Key Points
Question
Among patients with subclinical hypothyroidism, is the use of thyroid hormone therapy associated with improvements in general quality of life or thyroid-related symptoms?
Findings
In this meta-analysis of 21 randomized clinical trials including 2192 participants with subclinical hypothyroidism, thyroid hormone therapy was not significantly associated with improvements in general quality of life (standardized mean difference, −0.11) or thyroid-related symptoms (standardized mean difference, 0.01).
Meaning
These findings do not support the routine use of thyroid hormone therapy in adults with subclinical hypothyroidism.
Abstract
Importance
The benefit of thyroid hormone therapy for subclinical hypothyroidism is uncertain. New evidence from recent large randomized clinical trials warrants an update of previous meta-analyses.
Objective
To conduct a meta-analysis of the association of thyroid hormone therapy with quality of life and thyroid-related symptoms in adults with subclinical hypothyroidism.
Data Sources
PubMed, EMBASE, ClinicalTrials.gov, Web of Science, Cochrane Library, CENTRAL, Emcare, and Academic Search Premier from inception until July 4, 2018.
Study Selection
Randomized clinical trials that compared thyroid hormone therapy with placebo or no therapy in nonpregnant adults with subclinical hypothyroidism were eligible. Two reviewers independently evaluated eligibility based on titles and abstracts of all retrieved studies. Studies not excluded in this first step were independently assessed for inclusion after full-text evaluation by 2 reviewers.
Data Extraction and Synthesis
Two independent reviewers extracted data, assessed risk of bias (Cochrane risk-of-bias tool), and evaluated the quality of evidence (GRADE tool). For synthesis, differences in clinical scores were transformed (eg, quality of life) into standardized mean differences (SMDs; positive values indicate benefit of thyroid hormone therapy; 0.2, 0.5, and 0.8 correspond to small, moderate, and large effects, respectively). Random-effects models for meta-analyses were applied.
Main Outcomes and Measures
General quality of life and thyroid-related symptoms after a minimum follow-up of 3 months.
Results
Overall, 21 of 3088 initially identified publications met the inclusion criteria, with 2192 adults randomized. After treatment (range, 3-18 months), thyroid hormone therapy was associated with lowering the mean thyrotropin value into the normal reference range compared with placebo (range, 0.5-3.7 mIU/L vs 4.6 to 14.7 mIU/L) but was not associated with benefit regarding general quality of life (n = 796; SMD, −0.11; 95% CI, −0.25 to 0.03; I2=66.7%) or thyroid-related symptoms (n = 858; SMD, 0.01; 95% CI, −0.12 to 0.14; I2=0.0%). Overall, risk of bias was low and the quality of evidence assessed with the GRADE tool was judged moderate to high.
Conclusions and Relevance
Among nonpregnant adults with subclinical hypothyroidism, the use of thyroid hormone therapy was not associated with improvements in general quality of life or thyroid-related symptoms. These findings do not support the routine use of thyroid hormone therapy in adults with subclinical hypothyroidism.
This meta-analysis of randomized trials assesses the association of thyroid hormone therapy with quality of life (QOL) and thyroid-related symptoms in nonpregnant adults with subclinical hypothyroidism.
Introduction
Subclinical hypothyroidism, defined as elevated thyrotropin in combination with normal-range free thyroxine,1 is common.2,3 According to the NHANES III report,4 an estimated 13 million people have subclinical hypothyroidism in the United States. The prevalence is higher in women and in older people.2,3 Subclinical hypothyroidism is often treated with thyroid hormones (levothyroxine),5 particularly when it co-occurs with symptoms potentially attributable to hypothyroidism, such as tiredness, constipation, and unexplained weight gain.5
Relatively limited evidence exists from randomized clinical trials (RCTs) to guide therapy of subclinical hypothyroidism. Systematic reviews have been inconclusive and clinical practice guidelines have varied regarding recommendations for managing subclinical hypothyroidism.6,7,8,9,10 Two large randomized trials of levothyroxine therapy in patients with subclinical hypothyroidism were recently completed.11,12 This meta-analysis and systematic review incorporated recent trials and evaluated whether thyroid hormone therapy was associated with improved symptoms and other benefits in nonpregnant adults with subclinical hypothyroidism.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement13 and published the protocol of this systematic review in the PROSPERO database (identifier: CRD42017055536).
Eligibility Criteria, Literature Search, and Study Selection
We considered randomized trials that included nonpregnant adults with subclinical hypothyroidism. Subclinical hypothyroidism was defined as a thyrotropin level above the reference range in combination with a free thyroxine level within the reference range (according to center-specific reference ranges). The intervention had to consist of thyroid hormone therapy (triiodothyronine, thyroxine, or a combination of both) for at least 1 month, with a minimum follow-up of 3 months. The control group had to receive either placebo or no therapy. To be included, studies had to report quantitative data for at least 1 of the study’s primary or secondary outcomes: general quality of life, thyroid-related quality of life/hypothyroid symptoms, depressive symptoms, fatigue/tiredness, cognitive function, pain, muscle strength, blood pressure, body mass index, cardiovascular events (myocardial infarction, stroke, revascularization), mortality, or adverse effects (hyperthyroidism due to overdosing). Data had to be reported with effect estimates and measures of precision (standard deviations or standard errors). The primary outcomes were general quality of life and thyroid-related quality of life/hypothyroid symptoms, whereas depressive symptoms, fatigue/tiredness, cognitive function, pain, muscle strength, blood pressure, body mass index, cardiovascular events, mortality, and adverse effects were secondary outcomes. Studies that included only patients with subclinical hypothyroidism in combination with another specific condition (eg, patients with diabetic nephropathy and subclinical hypothyroidism) were excluded because this type of study population is not representative of most patients with subclinical hypothyroidism. We excluded studies that exclusively enrolled pregnant women or women who wanted to become pregnant. Pseudorandomization (eg, pre-post comparisons) did not qualify for inclusion.
We searched MEDLINE, EMBASE, Web of Science, Cochrane Library, CENTRAL, Emcare, and Academic Search Premier from inception until July 4, 2018, in cooperation with a trained librarian. Search terms were adapted according to the syntax of each specific database, and no language restrictions were applied. We searched trial registries on ClinicalTrials.gov for upcoming and not yet published trials on this research topic and asked authors for the status of the trial if not published yet. We screened references of key articles for additional potentially relevant articles. Details of the search strategy are presented in eAppendix 1 in Supplement 1.
Two researchers (M.S. and M.D.M.) evaluated eligibility independently based on titles and abstracts of all studies retrieved in the electronic search. Studies not excluded in this first step were independently assessed for inclusion after full-text evaluation by 2 reviewers (M.F. and M.S.). We manually screened bibliographies of the included studies as well as guidelines and review articles for additional studies. Discrepancies were resolved by consensus among the study team.
Data Extraction and Risk-of-Bias Assessment
A standard data extraction form was used, adapted from a template suggested by Cochrane (eAppendix 2 in Supplement 1).14 Two researchers (M.S. and E.M.) independently extracted bibliographic details, funding source, eligibility criteria, information about the study population and setting, study design, risk of bias, intervention/control intervention, and results and independently evaluated the quality of evidence using the GRADE tool.15 If a study reported more than 1 outcome measure for a specific outcome domain (eg, more than 1 cognition test to assess cognitive function), we chose measures that were most relevant to the largest number of patients, including international usage. This was determined by consensus among the study team. As an example, Parle et al16 reported 5 different cognition tests. We analyzed results from the Mini-Mental State Examination because it is used worldwide and because it is a more general assessment of cognitive function than more specific alternative tests, such as the Trail Making Test. When a study mentioned an outcome of interest without providing estimates (eg, the study reported no difference in body mass index between the intervention and the control group without providing data on mean differences and standard deviations), we contacted the author for the data. If studies reported results for an outcome at multiple time points during the intervention (eg, body mass index at 6 and 12 months), only the most recent measurement was used in statistical analyses. Data were extracted in duplicate by 2 independent reviewers (M.S. and E.M.) and differences were resolved by consensus.
Statistical Analyses
Study results were presented separately for each outcome with estimates as reported in the original publication and transformed into standardized mean differences (SMDs) when different scales were used for the same outcome domain. We coded SMDs such that positive values indicated benefit of thyroid hormone therapy, with 0.2, 0.5, and 0.8 corresponding to small, moderate and large effects, respectively.17 In contrast, for body mass index and blood pressure, negative values indicated a benefit of thyroid hormone therapy. For estimations of treatment effects, we used mean values and their standard deviations at the end of treatment in both groups, assuming balanced baseline values due to the randomized designs.
For outcomes on which studies reported treatment effects at different time points, we included only the estimate at the most recent follow-up time point, thereby avoiding counting a study twice in a formal meta-analysis. Overall results were calculated using random-effects models unless fewer than 5 studies were included for a meta-analysis because in that case, the between-study variance could not be estimated reliably, so a fixed-effects analysis was performed. For better clinical interpretation, overall SMDs were also back-transformed to one original scale according to a method proposed by the Cochrane Collaboration17 for general quality of life, thyroid-related quality of life/hypothyroid symptoms, depressive symptoms, cognitive function, and muscle strength. Heterogeneity was assessed visually with forest plots and quantified with I2 (low: 0%-40%; moderate: 40%-75%; and high: >75%). If substantial heterogeneity existed and a sufficient number of publications was available (n = 10), we aimed to explore potential sources of heterogeneity in protocol prespecified subgroup analyses (eg, restricting the analysis to high-quality studies). In addition, a post hoc sensitivity analysis was performed with the aim to evaluate heterogeneity after excluding studies showing a statistically significant benefit of placebo treatment. If a sufficient number of publications was available (n = 10), publication bias was assessed via funnel plots (visually) and more formally with the Egger test.18 Statistical significance testing was 2-sided and P<.05 was considered statistically significant. All analyses were conducted with Stata, release 14 (StataCorp).
Results
The systematic literature search retrieved 3086 studies, and 2 additional studies were retrieved after searching references of key articles. After removing 1438 duplicates, 2 reviewers (M.S. and M.D.M.) independently screened 1650 unique articles for potential eligibility based on title and abstract. Forty-nine potentially eligible studies were evaluated in full text independently by 2 reviewers (M.F. and M.S.). Among these, 25 studies did not meet the inclusion criteria. Three additional studies were excluded because of data presentation problems19,20,21; for 2 of these articles, the authors indicated that data were no longer available19,20; for one, the author could not be reached21 (eTable 1 in Supplement 1).
Twenty-one studies met the inclusion criteria11,12,16,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39 (eFigure in Supplement 1). Among the 21 studies, a total of 2192 adults were randomized (Table). Study sizes ranged from 20 to 737 participants; mean ages ranged from 32 to 74 years; percentages of women ranged from 46% to 100%; and baseline mean thyrotropin values ranged from 4.4 to 12.8 mIU/L. Two studies (99 participants) had a mean baseline thyrotropin level greater than 10 mIU/L.22,23 Seven studies provided information about hypothyroid symptoms at baseline, and in these studies, the burden of symptoms was mild to moderate (Table).12,22,23,24,25,26,27 In the thyroid hormone therapy groups, mean thyrotropin levels at the end of follow-up ranged between 0.5 and 3.7 mIU/L (eTable 2 in Supplement 1), indicating that treatment was associated with normalization of thyrotropin levels. In contrast, mean thyrotropin in the placebo/no intervention groups remained elevated at the end of follow-up, ranging from 4.6 to 14.7 mIU/L (eTable 2). The duration of the intervention (thyroid hormone therapy or placebo/no therapy) ranged from 3 months to 18 months. Three studies compared thyroid hormone therapy with no intervention and the other studies compared thyroid hormone therapy with placebo.11,28,29 Two studies were supported by industry (Table).22,30
Table. Characteristics of 21 Included Randomized Clinical Trials on Thyroid Hormone Therapy for Subclinical Hypothyroidism in Adults.
| Source | Country | Funding Source | Definition of Subclinical Hypothyroidism | No. of Participants | Age, Mean (SD), y | Women, No. (%) | Intervention | Control | Planned Follow-up Duration, mo | Outcomesa | Hypothyroid Symptoms at Baseline, Intervention vs Control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stott et al,12 2017 | The Netherlands, Switzerland, United Kingdom, Ireland | Nonindustry | Thyrotropin 4.6-19.99 mIU/L on 2 occasions and normal free thyroxine | 737 | 74 (6.3) | 396 (54) | Levothyroxine | Placebo | ≥12b | ThyPRO,40 EQ-5D,41 Letter-Digit Coding Test,42 hand-grip strength, blood pressure, BMI, cardiovascular events, mortality, adverse effects40 | ThyPRO hypothyroid symptom score: 17.5 (SD, 18.8) vs 16.9 (SD, 17.9) |
| Zhao et al,11 2016 | China | Nonindustry | Thyrotropin 4.2-10.0 mIU/L and normal free thyroxine on 2 occasions | 369 | 55 (7.6) | 270 (73) | Levothyroxine | No intervention | 15 | Blood pressure, BMI | NR |
| Najafi et al,24 2015 | Iran | Nonindustry | Thyrotropin >4.5 mIU/L, normal free thyroxine, and positive TPO-Ab | 60 | 34 (10.0) | 51 (85) | Levothyroxine | Placebo | 3 | BDI43 | Mean number of hypothyroid symptoms per participant (range, 0-12): 4.8 vs 5.1 |
| Ersoy et al,29 2012 | Turkey | Not declared | Thyrotropin 5.0-10.0 mIU/L and normal free thyroxine | 60 | 46 (13.1) | 58 (97) | Levothyroxine | No intervention | 6 | Blood pressure, BMI | NR |
| Aghili et al,25 2012 | Iran | Nonindustry | Thyrotropin >4.5 mIU/L, normal free thyroxine, and positive TPO-Ab | 60 | 34 (10.8) | 51 (85) | Levothyroxine | Placebo | 3 | Cognitive function (Wechsler memory scale44) | Mean number of hypothyroid symptoms per participant (range, 0-7): 3.2 vs 3.7 |
| Reuters et al,31 2012 | Brazil | Not declared | Thyrotropin >4.0 mIU/L and normal free thyroxine on 2 occasions | 71 | 50 (10.9) | 62 (87) | Levothyroxine | Placebo | 6 | Zulewski score,45 Short Form 36,46 BDI,43 quadriceps strength | Zulewski score (only change from baseline reported) |
| Cabral et al,28 2011 | Brazil | Not declared | Thyrotropin >4 mIU/L and normal free thyroxine on 2 occasions | 32 | 46 (9.0) | 32 (100) | Levothyroxine | No intervention | 12 | BMIc | NR |
| Parle et al,16 2010 | United Kingdom | Nonindustry | Thyrotropin >5.5 mIU/L and normal free thyroxine | 94 | 74 (5.8) | 57 (61) | Thyroxine | Placebo | 12 | HADS,47 cognitive function (MMSE,48 MEAMS,49 SCOLP,50 and Trail Making Test51) | NR |
| Nagasaki et al,36 2009 | Japan | Nonindustry | Increased thyrotropin and normal free triiodothyronine/free thyroxine | 95 | 65 (19.3) | 95 (100) | Levothyroxine | Placebo | 5 | Blood pressure, BMI | NR |
| Teixeira et al,30 2008 | Brazil | Industry supported | Thyrotropin >4 mIU/L and normal free thyroxine on ≥2 occasions | 60 | 48 (10.5) | 57 (95) | Levothyroxine | Placebo | 12 | BMI | NR |
| Razvi et al,32 2007 | United Kingdom | Nonindustry | Thyrotropin >4 mIU/L and normal free thyroxine on ≥2 occasions | 100 | 54 (12.6) | 82 (82) | Levothyroxine | Placebo | 3 | ThyDQoL,52 blood pressure, BMIc | ThyDQoL (only change from baseline reported) |
| Jorde et al,26 2006 | Norway | Nonindustry | Thyrotropin 3.5-10 mIU/L | 69 | 62 (11.9) | 32 (46) | Thyroxine | Placebo | 12 | GHQ-30,53 BDI,43 composite cognitive score26 | Mean number of hypothyroid symptoms per participant (range, 0-19): 4.0 vs 4.0 |
| Iqbal et al,37 2006 | Norway | Nonindustry | Thyrotropin 3.5-10 mIU/L on 2 occasions and normal free triiodothyronine/free thyroxine | 64 | 64 (12.2) | 31 (48) | Thyroxine | Placebo | 12 | BMI | NR |
| Caraccio et al,38 2005 | Italy | Nonindustry | Thyrotropin >3.6 mIU/L and normal free triiodothyronine | 23 | 32 (9.6) | 21 (91) | Levothyroxine | Placebo | 6 | BMI | NR |
| Yazici et al,35 2004 | Turkey | Not declared | Increased thyrotropin and normal free triiodothyronine/free thyroxine | 45 | 40 (7.9) | 38 (84) | Levothyroxine | Placebo | 12 | Blood pressure, BMI | NR |
| Monzani et al,34 2004 | Italy | Not declared | Thyrotropin >3.6 mIU/L | 45 | 37 (11.0) | 37 (82) | Levothyroxine | Placebo | 6 | Blood pressure, BMI | NR |
| Kong et al,27 2002 | United Kingdom | Not declared | Thyrotropin 5-10 mIU/L and normal free thyroxine | 40 | 50 (15.2) | 40 (100) | Thyroxine | Placebo | 6 | GHQ-30,53 HADS,47 BMI | Overall, 33/40 (83%) reported fatigue and 32/40 (80%) reported weight gain |
| Caraccio et al,39 2002 | Italy | Nonindustry | Thyrotropin >3.6 mIU/L on 2 occasions and positive TPO-Ab | 49 | 35 (9.1) | 42 (86) | Levothyroxine | Placebo | 6 | BMI | NR |
| Monzani et al,33 2001 | Italy | Not declared | Thyrotropin >3.6 mIU/L for >1 y and normal free thyroxine | 20 | 32 (12.1) | 18 (90) | Levothyroxine | Placebo | 6 | Blood pressure, BMI | NR |
| Meier et al,22 2001 | Switzerland | Nonindustry and industry supportedd | Thyrotropin >5 mIU/L on 2 consecutive blood tests and normal free thyroxine | 66 | 57 (10.6) | 66 (100) | Levothyroxine | Placebo | 12 | Billewicz score54,e | Billewicz score: −25.7 (SD, 5.2) vs −28.3 (SD, 14.1) |
| Cooper et al,23 1984 | United States | Nonindustry | Increased thyrotropin and normal free triiodothyronine/free thyroxine | 33 | 54 (10.1) | 32 (97) | Levothyroxine | Placebo | 12 | BMI | Mean number of hypothyroid symptoms per participant (range, 0-6): 2.1 vs 2.4 |
Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index; EQ-5D, Euro Quality of Life 5 Dimensions Questionnaire; GHQ-30, General Health Questionnaire (30 items); HADS, Hospital Anxiety and Depression Scale; MEAMS, Middlesex Elderly Assessment of Mental State; MMSE, Mini Mental State Examination; NR, not reported; SCOLP, Speed and Capacity of Language Processing Test; ThyDQoL, 18-Item Underactive Thyroid-Dependent Quality of Life; ThyPRO, Thyroid-Related Quality-of-Life Patient-Reported Outcome Measure (hypothyroid score: 4 items; range, 0-100; higher scores indicate more hypothyroid symptoms; tiredness score: 7 items); TPO-Ab, thyroid peroxidase antibody.
Only outcomes relevant to this systematic review are listed; ie, outcomes that were included in the study protocol and published in the PROSPERO database.
The Letter-Digit Coding Test outcome was available after 18 months of levothyroxine or placebo intervention; the other outcomes after 12 months.
Data obtained through direct communication with author.
This work was supported by the Swiss Research Foundation and by unconditional research grants from Henning Berlin, Sandoz Research, and Roche Research Foundations.
Billewicz score ranges from −47 to 67; higher scores indicate worse hypothyroid symptoms.
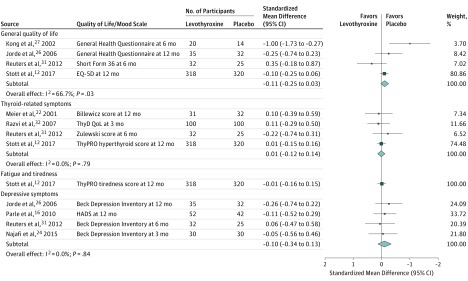
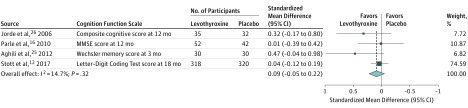
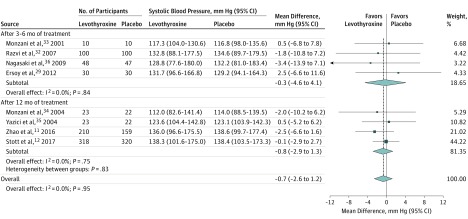
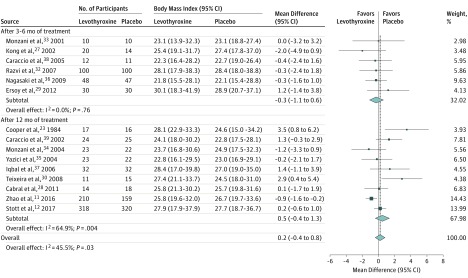
Thyroid hormone therapy was not associated with benefit for either of the 2 primary outcomes. Four studies including 796 participants evaluated general quality of life (SMD, −0.11; 95% CI, −0.25 to 0.03; I2=66.7%) (Figure 1).12,26,27,31 It is estimated that on the Euro Quality of Life 5 Dimensions Questionnaire (range, −0.59 to 1.00; higher scores indicate better quality of life), this SMD would represent a difference of 0.02 (95% CI, −0.01 to 0.05) in favor of placebo. Four studies including 858 participants evaluated thyroid-related quality of life/hypothyroid symptoms (SMD, 0.01; 95% CI, −0.12 to 0.14; I2=0.0%) (Figure 1).12,22,31,32 It is estimated that on the ThyPRO hypothyroid symptoms score (range, 0-100; higher scores indicate more hypothyroid symptoms), this SMD would represent a difference of 0.18 (95% CI, −2.10 to 2.45) in favor of levothyroxine. Similarly, thyroid hormone therapy was not associated with benefit regarding the secondary outcomes. For depressive symptoms (4 studies; 278 participants), the SMD was −0.10 (95% CI, −0.34 to 0.13; I2=0.0%) (Figure 1)16,24,26,31; on the Hospital Anxiety and Depression Scale (range, 0-21; higher scores indicate worse depressive symptoms), this SMD would represent a difference of 0.28 (95% CI, −0.36 to 0.95) in favor of placebo. For cognitive function (4 studies; 859 participants), the SMD was 0.09 (95% CI, −0.05 to 0.22; I2=14.7%) (Figure 2)12,16,25,26; on the Letter-Digit Coding Test (range, 0 or higher [no upper limit]; higher scores indicate better cognitive function), this SMD would represent a difference of 1.01 (95% CI, −0.56 to 2.46) in favor of levothyroxine. For muscle strength (2 studies; 695 participants), the SMD was 0.1 (95% CI, −0.1 to 0.2; I2=0.0%) (eTable 4 in Supplement 1)12,31; in hand-grip strength, this SMD would represent a difference of 1.12 kg (95% CI, −1.12 to 2.24 kg) in favor of levothyroxine. Systolic blood pressure (8 studies; 1372 participants) was −0.7 mm Hg (95% CI, −2.6 to 1.2 mm Hg; I2=0.0%) (Figure 3).11,12,29,32,33,34,35,36 Body mass index (calculated as weight in kilograms divided by height in meters squared) (15 studies; 1633 participants) was 0.2 (95% CI, −0.4 to 0.8; I2=45.5%) (Figure 4).11,12,23,27,28,29,30,32,33,34,35,36,37,38,39 Only the TRUST trial (the largest included study, with 737 participants randomized) evaluated fatigue/tiredness, cardiovascular events, mortality, and adverse effects.12 No beneficial or harmful association between thyroid hormone therapy and these outcomes was reported (Figure 1 and eTables 2 and 4 in Supplement 1). No study included pain as an outcome. Detailed results are summarized in eTables 2 and 4. Subgroup analyses were not performed because the number of studies for a single outcome was too small and/or there was low to moderate heterogeneity such that no exploration was indicated. The meta-analyses for general quality of life and body mass index showed moderate heterogeneity (I2=66.7% and I2=45.5%, respectively). Therefore, post hoc sensitivity analyses were performed excluding studies showing a statistically significant benefit of placebo.23,27,30 Results remained similar, but heterogeneity was lower (for general quality of life, SMD, −0.08 [95% CI, −0.22 to 0.06; I2=34.7%]; for body mass index, −0.2 [95% CI, −0.6 to 0.2; I2=1.6%]). We did not formally assess publication bias. Based on the negative results, there was no indication that positive studies were published while negative studies remained unpublished.
Figure 1. Randomized Clinical Trials of Levothyroxine Therapy in Subclinical Hypothyroidism Quality-of-Life and Mood-Related Outcomes.
BDI indicates Beck Depression Inventory; EQ-5D, Euro Quality of Life 5 Dimensions Questionnaire; HADS, Hospital Anxiety and Depression Scale; ThyDQoL, 18-Item Underactive Thyroid-Dependent Quality of Life; ThyPRO, Thyroid-Related Quality-of-Life Patient-Reported Outcome Measure. Mean values of the quality-of-life and mood-related outcome scales per study group are shown in eTable 2 in Supplement 1. Weights are derived from a fixed-effects meta-analysis of standardized mean differences. Sizes of data markers indicate weight of studies. All effect sizes are standardized. Standardized mean differences of 0.2, 0.5, and 0.8 correspond to small, moderate, and large clinical effects, respectively.55 See Table for descriptions of outcome scales. Numbers differ between participants randomized and participants with available outcome data in the studies by Kong et al,27 Jorde et al,26 Reuters et al,31 Stott et al,12 and Meier et al22 (see Table and eTable 2). The study by Razvi et al32 is a crossover study that included 100 participants.
Figure 2. Randomized Clinical Trials of Levothyroxine Therapy in Subclinical Hypothyroidism Outcomes on Cognitive Function.
MMSE indicates Mini Mental State Examination. Mean values of the cognition scale per study group are shown in eTable 2 in Supplement 1. Weights are derived from a fixed-effects meta-analysis of standardized mean differences. Sizes of data markers indicate weight of studies. Dashed vertical line represents overall mean effect. All effect sizes are standardized. Standardized mean differences of 0.2, 0.5, and 0.8 correspond to small, moderate, and large clinical effects, respectively.55 See Table for descriptions of outcome scales. Numbers differ between participants randomized and participants with available outcome data in the studies by Jorde et al26 and Stott et al12 (see Table and eTable 2).
Figure 3. Randomized Clinical Trials of Levothyroxine Therapy in Subclinical Hypothyroidism Outcomes on Systolic Blood Pressure.
Weights are derived from a fixed-effects meta-analysis of differences in blood pressure. Sizes of data markers indicate weight of studies. Dashed vertical line represents overall mean effect. Numbers differ between participants randomized and participants with available outcome data in the study by Stott et al12 (see Table and eTable 2 in Supplement 1). The study by Razvi et al32 is a crossover study that included 100 participants.
Figure 4. Randomized Clinical Trials of Levothyroxine Therapy in Subclinical Hypothyroidism Outcomes on Body Mass Index.
Weights are derived from a random-effects meta-analysis of differences in body mass index (calculated as weight in kilograms divided by height in meters squared). Sizes of data markers indicate weight of studies. Dashed vertical line represents overall mean effect. Numbers differ between participants randomized and participants with available outcome data in the studies by Kong et al,27 Teixeira et al,30 and Stott et al12 (see Table and eTable 2 in Supplement 1). The study by Razvi et al32 is a crossover study that included 100 participants.
The overall quality of the 21 included studies was good, with only 9 of 126 items judged to be at high risk of bias (eTable 3 in Supplement 1); 2 trials had low risk of bias for all criteria,12,22 including the largest and most recent trial,12 and only 1 trial, the second largest and second most recent, had a high risk of bias in 3 of 6 domains.11 Accordingly, the quality of evidence assessed with the GRADE tool was high regarding the main outcomes of general quality of life and thyroid-related symptoms, as well as regarding muscle strength, blood pressure, and body mass index (eTable 4 in Supplement 1). The quality of evidence was moderate for depressive symptoms, fatigue/tiredness, cognitive function, and adverse effects, whereas it was low for cardiovascular events and mortality (eTable 4).
Discussion
In this systematic review and meta-analysis of RCTs in nonpregnant adults with subclinical hypothyroidism, thyroid hormone therapy was not associated with benefit regarding general quality of life, thyroid-related symptoms, depressive symptoms, fatigue/tiredness, cognitive function, muscle strength, blood pressure, or body mass index.
Compared with prior systematic reviews and meta-analyses published between 20078 and 2015,9 this meta-analysis included 2 recent randomized trials, which were the largest trials published to date on this topic.11,12 Overall, the quality of evidence reported herein was moderate to high. Quality of evidence was high regarding the primary outcomes of this review (general quality of life and thyroid-related symptoms). Results of this review consistently demonstrated no association of thyroid replacement therapy with improved outcomes, including a relatively large number of diverse outcomes. Most outcomes, except cardiovascular events and mortality, had narrow confidence intervals. In addition, this meta-analysis focused on patient-centered outcomes such as quality of life and fatigue, which are the most common symptoms that prompt therapy in general practice.56
Although current guidelines are at first sight cautious with treatment recommendations, more than 90% of persons with subclinical hypothyroidism and a thyrotropin level of less than 10 mIU/L would actually qualify for treatment.6,10,57 However, results of this meta-analysis are not consistent with these guideline recommendations. In addition to absence of an association of thyroid hormone therapy with improved outcomes, thyroid hormone therapy is associated with adverse effects when overtreatment occurs.5,58,59
Limitations
This study has several limitations. First, the RCTs included in this meta-analysis used different questionnaires and/or tests for a given outcome in combination with different treatment durations (eg, 4 different cognitive tests in the 4 studies examining cognitive function, with treatment durations ranging from 3 to 18 months). However, little heterogeneity across the study results was observed except for general quality of life and body mass index. For these outcomes, heterogeneity resulted from 3 studies that showed a statistically significant benefit of placebo.23,27,30 After excluding these studies in post hoc sensitivity analyses, thyroid hormone therapy remained unassociated with benefit for general quality of life and body mass index, and heterogeneity was lower. Therefore, it seems unlikely that this meta-analysis missed a potential beneficial association between thyroid hormone therapy and any outcome analyzed due to inappropriate pooling of overly heterogeneous studies.
Second, only 1 RCT reported on major adverse cardiovascular events. Therefore, definitive evidence is lacking regarding the association of therapy for subclinical hypothyroidism with reduced cardiovascular event rates.12 Third, RCTs that reported results only qualitatively were excluded from analyses. Fourth, mean thyrotropin values at baseline were less than 7.0 mIU/L in 11 of 21 included RCTs, and only 2 RCTs examined participants with a mean baseline thyrotropin level higher than 10 mIU/L.22,23 Therefore, the current findings may not be generalizable to people with subclinical hypothyroidism and a thyrotropin level higher than 10 mIU/L. Fifth, the highest mean age in the included studies was 74 years.12,16 Therefore, these results may not be generalizable to people older than 80 years. Sixth, only 7 of 21 trials (33%) reported hypothyroid symptoms at baseline, and the burden of symptoms was mild to moderate in these trials. The other 14 trials did not describe symptoms at baseline. It is possible that the subgroup of people with subclinical hypothyroidism and a high burden of symptoms would still benefit from treatment. Seventh, patients with subclinical hypothyroidism and “severe” symptoms of hypothyroidism may be underrepresented in clinical trials because they may be treated immediately with levothyroxine and not included in clinical trials.60 Therefore, results reported herein may not be generalizable to patients with subclinical hypothyroidism who have severe symptoms. Eighth, 2 RCTs (n = 831) included participants with a mean age older than 65 years.12,16 Their mean thyrotropin level at baseline was between 6.0 and 7.0 mIU/L. Given the possibility that the upper thyrotropin reference limit may increase with age,61 the 2 studies may have included older individuals with mildly elevated thyrotropin levels who do not represent subclinical hypothyroidism, although current international guidelines do not use different thyrotropin levels according to age to define subclinical hypothyroidism.6,10,62 However, this phenomenon may have biased the results toward the null. Ninth, it is possible that thyroid hormone therapy is associated with benefit regarding outcomes that were not examined in this meta-analysis (eg, carotid intima-media thickness, various lipid fractions). Tenth, it is possible that treatment of subclinical hypothyroidism may be beneficial in study populations not included in these analyses (eg, patients with subclinical hypothyroidism and renal impairment). Eleventh, the largest RCT to date12 contributed substantially to the results of this meta-analysis because of the large sample size relative to the other trials (737 of 2192 participants [33.6%]). However, the mean age of participants in the largest trial was 74 years, while the mean age of participants in the studies included herein ranged from 32 to 74 years.
Conclusions
Among nonpregnant adults with subclinical hypothyroidism, the use of thyroid hormone therapy was not associated with improvements in general quality of life or thyroid-related symptoms. These findings do not support the routine use of thyroid hormone therapy in adults with subclinical hypothyroidism.
eAppendix 1. Literature Search
eFigure. Flow Diagram of the Systematic Review (Study Selection)
eTable 1. Description of 28 Excluded Studies (After Independent Evaluation by Two Reviewers)
eTable 2. Detailed Results of 21 Included RCTs on Thyroid Hormone Replacement Therapy in Non-pregnant Adults With Subclinical Hypothyroidism, Stratified by Outcomes
eTable 3. Risk of Bias Assessment of the 21 Included Randomized Controlled Trials, Adapted From a Template Suggested by the Cochrane Collaboration
eTable 4. Summary of Findings and Quality of Evidence (GRADE) on the Effect of Thyroid Hormone Therapy in Non-pregnant Adults With Subclinical Hypothyroidism
eReferences
eAppendix 2. Adapted Data Collection Form
Data Sharing Statement
References
- 1.Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376(26):2556-2565. doi: 10.1056/NEJMcp1611144 [DOI] [PubMed] [Google Scholar]
- 2.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76-131. doi: 10.1210/er.2006-0043 [DOI] [PubMed] [Google Scholar]
- 3.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142-1154. doi: 10.1016/S0140-6736(11)60276-6 [DOI] [PubMed] [Google Scholar]
- 4.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. doi: 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM, Brito JP. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol. 2017;5(4):246-248. doi: 10.1016/S2213-8587(16)30276-5 [DOI] [PubMed] [Google Scholar]
- 6.Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215-228. doi: 10.1159/000356507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab. 2005;90(1):581-585. doi: 10.1210/jc.2004-1231 [DOI] [PubMed] [Google Scholar]
- 8.Villar HC, Saconato H, Valente O, Atallah AN. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst Rev. 2007;(3):CD003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US Preventive Services Task Force. Ann Intern Med. 2015;162(1):35-45. doi: 10.7326/M14-1456 [DOI] [PubMed] [Google Scholar]
- 10.Garber JR, Cobin RH, Gharib H, et al. ; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults . Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235. doi: 10.1089/thy.2012.0205 [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, Liu L, Wang F, et al. A worthy finding: decrease in total cholesterol and low-density lipoprotein cholesterol in treated mild subclinical hypothyroidism. Thyroid. 2016;26(8):1019-1029. doi: 10.1089/thy.2016.0010 [DOI] [PubMed] [Google Scholar]
- 12.Stott DJ, Rodondi N, Kearney PM, et al. ; TRUST Study Group . Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376(26):2534-2544. doi: 10.1056/NEJMoa1603825 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochrane Effective Practice and Organisation of Care Data collection form: EPOC resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2017. https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/good_practice_data_extraction_form.doc. Accessed July 6, 2018.
- 15.Schünemann HBJ, Guyatt G, Oxman A, eds. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. 2013. https://gradepro.org/handbook. Accessed July 6, 2018.
- 16.Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid Study. J Clin Endocrinol Metab. 2010;95(8):3623-3632. doi: 10.1210/jc.2009-2571 [DOI] [PubMed] [Google Scholar]
- 17.GRADE Working Group About Re-expressing SMD https://pregnancy.cochrane.org. Accessed August 7, 2018.
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Helalah M, Law MR, Bestwick JP, Monson JP, Wald NJ. A randomized double-blind crossover trial to investigate the efficacy of screening for adult hypothyroidism. J Med Screen. 2010;17(4):164-169. doi: 10.1258/jms.2010.010057 [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke R, Guyatt G, Gerstein H, et al. Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J Gen Intern Med. 1996;11(12):744-749. doi: 10.1007/BF02598988 [DOI] [PubMed] [Google Scholar]
- 21.Nyström E, Caidahl K, Fager G, Wikkelsö C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with “subclinical” hypothyroidism. Clin Endocrinol (Oxf). 1988;29(1):63-75. doi: 10.1111/j.1365-2265.1988.tb00250.x [DOI] [PubMed] [Google Scholar]
- 22.Meier C, Staub JJ, Roth CB, et al. TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid Study). J Clin Endocrinol Metab. 2001;86(10):4860-4866. doi: 10.1210/jcem.86.10.7973 [DOI] [PubMed] [Google Scholar]
- 23.Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism: a double-blind, placebo-controlled trial. Ann Intern Med. 1984;101(1):18-24. doi: 10.7326/0003-4819-101-1-18 [DOI] [PubMed] [Google Scholar]
- 24.Najafi L, Malek M, Hadian A, Ebrahim Valojerdi A, Khamseh ME, Aghili R. Depressive symptoms in patients with subclinical hypothyroidism—the effect of treatment with levothyroxine: a double-blind randomized clinical trial. Endocr Res. 2015;40(3):121-126. doi: 10.3109/07435800.2014.896924 [DOI] [PubMed] [Google Scholar]
- 25.Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler Memory Scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci. 2012;8(6):1096-1101. doi: 10.5114/aoms.2012.32423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91(1):145-153. doi: 10.1210/jc.2005-1775 [DOI] [PubMed] [Google Scholar]
- 27.Kong WM, Sheikh MH, Lumb PJ, et al. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am J Med. 2002;112(5):348-354. doi: 10.1016/S0002-9343(02)01022-7 [DOI] [PubMed] [Google Scholar]
- 28.Cabral MD, Teixeira P, Soares D, Leite S, Salles E, Waisman M. Effects of thyroxine replacement on endothelial function and carotid artery intima-media thickness in female patients with mild subclinical hypothyroidism. Clinics (Sao Paulo). 2011;66(8):1321-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ersoy I, Banu KK, Bagci O, et al. Effects of levothyroxine treatment on cardiovascular risk profile and carotid intima media thickness in patients with subclinical hypothyroidism. Acta Endocrinol (Buc). 2012;8(3):433-442. doi: 10.4183/aeb.2012.433 [DOI] [Google Scholar]
- 30.Teixeira PF, Reuters VS, Ferreira MM, et al. Lipid profile in different degrees of hypothyroidism and effects of levothyroxine replacement in mild thyroid failure. Transl Res. 2008;151(4):224-231. doi: 10.1016/j.trsl.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 31.Reuters VS, Almeida CP, Teixeira PF, et al. Effects of subclinical hypothyroidism treatment on psychiatric symptoms, muscular complaints, and quality of life. Arq Bras Endocrinol Metabol. 2012;56(2):128-136. doi: 10.1590/S0004-27302012000200006 [DOI] [PubMed] [Google Scholar]
- 32.Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007;92(5):1715-1723. doi: 10.1210/jc.2006-1869 [DOI] [PubMed] [Google Scholar]
- 33.Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab. 2001;86(3):1110-1115. doi: 10.1210/jcem.86.3.7291 [DOI] [PubMed] [Google Scholar]
- 34.Monzani F, Caraccio N, Kozàkowà M, et al. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo- controlled study. J Clin Endocrinol Metab. 2004;89(5):2099-2106. doi: 10.1210/jc.2003-031669 [DOI] [PubMed] [Google Scholar]
- 35.Yazici M, Gorgulu S, Sertbas Y, et al. Effects of thyroxin therapy on cardiac function in patients with subclinical hypothyroidism: index of myocardial performance in the evaluation of left ventricular function. Int J Cardiol. 2004;95(2-3):135-143. doi: 10.1016/j.ijcard.2003.05.015 [DOI] [PubMed] [Google Scholar]
- 36.Nagasaki T, Inaba M, Yamada S, et al. Decrease of brachial-ankle pulse wave velocity in female subclinical hypothyroid patients during normalization of thyroid function: a double-blind, placebo-controlled study. Eur J Endocrinol. 2009;160(3):409-415. doi: 10.1530/EJE-08-0742 [DOI] [PubMed] [Google Scholar]
- 37.Iqbal A, Jorde R, Figenschau Y. Serum lipid levels in relation to serum thyroid-stimulating hormone and the effect of thyroxine treatment on serum lipid levels in subjects with subclinical hypothyroidism: the Tromsø Study. J Intern Med. 2006;260(1):53-61. doi: 10.1111/j.1365-2796.2006.01652.x [DOI] [PubMed] [Google Scholar]
- 38.Caraccio N, Natali A, Sironi A, et al. Muscle metabolism and exercise tolerance in subclinical hypothyroidism: a controlled trial of levothyroxine. J Clin Endocrinol Metab. 2005;90(7):4057-4062. doi: 10.1210/jc.2004-2344 [DOI] [PubMed] [Google Scholar]
- 39.Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002;87(4):1533-1538. doi: 10.1210/jcem.87.4.8378 [DOI] [PubMed] [Google Scholar]
- 40.Watt T, Hegedüs L, Groenvold M, et al. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur J Endocrinol. 2010;162(1):161-167. doi: 10.1530/EJE-09-0521 [DOI] [PubMed] [Google Scholar]
- 41.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 42.Houx PJ, Shepherd J, Blauw GJ, et al. Testing cognitive function in elderly populations: the PROSPER study. J Neurol Neurosurg Psychiatry. 2002;73(4):385-389. doi: 10.1136/jnnp.73.4.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D. Wechsler Memory Scale. 3rd ed San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 45.Zulewski H, Müller B, Exer P, Miserez AR, Staub JJ. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab. 1997;82(3):771-776. [DOI] [PubMed] [Google Scholar]
- 46.Ciconelli RM, Ferraz MB, Santos W. Brazilian-Portuguese version of the SF-36: a reliable and valid quality of life outcome measure. Rev Bras Reumatol. 1999;(39):143-150. [Google Scholar]
- 47.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale—a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17-41. doi: 10.1016/S0022-3999(96)00216-4 [DOI] [PubMed] [Google Scholar]
- 48.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 49.Golding E. The Middlesex Elderly Assessment of Mental State. Bury St Edmonds, England: Thames Valley Test; 1989. [Google Scholar]
- 50.Medical Research Council The Speed and Capacity of Language-Processing Test. Bury St Edmunds, England: Thames Valley Test; 1992. [Google Scholar]
- 51.Kortte KB, Horner MD, Windham WK. The Trail Making Test, part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol. 2002;9(2):106-109. doi: 10.1207/S15324826AN0902_5 [DOI] [PubMed] [Google Scholar]
- 52.McMillan CV, Bradley C, Woodcock A, Razvi S, Weaver JU. Design of new questionnaires to measure quality of life and treatment satisfaction in hypothyroidism. Thyroid. 2004;14(11):916-925. doi: 10.1089/thy.2004.14.916 [DOI] [PubMed] [Google Scholar]
- 53.Huppert FA, Walters DE, Day NE, Elliott BJ. The factor structure of the General Health Questionnaire (GHQ-30): a reliability study on 6317 community residents. Br J Psychiatry. 1989;155:178-185. doi: 10.1192/bjp.155.2.178 [DOI] [PubMed] [Google Scholar]
- 54.Billewicz WZ, Chapman RS, Crooks J, et al. Statistical methods applied to the diagnosis of hypothyroidism. Q J Med. 1969;38(150):255-266. [PubMed] [Google Scholar]
- 55.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. https://handbook-5-1.cochrane.org/. Accessed September 5, 2018.
- 56.Allport J, McCahon D, Hobbs FD, Roberts LM. Why are GPs treating subclinical hypothyroidism? case note review and GP survey. Prim Health Care Res Dev. 2013;14(2):175-184. doi: 10.1017/S1463423612000230 [DOI] [PubMed] [Google Scholar]
- 57.Rosario PW, Calsolari MR. How selective are the new guidelines for treatment of subclinical hypothyroidism for patients with thyrotropin levels at or below 10 mIU/L? Thyroid. 2013;23(5):562-565. doi: 10.1089/thy.2012.0502 [DOI] [PubMed] [Google Scholar]
- 58.Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab. 2009;94(4):1342-1345. doi: 10.1210/jc.2008-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baumgartner C, da Costa BR, Collet TH, et al. ; Thyroid Studies Collaboration . Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 2017;136(22):2100-2116. doi: 10.1161/CIRCULATIONAHA.117.028753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korevaar TIM, Chaker L, Peeters RP. Improving the clinical impact of randomised trials in thyroidology. Lancet Diabetes Endocrinol. 2018;6(7):523-525. doi: 10.1016/S2213-8587(17)30316-9 [DOI] [PubMed] [Google Scholar]
- 61.Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63(8):1663-1673. doi: 10.1111/jgs.13532 [DOI] [PubMed] [Google Scholar]
- 62.Jonklaas J, Bianco AC, Bauer AJ, et al. ; American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751. doi: 10.1089/thy.2014.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Literature Search
eFigure. Flow Diagram of the Systematic Review (Study Selection)
eTable 1. Description of 28 Excluded Studies (After Independent Evaluation by Two Reviewers)
eTable 2. Detailed Results of 21 Included RCTs on Thyroid Hormone Replacement Therapy in Non-pregnant Adults With Subclinical Hypothyroidism, Stratified by Outcomes
eTable 3. Risk of Bias Assessment of the 21 Included Randomized Controlled Trials, Adapted From a Template Suggested by the Cochrane Collaboration
eTable 4. Summary of Findings and Quality of Evidence (GRADE) on the Effect of Thyroid Hormone Therapy in Non-pregnant Adults With Subclinical Hypothyroidism
eReferences
eAppendix 2. Adapted Data Collection Form
Data Sharing Statement