Key Points
Question
Is radial hemorrhage in the outer plexiform layer of Henle characteristic of deep retinal capillary plexus (DCP) pathology, as in macular telangiectasia type 2 (MacTel 2)?
Findings
In this case series, 3 patients with sudden vision loss presented with characteristic radial hemorrhage in Henle layer. Optical coherence tomography angiography illustrated microvascular abnormalities in the DCP consistent with MacTel 2 in the absence of subretinal neovascularization.
Meaning
MacTel 2 is associated with microvascular abnormalities in the DCP pathology and may be complicated by sudden radial hemorrhage in the outer plexiform layer of Henle independent of subretinal neovascularization.
This case series describes the multimodal imaging findings of radial hemorrhage in the outer plexiform layer of Henle, which may be a complication of macular telangiectasia type 2.
Abstract
Importance
Radial hemorrhage in the outer plexiform layer of Henle may be a complication of macular telangiectasia type 2 (MacTel 2) and may occur because of microvascular abnormalities of the deep retinal capillary plexus in the absence of subretinal neovascularization.
Objective
To describe the multimodal imaging findings, including cross-sectional and en face optical coherence tomography (OCT), of radial hemorrhage in the outer plexiform layer of Henle, which may be a complication of MacTel 2.
Design, Setting, and Participants
This retrospective case series from 2 tertiary referral centers (Stein Eye Institute, Los Angeles, California; New England Eye Center, Boston, Massachusetts) between January 1, 2012, and December 31, 2017, describes 3 patients with MacTel 2 complicated by characteristic radial hemorrhage in the outer plexiform layer of Henle.
Main Outcomes and Measures
Color fundus photography, cross-sectional and en face OCT, OCT angiography (OCTA), fundus autofluorescence, and fluorescein angiography.
Results
Three male patients presented with sudden vision loss in the right eye. A characteristic radial pattern of hemorrhage was noted with color fundus photography. Cross-sectional and en face OCT and OCTA localized the hemorrhage to the outer plexiform layer of Henle in the absence of subretinal neovascularization. Optical coherence tomography findings consistent with MacTel 2 were identified in the fellow eye in each patient. At the follow-up visit 1 to 2 months after presentation, spontaneous resolution of the hemorrhage was noted in all 3 patients, and OCTA illustrated underlying microvascular abnormalities in the deep retinal capillary plexus in 2 patients.
Conclusions and Relevance
This report describes 3 patients with MacTel 2 complicated by characteristic radial hemorrhage in the outer plexiform layer of Henle, which may represent a characteristic finding in MacTel 2 that may develop as a result of microvascular abnormalities of the deep retinal capillary plexus in the absence of subretinal neovascularization.
Introduction
The era of optical coherence tomography angiography (OCTA) has led to a greater understanding of the importance of the deep retinal capillary plexus (DCP) in macular disease.1 Optical coherence tomography angiography provides depth-resolved visualization of the DCP, and recent studies2 with OCTA have confirmed the original findings by Gass3 that the DCP is the principal level of microvascular pathology in eyes with macular telangiectasia type 2 (MacTel 2).
We report 3 patients with MacTel 2, each of whom presented with sudden visual loss due to spontaneous macular hemorrhage in the absence of associated subretinal neovascularization (SRNV). The hemorrhage displayed a characteristic radial pattern in the outer plexiform layer (OPL) of Henle.
Methods
This study was approved by the University of California, Los Angeles Institutional Review Board, complied with the Health Insurance Portability and Accountability Act of 1996, and followed the tenets of the Declaration of Helsinki. Informed consent was waived because deidentified data were used. A retrospective case series from 2 tertiary referral centers (Stein Eye Institute, Los Angeles, California; New England Eye Center, Boston, Massachusetts) was conducted. Patients were evaluated between January 1, 2012, and December 31, 2017, and were included in the study if they presented with radial heme in the OPL. Multimodal imaging, including color fundus photography, cross-sectional optical coherence tomography (OCT), en face OCT, OCTA, fundus autofluorescence, and fluorescein angiography (FA), were analyzed. Outer plexiform layer heme was established by (1) presence of radial heme on color fundus photography and (2) localization of heme in the OPL of Henle on cross-sectional OCT. Absence of SRNV was verified on FA and OCTA. Other data collected included age, sex, medical history, current medications, and treatment history. Visual acuity (VA) and results of ocular and retinal examination were also recorded.
Report of Cases
Case 1
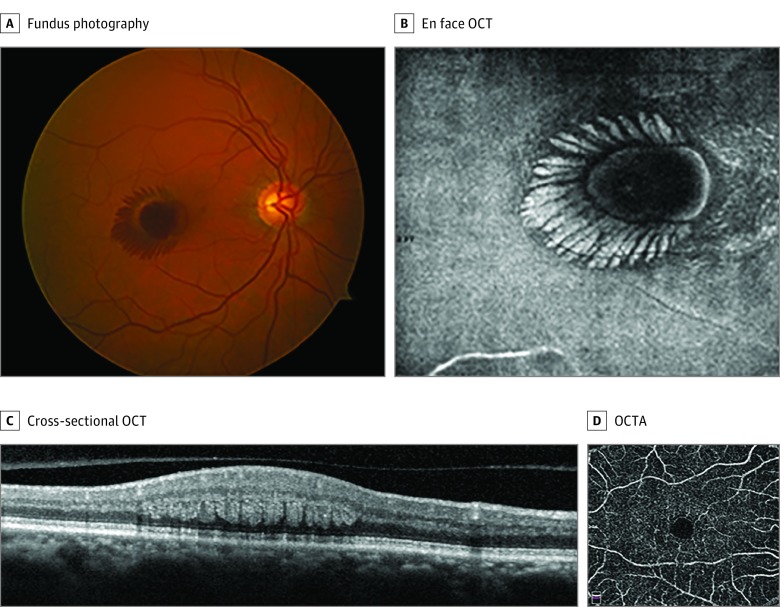
A man with obesity in his early 70s presented with sudden vision loss in the right eye on waking and denied any Valsalva-like maneuvers. His medical history was remarkable for obstructive sleep apnea, hypertension, diabetes, and pulmonary embolism. His VA was 20/200 OD and 20/25 OS. Retinal examination displayed central macular hemorrhage in a characteristic radial pattern in the right eye (Figure 1A) that illustrated radial hyperreflectivity, with en face OCT at the level of the OPL of Henle (Figure 1B) and blockage with fundus autofluorescence and FA. Optical coherence tomography displayed intraretinal hemorrhage radiating in the OPL of Henle (Figure 1C). The left eye was notable for retinal crystals in the fovea. Cystoid cavitation associated with ellipsoid loss temporal to the fovea was identified with cross-sectional spectral-domain OCT in the left eye. No evidence of SRNV was present with OCTA in either eye. The patient was diagnosed as having nonproliferative MacTel 2 complicated by spontaneous macular hemorrhage at the level of Henle layer in the right eye, presumably originating from the DCP.
Figure 1. Case 1.
A, Fundus photography of the right eye at presentation displayed central macular hemorrhage in a characteristic radial pattern. B, En face optical coherence tomography (OCT) of the right eye at presentation illustrated radial hyperreflectivity at the level of the outer plexiform layer of Henle. C, Cross-sectional OCT of the right eye displayed intraretinal hemorrhage radiating in the outer plexiform layer of Henle. D, At the 6-week follow-up visit, there was remarkable improvement of the radial hemorrhage, and OCT angiography (OCTA) of the deep retinal capillary plexus illustrated microvascular abnormalities consistent with macular telangiectasia type 2.
At the 4-week follow-up visit, the hemorrhage improved. By week 6, complete resolution of the hemorrhage was noted, with improvement in VA to 20/40 OD. En face OCTA at 8 weeks illustrated microvascular abnormalities in the DCP, including dilation and tortuosity of each eye (Figure 1D), consistent with a diagnosis of MacTel 2.
Case 2
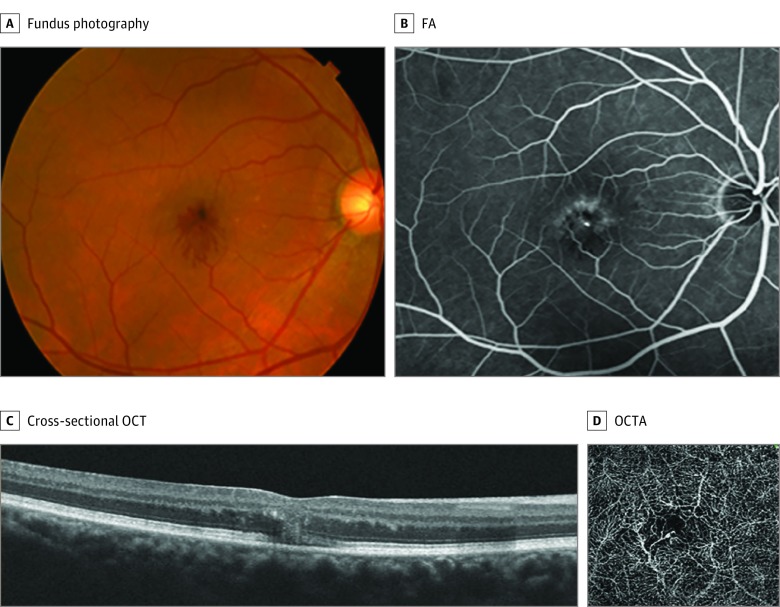
A man with obesity in his mid 50s presented with a 1-week history of reduced vision in his right eye. His medical history was pertinent for diabetes and hyperlipidemia. A remote history of spontaneously resolved macular hemorrhage in his right eye was noted 1.5 years prior. His VA was 20/70 OD and 20/25 OS. Retinal examination displayed intraretinal hemorrhage in the fovea with radial fingerlike projections extending inferiorly in the right eye (Figure 2A) and retinal pigment epithelial clumping in the left eye. Fluorescein angiography showed radial blockage (Figure 2B), and cross-sectional OCT illustrated intraretinal hemorrhage in Henle layer in the right eye (Figure 2C). Optical coherence tomography of the left eye displayed central foveal cystoidlike cavitation, and FA of the left eye illustrated perifoveal telangiectasia. While there was a questionable flow signal with OCTA, SRNV was not identified, and OCTA illustrated microvascular tortuosity and microvascular abnormalities of the DCP in each eye (Figure 2D). The patient was diagnosed as having nonproliferative MacTel 2 in each eye complicated by spontaneous hemorrhage at the level of OPL of Henle in the right eye, presumably originating from the DCP in the right eye in the absence of SRNV in each eye.
Figure 2. Case 2.
A, Fundus photography of the right eye at presentation illustrated intraretinal hemorrhage in the outer plexiform layer of Henle with radial fingerlike projections extending inferiorly. B, Fluorescein angiography (FA) of the right eye at presentation showed radial blockage but no associated leakage in the outer plexiform layer of Henle. C, Cross-sectional optical coherence tomography (OCT) of the right eye at presentation illustrated radial hemorrhage in the outer plexiform layer of Henle. D, Optical coherence tomography angiography (OCTA) illustrated microvascular abnormalities in the deep retinal capillary plexus consistent with macular telangiectasia type 2.
The radial OPL hemorrhage resolved in the right eye with commensurate VA improvement. The patient returned 1.5 years later with recurrent acute vision loss in the right eye. Optical coherence tomography angiography illustrated SRNV in the right eye that was judged to be unrelated to the patient’s original OPL hemorrhage at baseline (data not shown).
Case 3
A man with obesity in his mid 60s with a medical history pertinent for coronary artery bypass grafting surgery presented with decreased vision in his right eye. His VA was 20/400 OD, and retinal examination illustrated remarkable radial intraretinal hemorrhage localized to the temporal portion of the fovea (eFigure 1A in the Supplement). Right-angle venules and crystalline deposits were noted in the perifoveal region in the fellow left eye. Cross-sectional OCT of the right eye illustrated intraretinal hemorrhage in the OPL of Henle. Fluorescein angiography exhibited early blockage corresponding to the hemorrhage in the right eye and late leakage temporal to the fovea in the left eye (eFigure 1B in the Supplement). The patient was diagnosed as having MacTel 2 complicated by Henle layer hemorrhage in the right eye, presumably originating from the DCP in the right eye.
At 1 month, the hemorrhage had improved but not completely resolved on retinal and OCT examination. At 5 months, VA had improved to 20/60 OD. Fundus photography and OCT of the right and left eyes illustrated complete resolution of the radial hemorrhage (eFigure 1C and D in the Supplement).
Discussion
Although the pathogenesis of MacTel 2 is unknown, histopathologic analysis implicates a principal role of Müller cells.4 Müller cells exhibit many essential metabolic and structural functions and maintain the integrity of the blood-ocular barrier in the DCP.5,6 Disruption or degeneration in Müller cells may lead to retinal thinning, cystoid cavitation, and the development of telangiectatic microvascular abnormalities that are characteristic in this disorder.
Several studies have reported the association of sudden macular hemorrhage in eyes with MacTel 2.7,8,9,10 While SRNV may complicate this disorder and is an important cause of vision loss, the 3 patients in this report notably lacked associated neovascularization. While prior reports have identified hemorrhage in the inner and middle retinal planes,7,9 the interesting radial pattern with cross-sectional and en face OCT in the 3 patients included in this article and in 1 other case in the literature9 are indicative of pathology at the level of the OPL of Henle and may represent a characteristic finding of MacTel 2. In all cases, the hemorrhage spontaneously resolved, and VA substantially improved. Optical coherence tomography angiography in 2 cases identified microvascular abnormalities of the DCP consistent with MacTel 2 in each eye and excluded the presence of SRNV.
The pathogenic development of Henle layer hemorrhage in eyes with MacTel 2 may relate to the effect of increased hydrostatic pressure on the abnormal DCP, given the OPL location of the blood and the absence of neovascularization. The DCP, a primarily venous outflow tract, may be at high risk of bleeding in the setting of increased central venous pressure.11 The 3 patients in this report were noted to have risk factors for increased central venous pressure, including obesity and sleep apnea. Further, there may be a greater pressure gradient imbalance between the right and left venous tracts, as the right internal jugular vein directly enters the superior vena cava in the intrathoracic region. This may explain why radial Henle hemorrhage developed in the right eye in all 3 patients and in the 2 patients reported in the literature.7,9 An integrated effect with diurnal or positional fluctuations in venous pressure may have been sufficient to cause the intraretinal hemorrhage. However, the etiology of the hemorrhage could be coincidental to obesity or increased central venous pressure, given the high prevalence of obesity in the general population.
Limitations
This study has limitations. The study included a small sample size, used a retrospective study design, and lacked a control group. Therefore, the study is prone to selection bias, and future controlled studies and correlative histopathology are necessary. Regardless, this case series presents a novel characteristic finding specific to MacTel 2 and suggests the involvement of the DCP in its pathologic development.
Conclusions
Although the development of spontaneous macular hemorrhage in MacTel 2 may be rare, this study reported 3 cases of a remarkable radial pattern of OPL of Henle hemorrhage in patients with MacTel 2 in the absence of neovascularization. The origin of the bleeding is likely from the DCP, the principle layer of microvascular disease in MacTel 2. Future reports may illustrate this signature pattern of radial hemorrhage in the OPL of Henle in other disorders primarily associated with abnormalities in the DCP.
eFigure. Case 3.
References
- 1.Nemiroff J, Kuehlewein L, Rahimy E, et al. Assessing deep retinal capillary ischemia in paracentral acute middle maculopathy by optical coherence tomography angiography. Am J Ophthalmol. 2016;162:121-132.e1. doi: 10.1016/j.ajo.2015.10.026 [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers in macular telangiectasia type 2 imaged by optical coherence tomographic angiography. JAMA Ophthalmol. 2015;133(1):66-73. doi: 10.1001/jamaophthalmol.2014.3950 [DOI] [PubMed] [Google Scholar]
- 3.Gass JDM. Stereoscopic Atlas of Macular Disease: Diagnosis and Treatment. 4th ed Maryland Heights, MO: Mosby; 1997. [Google Scholar]
- 4.Powner MB, Gillies MC, Tretiach M, et al. Perifoveal Müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117(12):2407-2416. doi: 10.1016/j.ophtha.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bringmann A, Pannicke T, Grosche J, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397-424. doi: 10.1016/j.preteyeres.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 6.Tout S, Chan-Ling T, Holländer H, Stone J. The role of Müller cells in the formation of the blood-retinal barrier. Neuroscience. 1993;55(1):291-301. doi: 10.1016/0306-4522(93)90473-S [DOI] [PubMed] [Google Scholar]
- 7.Chandra V, Merani R, Hunyor AP, Ho IV, Gillies M. Macular telangiectasia type 2 with spontaneous resolution of intraretinal/sub-internal limiting membrane hemorrhage in the absence of neovascularization. J Vitreoretin Dis. 2017;1(6):415-419. doi: 10.1177/2474126417731478 [DOI] [Google Scholar]
- 8.Osher JM, Sisk RA, Petersen MR. Preretinal hemorrhage as a presenting sign of idiopathic macular telangiectasia type 2. Clin Ophthalmol. 2015;9:1417-1420. doi: 10.2147/OPTH.S83335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahimy E, Vander J. I see a seashell in my right eye. JAMA Ophthalmol. 2014;132(12):1413. doi: 10.1001/jamaophthalmol.2014.581 [DOI] [PubMed] [Google Scholar]
- 10.Friedman SM, Mames RN, Stewart MW. Subretinal hemorrhage after grid laser photocoagulation for idiopathic juxtafoveolar retinal telangiectasis. Ophthalmic Surg. 1993;24(8):551-553. [PubMed] [Google Scholar]
- 11.Garrity ST, Paques M, Gaudric A, Freund KB, Sarraf D. Considerations in the understanding of venous outflow in the retinal capillary plexus. Retina. 2017;37(10):1809-1812. doi: 10.1097/IAE.0000000000001784 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Case 3.