This population-based study investigates the association of inner retinal layer thickness with prevalent and incident dementia in a general population of Dutch adults.
Key Points
Question
What is the association of thicknesses of retinal layers with incident dementia in a general population of Dutch adults?
Findings
In this population-based study, thinner retinal nerve fiber layer at baseline, assessed on optical coherence tomography imaging, was associated with an increased risk of developing dementia, including Alzheimer disease.
Meaning
Thinner retinal nerve fiber layer may be a novel biomarker for dementia, specifically for Alzheimer disease.
Abstract
Importance
Retinal structures may serve as a biomarker for dementia, but longitudinal studies examining this link are lacking.
Objective
To investigate the association of inner retinal layer thickness with prevalent and incident dementia in a general population of Dutch adults.
Design, Setting, and Participants
From September 2007 to June 2012, participants from the prospective population-based Rotterdam Study who were 45 years and older and had gradable retinal optical coherence tomography images and at baseline were free from stroke, Parkinson disease, multiple sclerosis, glaucoma, macular degeneration, retinopathy, myopia, hyperopia, and optic disc pathology were included. They were followed up until January 1, 2015, for the onset of dementia.
Exposures
Inner retinal layer thicknesses (ie, retinal nerve fiber layer [RNFL]) and ganglion cell–inner plexiform layer (GC-IPL) thicknesses measured on optical coherence tomography images.
Main Outcomes and Measures
Odds ratios and hazard ratios for incident dementia per SD decrease in retinal layer thickness adjusted for age, sex, education, and cardiovascular risk factors.
Results
Of 5065 individuals eligible for optical coherence tomography scanning, 3289 (64.9%) (mean [SD] age 68.9 [9.9] years, 1879 [57%] women) were included in the analysis. Of these 3289 individuals, 41 (1.2%) already had dementia. Thinner GC-IPL was associated with prevalent dementia (odds ratio per SD decrease in GC-IPL, 1.37 [95% CI, 0.99-1.90]). No association was found of RNFL with prevalent dementia. During 14 674 person-years of follow-up (mean [SD], 4.5 [1.6] years), 86 individuals (2.6%) developed dementia of whom 68 (2.1%) had Alzheimer disease. Thinner RNFL at baseline was associated with an increased risk of developing dementia (hazard ratio per SD decrease in RNFL, 1.44 [95% CI, 1.19-1.75]), which was similar for Alzheimer disease (hazard ratio, 1.43 [95% CI, 1.15-1.78]). No association was found between GC-IPL thickness and incident dementia (hazard ratio, 1.13 [95% CI, 0.90-1.43]).
Conclusions and Relevance
Thinner RNFL is associated with an increased risk of dementia, including Alzheimer disease, suggesting that retinal neurodegeneration may serve as a preclinical biomarker for dementia.
Introduction
Dementia is a major cause of morbidity and mortality among elderly populations worldwide.1 Alzheimer disease (AD) is the most common type of dementia, characterized by the accumulation of misfolded amyloid-β and tau protein in the brain during a long preclinical period.2 Studies have shown that the accumulation of amyloid-β deposits also occurs in the retina of patients with AD and in mouse models of AD.3,4 Hence, these findings not only underpin the involvement of the retina and the visual pathways in AD pathology, but also show the potential to use retinal structures as a biomarker for AD.5,6,7
In recent years, noninvasive optical imaging techniques have increasingly been used to study neurodegenerative disorders of the eye, such as macular degeneration and glaucoma, as well as neurodegeneration in the brain. In particular, optical coherence tomography (OCT) provides an excellent opportunity to visualize retinal nerve tissue in vivo with biopsy-like precision (spatial resolution, <10 μm) and to detect neuro-axonal degeneration. Several cross-sectional studies using OCT have shown that patients with AD have thinner retinal nerve fiber layer (RNFL) and ganglion cell–inner plexiform layer (GC-IPL) compared with controls.8,9,10,11,12 Additionally, recent studies have shown that thinner RNFL and GC-IPL were associated with magnetic resonance imaging markers of brain atrophy, suggesting that neuronal damage indeed occurs simultaneously in the retina and throughout the brain.13,14 Although cross-sectional studies have suggested that retinal thinning may serve as a biomarker for dementia, it remains unclear whether retinal thinning precedes the occurrence of dementia or occurs once the disease has become clinically manifest. Longitudinal studies examining the link between retinal layers and incident dementia are lacking and are crucial to disentangle the temporal relation. Therefore, we investigated the association of RNFL and GC-IPL thicknesses on OCT with prevalent and incident dementia in a general adult Dutch population.
Methods
Study Population and Setting
This study was embedded within the Rotterdam Study, a prospective population-based cohort study among individuals 45 years or older, residing in Ommoord, a district in Rotterdam, the Netherlands.15 The Rotterdam Study has been approved by the medical ethics committee according to the Population Study Act, executed by the Ministry of Health, Welfare and Sports of the Netherlands. All individuals gave written informed consent.
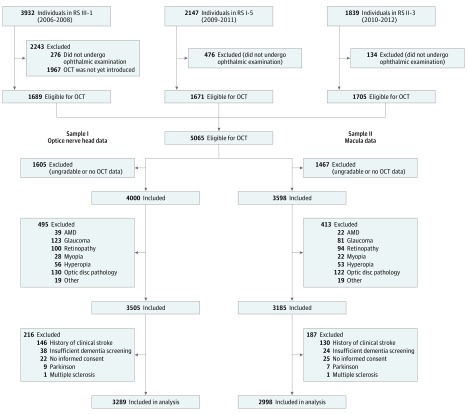
The cohort started in January 1990 (n = 7983) and was extended in February 2000 (n = 3011) and February 2006 (n = 3932). Follow-up examinations took place every 3 to 4 years. In September 2007, spectral-domain OCT scanning was added to the protocol and was performed at the fifth follow-up of the first cohort, the third follow-up of the second cohort, and in about half of the individuals at the first visit of the third cohort (Figure 1).
Figure 1. Flow Diagram of the Study Population.
AMD indicates age-related macular degeneration; OCT, optical coherence tomography; RS, Rotterdam Study.
A total of 5065 individuals were eligible for OCT scanning. Individuals were excluded if they did not undergo OCT scanning or had ungradable OCT scans owing to poor-quality scans. We also excluded individuals with age-related macular degeneration, glaucoma, hypertensive or diabetic retinopathy, myopia (<−6 diopter), hyperopia (>6 diopter), optic disc pathology (ie, tilted optic disc, ischemic optic neuropathy, and peripapillar atrophy), and other eye pathology (eg, macular hole, macular edema). Next, we excluded individuals with a history of clinical stroke, who underwent insufficient dementia screening, who did not provide informed consent to access medical records, with Parkinson disease, and with multiple sclerosis. These exclusions resulted in 3289 individuals (64.9%) for analysis with the RNFL, and in 2998 individuals (59.2%) for analysis with the GC-IPL, from which primarily the right eye (3126 [95%] for RNFL and 2827 [94%] for GC-IPL measurements) was chosen for further analysis. From the macula toward the optic nerve, the RNFL becomes thicker, whereas the GC-IPL becomes thinner. Therefore, we measured the RNFL in the peripapillary region and the GC-IPL in the perimacular region as these layers are the thickest in those regions.
Retinal Layer Thickness
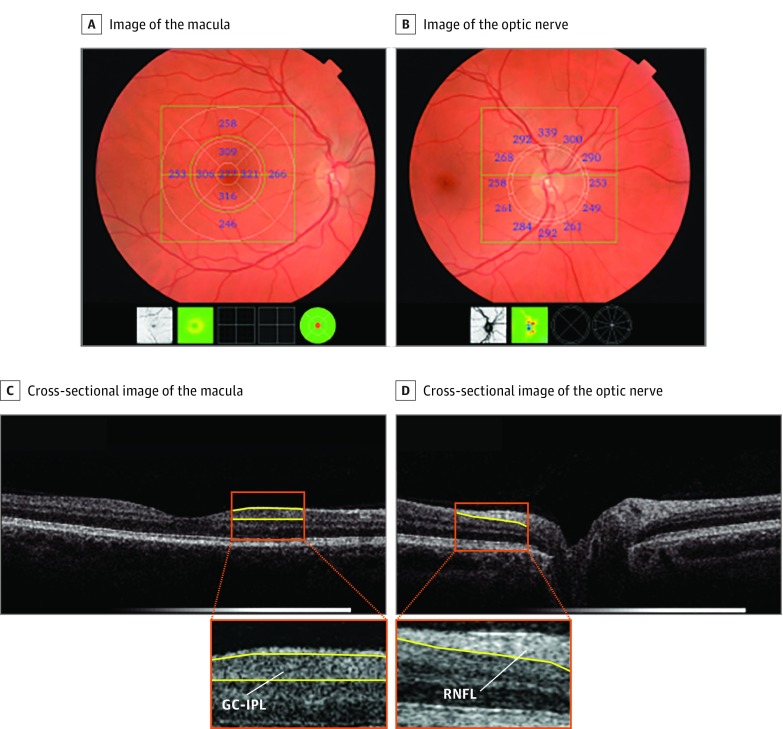
From September 2007 to June 2012, participants underwent complete eye examination including visual acuity, refraction, slitlamp examination, and retinal imaging. Retinal imaging (ie, fundus photography and OCT scanning) was performed on both eyes of each participant after pharmacological mydriasis according to a standard protocol. This resulted in a well-illuminated fundus, which is required for good visible retinal structures. Fundus photographs were graded for the presence of any retinal lesions. Hence, eyes with pathology that could potentially affect the OCT measurements were assessed and excluded. Eyes were scanned with the spectral-domain OCT-1000 (Topcon) with 2678 in sample I and 2390 in sample II. From August 2011 onwards, this device was replaced with the spectral-domain OCT-2000 owing to an update following the start of the study, with 611 in sample I and 608 in sample II. The macula and optic nerve head were scanned in the horizontal direction in an area of 6 × 6 × 1.68 mm with 512 × 512 × 480 voxels (using OCT-1000) and 6 × 6 × 2.30 mm with 512 × 512 × 885 voxels (using OCT-2000), enabling us to detect structures with 5-μm resolution (Figure 2). Peripapillary RNFL thickness was measured automatically by Topcon’s built-in segmentation algorithm. This was done in 12 peripapillary segments of 30° each, and the mean RNFL thickness was derived from the calculation circle. For the macula, volumes were segmented using Iowa Reference Algorithms, version 3.6 (available at https://www.iibi.uiowa.edu/content/shared-software-download). The intersession repeatability of segmentations and agreement between OCT devices of this algorithm has been previously validated.16,17 Macular GC-IPL thickness was measured in 9 regions of the Early Treatment Diabetic Retinopathy Study Grid, and the mean thickness was calculated.
Figure 2. Output of the Retinal Optical Coherence Tomography.
Focusing on the macula (A) and optic nerve (B), with corresponding cross-sectional view of the retina (C, and D, respectively). GC-IPL indicates ganglion cell–inner plexiform layer; RNFL, retinal nerve fiber layer.
Indices of quality control were used to preserve good-quality images (high signal, low noise) and to exclude scans with segmentation errors. We followed published recommendations on quality control according to the OSCAR-IB consensus criteria (O, obvious problems including violation of the protocol; S, poor signal strength defined as <15 dB; C, wrong centration of scan; A, algorithm failure; R, retinal pathology other than multiple sclerosis–related; I, illumination; and B, beam placement) and APOSTEL guidelines (Advised Protocol for OCT Study Terminology and Elements).18,19
Scans included in our study had a segmentability index of 30% or more and an undefined region of 20% or less (measures of segmentation/algorithm failures), and a quality factor of 30 or more (measure of signal strength). These quality metrics have been described previously.17,20,21 At the beginning of the study, only the right eyes (n = 883) were scanned owing to time constraints. To maintain consistency, we chose primarily the right eye.
Dementia
Participants were screened for dementia at baseline and subsequent visits to the study center, using the Mini-Mental State Examination and the Geriatric Mental State organic level. Those with a Mini-Mental State Examination score lower than 26 or GMS score above 0 underwent further investigation, including the Cambridge Examination for Mental Disorders of the Elderly. Additionally, the entire cohort was continuously being monitored for dementia through electronic linkage of the study center with medical records from general practitioners and the regional institute for outpatient mental health care. Available information on cognitive testing and clinical neuroimaging was used when required for diagnosis of dementia subtype. A consensus panel led by a consultant neurologist established the final diagnosis according to a standard criteria for dementia (using Diagnostic and Statistical Manual of Mental Disorders [Third Edition Revised]), AD (using National Institute of Neurological and Communicative Diseases and Stroke–Alzheimer’s Disease and Related Disorders Association criteria), and vascular dementia (using National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherce et l’Enseignement en Neurosciences criteria).22,23 On the basis of the Clinical Dementia Rating Scale, the severity of symptoms of dementia was quantified.24 Follow-up until January 1, 2015, was virtually complete (95% of potential person-years). Participants were censored at date of dementia diagnosis, death, loss to follow-up, or January 1, 2015, whichever came first.
Cognition
Participants underwent routine cognitive assessment during their visit to the center, including verbal fluency test (animal categories), 15-word learning test (immediate and delayed recall and recognition), letter-digit substitution test, Stroop test (reading and interference task, color naming task), and the Purdue pegboard test (sum score of both hands simultaneously).25 We transformed the distribution of all tests to a normal distribution and calculated z scores. We used the g factor for global cognition, ie, a standardized compound score that is calculated using the principal component analysis in which for tests with multiple subtasks, only 1 subtask was included to prevent highly correlated tasks distorting the factor loadings. The tests included were verbal fluency test, 15-word learning test, letter-digit substitution test, Stroop test, and Purdue pegboard test.
Statistical Analysis
Statistical analysis is presented in the eMethods in the Supplement. Briefly, in cross-sectional analysis, we assessed the association of RNFL and GC-IPL with prevalent dementia using logistic regression. In longitudinal analysis, we excluded individuals with prevalent dementia at baseline (at time of OCT scanning) and assessed the association of RNFL and GC-IPL with the risk of developing dementia and AD using Cox proportional hazards regression model. We adjusted the cross-sectional and longitudinal analyses for the same set of covariates (ie, covariates that are generally considered to be important confounders for dementia). Assessment of covariates is presented in the eMethods in the Supplement. In model 1, we adjusted for age, sex, subcohort, and education. In model 2, we additionally adjusted for systolic blood pressure, diastolic blood pressure, blood pressure-lowering medication use, body mass index (calculated as weight in kilograms divided by height in meters squared), total cholesterol, high-density lipoprotein cholesterol, diabetes mellitus, and smoking. All analyses were performed at the significance level of .05 (2-tailed) using SPSS, version 21.0 (IBM Corporation).
Results
Of 5065 individuals eligible for OCT scanning, 3289 individuals (64.9%) (mean [SD] age 68.9 [9.9] years, 1879 [57%] women) were included in the analysis with RNFL thickness, and 2998 individuals (59.9%) (mean [SD] age 68.2 [9.9] years, 1717 [57%] women) were included in the analysis with GC-IPL thickness. Table 1 shows the baseline characteristics of the study population. Compared with individuals included in the analysis, those who were excluded were substantially older, had a worse cardiovascular risk profile, and had higher prevalent dementia cases.
Table 1. Baseline Characteristics of the Study Population.
| Characteristic, Mean (SD) | Sample I | Sample II | ||
|---|---|---|---|---|
| Included (n = 3289) | Excluded (n = 1776)a | Included (n = 2998) | Excluded (n = 2067)a | |
| Age, y | 68.9 (9.9) | 71.8 (11.1)b | 68.2 (9.9) | 72.5 (10.7)b |
| Female , No. (%) | 1879 (57) | 1034 (58) | 1717 (57) | 1196 (58) |
| Systolic blood pressure, mm Hg | 145.9 (22.1) | 146.9 (24.0) | 145.6 (22.1) | 147.1 (23.7)b |
| Diastolic blood pressure, mm Hg | 84.7 (11.1) | 84.0 (11.7)b | 84.9 (11.1) | 83.7 (11.5)b |
| Blood pressure–lowering medication | 1450 (44) | 938 (53)b | 1283 (43) | 1107 (54)b |
| Body mass indexc | 27.5 (4.3) | 27.7 (4.5)b | 27.5 (4.2) | 27.6 (4.5) |
| Diabetes mellitus, No. (%) | 376 (11) | 250 (14)b | 337 (11) | 289 (14)b |
| Total cholesterol, mg/dL | 212.36 (42.47) | 204.63 (42.47)b | 212.36 (42.47) | 204.63 (42.47)b |
| High-density lipoprotein cholesterol, mg/dL | 57.92 (15.44) | 54.05 (15.44)b | 57.92 (15.44) | 54.05 (15.44)b |
| Smoking status, No. (%) | ||||
| Nonsmoker | 1032 (31) | 569 (32) | 951 (32) | 652 (32) |
| Former smoker | 1725 (52) | 892 (50)b | 1556 (52) | 1057 (51)b |
| Current smoker | 532 (16) | 306 (17)b | 491 (16) | 347 (17)b |
| Education | ||||
| Lower education | 1029 (31) | 624 (35)b | 964 (30) | 739 (36)b |
| Intermediate education | 1576 (48) | 811 (46) | 1545 (48) | 947 (46) |
| Higher education | 684 (21) | 310 (18)b | 705 (22) | 348 (17)b |
| Right eyes, No. (%) | 3126 (95) | NA | 2827 (94) | NA |
| Peripapillary retinal nerve fiber layer, μm | 95.3 (15.7) | NA | NA | NA |
| Macular ganglion cell, inner plexiform layer, μm | NA | NA | 70.4 (7.8) | NA |
| Prevalent dementia, No. (%) | 41 (1.2) | 54 (3.0)b | 34 (1.2) | 61 (3.0)b |
Abbreviation: NA, not applicable.
SI conversion factor: To convert cholesterol from mg/dL to mmol/L, multiple by 0.0259.
Missing values were not imputed. Percentage of missing values for all variables was less than 2%.
Age- and sex-adjusted mean differences (P < .05).
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Table 2 shows the association of retinal layer thickness with prevalent dementia. Thinner GC-IPL was strongly, but not substantially, associated with prevalent dementia (model 1: odds ratio per SD decrease in GC-IPL, 1.38 [95% CI, 0.99-1.92]). For RNFL, the odds ratio was 1.06 (95% CI, 0.78-1.43). Mean thickness (μm) with age- and sex-adjusted mean difference of individuals with and without dementia was 92.71 vs 95.36; −2.38 (95% CI, −7.24 to 2.48) for RNFL and 66.31 vs 70.46; −2.22 (95% CI, −4.79 to 0.33) for GC-IPL. We did not find significant differences in RNFL and GC-IPL thicknesses between the left and right eyes (eFigure in the Supplement). According to the Clinical Dementia Rating Scale, most prevalent dementia cases were classified as moderate dementia (60% [25 of 61]), whereas most incident dementia cases were classified as mild dementia (50% [40 of 86]) (eTable 1 in the Supplement).
Table 2. Association of Inner Retinal Layer Thickness With Prevalent Dementia.
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Odds Ratio (95% CI)a | P Value | Odds Ratio (95% CI)b | P Value | |
| Retinal nerve fiber layerc | ||||
| Per SD decrease | 1.06 (0.78-1.43) | .73 | 1.04 (0.77-1.41) | .79 |
| Per 1-μm decrease | 1.00 (0.98-1.01) | .58 | 1.00 (0.98-1.02) | .64 |
| Ganglion cell–inner plexiform layerd | ||||
| Per SD decrease | 1.38 (0.99-1.92) | .05 | 1.37 (0.99-1.90) | .06 |
| Per 1-μm decrease | 1.04 (1.00-1.09) | .05 | 1.03 (1.00-1.09) | .06 |
Model 1 was adjusted for age, sex, subcohort, and education.
Model 2 was adjusted as in model 1 and additionally adjusted for systolic blood pressure, diastolic blood pressure, blood pressure–lowering medication, body mass index (calculated as weight in kilograms divided by height in meters squared), total cholesterol, high-density lipoprotein cholesterol, diabetes mellitus, and smoking.
Of 3289 individuals, 41 (1.2%) developed dementia.
Of 2998 individuals, 34 (1.1%) developed dementia.
Table 3 shows the association of retinal layer thickness with incident dementia. Thinner RNFL at baseline was significantly associated with a higher risk of developing dementia (hazard ratio [HR] per SD decrease in RNFL, 1.51 [95% CI, 1.25-1.82]). After adjusting for cardiovascular risk factors, this association attenuated but remained statistically significant (HR, 1.44 [95% CI, 1.19-1.75]). Similar associations were observed for AD (adjusted HR, 1.43 [95% CI, 1.15-1.78]). We found no significant association for GC-IPL thickness with incident dementia.
Table 3. Association of Inner Retinal Layer Thickness With Incident Dementia.
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI)a | P Value | Hazard Ratio (95% CI)b | P Value | |
| All dementia | ||||
| Retinal nerve fiber layerc | ||||
| Per SD decrease | 1.51 (1.25-1.82) | <.001 | 1.44 (1.19-1.75) | <.001 |
| Per 1-μm decrease | 1.03 (1.01-1.04) | <.001 | 1.02 (1.01-1.04) | <.001 |
| Ganglion cell–inner plexiform layerd | ||||
| Per SD decrease | 1.13 (0.89-1.42) | .31 | 1.13 (0.90-1.43) | .29 |
| Per 1-μm decrease | 1.02 (0.99-1.05) | .33 | 1.02 (0.99-1.05) | .29 |
| Alzheimer disease | ||||
| Retinal nerve fiber layere | ||||
| Per SD decrease | 1.49 (1.20-1.84) | <.001 | 1.43 (1.15-1.78) | .001 |
| Per 1-μm decrease | 1.03 (1.01-1.04) | <.001 | 1.02 (1.01-1.04) | .001 |
| Ganglion cel–inner plexiform layerf | ||||
| Per SD decrease | 1.15 (0.90-1.48) | .28 | 1.16 (0.90-1.48) | .25 |
| Per 1-μm decrease | 1.02 (0.99-1.05) | .28 | 1.02 (0.99-1.05) | .25 |
Model 1 was adjusted for age, sex, subcohort, and education.
Model 2 was adjusted as in model 1 and additionally adjusted for systolic blood pressure, diastolic blood pressure, blood pressure–lowering medication, body mass index (calculated as weight in kilograms divided by height in meters squared), total cholesterol, high-density lipoprotein cholesterol, diabetes mellitus, and smoking.
Of 3248 individuals, 86 (2.6%) developed dementia.
Of 2964 individuals, 63 (2.1%) developed dementia.
Of 3248 individuals, 68 (2.4%) developed dementia.
Of 2964 individuals, 52 (1.8%) developed dementia.
eTable 2 in the Supplement shows the association of retinal layer thickness in quartiles with incident dementia. Individuals in the lowest quartile of RNFL thickness had a higher risk of developing dementia compared with individuals in the highest quartile (HR, 2.58; 95% CI, 1.38-4.83).
eTable 3 in the Supplement shows the association of retinal layer thickness with prevalent and incident dementia after adjusting the retinal layers for each other. Pearson correlation between RNFL and GC-IPL was 0.328. Using measurements from primarily the left eyes, the unadjusted HRs per SD decrease were 1.51 (95% CI, 1.23-1.85) for RNFL and 1.10 (95% CI, 0.86-1.39) for GC-IPL.
When combining the retinal layers (ie, RNFL plus GC-IPL), the unadjusted odds ratio was 1.12 (95% CI, 0.79-1.58), indicating some interaction between the retinal structures, and the unadjusted hazard ratio was 1.46 (95% CI, 1.17-1.83) showing that the association was primarily driven by RNFL. Of all dementia cases, 6 (sample I) and 5 (sample II) cases were preceded by a stroke, for both samples, a median 2.5 years before diagnosis of dementia. After censoring for stroke, the associations of RNFL thickness with all dementia and AD, if anything, became stronger, adjusted HR for dementia and AD: 1.56 (95% CI, 1.28-1.89) and 1.50 (95% CI, 1.23-1.83), respectively.
eTable 4 in the Supplement shows the associations of retinal layer thickness with cognition. In general, thinner RNFL and GC-IPL were associated with various cognitive tests, but these associations were not consistently seen on follow-up.
Discussion
In this prospective population-based cohort study, we found that thinner RNFL was associated with a higher risk of developing dementia, including AD, independent of cardiovascular risk factors. Thus far, evidence for retinal involvement in AD pathology comes primarily from histopathological studies, in which postmortem specimens were examined. Those early studies have shown that people with AD had substantial loss of retinal ganglion cells and thinner RNFL compared with controls.26,27 Subsequently, clinical-based studies have consistently observed RNFL thinning among patients with AD using optical imaging techniques in vivo.8,9 Advances in OCT technology allow us to observe the retina in greater detail, enabling automated segmentation of other retinal layers as well. Hence, studies have shown that patients with AD had, apart from thinner RNFL, also thinner GCL-IPL compared with cognitively healthy controls, suggesting that thinner GC-IPL is accompanied by thinner peripapillary RNFL.10,11,12 However, owing to the cross-sectional nature of these studies, it remained unclear whether thinner retinal layers preceded the clinical manifestation of AD or were only seen after a diagnosis had been made. In line with findings from previous studies, we found that particularly thinner GC-IPL tend to be statistically significantly associated with prevalent dementia. More importantly, our study is the first that shows an association between thinner RNFL and the risk of dementia, including AD, to our knowledge.
In patients with dementia, damage to brain regions covering the visual tract may cause retrograde degeneration of the optic nerve by affecting the neuronal connections of the visual tract. Subsequently, this neurodegenerative process may manifest itself in the retina initially as thinner RNFL, after which thinning of the GC-IPL follows. A 2016 study has shown that a reduction in GCL-IPL at baseline was associated with AD progression as measured by the Clinical Dementia Rating Scale.11 Hence, given our findings, it is possible that thinner GC-IPL is reflecting more advanced stages of AD pathology. However, an important question remains why thinner RNFL was not associated with prevalent dementia with a similar effect magnitude as the GC-IPL. Apart from loss of power owing to smaller number of prevalent dementia cases, we speculate that the time-course when dementia occurs might play a role. For instance, damage to the optic nerve causes swelling and gliosis formation of the axons.28,29,30 In fact, there appears to be a time delay between optic nerve degeneration and retinal ganglion cell loss, during which swelling or gliosis formation of structures can occur, and after which structural losses become more evident. Another possibility might be that differences in characteristics between those included and excluded may have introduced selection bias in the cross-sectional analyses and thus may underlie the lack of an association between RNFL and prevalent dementia. In contrast, in the longitudinal analyses, selection bias was not an issue because our follow-up for incident dementia was virtually complete. Furthermore, that we did not find an association of RNFL with prevalent dementia, but several clinical-based studies did, may be due to publication bias. Future meta-analyses on this topic should include a funnel plot.
Apart from retrograde degeneration as a possible explanation for our findings, another explanation might be that a common pathogenesis underlies the association between RNFL and dementia. Studies have observed the accumulation of amyloid-β deposits both in the retina and brain of transgenic mouse models and corroborated those findings in postmortem studies in patients with AD.3,4,31,32 Although exact mechanisms that underlie the formation of amyloid plaques are unknown, factors associated with the formation of these plaques such as inflammatory markers and genetic variants, may partly explain our findings. However, we were not able to control for such confounding factors. It should be noted that there is insufficient evidence to conclude whether retinal amyloid plaques are really reflective of dementia pathogenesis, and whether retinal amyloid-β can be used as a diagnostic tool for dementia.33 To this end, future studies are needed.
While we exclusively have shown an association of RNFL and GC-IPL with dementia, thickness of these layers has already been more extensively studied in other neurologic disorders, particularly multiple sclerosis. Hence, RNFL and GC-IPL thicknesses may provide information on neurodegeneration in general rather than specifically on dementia.
Further, we observed cross-sectional associations of the RNFL and in particular GC-IPL with various cognitive tests and global cognition, but these associations were not consistently seen on follow-up. However, these longitudinal analyses were hampered by substantial attrition (23%). As participants without cognitive reassessment were on average older and had thinner nerve fiber layers, this may have introduced selection bias that most likely led to underestimation of a true association. In contrast, our dementia follow-up is nearly complete (95% of potential person-years) because of continuous linkage with medical records in addition to in-person screening.
Limitations and Strengths
Several methodological aspects of our study need to be discussed. First, individuals excluded from our study had poor health, resulting in selection of relatively healthy individuals in our analysis. Second, participants were mainly middle-class white individuals, which limits the generalizability of our findings. Another limitation may be that we performed OCT on the right eyes. Studies have suggested that there might be structural and functional differences between eyes.34 However, we did not find significant differences in retinal layer thicknesses between left and right eyes, but possible explanations for intereye differences may be sampling variability, measurement error, and differences in mydriasis, illumination, refractive error, or signal strength. Moreover, the neurodegenerative process underlying dementia is generalized and should not be limited to 1 eye. Hence, we do not think that differences between eyes will have influenced the associations we found with dementia. Next, we did not use other retinal layers because the reliability of the segmentation for outer layers were poor and the thicknesses were not comparable across the 2 OCT devices. Finally, owing to the relatively small number of incident dementia cases, our current study is underpowered to properly perform prediction modeling for dementia. For dementia prediction, although researchers are in the process of developing such models, there is currently no established prediction models available, such as the Framingham Stroke Risk Score for stroke, but future studies should consider to take retinal structures into account. Strengths of our study include the population-based setting, longitudinal design, and thorough collection of events.
Conclusions
In conclusion, we found that thinner GCL was associated with prevalent dementia and that thinner RNFL was associated with an increased risk of developing dementia, including AD. These findings suggest that thinner RNFL may be a novel biomarker for dementia, specifically for AD. Moreover, there is an opportunity to use OCT in clinical or research settings as an accessible and noninvasive tool to help clinicians or researchers in eligibility determination for clinical trials, in monitoring disease progression or in evaluating treatment response.
eMethods. Assessment of covariates and statistical analysis
eTable 1. Number of persons classified according to the Clinical Dementia Rating Scale
eTable 2. Association of retinal layer thickness in quartiles with risk of dementia and Alzheimer’s disease
eTable 3. Association of retinal layer thickness with prevalent and incident dementia adjusting the retinal layers for each other
eTable 4. Association of retinal layer thickness with cognition
eFigure. Scatterplots of retinal layer thickness between left and right eyes.
eReferences
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63-75.e2. [DOI] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(suppl 1):S204-S217. doi: 10.1016/j.neuroimage.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koronyo Y, Biggs D, Barron E, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2(16):93621. doi: 10.1172/jci.insight.93621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrory S, Cameron JR, Pellegrini E, et al. The application of retinal fundus camera imaging in dementia: a systematic review. Alzheimers Dement (Amst). 2016;6:91-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart NJ, Koronyo Y, Black KL, Koronyo-Hamaoui M. Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathol. 2016;132(6):767-787. doi: 10.1007/s00401-016-1613-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javaid FZ, Brenton J, Guo L, Cordeiro MF. Visual and ocular manifestations of Alzheimer’s disease and their use as biomarkers for diagnosis and progression. Front Neurol. 2016;7:55. doi: 10.3389/fneur.2016.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppola G, Di Renzo A, Ziccardi L, et al. Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PLoS One. 2015;10(8):e0134750. doi: 10.1371/journal.pone.0134750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Haan J, Verbraak FD, Visser PJ, Bouwman FH. Retinal thickness in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement (Amst). 2017;6:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung CY, Ong YT, Hilal S, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2015;45(1):45-56. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Park SJ, Kim NR. Macular ganglion cell -inner plexiform layer thickness is associated with clinical progression in mild cognitive impairment and Alzheimers disease. PLoS One. 2016;11(9):e0162202. doi: 10.1371/journal.pone.0162202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marziani E, Pomati S, Ramolfo P, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(9):5953-5958. doi: 10.1167/iovs.13-12046 [DOI] [PubMed] [Google Scholar]
- 13.Ong YT, Hilal S, Cheung CY, et al. Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neurosci Lett. 2015;584:12-16. doi: 10.1016/j.neulet.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 14.Mutlu U, Bonnemaijer PWM, Ikram MA, et al. Retinal neurodegeneration and brain MRI markers: the Rotterdam Study. Neurobiol Aging. 2017;60:183-191. doi: 10.1016/j.neurobiolaging.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 15.Hofman A, Brusselle GG, Darwish Murad S, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol. 2015;30(8):661-708. doi: 10.1007/s10654-015-0082-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry L, Cassels N, Lu K, et al. Automated retinal layer segmentation using spectral domain optical coherence tomography: evaluation of inter-session repeatability and agreement between devices. PLoS One. 2016;11(9):e0162001. doi: 10.1371/journal.pone.0162001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K, Buitendijk GH, Bogunovic H, et al. Automated segmentability index for layer segmentation of macular SD-OCT images. Transl Vis Sci Technol. 2016;5(2):14. doi: 10.1167/tvst.5.2.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. doi: 10.1371/journal.pone.0034823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. ; IMSVISUAL consortium . The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303-2309. doi: 10.1212/WNL.0000000000002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel PJ, Foster PJ, Grossi CM, et al. ; UK Biobank Eyes and Vision Consortium . Spectral-domain optical coherence tomography imaging in 67 321 adults: associations with macular thickness in the UK Biobank Study. Ophthalmology. 2016;123(4):829-840. doi: 10.1016/j.ophtha.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 21.Keane PA, Grossi CM, Foster PJ, et al. ; UK Biobank Eye Vision Consortium . Optical coherence tomography in the UK Biobank Study: rapid automated analysis of retinal thickness for large population-based studies. PLoS One. 2016;11(10):e0164095. doi: 10.1371/journal.pone.0164095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 23.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN international workshop. Neurology. 1993;43(2):250-260. doi: 10.1212/WNL.43.2.250 [DOI] [PubMed] [Google Scholar]
- 24.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566-572. doi: 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- 25.Hoogendam YY, Hofman A, van der Geest JN, van der Lugt A, Ikram MA. Patterns of cognitive function in aging: the Rotterdam Study. Eur J Epidemiol. 2014;29(2):133-140. doi: 10.1007/s10654-014-9885-4 [DOI] [PubMed] [Google Scholar]
- 26.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315(8):485-487. doi: 10.1056/NEJM198608213150804 [DOI] [PubMed] [Google Scholar]
- 27.Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 1989;501(2):364-372. doi: 10.1016/0006-8993(89)90653-7 [DOI] [PubMed] [Google Scholar]
- 28.Rovere G, Nadal-Nicolás FM, Agudo-Barriuso M, et al. Comparison of retinal nerve fiber layer thinning and retinal ganglion cell loss after optic nerve transection in adult albino rats. Invest Ophthalmol Vis Sci. 2015;56(8):4487-4498. doi: 10.1167/iovs.15-17145 [DOI] [PubMed] [Google Scholar]
- 29.Abbott CJ, Choe TE, Lusardi TA, Burgoyne CF, Wang L, Fortune B. Evaluation of retinal nerve fiber layer thickness and axonal transport 1 and 2 weeks after 8 hours of acute intraocular pressure elevation in rats. Invest Ophthalmol Vis Sci. 2014;55(2):674-687. doi: 10.1167/iovs.13-12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal-Sanz M, Galindo-Romero C, Valiente-Soriano FJ, et al. Shared and differential retinal responses against optic nerve injury and ocular hypertension. Front Neurosci. 2017;11:235. doi: 10.3389/fnins.2017.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koronyo Y, Salumbides BC, Black KL, Koronyo-Hamaoui M. Alzheimer’s disease in the retina: imaging retinal aβ plaques for early diagnosis and therapy assessment. Neurodegener Dis. 2012;10(1-4):285-293. doi: 10.1159/000335154 [DOI] [PubMed] [Google Scholar]
- 32.Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49(11):5136-5143. doi: 10.1167/iovs.08-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang J, Wang H, Li W, Cao X, Li C. Amyloid plaques in retina for diagnosis in Alzheimer’s patients: a meta-analysis. Front Aging Neurosci. 2016;8:267. doi: 10.3389/fnagi.2016.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron JR, Megaw RD, Tatham AJ, et al. Lateral thinking: interocular symmetry and asymmetry in neurovascular patterning, in health and disease. Prog Retin Eye Res. 2017;59:131-157. doi: 10.1016/j.preteyeres.2017.04.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Assessment of covariates and statistical analysis
eTable 1. Number of persons classified according to the Clinical Dementia Rating Scale
eTable 2. Association of retinal layer thickness in quartiles with risk of dementia and Alzheimer’s disease
eTable 3. Association of retinal layer thickness with prevalent and incident dementia adjusting the retinal layers for each other
eTable 4. Association of retinal layer thickness with cognition
eFigure. Scatterplots of retinal layer thickness between left and right eyes.
eReferences