Abstract
Background:
The migration of tumor cells is critical in spreading cancers through the lymphatic nodes and circulatory systems. Although arachidonic acid (AA) and its soluble metabolites have been shown to induce the migration of breast and colon cancer cells, the mechanism by which it induces such migration has not been fully understood.
Objective:
The effect of AA on migratory responses of the MDA-MB-231 cell line (a triple-negative breast cancer cell) was examined and compared with MCF-7 (estrogen-receptor positive) breast cancer cells to elucidate the mechanism of AA-induced migration.
Methods:
Migrations of breast cancer cells were examined with the help of wound-healing assays. AA-induced eicosanoid synthesis was monitored by RP-HPLC. Cellular localizations of lipoxygenase and lipid rafts were assessed by immunoblot and confocal microscopy.
Results:
AA treatment stimulated the synthesis of leukotriene B4 (LTB4) and HETE-8, but lowered the levels of prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), and HETE-5 in MDA-MB-231 cells. Further analysis indicated that AA increased the expression of 5-lipoxygenase (5-LOX) in this cell line and inhibiting its expression by small molecule inhibitors lowered the production of LTB4 and reduced migration. In contrast, MCF-7 cells did not show any appreciable changes in eicosanoid synthesis, 5-LOX expression, or cellular migration.
Conclusion:
Our results suggest that AA treatment activates the BLT1 receptor (present in membrane microdomains) and stimulates the synthesis of LTB4 production, which is likely to be associated with the migration of MDA-MB-231 cells.
Keywords: Breast cancer, MDA-MB-231, MCF-7, eicosanoids, leukotrienes, lipid rafts
Introduction
Breast cancer is a common cancer among women across the globe. Epidemiological studies suggest that the intake of red meat or other high-fat foods that contain arachidonic acid (AA) or other omega 6-polyunsaturated fatty acids (ω6-PUFA) can increase the risk of various types of cancers, including breast cancer [1–3]. AA is initially incorporated into the plasma membrane and then released by the action of phospholipase A2s (PLA2s). Free and unesterified AAs are then utilized by the cells to synthesize various inflammatory eicosanoid molecules such as leukotrienes (LTs), prostaglandins (PGs), thromboxanes (TXs), and hydroxyeicosatetraenoic acids (HETEs) [2]. All of these molecules are involved in the immune response and modulation and, in many cases, excess production of these eicosanoids can lead to cellular proliferation and malignancy [4]. Studies suggest that AA not only promotes the adhesion and migration of endothelial cells, but it can also act as a regulator of wound healing and angiogenesis [5]. The level of prostaglandin E2 (PGE2), a major product of cycloxygenase-2 (COX-2) reaction, is elevated in breast cancer cells and functions as a pro-tumorigenic agent [6,7]. COX-2-dependent PG synthesis has been shown to be involved in maintaining the motility and invasion of breast cancer cells [8]. Inhibitors of COX-2 could block the radiation-induced migration of MDA-MB-231 cells [9], which suggests that the COX-2 pathway is important and involved in the migration of breast cancer cells.
Like COX pathways, lipoxygenase (LOX)-derived eicosanoids have been implicated in tumor formation and malignancy [10], although their role in cancer metastasis is not well understood. LOX enzymes use AA as a substrate (like COX) to produce LTs, HETEs, and hydroperoxyeicosatetraenoic acids (HPETEs). There are several types of LOXs—i.e., 5-LOX, 8-LOX, 12-LOX, and 15-LOX—and they are classified according to their positional specificity (e.g., 5, 8, 12, and 15) toward unsaturation in the AA molecule. One of the important enzymes of the LOX group, 5-LOX, synthesizes HETEs and various LTs, including leukotriene A4 (LTA4), leukotriene B4 (LTB4), leukotriene C4 (LTC4), leukotriene D4 (LTD4), and leukotriene E4 (LTE4) [10]. Recent reports indicate a direct association of increased 5-LOX activity with the progression of hepatocellular carcinoma, intestinal polyposis, and breast carcinoma [11, 12].
LTB4, a 4 series of eicosanoids, is synthesized from unstable LTA4 by the action of LTA4 hydrolase. This is a powerful bioactive lipid that has been implicated in various physiological functions including inflammation, tumor promotion, and metastasis. LTB4, which also has a role in anaphylactic reactions, is responsible for different lung cancer pathologies [13], and it can stimulate the proliferation of pancreatic cancer cells [14], suggesting the importance of LTB4 in promoting lung and pancreatic cancers. In a recent study, high concentrations of exhaled LTB4 and IL-8 were shown to be associated with neutrophilic inflammation in the airways of lung cancer patients [13]. In addition, a pro-tumorigenic effect of LTB4 in melanoma cells has also been reported [15].
There is evidence that AA and its metabolites can promote invasion/migration via the Rho and PI3K/Akt-dependent pathways [16]. Involvement of Stat-5 in AA-mediated migration of MDA-MB-231 cells has also been proposed [17]. Oleic acid has been shown to promote migration through AA-dependent pathways [18].
Despite all of this, it is not known if AA activates the same or a different set of molecules in two different breast cancer cell lines—MCF-7 and MDA-MB-231. Because it has been previously shown that lipid rafts (LRs), or caveolae-based signaling, is important for tumor progression and malignancy [19], we asked whether LRs could be involved in regulating AA-induced migration of basal-like cell line MDA-MB-231 and luminal-type MCF-7 cells. These two cell lines (MDA-MB-231 and MCF-7) differ in genome aberrations, transcriptional profiles, invasive properties, and therapeutic targeting [20]. In this paper, we demonstrate the following: (1) AA treatment induces the production of LTB4 and HETE8 in MDA-MB-231 cells; (2) AA suppresses the production of PGs, LTs, and HETE molecules in MCF-7 cells; (3) LOX inhibitors block the migration of MDA-MB-231 cells in culture and (4) methyl-β cyclodextrin (MBCD), a common LR disruptor, interferes with LR assembly, BLT1 receptor immunostaining, caveolin 1 expression, and the migration of MDA-MB-231 cells.
Materials and Methods
Cell culture and treatments
MDA-MB-231 and MCF-7 cells were cultured in RPMI-1640 and DMEM/Ham’s F-12 (Gibco Invitrogen, Carlsbad, CA) media, respectively. Both RPMI and DMEM were supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 1% penicillin/streptomycin (Hyclone, Logan, UT). Breast cancer cells were grown in a humidified atmosphere (95% air and 5% CO2) until they reached ~80%–90% confluency. Confluent cells were treated with trypsin (0.25% Trypsin-EDTA, Hyclone), harvested by centrifugation, plated on a 6-well plate (~0.5 × 106 cells per well), and treated with AA (sodium salt, 100 μM) (Sigma Chemicals, St. Louis) for 24 h with and without prior treatment with the 5-LOX inhibitor, nordihydroguaiaretic acid (NDGA, 5 μM; Sigma Chemicals), and zileuton (100 μM; Sigma Chemicals). Control and treated cells were collected, washed, and subject to further analyses as described in the text.
Cell viability assay
The cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium salt (MTS) assay. Briefly, cells were treated with various concentrations of AA or inhibitors (NDGA, zileuton) and the cytotoxic effects were determined following the method described by Malich et al [21].
Analysis of eicosanoids by reverse-phase, high-performance, and liquid chromatography (RP-HPLC)
MDA-MB-231 and MCF-7 cells were grown in tissue-culture flasks (T75 vented cap, BD Biosciences, CA), harvested, and cultured in 6-well plates (~0.5×106 cells/each well) for 24 h. Fresh media (2 ml) was added to each well and incubated for an additional 24 h in the presence of AA (100 μM) and NDGA (5 μM). The media from control and treated wells was collected by centrifugation (12,000 rpm, 10 min), and eicosanoids (secreted into the medium) were extracted using ethyl acetate following the protocol previously described by Franchi et al [22]. Samples were vortexed and centrifuged at 3000 rpm for 10 min, and the top layer containing eicosanoids was transferred to a Teflon-lined vial and dried under N2 gas. The dried samples were resuspended in 40 μl of methanol and stored at −20°C for further analysis by HPLC (Waters Inc., Milford, MA). Samples were mixed with acetonitrile (1:1000) and subjected to HPLC following the method of Moraes et al. [23] using a reverse-phase C18 column (Supplecon, St. Louis, MO). Eicosanoids were eluted with the help of trifluoroacetic acid (0.1%)-acetonitrile solvent system (flow rate: 0.4 ml/min) and analyzed by HPLC. Individual eicosanoid peaks were identified by comparison with the respective standards (i.e., PGE2, PGD2, LTB4, HETE5, and HETE8) that were purchased from Cayman Chemical (Ann Arbor, MI). The elution times of the eicosanoids were as follows: PGE2: 60 min; PGD2: 64 min; LTB4: 72 min; HETE5–5: 85 min; and HETE8: 90 min. Eicosanoids from control and treated samples were quantified by calculating the areas covered under each peak using EMPOWER software (Water Inc., Milford, MA).
Immunoblot analysis
Control and treated cells were harvested, washed in cold PBS, lysed by freeze-thaw in a lysis buffer followed by measuring proteins using a Bio-Rad Protein Assay Kit. Approximately 40-μg of protein was applied to each lane and subjected to electrophoresis in SDS-PAGE (10%), followed by immunoblot analyses on the polyvinylidene fluoride (PVDF) membranes, as previously described by Albert et al [24]. The PVDF membranes were incubated overnight (6–10°C) with anti-5-LOX antibody or BLT1 receptor polyclonal antibody (rabbit polyclonal, Cayman Chemical, Ann Arbor, MI), anti-caveolin-1 antibody (rabbit polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, monoclonal, Cell Signaling, Danvers, MA). The membranes were washed with 0.05% Tween-20 in Tris-Buffered saline (TBST) and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Thermo Scientific, Rockford, IL) and/or goat anti-rabbit IgG (KPL Biomedical, Chantilly, VA). An enhanced chemi-luminescence (ECL) technique was used for the detection of protein bands. The intensities of protein bands were measured using Labworks software (UVP Lab Products, Upland, CA).
Confocal microscopy
Cells were grown overnight in 4-well, chambered Lab-TEK II slides containing either an RPMI-1640 or DMEM/F12 medium, and were treated with AA (100 μM) and/or NDGA (5 μM) for 24 h, fixed with methanol (100%, chilled) for 5 min at −20°C before blocking with normal goat serum (NGS, 5%; Sigma-Aldrich, St. Louis, MO) for 1 h. As described in the figure legends, 4% paraformaldehyde-fixed cells were incubated overnight in the 4°C with antibodies. The slides were washed three times with PBS and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG or Alexa Fluor 568 conjugated goat anti-rabbit secondary antibody (1:500). The cells were mounted with ProLong® Gold Antifade Reagent with DAPI (Invitrogen, Carlsbad, CA). The confocal images were captured with the help of an LSM 700 Zeiss confocal microscope and analyzed using Zen 2009 software (Carl Zeiss, Thornwood, NY). Each experiment was repeated three times. Cells were randomly selected from each slide using the same resolution, laser power, and detector-gain.
Cell migration assays
Migration of breast cancer cells was examined with the help of wound-healing assays, which is standard procedure in monitoring cell migration [5]. Approximately 0.8 ×106 cells were plated in each well of a 6-well plate and incubated overnight in a medium (RPMI-1640 or DMEM) containing fetal bovine serum (FBS, 10%). Cells were allowed to incubate overnight in 5% FBS-supplemented medium. It was observed that 5% FBS (instead of 0 or 10%) was optimal to monitor the effect of AA-induced migration of MDA-MB-231 cells. The monolayers were wounded by scratching with a sterile 10-μL pipette tip [5] and treated individually or in combination with AA (100 μM), NDGA (5μM), zileuton (100 μM), LTB4 (100 nM) PGD2 (10 ng/ml), and methyl-β-cyclodextrin (MBCD, 1 mM) for 24 h. The cell images that had migrated between wounded regions were captured using a Nikon-TMS microscope equipped with a Nikon F-601 camera and then counted. The cells treated with NDGA or AA+NDGA were also supplemented with either PGD2 or LTB4.
Lipid rafts and leukotriene B4 receptors
Lipid rafts (LRs) were labeled with an LR labeling kit (Vybrant Alexa Fluor 488) containing cholera toxin subunit B (CTXB) that was purchased from Invitrogen (Carlsbad, CA). Live control and treated cells were labeled with the CTXB (1 μg/ml) conjugated to Alexa Fluor 488 for 10 min followed by labeling with anti-CTXB antibody (1:200 dilution) for 15 min. The cells were then fixed with 4% paraformaldehyde followed by blocking with NGS. For the identification of leukotriene B4 receptors (BLT1), polyclonal antibody (1:200; rabbit, Cayman Chemical, Ann Arbor, MI) was used followed by Alexa Fluor 568 conjugated goat anti-rabbit secondary antibody (1:500). The cells were mounted with ProLong Gold Antifade Reagent (Invitrogen, Carlsbad, CA), counterstained with DAPI, and analyzed by an LSM 700 confocal laser-scanning microscope (Carl Zeiss, Inc., Thornwood, NY).
Colocalization analysis
The quantitative evaluation of co-localization between CTXB and BLT1 was performed using ZEN 2009 software. Regions of interest (ROIs) were defined manually within cells to reduce background fluorescence contribution. The degree of colocalization of the two fluorophores was determined using a squared Manders’ overlap coefficient (OC) of fluorescent signals (green and red for CTXB and BLT1, respectively), implemented on a pixel-by-pixel basis [23]. According to Manders et al. [25], OC ranges from 0 to 1, whereas 0 indicates no pixels and 1 indicates perfectly colocalized pixels in the selected ROI. The values for selected ROIs were acquired from images taken from 10–12 cells from different microscope fields, using ZEN 2009 software.
Cytokines assay
Various pro- and anti-inflammatory cytokines (i.e., IL-1A, IL-1B, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 IL-17A, IFNγ, and TNFα) were measured using a commercially available ELISA assay kit (SA Biosciences, Qiagen, Frederick, MD). The concentrations of different cytokines were quantitated from the supernatants isolated from control and AA-treated MCF-7 and MDA-MB-231 cells following the manufacturer’s protocol. Briefly, ~1 × 105 cells were plated in 12-well plates and treated with AA, AA+NDGA, or NDGA for 24 h, as previously mentioned. The supernatants were removed and centrifuged at 12,000 rpm for 5 min. Concentrations of cytokines in the culture supernatants were analyzed by a commercially available enzyme–linked immunoassay kit (Qiagen) according to the manufacturer’s instructions. The absorbance was measured using a microplate reader (Bio-Rad) at 450 nm, and the data are represented as absorbance values with proper statistical evaluations.
Measuring the level of phospho-NFκΒ
To measure the levels of phospho-NFκΒ p65, we used the Multispecies InstantOne ELISA Kit, (Thermo Fisher). Approximately, 0.5 × 106 cells were plated in a 6-well plate and grown overnight for attachment. The cells were incubated for an additional 24 h in the presence of AA (100 μM), AA (100 μM) + NDGA (5 μM), or NDGA (5 μM). Cell lysates were prepared using the lysis buffer provided with the kit, and equal concentrations of proteins were loaded in an antibody-coated 96-well plate following the manufacturers’ instructions. The samples’ absorbance was measured using a microplate reader (Bio-Rad Laboratories) set at 450 nm. Values are expressed as relative optical density (OD).
Statistical analyses
To calculate differences between the treatment and the control sets, one-way analyses of variance (ANOVAs) followed by the Holm-Šídák methods were performed using Sigma Plot (version 12) software. Student’s t tests were performed for the results shown in Fig. S1, and a P value of < 0.05 was considered statistically significant [26]. The results obtained from HPLC data (Fig. 1) and Fig. S2 were expressed as the mean ± SE, and statistical analysis was performed using Microsoft Excel (2007) software. The student’s t tests between the treatment and control groups were carried out using the GraphPad Software. Results were considered to be statistically significant when p values were less than 0.05 (< 0.05).
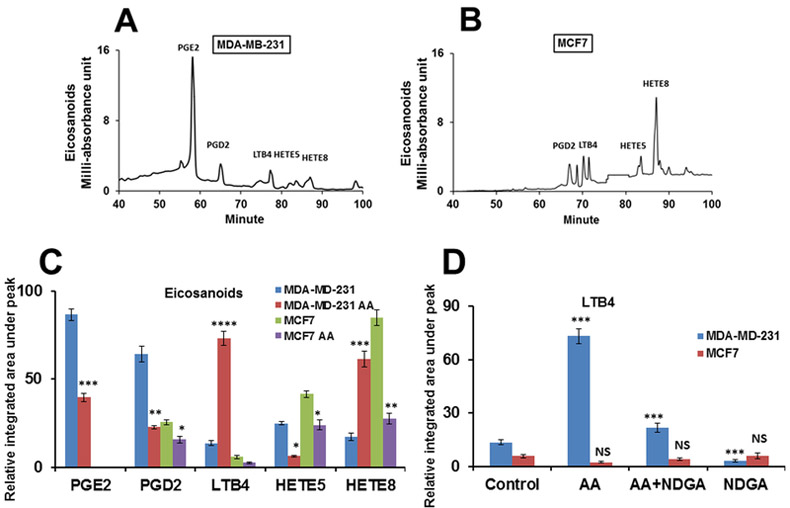
Fig. (1). Arachidonic acid influences the secretion of eicosanoids by MDA-MB-231 and MCF-7 cells.
Panels A and B show the HPLC profiles of eicosanoids in MDA-MB-231 and MCF-7 cells without any treatment. Panel C demonstrates the effects of AA (100 μM) on the syntheses of PGD2, PGE2, LTB4, HETE5, and HETE8 by MDA-MB-231 and MCF-7 cells. For statistical analysis, control samples were compared with AA treatments of respective cells (i.e., MDA-MB-231 and MCF-7). Panel D represents the LTB4 levels in the two cell lines after AA, AA+NDGA, and NDGA treatments. For significance calculation, control MDA-MB-231 was compared with AA-treated MDA-MB-231 cells (blue) and control MCF-7 (magenta) was matched with AA-treatment. Experiments (panels C and D) were repeated at least three times in triplicate. Results were given as mean ± SE and for the significance calculations GraphPad software was employed. *p < 0.05, ** p<0.001, *** p<0.0001, **** p<0.00001. AA, arachidonic acid, NDGA, norhydroguaiaretic acid; PGE2, prostaglandin E2; PGD2, prostaglandin D2; LTB4, leukotriene B4; HETE, hydroxyeicosatetraenoic acid. Results shown in Panel C were analyzed by Student’s t-tests. For panel D, the p values were analyzed using One-way ANOVA test (Holm-Šídák methods). ** p < 0.01; *** p < 0.001, NS = Not significant.
Results
Arachidonic acid induces LTB4 and HETE8 production in MDA-MB-231 cells
The incorporation of AA to membrane phospholipids is critical for arachidonoyl-phospholipid (AA-PL) synthesis, which in turn releases free AA and serves as a precursor of eicosanoid molecules [27]. However, it is not clear how AA and its metabolites regulate the invasiveness of breast cancer cells. Therefore, in an effort to understand the possible role of AA-induced eicosanoid production, we evaluated how AA modulates eicosanoid synthesis in both invasive (MDA-MB-231) and mildly invasive (MCF-7) breast cancer cells. First, a dose-response assay was conducted to evaluate cell viability in the presence of AA. The cells were briefly treated with various concentrations of AA (10, 25, 40, 100, 200, and 300 μM) for 24 h and then subjected to an MTS assay to evaluate the cytotoxic effects of AA exposure [21]. We found that the IC50 of AA was ~200 μM in both MDA-MB-231 and MCF-7 cells (Fig. S1). Therefore, the experiments in this present study were conducted using 100 μM of AA, a concentration at which the cells remain viable. It has been previously reported that at a 100-μM concentration, AA does not exhibit any toxic effects on cancer cells [28, 29].
In the current study, using a C18 reverse-phase column attached to an HPLC, eicosanoids were resolved and identified by comparing their positions with standard eicosanoids, and quantified by measuring the areas under each peak using the integration software EMPOWER 3 (Waters Corporation, Milford, MA). The results (Figs. 1A and1B) indicate that the eicosanoid profiles in MDA-MB-231 and MCF-7 cells are very different from each other. While the basal levels of the 5-LOX products—i.e., HETE5 and HETE8—are higher in MCF-7 cells than in MDA-MB-231 cells, the amount of PGE2 is several-fold higher in MDA-MB-231 cells (Figs. 1A and1B). Figure 1C shows the quantitative assessments of different lipid mediators (in the two cell lines) and how AA affects their syntheses. Notably, AA (100 μM) suppresses the synthesis of all eicosanoids in MCF-7 cells, but in MDA-MB-231 cells, such treatments down-regulate PGE2 and PGD2 and stimulate the syntheses of LTB4 (~ 4 fold) and HETE8 (~3–4 fold). To further understand if excess LTB4 production by MDA-MB-231 cells is linked to the LOX pathway, nordihydroguaiaretic acid (NDGA), a 5-LOX inhibitor [30], was tested. Figure 1D shows that AA induced-LTB4 production in MDA-MB-231 could be blocked by NDGA (5 μM). Interestingly, NDGA by itself also inhibited endogenous LTB4 synthesis. Additionally, we found that NDGA inhibited HETE8 production as well (data not shown). As LTB4 has been shown to be involved in simulating the growth and proliferation of various forms of cancer [14, 15, 30]—and we found that AA stimulates the production of LTB4 (Figs. 1C and D)—we mainly focused on the role of LTB4 in inducing the production of inflammatory cytokines and promoting the migration of MDA-MB-231 cells in the current investigation.
Arachidonic acid stimulates the expression of 5-LOX in MDA-MB-231 cells
5-LOX is an essential enzyme of the AA pathway, and it is involved in the synthesis of LTB4 and various cysteinyl-LTs including LTC4, LTD4, and LTE4 [10]. Because AA treatment increases the production of LTB4 in MDA-MB-231 cells, we examined whether AA stimulates the expression of the 5-LOX in MDA-MB-231 cells. To address this, AA-treated cells were lysed, and total protein preparations were analyzed by immunoblot. Results show that the expression of 5-LOX (~79kD) in MDA-MB-231 cells increases (~25%) with AA treatment and are inhibited by a LOX inhibitor, nordihydroguaiaretic acid (NDGA) (Figs. 2A and2B). It is also apparent that the inhibition of 5-LOX by NDGA can be reversed in part by AA. Unlike MDA-MB-231, MCF-7 cells do not show any appreciable changes by AA or NDGA. Confocal microscopy results (Figs. 3A and3B) show that although both cells express the 5-LOX, only MDA-MB-231 responded to AA treatment significantly as evidenced by increased immunostaining with the LOX antibody. Figure 3A shows that 5-LOX in MDA-MB-231 cells is mostly localized in the cytoplasm (Image a, red). Upon stimulation by AA, the expression of 5-LOX increases ~2 fold compared to the control and extends throughout the cytoplasm (Image b). As shown in Images c and d (Fig. 3A), NDGA reduces the AA-stimulated 5-LOX reactivity by ~2 fold. On the other hand, 5-LOX expression increases slightly by AA treatment in the MCF-7 cells. Interestingly, NDGA shows a minimal effect on 5-LOX reactivity in MCF-7 cells (Images e–h, Fig. 3A). Figure 3B shows the quantitative analysis of immunofluorescence images. Although NDGA is known to inhibit the arachidonic acid-lipoxygenase pathway and thereby reduces inflammatory reactions, it also functions as an antioxidant agent and has been shown to block the protein trafficking from the ER to Golgi, which cause redistributions of Golgi proteins into the ER and affects the intracellular levels of calcium (reviewed by Lü et al. 2010 ref. 32). Therefore, it is likely that NDGA directly or indirectly interferes with the synthesis and trafficking of 5-LOX and thereby reduces the expression of this enzyme as shown in Fig. 3.
Fig. (2). Arachidonic acid stimulates 5-lipooxygense expression in MDA-MB-231 cells.
Panel A: the expression 5-LOX (~79 KD) by MDA-MB-231 and MCF-7 cells in the presence of AA (100 μM), NDGA (5 μM), or a mixture of AA+NDGA. GAPDH was used as the loading control. Panel B shows the densitometric analysis of each band shown in panel A. All data represent the average (mean ±SE) of the three independent experiments. Statistical significance was calculated using One-way ANOVA test followed by the Holm-Šídák methods (panel B). ** p < 0.01; NS = Not significant.
Fig. (3). Arachidonic acid increases the expression of 5-lipooxygense enzyme.
Panel A: Image a: MDA-MB-231, control; Image b: AA-treatment; Image c: treatment with AA+NDGA; Image d: NDGA treatment; Image e: MCF-7, control; Image f: AA treatment; Image g: treatment with AA+NDGA; and Image h: treatment with NDGA. The enlarged images of the white box (a, b, c, and d) show the distinct localization of 5-LOX in the cytoplasm (a′, b′, c′, and d′ respectively). The arrow denotes the cytoplasm, and the asterisk indicates the nucleus. Bar: 10 μM. Panel B: The quantitative assessments of the mean fluorescence intensities of 5-LOX in MDA-MB-231 (magenta) and MCF-7 (blue). All data represent the average of the three independent experiments. The significance was analyzed using One-way ANOVA test (Holm-Šídák methods). ** p < 0.01; NS = Not significant.
Inhibitors of LOX enzyme inhibit the migration of MDA-MB-231 cells
The migratory behavior of cancer cells is associated with the cellular interactions of the extracellular matrix as well as signals that promote cell motility [33–35]. Therefore, we tested whether AA-induced LOX-5 activation and LTB4 synthesis is linked to the migration of MDA-MB-231 cells. In addition to NDGA, we also included zileuton in this experiment. Zileuton is a known inhibitor of 5-LOX and suppresses PG biosynthesis by blocking the release of AA in macrophages and other cells [36]. Briefly, MDA-MB-231 cells were placed in a 6-well plate and incubated overnight as described in the Materials and Methods section. The monolayers were wounded and thereafter cultured for 24 h in the presence of two LOX inhibitors—i.e., NDGA and zileuton. The dose-dependent response of NDGA and zileuton in the migration of MDA-MB-231 and MCF-7 is shown in Fig. S2. These two inhibitors were used individually or in combination with AA, LTB4, and PGD2. PGD2, produced by COX-2, was used to compare and evaluate the effect of LTB4 produced by the 5-LOX. Briefly, cells were plated in each well of a 6-well plate and incubated overnight in a medium (RPMI-1640 or DMEM) containing fetal bovine serum. The monolayers were wounded by scratching with a sterile 10-μL pipette tip [5] and treated individually or in combination with AA (100 μM), NDGA (5μM), zileuton (100 μM), and LTB4 (100 nM), and PGD2 (10 ng/ml) for 24 h, followed by a count of the cells that had migrated in the scratched areas. Figure 4A shows the number of migratory cells (MDA-MB-231) across the wounded region after 24 h of incubation in control and AA-treated plates. While the AA treatment increased (Image b), NDGA and zileuton blocked the migration significantly (Images d and f). NDGA and zileuton were also effective in reducing the AA-induced migration of MDA-MB-231 (Images e and g). Although AA treatment in addition to LTB4 (Image h) promoted the migration to a large extent, PGD2 (Image i) supplement, along with AA treatment, showed a significant reduction in migration. As expected, MCF-7 cells showed little or no migration across the wounded region under these experimental settings (Fig. 4B). Values shown in Table-1 represent the effects of AA, inhibitors, and LTB4 and PGD2 on the migration of MDA-MB-231 and MCF-7 cells.
Fig. (4). Arachidonic acid induces the migration of MDA-MB-231 cells.
The cells (~70–80% confluent monolayer) were scratch-wounded using a 10-μM pipette tip, and treated for 24 h with AA (100 μM), AA (100 μM) + NDGA (5 μM), or NDGA (5 μM). For MDA-MB-231 and MCF-7, respectively: Image a, a′: at 0 h wound-scratch; Image b, b′: at 24 h; Image c, c′: AA (100 μM); Image d, d′: NDGA (5 μM); Image e, e′: AA+NDGA (100 μM + 5 μM); Image f, f′: Zileuton (100 μM); Image g, g′: AA+Zil (100 μM +100 μM); Image h, h′: AA+LTB4 (100 μM+ 100 nM); Image i, i′: AA+PGD2 (100 μM + 10 ng/ml). MDA-MB-231 cells that migrated into the wound-region were counted and results are shown in Table-1.
Table 1.
Migration analysis of MDA-MB-231 cells after the treatment with 5-LOX inhibitors
| Treatment | MDA-MB-231, 0 h | MDA-MB-231, 24 h |
|---|---|---|
| Mean ± SD | Mean ±SD | |
| Control | 2.3 ± 1.3 | 79 ± 7.6 |
| AA | 3.4 ± 2.2 | 119.7 ±18.9 |
| NDGA | 2±1.4 | 9 ± 1.7 |
| AA + NDGA | 4±1.5 | 84.7±18.9 |
| Zil | 2.2 ± 1.1 | 40 ± 5.2 |
| AA + Zil | 2.3 ± 1 | 69 ± 10 |
| AA + LTB4 | 1.6 ±1.2 | 138.3 ± 4.7 |
| LTB4 + NDGA | 1 ± 1.3 | 48.3 ± 1.7 |
| AA + NDGA+ LTB4 | 2.3 ± 2.2 | 67.3 ±10.8 |
| LTB4 + Zil | 0.3 ±1.1 | 62.7± 5.8 |
| AA + Zil + LTB4 | 2.6 ±1.5 | 68 ±12.4 |
| AA + PGD2 | 4.3±1 | 62 ± 6.2 |
| PGD2 + NDGA | 2.3±1.5 | 44.3± 5.2 |
| AA + NDGA+ PGD2 | 1.5 ±2.6 | 58.7 ±18.1 |
| PGD2 + Zil | 1.6±2.3 | 61.6 ±15.7 |
| AA + Zil + PGD2 | 3.6 ± 1.2 | 43 ±14.8 |
Results shown here are the average values (± SD) obtained from migration assays after various treatments. The experiments were repeated twice in triplicate on two different days. SD, Standard deviation; LTB4, leukotriene B4; NDGA, nordihydroguaiaretic acid; PGD2, prostaglandin D2.
The disruption of lipid rafts interferes with the migration of MDA-MB-231 cells
LTB4 or other cysteinyl LTs synthesized by LOX pathways are secreted from the cells and act via G protein-coupled BLT receptors [35]. It has been reported that BLT receptors (i.e., BLT-1 and BLT-2) are located within the LRs of human neutrophils and that disruption of LRs suppresses LTB4 syntheses [37]. Since AA stimulated LTB4 production in MDA-MB-231 cells (Fig. 1C), and the inhibitors of the LOX enzyme block cellular migration (Fig. 4), we asked whether LTB4-mediated cellular migration occurs through the LRs. Earlier reports have suggested that the integrity of raft microdomains could be critical for the survival of triple-negative breast cancer cells, as well as for the development of malignancy [38, 39]. Therefore, we performed migration assays to examine whether the disassembly of raft microdomains interferes with the mobility of MDA-MB-231 cells. For this, we used methyl-β-cyclodextrin (MBCD), a commercially available LR disruptor that eliminates cholesterol from membranes and raft domains [38]. Briefly, the migrations of MDA-MB-231 and MCF-7 cells across the scratched wound were carried out in the presence and absence of MBCD (1 mM) as previously described. Results show that MBCD reduces the cellular migration in MDA-MB-231 cells significantly (Figs. 5A and5B).
Fig. (5). Methyl-β-cyclodextrin blocks the migration of MDA-MB-231 cells.
Approximately 1×105 cells were grown in a 12-well plate in growth medium supplemented with 5% FBS and treated with AA (100 μM), AA (100 μM) + NDGA (5 μM), NDGA, or MBCD (1 mM) for 24 h. Panel A: Effect of MBCD (1 mM) on the migration of MDA-MB-231 (Images a and b) and MCF-7 (Images c and d) cells. Panel B: Graphical representation of migration results shown in panel A. Data represent the average (mean ± SE) of the two separate experiments, and each experiment was carried out in duplicate. MBCD-treated samples were compared with controls and P values were calculated based on Student’s t test using the Graph Pad (** p < 0.001).
Because LRs and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells [40], and since MBCD blocks the migration of MDA-MB-231 cells (Fig. 5), we asked if caveolin-1 (a marker of LRs) expression is also altered by AA treatment. Anti-caveolin-1 antibody was used to evaluate the expression of caveolin-1 by immunoblot and confocal microscopy analysis (Fig. 6). Results show that the expression of caveolin-1 is increased slightly by AA treatment and that NDGA reduces the caveolin-1 expression (Figs. 6A and6B). Figure 6C shows the caveolin-1 expression (green) in MDA-MB-231 cells, which is predominantly concentrated in the plasma membrane (Image a, Fig. 6C). While AA treatment increased the intensity of staining significantly both in membrane and cytoplasm (~ 3 fold, Image b), NDGA reduced the expression (Image c). The NDGA-mediated reduction of caveolin-1 expression could be reversed partially by co-treatment with AA (Image d). The quantitative analysis of immunostaining is shown in Fig. 6D. In a separate experiment, we tested the reactivity of anti-caveolae antibody on MCF-7 cells. The cells showed weak reactivity against caveolin-1 antibody, and AA-treatment did not change the staining pattern (not shown).
Fig. (6). Arachidonic acid induces the expression of caveolin-1 in MDA-MB-231 cells.
Panel A: Immunoblot analysis using caveolin-1 antibody. Tubulin antibody (Sigma) was used as a loading control. Panel B: Densitometric analysis of the immunoblot. Panel C: Immunostaining of caveolae using anti-caveolin-1 antibody. Image a: control; image b: AA (100 μM) treatment; Image c: treatment with NDGA (5 μM); and Image d: treatment with AA + NDGA (100 μM+5 μM). The arrow denotes the membrane, and the asterisk indicates the nucleus. Bar: 10 μM. Panel D: analysis of fluorescence intensities using Zeiss ZEN confocal software (2009). Experiments were repeated three times, and the results expressed in mean ± SE. p values shown in panel B and D were calculated based on Student’s t test and compared with the control. *p < 0.05.
Arachidonic acid promotes the colocalization of BLT1 with lipid rafts
Because AA treatment increases the expressions of LTB4 and LOX (Figs. 1–3), and the BLT1 receptor is located in LRs [37], we asked whether AA stimulates the expression of BLT1 and increases its colocalization with LRs. To investigate this, BLT receptors of MDA-MB-231 and MCF-7 cells were immunostained with an antibody against the BLT1 receptor (red) and LRs were identified by labeling with cholera toxin subunit B (CTXB, green). Images a–f (Fig. 7A, red) show immunostaining with the BLT1 antibody (red), images a′–f′ (green) depict the labeling with CTXB (recognizes LRs), and images a′′–f′′ (yellow continuous or punctate appearances) demonstrate the colocalization of BLT1 with CTXB with DAPI overlay. The AA treatment in MDA-MB-231 increases the labeling intensity significantly for the BLT1 antibody (~3 fold, image b) and CTXB staining (~2 fold, image b′). The merged image shows bright and well-defined colocalization of the two antibodies (Image b′′). However, the changes of intensity in the AA-treated MCF-7 cells are low and not significant. MBCD has a profound effect in reducing the colocalization of BLT1 and CTX B (Image c′′, MDA-MB-231 and f′′, MCF-7 cells). The mean intensity/unit areas of CTXB and BLT1 for the MDA-MB-231 and MCF-7 cells are shown in Fig. 7B. These results support the idea that AA not only activates the BLT receptor located in the LRs of MDA-MB-231 cells, it also stimulates the synthesis of LTB4 and cellular migration. To evaluate the degree of colocalization among CTXB and BLT1 along the cell membrane, a part of the cell membrane was outlined using a white contour (not shown in the figure) as a region of interest (ROI) and the co-localization scattergram (using Zeiss ZEN 2009 software) is shown in Fig. 7C, in which green (CTXB) and red (BLT1) signals are assigned to the x and y axes, respectively. Each pixel is represented as a dot, and pixels with well co-localized signals appear as a scattered diagonal line [41, 42]. The average Manders’ overlap coefficients for MCF-7 cells (0.41 ± 0.013) and MDA MB 231 cells (0.90 ± 0.045) suggest strong colocalization between CTXB and BLT1 along the cell membrane of MDA MB 231. Almost ~90% of MDA MB 231 cells show strong colocalization of CTXB and BLT1 (Manders’ overlap coefficients 0.9 or above).
Fig. (7). Arachidonic acid promotes the colocalization of BLT1 receptors with lipid rafts in MDA-MB-231 cells.

Panel A: Cells were treated with AA (100 μM) and MBCD (1mM) for 24 h followed by staining of MDA-MB-231 and MCF-7 cells with anti-BLT1 antibody (red, images a–f) and alexa fluor 488-conjugated CTXB for LRs (green, Images a′-f′). The colocalization of CTX B and BLT1 antibody with DAPI are shown in images a′′–f′′. CM, cell membrane; N, nucleus (stained with DAP, blue). Bar: 10 μM. Panels B and C: The fluorescence intensities of CTXB and BLT1 for control and treated cells (MDA-MB-231 and MCF-7) were measured using Zeiss ZEN confocal software (2009). AA and MBCD treated samples were compared with their respective control cells (MDA-MB-231 or MCF-7 immunostained with CTXB or BLT1 antibody, respectively). Results were expressed in mean ± SE and p values were analyzed by One-way ANOVA test followed by the Holm-Šídák method. *p < 0.05, ** p < 0.01; *** p < 0.001; NS = Not significant. Panel D: Scattergrams showing colocalization of the CTXB and BLT1 in MDA-MB-231 and MCF-7 cell lines.
Arachidonic acid elevates the levels of NFκΒ, IL-6 and IL-8 in MDA-MB-231 cells
Some of the key molecules that are shown to be associated with cancer inflammation include various transcription factors and pro-inflammatory cytokines [43]. The transcriptional factor NFκΒ, a potential tumor promoter, is considered to be an important modulator of innate immunity and inflammation [44]. For example, NFκΒ is responsible for the activation of IL-6 and IL-8, which are two pro-inflammatory cytokines in tumor cells [45, 46]. Because phospho-NFκΒ p65 is known to be linked to the expression of cytokines, and AA activates 5-LOX expression (Figs. 2 and 3), we asked whether AA also modulates the levels of phosphorylated NFκΒ and pro-inflammatory cytokines. Figure 8A shows that AA stimulates (~2 fold) the production of phospho-NFκΒ p65 (ser 536) and NDGA blocks this stimulation. Compared to MDA-MB-231 cells, however, MCF-7 cells exhibit a low level of phospho-NFκΒ expression and remains unaltered by AA or NDGA. Since this transcription factor is linked to the expression of various cytokines, we tested whether AA modulates a variety of interleukins and other inflammatory molecules. Various pro- and anti-inflammatory cytokines (i.e., IL-1A, IL-1B, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 IL-17A, IFNγ, and TNFα) were measured using a commercially available ELISA assay kit, as mentioned in the Materials and Methods section (data not shown). We observed that, with the exception of IL-6 and IL-8, the majority of cytokines that we measured were either very low or could not be detected (data not shown). Our results show that MDA-MB-231 cells produce higher levels of IL-6 and IL-8 (~2 and ~5 fold, respectively) than MCF-7 cells. Furthermore, in MDA-MB-231 cells, AA increases the production of IL-6 and IL-8 (Figs. 8B and8C). Interestingly, the induction of IL-6 by AA is higher (~3 fold) than that of IL-8. Following AA treatment, NDGA prevented the increase of both cytokines. In addition, NDGA alone caused the reduction of IL-6 and IL-8 significantly, which could be because NDGA inhibits the metabolism of endogenous LOX products and cytokines. These experiments suggest that AA-induced 5-LOX activation and LTB4 production are linked to IL-6 and IL-8 synthesis.
Fig. (8). Arachidonic acid stimulates the production of phospho NFκΒ (p65), IL-6 and IL-8 in MDA-MB-231 cells.

MDA-MB-231 and MCF-7 cells were cultured and treated with AA, AA+NDGA, or NDGA before measuring the phospho-NFkB, IL-6, and IL-8 levels using commercially available assay kits, as described in Materials and Methods. Data (mean ± SE) represent the average of four independent experiments carried out in triplicate. Treated and control samples of respective cells (MDA-MB-231 or MCF-7) were compared and p values were calculated by One-way ANOVA test followed by the Holm-Šídák method. *p < 0.05; NS = Not significant.
Discussion
Understanding the mechanism of the metastatic migration of breast cancer cells is a matter of great interest. Reports indicate that while unsaturated free fatty acids like oleic acid (OA) and linoleic acid (LA) stimulate cellular proliferation and migration of MDA-MB-231 cells, saturated fatty acids inhibit cell proliferation and apoptosis [47–49]. The activation of CCR10, a member of the chemokine receptor family, was reported to stimulate the migration of breast cancer cells through the ERK1/2/MMP-7 signaling pathway, and that EGF promotes the migration of MDA-MB-231 cells by modulating the functions of phosphatidylinositol kinase and phospholipase C [50]. In the current study, we investigated the mechanism of AA-dependent cellular migration of MDA-MB-231 cells. Since AA is the precursor of eicosanoid molecules, we tested the hypothesis that altering the synthesis of eicosanoids could be linked to the migration of breast cancer cells.
We used two well-studied breast cancer cell lines, MDA-MB-231 and MCF-7, to evaluate the effect of AA on eicosanoid-dependent cellular migrations. These two functionally distinct cell lines differ from each other in morphologic, genomic, transcriptomic, migratory, and therapeutic responses properties [20]. Although both cells have epithelial-like structures, MDA-MB-231 cells are spindle-shaped with an invasive phenotype while MCF-7 exhibits dome-like morphologies. Genomic analyses have revealed the presence of substantial genomic heterogeneity/abnormalities and a transcriptional profile identified luminal and basal-like clustered genes in both MDA-MB-231 and MCF-7 cells. Reports also have suggested that these two breast cancer cells differ in their glycosphingolipid (GSL) compositions [51]. Glycolipids (GLs) and GSLs (i.e., gangliosides) are involved in cell growth, cellular recognition, and metastasis. Although the amount of total GLs is higher in MCF-7 cells, gangliosides are higher in MDA-MB-231 cell lines. Among all gangliosides, the most striking difference is found in the case of GM3 (mono-sialo dihexosyl-ganglioside). GM3, which regulates the growth factor function, is ~18 fold higher in MDA-MB-231 [51].
Our results show that AA treatment suppresses the syntheses of PGs in MDA-MB-231 cells and stimulates the syntheses of LOX products (e.g., LTB4 and HETE8). Furthermore, AA stimulates cellular migration, which is blocked by LOX inhibitors (NDGA and zileuton), thus suggesting that the LOX pathway is equally important and activated during the migration of breast cancer cells. The increased synthesis of LTB4 in MDA-MB-231 cells under the influence of AA could be an indication of LOX’s catalytic function changes, which enable these groups of enzymes to become hyperactive. In our study, the involvement of LOX, especially the 5-LOX, in stimulating the LTB4 synthesis was evidenced by the fact that the expression of 5-LOX activity is up-regulated by AA treatment and that NDGA, a LOX inhibitor, lowers this increased expression (Figs. 2 and 3). Zileuton, another 5-LOX inhibitor, also reduces the migration of triple-negative breast cancer cells (Fig. 4, Table-1). BLT1, the LTB4 receptor, resides in lipid rafts, and thus assembly and disassembly of lipid rafts alter the receptor-specific LTB4 secretion, which results in migrating MDA-MB-231 cells in culture. The importance of 5-LOX in breast cancer malignancy can be further supported by reports on other cancers. For example, elevated activity of 5-LOX is associated with the progression of hepatocellular carcinoma [12] and also promotes polyposis formation in the intestine [11]. These reports are consistent with findings described in this study.
It has been previously proposed that malignant transformation is associated with the secretion of inflammatory molecules, including tumor-necrosis factor, interleukins, and chemokines. Among the interleukins, IL-1 was found to be correlated with gastric carcinoma [52], IL-6 with hematological malignancies [53], and IL-8 with melanomas [54]. In glioblastoma, the activation of the transcription factor NFkΒ was shown to be connected with the expression of IL-8 [55]. Our results showing the AA-induced increased expression of IL-6 and IL-8 productions (Figs. 8B and8C) clearly demonstrate that these two pro-inflammatory cytokines could act as contributing factors to the development and progression of invasive breast cancers and that the LOX pathway could play an important role in this process.
In regards to LRs, it has been previously reported that the disassembly of membrane microdomains interferes with survival, invadopodia formation, and ECM (extracellular matrix) degradation by MDA-MB-231 cells [38, 56]. In our study, we found that the disruption of raft domains by MBCD interferes with the migration of MDA-MB-231 cells (Fig. 5) most likely through binding with cholesterol, one of the major constituents of LRs. This observation further supports the idea that the cholesterol-dependent assembly of LRs is important for AA-mediated LOX activation, LTB4 production, and the migration of MDA-MB-231 cells. LTB4, which is synthesized from AA by the action of 5-LOX, is released from cells and re-internalized by BLT1 receptors present in the LR.
LTB4 subsequently stimulates the cells by activating the transcription factor NFκΒ [57] that up-regulates the expression of cytokines such as IL-6 and IL-8. The increased production of these inflammatory cytokines is either directly or indirectly linked to the migration by MDA-MB-231 cells. Others have previously reported that AA causes an increase in the migration of MDA-MB-231 cells by activating focal adhesion kinase (FAK) [18]. Thus, it is possible that AA induces the migration of invasive breast cancer cells by activating FAK via LTB4 and IL-6/IL-8 cytokines. Wen et al. [30] have reported that AA induces angiogenesis of mammary tumors by activating LOX and mTOR pathways but not COX-2, further supporting our hypothesis that LOX is important for breast cancer metastasis. Currently, we are in the process of generating 5-LOX knockout MDA-MB-231 cells, as well as variants that expressing hemagglutinin-tagged 5-LOX. This research enables us to conduct an in-depth investigation to determine the precise role of the LOX pathway in breast cancer cell movement.
Conclusion
Our studies show that AA-induced migration of MDA-MB-231 cells could be linked to the stimulation of LOX enzyme and LTB4 synthesis. Free AA served as a precursor of eicosanoid molecules including LTB4, which is secreted and reabsorbed by cells via BLT1 receptors located in lipid rafts. In the case of MCF-7 cells, however, it appears that AA treatment does not influence the LTB4 production and migration in culture. However, in the current study, only the total LOX enzyme from the whole cell lysate was used. Future experiments will include active LOX enzymes of cytoplasmic or nuclear origin [58]. An attempt will also be made to overexpress LOX in MCF-7 cells and determine if excess LOX accelerates the migration. The broader aspect of our results could be attributed to the inflammatory and metastatic migration of basal and luminal tumors induced by a fatty diet containing AA and other PUFAs. We propose that LTB4 synthesis could be targeted for designing novel chemotherapeutic agents to treat breast cancers in the future.
Supplementary Material
MDA-MB-231 and MCF-7 cells were grown in 96-well plates and treated with various concentrations of AA (indicated in the figure) for 24 h, and the viability was tested by MTS assay. (A) MDA-MB-231 cells and (B) MCF-7 cells. Viabilities are shown as percent control. Hydrogen peroxide was used as a positive control. Results shown here are the average (mean ±SE) of three biological experiments carried out in duplicate. For statistical significance calculation (GraphPad), treated samples were compared with untreated controls using Student’s t test. **p<0.001.
(A) Cells were treated with various concentrations of NDGA (2.5, 5 and 10 μM) and zileuton (100, 200, 300 and 400 μM) for 24h followed by the migration assay as described in Materials and Methods. Image a: control, 0h; Image b, control, 24h; Image c, NDGA (2.5 μM); Image d, NDGA (5 μM); Image e, NDGA (10 μM); Image a′: control, 0h; Image b′, control, 24h; Image c′, zileuton (100 μM); Image d′, zileuton (200 μM); Image e′, zileuton (300 μM) and image f′, zileuton (400 μM). (B) Migration analysis: the cells that migrated into the wound-region were counted and analyzed. The data shown here are the mean (± SE) of the two independent experiments. Experiments were repeated three times, p values shown in panel B and C were calculated based on Student’s t test and compared with the control. *p < 0.05; **p<0.001; *** p < 0.001.
Acknowledgments
The National Institutes of Health (NIH) Grant 1R01AI 095667–01 supported part of this work to SD. DR received support from the HHMI grant (UTEP) and was supported in part by the Cotton Memorial Graduate Fellowship. High-performance liquid chromatography, confocal microscopy, and statistical analyses were carried out in the Biomolecule Analysis, Genomic Analysis, Cytometry, Screening and Imaging and Statistical Consulting Core Facilities at the Border Biomedical Research Center at UTEP with the support of a grant (G12MD007592) from the National Institutes of Minority and Health Disparity of the National Institutes of Health (NIMHD, NIH).
Abbreviations:
- AA
arachidonic acid
- BLT
B leukotriene receptor
- COX
cyclooxygenase
- HETE
hydroxyeicosatetraenoic acid
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- GSL
(glycosphingolipid)
- IL
interleukin
- LA
linoleic acid
- LT
leukotriene
- LR
lipid raft
- LOX
lipoxygenase
- MBCD
methyl-beta-cyclodextrin
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium salt
- NDGA
nordihydroguaiaretic acid
- NFκΒ
nuclear factor kappa beta
- NGS
normal goat serum
- OA
oleic acid
- OC
overlap coefficient
- PLA2
phospholipase A2
- PG
prostaglandin
- PUFA
polyunsaturated fatty acid
- ROI
region of interest
- TX
thromboxanes
- zil
zileuton
References
- 1.Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW.. Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. Br J Cancer 2006; 94:842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones R, Adel-Alvarez LA, Alvarez OR, Broaddus R, Das S.. Arachidonic acid and colorectal carcinogenesis. Mol Cell Biochem 2003; 253:141–9. [DOI] [PubMed] [Google Scholar]
- 3.Yang K, Li H, Dong J, Dong Y, Wang CZ, 2015. Expression profile of polyunsaturated fatty acids in colorectal cancer. World J. Gastroenterol 21, 2405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Dubois RN.. Eicosanoids and cancer. Nat Rev Cancer 2010; 10:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossen NS, Hansen AJ, Selhuber-Unkel C, Oddershede LB. Arachidonic acid randomizes endothelial cell motion and regulates adhesion and migration. PLoS One 2011; 6:e25196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res 2007; 9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maloberti PM, Duarte AB, Orlando UD, Pasqualini ME et al. Functional interaction between acyl-CoA synthetase 4, lipooxygenases and cyclooxygenase-2 in the aggressive phenotype of breast cancer cells. PLoS One 2010; 5:e15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer 2006; 6:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paquette B, Therriault H, Desmarais G, Wagner R, Royer R, Bujold R.. Radiation-enhancement of MDA-MB-231 breast cancer cell invasion prevented by a cyclooxygenase-2 inhibitor. Br J Cancer 2011; 105:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider C, Pozzi A. Cyclooxygenases and lipoxygenases in cancer. Cancer Metastasis Rev 2011; 30:277–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheon EC, Khazaie K, Khan MW, Strouch MJ et al. Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice. Cancer Res 2011; 71:1627–36. [DOI] [PubMed] [Google Scholar]
- 12.Xu XM, Deng JJ, Yuan GJ, Yang F, Guo HT, Xiang M, Ge W, Wu YG. 5-Lipoxygenase contributes to the progression of hepatocellular carcinoma. Mol Med Report 2011; 4:1195–200. [DOI] [PubMed] [Google Scholar]
- 13.Carpagnano GE, Palladino GP, Lacedonia D, Koutelou A, Orlando S, Foschino-Barbaro MP. Neutrophilic airways inflammation in lung cancer: the role of exhaled LTB-4 and IL-8. BMC Cancer. 2011; 11, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong WG, Ding XZ, Talamonti MS, Bell RH, Adrian TE. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res Commun 2005; 335:949–56. [DOI] [PubMed] [Google Scholar]
- 15.Bachi AL, Kim FJ, Nonogaki S, Carneiro CR, Lopes JD, Jasiulionis MG, Correa M.. Leukotriene B4 creates a favorable microenvironment for murine melanoma growth. Mol Cancer Res 2009; 7:1417–24. [DOI] [PubMed] [Google Scholar]
- 16.Villegas-Comonfort S, Castillo-Sanchez R, Serna-Marquez N, Cortes-Reynosa P, Salazar EP. Arachidonic acid promotes migration and invasion through a PI3K/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Prostaglandins Leukot Essential Fatty Acids. 2014; 90, 169–77. [DOI] [PubMed] [Google Scholar]
- 17.Soto-Guzman A, Villegas-Comonfort S, Cortes-Reynosa P, Perez Salazar E. Role of arachidonic acid metabolism in Stat5 activation induced by oleic acid in MDA-MB-231 breast cancer cells. Prostaglandins Leukot Essential Fatty Acids 2013; 88, 243–918. [DOI] [PubMed] [Google Scholar]
- 18.Navarro-Tito N, Robledo T, Salazar EP. Arachidonic acid promotes FAK activation and migration in MDA-MB-231 breast cancer cells. Exp Cell Res 2008; 314:3340–55. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer 2015; 15, 225–37. [DOI] [PubMed] [Google Scholar]
- 20.Neve RM, Chin K, Fridlyand J, Yeh J et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006; 10, 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malich G, Markovic B, Winder C.. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997;124:179–92. [DOI] [PubMed] [Google Scholar]
- 22.Franchi AM, Chaud M, Rettori V, Suburo A, McCann SM, Gimeno M.. Role of nitric oxide in eicosanoid synthesis and uterine motility in estrogen-treated rat uteri. Proc Natl Acad Sci U S A 1994; 91:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraes LA, Giner RM, Paul-Clark MJ, Perretti M, Perrett D. An isocratic HPLC method for the quantitation of eicosanoids in human platelets. Biomed Chromatogr. 2004. 18:64–8. [DOI] [PubMed] [Google Scholar]
- 24.Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther 2006. 5:1183–9. [DOI] [PubMed] [Google Scholar]
- 25.Manders EM, Verbeek FJ, Aten JA. Measurment of co-localization of objects in dual-color confocal images. J. Microsc 1993. 169, 375–382. [DOI] [PubMed] [Google Scholar]
- 26.De Chatterjee A, Mendez TL, Roychowdhury, S, Das. The Assembly of GM1 Glycolipid-and Cholesterol-Enriched Raft-Like Membrane Microdomains Is Important for Giardial Encystation. Infect Immun 2015;.83:2030 –2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das S, Robles-Martinez L, Ray S. Phospholipid remodeling and eicosanoid signaling in colon cancer cells. Ind J Biochem Biophys 2014; 51, 512–519. [PMC free article] [PubMed] [Google Scholar]
- 28.Connell E, Darios F, Broersen K, Gatsby N, Peak-Chew SY, Rickman C, Davletov B. 2007. Mechanism of arachidonic acid action on syntaxin-Munc18. EMBO Rep 8:414–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trombetta A, Maggiora M, Martinasso G, Cotogni P, Canuto RA, Muzio G. 2007. Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem Biol Interact 165:239–50. [DOI] [PubMed] [Google Scholar]
- 30.Wen ZH, Su YC, Lai PL, Zhang Y, Xu YF, Zhao A, Yao GY, Jia CH, Lin J, Xu S, Wang L, Wang XK, Liu AL, Jiang Y, Dai YF, Bai XC. 2012. Critical role of arachidonic acid-activated mTOR signaling in breast carcinogenesis and angiogenesis. Oncogene 32, 160–70 [DOI] [PubMed] [Google Scholar]
- 31.Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci 2007; 103, 24–32 [DOI] [PubMed] [Google Scholar]
- 32.Lü JM, Nurko J, Weakley SM, Jiang J et al. Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives:an update. Med Sci Monit 2010;16: RA93–100 [PMC free article] [PubMed] [Google Scholar]
- 33.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992; 69:11–25. [DOI] [PubMed] [Google Scholar]
- 34.Lauffenburger DA, Horwitz AF. 1996. Cell migration: a physically integrated molecular process. Cell 84:359–69. [DOI] [PubMed] [Google Scholar]
- 35.Liu M and Yokomizo T The role of leukotrienes in allergic diseases. Allergology Int 2015; 64, 17–26. [DOI] [PubMed] [Google Scholar]
- 36.Rossi A, Pergola, C, Koeberle A, Hoffmann, M. The 5-lipoxygenase inhibitor, zileuton, suppresses prostaglandin biosynthesis by inhibition of arachidonic acid release in macrophages. Brit. J. Pharmacol 2010; 161, 555–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sitrin RG, Emery SL, Sassanella TM, Blackwood RA, Petty HR. Selective localization of recognition complexes for leukotriene B4 and formyl-Met-Leu-Phe within lipid raft microdomains of human polymorphonuclear neutrophils. J Immunol 2006; 177:8177–84. [DOI] [PubMed] [Google Scholar]
- 38.Badana A, Chintala M, Varikuti G, Pudi N, Kumari S, Kappala VR, Malla RR. Lipid Raft Integrity Is Required for Survival of Triple Negative Breast Cancer Cells. J Breast Cancer 2016; 19, 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta 2008; 1785:182–206. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z et al. Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res 2009; 69, 8594–602. [DOI] [PubMed] [Google Scholar]
- 41.Sierra-Fonseca JA, Najera O, Martinez-Jurado J, Walker EM, Varela-Ramirez A, Khan AM, Varela-Ramirez A, Khan AM, Miranda M, Lamango NS, Roychowdhury S,. Nerve growth factor induces neurite outgrowth of PC12 cells by promoting Gβγ-microtubule interaction. BMC Neurosci 2014; 15, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gant VU Jr, Moreno S, Varela-Ramirez A, Johnson KL. Two membrane-associated regions within the Nodamura virus RNA-dependent RNA polymerase are critical for both mitochondrial localization and RNA replication. J Virol 2014; 88, 5912–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436–44. [DOI] [PubMed] [Google Scholar]
- 44.Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 2006; 25:6831–43 [DOI] [PubMed] [Google Scholar]
- 45.Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res 2005; 65:11001–9. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y, Cai Z, Xiao G, Keller ET et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res 2007; 67:3646–53. [DOI] [PubMed] [Google Scholar]
- 47.Ford JH. Saturated fatty acid metabolism is key link between cell division, cancer, and sensecence in cellular and whole organism aging. Age (Dordr) 2010; 32: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu J, Wu KI, Li JJ, Chen C et al. Autophagy mediates free fatty acid effects on MDA-MB-231 cell proliferation, migration and invasion. Oncol Lett 2017; 14: 4715–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Reys C, Marcial-Medina C, Cervantes-Anaya N, Cortes-Reynosa P, Salazar EP. Migration and inavsion induced by linoleic acid are mediated through fascin in MDA-MB-231 breast cancer cells. Mol Cell Biochem 2017. (in press) DOI 10.1007/s11010-017-3205-8. [DOI] [PubMed] [Google Scholar]
- 50.Price JT, Tiganis T, Agarwal A, Djakiew D et al. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3-kinase and phospholipase C-dependent mechanism. Cancer Res 1999; 59: 5475–5478. [PubMed] [Google Scholar]
- 51.Nohara K, Wang F, Spiegel S. Glycosphingolipid composition of MDA-MB-231 and MCF-7 human breast cancer cell lines. Breast Cancer Res Treat 1998; 48: 149–157. [DOI] [PubMed] [Google Scholar]
- 52.Kamangar F, Cheng C, Abnet CC, Rabkin CS. Interleukin-1B polymorphisms and gastric cancer risk--a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1920–8. [DOI] [PubMed] [Google Scholar]
- 53.Jongen-Lavrencic M, Peeters HR, Rozemuller H, Rombouts WJ et al. IL-6-induced anaemia in rats: possible pathogenetic implications for anemia observed in chronic inflammations. Clin Exp Immunol 1996; 103:328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Fu T, McGettigan S, Kumar S et al. IL8 and Cathepsin B as Melanoma Serum Biomarkers. Int J Mol Sci 2011, 12:1505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raychaudhuri B, Vogelbaum MA. IL-8 is a mediator of NF-kappaB induced invasion by gliomas. J Neurooncol 2011; 101:227–35. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Guan L, Li S, Jiang Y et al. Mechanosensitive caveolin-1 activation-induced PI3K/Akt/mTOR signaling pathway promotes breast cancer motility, invadopodia formation and metastasis in vivo. Oncotarget 2016. 7(13):16227–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Galan E, Gomez-Hernandez A, Vidal C, Martin-Ventura JL et al. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res 2009; 81:216–25. [DOI] [PubMed] [Google Scholar]
- 58.Peters-Golden M, Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids 2003; 69: 99–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MDA-MB-231 and MCF-7 cells were grown in 96-well plates and treated with various concentrations of AA (indicated in the figure) for 24 h, and the viability was tested by MTS assay. (A) MDA-MB-231 cells and (B) MCF-7 cells. Viabilities are shown as percent control. Hydrogen peroxide was used as a positive control. Results shown here are the average (mean ±SE) of three biological experiments carried out in duplicate. For statistical significance calculation (GraphPad), treated samples were compared with untreated controls using Student’s t test. **p<0.001.
(A) Cells were treated with various concentrations of NDGA (2.5, 5 and 10 μM) and zileuton (100, 200, 300 and 400 μM) for 24h followed by the migration assay as described in Materials and Methods. Image a: control, 0h; Image b, control, 24h; Image c, NDGA (2.5 μM); Image d, NDGA (5 μM); Image e, NDGA (10 μM); Image a′: control, 0h; Image b′, control, 24h; Image c′, zileuton (100 μM); Image d′, zileuton (200 μM); Image e′, zileuton (300 μM) and image f′, zileuton (400 μM). (B) Migration analysis: the cells that migrated into the wound-region were counted and analyzed. The data shown here are the mean (± SE) of the two independent experiments. Experiments were repeated three times, p values shown in panel B and C were calculated based on Student’s t test and compared with the control. *p < 0.05; **p<0.001; *** p < 0.001.