Abstract
An asymmetric synthesis of the C10–C24 fragment of the potent antitumor macrolide, peloruside A is described. All three stereogenic centers have been enantioselectively constructed utilizing Evans alkylation, Brown asymmetric allylboration, and a substrate controlled epoxide formation. Other key reactions involved Grubbs’s ring-closing olefin metathesis and Ando’s Z-selective olefination reaction.
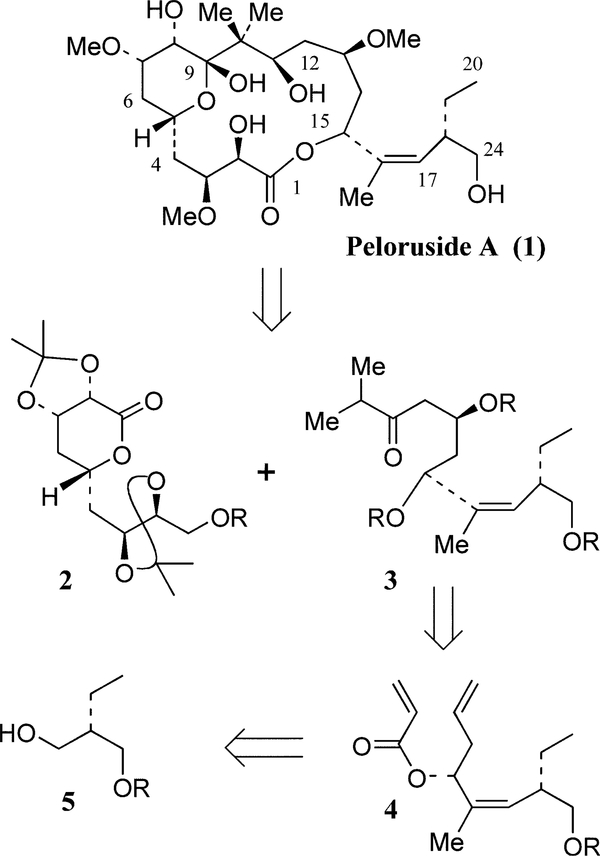
Peloruside A, a 16-membered macrolide, was recently isolated from the New Zealand marine sponge Mycale hentscheli.1 It exhibited potent cytotoxicity against P388 murine leukemia cells with an IC50 value of 10 ng/mL.1 Like paclitaxel, peloruside A has shown microtubule-stabilizing activity and arrests cells in the G2-M phase.2 It has structural resemblance to epothilones which are undergoing clinical trials.3 Important antitumor activities of peloruside A along with its unique structural features have stimulated immense interest in the synthesis and structure–function studies. Peloruside A’s initial structure and relative stereochemistry were established by NMR studies.1 However, its absolute stereochemistry was conclusively established only recently after the first total synthesis4 followed by its chemical and biological correlation with the natural peloruside A. To date, De Brabander and co-workers have reported the only total synthesis. Synthetic approaches toward fragments of peloruside A have been reported by Paterson et al.5 We recently reported an enantioselective synthesis of the C1–C9 segment of peloruside A.6 In continuation of our on-going studies, we now report a stereocontrolled route to the C10–C24 segment (3) of peloruside A where all three stereocenters have been created by asymmetric synthesis.
As shown in Figure 1, our convergent synthetic plan for peloruside A involves the assembly of C1–C9 segment (2) and C10–C24 (3) by an aldol reaction followed by a macrolactonization between the C15-hydroxyl group and the C1-carboxylic acid. We plan to synthesize the fragment 3 by a nucleophilic addition of isopropylmagnesium halide to the functionalized δ -lactone derived from acrylate ester 4. The corresponding homoallyl alcohol will be derived from protected alcohol 5 by oxidation, olefination and subsequent asymmetric allylboration of the resulting aldehyde utilizing Brown’s protocol. Protected alcohol 5 will be readily available by Evans’ asymmetric alkylation reaction.7
Figure 1.
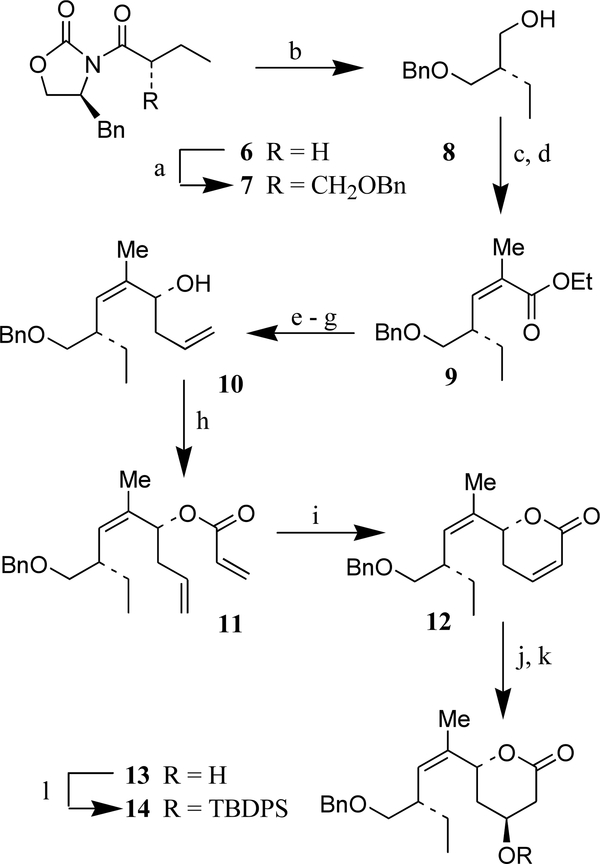
As depicted in Scheme 1, asymmetric alkylation of chiral imide 6 was carried out with benzyloxymethyl chloride using Evans’ protocol7a to provide the alkylated product 7 as a single isomer in 80% yield (from benzyloxazolidinone). Reduction of imide 7 by LiBH4 in THF–MeOH at 23°C afforded alcohol 8. Swern oxidation of 8 and subsequent Horner–Emmons reaction of the resulting aldehyde with sodium enolate of (o-cresol)2P=O(CH3)CHCO2Et as described by Ando8 furnished tri-substituted Z-olefin 9 in 90% yield over two steps. The Z-selectivity was >99:1 as revealed by 1H and 13C NMR analysis. Our next synthetic plan was to install an α,β-unsaturated δ-lactone with appropriate stereochemistry and then elaborate the syn-1,3-diol functionality by a substrate-controlled diastereoselective epoxidation followed by reductive opening of the resulting epoxide. For the synthesis of α,β-unsaturated δ-lactone, ester 9 was first reduced by Dibal-H to deliver the corresponding alcohol. Dess-Martin periodinane oxidation provided the aldehyde which was subjected to the Brown asymmetric allylboration protocol9 with allyldiisopinocampheylborane to afford homoallylic alcohol 10 as the major diastereomer (dr=93:7 by 1H and 13C NMR). Alcohol 10 was obtained in 65% yield over two steps after silica gel chromatography.
Scheme 1. Reagents and conditions:
(a) TiCl4, Et3N, CH2Cl2, PhCH2OCH2Cl, 0°C, 1.5 h; (b) LiBH4, MeOH, THF, 23°C, 1 h (94%); (c) (COCl)2, DMSO, Et3N, CH2Cl2, −60°C, 45 min; (d) (o-cresol)2PO(CH3)CHCO2Et, NaH, THF, −78 to −20°C, 2 h (90% over two steps); (e) Dibal-H, CH2Cl2, −78° to −40°C, 1 h (96%); (f) Dess–Martin periodinane, NaHCO3, CH2Cl2, 23°C, 1.5 h; (g) CH2CHCH2B[(+)-Ipc]2, Et2O, −80°C, 3 h (65% over two steps); (h) CH2CHCOCl, Et3N, CH2Cl2, 0°C, 2 h (77%); (i) Cl2(Pcy)2RuCHPh, CH2Cl2, 40°C, 12 h (83%); (i) H2O2, 6N-aq NaOH, MeOH, 1.5 h (74%); (k) NaBH4, PhSeSePh, AcOH, iPrOH, 0°C, 30 min (quant.); (l) TBDPSCl, imidazole, DMAP, DMF, 23°C, 13 h (quant.).
For synthesis of α,β-unsaturated δ-lactone, we relied upon ring-closing olefin metathesis protocol described in our recent work.10 Reaction of 10 with acryloyl chloride and triethylamine at 0°C furnished acryloyl ester 11. Exposure of 11 to a catalytic amount (10 mol%) of first generation commercial Grubb’s catalyst11 in CH2Cl2 at reflux for 12 h provided α,β-unsaturated δ-lactone 12 in 83% yield after one recycle of the recovered starting material.12 The use of titanium tetra-isopropoxide as a co-catalyst (40 mol%) significantly lowered the yield (62%) of δ-lactone 12. Reaction with second generation Grubb’s catalyst did not improve the overall yield (74%) either. For elaboration of the hydroxy γ-lactone with appropriate stereochemistry, δ-lactone 12 was exposed to nucleophilic epoxidation with alkaline hydrogen peroxide in methanol at 0°C to furnish the corresponding epoxide in 74% yield as a single isomer. Treatment of the resulting epoxide with diphenyldiselenide and sodium borohydride in 2-propanol afforded exclusively hydroxylactone 13 in quantitative yield.13 Lactone 13 possesses the required syn-1,3-diol stereochemistry necessary for peloruside A synthesis. Protection of alcohol 13 under standard condition provided TBDPS ether 14 in quantitative yield.
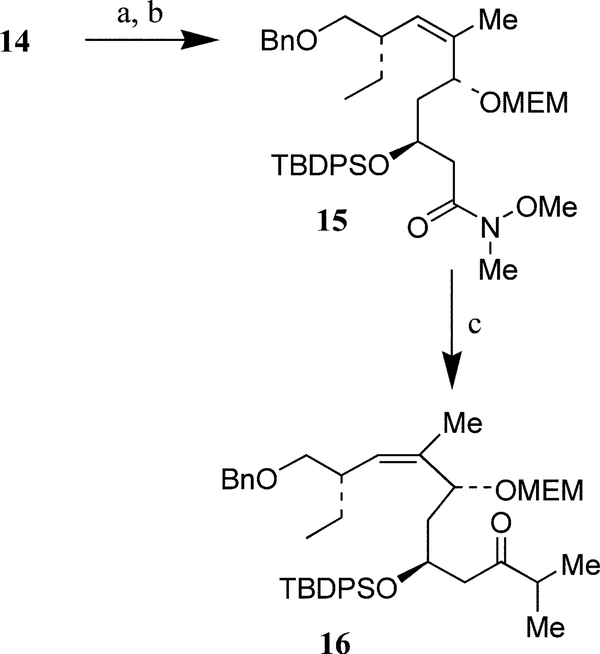
For conversion of -lactone 14 to the C10–C24 fragment, we first attempted direct opening of lactone ring with isopropylmagnesium chloride. However, reaction of 14 with excess isopropylmagnesium chloride under a variety of reaction conditions did not provide the desired ketone. In an alternative approach, δ-lactone 14 was transformed into C10–C24 fragment ketone 16 as follows (Scheme 2). Reaction of 14 with N,O-dimethylhydroxylamine hydrochloride in the presence of trimethylaluminum in CH2Cl2 according to Weinreb procedure14 furnished the corresponding hydroxy Weinreb amide. Protection of the resulting alcohol as MEM–ether provided Weinreb amide 15 in 86% yield (from 14). Treatment of 15 with isopropylmagnesium chloride (5 equiv.) in THF at 23°C for 5 h afforded C10–C24 fragment ketone 1615 in 61% yield. It turned out that both MEM-and TBDPS-protecting groups were critical to form 16. Replacement of the MEM-group with a PMB-group resulted in substantial β-elimination as well as degradation of starting material.
Scheme 2. Reagent and conditions:
(a) AlMe3, HN(OCH3)-CH3·HCl, CH2Cl2, 23°C, 2.5 h (93%); (b) MEMCl, DIPEA, 23°C, 9 h (92%); (c) iPrMgCl, THF, 23°C, 5 h (61%).
In summary, a highly stereoselective synthesis of C10–C24 segment of peloruside A has been accomplished. The key steps involved an Evans asymmetric alkylation, Ando’s Z-selective olefination, Brown asymmetric allylboration, Grubbs’s ring-closing olefin metathesis, and substrate controlled stereoselective epoxidation. Work toward the total synthesis of peloruside A is in progress.
Acknowledgements
Partial financial support for this work was provided by the National Institutes of Health.
References
- 1.West LM; Northcote PT J. Org. Chem 2000, 65, 445. [DOI] [PubMed] [Google Scholar]
- 2. (a).Hood KA; Backstorm BT; West LM; Northcote PT; Berridge MV; Miller JH Anti-Cancer Drug Design 2001, 16, 155; [PubMed] [Google Scholar]; (b) Hood KA; West LM; Rouwe B; Northcote PT; Berridge MV; St. Wakefield J; Miller JH Cancer Res. 2002, 62, 3356. [PubMed] [Google Scholar]
- 3. (a).Bollag DM; McQueney PA; Zhu J; Hensens O; Koupal L; Liesch J; Goetz M; Lazarides E; Woods CM Cancer Res. 1995, 55, 2325; [PubMed] [Google Scholar]; (b) Nicolaou KC; Rosechanger F; Vourloumis D Angew. Chem 1998, 37, 2014; [DOI] [PubMed] [Google Scholar]; (c) Stachel SJ; Biswas K; Danishefsky SJ Curr. Pharm. Des 2001, 7, 1277 and references cited therein. [DOI] [PubMed] [Google Scholar]
- 4.Liao X; Wu Y; De Brabander JK Angew. Chem., Int. Ed 2003, 42, 1648. [DOI] [PubMed] [Google Scholar]
- 5.Paterson I; Di Francesco ME; Kuhn T Org. Lett 2003, 5, 599. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh AK; Kim J-H Tetrahedron Lett 2003, 44, 3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. (a).Evans DA; Chapman KT; Huang DT; Kawaguchi AT Angew. Chem., Int. Ed. Engl 1987, 26, 1184; [Google Scholar]; (b) Evans DA; Urpi F; Somers TC; Cleark JS; Bilodeau MT J. Am. Chem. Soc 1990, 112, 8215; [Google Scholar]; (c) Ihara M; Katsumata A; Setsu F; Tokunaga Y; Fukumoto K J. Org. Chem 1996, 61, 677. [DOI] [PubMed] [Google Scholar]
- 8.Ando K J. Org. Chem 1998, 63, 8411. [Google Scholar]
- 9. (a).Jadhav PK; Bhat KS; Perumaal T; Brown HC J. Org. Chem 1986, 51, 432; [Google Scholar]; (b) Racgerlaa US; Brown HC J. Org. Chem 1991, 56, 401. [Google Scholar]
- 10. (a).Ghosh AK; Cappiello J; Shin D Tetrahedron Lett. 1998, 39, 4651; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK; Liu C Chem. Commun 1999, 1743; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ghosh AK; Wang Y J. Am. Chem. Soc. 2002, 122, 11027; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ghosh AK; Wang Y; Kim JT J. Org. Chem 2001, 66, 8973. [DOI] [PubMed] [Google Scholar]
- 11.Grubbs RH; Chang S Tetrahedron 1998, 54, 4413. [Google Scholar]
- 12. Initially, lactone 12 was obtained in 68% along with 23% of unreacted starting material. After one recycling of starting material overall isolated yield was 83%.
- 13. (a).Ghosh AK; Lei H J. Org. Chem 2000, 65, 4779; [DOI] [PubMed] [Google Scholar]; (b) Ghosh AK; Lei H Synthesis 2002, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basha A; Lipton M; Weinreb SM Tetrahedron Lett. 1977, 18, 4171. [Google Scholar]
- 15.All new compounds gave satisfactory spectroscopic andanalytical results. 16: =+87.5 (c 0.4, CHCl3); IR (thin film) 2932, 1713, 1429, 1039 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.72–7.67 (m, 4H), 7.42–7.26 (m, 11H), 5.06 (d, J=10.3 Hz, 1H), 4.54 (m, 1H), 4.49–4.42 (m, 4H), 4.34 (d, J=6.8 Hz, 1H), 3.57 (m, 1H), 3.43–3.27 (m, 8H), 2.75 (dd, J=16.0, 4.6 Hz, 1H), 2.63(dd, J=6.0, 7.3 Hz, 1H), 2.59–2.52 (m, 2H), 1.95 (m, 1H), 1.56 (m, 1H), 1.46 (m, 1H), 1.42 (d, J=0.9 Hz, 3H), 1.15 (m, 1H), 1.06–1.01 (m, 15H), 0.79 (t, J=7.5 Hz, 3H); 13C NMR (125 MHz) δ 213.2, 139.2, 136.4, 136.3, 135.6, 134.3, 131.5, 130.0, 129.9, 128.7, 127.9, 127.8 (2C), 92.5, 74.2, 73.3, 72.2, 70.5, 68.4, 67.6, 59.4, 46.9, 42.1, 41.1, 39.5, 27.4 (3C), 25.4, 19.7, 18.3, 18.2, 12.1; HRMS (ESI) m/z calcd for C42H60O6Si (M++Na) 711.4057, found 711.4045.