This randomized clinical trial examines whether calcitonin gene-related peptide induces cluster headache attacks in episodic cluster headache in active phase, episodic cluster headache in remission phase, and chronic cluster headache.
Key Points
Question
Does calcitonin gene-related peptide play a role in initiating cluster headache attacks in patients with episodic cluster headache in active phase, episodic cluster headache in remission, and chronic cluster headache?
Findings
In this randomized cross-over trial of 32 patients with cluster headache, Calcitonin gene-related peptide provoked cluster headache attacks in patients with episodic cluster headache exclusively during active phase, not in remission phase, and provokes attacks in chronic cluster headache.
Meaning
Calcitonin gene-related peptide plays a pivotal role in initiation of cluster headache attacks, possibly indicating efficacy of calcitonin gene-related peptide antagonism in the treatment of cluster headache.
Abstract
Importance
Signaling molecule calcitonin gene-related peptide (CGRP) induces migraine attacks and anti-CGRP medications abort and prevent migraine attacks. Whether CGRP provokes cluster headache attacks is unknown.
Objective
To determine whether CGRP induces cluster headache attacks in episodic cluster headache in active phase, episodic cluster headache in remission phase, and chronic cluster headache.
Design, Setting, and Participants
A randomized, double-blind, placebo-controlled, 2-way crossover study set at the Danish Headache Center, Rigshospitalet Glostrup, in Denmark. Analyses were intent to treat. Inclusion took place from December 2015 to April 2017. Inclusion criteria were diagnosis of episodic/chronic cluster headache, patients aged 18 to 65 years, and safe contraception in women. Exclusion criteria were a history of other primary headache (except episodic tension-type headache <5 days/mo), individuals who were pregnant or nursing; cardiovascular, cerebrovascular, or psychiatric disease; and drug misuse.
Interventions
Thirty-seven patients with cluster headaches received intravenous infusion of 1.5 μg/min of CGRP or placebo over 20 minutes on 2 study days.
Main Outcomes and Measures
Difference in incidence of cluster headache–like attacks, difference in area under the curve (AUC) for headache intensity scores (0 to 90 minutes), and difference in time to peak headache between CGRP and placebo in the 3 groups.
Results
Of 91 patients assessed for eligibility, 32 patients (35.2%) were included in the analysis. The mean (SD) age was 36 (10.7) years (range, 19-60 years), and the mean weight was 78 kg (range, 53-100 kg). Twenty-seven men (84.4%) completed the study. Calcitonin gene-related peptide induced cluster headache attacks in 8 of 9 patients in the active phase (mean, 89%; 95% CI, 63-100) compared with 1 of 9 in the placebo group (mean, 11%; 95% CI, 0-37) (P = .05). In the remission phase, no patients with episodic cluster headaches reported attacks after CGRP or placebo. Calcitonin gene-related peptide–induced attacks occurred in 7 of 14 patients with chronic cluster headaches (mean, 50%; 95% CI, 20-80) compared with none after placebo (P = .02). In patients with episodic active phase, the mean AUC from 0 to 90 minutes for CGRP was 1.903 (95% CI, 0.842-2.965), and the mean AUC from 0 to 90 minutes for the placebo group was 0.343 (95% CI, 0-0.867) (P = .04). In patients with chronic cluster headache, the mean AUC from 0 to 90 minutes for CGRP was 1.214 (95% CI, 0.395-2.033), and the mean AUC from 0 to 90 minutes for the placebo group was 0.036 (95% CI, 0-0.114) (P = .01). In the remission phase, the mean AUC from 0 to 90 minutes for CGRP was 0.187 (95% CI, 0-0.571), and the mean AUC from 0 to 90 minutes for placebo was 0.019 (95% CI, 0-0.062) (P > .99).
Conclusions and Relevance
Calcitonin gene-related peptide provokes cluster headache attacks in active-phase episodic cluster headache and chronic cluster headache but not in remission-phase episodic cluster headache. These results suggest anti-CGRP drugs may be effective in cluster headache management.
Trial Registration
ClinicalTrials.gov (NCT02466334).
Introduction
Cluster headache is a primary headache disease with an estimated prevalence at 0.5 to 1.0 of 1000 of the general population.1 Cluster headache attacks feature a striking combination of extreme unilateral pain in the periorbital area accompanied by prominent ipsilateral cephalic autonomic symptoms (CAS), such as tearing, conjunctival redness, rhinorrhea or nasal congestion, ptosis, as well as sense of agitation or restlessness.2 The disease shows remarkable periodicity with most patients experiencing episodic cluster headache with month-long attack periods separated by remission periods.3 The remaining 10% to 15% of patients experience chronic cluster headache. With attacks lasting up to 3 hours occurring up to 8 times daily,2 cluster headache greatly burdens individuals as well as society with increased health care expenses and long sick-leave periods.1 The signaling pathways responsible for initiating cluster periods and individual attacks are unknown.3 Not surprisingly, treatment options are limited, and no disease-specific preventive medication exists.4
In migraine, signaling molecule calcitonin gene-related peptide (CGRP) has gained considerable attention for its migraine-inducing abilities.5 Furthermore, CGRP antagonism with receptor antagonists or monoclonal antibodies aborts and prevents migraine.6 Although phenotypically different, migraine and cluster headache share some features like response to triptans,7,8 attack induction by glyceryl trinitrate,9,10 and elevated plasma levels of CGRP.11,12 Elucidating the role of CGRP in cluster headache attack induction will provide novel insights to mechanisms underlying the disease. A role of CGRP in attack generation would provide novel opportunities for targeted treatment of this severely disabling disease.
We hypothesized intravenous infusion of CGRP would provoke cluster headache attacks in episodic cluster headache during the active phase but not during the remission phase. We also hypothesized CGRP would provoke cluster headache attacks in patients with chronic cluster headache. To test these hypotheses, we conducted a randomized, double-blind, placebo-controlled, 2-way crossover study.
Methods
Study Design and Participants
We recruited participants from the Danish Headache Center outpatient clinic between December 2015 and April 2017. Participants aged 18 to 65 years were eligible when having a verified diagnosis of episodic or chronic cluster headache according to the International Classification of Headache Disorders.2 Patients were defined as having active-phase episodic cluster headache when experiencing usual attacks within the last 30 days, remission-phase episodic cluster headache when attack-free for a minimum of 30 days, or chronic cluster headache when no more than 30 consecutive attack-free days during the previous 12 months or longer. Cluster headache preventive medications were allowed. Use of safe contraceptive methods if a woman of childbearing potential was required. Exclusion criteria were any history of other primary headache (except episodic tension-type headache <5 days per month), pregnant or nursing women, cardiovascular or cerebrovascular disease, psychiatric disease, or drug misuse. Enrollment was done at Righospitalet Glostrup. The Regional Health Research Ethics Committee of the Capital Region approved the study (H-15006836). All participants gave written consent after receiving detailed oral and written information. The study was conducted at the Danish Headache Center in accordance with the Declaration of Helsinki,13 with later revisions. The study was registered at ClinicalTrials.gov (identifier NCT02466334) and approved by the Danish Data Protection Agency. The full trial protocol is available in Supplement 1.
Experimental Design
We randomly allocated participants to receive continuous intravenous infusion of 1.5 μg/min of CGRP or placebo during 20 minutes on 2 study days separated by at least 7 days. Calcitonin gene-related peptide dose was identical to that in migraine provocation studies.14,15 Participants were informed that CGRP might induce headache, but timing and type of headache, including if it would mimic their usual cluster headache attacks, was not discussed. No study individuals had previously participated in provocation studies. All procedures were performed with individuals in supine position, a venous catheter inserted into an antecubital vein, and CGRP infused over 20 minutes using a time- and volume-controlled infusion pump. For details on balanced allocation, blinding, and randomization, see eAppendix in Supplement 2.
All patients arrived nonfasting during the daytime. On study day 1, a brief medical and neurological examination and 12-lead electrocardiogram was performed before experiment start. Patients with episodic cluster headache in active phase and patients with chronic cluster headache were at least 3 hours attack-free. All patients with remission-phase episodic cluster headache were, apart from being cluster headache attack-free for a minimum of 30 days, also free of any other type of headache for at least 8 hours at infusion start. Pregnancy tests were completed in women before each infusion. After 15 minutes of rest, baseline (10 minutes before infusion start and at the time of infusion start) headache intensity and vital signs were measured.
Headache Intensity and Questionnaire
If headache of any type occurred, headache intensity was recorded. This was done at baseline (10 minutes before infusion start and at the time of infusion start) and after the intervention every 10 minutes until 1.5 hours (90 minutes after infusion start) on an 11-point numerical scale from 0 to 10, with 0 representing no headache; 1, a very mild headache (including sensations of pressing, throbbing, or otherwise altered sensation in the head not associated with pain); 5, headache of moderate intensity; and 10, the worst headache imaginable. If participants reported a cluster headache attack at 70 minutes after infusion start and later, we extended the recording period with at least 30 minutes to ensure complete capture of attack development. Participants were asked to characterize any headache occurring during the observation period as resembling usual cluster headache or not. Additionally, headache characteristics (throbbing, stabbing, pressing), headache localization, and intake of rescue medication including intake time and dosage were recorded.
Cluster Headache–like Attack Criteria
The applied criteria for an experimentally provoked clusterlike attack in the present study are shown in the Box (see eAppendix in Supplement 2 for background on criteria). Based on previous cluster headache provocation studies,10,16,17 we defined attacks as provocation-related when occurring 0 to 90 minutes after infusion start. On study day 1 before infusion, we asked participants to retrospectively estimate their attack frequency the preceding 30 days. For measured vital signs, nonheadache, and CAS, see eAppendix in Supplement 2.
Box. Cluster Headache–like Attack Criteria.
The following criteria were used for an experimentally induced clusterlike attack. Clusterlike headache attack must fulfill either:
Headache described as mimicking the patient’s usual cluster headache attack (with or without cephalic autonomic symptom).
-
Headache fulfilling criteria A and B for cluster headache according to International Classification of Headache Disorders criteria2:
Severe unilateral pain lasting 15 to 180 minutes.
-
Either or both of the following:
At least 1 cephalic autonomic symptom ipsilateral to the headache.
A sense of restlessness or agitation.
Statistical Analyses
Headache intensity scores are presented as medians and time to onset of cluster headache–like attacks, and autonomic symptoms after CGRP infusion are presented as medians, interquartiles, and range. Heart rate and mean arterial pressure data are presented as median values. Sample size was chosen based on previous similar migraine studies showing migraine attack induction in 60% to 70% of patients after administration of CGRP and 20% after placebo at 5% significance with 80% power.5 We calculated that 15 participants in each group would be sufficient to show a difference between 2 experimental days (eAppendix in Supplement 2). The primary end points were difference in incidence of cluster headache–like attacks, difference in area under the curve (AUC) for headache intensity scores (0 to 90 minutes), and difference in time to peak headache between CGRP and placebo in the 3 groups. Secondary end points were differences in AUC for mean arterial pressure and heart rate during the in-hospital phase (0 to 90 minutes). Incidence of clusterlike attacks and CAS in each group of patients was analyzed as categorical data using McNemar test. We calculated AUC according to the trapezium rule to obtain a summary measure. Area under the curve from 0 to 90 minutes for headache scores, mean arterial pressure, and heart rate were compared using Wilcoxon signed-rank test. Incidence of attacks and AUC from 0 to 90 minutes of headache are presented with means and 95% CI. We tested for carryover effects of randomization to CGRP vs placebo on study day 1 with Fisher exact test. Difference in time to peak headache between CGRP and placebo was not calculated owing to a total of only 1 cluster headache attack occurring with placebo. All analyses were performed with GraphPad Prism Statistics, version 7, for Windows (GraphPad Software, Inc). We made no adjustment for multiple analyses. Thus, the level of significance at .05 was accepted for each comparison.
Results
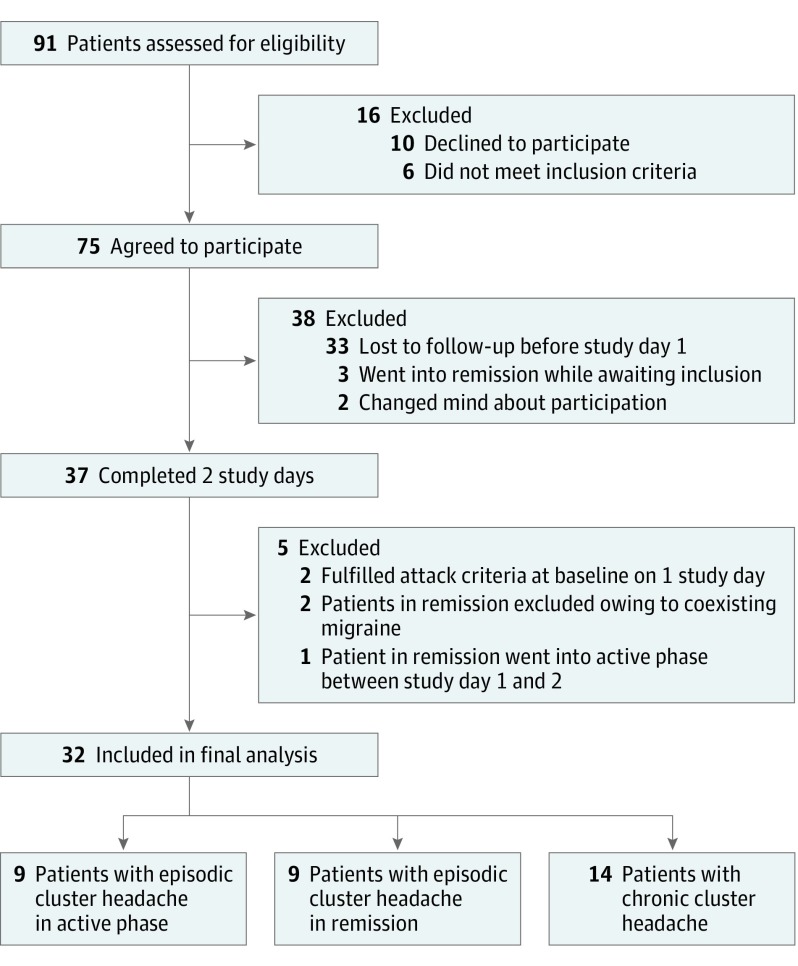
Of 37 recruited participants, 32 (86.5%) were included in the final analysis (27 men [84.4%] and 5 women [15.6%]) (Figure 1). The mean (SD) age was 36 (10.7) (range, 19-60 years) years, and the mean weight was 78 kg (range, 53-100 kg). Enrollment ended before reaching 45 participants owing to recruitment difficulties. Nine patients (28.1%) with episodic cluster headache were included during active phases and 9 (28.1%) were included during remission. Fourteen patients (43.8%) had chronic cluster headache. Detailed descriptions of usual attacks, attack treatment, preventive medication, and study day information are listed in the Table. For analysis of carryover effect and details on intake of preventive medication, patients in active disease phase, and vital signs, see eAppendix in Supplement 2.
Figure 1. Flowchart of Participant Recruitment of Patients With Episodic and Chronic Cluster Headache .
Table. Attack Characteristics of Patients With Cluster Headachesa.
| Patient No./Sex | Type of Intervention | Headache Characteristics/Peak Intensityb | Autonomic Symptoms/Agitation | Time to Onset (Duration), min | Mimics Usual Cluster Attackc | Time to Peak From Onset, min | Immediate Rescue Therapy (Time, d)/Effectd | Preventive Treatment | Disease Duration, y |
|---|---|---|---|---|---|---|---|---|---|
| Episodic Cluster Headache in Active Phase Group | |||||||||
| ECHA01/M | Usual | Right/10 | Conjunctival redness; lacrimation; rhinorrhea | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection | Verapamil, 400 mg | 28 |
| CGRPe | Left/1 | Lacrimation; ptosis; miosis | 70 (15) | Yes | 0 | No | Verapamil, 400 mg | NA | |
| Placebo | None | None | NA | NA | NA | NA | Verapamil, 400 mg | NA | |
| ECHA 02/M | Usual | Left/9 | Conjunctival redness; lacrimation; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; oxygen | None | 7 |
| CGRP | None | None | NA | NA | NA | NA | None | NA | |
| Placeboe | Left/2 | Lacrimation; agitation | 20 (80) | Yes | 0 | No | None | NA | |
| ECHA 03/M | Usual | Left/10 | Conjunctival redness; miosis; forehead and facial sweating; ptosis; agitation | NA | NA | NA | None | None | 3 |
| CGRPe | Left/3 | Ptosis | 30 (10) | Yes | 0 | No | None | NA | |
| Placebo | None | None | NA | NA | NA | NA | None | NA | |
| ECHA 04/F | Usual | Right/10 | Conjunctival redness; lacrimation; rhinorrhea; nasal congestion; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection | None | 4 |
| CGRPe | Right/10 | Nasal congestion; ptosis; agitation | 20 (40) | Yes | 30 | Sumatriptan, 6-mg subcutaneous injection; oxygen (14, 16)/yes | None | NA | |
| Placebo | None | None | NA | NA | NA | NA | None | NA | |
| ECHA 05/F | Usual | Right/10 | Conjunctival redness; lacrimation; rhinorrhea; agitation | NA | NA | NA | Oxygen | None | 15 |
| CGRPe | Right/10 | Nasal congestion; ptosis; agitation | 60 (70) | Yes | 10 | Oxygen (63)/NA | None | NA | |
| Placebo | None | None | NA | NA | NA | NA | None | NA | |
| ECHA 06/F | Usual | Right/10 | Conjunctival redness; miosis; ptosis; forehead and facial sweating | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; rizatriptan, 10 mg | Verapamil, 560 mg | 2 |
| CGRPe | Right/10 | Conjunctival redness; lacrimation; forehead and facial sweating; ptosis; agitation | 13 (40) | Yes | 20 | Sumatriptan, 6-mg subcutaneous injection (18)/yes | Verapamil, 560 mg | NA | |
| Placebo | None | None | NA | NA | NA | NA | Verapamil, 560 mg | NA | |
| ECHA 09/M | Usual | NA/8 | Conjunctival redness; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection | Greater occipital nerve blockade | 16 |
| CGRPe | Left/7 | Lacrimation; ptosis; agitation | 20 (60) | Yes | 30 | Sumatriptan, 6-mg subcutaneous injection (45, 65)/yes | None | NA | |
| Placebo | None | Lacrimation | NA | NA | NA | NA | None | NA | |
| ECHA 10/M | Usual | Right/8 | Conjunctival redness; eyelid edema; rhinorrhea; ptosis; forehead and facial sweating | 30 (40) | Yes | 10 | None | None | 20 |
| CGRPe | Right/3 | Ptosis | 30 (40) | Yes | 10 | No | None | NA | |
| Placebo | Right/1 | None | 10 (110) | No | 0 | None | None | NA | |
| ECHA 11/M | Usual | Right/10 | Conjunctival redness; lacrimation; miosis; nasal congestion rhinorrhea; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; oxygen | Verapamil, 400 mg | 5 |
| CGRPe | Right/4 | Agitation | 60 (20) | Yes | 10 | Oxygen (64)/yes | Verapamil, 400 mg | NA | |
| Placebo | None | None | NA | NA | NA | NA | Verapamil, 400 mg | NA | |
| Chronic Cluster Headache Group | |||||||||
| CCH01/M | Usual | Right/9 | Agitation | NA | NA | NA | Oxygen | Verapamil, 240 mg | 6 |
| CGRP | NA | NA | NA | NA | NA | NA | Verapamil, 240 mg | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | Verapamil, 240 mg | NA | |
| CCH02/M | Usual | Left/6 | Conjunctival redness; eyelid edema; nasal congestion; rhinorrhea; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; voltaren, 25-mg subcutaneous injection | None | 27 |
| CGRPe | Left/10 | Lacrimation; rhinorrhea; eyelid edema; forehead and facial sweating; agitation | 20 (15) | Yes | 20 | Voltaren, 25-mg subcutaneous injection | None | NA | |
| Placebo | NA | Eyelid edema | NA | NA | NA | NA | None | NA | |
| CCH03/M | Usual | Left/10 | Conjunctival redness; miosis; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; oxygen | Verapamil, 440 mg | 11 |
| CGRP | NA | NA | NA | NA | NA | NA | Verapamil, 440 mg | NA | |
| Placebo | NA | Eyelid edema | NA | NA | NA | NA | Verapamil, 440 mg | NA | |
| CCH04/M | Usual | Right/1 0 | Conjunctival redness; lacrimation; nasal congestion; rhinorrhea; agitation | NA | NA | NA | None | Verapamil, 480 mg | 12 |
| CGRPe | Right/3 | Conjunctival redness; nasal congestion | 20 (30) | Yes | 0 | None | Verapamil, 480 mg | NA | |
| Placebo | Right/1 | None | 20 (20) | No | 0 | None | Verapamil, 480 mg | NA | |
| CCH05/F | Usual | Right/NA | Lacrimation; nasal congestion; rhinorrhea; agitation | NA | NA | NA | Oxygen | Verapamil, 100 mg | 2 |
| CGRPe | Right/10 | Conjunctival redness; lacrimation; nasal congestion; eyelid edema; ptosis; agitation | 10 (30) | Yes | 10 | Sumatriptan, 6-mg subcutaneous injection; oxygen (35)/yes | Verapamil, 100 mg | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | Verapamil, 100 mg | NA | |
| CCH06/F | Usual | Left/9 | Conjunctival redness; lacrimation; miosis; eyelid edema; rhinorrhea; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; oxygen; sumatriptan, 100 mg orally | NA | 10 |
| CGRP | NA | NA | NA | NA | NA | NA | NA | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | NA | NA | |
| CCH07/M | Usual | Right/7 | Conjunctival redness; nasal congestion; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection | Verapamil, 400 mg | 13 |
| CGRP | NA | NA | NA | NA | NA | NA | Verapamil, 400 mg | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | Verapamil, 400 mg | NA | |
| CCH08/M | Usual | Left/10 | Conjunctival redness; lacrimation; rhinorrhea; ptosis; agitation | NA | NA | NA | Oxygen | Verapamil, 240 mg | 4 |
| CGRP | NA | NA | NA | NA | NA | NA | Verapamil, 240 mg | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | Verapamil, 240 mg | NA | |
| CCH09/M | Usual | Left/5 | Lacrimation; conjunctival redness; ptosis; agitation | NA | NA | NA | Oxygen | Verapamil, 600 mg | 3 |
| CGRPe | Left/3 | Conjunctival redness; lacrimation; agitation | 30 (70) | Yes | 10 | None | Verapamil, 600 mg | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | Verapamil, 600 mg | NA | |
| CCH10/M | Usual | Left/9 | Lacrimation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; oxygen | None | 6 |
| CGRP | Bilateral/1 | None | 10 (20) | No | 0 | None | None | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | None | NA | |
| CCH11/M | Usual | Left,right/8 | Nasal congestion; rhinorrhea; agitation | NA | NA | NA | Oxygen; sumatriptan, 100-mg orally | Verapamil, 240 mg; lithium, 200 mg |
35 |
| CGRPe | NA/1 | None | 60 (30) | Yes | 0 | None | Verapamil, 240 mg; lithium, 200 mg |
NA | |
| Placebo | NA | NA | NA | NA | NA | NA | Verapamil, 240 mg; lithium, 200 mg |
NA | |
| CCH12/F | Usual | Left/NA | Lacrimation; eyelid edema; rhinorrhea; nasal congestion; forehead and facial sweating; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; sphenopalatine ganglion neurostimulator | Melatonin, 4 mg | 10 |
| CGRPe | Left/10 | Conjunctival redness; lacrimation; rhinorrhea; nasal congestion; eyelid edema; forehead and facial sweating; ptosis; agitation | 13 (40) | Yes | 10 | Sphenopalatine ganglion neurostimulator | Melatonin, 4 mg | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | Melatonin, 4 mg | NA | |
| CCH13/M | Usual | Right/9 | Lacrimation; conjunctival redness; eyelid edema; nasal congestion; rhinorrhea; forehead and facial sweating; ptosis; agitation | NA | NA | NA | Oxygen | None | 26 |
| CGRP | NA | NA | NA | NA | NA | NA | None | NA | |
| Placebo | NA | Agitation | NA | NA | NA | NA | None | NA | |
| CCH14/M | Usual | Right/10 | Conjunctival redness; lacrimation; nasal congestion; rhinorrhea; agitation | NA | NA | NA | Sumatriptan, 100-mg orally | None | 5 |
| CGRPe | Right/5 | Conjunctival redness | 60 (30) | Yes | 10 | Oxygen (60)/yes | None | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | None | NA | |
| Episodic Cluster Headache in Remission Group | |||||||||
| ECHR01/M | Usual | Right/NA | Conjunctival redness; lacrimation; rhinorrhea; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection | None | 1 |
| CGRP | Bilateral/1 | Lacrimation | 20 (10) | No | 0 | None | None | NA | |
| Placebo | Bilateral/1 | None | 20 (10) | No | 0 | None | None | NA | |
| ECHR02/M | Usual | Right/10 | Conjunctival redness; lacrimation; miosis; eyelid edema; rhinorrhea; nasal congestion; forehead and facial sweating; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; oxygen | Verapamil, 400 mg | 4 |
| CGRP | NA | NA | NA | NA | NA | NA | Verapamil, 400 mg | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | Verapamil, 400 mg | NA | |
| ECHR03/M | Usual | Right/10 | Conjunctival redness; eyelid edema; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection | None | 12 |
| CGRP | NA | Nasal congestion | NA | NA | NA | NA | None | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | None | NA | |
| ECHR04/M | Usual | Left/10 | Conjunctival redness; miosis; forehead and facial sweating; ptosis; agitation | NA | NA | NA | None | None | 3 |
| CGRP | NA | NA | NA | NA | NA | NA | None | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | None | NA | |
| ECHR05/M | Usual | Right/NA | Conjunctival redness; lacrimation; rhinorrhea; ptosis; agitation | NA | NA | NA | sumatriptan, 6-mg subcutaneous injection; oxygen | None | 14 |
| CGRP | NA | NA | NA | NA | NA | NA | None | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | None | NA | |
| ECHR06/ M | Usual | Right/9 | Conjunctival redness; lacrimation; miosis; nasal congestion; forehead and facial sweating; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection | Verapamil, 800 mg | 15 |
| CGRP | NA | Ptosis | NA | NA | NA | NA | Verapamil, 800 mg | NA | |
| Placebo | NA | Ptosis | NA | NA | NA | NA | Verapamil, 800 mg | NA | |
| ECHR08/M | Usual | Right/7 | Lacrimation; ptosis; agitation | NA | None | None | 17 | ||
| CGRP | NA | NA | NA | NA | NA | NA | None | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | None | NA | |
| ECHR10/M | Usual | Right/9 | Conjunctival redness; lacrimation; eyelid edema; rhinorrhea; ptosis; agitation | NA | NA | NA | Sumatriptan, 6-mg subcutaneous injection; oxygen | None | 15 |
| CGRP | NA | Eyelid edema | NA | NA | NA | NA | None | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | None | NA | |
| ECHR12/M | Usual | Right/9 | Conjunctival redness; lacrimation; rhinorrhea; agitation | NA | NA | NA | None | None | 8 |
| CGRP | Right/2 | None | 40 (50) | No | 0 | None | None | NA | |
| Placebo | NA | NA | NA | NA | NA | NA | None | NA | |
Abbreviations: CCH, chronic cluster headache; CGRP, calcitonin gene-related peptide; ECHA, episodic cluster headache in active phase; ECHR, episodic cluster headache remission phase; NA, not applicable.
Cerebral autonomic symptoms, restlessness, and immediate treatment is reported from 0 to 90 minutes after 20 minutes of intravenous infusion of calcitonin CGRP (1.5 μg/min) or placebo.
Patients rated the intensity of their headache on an 11-point scale, with 0 indicating no headache; 1, a very mild headache (including a sensation of pressing or throbbing or otherwise altered sensation in the head not associated with pain); 5, headache of moderate intensity; and 10, the worst headache imaginable.
Clusterlike attack is defined according to criteria described in the Methods section.
Therapy effect: more than 50% reduction in pain intensity over 2 hours.
Patient developed a clusterlike attack on study days.
Patients With Active-Phase Episodic Cluster Headache
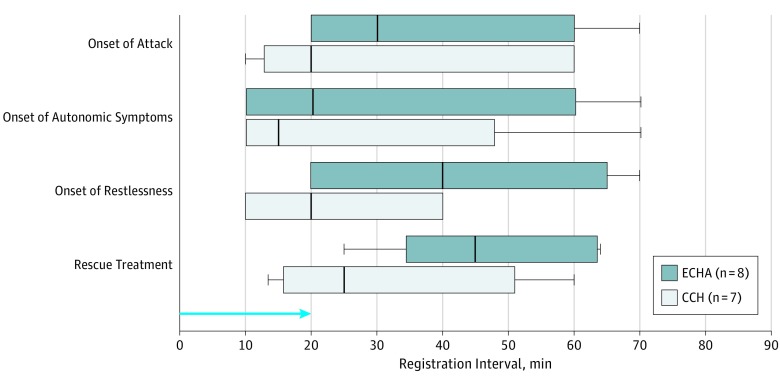
During the 90-minute in-hospital phase, 8 of 9 patients with active-phase episodic cluster headache reported a clusterlike attack after CGRP (mean, 89%; 95% CI, 63%-100%) compared with 1 of 9 after placebo (mean, 11%; 95% CI, 0%-37%) (P = .05) (Table). All except 1 attack occurred on the patient’s usual side, and no bilateral attacks occurred (Table). During CGRP-induced attacks, all 8 patients with episodic cluster headache in active phase experienced CAS and/or agitation (Table). The mean AUC from 0 to 90 minutes for CGRP was 1.903 (95% CI, 0.842-2.965), and the mean AUC from 0 to 90 minutes for placebo was 0.343 (95% CI, 0-0.867) (P = .04) (Figure 2).
Figure 2. Onset of Clusterlike Attack, Cephalic Autonomic Symptoms, Restlessness, and Intake of Rescue Medication .
Medians, interquartiles, and ranges from 0 to 90 minutes after 20-minute intravenous infusion of calcitonin gene-related peptide (1.5 μg/min) are reported. The blue arrow indicates the duration of infusion. CCH indicates chronic cluster headache, ECHA, episodic cluster headache in active phase.
Patients With Remission-Phase Episodic Cluster Headache
During the 90-minute in-hospital phase, no patients with remission-phase episodic cluster headache reported clusterlike attacks after CGRP or placebo (Table). In addition, no patients with remission-phase episodic cluster headache reported attacks the following 24 hours. The mean AUC from 0 to 90 minutes for CGRP was 0.187 (95% CI, 0-0.571), and the mean AUC from 0 to 90 minutes for placebo was 0.019 (95% CI, 0-0.062) (P > .99) (Figure 2). The eAppendix in Supplement 2 includes a description of headache in the 2 patients excluded for coexisting migraine.
Patients With Chronic Cluster Headache
During the 90-minute in-hospital phase, 7 of 14 patients with chronic cluster headache reported a clusterlike attack after CGRP (mean, 50%; 95% CI, 20%-80%) compared with none after placebo (mean, 0; 95% CI, 0%) (P = .02) (Table). All attacks occurred on the patient’s usual attack side, and no patients reported bilateral attacks (Table). During CGRP-induced attacks, 6 of 7 patients with chronic cluster headache experienced CAS (Table). The median time to onset of attack, autonomic symptoms, restlessness, and intake of medication are shown in Figure 3.
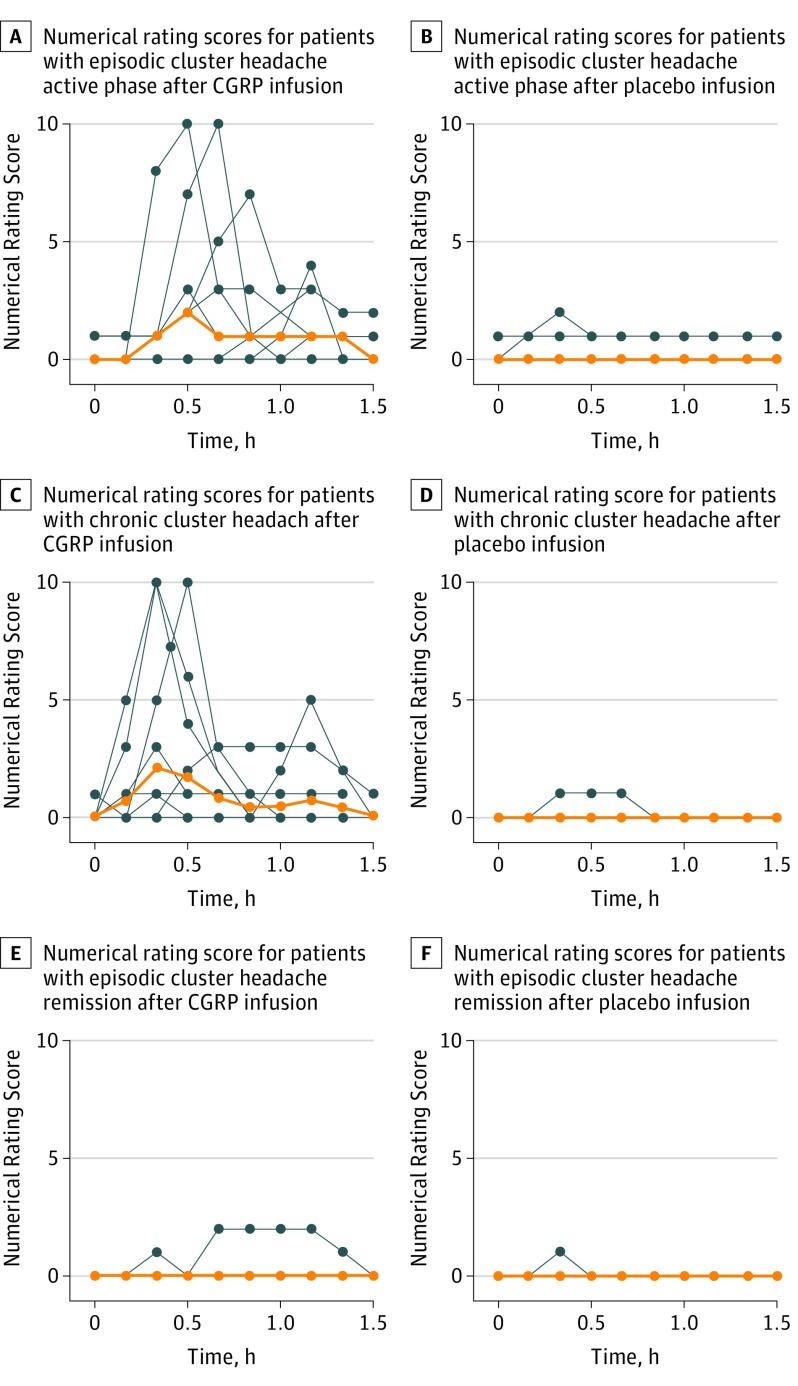
Figure 3. Numerical and Verbal Rating Scores of Patients.
The median is indicated by orange lines, and individuals are indicated by blue lines. Headache intensity was rated on an 11-point numerical scale from 0 to 10 for 9 patients with episodic cluster headache in active phase, 14 patients with chronic cluster headache, and 9 patients with episodic cluster headache in remission from 0 to 90 minutes after 20-minute intravenous infusion of calcitonin gene-related peptide (CGRP) (1.5 μg/min) and placebo. In episodic active phase, the area under the curve (AUC) 0 to 90 minutes of headache intensity after CGRP was larger than placebo (P = .04), the median peak attack pain intensity after CGRP was 3.5 (range, 1-10), mean of AUC from 0 to 90 minutes for CGRP was 1.903 (95% CI, 0.842-2.965), and the mean AUC from 0 to 90 minutes of placebo was 0.343 (95% CI, 0-0.867). In chronic cluster headache, the AUC from 0 to 90 minutes of headache intensity after CGRP was larger than placebo (P = .01), the mean of AUC from 0 to 90 minutes for CGRP was 1.214 (95% CI, 0.395-2.033), and mean AUC from 0 to 90 minutes of placebo was 0.036 (95% CI, 0-0.114). In episodic remission phase there was no difference in the AUC from 0 to 90 minutes of headache intensity between CGRP and placebo (P > .99, mean of AUC from 0 to 90 minutes for CGRP was 0.187 (95% CI, 0-0.571), and mean AUC from 0 to 90 minutes of placebo was 0.019 (95% CI, 0-0.062).
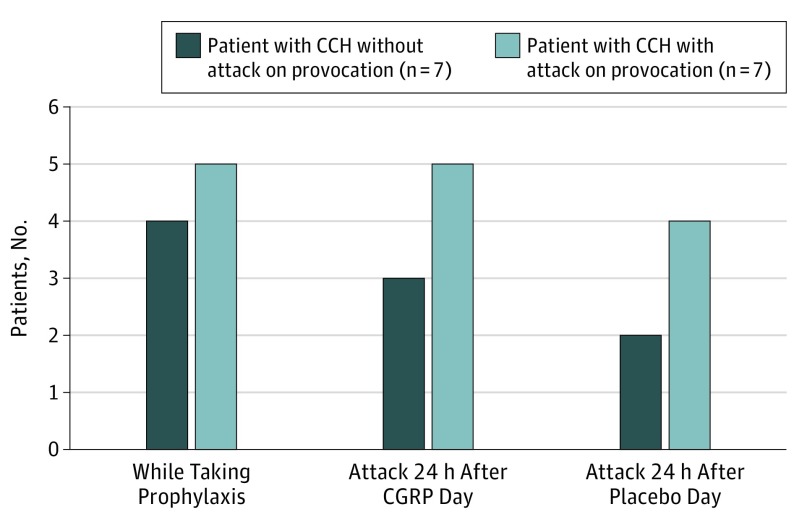
In the 30 days before study day 1, the 7 patients who did report a clusterlike attack after CGRP reported a median attack frequency of 33. The 7 patients who did not report attack after CGRP had a median attack frequency of 7.5 (Figure 4). The mean AUC from 0 to 90 minutes for CGRP was 1.214 (95% CI, 0.395-2.033), and the mean AUC from 0 to 90 minutes for placebo was 0.036 (95% CI, 0-0.114) (P = .01) (Figure 2).
Figure 4. Number of Patients With Chronic Cluster Headache (CCH) Taking Preventive Treatment and Experiencing Usual Attacks .
Usual attacks happened 24 hours after completing study days 1 and 2. Data are reported 0 to 90 minutes after 20-minute intravenous infusion of calcitonin gene-related peptide (CGRP) (1.5 μg/min) and placebo.
Discussion
A key novel finding of the present study was that CGRP provoked cluster headache attacks only in patients with episodic cluster headache during the active phase and not in patients in remission. Another important finding was the influence that attack frequency (periodicity) had on incidence of CGRP-induced attacks in patients with chronic cluster headache.
Overall, our findings suggest that (1) CGRP plays a pivotal role in initiation of a single cluster headache attack and (2) that CGRP induces cluster attacks may suggest a possible clinical efficacy of pharmacological interventions that block the effects of CGRP (eg, specific monoclonal antibodies). In episodic and chronic cluster headache, monoclonal CGRP antibodies are currently being investigated in phase III studies as preventive treatment with study completion expected in 2018.18
Our findings raised an important question of how infusion of CGRP induces cluster headache attacks in active disease phase (ie, patients with episodic cluster headache in active phase and patients with chronic cluster headache). Studies in patients with cluster headache reported elevated ictal plasma levels of CGRP, which normalized after oxygen inhalation and subcutaneous sumatriptan injection.11 Interestingly, plasma CGRP is also elevated in patients during active phase compared with patients during remission.17
Calcitonin gene-related peptide is one of the most powerful vasodilators known, and CGRP immunoreactive nerves innervate human cranial arteries.19 The intracellular signaling cascade after CGRP receptor activation is mediated by cyclic adenosine monophosphate,19 a pathway shared by other known headache/migraine–inducing molecules.20 Calcitonin gene-related peptide modulates the activity of nociceptive trigeminal neurons as well as central structures that process trigeminal pain.21 Neurons in the trigeminal19 and sphenopalatine22 ganglia express CGRP, and CGRP is released on the thermocoagulation of the trigeminal ganglion.23 This suggests that CGRP is optimally located to play a role at both the nociceptive and parasympathetic end of the trigemino-autonomic reflex. Since CGRP has very limited passage of the blood-brain barrier,24 the triggering site is likely to be peripheral. Calcitonin gene-related peptide may exert its cluster headache–inducing abilities in 3 distinctive ways. First, this may happen via vascular effects of CGRP, likely involving neurogenic inflammation.25 Second, CGRP receptor components are also found in the human trigeminal ganglion,26,27 which has been suggested as the possible site of action for the CGRP receptor antagonists in migraine treatment.26 Third, neurons in sphenopalatine ganglion express CGRP and its receptor components.22 Efferent outflow from sphenopalatine ganglia is suggested as initiating mechanism of cluster headache attacks, and on-demand sphenopalatine ganglia stimulation is an effective novel therapy for individuals with cluster headache with dual effects, acute pain relief, and attack prevention.28 Interestingly, in the present study, the median time to onset of parasympathetic symptoms preceded median onset of head pain. These data indicate that CGRP may induce parasympathetic outflow and trigger cluster headache attacks. Collectively, these data suggest that mechanisms behind CGRP-induced cluster headache attacks likely involve peripheral triggering through trigeminal afferents and parasympathetic efferents.
Previous nonplacebo-controlled studies showed glyceryl trinitrate induced cluster headache during active phase in 33% to 100% of patients.10,12,17,29 In chronic cluster headache, glyceryl trinitrate induced attacks in 20% to 78% of patients29,30 and no attacks in remission-phase episodic cluster headache.10,12,17 Our results extend these findings showing, placebo-controlled, that CGRP did not provoke cluster headache attacks during remission. Interestingly, compared with migraine provocation studies9,20 the observed placebo response rate was extremely low (only 1 patient with active phase episodic cluster headache after saline). These data suggest peripheral mechanisms alone cannot explain CGRP-induced attacks and central mechanisms altering the provocability threshold by CGRP should be considered. The remarkable circadian/circannual periodicity in cluster headache as well as neuroendocrine changes hint to the hypothalamus as responsible for altering the provocability threshold.3,4 Brain imaging studies have yielded substantial support for this hypothesis. Activation of the posterior hypothalamus is seen during cluster headache attacks.31 Additionally, structural abnormalities in the same region were detected using voxel-based morphometry magnetic resonance imaging in patients with cluster headache.32 However, later larger studies were unable to reproduce this.33 These controversies notwithstanding, deep brain stimulation studies showed that continuous posterior hypothalamus stimulation aborts clusters.34 Remarkably, though, relief is not instant34 as deep brain stimulation has no effect on acute attacks but prevents cluster headache attacks after a lag time of days to weeks. This suggests that simple direct neuronal inhibition in the hypothalamus is not responsible for the success of deep brain stimulation.35 Rather, the hypothalamus may modulate the system lowering the provocability threshold down to allowing a peripheral trigger to set off attacks.
In the present study, CGRP infusion induced cluster headache attacks in 7 (5 men and 2 women) of 14 (11 men and 3 women) patients with chronic cluster headache. Intake of preventive medications was similar in those who developed attack (5 of 7) and those who did not (4 of 7). Thus, intake of preventive medication seems insufficient in explaining provocability in patients with chronic cluster headache. Interestingly, exploratory analyses showed that in the month before provocation, patients with chronic cluster headache who experienced attacks after CGRP reported a median of 33 attacks, contrasting 7.5 attacks in patients who were not triggered by CGRP. This could indicate (1) those with fewer attacks may benefit more from their preventive medication than those with more attacks, possibly explaining their insusceptibility to CGRP provocation; (2) those with fewer attacks may have higher thresholds for CGRP-induced attacks; and (3) high attack frequency in those who developed attack after CGRP may indicate increased disease activity and low threshold for triggering because of possible periodicity in chronic cluster headache. In support, circannual periodicity of chronic cluster headache has been suggested in some patients with chronic cluster headache.36 Participants were asked about attack frequency in the month before provocation before infusion start on study day 1. Although participants at this time were unaware of their provocability status, we cannot rule out recall bias.
Limitations
We acknowledge some of the limitations of the present study. The differences in CGRP-induced attacks in patients with chronic cluster headache based on usual attack frequency should be interpreted cautiously because baseline (1 month before experimental day 1) attack frequency was collected retrospectively. Ideally, data should have been collected during 1-month run-in period by electronic diary. Furthermore, patients were allowed to use preventive medication, which could have influenced the threshold to induce cluster headache attacks. However, we found similar numbers of patients using preventive medication in patients with chronic cluster headaches who developed attacks and in patients with chronic cluster headaches who did not develop attacks. One might argue that some provoked attacks were mild to moderate in pain intensity. However, patients may report variability in pain intensity during spontaneous attacks, which is noted in the International Classification of Headache Disorders, 3rd edition, cluster headache criteria.2
Conclusions
We demonstrated that CGRP provokes cluster headache attacks in patients with cluster headache exclusively during active phase in episodic cluster headache and in chronic cluster headache. We hypothesize that this difference hails from the hypothalamus modulating the provocability threshold of the system allowing a peripheral trigger to set off attacks. Our results also cautiously suggest efficacy of CGRP antagonism in the treatment of cluster headache and current phase III trials elucidating this18 will emerge in coming years.
Trial protocol.
eAppendix. Supplementary methods and results
References
- 1.Jensen RM, Lyngberg A, Jensen RH. Burden of cluster headache. Cephalalgia. 2007;27(6):535-541. doi: 10.1111/j.1468-2982.2007.01330.x [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. doi: 10.1177/0333102413485658 [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1(4):251-257. doi: 10.1016/S1474-4422(02)00104-7 [DOI] [PubMed] [Google Scholar]
- 4.May A. Cluster headache: pathogenesis, diagnosis, and management. Lancet. 2005;366(9488):843-855. doi: 10.1016/S0140-6736(05)67217-0 [DOI] [PubMed] [Google Scholar]
- 5.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54-61. doi: 10.1046/j.1468-2982.2002.00310.x [DOI] [PubMed] [Google Scholar]
- 6.Dodick DW, Goadsby PJ, Spierings ELH, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13(9):885-892. doi: 10.1016/S1474-4422(14)70128-0 [DOI] [PubMed] [Google Scholar]
- 7.Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358(9294):1668-1675. doi: 10.1016/S0140-6736(01)06711-3 [DOI] [PubMed] [Google Scholar]
- 8.Ekbom K, Monstad I, Prusinski A, Cole JA, Pilgrim AJ, Noronha D; The Sumatriptan Cluster Headache Study Group . Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. Acta Neurol Scand. 1993;88(1):63-69. doi: 10.1111/j.1600-0404.1993.tb04189.x [DOI] [PubMed] [Google Scholar]
- 9.Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol. 1994;1(1):73-80. doi: 10.1111/j.1468-1331.1994.tb00053.x [DOI] [PubMed] [Google Scholar]
- 10.Ekbom K. Nitrolglycerin as a provocative agent in cluster headache. Arch Neurol. 1968;19(5):487-493. doi: 10.1001/archneur.1968.00480050057005 [DOI] [PubMed] [Google Scholar]
- 11.Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache: neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117(Pt 3):427-434. doi: 10.1093/brain/117.3.427 [DOI] [PubMed] [Google Scholar]
- 12.Fanciullacci M, Alessandri M, Figini M, Geppetti P, Michelacci S. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain. 1995;60(2):119-123. doi: 10.1016/0304-3959(94)00097-X [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Asghar MS, Hansen AE, Kapijimpanga T, et al. . Dilation by CGRP of middle meningeal artery and reversal by sumatriptan in normal volunteers. Neurology. 2010;75(17):1520-1526. doi: 10.1212/WNL.0b013e3181f9626a [DOI] [PubMed] [Google Scholar]
- 15.Asghar MS, Hansen AE, Amin FM, et al. . Evidence for a vascular factor in migraine. Ann Neurol. 2011;69(4):635-645. doi: 10.1002/ana.22292 [DOI] [PubMed] [Google Scholar]
- 16.Bogucki A. Studies on nitroglycerin and histamine provoked cluster headache attacks. Cephalalgia. 1990;10(2):71-75. doi: 10.1046/j.1468-2982.1990.1002071.x [DOI] [PubMed] [Google Scholar]
- 17.Fanciullacci M, Alessandri M, Sicuteri R, Marabini S. Responsiveness of the trigeminovascular system to nitroglycerine in cluster headache patients. Brain. 1997;120(Pt 2):283-288. doi: 10.1093/brain/120.2.283 [DOI] [PubMed] [Google Scholar]
- 18.Khan S, Olesen A, Ashina M. CGRP, a target for preventive therapy in migraine and cluster headache: Systematic review of clinical data [published online January 1, 2017]. Cephalalgia. doi: 10.1177/0333102417741297 [DOI] [PubMed] [Google Scholar]
- 19.Russell FA, King R, Smillie S-J, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099-1142. doi: 10.1152/physrev.00034.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schytz HW, Schoonman GG, Ashina M. What have we learnt from triggering migraine? Curr Opin Neurol. 2010;23(3):259-265. doi: 10.1097/WCO.0b013e328337b884 [DOI] [PubMed] [Google Scholar]
- 21.Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol. 2004;142(7):1171-1181. doi: 10.1038/sj.bjp.0705807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Csati A, Tajti J, Tuka B, Edvinsson L, Warfvinge K. Calcitonin gene-related peptide and its receptor components in the human sphenopalatine ganglion: interaction with the sensory system. Brain Res. 2012;1435:29-39. doi: 10.1016/j.brainres.2011.11.058 [DOI] [PubMed] [Google Scholar]
- 23.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23(2):193-196. doi: 10.1002/ana.410230214 [DOI] [PubMed] [Google Scholar]
- 24.Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther. 2005;77(3):202-213. doi: 10.1016/j.clpt.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 25.Buzzi MG, Carter WB, Shimizu T, Heath H III, Moskowitz MA. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology. 1991;30(11):1193-1200. doi: 10.1016/0028-3908(91)90165-8 [DOI] [PubMed] [Google Scholar]
- 26.Eftekhari S, Edvinsson L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci. 2011;12(1):112. doi: 10.1186/1471-2202-12-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller S, Liu H, Warfvinge K, et al. . Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience. 2016;328:165-183. doi: 10.1016/j.neuroscience.2016.04.046 [DOI] [PubMed] [Google Scholar]
- 28.Schoenen J, Jensen RH, Lantéri-Minet M, et al. . Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment: pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33(10):816-830. doi: 10.1177/0333102412473667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa A, Pucci E, Antonaci F, et al. . The effect of intranasal cocaine and lidocaine on nitroglycerin-induced attacks in cluster headache. Cephalalgia. 2000;20(2):85-91. doi: 10.1046/j.1468-2982.2000.00026.x [DOI] [PubMed] [Google Scholar]
- 30.Hannerz J, Jogestrand T.. Chronic cluster headache: provocation with carbon dioxide breathing and nitroglycerin. Headache. 1996;36(3):174-177. [DOI] [PubMed] [Google Scholar]
- 31.May A, Bahra A, Büchel C, Frackowiak RSJ, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352(9124):275-278. doi: 10.1016/S0140-6736(98)02470-2 [DOI] [PubMed] [Google Scholar]
- 32.May A, Ashburner J, Büchel C, et al. . Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med. 1999;5(7):836-838. doi: 10.1038/10561 [DOI] [PubMed] [Google Scholar]
- 33.Yang FC, Chou KH, Fuh JL, et al. . Altered gray matter volume in the frontal pain modulation network in patients with cluster headache. Pain. 2013;154(6):801-807. doi: 10.1016/j.pain.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 34.Leone M, Franzini A, Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med. 2001;345(19):1428-1429. doi: 10.1056/NEJM200111083451915 [DOI] [PubMed] [Google Scholar]
- 35.Leone M, Franzini A, Proietti Cecchini A, Bussone G. Success, failure, and putative mechanisms in hypothalamic stimulation for drug-resistant chronic cluster headache. Pain. 2013;154(1):89-94. doi: 10.1016/j.pain.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 36.Barloese MCJ. Neurobiology and sleep disorders in cluster headache. J Headache Pain. 2015;16(1):562. doi: 10.1186/s10194-015-0562-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eAppendix. Supplementary methods and results