Abstract
Background: Most women with ductal carcinoma in situ (DCIS) will receive breast-conserving surgery (BCS) and radiation (RT). RT can be omitted for women at low risk of local recurrence (LR). The Oncotype DX DCIS score (DS) predicts LR risk after BCS alone. This study assesses the impact of RT and DS on LR risk.
Methods: Population-based cohort analysis of individuals with DCIS treated by BCS ± RT from 1994–2003. Treatment and outcomes were determined by linkage and chart review. We used a propensity score–adjusted multivariable model to examine associations between DS and LR and evaluate the impact of RT. All statistical tests were two-sided.
Results: The cohort includes 571 individuals treated by BCS alone, 689 cases treated with BCS + RT. Median follow-up was 9.4 years. On multivariable analysis, factors associated with LR include RT, age at diagnosis, tumor size, and multifocality. Adjusting for these factors, the DS risk group was statistically significantly associated with LR risk (hazard ratio high/intermediate = 1.75, 95% confidence interval = 1.28 to 2.41, P < .001). Women with a low-risk DS treated by BCS alone had an LR risk of 10.6% at 10 years and a small benefit from RT, while those with a high DS had a higher risk of LR (25.4%) after BCS alone and greater benefit from RT. A subgroup of patients with favorable clinicopathological features had a high-risk DS; these patients had a higher than expected risk of LR after BCS alone and a greater benefit with RT.
Conclusions: The DS molecular assay improves risk stratification and estimates of RT benefit in individuals with DCIS treated with breast-conserving therapy.
The management of ductal carcinoma in situ (DCIS) continues to be a challenge. DCIS is predominantly diagnosed in healthy women and is associated with a low risk of mortality (1–3). On the other hand, it is well documented that some women with DCIS will develop ipsilateral invasive breast cancer, which is associated with a seven- to 17-fold increased risk of dying of breast cancer (4,5). Our inability to identify patients at low risk of recurrence results in recommendations that all women with DCIS undergo treatment.
Most women will be treated with breast-conserving surgery (BCS), usually followed by whole breast radiation (RT), because of its proven efficacy to reduce local recurrence (LR) risk (2). There is agreement that RT can be omitted for women at low risk of recurrenc; however, clinical and pathologic features have not reliably identified patients at low risk of LR following BCS alone, leading to variability in treatment and outcomes of women with DCIS (6,7). The long-term results of two prospective cohort studies of selected women with low-risk features of DCIS treated by BCS alone without RT were recently reported. The ECOG E5194 study included cases with clear margins (>3 mm), with either low- or intermediate-grade DCIS measuring less than 2.5 cm (cohort 1) or small (≤1 cm) high-grade lesions (cohort 2). The corresponding 12-year local recurrence risks were 14.4% for cohort 1 and 24.6% for cohort 2 (6). A second prospective cohort study of similar patients (margins ≥ 1 cm) reported a 10-year risk of LR of 15.6% (7). A population-based analysis including 1867 women with DCIS treated by BCS alone found that the 10-year risk of LR was 19.2% (8). These studies demonstrate that clinicopathological features do not reliably identify the individuals with DCIS at low risk of LR after BCS alone, culminating in unnecessary treatment of those at low risk of recurrence and undertreatment of patients with higher-risk disease in whom additional efficacious treatment such as RT was not administered. Molecular biomarkers are needed to improve recurrence risk assessment and treatment decision-making in DCIS.
The Oncotype DCIS score (DS) is a multigene expression assay for DCIS patients that generates individualized estimates of 10-year risk of LR (DCIS or invasive) following treatment by BCS alone (9). The DS includes 12 of the 21 genes of the recurrence score assay for invasive carcinoma (10). The DCIS score was recently shown to predict an individual's risk of LR in the E5194 cohort study and was further validated in a population cohort treated by BCS alone (9,11). However, the impact of the assay in individuals treated by BCS + RT is unknown. In the current study, we determine if the DCIS score is an independent predictor of local recurrence in a population of individuals with DCIS treated with BCS + RT and assess the impact of RT according to the DCIS score, adjusting for statistically significant clinicopathological covariates.
Methods
Ontario DCIS Cohort
The methods used to establish the Ontario population-based DCIS cohort have been previously described (8). Cases treated by mastectomy, those diagnosed with invasive breast cancer or who died within six months of DCIS, and those with bilateral DCIS were excluded. The population includes 3320 cases with pure DCIS treated by BCS, 1658 treated by BCS alone and 1662 by BCS + RT. We obtained tissue blocks in 1751 cases (n = 828, BCS alone; n = 923, BCS + RT). One hundred eighty-six cases were excluded from analysis because of the presence of invasive carcinoma (n = 9), no tumor present (n = 20), insufficient RNA (n = 108), poor quantitative polymerase chain reaction (qPCR) sample quality (n = 49), or absence of clear margins (n = 304). The cohort includes 571 individuals treated by BCS and 689 individuals treated by BCS + RT. This study was approved by the institutional review board at Sunnybrook Health Sciences Centre, Toronto, Canada.
Pathology
Pathology review was performed on all original hematoxylin and eosin (H&E) slides from each BCS specimen or recuts as described (11,12). Nuclear grade, comedo necrosis, multifocality, tumor size (DCIS greatest dimension, mm), and margin status were predefined and assessed (13). Multifocal lesions were defined as having more than one distinct focus of DCIS, with at least 5.0 mm of intervening benign tissue confined to a single quadrant of the breast (14). Tumor size and margin width could not be assessed in cases without complete sets of slides or where the gross description was incomplete.
Treatment
Methods used to obtain data on treatment and outcomes were previously described (8). Deterministic linkage was performed with the Canadian Institute for Health Information (CIHI) database of discharge summaries, the Ontario Health Insurance Plan (OHIP) database of physician billings, the Registered Persons Database (RPDB), and the Ontario Cancer Registry (OCR) database. Breast surgical procedures and administration of RT were validated by chart review. The date of diagnosis is the date of the initial breast surgery associated with the DCIS diagnosis. All breast surgical procedures performed on the same breast within six months of DCIS diagnosis were considered part of initial treatment. Tamoxifen usage was not available for the cohort.
Outcomes
To identify subsequent breast events, we first identified all breast surgical procedures performed six months or more after diagnosis of the index DCIS lesion. We linked with the OCR and CIHI databases and reviewed available pathology reports to determine recurrence laterality and histology of the subsequent breast event. Outcomes were determined from the date of DCIS diagnosis. LR is defined as invasive breast cancer or DCIS in the ipsilateral breast six months or more after DCIS diagnosis. Invasive LR is defined as the development of an invasive breast cancer in the ipsilateral breast whether it was a first or subsequent recurrence. Contralateral breast cancer is defined by the presence of DCIS or invasive breast cancer in the opposite breast. The date of death was determined from the RPDB. The last date of follow-up is March 31, 2010. Because LR risks after BCS for DCIS have decreased over time, we evaluated outcomes for the whole study period and in those treated in the most contemporary years, 2000–2003.
Gene Assay
The DS was previously described (10,15). Cases with DCIS lesions smaller than 2 mm were excluded (9,11). RNA was extracted from 30 µm sections if DCIS measured 5.5 mm or larger, or from 60 µm sections if DCIS measured smaller than 5.5 mm. The DS is scaled as a continuous variable from 0 to 100 or in three risk categories as used in prior studies: 1) low risk (DS < 39), 2) intermediate risk (DS = 39 – 54), and 3) high risk (DS ≥ 55) (8,11).
Statistical Analysis
Descriptive analyses were conducted by examining the distributions of patient characteristics of women treated by BCS alone or BCS + RT. Categorical characteristics were summarized using frequencies and percentages, and chi-square tests were used to investigate associations. The first objective was to examine the association between DS and hazard of LR among patients treated with BCS + RT with clear margins. We implemented two multivariable Cox proportional hazards regression models, one in which DS was treated as a continuous measure and another in which it was treated as a three-level categorical variable (low-, intermediate-, high-risk groups). These models were adjusted for tumor size (>1 cm or ≤1 cm) and multifocality (yes/no). For cases where tumor size was missing, size was imputed using a linear regression model (tumor size = 4.17*min (#blocks involved,10)-0.215*min (#blocks involved,10)+0.35*largest focus; correlation between imputed values and observed values = 0.84). The assumption of proportionality was verified using a Kolmogorov-type supremum test.
The subsequent aim was to examine the association between DS and hazard of LR among the entire cohort of women with clear margins. To account for systematic differences between women treated with BCS alone vs BCS + RT (and to obtain an unbiased estimate of the effect of the DCIS score on the risk of LR), we used a propensity score adjustment approach (16). A propensity score was calculated for each patient, where the score is the linear component of the logistic regression model and measures each woman’s probability of receiving RT, conditional on measured covariates: tumor size, margins, surgery year, age (linear, quadratic, and cubic terms), subtype, multifocality, nuclear grade, comedo necrosis, estrogen receptor status, human epidermal growth factor receptor 2 status. Conditional on the propensity score, the distribution of observed baseline covariates will be similar between treatment groups (16). A multivariable Cox regression model was implemented to identify factors associated with LR. We used a forward selection approach (adjusted for propensity score) and included the following covariates: age at diagnosis (< 50, ≥50 years), tumor size (>1 cm, ≤1cm), nuclear grade (low/intermediate, high), comedo necrosis (yes/no), subtype (solid, cribriform, other), RT (yes/no), surgery year, and the DCIS score. The DS was evaluated separately as a continuous variable (per 50 units) and as a categorical risk group (low, intermediate, high).
Multivariable Cox models, adjusted for propensity score, were used to estimate the absolute risk of LR at 10 years with differences between curves compared using log-rank test. To compute the 10-year estimates for a subgroup of interest with a fixed set of covariates, a prediction was made for each of 202 possible combinations of treatment and the integer DS, while holding the propensity score constant at the average value for that subgroup. In the absence of a statistically significant interaction, the model assumes all effects are constant between treatment groups.
A P value of less than .05 was considered to be statistically significant. All statistical tests were two-sided.
Results
Population-Based Cohort
The cohort includes 571 individuals treated by BCS and 689 individuals treated by BCS + RT. Median follow-up was 9.4 years (9.6 years for individuals treated by BCS alone and 9.2 years for those treated with BCS + RT).
Among cases treated by BCS alone, about 62.2% (n = 355) had a low-risk DS, 16.6% (n = 95) had an intermediate-risk score, and 21.2% (n = 121) had a high-risk DS. There were 100 local recurrences (57 invasive, 44 DCIS [one invasive developed after DCIS]). The 10-year cumulative risk of LR for women treated by BCS alone was 19.2%. Individuals in the cohort who received RT had more adverse features than those treated by BCS alone (Table 1). Forty-eight point one percent of patients treated by BCS + RT (n = 332) had a low-risk DS, 22.5% (n = 155) had an intermediate-risk score, and 29.3% (n = 202) had a high-risk score. There were 86 local recurrences among those treated with RT (55 invasive, 32 DCIS). The 10-year cumulative risk of LR for women treated by BCS + RT was 12.7% (95% CI = 10.2% to 15.8%).
Table 1.
Patient characteristics for women who received breast-conserving surgery alone or BCS and radiation
| Variable | BCS only (n = 571) | BCS + RT (n = 689) | P* |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Age at diagnosis, y | <.001 | ||
| <50 | 110 (19.3) | 164 (23.8) | |
| 50–59 | 153 (26.9) | 248 (36.0) | |
| 60–69 | 134 (23.6) | 177 (25.7) | |
| ≥70 | 172 (30.2) | 100 (14.5) | |
| Tumor size, mm | .02 | ||
| ≤5 | 77 (13.7) | 62 (9.2) | |
| 5.1–10 | 127 (22.6) | 138 (20.4) | |
| >10 | 358 (63.7) | 477 (70.5) | |
| Unknown | 9 | 12 | |
| Multifocality | .02 | ||
| No | 457 (80.0) | 512 (74.3) | |
| Yes | 114 (20.0) | 177 (25.7) | |
| Tumor type | .002 | ||
| Solid | 358 (62.7) | 496 (72.0) | |
| Cribriform | 175 (30.7) | 160 (23.2) | |
| Other | 38 (6.7) | 33 (4.8) | |
| Nuclear grade | <.001 | ||
| Low | 55 (9.6) | 40 (5.8) | |
| Moderate | 332 (58.1) | 353 (51.2) | |
| High | 184 (32.2) | 296 (43.0) | |
| Comedo necrosis | <.001 | ||
| Absent | 221 (38.7) | 196 (28.4) | |
| Present | 350 (61.3) | 493 (71.6) |
Pearson’s chi-square, two-sided. BCS = breast conserving surgery; RT = radiation therapy.
Predictors of Local Recurrence After BCS Alone or BCS + RT in Patients With Clear Margins
We previously reported that factors associated with LR in women treated by BCS alone include the DS (hazard ratio [HR] = 1.68, 95% confidence interval [CI] = 1.08 to 2.62), multifocality (HR = 1.97, 95% CI = 1.27 to 3.02), tumor size larger than 1 cm (HR = 2.07, 95% CI = 1.15 to 3.83), age at diagnosis (HR = 1.75, 95% CI = 1.07 to 2.76), and subtype (HR = 1.63 solid vs cribriform, 95% CI = 0.97 to 2.88) (11). Among patients treated with BCS + RT with clear margins, factors associated with LR include tumor size larger than 1 cm (HR = 2.34, 95% CI = 1.26 to 4.34, P = .001) and multifocality (HR = 1.91, 95% CI = 1.22 to 2.98, P = .006) (Table 2). Adjusting for these covariates, the DS was statistically significantly associated with the development of LR both when evaluated as a continuous variable (HR per 50 units = 3.10, 95% CI = 1.87 to 5.16, P < .001) or categorical DS risk group. The hazards ratio for individuals with a high-risk score was 2.53 (95% CI = 1.48 to 4.35, P < .001) and for those with an intermediate-risk score was 1.62 (95% CI = 0.88 to 2.92) compared with those with a low-risk score (P = .11) (Table 2). The 10-year risks of LR were 20.5% (95% CI = 15.1% to 27.5%) for those with a high-risk score, 13.6% (95% CI = 8.6% to 21.2%) for those with an intermediate-risk score, and 7.5% (95% CI = 4.9% to 11.2%) for those with a low-risk score (P < .001) (Figure 1).
Table 2.
Multivariable analysis examining factors associated with the hazard of local recurrence after breast-conserving surgery + radiation in cases with clear margins
| Variable | HR (95% CI) | P* |
|---|---|---|
| Model with continuous score | ||
| Tumor size > 1 cm | 2.34 (1.26 to 4.34) | .01 |
| Multifocality | 1.91 (1.22 to 2.98) | .006 |
| High nuclear grade vs low/intermediate | 0.74 (0.46 to 1.18) | .21 |
| DCIS score (HR/50 units) | 3.10 (1.87 to 5.16) | <.001 |
| Model with categorical score | ||
| Tumor size > 1 cm | 2.32 (1.30 to 4.53) | .01 |
| Multifocality | 1.82 (1.16 to 2.82) | .01 |
| High nuclear grade vs low/intermediate | 0.85 (0.53 to 1.36) | .49 |
| DCIS risk group | .003 | |
| Low | 1.00 (referent) | – |
| High | 2.53 (1.48 to 4.35) | <.001† |
| Intermediate | 1.62 (0.88 to 2.92) | .11† |
Likelihood ratio P value, except where noted otherwise, two-sided. CI = confidence interval; DCIS = ductal carcinoma in situ; HR = hazard ratio.
Wald chi-Square P value, two-sided.
Figure 1.
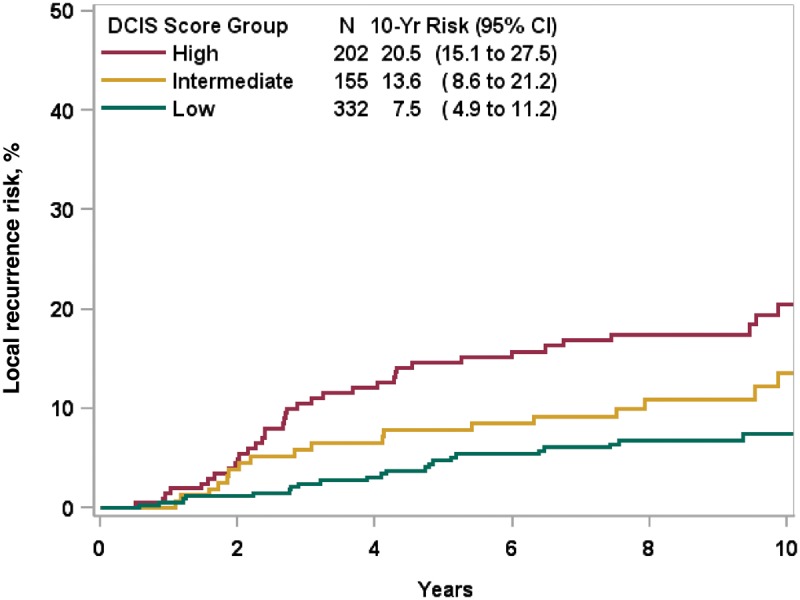
Ten-year Kaplan-Meier risk estimates of local recurrence in patients with ductal carcinoma in situ treated with breast-conserving surgery + radiation.
Whole Cohort Analysis
We combined the two treatment groups and used a propensity score–adjusted multivariable model to identify pertinent factors associated with the development of LR among individuals treated by BCS with or without RT. We found age at diagnosis younger than 50 years (HR = 1.54, 95% CI = 1.10 to 2.14, P = .01), tumor size larger than 1 cm (HR = 1.98, 95% CI = 1.35 to 2.99, P = .001), the presence of multifocality (HR = 1.88, 95% CI = 1.37 to 2.55, P < .001), solid subtype (HR = 1.43, 95% CI = 0.96 to 2.18, P = .09), and the administration of RT (HR = 0.56, 95% CI = 0.42 to 0.76, P < .001) were associated with LR (Table 3). Adjusting for these covariates and year of diagnosis, the DS remained a statistically significant predictor of LR (HR = 1.97 per 50 units, 95% CI = 1.37 to 2.84, P < .001). The hazard ratio for the high-risk DS group (HR = 1.77, 95% CI = 1.24 to 2.35) and the intermediate-risk group (HR = 1.73, 95% CI = 1.17 to 2.52) were statistically (HR for high/intermediate groups combined = 1.75, 95% CI = 1.28 to 2.41) significantly higher compared with the low-risk DS group. There was no statistically significant interaction between the DS and RT (data not shown, P = .44). Nuclear grade and the presence of comedo necrosis were not associated with LR (Table 3).
Table 3.
Multivariable analysis examining factors associated with the hazard of local recurrence after breast-conserving surgery with or without RT in cases with clear margins
| Variable | HR (95% CI) | P* |
|---|---|---|
| Age < 50 y | 1.54 (1.10 to 2.14) | .01 |
| Tumor Size > 1 cm | 1.98 (1.35 to 2.99) | .001 |
| Multifocality | 1.88 (1.37 to 2.55) | <.001 |
| Tumor type | .04 | |
| Solid vs cribriform | 1.43 (0.96 to 2.18) | .09† |
| Other vs cribriform | 2.28 (1.16 to 4.27) | .01† |
| High nuclear grade vs low/intermediate | 0.84 (0.60 to 1.19) | .33 |
| Comedo necrosis | 1.00 (0.69 to 1.48) | .99 |
| Radiation | 0.56 (0.42 to 0.76) | <.001 |
| DCIS score per 50 units | 1.97 (1.37 to 2.84) | <.001 |
Likelihood ratio P value, except where noted otherwise, two-sided. CI = confidence interval; DCIS = ductal carcinoma in situ; HR = hazard ratio.
Wald Chi-Square P value, two-sided.
Figure 2 illustrates the predicted risks of local recurrence by DS following treatment by BCS alone or BCS + RT, adjusted for propensity score and year of diagnosis. For individuals with a low-risk DS, the 10-year risk of LR after BCS alone was 16.0% (95% CI = 12.2% to 20.9%) and 9.4% (95% CI = 7.0% to 12.5%) after BCS + RT (absolute reduction with RT = 6.6%, 95% CI = 5.2% to 8.4%) (Figure 2A). The 10-year risks of invasive LR were 9.7% (95% CI = 6.7% to 13.9%) after BCS alone and 6.8% (95% CI = 4.7% to 9.7%) after BCS + RT (absolute reduction with RT = 2.9%, 95% CI = 2.0% to 4.2%, data not shown). For cases with a low-risk DCIS score treated in the most contemporary years of the study period (years 2000–2003), the risks of LR were lower (Figure 2B). The 10-year risks of LR after BCS alone was 10.6% (95% CI = 5.6% to 17.0%) and 5.0% (95% CI = 2.9% to 8.6%) for those treated with BCS + RT (absolute reduction with RT = 5.6%, 95% CI = 2.7% to 8.4%).
Figure 2.
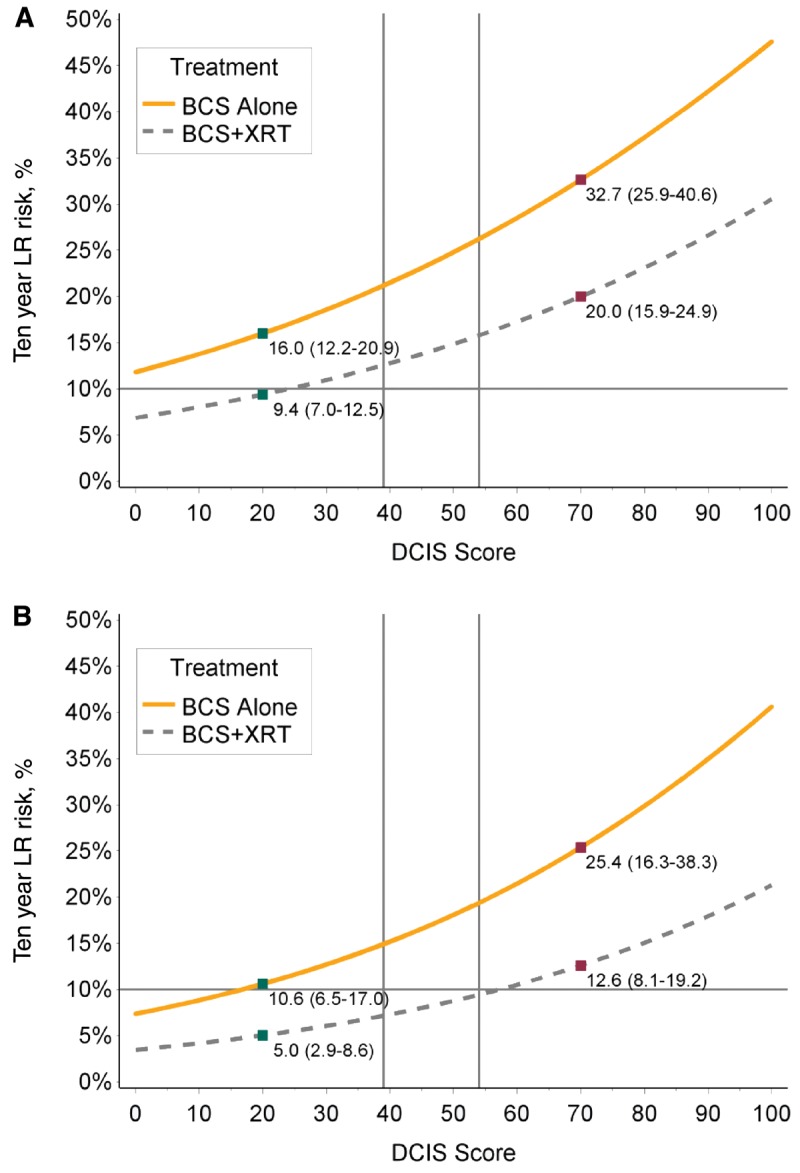
Predictive 10-year local recurrence risk by ductal carcinoma in situ (DCIS) score in patients with DCIS treated by breast-conserving surgery (BCS) alone or BCS + radiation (RT). A) Patients treated from 1994–2003. B) Patients treated from 2000–2003. The predicted risks of local recurrence by DS following treatment by BCS alone or BCS + RT, adjusted for propensity score and year of diagnosis, for the whole study period (A) and for individuals treated in years 2000–2003 (B). BCS = breast-conserving surgery; LR = local recurrence; XRT = radiation.
Individuals with a high-risk DS experienced higher risks of LR and invasive LR and had a greater absolute benefit with RT compared with those with a low-risk score. Adjusting for propensity score and year of diagnosis, the 10-year risk of LR was 32.7% (95% CI = 25.9% to 40.6%) after BCS alone and 20.0% (95% CI = 15.9% to 24.9%) after BCS + RT (absolute reduction with RT = 12.7%, 95% CI = 10.0% to 15.7%) (Figure 2). The corresponding risks of invasive LR were 17.0% (95% CI = 11.9% to 23.8%) and 11.9% (95% CI = 8.7% to 16.2%; absolute reduction with RT = 5.1%, 95% CI = 3.2% to 7.6%, data not shown). Among cases diagnosed in years 2000–2003 with a high-risk DS, the 10-year risks of local recurrence were 25.4% (95% CI = 16.3% to 38.3%) after BCS alone and 12.6% (95% CI = 8.1% to 19.2%) after BCS + RT (absolute reduction = 12.8%, 95% CI = 8.2% to 19.1%) (Figure 2).
Impact of Multigene Assay in Cases With Low-risk Clinicopathological Features of DCIS
The eligibility criteria used in studies of “low-risk DCIS” include individuals with low– or intermediate–nuclear grade DCIS, wide clear margins, and tumor size smaller than 2.5 cm (6,7,17). Most participants were age 50 years or older at diagnosis. We assessed the predicted risks of LR in cases from the population cohort with similar clinicopathological features and evaluated the impact of the DCIS score on LR risks. Within the whole population cohort meeting these criteria (n = 286), three-quarters (74.1%) had a low-risk DS and for these women the 10-year risk of LR after BCS alone was 13.9%. However, 9.8% of women with these favorable clinico-pathological features had a high-risk DS, and for these cases the 10-year risk of LR following BCS alone was 28.9%. Restricting our analysis to cases treated after the year 2000, 80.0% had a low-risk DS, and for these women the 10-year risk of LR after BCS alone was 10.1%. Similarly, 8.5% of cases had a high-risk DS, and for these individuals the 10-year risk of LR after BCS alone was 19.6%. Overall, the absolute reduction in 10 year LR risk with RT for women with favorable clinico-pathological features and a low risk DS was 10.1% (95% CI = 6.9% to 14.8%) vs. 6.0% (95% CI = 4.1% to 8.9%) and 19.6% (95% CI = 12.8% to 29.5%) vs. 11.9% (95% CI = 7.8% to 18.0%) for those with a high risk DCIS score (Figure 3).
Figure 3.
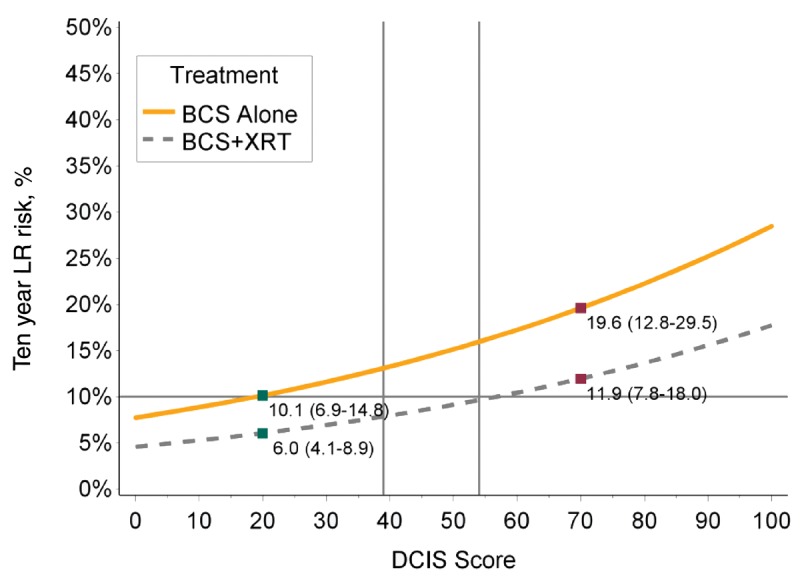
Predictive local recurrence curves after breasts-conserving surgery (BCS) alone or BCS + radiation (RT) in cases with low-risk clinico-pathological features of ductal carcinoma in situ (DCIS). The predicted risks of local recurrence at 10 years following treatment by BCS alone or BCS + RT in patients with low-risk clinicopathological features of DCIS (age at diagnosis ≥ 50 years, tumor size ≤ 2.5 cm, non–high grade, clear margins). BCS = breast-conserving surgery; LR = local recurrence; XRT = radiation.
Discussion
The inability to reliably identify lesions at low risk of recurrence after surgical excision has hampered attempts to deescalate therapy for certain women with DCIS while ensuring adherence to recommended RT for those at higher risk. We found that the DS molecular assay predicts the risk of LR following treatment by breast-conserving surgery and improves risk stratification and estimates of RT benefit in conjunction with and beyond clinico-pathological characteristics. We used a propensity score–weighted analysis to adjust for imbalances in known adverse risk factors between treatment groups. The molecular assay is independently associated with LR risk. Individuals with a high-risk (≥55) or an intermediate-risk score (39–54) had a nearly twofold increased risk of LR compared with those with a low-risk score. Other pertinent factors associated with LR include tumor size larger than 1 cm, the presence of multifocality, solid subtype, and the administration of RT. Nuclear grade and the presence of comedo necrosis were not associated with LR. The lack of statistical significance of nuclear grade was also reported in the recent E5194 analysis (6). Further research is needed to determine whether the integrated assessment of grade, which included nuclear grade, comedo necrosis, and histologic subtype, is more informative as a predictor of LR than nuclear grade.
The RTOG 9804 trial (17), E5194 trial (6), and Boston studies (7) included patients with favorable clinico-pathological features of DCIS deemed to be associated with a low risk of LR after BCS alone (age ≥ 50 years, tumor size ≤ 2.5 cm, nuclear grade 1 or 2, and margins ≥ 3 mm). We identified cases in the population cohort treated in 2000–2003 with similar features (age ≥ 50 years, tumor size ≤ 2.5 cm, nuclear grade 1 or 2) but with clear margins (we did not have data on margin width) treated by BCS alone. Most cases with these low-risk clinico-pathological features had a low-risk DS, and for these cases treatment by BCS alone was associated with the expected low risk of LR (10.1% at 10 years). However, the molecular assay identified a subset of patients (11%) with these same features treated during the same time period who had a high-risk score, and for these patients treatment by BCS alone was associated with a significantly higher risk of LR (19.6% at 10 years). This suggests the molecular assay improves the assessment of recurrence risk after treatment by BCS beyond clinico-pathological features.
Our study was not without limitations. Patients were not randomized and were selected for treatment based on clinico-pathologic features and patient preference. During the time interval of this study, many pathology reports lacked tumor size and resection margin width information (18,19). Therefore, margin width and tumor size data were incomplete. In addition, data on clinical presentation or family history of breast cancer that may predict for LR were not available. Complete data on tamoxifen usage were not available; however, tamoxifen utilization during the time period of this study was infrequently used. There was no statistically significant interaction between the DS and RT, suggesting that the proportional reduction in the risk of LR related to RT may be similar for women with a low-risk or high-risk DCIS score. However, women with a low-risk score derive a small absolute benefit from RT. Individuals with a high-risk score experienced significantly higher risks of LR after treatment by BCS alone (10-year risk of LR = 32.7%) and derived a greater benefit from RT (8% absolute reduction in LR). Among cases diagnosed in years 2000–2003 with a high-risk DS, the 10-year risks of local recurrence were 25.4% after BCS alone and 12.6% after BCS + RT. Patients with a high-risk score and other adverse features had a higher risk of LR; however, the number of cases in the subset is small. Additional data are needed to evaluate the outcomes and optimize the management of women with high-risk DCIS.
The DS molecular assay can improve treatment recommendations in DCIS. It provides individualized estimates of LR risk after BCS alone, improved identification of patients at low risk of recurrence in whom the absolute benefit of RT is small, and those at higher risk who might benefit from additional treatment. Women age 50 years or older at diagnosis with tumor size of 2.5 cm or smaller and nuclear grade 1 or 2 with clear margins comprise the majority of women with DCIS today and represent the subgroup with which clinicians are most concerned about overdiagnosis and overtreatment. Most women with these features had a low-risk DS, and for these women the 10-year risk of LR after BCS alone was low and the absolute benefit from RT was very small. On the other hand, 10% of women with these same favorable clinico-pathological features had a high-risk molecular score, and these women had a higher risk of LR after BCS alone and a greater benefit from RT. Most cases in this population cohort did not receive tamoxifen (90%), which may lead to further lowering of the risks of recurrence, although adherence is inconsistent (20). Further research is needed to integrate the impact of other relevant risk factors and to optimize the treatment of women with high-risk DCIS by evaluating the impact of higher doses of radiation, systemic therapies, and mastectomy.
Funding
This work was supported in part by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC), and in part by funding from the Canadian Cancer Society Research Initiative (Award # 018491), the Ontario Institute for Cancer Research (Award # SPI-DCIS-1011), and Genomic Health Inc.
Notes
The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. These data sets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences. Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not necessarily those of CIHI. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO, ICES, or the Ontario MOHLTC is intended or should be inferred.
The funders had no role the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Presented in part at the 57th Annual American Society for Therapeutic Radiation Oncology (ASTRO) meeting, 2015, San Antonio, Texas.
Dr. Rakovitch is the LC Campbell Chair for Breast Cancer Research. We would like to thank Dr. S. Robertson at the Ottawa Hospital (EORLA) for their contribution in pathology review, specimen collection, and facilitation of ethics approvals.
References
- 1. Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;17:888–896. [DOI] [PubMed] [Google Scholar]
- 2. EBCTCG, Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ernster VL, Barclay J, Kerlikowske K, et al. Mortality among women with ductal carcinoma in situ of the breast in the population-based Surveillance, Epidemiology and End Results Program. Arch Intern Med. 2000;160:953–958. [DOI] [PubMed] [Google Scholar]
- 4. Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;1036:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;3132:4054–4059. [DOI] [PubMed] [Google Scholar]
- 6. Solin LJ, Gray R, Hughes LL, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 Study. J Clin Oncol. 2015;3333:3938–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS). Breast Cancer Res Treat. 2014;1432:343–350. [DOI] [PubMed] [Google Scholar]
- 8. Rakovitch E, Nofech-Mozes S, Narod SA, et al. Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res Treat. 2013;1382:581–590. [DOI] [PubMed] [Google Scholar]
- 9. Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;10510:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;35127:2817–2826. [DOI] [PubMed] [Google Scholar]
- 11. Rakovitch E, Nofech-Mozes S, Hanna W, et al. A population-based validation study of the DCIS score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;1522:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rakovitch E, Nofech-Mozes S, Narod S, et al. Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res Treat. 2013;1382:581–590. [DOI] [PubMed] [Google Scholar]
- 13. Lester SC, Bose S, Chen YY, et al. Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch Pathol Lab Med. 2009;1331:15–25. [DOI] [PubMed] [Google Scholar]
- 14. Sikand K, Lee AH, Pinder SE, et al. Sections of the nipple and quadrants in mastectomy specimens for carcinoma are of limited value. J Clin Pathol. 2005;585:543–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;536:1084–1091. [DOI] [PubMed] [Google Scholar]
- 16. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;463:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCormick B, Winter K, Hudis C, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015;337:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Staradub VL, Messenger KA, Hao N, et al. Changes in breast cancer therapy because of pathology second opinions. Ann Surg Oncol. 2002;910:982–987. [DOI] [PubMed] [Google Scholar]
- 19. Rakovitch E, Mihai A, Pignol JP, et al. Is expert breast pathology assessment necessary for the management of ductal carcinoma in situ? Breast Cancer Res Treat. 2004;873:265–272. [DOI] [PubMed] [Google Scholar]
- 20. Nichols HB, Bowles EJA, Islam J, et al. Tamoxifen initiation after ductal carcinoma in situ. Oncologist. 2016;212:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]


