100% in AH samples. Specificity was 100% for both ocular fluids.
Table 3. Analysis of MYD88 L265P by ddPCR of 12 Paired Vitreous Fluid and Aqueous Humor Samples of 11 Patients With Vitreoretinal Lymphoma (VRL) and 5 Paired Samples of 5 Patients With Uveitis.
| VRL | Aqueous Humor Samples, No. (%) | |||
|---|---|---|---|---|
| Patients With Vitreoretinal Lymphoma | Patients With Uveitis | |||
| Positive for MYD88 L265P | Negative for MYD88 L265P | Positive for MYD88 L265P | Negative for MYD88 L265P | |
| Positive for MYD88 L265P | 8 (89) | 1 (11) | 0 | 0 |
| Negative for MYD88 L265P | 0 | 3 (100) | 0 | 5 (100) |
Abbreviations: ddPCR, droplet digital polymerase chain reaction; MYD88, myeloid differentiation primary response gene 88.
To determine the association of therapy with mutation detection, we monitored the mutation in 5 MYD88 L265P–positive patients with VRL after intravitreal or systemic treatment for a median of 2.5 months (range, 1 week to 4.0 months). We found that the mutation was no longer detectable in any of the ocular fluids collected after treatment (eTable 1 in the Supplement). Characteristics and test results of each included patient with VRL are outlined in eTable 4 in the Supplement.
MYD88 L265P Performance in VF and AH Samples vs Standard Diagnostic Tests
To determine performance of MYD88 testing in the context of other laboratory tests, we compared MYD88 results from investigated patients with routine laboratory tests that are part of the diagnostic workup for VRL in the Netherlands (Table 4). Cytomorphology data and MYD88 L265P data for the same sample were present for 20 VF and 12 AH samples of patients with VRL. Cytomorphology was positive in 6 of 20 VF samples (30%) and 4 of 12 AH samples (33%), while MYD88 L265P was positive in 15 of 20 VF samples (75%) and 7 of 12 AH samples (58%) of patients with VRL (Table 4). Although MYD88 L265P status was about twice as often positive in VF and AH samples as in cytomorphology, this was not sufficient to detect all lymphoma cases. In VF samples, MYD88 L265P testing had a sensitivity of 75% (95% CI, 55%-90%) compared with a sensitivity of 30% (95% CI, 10%-50%) for VF cytomorphology.
Table 4. Analysis of MYD88 L265P in Samples of Patients With Vitreoretinal Lymphoma Compared With Routine Laboratory Tests in Diagnostic Workup of Vitreoretinal Lymphoma.
| Variable | Samples, No. (%) | |||
|---|---|---|---|---|
| Patients With Vitreoretinal Lymphoma | Patients With Uveitis | |||
| Positive for MYD88 L265P | Negative for MYD88 L265P | Positive for MYD88 L265P | Negative for MYD88 L265P | |
| Vitreous fluid | ||||
| Cytomorphologya | ||||
| Positive for MYD88 L265P | 5 (83) | 1 (17) | 0 | 0 |
| Negative for MYD88 L265P | 10 (71) | 4 (29) | 0 | 9 (100) |
| Flow cytometrya | ||||
| Positive for MYD88 L265P | 7 (70) | 3 (30) | 0 | 0 |
| Negative for MYD88 L265P | 5 (71) | 2 (29) | 0 | 12 (100) |
| Aqueous humorb | ||||
| Cytomorphologya | ||||
| Positive for MYD88 L265P | 3 (75) | 1 (25) | 0 | 0 |
| Negative for MYD88 L265P | 4 (50) | 4 (50) | 0 | 1 (100) |
| Flow cytometrya | ||||
| Positive for MYD88 L265P | 5 (63) | 3 (38) | 0 | 0 |
| Negative for MYD88 L265P | 4 (80) | 1 (20) | 0 | 2 (100) |
Abbreviations: ddPCR, droplet digital polymerase chain reaction; MYD88, myeloid differentiation primary response gene 88.
Samples of vitreous fluid used for cytomorphology came from 20 patients, vitreous fluid samples for flow cytometry were from 18 patients, aqueous humor samples for cytomorphology were from 18 patients, and aqueous humor samples for flow cytometry were from 18 patients. In several cases, multiple samples were used from single patients.
Since cytomorphology and flow cytometry are usually not performed on aqueous humor samples, the performance of MYD88 analysis in aqueous humor was compared with the simultaneous evaluation of cytomorphology and flow cytrometry in vitreous fluid. An overview of test results per patient are outlined in eTable 5 in the Supplement.
Because AH cytomorphology is rarely performed, we compared MYD88 L265P in AH samples with simultaneously performed VF cytomorphology. In AH samples, MYD88 L265P analysis demonstrated a sensitivity of 58% (95% CI, 33%-83%), compared with 33% (95% CI, 8%-58%) for VF cytomorphology. Similar relative performance of MYD88 L265P testing was observed compared with detection of clonal B-cell populations by flow cytometry of VF samples (Table 4; eTable 5 in the Supplement). Among samples with parallel MYD88 L265P and flow cytometry data, 12 of 17 VF samples (71%) and 9 of 13 AH samples (69%) were positive for MYD88 L265P, while flow cytometry was positive in 10 of 17 VF samples (59%) and 8 of 13 AH samples (62%).
MYD88 L265P Detection in Cell-Free DNA of Diluted VF Samples
Finally, to further explore the high-detection sensitivity of the ddPCR platform and to provide an additional avenue (eg, another type of ocular fluid) for MYD88 L265P detection, we analyzed diluted VF samples from 3 patients suspected to have VRL. We found that MYD88 L265P was detectable in cell-free DNA from all highly diluted (>100 times) VF samples (eFigure 2 in the Supplement).
Discussion
In this study, we used ultrasensitive ddPCR, which in principle is not limited by DNA input. In addition, the absolute mutation frequency can be quantified by ddPCR, which enables unique and minimally invasive follow-up of patients with VRL for monitoring of treatment response or detection of early relapse.
Recently, we devised and validated a robust MYD88 L265P ddPCR assay for liquid biopsy analysis.14 Using this ddPCR approach, we demonstrated that MYD88 L265P can be detected in AH samples and that results are highly comparable with VF analysis: of the 12 paired samples from patients with VRL, there was only 1 discrepant case in which MYD88 L265P could not be detected in the paired AH sample. As expected, this MYD88 L265P–negative AH sample harbored only very little DNA. Three patients who were negative for MYD88 L265P in VF samples had sufficient DNA for a valid result as indicated by a mean of 661 wild-type droplets (range, 173-1476 droplets) detected by ddPCR.
No single diagnostic test has adequate sensitivity or specificity to accurately detect VRL. Therefore, an ensemble of laboratory tests is currently used to aid in the workup of patients whose clinical presentation suggested a high suspicion of VRL.5,7,17,18,19,20 In addition to VF analysis, a fine-needle aspiration biopsy of the retina can be performed when there are sizable subretinal infiltrates.21 However, a retinal biopsy is performed only in highly suspect cases when analysis of ocular fluids is inconclusive because of the considerable risk for surgical complications (eg, hemorrhage, infection, and retinal detachment). The advantage of using a VF biopsy for the diagnostic workup of VRL has been thoroughly reviewed22,23 and has dramatically improved the ability to identify patients with VRL. Nonetheless, a VF biopsy is not always feasible, because abnormalities may not always be clearly discernable. In addition, a biopsy may be difficult because of the location of the lymphoma. Thus, there remains a need to further reduce the common diagnostic delay caused by the frequent atypical presentation, while trying to minimize burden for the patient.
Aqueous humor paracentesis is considerably less invasive with a low risk of complications.11,12 This makes AH not only attractive for additional, minimally invasive diagnostic testing, but also for monitoring of treatment response or disease relapse. Although anatomically AH is more distant from the retina, which is the affected tissue in VRL, the advent of highly sensitive molecular technologies facilitate AH-based detection of VRL. Previously, we4 and others24,25,26,27 have shown that AH contains clinically useful nucleic acids (eg, tumor DNA) and protein compositions that reflect signatures found in VF, underpinning the robustness of AH profiling to monitor vitreoretinal diseases.
The detection of MYD88 L265P in VF has been reported in approximately 70% of cases of VRL,2,28 which is in line with our observations. Building on this observation, the aim of this study was to provide proof of concept for AH-based mutation analysis for detection of VRL. Recently, a proof-of-concept study for AH-based mutation analysis in 3 patients with retinoblastoma demonstrated the potential of AH to aid in diagnosis of malignant conditions of the eye.26 However, all included patients had advanced tumors and therefore detectable tumor DNA.
In contrast, hardly any of the AH samples in our study contained detectable DNA by standard fluorometric quantification. This has hitherto been a major limitation for molecular analysis of AH in general, which usually harbors very few cells and little DNA.29 Nonetheless, we were able to detect MYD88 L265P by ddPCR in 89% of the AH samples found to be positive for MYD88 L265P in biopsies of VF.
The sensitivity and specificity of MYD88 ddPCR in AH samples compared with other laboratory tests was proportional to the performance of MYD88 analysis and these parameters in VF samples. Therefore, MYD88 analysis in AH samples may provide complementary or relatively comparable performance with VF samples when compared with other laboratory tests.
We would like to emphasize that the detection of malignant cells by cytomorphology is central to the diagnosis of VRL, but cytomorphology has a low sensitivity for VRL detection. Indeed, we demonstrated that about half of the cases with a MYD88 L265P mutation were initially negative by cytomorphology, while no false-positive cases were found in this study. This is in line with the observation that, in adults, the oncogenic L265P mutation in MYD88 is restricted to hematological malignant conditions and presents in nearly all patients with VRL (74% in this study). The clinical relevance of this mutation is further supported by the unique susceptibility of patients harboring the MYD88 L265P mutation (and not in MYD88 wild-type cases) to the tyrosine kinase inhibitor ibrutinib.30 Because not all cases carry the MYD88 mutation, ddPCR for this hotspot mutation cannot substitute cytomorphologic evaluation.
To explore future possibilities for MYD88 analysis in other clinical samples, we tested MYD88 L265P detection in diluted vitreous aspiration fluid obtained during diagnostic vitrectomy. Although these cases probably represent patients with relatively advanced tumors (as was made clear by the easily detectable tumor cells by cytomorphology), we demonstrated feasibility of ddPCR for MYD88 L265P detection on cell-free DNA in VF samples diluted more than 100 times, which provides rationale for using this material when limited quantities of undiluted VF are available for testing.
Limitations
This was an exploratory performance analysis, and we deliberately withheld from detailed comparison of performance statistics of other laboratory tests in VF and AH samples. Although outside the scope of this study, this can only be examined in detail when these tests (eg, flow cytometry or cytomorphology) are performed in both AH and VF samples and a sufficient number of paired samples are available, which was limited in the current study. Therefore, these results need to be validated in subsequent larger cohorts of samples and patients, most likely via an international consortium, which will aid in providing more robust estimates of the accuracy and added value of MYD88 testing in AH and VF samples from patients suspected of having VRL. Regardless, the high concordance rate of paired AH and VF samples in this study demonstrates the potential of AH-based mutation analysis.
Another limitation of this study is the relatively large number of included samples from patients with infectious uveitis, while the differential diagnosis with VRL is usually noninfectious uveitis. However, we deliberately included cases with infectious uveitis because this type of uveitis and viral retinitis can present with typical VRL features, such as B-cell clonality, atypical cells by cytomorphology, and increased interleukin 10 levels in ocular fluids.31 In contrast to these features, we demonstrated that MYD88 L265P is strictly observed in patients with VRL.
Conclusions
In conclusion, it is feasible to detect the hotspot mutation in MYD88 via ddPCR of AH samples, and this approach may provide a valuable additional tool for minimally invasive liquid biopsy analysis of patients suspected of having VRL. As molecular techniques are evolving rapidly, this may enable the role of AH analysis in routine diagnostic workup of patients who are presenting with a differential diagnosis, including VRL.
eTable 1. MYD88 p.(L265P) analysis (ddPCR) in treated VRL patients: all ocular fluids were negative after treatment.
eTable 2. Etiology of investigated uveitis patients in this study.
eTable 3. Ophthalmological findings and demographics of each of the investigated VRL patients (n=23).
eTable 4. Characteristics and test results of each of the investigated VRL patients (n=23).
eTable 5. Sensitivity and specificity of MYD88 analysis in VF (upper panel) and AH (lower panel) compared to routine laboratory tests in diagnostic work-up of VRL.
eFigure 1. Flow chart of MYD88 p.(L265P) analysis on cell pellet and cfDNA harvested from diluted and non-diluted VF of patients suspected with VRL.
eFigure 2. ddPCR results of a MYD88 p.(L265P)-positive diluted VF sample (cfDNA; mutation frequency 48.0%).
References
- 1.Sagoo MS, Mehta H, Swampillai AJ, et al. Primary intraocular lymphoma. Surv Ophthalmol. 2014;59(5):503-516. doi: 10.1016/j.survophthal.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 2.Bonzheim I, Giese S, Deuter C, et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood. 2015;126(1):76-79. doi: 10.1182/blood-2015-01-620518 [DOI] [PubMed] [Google Scholar]
- 3.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367(9):826-833. doi: 10.1056/NEJMoa1200710 [DOI] [PubMed] [Google Scholar]
- 4.Kuiper J, Ten Dam-van Loon N, Domanian A, et al. Correlation between measurement of IL-10 and IL-6 in paired aqueous humour and vitreous fluid in primary vitreoretinal lymphoma. Acta Ophthalmol. 2015;93(8):e680-e681. doi: 10.1111/aos.12733 [DOI] [PubMed] [Google Scholar]
- 5.Sugita S, Takase H, Sugamoto Y, Arai A, Miura O, Mochizuki M. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn J Ophthalmol. 2009;53(3):209-214. doi: 10.1007/s10384-009-0662-y [DOI] [PubMed] [Google Scholar]
- 6.Raja H, Salomão DR, Viswanatha DS, Pulido JS. Prevalence of MYD88 L265P mutation in histologically proven, diffuse large B-cell vitreoretinal lymphoma. Retina. 2016;36(3):624-628. doi: 10.1097/IAE.0000000000000996 [DOI] [PubMed] [Google Scholar]
- 7.Kase S, Namba K, Iwata D, et al. Diagnostic efficacy of cell block method for vitreoretinal lymphoma. Diagn Pathol. 2016;11(29):29. doi: 10.1186/s13000-016-0479-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulagnon C, Ducasse A, Patey M, Diebold MD, Arndt C. Cytopathology of vitreous humor samples in routine practice. Acta Cytol. 2016;60(1):65-73. doi: 10.1159/000444576 [DOI] [PubMed] [Google Scholar]
- 9.Davis JL, Miller DM, Ruiz P. Diagnostic testing of vitrectomy specimens. Am J Ophthalmol. 2005;140(5):822-829. doi: 10.1016/j.ajo.2005.05.032 [DOI] [PubMed] [Google Scholar]
- 10.Gupta OP, Weichel ED, Regillo CD, et al. Postoperative complications associated with 25-gauge pars plana vitrectomy. Ophthalmic Surg Lasers Imaging. 2007;38(4):270-275. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi D, Denniston AK, Murray PI. Safety profile of anterior chamber paracentesis performed at the slit lamp. Clin Exp Ophthalmol. 2011;39(8):725-728. doi: 10.1111/j.1442-9071.2011.02565.x [DOI] [PubMed] [Google Scholar]
- 12.Chronopoulos A, Roquelaure D, Souteyrand G, Seebach JD, Schutz JS, Thumann G. Aqueous humor polymerase chain reaction in uveitis—utility and safety. BMC Ophthalmol. 2016;16(1):189. doi: 10.1186/s12886-016-0369-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Q, Huang F, Zhang M, et al. Multiplex picoliter-droplet digital PCR for quantitative assessment of EGFR mutations in circulating cell-free DNA derived from advanced non-small cell lung cancer patients. Mol Med Rep. 2017;16(2):1157-1166. doi: 10.3892/mmr.2017.6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiemcke-Jiwa LS, Minnema MC, Radersma-van Loon JH, et al. The use of droplet digital PCR in liquid biopsies: a highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol Oncol. 2017;36(2):429-435. doi: 10.1002/hon.2489 [DOI] [PubMed] [Google Scholar]
- 15.De Groot-Mijnes JD, Rothova A, Van Loon AM, et al. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol. 2006;141(2):313-318. doi: 10.1016/j.ajo.2005.09.017 [DOI] [PubMed] [Google Scholar]
- 16.Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group . Standardization of uveitis nomenclature for reporting clinical data: results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509-516. doi: 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiewe P, Fischer L, Martus P, Thiel E, Korfel A. Meningeal dissemination in primary CNS lymphoma: diagnosis, treatment, and survival in a large monocenter cohort. Neuro Oncol. 2010;12(4):409-417. doi: 10.1093/neuonc/nop053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroers R, Baraniskin A, Heute C, et al. Diagnosis of leptomeningeal disease in diffuse large B-cell lymphomas of the central nervous system by flow cytometry and cytopathology. Eur J Haematol. 2010;85(6):520-528. doi: 10.1111/j.1600-0609.2010.01516.x [DOI] [PubMed] [Google Scholar]
- 19.Ohta K, Sano K, Imai H, Kikuchi T. Cytokine and molecular analyses of intraocular lymphoma. Ocul Immunol Inflamm. 2009;17(3):142-147. doi: 10.1080/09273940802702553 [DOI] [PubMed] [Google Scholar]
- 20.Merle-Béral H, Davi F, Cassoux N, et al. Biological diagnosis of primary intraocular lymphoma. Br J Haematol. 2004;124(4):469-473. doi: 10.1046/j.1365-2141.2003.04800.x [DOI] [PubMed] [Google Scholar]
- 21.Pavan PR, Oteiza EE, Margo CE. Ocular lymphoma diagnosed by internal subretinal pigment epithelium biopsy. Arch Ophthalmol. 1995;113(10):1233-1234. doi: 10.1001/archopht.1995.01100100021014 [DOI] [PubMed] [Google Scholar]
- 22.Missotten T, Tielemans D, Bromberg JE, et al. Multicolor flowcytometric immunophenotyping is a valuable tool for detection of intraocular lymphoma. Ophthalmology. 2013;120(5):991-996. doi: 10.1016/j.ophtha.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 23.Baehring JM, Androudi S, Longtine JJ, et al. Analysis of clonal immunoglobulin heavy chain rearrangements in ocular lymphoma. Cancer. 2005;104(3):591-597. doi: 10.1002/cncr.21191 [DOI] [PubMed] [Google Scholar]
- 24.Kuiper JJ, Beretta L, Nierkens S, et al. An ocular protein triad can classify four complex retinal diseases. Sci Rep. 2017;7(41595):41595. doi: 10.1038/srep41595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunmire JJ, Lagouros E, Bouhenni RA, Jones M, Edward DP. MicroRNA in aqueous humor from patients with cataract. Exp Eye Res. 2013;108:68-71. doi: 10.1016/j.exer.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 26.Berry JL, Xu L, Murphree AL, et al. Potential of aqueous humor as a surrogate tumor biopsy for retinoblastoma. JAMA Ophthalmol. 2017;135(11):1221-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harbour JW. Liquid biopsy in retinoblastoma. JAMA Ophthalmol. 2017;135(11):1231. doi: 10.1001/jamaophthalmol.2017.4094 [DOI] [PubMed] [Google Scholar]
- 28.Cani AK, Hovelson DH, Demirci H, Johnson MW, Tomlins SA, Rao RC. Next generation sequencing of vitreoretinal lymphomas from small-volume intraocular liquid biopsies: new routes to targeted therapies. Oncotarget. 2017;8(5):7989-7998. doi: 10.18632/oncotarget.14008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96(8):1078-1083. doi: 10.1136/bjophthalmol-2011-301450 [DOI] [PubMed] [Google Scholar]
- 30.Treon SP, Xu L, Hunter Z. MYD88 mutations and response to ibrutinib in Waldenström’s macroglobulinemia. N Engl J Med. 2015;373(6):584-586. doi: 10.1056/NEJMc1506192 [DOI] [PubMed] [Google Scholar]
- 31.Gooi P, Farmer J, Hurley B, Brodbaker E. Cytomegalovirus retinitis mimicking intraocular lymphoma. Clin Ophthalmol. 2008;2(4):969-971. doi: 10.2147/OPTH.S4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. MYD88 p.(L265P) analysis (ddPCR) in treated VRL patients: all ocular fluids were negative after treatment.
eTable 2. Etiology of investigated uveitis patients in this study.
eTable 3. Ophthalmological findings and demographics of each of the investigated VRL patients (n=23).
eTable 4. Characteristics and test results of each of the investigated VRL patients (n=23).
eTable 5. Sensitivity and specificity of MYD88 analysis in VF (upper panel) and AH (lower panel) compared to routine laboratory tests in diagnostic work-up of VRL.
eFigure 1. Flow chart of MYD88 p.(L265P) analysis on cell pellet and cfDNA harvested from diluted and non-diluted VF of patients suspected with VRL.
eFigure 2. ddPCR results of a MYD88 p.(L265P)-positive diluted VF sample (cfDNA; mutation frequency 48.0%).