Abstract
Objective:
The authors tested whether clonidine blocks stress-induced seeking of heroin and cocaine. The study was also intended to confirm translational findings from a rat model of drug relapse by using ecological momentary assessment of patients’ stress to test hypotheses about clonidine’s behavioral mechanism of action.
Method:
The authors conducted a randomized double-blind placebo-controlled clinical trial with 208 opioid-dependent patients at an outpatient buprenorphine clinic. The 118 participants (57%) who maintained abstinence during weeks 5–6 were continued on buprenorphine and randomly assigned to receive clonidine (N=61) or placebo (N=57) for 14 weeks. Urine was tested thrice weekly. Lapse was defined as any opioid-positive or missed urine test, and relapse as two or more consecutive lapsesTime to lapse and relapse were examined with Cox regressions; longest period of abstinence was examined with a t test, and ecological momentary assessment data was examined with generalized linear mixed models.
Results:
In an intent-to-treat analysis, clonidine produced the longest duration (in consecutive days) of abstinence from opioids during the intervention phase (34.8 days [SD=3.7] compared with 25.5 days [SD=2.7]; Cohen’s d=0.38). There was no group difference in time to relapse, but the clonidine group took longer to lapse (hazard ratio=0.67, 95% CI=0.45–1.00). Ecological momentary assessment showed that daily-life stress was partly decoupled from opioid craving in the clonidine group, supporting the authors’ hypothesized mechanism for clonidine’s benefits.
Conclusions:
Clonidine, a readily available medication, is useful in opioid dependence not just for alleviation of withdrawal signs, but also as an adjunctive maintenance treatment that increases duration of abstinence. Even in the absence of physical withdrawal, it decouples stress from craving in everyday life.
Despite effective treatments for opioid addiction (e.g., buprenorphine and methadone) (1), patients still relapse to opioid misuse (2–4). Relapses, briefer lapses, and episodes of craving can be triggered by acute precipitants: stress, exposure to drug-associated cues, or the use of an initially small amount, or priming dose, of drug (5, 6). Buprenorphine maintenance might be improved with the addition of a treatment that specifically buffers the effects of one or more triggers.
In search of such a treatment, we turned to findings from the rat reinstatement model, in which extinguished drug seeking resumes after exposure to a stressor, a drug cue, or a priming dose of drug (7, 8). Among the clinically available medications, alpha-2 adrenoceptor agonists such as lofexidine, guanfacine, and clonidine (9, 10) selectively block stress-induced reinstatement of heroin, cocaine, alcohol, and nicotine seeking (9–13). Thus, alpha-2 agonists may act on a final common pathway of stress-induced and possibly cue-induced relapse (14), which are relevant with multiple drugs of abuse. This notion is supported by results from several human laboratory studies in which we and the Sinha group reported that alpha-2 agonists decrease both stress- and cue-induced drug craving (15–20). In the rat reinstatement model, chronic buprenorphine or methadone administration blocks both heroin- and cocaine-primed reinstatement of drug seeking, but not stress-induced reinstatement (21, 22).
Taken together, these preclinical data and human laboratory studies suggest an attractive hypothesis for translational research: clonidine maintenance treatment combined with buprenorphine treatment should help prevent stress-induced lapses. This would be a new treatment approach with clonidine, unrelated to its well-established use as an adjunctive medication during opioid detoxification (23, 24).
We tested clonidine in a clinical trial in which we incorporated two innovative features. First, we took a literal approach to relapse prevention: we randomized participants to clonidine or placebo only after they had achieved a period of outpatient abstinence from opioids, because cessation of ongoing drug use is probably mediated by neural processes different from those involved in avoidance of relapse (25, 26). Second, to address mechanistic questions about whether clonidine would prevent only certain types of relapses, we incorporated ecological momentary assessment, a method of collecting field data with handheld computers on which participants report, in real time, their activities and moods (27). Ecological momentary assessment is essential for addressing questions about the order in which events happen and the extent to which one event is specifically associated with another (5); retrospective assessments of the same questions are fraught with illusory correlations that result from recall biases (28, 29).
Thus, we tested the hypothesis that clonidine maintenance treatment as an adjunct to buprenorphine maintenance treatment would decrease lapses and relapses, increase duration of abstinence, and decrease opioid misuse, and that clonidine’s effect would be associated with decreases in stress-induced craving and drug use, as suggested by the preclinical data described above.
METHOD
This was a double-blind placebo-controlled randomized clinical trial with a parallel design testing clonidine’s efficacy as an adjuvant to buprenorphine to increase duration of opioid abstinence, time to opioid lapse and relapse, and percent opioid-negative urine tests.
Participants and Setting
Participants were treatment-seeking heroin-dependent or prescription opioid-dependent outpatient volunteers recruited from October 2006 to August 2013. Study candidates were evaluated with standardized interviews (30, 31), physical examination, and laboratory screenings. Inclusion criteria were physical dependence on opioids and age between 18 and 60 years. Exclusion criteria were a current psychotic disorder, a history of bipolar disorder or schizophrenia, current major depression, current dependence on alcohol or sedatives, cognitive impairment that would preclude informed consent or valid self-report, medical illness that would compromise participation, pregnancy or breastfeeding, and use of contraindicated medications, such as beta-blockers.
Buprenorphine maintenance treatment began at enrollment and continued for up to 28 weeks at our outpatient treatment research clinic in Baltimore. Throughout the study, participants attended the clinic 7 days a week for sublingual buprenorphine (8–24 mg/day) and received individual counseling once a week. Participants provided urine and breath samples under observation thrice weekly; samples were tested for opioids, cocaine, marijuana, amphetamines, barbiturates, benzodiazepines, and alcohol.
The Institutional Review Board of the National Institute on Drug Abuse Intramural Research Program approved the study, and all participants gave written informed consent. Power analyses indicated a required sample size of 120 (32).
Study Design
Abstinence initiation and group assignment.
To facilitate opioid abstinence, we used a standard contingency-management procedure. During weeks 1 through 8, all participants earned a voucher exchangeable for goods and services consistent with treatment goals for each opioid-negative urine test. The value of the vouchers started at $4 and increased by $4 for each consecutive opioid-negative urine test. If a test was opioid positive or was missed, no voucher was given, and the value of the next voucher was reset to $4.
Participants who were abstinent from illicit opioids during weeks 5 and 6 (verified by six opioid-negative urine tests) were randomly assigned to receive either clonidine or placebo. Randomization was conducted by an investigator who had no contact with participants (K.L.P.), using a computerized algorithm stratified by age, sex, race, and cocaine and opioid use during the 6-week baseline periodAll other staff and participants were blind to study group assignment. Participants who did not meet the abstinence criterion were switched to methadone for 4 weeks followed by an 8-week medication taper, or were helped to transfer to a community treatment program.
Clonidine/Placebo Induction
Beginning at week 7, our study nurses administered three identical capsules daily to each randomized participant, concurrent with the daily dose of buprenorphine. The capsules, taken while a nurse watched, contained either placebo or clonidine (0.1 mg per capsule). The schedule for clonidine induction was 0.1 mg for 7 days, 0.2 mg for the next 7 days, and 0.3 mg from weeks 9 through 20 (considered the intervention phase). Nurses interviewed participants for adverse events weekly, and, if indicated, clonidine/placebo dosages were adjusted in 0.1-mg increments at the discretion of a physician (J.P.S. or K.A.P.) who was blind to assignment. Research pharmacists adjusted dosages as ordered.
Intervention Phase (Weeks 9–20)
Throughout the intervention phase, participants received daily clonidine or placebo and were maintained on a stable dosage of buprenorphine. For ecological momentary assessment, we issued one of three devices to participants (a PalmOne Zire 21, a Palm Tungsten E2, or an HTC TyTN II smartphone) to be used as an electronic diary. The electronic diary emitted an audible prompt at four randomly chosen times during the participant’s typical waking hours; at each prompt, the participant was asked to report on stress, craving, mood, and drug-related environmental cues. Participants answered stress, craving, and mood items with the response options “NO!!”, “no??”, “yes??”, and “YES!!”. Drug-related cues were assessed by asking whether the cue had been encountered in the hour before the prompt (5). We also asked participants to make an entry in their electronic diary each time they used a drug (data not reported).
Maintenance Phase (Weeks 21–28)
The clonidine dosage was tapered to zero over the first 14 days of the 8-week maintenance phase. Ecological momentary assessment continued throughout the maintenance phase. After week 28, participants’ buprenorphine dosages were tapered over another 8 weeks, or participants were helped to transfer to another treatment program.
Monitoring of Vital Signs, Liver Function, and Adverse Events
We assessed orthostatic vital signs weekly and also on the first day of clonidine/placebo dosing. To monitor liver function, we drew blood from participants 4 weeks into the intervention phase, after buprenorphine stabilization. Data on adverse events were collected weekly in interviews with nurses and as spontaneously reported by participants. Urine pregnancy tests were conducted once a month in female participants.
Data Analysis
We transformed continuous variables with highly skewed distributions into categorical variables. In preliminary analyses, we compared participants who were randomized with those who were not. Among those randomized, we checked for differences between the clonidine and placebo groups in demographic characteristics, drug use history, and drug use in the 6-week baseline period. These were bivariate analyses using t tests, chi-square tests, and Fisher’s exact tests, as appropriate.
Primary outcome analyses included tests of time to lapse and time to relapse from the beginning of induction; these were tested in Cox proportional hazards models (using SAS Proc PHreg). We defined lapse as any positive or missed urine test, and relapse as two or more consecutive lapses. Dropouts were considered to have lapsed and relapsed. The majority of dropouts (76%) occurred because the participant ceased coming to the clinic. Those who remained abstinent at the end of the intervention phase were coded as right censored. Analyses controlled for demographic or baseline variables associated with group or with a specific outcome measure. When the proportional hazards assumption was unmet, we added an interaction with time.
We also included two other co-primary analyses of outcome. The first was a repeated-measures group comparison on the sample-by-sample likelihood of an opioid-negative urine test (using SAS Proc Glimmix). This model controls for the number of urine tests provided by each participant and for each participant’s percentage of negative urine tests during the 6-week baseline period. The second co-primary outcome measure was the mean longest period of abstinence across groups during the intervention phase (assessed by independent-samples t test).
In secondary outcome analyses, we assessed the likelihoods of urine tests negative for cocaine and for tetrahydrocannabinol (THC) (using generalized linear mixed models [SAS Proc Glimmix]) and the longest period of abstinence from cocaine (using independent-samples t tests).
The aforementioned analyses had been specified before data collection. Analyses of the ecological momentary assessment data were exploratory, because although we intended from the outset to assess clonidine’s effect on stress-induced relapse, we were not sure how to approach the analysis before seeing the distribution of the data. Using a generalized linear mixed model.we used stress, mood, or cue exposure (each treated as a time-varying predictor) to predict a dichotomous outcome—either the likelihood of reporting craving for heroin in the same response (a response of “yes??” or “YES!!”) or reporting no craving (a responses of “no??” or “NO!!”). Analyses used a first-order autoregressive error structure and included a term for the number of responses given by each participant.
For all analyses, we used a two-tailed alpha of 0.05, with trends noted at 0.10 (a criterion we specified prior to starting data collection).
RESULTS
Of 208 enrollees, 118 (57%) were randomly assigned to receive clonidine or placebo. Of the 90 participants who were not randomized, only 32 (35%) failed the 2-week abstinence requirement; most of the others dropped out (see Figure S1 in the data supplement that accompanies the online edition of this article). Randomized participants were older on average (a mean age of 38.7 years [SD=8.1] compared with 36.3 years [SD=8.7]; t=2.1, df=206, p≤0.05) and used opioids on fewer days during the month preceding enrollment (24.7 days [SD=7.9] compared with 26.8 days [SD=6.8]; t=2.0, df=203, p≤0.05) (see Table S1 in the data supplement). Among randomized participants, there were no significant differences in demographic and drug use characteristics between the clonidine and placebo groups (Table 1). Most participants completed the intervention phase (clonidine group, 44 of 61; placebo group, 41 of 57) (see Figure S1). Four participants (two in each group) stopped taking the study drug. The study was completed without early termination in February 2014. Primary outcome analyses were intent-to-treat and include all randomized participants.
TABLE 1.
Demographic and Drug Use Characteristics of Patients Who Received Placebo or Clonidine in Addition to Buprenorphinea
| Variable | Placebo Group (N=57) | Clonidine Group (N=61) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 38.3 | 8.5 | 39.2 | 7.8 |
| Education (years) | 12.0 | 2.0 | 11.9 | 1.3 |
| Employment (days paid for work in the last 30) | 8.6 | 9.3 | 8.8 | 9.1 |
| Heroin or prescription opioid use (years) | 11.1 | 6.5 | 12.9 | 8.4 |
| Heroin or prescription opioid use (days in the last 30) | 24.4 | 7.9 | 25.1 | 8.0 |
| Cocaine use (years) | 3.8 | 5.3 | 3.7 | 6.4 |
| Cocaine use (days in the last 30) | 3.4 | 7.5 | 3.4 | 7.3 |
| Number of past drug treatments | 1.8 | 1.8 | 1.7 | 1.4 |
| Money spent on drugs ($ per month) | 1,179 | 1,407 | 1,038 | 940 |
| N | % | N | % | |
| Male | 46 | 80.7 | 46 | 75.4 |
| Race | ||||
| Black | 31 | 54.4 | 40 | 65.6 |
| White | 25 | 43.9 | 19 | 31.1 |
| Other | 1 | 1.8 | 2 | 3.3 |
| Major substance use problem | ||||
| Heroin use | 41 | 71.9 | 46 | 75.4 |
| Polysubstance use | 9 | 15.8 | 11 | 18.0 |
| Prescription opioid use | 7 | 12.3 | 4 | 6.6 |
| Heroin or prescription opioid route | ||||
| Inhalation | 32 | 56.1 | 39 | 63.9 |
| Intravenous injection | 19 | 33.3 | 16 | 29.7 |
| Oral | 4 | 7.0 | 4 | 6.6 |
| Smoked | 2 | 3.5 | 2 | 3.3 |
| Cocaine route | ||||
| Smoked | 18 | 51.4 | 22 | 61.1 |
| Inhalation | 8 | 22.9 | 8 | 22.2 |
| Intravenous injection | 9 | 25.7 | 6 | 16.7 |
No significant differences between treatment groups on any measure.
Adverse Events
Clonidine was well tolerated. During the intervention phase, the dosage was reduced from 0.3 mg to 0.2 mg in 10 participants and to 0.1 mg in five participants. Table S2 in the online data supplement summarizes adverse events during the induction and intervention phases that were reported by at least 5% of participants, and any event that differed in frequency between groups. Participants in the clonidine group were more likely to report an adverse event (N=58 [95.1%] compared with N=48 [84.2%]; χ2=3.8 p≤0.05), but the only specific symptom they were more likely to report was dry mouth (N=6 [9.8%] compared with N=0; Fisher’s exact test, p≤0.05). The events most expected to be problematic—sedation (clonidine group, N=17 [27.9%]; placebo group, N=8 [14.0%]; χ2=3.4 p=0.07), hypotension (clonidine group, N=8 [13.1%]; placebo group, N=3 [5.3%]; χ2=2.1 p=0.14), and dizziness (clonidine group, N=2 [3.3%]; placebo group, N=3 [5.3%]; Fisher’s exact test, p=0.21)—did not differ significantly between groups. Bradycardia was symptomatic in only one case, in which it occurred with hypovolemia.
Primary Outcome Measures
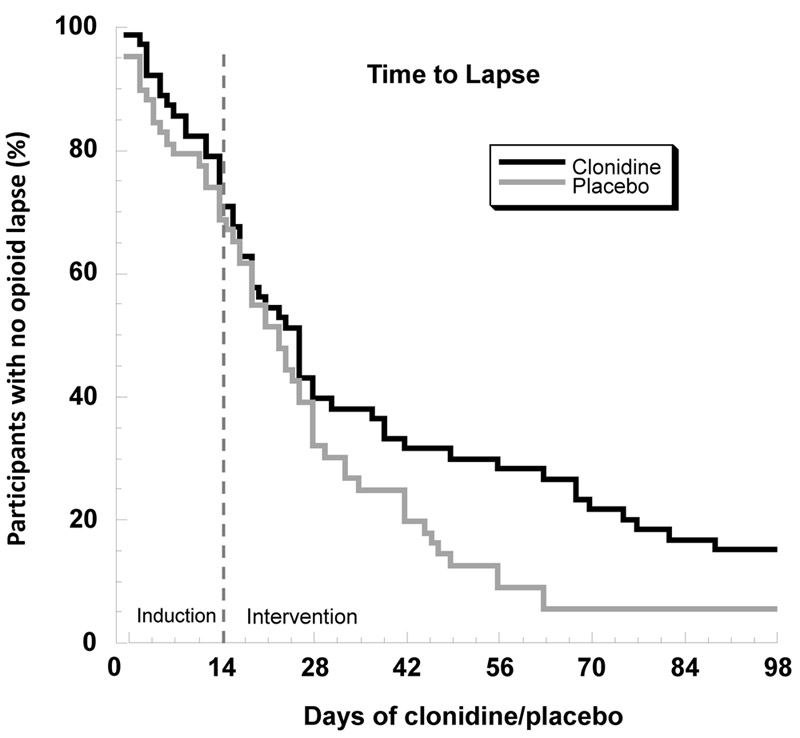
In a Cox proportional hazards model, clonidine use increased the time to initial opioid lapse, although the difference did not reach statistical significance (hazard ratio=0.70, 95% CI=0.47–1.03, p=0.07) (Figure 1). However, time to opioid lapse was also predicted by frequency of cocaine use during the 6-week baseline period (see Figure S1 in the data supplement). Therefore, in a final model, we controlled for baseline period cocaine-use category and its interaction with time. We had expected the nearly significant effect of clonidine to become nonsignificant in this model; instead, we found that clonidine use now significantly increased the time to initial opioid lapse (p≤0.05) (Table 2).
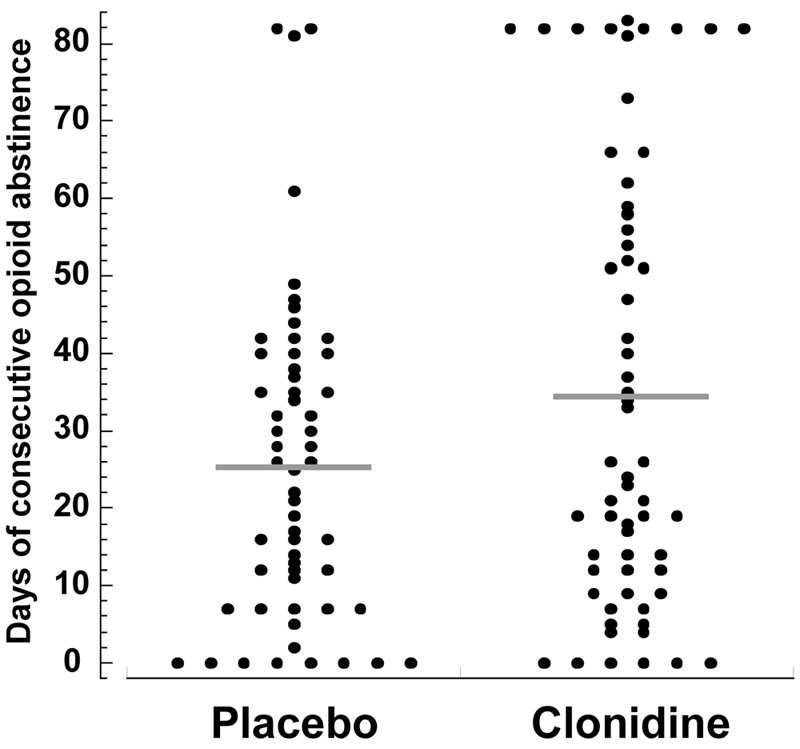
FIGURE 1.
Longest Period of Consecutive Opioid Abstinence in the Intervention Phase Among Patients Who Received Placebo or Clonidine in Addition to Buprenorphinea
a Each circle represents a single participant’s longest period of abstinence during the intervention phase (weeks 9–20, following a 2-week induction phase; the induction phase followed a 6-week baseline period of buprenorphine treatment alone). Horizontal bars show the group means. The clonidine group maintained continuous abstinence longer than the placebo group (p≤0.05).
TABLE 2.
Time to Lapse Among Patients Who Received Placebo or Clonidine in Addition to Buprenorphine: Final Modela
| Parameter | Parameter Estimate |
Hazard Ratio | 95% CI | p |
|---|---|---|---|---|
| Clonidine | −0.39 | 0.67 | 0.45, 1.00 | 0.05 |
| No cocaine use | −0.91 | 0.40 | 0.18, 0.86 | 0.02 |
| Low cocaine use | −0.64 | 0.53 | 0.31, 0.91 | 0.02 |
| High cocaine use | Reference | 1.00 | ||
| Cocaine-by-time interaction | −0.01 | 1.00 | 0.43 |
Final Cox proportional hazards model predicting time to opioid lapse. The model controls for cocaine use during the 6-week baseline period and its interaction with time.
In a separate proportional-hazards model, testing time to opioid relapse rather than lapse, clonidine had no effect (hazard ratio=0.84, 95% CI=0.54–1.28).
Clonidine produced the longest duration of consecutive days of abstinence for opioids during the intervention phase (clonidine group, 34.8 days [SD=3.7]; placebo group, 25.5 days [SD=2.7]; t=2.04, df=109, p≤0.05; Cohen’s d=0.375) (Figure 2).
FIGURE 2.
Survival Curve for Time Until Lapse to Opioid Use Among Patients Who Received Placebo or Clonidine in Addition to Buprenorphinea
a Participants in the clonidine group tended to maintain abstinence longer before an initial lapse (p=0.07); controlling for the effect of cocaine use during the 6-week baseline period., the effect was statistically significant (p≤0.05). We had included this control term expecting it to eliminate, not strengthen, the effect of group.
In the generalized linear mixed models, we found no group difference in the likelihood of providing an opioid-negative urine test (clonidine group, 82.1%, 95% CI=78.3–85.3; placebo group, 79.6%, 95% CI=75.6–83.2).
Secondary Outcome Measures
During the intervention phase, the clonidine group was more likely than the placebo group to test negative for THC, in models that controlled for baseline-phase THC positive tests (clonidine group, 88.5%, 95% CI=83.8–92.0; placebo group, 79.8%, 95% CI=73.8–84.7; F=6.32, df=1, 108, p≤0.01; Cohen’s d=0.48). There was no significant difference in the percentage of cannabis smokers at baseline in each group (62.3% in the clonidine group, 61.4% in the placebo group) or the mean percentage of negative urine tests at baseline (clonidine group, 70.5% [SD=5.1]; placebo group, 78.7% [SD=4.2]). There was no group difference in cocaine use during the intervention phase (clonidine group, 81.2%, 95% CI=75.7–85.7; placebo group, 84.1%, 95% CI=78.6–88.4). There was also no group difference in longest duration of abstinence from cocaine (clonidine group, 32.7 days [SD=3.8]; placebo group, 30.6 days [SD=3.4).
Ecological Momentary Assessment
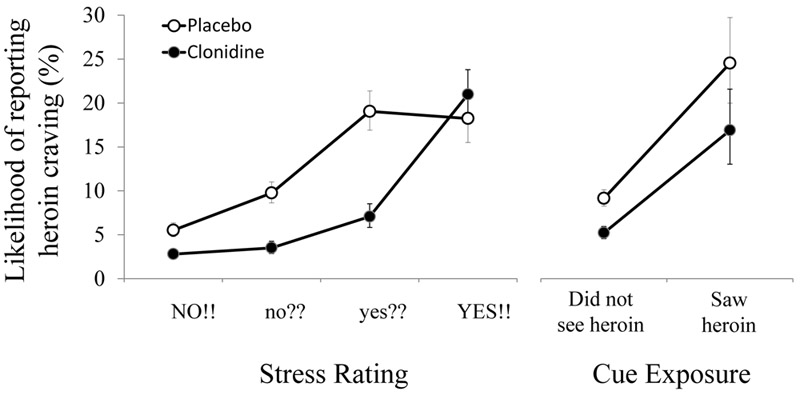
Figure 3 (left panel) suggests that increases in daily-life stress were accompanied by increased heroin craving, but that this relationship was blunted for the clonidine group at all but the highest level of stress. A generalized linear mixed model confirmed that clonidine use reduced the likelihood of heroin-craving reports overall (6.3% [95% CI=5.6–7.1] compared with 11.8% [95% CI=10.9–13.0]; F=71.5, df=1, 105, p≤0.001; Cohen’s d=1.61), that stress was associated with craving (F=65.6, df=3, 257, p≤0.001), and that there was a decoupling of stress from craving in the clonidine group (group-by-stress interaction, F=8.8, df=3, 257, p≤0.001). This effect is best seen in the difference between clonidine and placebo when stress is reported as “yes??” (7.1% [95% CI=5.8–8.5] compared with 19.0% [95% CI=16.9–21.4]; Cohen’s d=1.79).
FIGURE 3.
Association of Stress and Cues With Likelihood of Reporting Heroin Craving Among Patients Who Received Placebo or Clonidine in Addition to Buprenorphinea
a The left panel shows the interaction of stress and group (F=8.8, df=3, 257, p≤0.001) on likelihood of reporting craving: at the moderate (though not the highest) level of stress, there was a decoupling of stress from craving with clonidine treatment. The right panel shows that there was no such interaction for exposure to heroin cues. Error bars represent 95% confidence intervals.
In similar analyses (see the online data supplement), other mood ratings were also associated with ratings of heroin craving, and again, these associations were weaker in the clonidine group.
The decoupling effect of clonidine was specific to stress and mood. Although craving increased after cue exposures, such as seeing heroin (F=152.7, df=3, 257, p≤0.001) (Figure 3) or seeing cocaine (F=74.4, df=3, 257, p≤0.001), neither of these associations interacted with the clonidine group.
None of the ecological momentary assessment variables tested (stress, mood, or cue exposure) predicted the likelihood that the next urine test would be opioid positive.
DISCUSSION
Our main finding is that clonidine maintenance treatment as an adjuvant to buprenorphine maintenance treatment in outpatients who had become abstinent delayed initial opioid lapses (isolated positive urine tests), but not relapses (series of positive urine tests). In addition, using real-time ambulatory assessment (ecological momentary assessment), we showed that clonidine partly decoupled daily-life stress, but not daily-life drug-cue exposure, from increases in drug craving, just as predicted by the animal models that led to our clinical trial.
The clinical benefit of delaying initial lapses seems clear in the context of findings that “abstinence begets abstinence” (33)—in other words, that the longer the duration of abstinence, the greater the likelihood that it will be sustained. Our findings do not support this unequivocally, but they do support clonidine’s ability to increase participants’ mean durations of abstinence.
The ability of clonidine to increase the duration of opioid abstinence has important clinical implications, because all medications currently used to treat opioid addiction are either agonists or antagonists at mu-opioid receptors (34). Those medications have drawbacks not shared by clonidine or other alpha-2 adrenoceptor agonists. Clonidine is especially appealing because it carries no special prescribing requirements like those imposed on buprenorphine and methadone, and it is known to be well tolerated in opioid users because it is used regularly for short-term amelioration of withdrawal signs (35). Although a new standard of care cannot be derived from the findings of just one clinical trial, we think our results provide support for a potential new off-label use for an old medication, expanding the addiction-treatment armamentarium of opioid-agonist clinics, and perhaps also of psychiatrists and internists who are not prescribing opioid agonists.
The effect of clonidine on lapses, but not relapses, may be understandable in terms of each drug’s mechanism of action (and in the way we defined “lapse” and “relapse”). As we discussed, reinstatement studies in rodents and human laboratory studies have shown that alpha-2 adrenoceptor agonists can block drug seeking and craving induced by stress (9–11, 15) and possibly by drug cues (14–16, 18), but not by priming doses of drug (9). By definition, relapse in our trial (two or more consecutive opioid-positive urine tests) may have been at least partly primed by recent opioid use; lapse (an isolated opioid-positive urine test) need not have been. Both groups in our trial were presumably somewhat protected from priming effects, because buprenorphine maintenance confers that protection (21). Thus, in the context of buprenorphine’s likely ability to stem opioid-primed use, the additional protection conferred by clonidine may have been most detectable in terms of propensity toward initial lapses.
The clearest test of clonidine’s action was a test of whether it buffered the risk associated with stress—as ecological momentary assessment data showed it did. Participants in the clonidine group were less likely than those in the placebo group to report heroin craving at moderately high levels of stress, reaching the same likelihood of heroin craving only at the highest level of stress. We saw no such buffering effect in self-reported exposure to drug-associated cues, suggesting that clonidine’s protection was specific, as preclinical studies using the reinstatement model had predicted. These results highlight an advantage of using ecological momentary assessment in the context of a clinical trial. Studies of nicotine replacement for smoking cessation using ecological momentary assessment have shown, for example, how hedonic responses to a lapse predict a second lapse (36–39). Our findings are, to our knowledge, the first to extend such mechanistic analyses to illicit drug use.
We do not know whether a higher dosage of clonidine would decrease craving in response to extreme stressors. With our once-daily observed dosing, a higher dosage would have been problematic. With divided daily dosing, the question could be addressed.
The ecological momentary assessment results allow this clinical trial to have a bidirectional translational impact (19, 40). First, for clinicians, the ecological momentary assessment results identify the subpopulation of patients who would benefit most from clonidine maintenance: those who are susceptible to stress-induced lapses and those who have persistent though moderate stress. This is the sort of individualized approach to treatment that could greatly improve outcomes. For laboratory animal researchers, the ecological momentary assessment data provide insight into the animal models themselves. Like most animal models, the reinstatement model has been the subject of vigorous debate about how well it can predict real-world human behavior. It is reasonable to be skeptical about whether an electrical shock or a yohimbine injection administered to a rat will relate to the myriad of cognitive and social stressors (e.g., marital, financial, housing) that are common in humans. Our ecological momentary assessment results suggest that the seemingly contrived stressors used in the reinstatement model can indeed stand in for stressors encountered by humans—not just stressors administered to humans in behavioral laboratories, but stressors encountered spontaneously, outside the control of the investigator.
We did not design the study to determine whether clonidine would also prevent relapse to misuse of other drugs, as animal models suggest it will. Even so, we found that clonidine showed some efficacy in reducing marijuana use as assessed by urine testing. This finding is promising for the six other active studies listed at clinicaltrials.gov in which alpha-2 agonists are being tested for relapse prevention, with the targeted drugs being opioids, cocaine, cannabis, nicotine, and alcohol (NCT00585754, NCT01020019, NCT01467999, NCT01598896, NCT01863186, NCT02051309). These studies will provide clearer evidence of whether alpha-2 agonists prevent relapse through some common mechanism, not limited to one or two drugs of abuse.
One limitation of our study is that we cannot draw conclusions about the effect of clonidine maintenance outside the context of buprenorphine maintenance. It is possible that clonidine will help maintain opioid abstinence only during opioid agonist maintenance treatment. However, as discussed earlier, it is just as likely that the clinical benefits of buprenorphine obscured what would have been a larger effect of clonidine.
Another limitation lies in our event-contingent ecological momentary assessment data. We rewarded responding to random prompts, but not ecological momentary assessment reporting of drug use. Opioid use reports were well below what would be expected given the results of the urine screens. Thus, we cannot rely on that portion of our ecological momentary assessment data to examine whether stress was reliably decoupled from individual instances of drug use. In ongoing studies, we now reward ecological momentary assessment reporting of drug use to increase the completeness of those reports.
In summary, this study is the first to demonstrate efficacy of clonidine for relapse prevention in treatment-seeking opioid users, which would be a novel and important indication for clonidine, adding to its current use in opioid detoxification. In addition, we can conclude that clonidine is modestly effective in decoupling stress from lapses, and that ecological momentary assessment is useful not only for descriptive studies, but also for testing mechanistic hypotheses in randomized controlled trials and corroborating predictions from animal and laboratory work about how humans will behave in the real world.
Supplementary Material
Footnotes
The authors report no financial relationships with commercial interests.
Clinicaltrials.gov identifier: NCT00295308.
REFERENCES
- 1.American Psychiatric Association: Practice Guideline for the Treatment of Patients With Substance Use Disorders, 2nd ed. Am J Psychiatry 2006; 163(Aug suppl) [Google Scholar]
- 2.Bickel WK, Amass L, Higgins ST, et al. : Effects of adding behavioral treatment to opioid detoxification with buprenorphine. J Consult Clin Psychol 1997; 65:803–810 [DOI] [PubMed] [Google Scholar]
- 3.Kosten T, Oliveto A, Feingold A, et al. : Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend 2003; 70:315–325 [DOI] [PubMed] [Google Scholar]
- 4.Silverman K, Wong CJ, Umbricht-Schneiter A, et al. : Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol 1998; 66:811–824 [DOI] [PubMed] [Google Scholar]
- 5.Epstein DH, Willner-Reid J, Vahabzadeh M, et al. : Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry 2009; 66:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marlatt GA, Gordon JRE: Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, Guilford, 1985 [Google Scholar]
- 7.Shaham Y, Shalev U, Lu L, et al. : The reinstatement model of drug relapse: history, methodology, and major findings. Psychopharmacology (Berl) 2003; 168:3–20 [DOI] [PubMed] [Google Scholar]
- 8.Bossert JM, Marchant NJ, Calu DJ, et al. : The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013; 229:453–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erb S, Hitchcott PK, Rajabi H, et al. : Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 2000; 23:138–150 [DOI] [PubMed] [Google Scholar]
- 10.Shaham Y, Highfield D, Delfs J, et al. : Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 2000; 12:292–302 [DOI] [PubMed] [Google Scholar]
- 11.Highfield D, Yap J, Grimm JW, et al. : Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology 2001; 25:320–331 [DOI] [PubMed] [Google Scholar]
- 12.Yamada H, Bruijnzeel AW: Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology 2011; 60:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lê AD, Harding S, Juzytsch W, et al. : Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005; 179:366–373 [DOI] [PubMed] [Google Scholar]
- 14.Smith RJ, Aston-Jones G: α(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry 2011; 70:712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jobes ML, Ghitza UE, Epstein DH, et al. : Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berl) 2011; 218:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox HC, Seo D, Tuit K, et al. : Guanfacine effects on stress, drug craving, and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol 2012; 26:958–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox H, Sinha R: The role of guanfacine as a therapeutic agent to address stress-related pathophysiology in cocaine-dependent individuals. Adv Pharmacol 2014; 69:217–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha R, Kimmerling A, Doebrick C, et al. : Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology (Berl) 2007; 190:569–574 [DOI] [PubMed] [Google Scholar]
- 19.Sinha R, Shaham Y, Heilig M: Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011; 218:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haney M, Hart CL, Vosburg SK, et al. : Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008; 197:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorge RE, Rajabi H, Stewart J: Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology 2005; 30:1681–1692 [DOI] [PubMed] [Google Scholar]
- 22.Leri F, Tremblay A, Sorge RE, et al. : Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology 2004; 29:1312–1320 [DOI] [PubMed] [Google Scholar]
- 23.Gossop M: Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend 1988; 21:253–259 [DOI] [PubMed] [Google Scholar]
- 24.Gold MS, Redmond DE Jr, Kleber HD: Noradrenergic hyperactivity in opiate withdrawal supported by clonidine reversal of opiate withdrawal. Am J Psychiatry 1979; 136:100–102 [DOI] [PubMed] [Google Scholar]
- 25.Shalev U, Grimm JW, Shaham Y: Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 2002; 54:1–42 [DOI] [PubMed] [Google Scholar]
- 26.Kalivas PW, Volkow ND: The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005; 162:1403–1413 [DOI] [PubMed] [Google Scholar]
- 27.Shiffman S, Gwaltney CJ, Balabanis MH, et al. : Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol 2002; 111:531–545 [DOI] [PubMed] [Google Scholar]
- 28.Shiffman S, Hufford M, Hickcox M, et al. : Remember that? A comparison of real-time versus retrospective recall of smoking lapses. J Consult Clin Psychol 1997; 65:292–300 [DOI] [PubMed] [Google Scholar]
- 29.Schwarz N: Retrospective and concurrent self-reports: The rationale for real-time data capture, in The Science of Real-Time Data Capture: Self-Reports in Health Research Edited by Stone AA, Shiffman S, Atienza AA, et al. New York, Oxford University Press, 2007, pp 11–28 [Google Scholar]
- 30.McLellan AT, Luborsky L, Cacciola J, et al. : New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis 1985; 173:412–423 [DOI] [PubMed] [Google Scholar]
- 31.Robins LN, Cottler LB: Making a structured psychiatric diagnostic interview faithful to the nomenclature. Am J Epidemiol 2004; 160:808–813 [DOI] [PubMed] [Google Scholar]
- 32.Lachin JM, Foulkes MA: Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics 1986; 42:507–519 [PubMed] [Google Scholar]
- 33.Higgins ST, Badger GJ, Budney AJ: Initial abstinence and success in achieving longer term cocaine abstinence. Exp Clin Psychopharmacol 2000; 8:377–386 [DOI] [PubMed] [Google Scholar]
- 34.Bart G: Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis 2012; 31:207–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigmon SC, Bisaga A, Nunes EV, et al. : Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse 2012; 38:187–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson SG, Shiffman S: Effect of high-dose nicotine patch on craving and negative affect leading up to lapse episodes. Psychopharmacology (Berl) 2014; 231:2595–2602 [DOI] [PubMed] [Google Scholar]
- 37.Ferguson SG, Shiffman S: Effect of high-dose nicotine patch on the characteristics of lapse episodes. Health Psychol 2010; 29:358–366 [DOI] [PubMed] [Google Scholar]
- 38.Shiffman S, Ferguson SG: The effect of a nicotine patch on cigarette craving over the course of the day: results from two randomized clinical trials. Curr Med Res Opin 2008; 24:2795–2804 [DOI] [PubMed] [Google Scholar]
- 39.Shiffman S, Ferguson SG, Gwaltney CJ: Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology (Berl) 2006; 184:608–618 [DOI] [PubMed] [Google Scholar]
- 40.Kreek MJ, Schlussman SD, Reed B, et al. : Bidirectional translational research: progress in understanding addictive diseases. Neuropharmacology 2009; 56(suppl 1):32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.