Abstract
The use of several variants of the asymmetric aldol reaction as key steps in the syntheses of bioactive target molecules is described.
Keywords: natural products, asymmetric synthesis, chiral auxiliary, syn-aldol, anti-aldol, indanolamine
For centuries, natural products have been a major source of inspiration to researchers in the fields of chemistry, biology, and medicine. Numerous natural products and their derivatives have received approval as front-line therapies for the treatment of a variety of human diseases. The seemingly endless structural diversity, complexity, and important biological functions of natural products have provided an impetus for the development of novel methodologies for their synthesis. Furthermore, drug-discovery researchers frequently attempt to reproduce various structural motifs and stereochemically defined functionalities from natural products. This has further stimulated the design and development of new and practical synthetic technologies, and has greatly enhanced medicinal research investigations in academia and industry.
Over the years, we have been involved in the synthesis of a range of structurally diverse natural products with a variety of biological activities. Our objectives have been to synthesize these scarce natural products efficiently, to carry out further biological evaluation, to perform structure-activity studies, and to prepare structural variants for biological studies. In this context, we have developed a variety of methods for asymmetric synthesis, including highly diastereoselective syn- and anti-aldol reactions that rely on ester-derived titanium enolates.
The aldol reaction is one of the most powerful reactions for forming carbon–carbon bonds that is available in organic synthesis. Asymmetric aldol reactions have proved their versatility and utility in organic synthesis, especially for the construction of architecturally complex natural products. Here we report our attempts to use the asymmetric aldol reaction as a key step in the synthesis of a variety of bioactive target molecules (Figure 1). In these studies, we used several asymmetric aldol reactions, including Evans’s syn-aldol protocol,1 Mukaiyama’s aldol reaction,2 our own chiral ester-based asymmetric aldol reaction, and a nitro-aldol reaction.3 We will also describe a recent asymmetric reductive aldol process developed in our laboratory in the context of the synthesis of peloruside A, a potent anticancer agent with clinical potential.4,5 Furthermore, we report an asymmetric aldol-based synthesis of a stereochemically defined bis-tetrahydrofuran (bis-THF) scaffold present in ginkolide natural products.6 This scaffold has been successfully incorporated in the design and development of Darunavir, a HIV-1 protease inhibitor for the treatment of multi-drug-resistant HIV-1 variants that has recently been approved by the US Food and Drug Administration (FDA).
Figure 1.
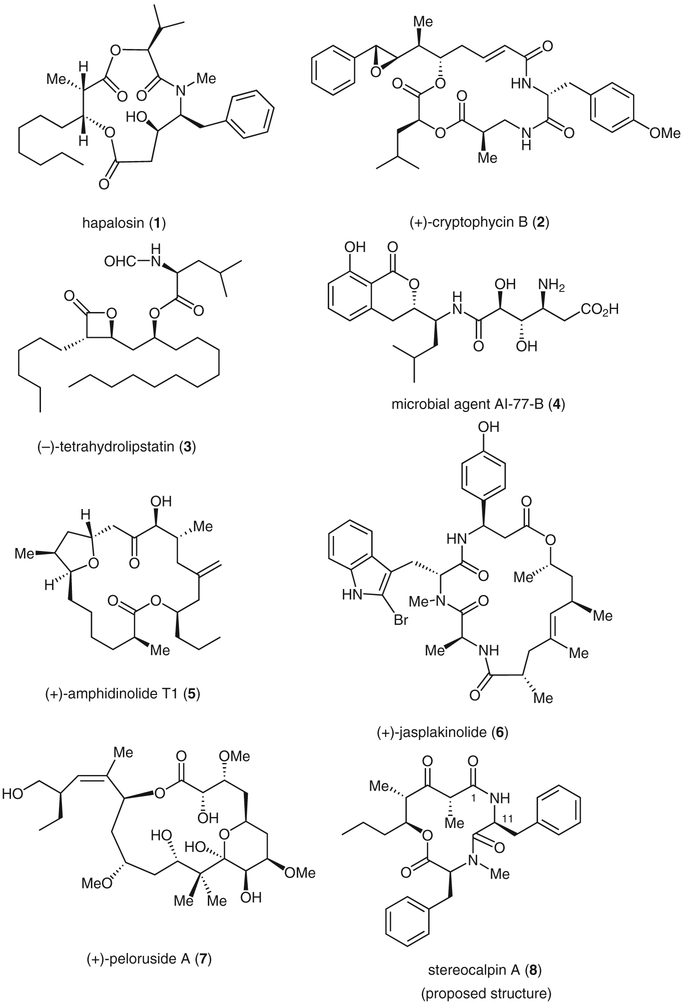
Some natural products synthesized by aldol reaction strategies
Hapalosin
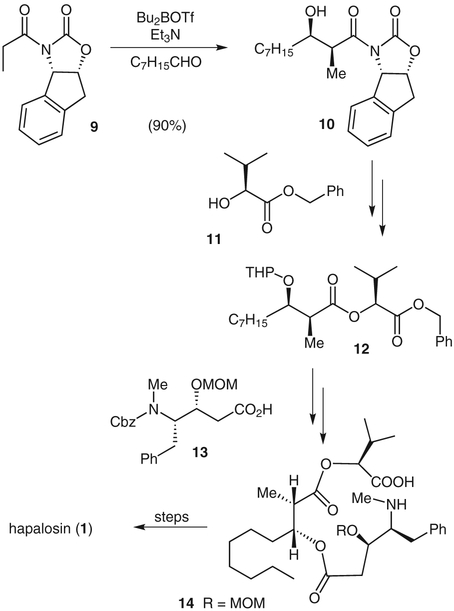
Multi-drug resistance (MDR) is a phenomenon whereby many cancers develop resistance to chemotherapeutic drugs, and it is a major factor for treatment failure. It appears that MDR is caused by overexpression of P-glyco-protein, a 170–200 kDa transmembrane protein that acts as an ATP-dependent drug efflux pump.7 Hapalosin (1), isolated from the blue-green alga Hapalosiphon welwitschii,8 has shown a reversing effect on MDR in tumor cells.9 We reported the first total synthesis of hapalosin in 1996. In this synthesis, we used Evans’s asymmetric syn-aldol reaction to install two key stereo-centers.10 This aldol strategy involved the use of an aminoindanol-based chiral oxazolidinone as the chiral auxiliary. As shown in Scheme 1, the reaction of oxazolidinone 911 and octanal with dibutylboryl triflate and triethylamine at −78 °C gave the aldol adduct 10 in 90% yield. The resulting aldolate was protected as its tetrahydropyranyl ether, and the chiral auxiliary was removed to give the corresponding acid. Coupling of this acid with alcohol 11 gave diester 12. The tetrahydropyranyl group was removed and the diester was coupled with acid 13 to provide the corresponding ester. Removal of the benzyl and benzyloxycarbonyl protecting groups, followed by cycloamidation of the resulting amino acid 14 with N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide and N-hydroxybenzotriazole afforded the corresponding cycloamide. Removal of the methoxymethyl protecting group then gave synthetic hapalosin.
Scheme 1.
Synthesis of hapalosin
(+)-Cryptophycin B
The synthesis of cryptophycins, which are a group of marine depsipeptides isolated from Nostoc sp., has attracted much attention over the years because of the significant clinical potential of these chemicals and their relatively low natural abundance.12 Members of the cryptophycin family, including arenastatin, inhibit tubulin polymerization in vitro, which may render these compounds or their derivatives important in cancer therapy.13
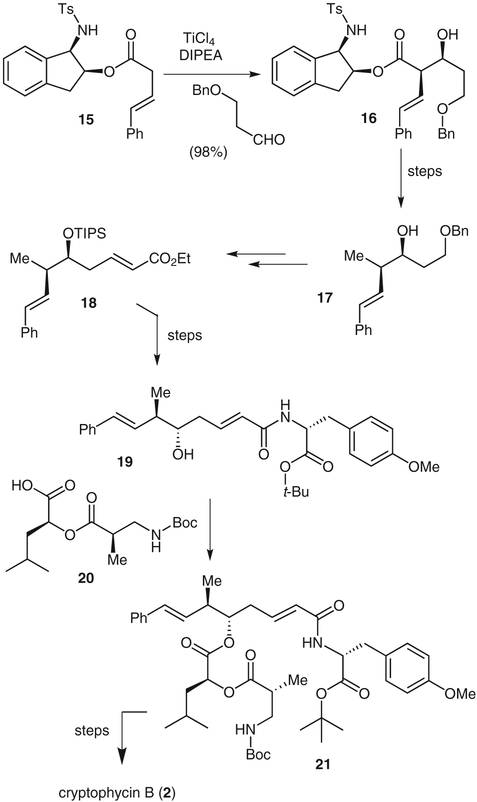
We carried out a convergent synthesis of cryptophycin B (2) by using aldol reactions based on asymmetric titanium enolates that we developed in our laboratory.14 As shown in Scheme 2, ester 15, obtained by coupling (3E)-4-phenylbut-3-enoic acid and 1-(N-tosylamino)indan-2-ol, was treated with titanium tetrachloride and N,N-diisopropylethylamine. The resulting enolate was treated with 3-(benzyloxy)propanal to provide the syn-aldol adduct 16 in 98% yield as a single diastereomer. Reduction of the aldol adduct with lithium aluminum hydride and selective conversion of the primary hydroxy group into the corresponding methyl group was accomplished in a one-pot, two-step sequence to give the alcohol intermediate 17. This was converted into the corresponding aldehyde followed by a Horner–Wadsworth–Emmons reaction with ethyl (diethoxyphosphono)acetate to give ester 18. This acid was coupled with teri-butyl O-methyl-D-tyrosinate to afford amide 19, which was then attached to the protected amino acid 20 by means of Yamaguchi coupling. Macrolactamization of the amino acid derived from the resulting derivative 21 was accomplished by using Yamaguchi’s protocol. Epoxidation with dimethyldioxirane gave the title compound as a 3:1 mixture of epoxides. We have since carried out a synthesis of cryptophycin-52 by a non-aldol process.15
Scheme 2.
Synthesis of cryptophycin B
(−)-Tetrahydrolipstatin
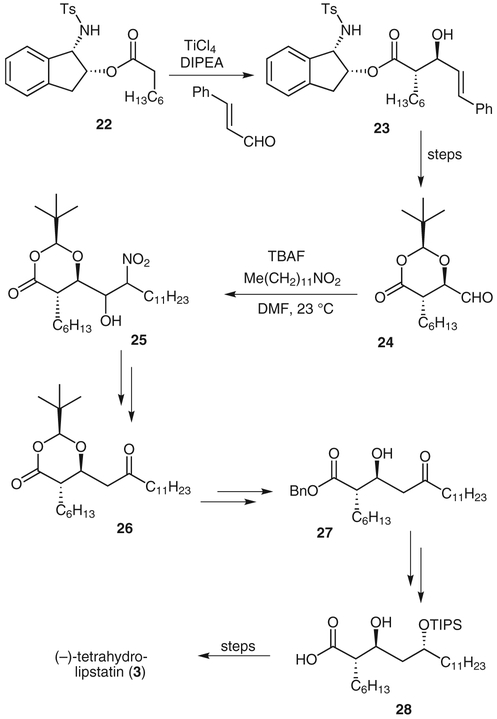
(−)-Tetrahydrolipstatin (3), a saturated derivative of lipstatin, was isolated in 1987, from the bacterium Streptomyces toxytricini.16,17 This compound, under the tradt name Xenical®, has been approved by the FDA as an anti obesity agent. It inhibits pancreatic lipase by irreversibl binding to the serine at the active site with its β-lactont moiety.17 We synthesized tetrahydrolipstatin using an asymmetric ester-derived titanium enolate anti-aldol reaction and a nitro-aldol reaction as key steps.18
Chiral ester 22, was treated with titanium tetrachloridt and N,N-diisopropylethylamine in dichloromethane at 0 °C to 23 °C. The resulting enolate was then cooled to(−78 °C and trans-cinnamaldehyde complexed with dibutylboryl triflate (premixed) was added to give the anti-aldol product 23 in 60% yield (6.1:1 dr). This aldol product was converted into the aldehyde 24. A nitro-aldol reaction of aldehyde 24 with nitrododecane in N,N-dimethylform amide containing a catalytic amount of tetrabutylammoni um fluoride provided the nitro-aldol product 25 in 82% yield as a mixture of diastereomers. After dehydration of the nitro-aldolate, the resulting nitrovinyl moiety was converted into the corresponding oxime, which was reduce to give ketone 26 as a single isomer. The dioxanone was hydrolyzed under Seebach’s conditions,19 and the result ing β-hydroxy acid was protected as a benzyl ester. The resulting ketone was reduced by using an anti-selectiv reduction protocol developed by Evans (selectivity 22:1).20 Selective protection of the less hindered hydroxy group gave the triisopropylsilyl ether 28. The synthesis of tetrahydrolipstatin was completed by treating acid 28 with benzenesulfonyl chloride in pyridine at 0 °C to give the β-lactone and then removal of the triisopropylsilyl ether and Mitsunobu esterification with N-formyl-L-leucine.
Pseudopeptide Microbial Agent AI-77-B
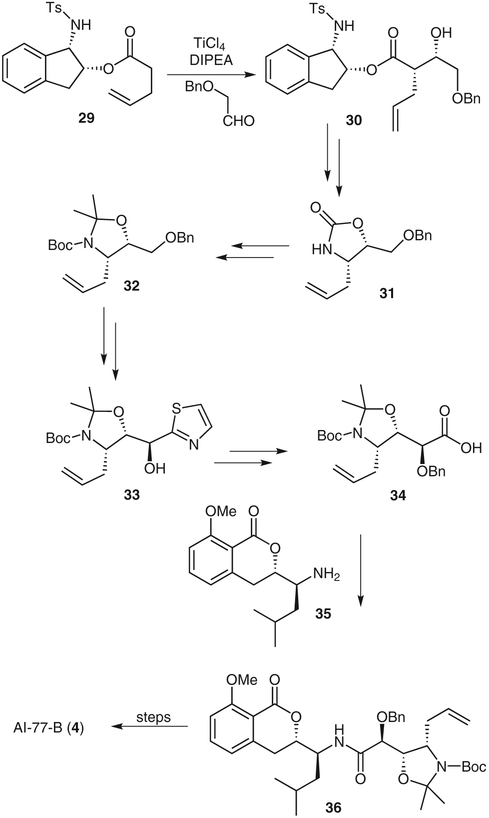
AI-77-B (4) was isolated from the fermentation broths of Bacillus pumilus AI-77 in 1982.21 It has unique gastroprotective properties with few side effects; however, its therapeutic potential has been limited because of its poor oral absorption properties.22 We synthesized the compound by using a titanium enolate-mediated syn-aldol reaction to generate the dihydroxyamino acid moiety.23 Other key reaction steps of our synthesis include a Curtius rearrangement, a regioselective Diels–Alder reaction, and a Dondoni homologation.
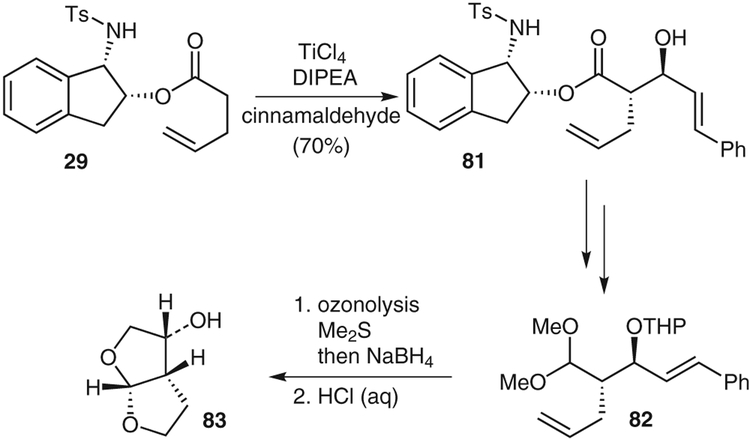
Chiral ester 29 was treated with titanium tetrachloride and N,N-diisopropylethylamine in dichloromethane at room temperature to give the corresponding titanium enolate, which was cooled to −78 °C and treated with (benzyloxy)acetaldehyde. The resulting aldol adduct 30 was isolated as a single diastereomer in 97% yield. The chiral auxiliary was removed and the resulting acid was converted into oxazolidinone 31 by a Curtius rearrangement. Oxazolidinone 31 was converted into the tertbutoxycarbonyl-protected acetonide 32. Benzyl deprotection and Swern oxidation gave an aldehyde that was subjected to stereoselective homologation, as developed by Dondoni and co-workers,24 to provide thiazole 33. This thiazole was transformed into the corresponding aldehyde, which was oxidized to give the acid analogue 34.Coupling of this acid with the isocoumarin fragment 35 provided amide 36.25 The terminal alkene group of amide 36 was oxidized to an acid and protected as its benzyl ester, which allowed a clean O-demethylation of the aromatic methoxy group. Removal of the protecting groups gave AI-77-B (4).
(+)-Amphidinolide T1
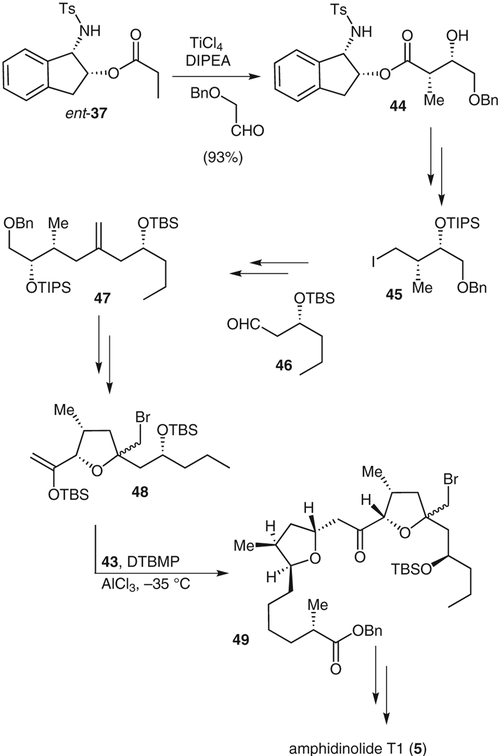
The Amphidinium species of marine dinoflagellates have developed metabolites that are quite potent against a variety of cancer cell lines.26 The 19-membered amphidinolide T1, (5) has shown potent activity against murine lymphoma L1210 and human epidermoid carcinoma KB cell lines.27 As a result of their low natural abundance, high biological activity, and unique structural features, the amphidinolides have attracted much attention and have been the subject of many synthetic and biological studies. We accomplished the first total synthesis of amphidinolidelide T1 utilizing diastereoselective aldol reactions, an oxocarbenium ion-mediated alkylation, cross-metathesis, and a novel exo-methylene group protection as the key steps.28
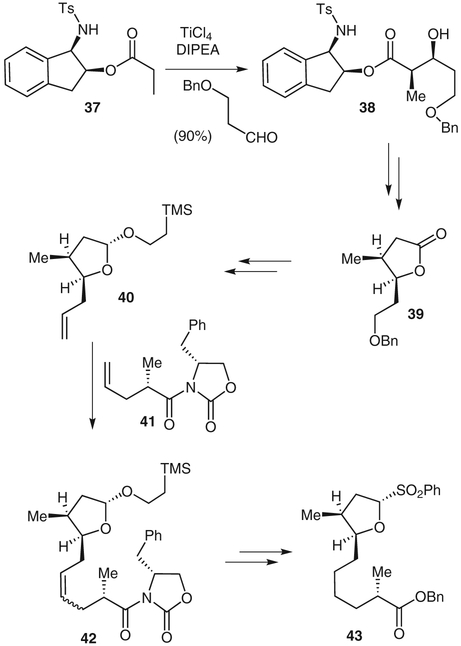
Aldol adduct 38 was generated from the condensation of the 1-(N-tosylamino)indan-2-ol ester 37 with 3-(benzyloxy)propanal (Scheme 5). The aldol adduct was obtained as a single diastereomer in 90% yield. Removal of the chiral auxiliary with lithium aluminum hydride gave the corresponding diol, which was converted into the γ-lactone 39 in three steps. Lactone 39 was, in turn, transformed into alkene 40. Cross-metathesis of 40 and oxazolidinone derivative 41 in the presence of Grubbs catalyst gave a 1:1 mixture of the E-and Z-isomers of the cross-coupled product 42. Sulfone 43 was obtained by treating the corresponding benzyl ester derivative of 42 with benzenesulfinic acid and calcium chloride.
Scheme 5.
Synthesis of sulfone fragment 43
Scheme 6 outlines the synthesis of the C11–C22 segment and the completion of the synthesis of amphidinolide T1. Ester-enolate aldol reaction of ent-37 with (benzyloxy)acetaldehyde gave the aldolate 44 as a single diastereomer in 95% yield. This was readily converted into iodide 45 by protecting the alcohol as a triisopropysilyl ether, reduction with diisobutylaluminum hydride, and conversion of the resulting alcohol into an iodide. Lithiation of iodide 45 followed by reaction with aldehyde 46 gave a 1:1 mixture of alcohols, which were oxidized to give a ketone that was converted into olefin 47 under Petasis’s conditions.29 After reductive removal of the triisopropysilyl and benzyl groups, the resulting diol was treated with N-bromosuccinimide to give a bromotetrahydrofuran that was subsequently converted into enol ether 48. A modified procedure, first developed by Ley,30 was used to carry out the key oxocarbenium ion-mediated alkylation of segments 43 and 48 to give compound 49. Deprotection of the coupled product 49 and subsequent Yamaguchi macrolactonization gave the macrolactone, which upon reductive unmasking of the bromoether gave amphidinolide T1.
Scheme 6.
Completion of the synthesis of amphidinolide T1; DTBMP = 2,6-di-tert-butyl-4-methylpyridine
(+)-Jasplakinolide
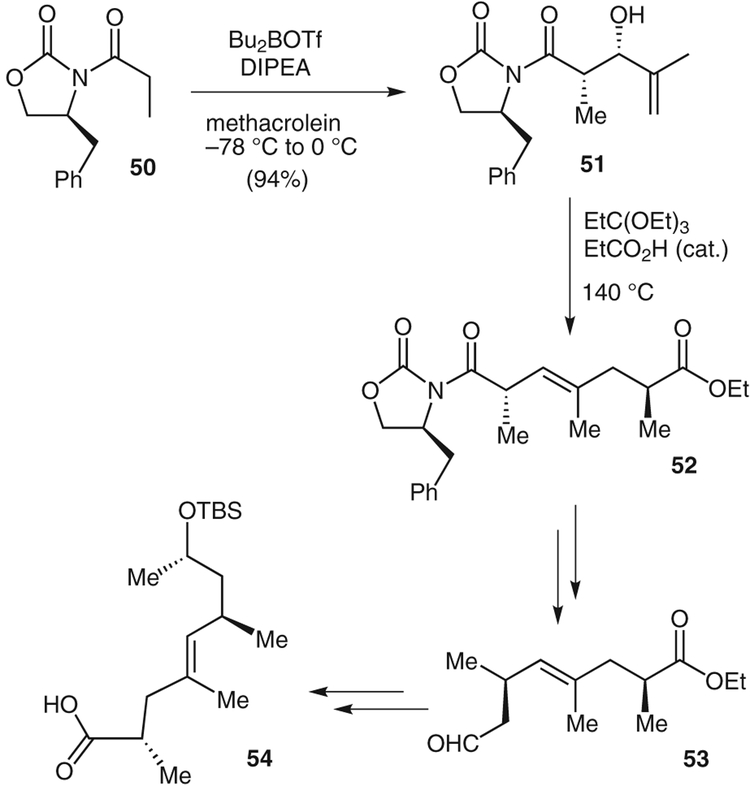
Jasplakinolide (6), a 19-membered cyclic depsipeptide, has attracted the attention of many synthesis groups since its isolation from the Fijian marine sponge Jaspis splendens in 1986.31 Jasplakinolide has shown excellent antitumor activity,32 and was a candidate for clinical development until this was stopped as a result of the compound’s toxicity.33 A number of syntheses of jasplakinolide have been reported.34 We recently reported a convergent synthesis of this molecule. The polypropionic acid segment was synthesized by using an aldol reaction followed by a Claisen rearrangement as the key steps.35
The initial C2-stereocenter was created by an Evans syn-aldol reaction, as shown in Scheme 7.1 The boron-enolate of oxazolidinone 50 was treated with methacrolein at −78 °C to 0 °C to give the aldol condensation product 51 in 94% yield. Allylic alcohol 51 was heated in 1,1,1-triethoxypropane at 140 °C in the presence of a catalytic amount of propionic acid to yield the Claisen rearrangement product, ester 52.36 Hydrolysis of the oxazolidinone afforded a primary hydroxy group that was converted into a nitrile group through a Mitsunobu reaction using acetone cyanohydrin. The resulting nitrile was reduced with Raney nickel catalyst to give aldehyde 53. Methyl Grignard addition to aldehyde 53, silylation, and ester hydrolysis gave acid 54. The mixture of diastereomers resulting from the Grignard reaction was readily separated, and the undesired diastereomer was converted into the desired alcohol by a two-step procedure.
Scheme 7.
Synthesis of protected 8-hydroxynonenoic acid 54
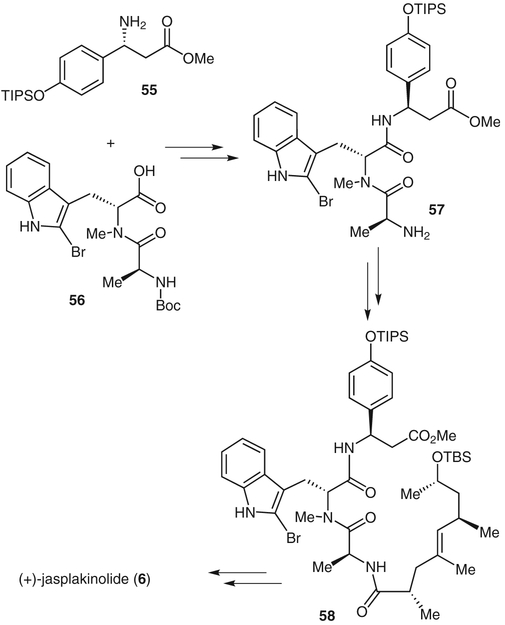
The β-tyrosine moiety was prepared by using chemistry developed by Davis and co-workers.37 Coupling of amino ester 55 and acid 56 gave the tripeptide 57 (Scheme 8). Further coupling of acid 54 to tripeptide 57 gave ester 58. Removal of the tert-butyl(dimethyl)silyl protecting group and ester hydrolysis followed by Yamaguchi macrolactonization gave the corresponding macrolactone. Subsequent removal of the phenolic triisopropylsilyl group gave synthetic jasplakinolide. Structural modifications with the goal of identifying less toxic and more potent derivatives are currently in progress in our laboratory.
Scheme 8.
Completion of (+)-jasplakinolide
Peloruside A
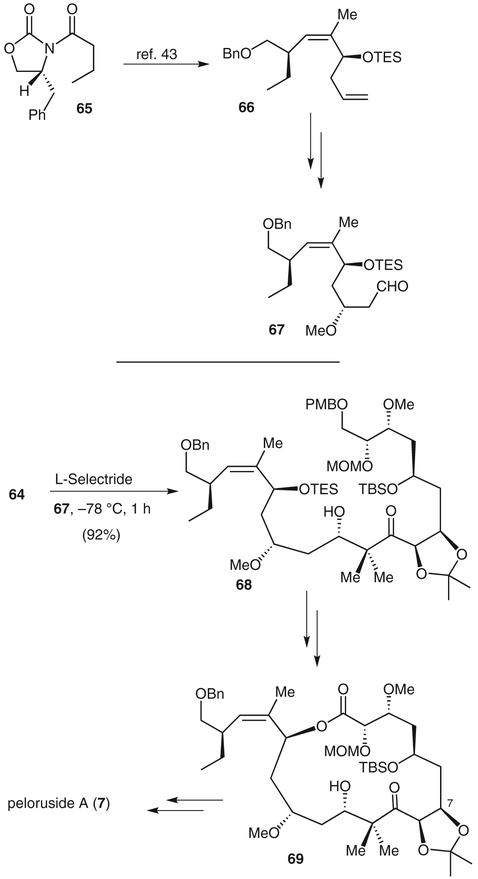
The microtubule-stabilizing agent peloruside A (7) was isolated from the marine sponge Mycale hentscheli in 2000.38 Like laulimalide, peloruside A shows a synergistic effect with taxol, arresting cells undergoing mitosis in the G2-phase by binding to a nontaxoid region of tubulin.39 Peloruside A represents a new class of antitumor agents, and it has shown excellent potency against P388 murine leukemia cells (IC50 = 10 nM).40 The potency, intriguing structure, and potential clinical applications have attracted much interest. Two total syntheses41 and several synthetic studies on peloruside have been published.42,43 We have reported a convergent synthesis of peloruside A that featured a novel aldol reaction mediated by L-Selectride [lithium tri(sec-butyl)borohydride] as one of the key steps.5
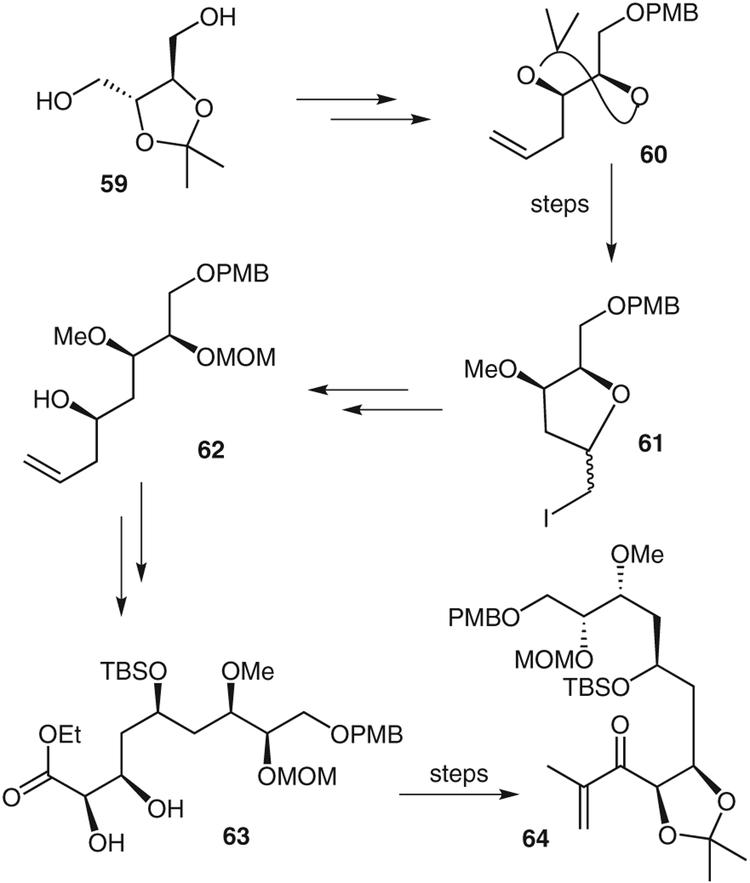
The synthesis of the C1–C10 segment is shown in Scheme 9. Commercially available isopropylidene-D-threitol (59) was converted into the isopropylidene product 60. Acid-catalyzed removal of the isopropylidene group followed by iodoesterification and subsequent O-methylation gave iodide 61. This was then converted into an aldehyde, which in turn was subjected to Brown’s asymmetric allylation to give alcohol 62.44 Alcohol 62 was converted into diol 63 by using an Ando Z-olefination45 and a Sharpless asymmetric dihydroxylation46 as the key steps. The diol was then converted into enone 64 by standard synthetic steps.
Scheme 9.
Synthesis of peloruside A intermediate 64
The completion of the synthesis of peloruside A is shown in Scheme 10. The protected homoallylic alcohol 66 was generated from chiral imide 65.43 The terminal olefin was oxidized and exposed to asymmetric allylation conditions to give a 5:1 mixture of diastereomers. The resulting alcohol was converted into a methyl ether, and oxidative cleavage of the terminal alkene gave aldehyde 67. Treatment of enone 64 with L-Selectride at −78 °C for 10 minutes, followed by addition of aldehyde 67, gave aldol adduct 68 as a 4:1 mixture of diastereomers in 92% yield. We recently investigated the scope of this reaction for a variety of chiral and achiral enones and aldehydes. Our exploration of this reductive aldol strategy showed that several representative aldol adducts can be prepared in good yield and high diastereoselectivity when the reactant aldehyde contains an α-chiral center.4 The synthesis of peloruside A was carried out by removal of the p-methoxybenzyl protecting group and oxidation of the primary alcohol to the corresponding acid, followed by Yamaguchi lactonization to provide macrolactone 69. This was then converted into (+)-peluroside A.
Scheme 10.
Synthesis of intermediate 67 and completion of the synthesis of peloruside A
Stereocalpin A
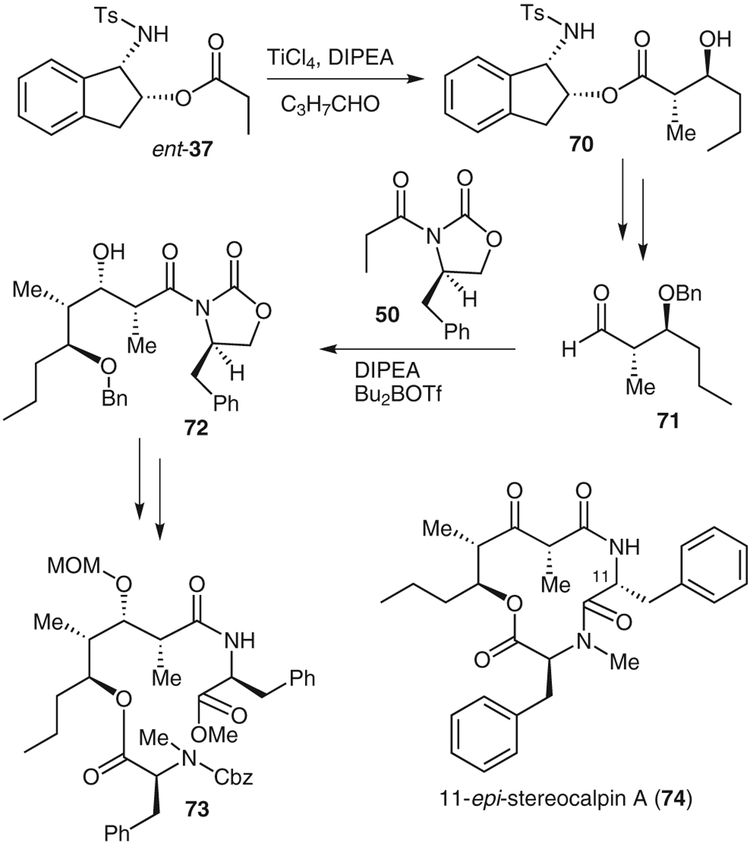
The antitumor depsipeptide stereocalpin A (8) was isolated from the dry lichen Ramalina terebrata in 2008.47 This 12-membered cycloamide shows good activity against human colon, skin, and liver solid tumor cell lines. In addition, stereocalpin displays protein tyrosine phosphatase 1B (PTP1B) inhibitory activity.47 We recently carried out a 14-step synthesis of the proposed structure of stereocalpin A, which involved syn- and anti-aldol reactions as key steps.48
As shown in Scheme 11, in a titanium tetrachloride-promoted anti-aldol reaction, the 1-(tosylamino)indan-2-ol ester ent-37 and N,N-diisopropylethylamine were added to a mixture of butanal (2 equiv), titanium tetrachloride (2 equiv), and acetonitrile (2 equiv) at −78 °C to give anti-aldol adduct 70 in 65% yield and excellent diastereoselectivity (37:1 by 1H NMR and 13C NMR spectroscopy). Benzyl protection of aldol adduct 70 followed by lithium aluminum hydride reduction and subsequent oxidation of the resulting alcohol gave aldehyde 71. The C2- and C3-stereocenters were installed by using an Evans syn-aldol procedure.1 The reaction between the chiral oxazolidinone 50 and aldehyde 71 in N,N-diisopropylethylamine containing dibutylboryl triflate gave the aldol product 72 in 89% yield (7:1 dr). Aldol product 72 was protected as a methoxymethyl ether, and the chiral auxiliary was removed. The resulting acid was coupled with L-phenylalanine and then esterified with N-benzyloxycarbonyl-N-methylphenylalanine. This provided the protected amino ester 73. Interestingly, initial attempts at macrolactamization resulted in complete epimerization at the C11 stereo-center. Other coupling reagents, including O-(benzotriazol-1-yl)-N,N,N′,N′,-tetramethyluronium tetrafluoroborate and (benzotriazole-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate, gave the same epimerized product. To overcome the steric constraints of the 12-membered ring, our strategy was altered to generate the ketone at C3 and install the C2-methyl group after macrolactamization.
Scheme 11.
Synthesis of 11-epi-stereocalpin A
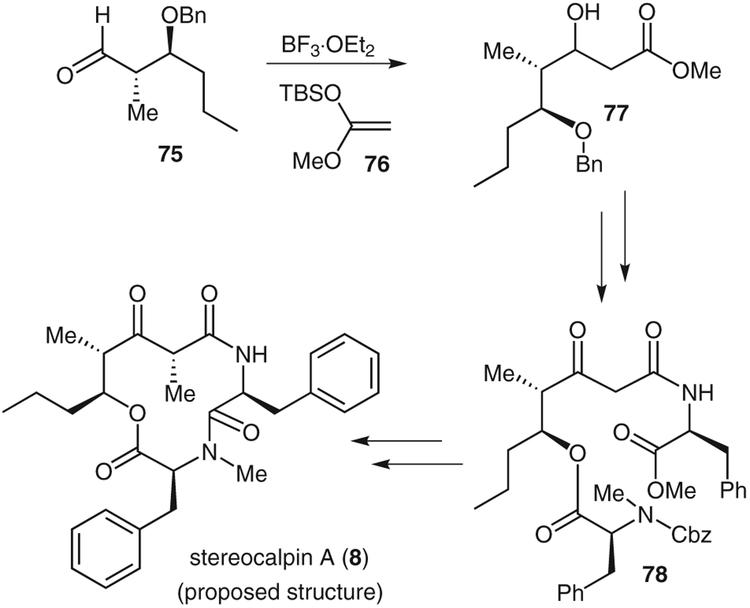
As shown in Scheme 12, aldehyde 75 was subjected to a Mukaiyama aldol protocol using ketene acetal 76 to give aldol product 77 in 72% yield (5:1 dr).2,49 Hydrolysis of methyl ester 77 followed by ethyl-N′-(3-dimethylaminopropyl)carbodiimide-mediated coupling with L-phenyl-alanine and subsequent oxidation gave the desired keto amide. Benzyl deprotection and esterification with N-benzyloxycarbonyl-N-methyl-L-phenylalanine gave ester 78. This was converted into the corresponding cycloamide in 47% yield, along with 10% of the epimerized product. Methylation from the less-hindered face using cesium carbonate and methyl iodide completed the synthesis of the compound with the proposed structure of stereocalpin A; however, our spectral data for the synthetic stereocalpin did not match those for natural stereocalpin, which led us to believe that the absolute stereochemistry of stereocalpin had been assigned incorrectly.47
Scheme 12.
Synthesis of the compound with the proposed structure of stereocalpin A
(3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-ol Ligand
The development and FDA approval of the first protease inhibitors (PIs) in 1996 marked a new era of HIV/AIDS management. HAART (highly active antiretroviral therapy) drug combinations containing PIs arrested the progression of HIV/AIDS by reducing the viral load and increasing the CD4+ lymphocyte cell count in HIV/AIDS patients.50 Gaining the upper hand in the battle against HIV/AIDS proved bittersweet, as it became apparent that these potent first-generation PIs had several drawbacks, including high toxicity and related side-effects, high therapeutic doses, and expensive treatment costs. The emergence of multi-drug-resistant HIV-1 variants also became a major problem. Drug-resistant HIV is responsible for nearly 40–50% of patients who have previously shown viral suppression to undetectable levels, to rapidly experience treatment failure.51 In addition, resistant HIV-1 strains have probably been transmitted, as 20–40% of previously untreated HIV-infected individuals have shown continued viral replication despite HAART treatment regimens.
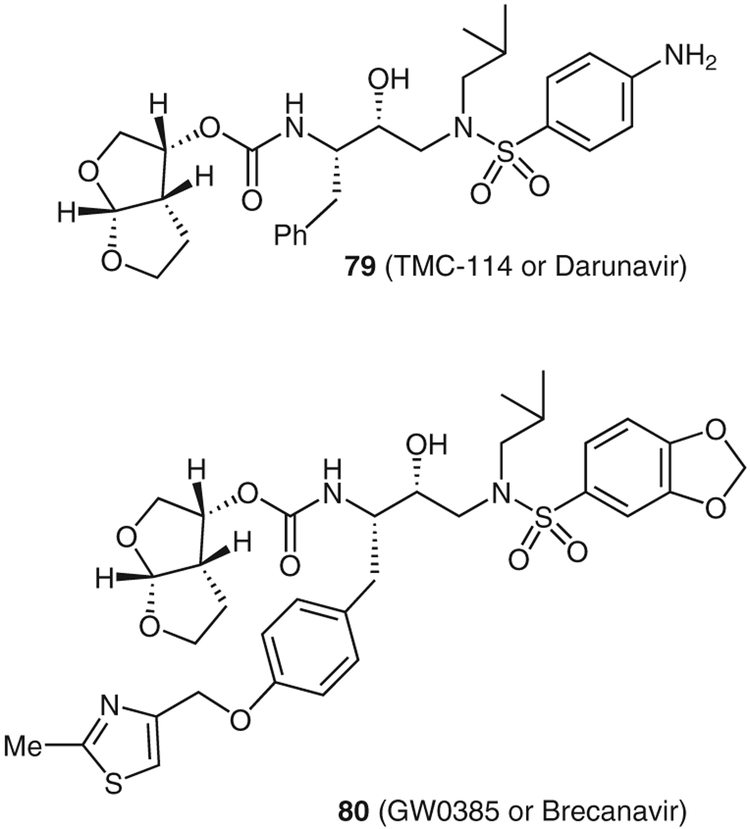
Recently, through a structure-based approach, we have designed a series of exceedingly potent nonpeptidyl HIV PIs.52 One inhibitor, TMC114 (79; Daranuvir) has been approved by the FDA for the treatment of drug-resistant HIV.53 A key feature of these potent inhibitors is the incorporation of a stereochemically defined bis-THF as the P2-ligand. This ligand was designed to maintain backbone hydrogen-bonding interactions at the active site of the protease. Another inhibitor, GW0385 (80, Brecanavir), which also contains the bis-THF ligand, has undergone phase II clinical trials.54
We have developed a number of synthetic routes for preparing the key bistetrahydrofuran ligand in an optically active form. Our previous method using diethyl (3R)-malate as a key starting material was overall not very efficient. We subsequently developed a procedure that required a lipase-catalyzed enzymatic resolution; this, however, resulted in optical purity in the range 92–96% ee.55 Synthesis of the ligand by means of a diastereoselective Michael addition reaction has been recently reported by Quaedflieg and co-workers.56 To obtain the bistetrahydrofuran ligand efficiently and in high optical purity, we carried out a synthesis using a titanium-enolate based anti-aldol reaction as the key step.6
As shown in Scheme 13, aldol condensation product 81 was obtained in 70% yield and a 96:4 anti/syn ratio by the reaction of ester 29 with cinnamaldehyde. Subsequent manipulations of the aldolate, including protection, reduction, Swern oxidation of the alcohol, and protection of the resulting aldehyde gave the methyl acetal 82. Ozonolysis of 82, followed by reduction of the resulting aldehydes, gave the corresponding diol. This diol was treated with aqueous hydrochloric acid, which resulted in deprotection and acid-catalyzed cyclization to provide the desired ligand 83 with a very high optical purity (>99% ee Mosher ester analysis).
Scheme 13.
Synthesis of a bistetrahydrofuran ligand
In conclusion, the expansion of aldol methodologies has contributed significantly to advancing the synthesis of natural products. Our titanium enolate mediated syn- and anti-aldol strategies have been a cornerstone of our syntheses of β-hydroxy α-alkyl carbonyl derivatives. We have incorporated this strategy into an efficient synthesis of an optically pure form of the bistetrahydrofuran ligand, which is a crucial component of potent HIV protease inhibitors. We have also used Evans aldol reactions in the syntheses of a number of natural products. Furthermore, we have recently developed a novel L-Selectride-mediated reductive aldol reaction and have demonstrated its usefulness by synthesizing the anticancer natural product peloruside A.
Natural products will continue to stimulate scientists in all disciplines. These natural masterpieces have been, and will continue to be, mirrored through the art of organic synthesis. The struggles and achievements on the path to the synthesis of bioactive natural products will continue to enrich the ever-expanding tapestry of chemical methodologies.
Example of an Evans syn-Aldol Reaction: (4R)-4-Benzyl-3-[(2R,3S,4R,5S)-5-(benzyloxy)-3-hydroxy-2,4-dimethyloctanoyl]-1,3-oxazolidin-2-one (72)
A 1 M soln of Bu2BOTf in CH2Cl2 (8.5 mL, 8.5 mmol) and DIPEA (1.6 mL, 9.3 mmol) were added sequentially to a soln of (4R)-4-benzyl-3-propionyl-1,3-oxazolidin-2-one (50; 1.81 g, 7.74 mmol) in CH2Cl2 (30 mL), and the clear soln was stirred at 0 °C for 30 min then cooled to −78 °C. A soln of aldehyde 71 (1.32 g, 5.96 mmol) in CH2Cl2 (10 mL) was then slowly added by syringe. The aldehyde flask was washed with CH2Cl2 (5 mL) and the remaining soln was also added to the enolate soln. The mixture was stirred at −78 °C for 10 min then warmed to 0 °C over 1 h. The reaction was quenched with pH 7 buffer (10 mL) and MeOH (15 mL). A 30% soln of H2O2 (10 mL) and MeOH (20 mL) were slowly added while the internal temperature was kept below 5 °C. The mixture was stirred at 0 °C for an additional 1 h then diluted with CH2Cl2 (40 mL). The aqueous layer was extracted with CH2Cl2 and the combined extracts were washed with brine, dried (Na2SO4), and concentrated under vacuum. The crude product was purified by flash column chromatography (silica gel, hexanes–EtOAc, 85:15); yield: 2.45 g (89%).
Example of a Titanium Enolate syn-Aldol Reaction: (1S,2R)-1-(Tosylamino)indan-2-yl (2S,3R)-4-(Benzyloxy)-3-hydroxy-2-methylbutanoate (44)
Neat TiCl4 (1.47 mL, 13.4 mmol) and DIPEA (7.75 mL, 44.5 mmol) were added to a soln of propionate ent-37 (4.0 g, 11.1 mmol) in CH2Cl2 (150 mL) at 0 °C. The resulting soln was warmed to 23 °C, stirred for 2 h, and then cooled to −78 °C. A mixture of TiCl4 (3.68 mL, 33.4 mmol) and BzOCH2CHO (3.13 mL, 22.3 mmol) in CH2Cl2 (20 mL) was slowly added, and the dark soln was stirred for 2 h at −78 °C. The reaction was quenched with sat. aq NH4Cl, and the mixture was warmed to 23 °C. The aqueous layer was separated and extracted with CH2Cl2. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated to give a brown residue that was purified by column chromatography to provide the pure product; yield: 5.27 g (93%).
Examples of Titanium Enolate anti-Aldol Reaction: (1S,2R)-1-(Tosylamino)indan-2-yl (1S,2R)-3-Hydroxy-2-methylhexanoate (70)
TiCl4 (4.34 mL, 39.6 mmol, 1.1 equiv) was added dropwise to a soln of propionate ent-37 (12.93 g, 36 mmol) in CH2Cl2 (180 mL) at 0 °C and the mixture was stirred at 0 °C for 15 min before DIPEA (23.8 mL, 137 mmol, 3.8 equiv) was added slowly. The dark soln was allowed to warm to 23 °C and stirred at 23 °C for 2 h. In a separate flask, TiCl4 (7.9 mL, 2 equiv) was added slowly to PrCHO (6.5 mL, 72 mmol, 2 equiv) in CH2Cl2 (180 mL) at −78 °C, followed by addition of anhyd MeCN (3.8 mL, 2 equiv). The mixture was stirred at −78 °C for 5 min while a white precipitate formed. The soln of the titanium enolate prepared from ent-37 was added to this mixture from a cannula, over 15 min. The resulting dark soln was stirred at −78 °C for 2 h, and then the reaction was quenched with sat. aq NH4Cl. The aqueous layer was extracted with CH2Cl2, and the combined organic phases were washed with brine and dried (Na2SO4). The solvent was removed under reduced pressure and the residue was purified by column chromatography (silica gel, hexanes–EtOAc, 9:1); yield: 10.1 g (65%; 37:1 dr).
(1S,2R)-1-(Tosylamino)indan-2-yl (3S,4E)-2-Allyl-3-hydroxy-5-phenylpent-4-enoate (81)
A 1.8 M soln of TiCl4 (13.8 mL, 24.8 mmol) was added dropwise to a stirred soln of pent-4-enoate 29 (7.89 g, 20.5 mmol) in CH2Cl2 (210 mL) at 0 °C under an inert atmosphere. The resulting soln was stirred for 5 min and then DIPEA (13.9 mL, 79.7 mmol) was added dropwise. The mixture was allowed to warm to 23 °C and stirred for 2 h. In a separate flask, a 1.8 M soln of TiCl4 in CH2Cl2 (11.5 mL, 20.7 mmol) was added dropwise to cinnamaldehyde (5.30 mL, 42 mmol) in CH2Cl2 (320 mL) at −78 °C, and the soln was stirred for 5 min at −78 °C. DIPEA (7.3 mL, 41.9 mmol) was then added dropwise and the resulting mixture was stirred for an additional 5 min. The titanium enolate soln prepared from pent-4-enoate 29 was added dropwise through an insulated cannula over 30 min, and the mixture was stirred at −78 °C for 3.5 h. The reaction was quenched with sat. aq NH4Cl and the mixture was allowed to warm to 23 °C. The layers were separated and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated to give a crude aldol product that was purified by chromatography (silica gel, 15% then 20% EtOAc–hexanes) to give diastereomerically pure anti-aldol product 81 as a gummy solid; yield: 7.43 g (70%).
Example of an L-Selectride-Mediated Reductive Aldol Reaction: Aldol Product 68
A 1.0 M soln of L-Selectride in THF (1.1 mL, 1.1 mmol) was added to a soln of enone 64 (645 mg, 1.06 mmol) in Et2O (200 mL) at −78 °C, and the mixture was kept at −78 °C for 10–15 min. A soln of aldehyde 67 (520 mg, 1.2 mmol) in Et2O (20 mL) was added at −78 °C, and the mixture was stirred at −78 °C for 1 h. The reaction was quenched with sat. aq NH4Cl, and the organic layer was separated. The aqueous layer was extracted with EtOAc, and the combined organic layers were washed with H2O and brine then dried (Na2SO4) and concentrated in vacuo. The crude product was purified by column chromatography to give the major isomer 68; yield: 823 mg (92%; dr 4:1).
Figure 2.
Structure of protease inhibitors 79 and 80
Scheme 3.
Synthesis of tetrahydrolipstatin
Scheme 4.
Synthesis of the pseudopeptide microbial agent AI-77-B
References
- (1).Evans DA; Fitch DM J. Org. Chem 1997, 62, 454. [DOI] [PubMed] [Google Scholar]
- (2).Mukaiyama T; Banno K; Narasaka KJ Am. Chem. Soc 1974, 96, 7503. [Google Scholar]
- (3) (a).Ghosh AK; Kim, J. Org. Lett 2003, 3, 1063. [DOI] [PubMed] [Google Scholar]; (b) Ghosh AK; Onishi MJ Am. Chem. Soc 1996, 118, 2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ghosh AK; Kass J; Anderson DD; Xu X; Marian C Org. Lett 2008, 21, 4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ghosh AK; Xu X; Kim J-H; Xu C-X Org. Lett 2008, 10, 1001. [DOI] [PubMed] [Google Scholar]
- (6).Ghosh AK; Li J-F Synthesis 2006, 3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7) (a).Kartner N; Riordan JR; Ling V Science 1983, 221, 1285. [DOI] [PubMed] [Google Scholar]; (b) Pastan I; Gottesman MM Annu. Rev. Med 1991, 42, 277. [DOI] [PubMed] [Google Scholar]
- (8).Stratmann K; Burgoyne DL; Moore RE; Patterson GML; Smith CD J. Org. Chem 1994, 39, 7219. [Google Scholar]
- (9).Endicott JA; Ling V Annu. Rev. Biochem 1989, 38, 137. [DOI] [PubMed] [Google Scholar]
- (10).Ghosh AK; Liu W; Xu Y; Chen Z Angew. Chem.,Int. Ed. Engl 1996, 33, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ghosh AK; Duong TT; McKee SPJ Chem. Soc., Chem. Commun 1992, 1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Schwarz RE; Hirsch CF; Sesin DF; Flor JE; Chartrain M; Fromtling RE; Harris GH; Salvatore MJ; Liesch JM; Yudin KJ Ind. Microbiol 1990, 3, 113. [Google Scholar]
- (13).Smith CD; Zhang X; Mooberry SL; Patterson GML; Moore RE Cancer Res 1994, 34, 3779. [PubMed] [Google Scholar]
- (14).Ghosh AK; Bischoff A Org. Lett 2000, 2, 1573. [DOI] [PubMed] [Google Scholar]
- (15).Ghosh AK; Swanson LJ Org. Chem 2003, 68, 9823. [DOI] [PubMed] [Google Scholar]
- (16) (a).Hochuli E; Kupfer E; Maurer R; Meiser W; Mercadel Y; Schmidt K J. Antibiot 1987, 40, 1086. [DOI] [PubMed] [Google Scholar]; (b) Weibel EK; Hadvary P; Kupfer E; Lengsfeld HJ Antibiot 1987, 40, 1081. [DOI] [PubMed] [Google Scholar]
- (17) (a).Borgstrom B Biochem. Biophys. Acta 1988, 962, 308. [DOI] [PubMed] [Google Scholar]; (b) Hadvary P; Sidler W; Meiser W; Vetter W; Wolfer HJ Biol. Chem 1991, 266, 2021. [PubMed] [Google Scholar]
- (18).Ghosh AK; Fidanze S Org. Lett 2000, 2, 2405. [DOI] [PubMed] [Google Scholar]
- (19).Knochel P; Seebach D Synthesis 1982, 1017. [Google Scholar]
- (20) (a).Evans DA; Chapman KT Tetrahedron Lett 1986, 27, 5939. [Google Scholar]; (b) Evans DA; Chapman KT; Carreira EM J. Org. Chem 1988, 33, 3560. [Google Scholar]
- (21).Shimojima Y; Hayashi H; Ooka T; Shibukawa M Agric. Biol. Chem 1982, 46, 1823. [Google Scholar]
- (22) (a).Shimojima Y; Hayashi H J. Med. Chem 1983, 26, 1370. [DOI] [PubMed] [Google Scholar]; (b) Shimojima Y; Shirai T; Baba T; Hayashi HJ Med. Chem 1985, 28, 3. [DOI] [PubMed] [Google Scholar]
- (23).Ghosh AK; Bischoff A; Cappiello J Org. Lett 2001, 3, 2677. [DOI] [PubMed] [Google Scholar]
- (24) (a).Dondoni A; Fantin G; Fogagnolo M; Medici A; Pedrini P Synthesis 1988, 685. [Google Scholar]; (b) Dondoni A; Fogagnolo M; Medici A; Pedrini P Tetrahedron Lett 1985, 26, 5477. [Google Scholar]; (c) Dondoni A; Perrone D; Semola MT J. Org. Chem 1995, 60, 7927. [Google Scholar]
- (25) (a).Bertelli L; Fiaschi R; Napolitano E Gazz. Chim. Ital 1993, 123, 669. [Google Scholar]; (b) Kotsuki H; Miyazaki A; Ochi M Chem. Lett 1992, 1255. [Google Scholar]; (c) Superchi S; Minutolo F; Pini D; Salvadori PJ Org. Chem 1996, 61, 3183. [DOI] [PubMed] [Google Scholar]
- (26).Kobayashi J In Comprehensive Natural Products Chemistry, Vol. 8; Barton DHR; Nakanishi K; Meth-Cohn O, Eds.; Elsevier: New York, 1999, 619. [Google Scholar]
- (27).Tsuda M; Endo T; Kobayashi JJ Org. Chem 2001, 66, 134. [DOI] [PubMed] [Google Scholar]
- (28).Ghosh AK; Liu CJ Am. Chem. Soc 2003, 123, 2374. [DOI] [PubMed] [Google Scholar]
- (29).Petasis NA; Bzowej EI J. Am. Chem. Soc 1990, 112, 6394. [Google Scholar]
- (30).Ley SV; Lygo B; Wonnacott A Tetrahedron Lett 1989, 26, 535. [Google Scholar]
- (31) (a).Crews P; Manes LV; Boehler M Tetrahedron Lett 1986, 27, 2797. [Google Scholar]; (b) Zabriskie TM; Klocke JA; Ireland CM; Marcus AH; Molinski TF; Faulkner DJ; Xu C; Clardy JC J. Am. Chem. Soc 1986, 108, 3123. [Google Scholar]
- (32).Inman W; Crews PJ Am. Chem. Soc 1986, 111, 2822. [Google Scholar]
- (33).Bubb MR; Senderowicz AMJ; Sausville EA; Duncan KLK; Korn ED J. Biol. Chem 1994, 269, 14869. [PubMed] [Google Scholar]
- (34) (a).Chu KS; Negrete GR; Konopelski JP J. Org. Chem 1991, 36, 5196. [Google Scholar]; (b) Grieco PA; Hon YS J. Am. Chem. Soc 1988, 110, 1630. [Google Scholar]; (c) Hanada Y; Shioiri T Tetrahedron Lett 2004, 33, 591. [Google Scholar]; (d) Hirai Y; Yokota K; Momose T Heterocycles 1994, 39, 603. [Google Scholar]; (e) Imaeda T; Rao AV; Gurjar MK; Nallaganchu BR; Bhandari A Tetrahedron Lett 1993, 34, 7085. [Google Scholar]
- (35).Ghosh AK; Moon DK Org. Lett 2007, 9, 2425. [DOI] [PubMed] [Google Scholar]
- (36).Ziegler FE Acc. Chem. Res 1977, 10, 227. [Google Scholar]
- (37).Davis FA; Reddy RE; Szewczyk JM J. Org. Chem 1995, 60, 7037. [Google Scholar]
- (38).West LM; Northcote PT; Battershill CN J. Org. Chem 2000, 63, 445. [DOI] [PubMed] [Google Scholar]
- (39) (a).Pryor DE; O’Brate A; Bilcer G; Diaz JF; Wang Y; Kabaki M; Jung MK; Andreu JM; Ghosh AK; Giannakakou P; Hamel E Biochemistry 2002, 41, 9109. [DOI] [PubMed] [Google Scholar]; (b) Gaitanos TN; Buey RM; Diaz JF; Northcote PT; Teesdale-Spittle P; Andreu JM; Miller JH Cancer Res 2004, 64, 5063. [DOI] [PubMed] [Google Scholar]
- (40).Hood KA; West LM; Rouwe B; Northcote PT; Berridge MV; Wakefield SJ; Miller JH Cancer Res 2002, 62, 3356. [PubMed] [Google Scholar]
- (41) (a).Jin M; Taylor RE Org. Lett 2005, 7, 1303. [DOI] [PubMed] [Google Scholar]; (b) Liao X; Wu Y; De Brabander JK Angew. Chem. Int. Ed 2002, 42, 1648. [DOI] [PubMed] [Google Scholar]
- (42) (a).Chen Z-L; Zhou WS Tetrahedron Lett 2006, 47, 5289. [Google Scholar]; (b) Engers DW; Bassindale MJ; Pagenkopf BL Org. Lett 2004, 6, 663. [DOI] [PubMed] [Google Scholar]; (c) Ghosh AK; Kim J-H Tetrahedron Lett 2003, 44, 3967. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gurjar MK; Pedduri Y; Ramana CV; Puranik VG; Gonnade RG Tetrahedron Lett 2004, 43, 387. [Google Scholar]; (e) Liu B; Zhou WS Org. Lett 2004, 6, 71. [DOI] [PubMed] [Google Scholar]; (f) Owen RM; Roush WR Org. Lett 2005, 7, 3941. [DOI] [PubMed] [Google Scholar]; (g) Paterson I; Di Francesco ME; Kuhn T Org. Lett 2003, 3, 599. [DOI] [PubMed] [Google Scholar]; (h) Roulland E; Ermolenko MS Org. Lett 2005, 7, 2225. [DOI] [PubMed] [Google Scholar]; (i) Taylor RE; Jin M Org. Lett 2003, 3, 4959. [DOI] [PubMed] [Google Scholar]
- (43).Ghosh AK; Kim J-H Tetrahedron Lett 2003, 44, 7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Jadhav PK; Bhat KS; Perumal PT; Brown HC J. Org. Chem 1986, 51, 432. [Google Scholar]
- (45).Ando KJ J. Org. Chem 1998, 63, 8411. [Google Scholar]
- (46).Kolb HC; Van Nieuwenhze MS; Sharpless KB Chem. Rev 1994, 94, 2483. [Google Scholar]
- (47).Seo C; Kim JH; Lee HK; Park SM; Sohn J-H; Oh H Tetrahedron Lett 2008, 49, 29. [Google Scholar]
- (48).Ghosh AK; Xu C-X Org. Lett 2009, 11, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49) (a).Mutou T; Suenaga K; Fujita T; Itoh T; Takada N; Hayamizu K; Kigoshi H; Yamada K Synlett 1997, 199. [Google Scholar]; (b) Paterson I; Smith JD J. Org. Chem 1992, 37, 3261. [Google Scholar]; (c) Paterson I; Smith JD Tetrahedron 1995, 31, 9413. [Google Scholar]
- (50).Sepkowiz KA N. Engl. J. Med 2001, 344, 1764. [DOI] [PubMed] [Google Scholar]
- (51) (a).Grabar S; Pradier C; Le Corfec E; Lancar R; Allavena C; Bentata M; Berlureau P; Dupont C; Fabbro-Peray P; Poizot-Martin I; Costagliola D AIDS 2000, 14, 141. [DOI] [PubMed] [Google Scholar]; (b) Pillay D; Bhaskaran K; Jurriaans S; Prins M; Masquelier B; Dabis F; Gifford R; Nielsen C; Pedersen C; Balotta C; Rezza G; Ortiz M; de Mendoza C; Kucherer C; Poggensee G; Gill J; Porter K AIDS 2006, 20, 21. [DOI] [PubMed] [Google Scholar]; (c) Wainberg MA; Friedland GJ Am. Med. Assoc 1998, 279, 1977. [DOI] [PubMed] [Google Scholar]
- (52) (a).Ghosh AK; Shin DW; Swanson L; Krishnan K; Cho H; Hussain KA; Walters DE; Holland L; Buthod J Farmaco 2001, 56, 29. [DOI] [PubMed] [Google Scholar]; (b) Ghosh AK; Kincaid JF; Cho W; Walters DE; Krishnan K; Hussain KA; Koo Y; Cho H; Rudall C; Holland L; Buthod J Bioorg. Med. Chem. Lett 1998, 8, 687. [DOI] [PubMed] [Google Scholar]; (c) Koh Y; Nakata H; Maeda K; Ogata H; Bilcer G; Devasamudram T; Kincaid JF; Boross P; Wang Y-F; Tie Y; Volarath P; Gaddis L; Harrison RW; Weber IT; Ghosh AK; Mitsuya H Antimicrob. Agents Chemother 2003, 47, 3123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yoshimura K; Kato R; Kavlick MF; Nguyen A; Maroun V; Maeda K; Hussain KA; Ghosh AK; Gulnik SV; Erickson JW; Mitsuya HJ Virol 2002, 26, 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Darunavir received FDA approval on June 23, 2006: see http://www.fda.gov/bbs/topics/NEWS/2006NEW01395.html
- (54).Miller JF; Andrews CW; Brieger M; Furfine ES; Hale MR; Hanlon MH; Hazen RJ; Kaldor I; McLean EW; Reynolds D; Sammond DM; Spaltenstein A; Tung R; Turner EM; Xu RX; Sherrill RG Bioorg. Med. Chem. Lett 2006, 16, 1788. [DOI] [PubMed] [Google Scholar]
- (55) (a).Ghosh AK; Kincaid JF; Walters DE; Chen Y; Chaudhuri NC; Thompson WJ; Culberson C; Fitzgerald PMD; Lee HY; McKee SP; Munson PM; Duong TT; Darke PL; Zugay JA; Schleif WA; Axel MG; Lin J; Huff JR J. Med. Chem 1996, 39, 3278. [DOI] [PubMed] [Google Scholar]; (b) Ghosh AK; Chen Y Tetrahedron Lett 1995, 36, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ghosh AK; Leshchenko S; Noetzel MJ J. Org. Chem 2004, 69, 7822. [DOI] [PubMed] [Google Scholar]
- (56).Quaedflieg PJLM; Kesteleyn BRR; Wigerinck PBTP; Goyvaerts NMF; Vijn RJ; Liebregts CSM; Kooistra JHMH; Cusan C Org. Lett 2005, 7, 5917. [DOI] [PubMed] [Google Scholar]