Abstract
Efficient and highly stereoselective syntheses of cryptophycin B and arenastatin A, potent cytotoxic agents, are described. An ester-derived titanium enolate mediated syn-aldol reaction was employed to generate the stereocenters C-5 and C-6. The route is convergent and provides a convenient access to the synthesis of structural variants of cryptophycins as well as members of its family.
Keywords: Natural products, Cryptophycins, Arenastatin A, Asymmetric synthesis, Aldols
Introduction
In 1990, while screening the extracts of blue-green algae for antitumor activity, researchers at Merck & Co., Inc. found a strong antifungal agent in the lipophilic extract of the cyanobacteria Nostoc sp. (ATCC 53789).[1] It was highly active against filamentous fungi and yeast of Cryptococcus sp. with MIC50 and MIC90 values of both 31 μg/mL and consequently, was given the name cryptophycin (1, Figure 1). Later Moore et al. isolated and identified 25 compounds from Nostoc sp. GSV 224 of which the four major constituents, named cryptophycin A (1), B (2), C (3) and D (4), showed excellent activities against solid tumors implanted in mice.[2–4] Cryptophycins A and B exhibited IC50 values of 5 and 7 pg/mL against KB cells and 3 and 0.2 pg/mL against LoVo cells, respectively.[2] In addition, the compounds were equally effective against drug-sensitive and drug-resistant tumor cells.[2,3] In vivo structure-activity relationship studies on all isolated cryptophycins showed that the exclusion of the chlorine atom from the D-tyrosine moiety generally reduced the cytoxicity 10-fold, although the potencies of cryptophycin A and B were almost identical.[4] Also, removal of the O-methyl group or elimination of the epoxide oxygen atom both resulted in loss in potency (up to 1000-fold). Interestingly, if the epoxide is converted into a chloro- or bromohydrin no decrease in cytotoxicity was observed.[6] Replacement of the isobutyl group of the L-leucic acid moiety by an n-propyl, isopropyl or sec-butyl group was accompanied by a considerable reduction in cytoxicity (10- to 100-fold). The lack of a methyl group adjacent to the epoxide group lessened the cytoxicity substantially.
Figure 1.
Structures of arenastatin A and cryptophycins
In 1994 another potent member of the cryptophycin family was isolated from the Okinawan marine sponge Dysidea arenaria and identified by Kitagawa et al.[7] Arenastatin A (5), named after its origin, showed an excellent cytotoxicity of 5 pg/mL against KB cell line (IC50).[7] Unfortunately, its potency is limited by the fact that it is subject to degradation in blood, a vulnerability caused by the high susceptibility of the ester linkages to hydrolysis.[8–11] Synthesis of triamide and carba analogs resulted in better in vivo stability, but reduced potency.[8]
One of the more extensively investigated cryptophycins is cryptophycin A. It is an antimitotic and antiproliferative agent exhibiting antitumor activity against mammary, colon and pancreatic adenocarcinomas in mice through its interaction with microtubules. Cryptophycin A binds in vitro to tubulin within the vinca domain and inhibits tubulin polymerization, causes tubulin to aggregate and depolymerizes microtubules to linear polymers.[12,13] At low nanomolar concentrations, in the absence of microtubule depolymerization, cryptophycin A effectively stabilized microtubule dynamics by binding reversibly and with high affinity to the ends of microtubules.[2,14] Its actions cause mitotic arrest accompanied by the formation of abnormal mitotic spindles and condensed chromatin without effecting interphase microtubule structures. In addition, cryptophycins overcome a common form of multidrug resitance, P-glycoprotein-mediated efflux, since it is not a substrate for Pglycoprotein. In view of the potency of cryptophycins and the need for analogs that have a higher stability toward in vivo hydrolysis, many synthetic analogs of cryptophycins have been synthesized.[2–5,8–11,15–19] Cryptophycin 52 (6), also known as LY355703, and initially synthesized by Lilly Research Laboratories, is currently under clinical trial phase 2. LY355703 was selected due to its high hydrolytic stability and, nevertheless, very potent activity against tumor cell lines in culture.[20]
The significant clinical potential of the cryptophycins and their relatively low natural abundance has attracted immense interest in their synthesis and structural modification. Several total syntheses and synthetic approaches to cryptophycins and arenastatin A have been described in recent years.[21–40] As part of our interest in the structurefunction studies of cryptophycins, we sought a flexible, enantioselective synthesis of cryptophycin B. Herein we report a convergent and stereocontrolled total synthesis of cryptophycin B and arenastatin A.
Results and Discussion
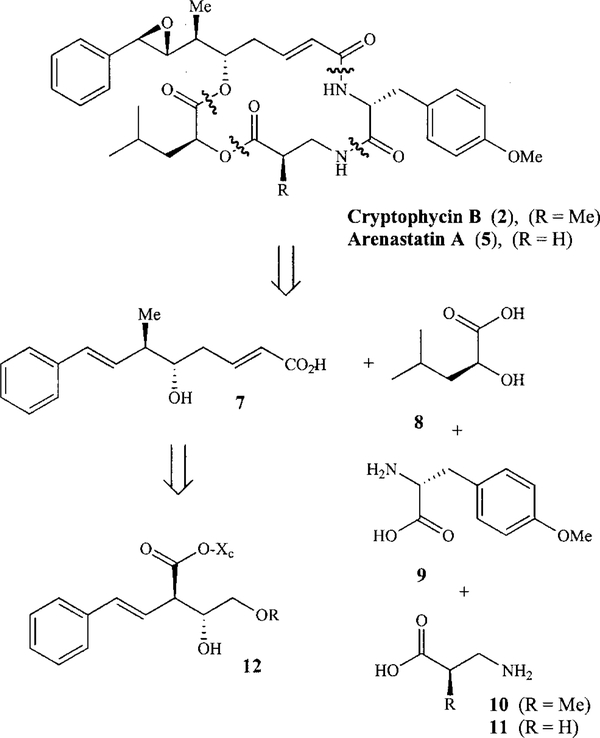
As outlined in Figure 2, we planned the assembly of cryptophycin B and arenastatin A in a convergent manner from octadienoic acid 7, hydroxyisocaproic acid 8, D-tyrosine derivative 9 and β-amino acid 10 or 11, respectively. The fragments would be connected by Yamaguchi esterification and macrolactamization reactions. Introduction of the sensitive epoxide functionality would be carried out at the final stage of the synthesis. The stereocenters on C-5 and C-6 of fragment 7 would derive from syn-aldol adduct 12 generated by means of a titanium enolate mediated aldol reaction.
Figure 2.
Retrosynthetic analysis
In order to set the two stereocenters on C-5 and C-6 of 7, an ester-derived titanium enolate mediated syn-aldol reaction was employed.[41,42] The aldol starting material, ester 14 containing the chiral auxiliary and the styryl moiety, was derived by consecutive tosylation and acylation of (1R,2S)1-aminoindan-2-ol (13) (Scheme 1). Thus, tosylation of the amine with TsCl and 2 equiv. of DMAP in DCM at 0 °C to room temperature for 1 h, followed by esterification with commercially available (E)-4-phenyl-3-butenoic acid and EDCI at ambient temperature for 6 h furnished 98% of indanyl ester 14. Exposure of ester 14 to TiCl4 and iPr2NEt in DCM at 0 °C to room temperature for 1 h to generate the (Z)-enolate and subsequent reaction with 3-(benzyloxy)propionaldehyde at −78 °C for 20 min gave aldol product 15 in 98% yield and 99% de.[41,42] When the enolate of 14 was treated with 3-[(p-methoxybenzyl)oxy]propionaldehyde the desired aldol product could not be obtained possibly due to the instability of the PMB group under the reaction conditions.
Scheme 1.
(a) TsCl, DMAP, CH2Cl2, 0 °C, 1 h, then PhCH = CHCH2CO2H, EDCI, 23 °C, 6 h (98%); (b) TiCl4, iPr2NEt, 0–23 °C, 1 h, then BnO(CH2)2CHO, −78 °C, 20 min (98%); (c) LAH, THF, 0 °C, 1 h (92%); (d) PhLi, THF, −78 °C, 30 min, then TsCl, −20 °C, 30 min, then LAH, 0 °C, 20 min (96%); (e) TIPSOTf, 2,6-lutidine, CH2Cl2, 23 °C, 20 min (99%); (f) BBr3, 0 °C, 5 min (45%); (g) BBr3, K2CO3, CH2Cl2, 0 °C, (83%); (h) PCC, MS (4 Å), CH2Cl2, 23 °C, 10 min (98%); (i) NaH, (EtO)2P(O)CH2CO2Et, THF, 0 °C, 30 min (92%); (j) LiOH, EtOH/H2O (1:1), 23 °C, 2 h (94%); (k) CH2(CO2H)2, Et3N, reflux, 4 h (33%)
The auxiliary was cleaved by reduction of ester 15 with LAH in THF at 0 °C for 1 h. The resulting 1,3-diol was obtained in 92% yield and the auxiliary was also recovered in 92% yield. The primary alcohol was to be converted into the methyl group in a one-pot procedure by tosylation and subsequent reduction with hydride. Thus, treatment of the 1,3-diol with phenyllithium in THF at −78 °C for 30 min, subsequent addition of TsCl at −78 °C and warming of the reaction mixture to −20 °C furnished the primary tosylate in situ. Hydride reduction with LAH at −20 to 0 °C for 20 min gave alcohol 16 in 96% yield. The use of Super Hydride® resulted in comparable yields. The free alcohol was then protected as triisopropylsilyl ether 17 in quantitative yield by treatment of 16 with TIPSOTf and 2,6-lutidine in DCM at room temperature for 20 min.
In order to install the α,β-unsaturated carboxylic acid, the benzyl protecting group had to be removed selectively. Treatment of 17 with lithium or sodium in liquid ammonia partially reduced the styryl double bond and gave the desired primary alcohol only as minor product. The use of alternative benzyl ether cleaving reagents, such as TMSI, FeCl3, AlCl3, BF3·Et2O and DDQ, in a variety of solvents rendered no practical amount of desired product. Only treatment with BBr3 in DCM at 0 °C cleanly removed the benzyl group, but unfortunately, converted the silyl ether into bromide 18 with inversion of configuration (only one isomer isolated). Silyloxy replacement might have been the consequence of the presence of HBr, therefore, in order to reduce the reactivity and acidity of BBr3, treatment of 17 with BBr3 was performed in DCM at 0 °C and in the presence of solid K2CO3. Rewardingly, the selectively unprotected alcohol was obtained in 83% yield. Oxidation to the corresponding aldehyde 19 was accomplished in 98% yield with PCC in DCM in the presence of molecular sieves (4 Å). A HornerEmmons olefination of 19 with the sodium salt of triethyl phosphonoacetate in THF at 0 °C proceeded with 92% yield and rendered (E)-α,β-unsaturated ester 20 as the only isomer. Ester hydrolysis with LiOH in a 1:1 mixture of EtOH/H2O gave octadienoic acid 21 in 94% yield. The conversion of aldehyde 19 to acid 21 could also be achieved in one step by treatment with malonic acid and Et3N in refluxing benzene, but in only 33% yield.
After generation of diene fragment 21, the remaining macrolide sections had to be generated and successively connected. First, commercially available N-Cbz-D-tyrosine (22) was converted into the methyl phenyl ether in 91% by treatment with Me2SO4 and NaOH in refluxing EtOH (Scheme 2).[43] Esterification of the acid with tBuOH was achieved using Yamaguchi’s conditions.[44] Thus, anhydride formation with trichlorobenzoyl chloride in the presence of Hünig’s base and subsequent reaction with tBuOH and DMAP in toluene rendered fully protected D-tyrosine. Removal of the Cbz protection group was performed under hydrogen with 5% Pd/C catalyst in MeOH for 2 h and afforded tyrosine fragment 23 in 95% yield. The coupling of octadienoic acid fragment 21 with 23 was accomplished by exposure to EDCI and DMAP at ambient temperature for 12 h and gave α,β-unsaturated amide 24 in 83% yield. Removal of the silyl ether protecting group with TBAF in THF at room temperature gave alcohol 25 in 99% yield.
Scheme 2.
(a) Me2SO4, NaOH, EtOH/H2O (50:1), reflux, 3 h (91%); (b) 2,4,6-Cl3C6H2COCl, iPr2NEt, THF, 23 °C, 30 min, then tBuOH, DMAP, toluene, 23 °C, 3 h (64%); (c) H2, 5% Pd/C, MeOH, 2 h (95%); (d) 21, EDCI, DMAP, 23 °C, 12 h (83%); (e) TBAF, THF, 23 °C, 6 h (99%)
Next, L-leucic acid (26) was O-benzylated by treatment with Cs2CO3 in MeOH/H2O (1:1) at ambient temperature for 30 min, followed by removal of the solvents and subsequent reaction with benzyl bromide in DMF at 0 °C to room temperature for 12 h (Scheme 3).[45] The synthesis of cryptophycin B required N-Boc-protected 3-amino-2-methylpropionic acid 30, whereas N-Boc-protected β-alanine 31 was required for arenastatin A. Methyl-propionic acid derivative 30 was derived from commercially available methyl (R)-2-methylpropionate (28) in a three-step sequence. The hydroxy group was tosylated by treatment with TsCl, Et3N and DMAP in DCM at 0 °C in 91% yield. Quantitative conversion of the tosylate to N-Boc-protected amine 29 was attained in a one-pot procedure by reaction of the tosylate with NaN3 in DMSO at room temperature and subsequent hydrogenation in the presence of 10% Pd/C and di-tert-butyl dicarbonate for 12 h. Saponification of ester 29 with LiOH in EtOH at room temperature for 30 min led to the free acid in quantitative yield. Acid 30 was then coupled with 27 using DCC and DMAP in DCM at room temperature for 12 h. The diester was obtained in 91% yield and debenzylated by hydrogenation in the presence of 5% Pd/C in EtOAc for 5 h to furnish desired fragment 32 in 95% yield. Fragment 33 for the synthesis of arenastatin A was obtained in two steps from known N-Boc-β-alanine[46] (31) (Scheme 3). Coupling of acid 31 with 27 was accomplished in 91% yield by reaction with DCC and DMAP in DCM at room temperature for 12 h. The resulting diester was debenzylated by hydrogenation in the presence of 10% Pd/C in EtOAc and fragment 33 was obtained in 97% yield.
Scheme 3.
(a) Cs2CO3, MeOH/H2O (5:1), 23 °C, 30 min, then BnBr, DMF, 0 °C, 12 h (quant.); (b) TsCl, Et3N, CH2H2, 0 °C, 1 h (99%); (c) NaN3, DMSO, 23 °C, 6 h; (d) Boc2O, H2, 10% Pd/C, EtOAc, 23 °C, 12 h (quant., 2 steps); (e) LiOH, EtOH, 23 °C, 30 min (quant.); (f) DCC, DMAP, CH2Cl2, 23 °C, 12 h (91%); (g) H2, 5% Pd/C, EtOAc, 23 °C, 5 h (95%); (h) H2, 10% Pd/C, EtOAc 23 °C, 1 h (97%)
With all essential pieces in hand, the macrolides of cryptophycin B and arenastatin A were constructed by linking fragment 25 with fragments 32 and 33, respectively, and subsequent macrolactamization between the D-tyrosine and β-amino acid segments. As summarized in Scheme 4, acid 32 was treated with 2,4,6-trichlorobenzoyl chloride and iPr2NEt in THF at room temperature for 2 h to form the corresponding anhydride. After evaporation of the solvent, the anhydride was treated with fragment 25 and DMAP in benzene at ambient temperature for 1 h. Cryptophycin B precursor 34 was obtained in 84% yield. Treatment of 34 with a solution of 50% TFA in DCM for 1 h rendered the free amine (crude NMR), while the tert-butyl ester was inert to these conditions. Therefore, 34 was converted into cryptophycin D (4) by tert-butyl removal with neat TFA at room temperature for 2 h (crude NMR of the concentrated mixture showed loss of both tert-butyl groups) and subsequent macrolactamization under Yamaguchi conditions by treatment of the resulting amino acid with 2,4,6-trichlorobenzoyl chloride, iPr2NEt and DMAP in benzene at room temperature for 1 h. This one-pot procedure afforded macrolide 4 in 74% yield. Spectroscopic and analytical data are in agreement with those reported { = +36.2 (c = 0.72, MeOH); ref.[2] = +36.7 (c 1.93, MeOH)}. The same coupling-deprotection-macrocyclization procedure was applied to fragment 33 to produce protected amino acid 35 and deoxyarenastatin A (36) in 80% and 81% yield, respectively.
Scheme 4.
(a) 2,4,6-Cl3C6H2COCl, iPr2NEt, THF, 23 °C, 2 h, then 25, DMAP, benzene, 23 °C, 1 h; (b) TFA, 23 °C, 2 h, then 2,4,6-(Cl3)PhCOCl, iPr2NEt, DMAP, THF, 23 °C, 1 h, then benzene, 23 °C, 12 h; (c) Me2C(O)2, CH2Cl2, −30 to 23 °C, 12 h
The epoxidation of the styryl double bond concluded the syntheses of cryptophycin B and arenastatin A. The most reliable and reproducable procedure previously published is the epoxidation with dimethyldioxirane.[21,29] Accordingly, the treatment of cryptophycin D (4) with dimethydioxirane[47] at 30 °C for 2 h and room temperature for 10 h furnished cryptophycin B (2) in 87% yield as a 3:1 mixture of diastereomers (determined by 1H NMR) (Scheme 4). Since both isomers could not be separated by flash column chromatography, clean separation of the major epoxide was accomplished by reversed phase HPLC (YMC-PACK ODAQ 5S 120Å 4.6 × 250 mm, MeOH/H2O, 3:1, 1 mL/ min).[33] Retention times for 2 and its minor isomer were 31.58 and 37.20 min, respectively. Unfortunately, the minor isomer could not be obtained in pure form. Spectroscopic and analytical data for cryptophycin B are in agreement with those reported { = +20.6 (c = 0.24, MeOH); ref.[2] = +20.4 (c = 0.54, MeOH)}. Epoxidation of deoxyarenastatin A (36) under the same conditions afforded arenastatin A (5) in 75% yield as a 3:1 mixture of diastereomers (determined by 1H NMR). Spectroscopic and analytical data for arenastatin A are in agreement with those reported { = + 48.1 (c = 0.09, CHCl3); ref.[33] = +48.7 (c = 0.87, CHCl3)}. In addition to the utilized epoxidation procedure, several other epoxidation protocols were tried. Shi’s fructose-based dioxirane,[48–50] N-sulfonyloxaziridine,[51,52] and Jacobsen’s catalyst[53] did not give a higher selectivity or any epoxy product at all. On the other hand, methyl(trifluoromethyl)dioxirane did epoxidize the olefin with comparable selectivities.[54,55]
The stereochemical outcome of the epoxidation with dimethyldioxirane could be rationalized according to Figure 3. The oxygen atom transfer from dimethyldioxirane to the olefin takes places by means of perpendicular approach of dimethyldioxirane.[56] This orientation of the transition state benefits from a stabilizing interaction of an oxygen lone pair with the π* orbital of the olefin. The sterically demanding isobutyl group is shielding the α-face of the double bond. This would favour an oxygen transfer from the β-face.
Figure 3.
Stereochemical model for epoxidation
Conclusion
In summary, high yielding total syntheses of the antitumor agents cryptophycin B and arenastatin A have been accomplished in a convergent manner. This is the first total synthesis of cryptophycin B. The syntheses utilized a highly stereoselective ester-derived syn-aldol reaction to control the absolute stereochemistry of the octadienoic acid fragment. Selective cleavage of a benzyl ether by use of a Lewis acid was achieved in presence of an acid-sensitive silyl ether group. The assembly of the macrolide fragments was accomplished by Yamaguchi- and Steglich-type esterification and amidation reactions. Ring-closing to the macrolides was performed by Yamaguchi lactamization. A stereoselective epoxidation installed the epoxy moieties of cryptophycin B and arenastatin A. Starting from 13 the overall yields for cryptophycin B and arenastatin A were 20 and 18%, respectively. The present synthesis provides convenient access to structural analogues of cryptophycins which are in great demand, considering the high clinical potential of cryptophycins.
Experimental Section
General:
Melting points are uncorrected. Anhydrous solvents and reagents were obtained as follows: tetrahydrofuran by distillation from sodium and benzophenone, dichloromethane by distillation from CaH2, triethylamine by distillation from CaH2. All other solvents were of HPLC grade. Flash column chromatography was performed with Whatman 240400 mesh silica gel under low pressure (510 psi). Thin-layer chromatography (TLC) was carried out with E. Merck silica gel 60 F-254 plates. All starting materials are commercially available from SigmaAldrich®.
(3E,1′R′,2′S)-1-[(p-Tolylsulfonyl)amino]indan-2′-yl 4-Phenylbut-3-enoate (14):
13 (2.69 g, 17.5 mmol) was dissolved in DCM (200 mL) and the solution was cooled to 0 °C. p-Toluenesulfonyl chloride (3.34 g, 17.5 mmol) and DMAP (4.27 g, 35.0 mmol) were added and the mixture was stirred at room temperature for 1 h. (3E)-4-Phenylbut-3-enoic acid (2.20 mL, 17.5 mmol) and EDCI (3.36 g, 17.5 mmol) were added and after stirring at room temperature for 6 h, the reaction mixture was successively washed with saturated aqueous NH4Cl and NaHCO3. Drying of the organic layer with Na2SO4 and concentration in vacuo yielded 14 (7.75 g, 98%) as a white solid. M.p. 105 °C. = +3.82 (c = 4.30, CHCl3). 1H NMR (CDCl3, 500 MHz): δ = 7.84 (d, 3J = 8.5 Hz, 2 H), 7.36–7.20 (m, 11 H), 6.44 (d, 3J = 16.0 Hz, 1 H), 6.19 (dt, 3J = 16.0, 3J = 7.2 Hz, 1 H), 5.60 (d, 3J= 10.5 Hz, 1 H), 5.18 (ddd, 1 H, 3J = 4.0, 3J = 4.0, 3J = 1.5 Hz), 5.05 (dd, 3J = 10.5, 3J = 5.0 Hz, 1 H), 3.13 (m, 3 H), 2.96 (d, 2J = 17.2 Hz, 1 H), 2.43 (s, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 171.0, 144.3, 140.1, 139.0, 138.3, 137.1, 134.1, 130.4, 129.1, 129.0, 128.1, 127.9, 127.3, 126.7, 125.5, 124.8, 121.6, 75.5, 60.0, 38.3, 37.9, 22.0 ppm. IR (film): = 3282 (br. s), 1738 (s), 1598 (w), 1447 (m), 1435 (s), 1335 (s), 1161 (s), 1093 (s) cm−1. HRMS (FAB): m/z calcd. C26H25NNaO4S [M + Na] 470.1402, found 470.1404. LRMS (FAB): m/z (%) 470.0 (52), 329.0 (27), 277.1 (19), 176.1 (100), 154.1 (68), 136.1 (51), 117.1 (29), 76.9 (25).
(2R,3E,1′S,1′′R,2′′S)-1-[(p-Tolylsulfonyl)amino]indan-2′′-yl 2-[3′-(Benzyloxy)-1′-hydroxypropyl]-4-phenylbut-3-enoate (15):
To a solution of 14 (2.00 g, 4.47 mmol) in DCM (50 mL) was added TiCl4 (1.0 M in DCM, 4.50 mL, 4.47 mmol) at 0 °C. After stirring for 15 min, iPr2NEt (2.57 mL, 14.7 mmol) was added dropwise at 0 °C and the resulting mixture was stirred at room temperature for 1 h. It was cooled to −78 °C and TiCl4 (1.0 M in DCM, 8.10 mL, 8.04 mmol) was added at once. Subsequently, 3-(benzyloxy)propionaldehyde (1.25 mL, 8.04 mmol) was added dropwise over a period of 5 min. After stirring at −78 °C for 20 min, the reaction was quenched with saturated aqueous NH4Cl and the organic layer was dried with Na2SO4. Concentration in vacuo (crude NMR showed > 99% de) and chromatographic purification (20% EtOAc in hexane) yielded 15 (2.69 g, 98%) as a white solid. M.p. 172 °C. = +32.5 (c = 2.83, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.81 (d, 3J = 7.5 Hz, 2 H), 7.387.19 (m, 16 H), 6.47 (d, 3J = 16.0 Hz, 1 H), 6.44 (d, 3J = 10.0 Hz, 1 H), 6.25 (dd, 3J = 16.0, 3J = 9.5 Hz, 1 H), 5.40 (dd, 3J = 4.5, 3J = 4.5 Hz, 1 H), 5.02 (dd, 3J = 10.0, 3J = 5.0 Hz, 1 H), 4.53 (s, 2 H), 4.25 (dd, 3J = 7.0, 3J = 3.7 Hz, 1 H), 3.65 (m, 3 H), 3.19 (dd, 3J = 10.0, 3J = 4.0 Hz, 1 H), 3.11 (dd, 2J = 17.0, 3J = 4.5 Hz, 1 H), 2.94 (d, 2J = 17.0 Hz, 1 H), 2.43 (s, 3 H), 1.82 (m, 1 H), 1.68 (m, 1 H) ppm. 13C NMR (CDCl3, 125 MHz): δ 172.0, 143.9, 140.6, 138.9, 138.6, 138.2, 136.9, 135.1, 130.2, 129.1, 129.0, 128.9, 128.8, 128.4, 128.3, 127.8, 127.4, 126.9, 125.3, 124.9, 123.2, 76.0, 73.8, 72.2, 68.7, 60.3, 56.2, 37.7, 34.6, 22.0 ppm. IR (film): = 3487 (br. s), 3274 (br. s), 3060 (m), 3028 (m), 2922 (m), 2867 (m), 1733 (s), 1598 (w), 1451 (s), 1335 (s), 1160 (s), 1119 (w), 1094 (m), 1032 (m), 972 (m), 814 (m), 748 (s) cm−1. HRMS (FAB): m/z calcd. C36H37NNaO6S [M + Na] 634.2239, found 634.2224. LRMS (FAB): m/z (%) = 634.2 (50), 329.1 (36), 176.1 (100), 154.0 (58), 136.1 (42), 91.1 (49).
(3S,4R,5E)-1-(Benzyloxy)-4-methyl-6-phenylhex-5-en-3-ol (16):
To a solution of 15 (2.62 g, 4.28 mmol) in THF (40 mL) at 0 °C was added LAH (0.34 g, 8.56 mmol). The reaction mixture was stirred for 1 h and aqueous NaHSO4 (2.5 M) was added until the solution turned clear. The organic layer was decanted and the aqueous phase was extracted once again with diethyl ether. The combined ether phases were dried with Na2SO4, concentrated in vacuo and the residue was chromatographically purified (30% EtOAc in hexane) to yield (2S,3S)-5-(benzyloxy)-2-[(E)-styryl]pentane-1,3-diol (1.23 g, 92%) as a white solid. M.p. 69 °C. = +12.7 (c = 0.55, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.447.33 (m, 10 H), 6.52 (d, 3J = 16.0 Hz, 1 H), 6.35 (dd, 3J = 16.0, 3J = 9.5 Hz, 1 H), 4.54 (s, 2 H), 4.19 (d, 3J = 10.5 Hz, 1 H), 3.89 (dd, 3J = 11.0, 3J = 7.0 Hz, 1 H), 3.82 (dd, 3J = 11.0, 3J = 6.0 Hz, 1 H), 3.74 (m, 1 H), 3.70 (m, 1 H), 3.58 (br. s, 2 H), 2.47 (m, 1 H), 1.93 (m, 1 H), 1.73 (m, 1 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 138.4, 137.6, 133.9, 129.0, 128.9, 128.2, 128.1, 127.8, 127.0, 126.7, 73.7, 72.4, 69.4, 65.0, 51.1, 35.0 ppm. IR (film): = 3393 (br. s), 3060 (w), 3027 (w), 2923 (m), 2869 (m), 1599 (w), 1452 (m), 1364 (m), 1093 (s), 1028 (m), 971 (m), 749 (s) cm−1. HRMS (CI): m/z calcd. C20H25O3 [M + H] 313.1804, found 313.1809. LRMS (CI): m/z (%) = 330.2 (75), 313.2 (100). Phenyllithium (1.8 M in cyclohexane/ diethyl ether, 3.90 mL, 7.04 mmol) was added dropwise to a solution of (2S,3S)-5-(benzyloxy)-2-[(E)-styryl]pentane-1,3-diol (2.20 g, 7.04 mmol) in THF (60 mL) at =78 °C. The reaction mixture was stirred at =78 °C for 30 min and TsCl (1.34 g, 7.04 mmol) was added in one portion. The reaction mixture was allowed to warm to =20 °C over a period of 30 min and was subsequently treated with LAH (0.85 g, 21.1 mmol). After 20 min, the reaction was quenched and the mixture washed with saturated aqueous NH4Cl, dried with Na2SO4 and concentrated in vacuo. The residue was chromatographically purified (20% EtOAc in hexane) to give 16 (2.01 g, 96%) as a colorless oil. = +25.2 (c = 6.61, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.417.20 (m, 10 H), 6.47 (d, 3J = 16.0 Hz, 1 H), 6.27 (dd, 3J = 16.0, 3J = 8.5 Hz, 1 H), 4.57 (s, 2 H), 3.843.70 (m, 3 H), 2.70 (br. s, 1 H), 2.45 (dd, 3J = 6.5, 3J = 1.5 Hz, 1 H), 1.83 (m, 2 H), 1.19 (d, 3J = 6.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 138.4, 137.9, 132.5, 131.2, 128.9, 128.2, 128.1, 127.5, 126.6, 126.7, 75.1, 73.8, 69.7, 43.9, 34.3, 16.9 ppm. IR (film): = 3448 (br. s), 3060 (m), 3027 (m), 2961 (m), 2926 (m), 2869 (m), 1595 (m), 1495 (m), 1453 (s), 1364 (m), 1095 (s), 1028 (m), 970 (m), 748 (s) cm−1. HRMS (CI): m/z calcd. C20H25O2 [M + H] 297.1855, found 297.1846. LRMS (CI): m/z (%) = 314.2 (100), 297.3 (81).
(1S,2R,3E)-({1-[2′-(Benzyloxy)ethyl]-2-methyl-4-phenylbut-3-enyl}-oxy)triisopropylsilane (17):
To a solution of 16 (1.60 g, 5.40 mmol) and 2,6-lutidine (1.26 mL, 10.8 mmol) in DCM (40 mL) was added TIPSOTf (2.18 mL, 8.10 mmol). The resulting solution was stirred at room temperature for 20 min, concentrated in vacuo and chromatographically purified (10% EtOAc in hexanes) to furnish 17 (2.42 g, 99%) as a colorless oil. = +41.8 (c = 3.20, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.427.35 (m, 9 H), 7.28 (t, 3J = 7.1 Hz, 1 H), 6.44 (d, 3J = 16.0 Hz, 1 H), 6.30 (dd, 3J = 16.0, 3J = 7.5 Hz, 1 H), 4.56 (q, 3J = 12.5 Hz, 2 H), 4.15 (m, 1 H), 3.64 (t, 3J = 7.0 Hz, 2 H), 2.63 (m, 1 H), 1.90 (m, 2 H), 1.24 (d, 3J = 7.0 Hz, 3 H), 1.18 (s, 21 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 139.0, 138.2, 133.0, 130.3, 128.9, 128.8, 128.1, 127.9, 127.4, 126.5, 73.9, 73.4, 67.9, 43.3, 34.4, 18.8, 15.6, 13.4 ppm. IR (film): = 3060 (m), 3027 (m), 2943 (s), 2866 (s), 1596 (m), 1493 (m), 1463 (s), 1365 (m), 1263 (m), 1104 (s), 1068 (m), 968 (m), 883 (m), 747 (m) cm−1. HRMS (FAB): m/z calcd. C29H44O2NaSi [M + Na+] 475.3008, found 475.2994. LRMS (FAB): m/z (%) = 321.3 (20), 215.2 (42), 157.2 (60), 131.2 (78), 115.2 (72), 91.1 (100), 73.0 (86), 60.9 (67).
(3R,4R,5E)-3-Bromo-4-methyl-6-phenylhex-5-en-1-ol (18):
A solution of 17 (30.0 mg, 66.3 μmol) in DCM (2 mL) was cooled to 0°C. BBr3 (1.0 M in DCM, 100 μL, 99.5 μmol) was added dropwise and after 5 min of stirring at 0 °C, the reaction was quenched and the mixture washed with saturated aqueous NaHCO3, dried with Na2SO4 and concentrated in vacuo. Chromatographic purification (10% EtOAc in hexanes) of the residue furnished 18 (8.0 mg, 45%) as a colorless oil. = +40.5 (c = 0.85, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.40 (d, 3J = 9.0 Hz, 2 H), 7.33 (dd, 3J = 10.5, 3J = 9.0 Hz, 2 H), 7.26 (d, 3J = 8.0 Hz, 1 H), 6.50 (d, 3J = 20.0 Hz, 1 H), 6.13 (dd, 3J = 20.0, 3J = 11.0 Hz, 1 H), 4.32 (dtd, 1 H, 3J = 8.5, 3J = 8.5, 3J = 3.5 Hz), 3.76 (m, 1 H), 3.67 (m, 1 H), 2.50 (qd, 3J = 8.5, 3J = 3.5 Hz, 1 H), 1.901.84 (m, 2 H), 1.70 (d, 3J = 8.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 137.3, 134.0, 129.2, 129.0, 128.0, 126.7, 60.8, 56.8, 47.4, 37.1, 24.7 ppm.
(3S,4R,5E)-4-Methyl-6-phenyl-3-[(triisopropylsilanyl)oxy]hex-5-enal (19):
To a solution of 17 (1.30 g, 2.87 mmol) in DCM (50 mL) was added K2CO3 (1.00 g). The resulting mixture was stirred at room temperature for 20 min and was then cooled to 0 °C. BBr3 (1.0 M in DCM, 3.12 mL, 3.12 mmol) was added dropwise and after 5 min of stirring at 0 °C, the reaction was quenched and the mixture washed with saturated aqueous NaHCO3, dried with Na2SO4 and concentrated in vacuo. Chromatographic purification (10% EtOAc in hexanes) of the residue furnished (3S,4R,5E)-4-methyl-6-phenyl-3-[(triisopropylsilanyl)oxy]hex-5-en-1-ol (865 mg, 83%) as a colorless oil. = +33.3 (c = 0.63, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.37 (d, 3J = 8.5 Hz, 2 H), 7.33 (dd, 3J = 10.5, 3J = 9.0 Hz, 2 H), 7.23 (dd, 3J = 8.5, 3J = 8.5 Hz, 1 H), 6.42 (d, 3J = 16.0 Hz, 1 H), 6.21 (dd, 3J = 16.0, 3J = 7.5 Hz, 1 H), 4.12 (m, 1 H), 3.80 (t, 3J = 6.5 Hz, 2 H), 2.65 (m, 1 H), 1.79 (m, 2 H), 1.19 (d, 3J = 7.0 Hz, 3 H), 1.13 (s, 21 H) ppm. 13C NMR (CDCl3, 125 MHz): δ 138.0, 132.9, 130.4, 128.9, 127.5, 126.4, 75.0, 61.0, 43.2, 35.9, 18.6, 14.9, 13.4 ppm. IR (film): = 3352 (br. s), 3025 (m), 2943 (s), 2866 (s), 1600 (w), 1463 (s), 1383 (s), 1246 (m), 1102 (s), 1061 (s), 968 (m), 883 (s), 748 (m) cm−1. HRMS (CI): m/z calcd. C22H39O2Si [M = H] 363.2719, found 363.2693. LRMS (CI): m/z (%) = 363.4 (43), 206.2 (52), 189.2 (100), 171.2 (30). To a solution of (3S,4R,5E)-4-methyl-6-phenyl-3-[(triisopropylsilanyl)oxy]hex-5-en-1-ol (800 mg, 2.21 mmol) in DCM (100 mL) was added molecular sieves (4 Å) (2.50 g). The resulting mixture was stirred at room temperature for 10 min and PCC (713 mg, 3.31 mmol) was added portionwise. After 10 min of stirring at room temperature, the reaction mixture was filtered through a pad of Celite© and the resulting filtrate was concentrated in vacuo. Chromatographic purification (50% diethyl ether in hexanes) of the residue afforded 19 (781 mg, 98%) as a colorless oil. = +27.1 (c = 1.62, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 9.85 (t, 3J = 2.0 Hz, 1 H), 7.387.32 (m, 4 H), 7.26 (dd, 3J = 8.5, 3J = 8.5 Hz, 1 H), 6.42 (d, 3J = 16.0 Hz, 1 H), 6.15 (dd, 3J = 16.0, 3J = 7.5 Hz, 1 H), 4.50 (td, 3J = 6.0, 3J 4.0 Hz, 1 H), 2.66 (m, 1 H), 2.61 (td, 3J = 4.0, 3J = 2.0 Hz, 2 H), 1.20 (d, 3J = 7.0 Hz, 3 H), 1.11 (s, 21 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 202.3, 137.7, 131.8, 131.3, 129.0, 127.7, 126.5, 72.0, 48.4, 43.8, 18.6, 14.8, 13.1 ppm. IR (film): = 3027 (w), 2943 (s), 2867 (s), 1725 (s), 1601 (w), 1463 (s), 1384 (m), 1284 (m), 1100 (s), 1068 (s), 883 (s), 749 (m) cm−1. HRMS (CI): m/z calcd. C22H37O2Si [M + H+] 361.2563, found 361.2570. LRMS (CI): m/z (%) = 393.4 (100) [M + Na+], 309.2 (83), 192.3 (62), 75.1 (74).
(2E,5S,6R,7E)-Ethyl 6-Methyl-8-phenyl-5-[(triisopropylsilanyl)oxy]octa-2,7-dienoate (20):
To a dispersion of NaH (60% dispersion in mineral oil, 306 mg, 12.8 mmol) in THF (15 mL) was added triethyl phosphonoacetate (633 μL, 3.19 mmol) and the resulting mixture was stirred at room temperature for 10 min. Then, the reaction mixture was cooled to 0 °C and 19 (1.15 g, 3.19 mmol) in THF (10 mL) was added. After stirring at 0 °C for 10 min, the reaction was quenched with saturated aqueous NH4Cl and the organic phase was dried with Na2SO4. Concentration in vacuo and chromatographic purification (5% EtOAc in hexane) of the residue yielded 20 (1.27 mg, 92%) as a single isomer by 1H NMR and as colorless oil. = +73.7 (c = 2.78, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.39 (d, 3J = 7.5 Hz, 2 H), 7.34 (dd, 3J = 8.0, 3J = 7.5 Hz, 2 H), 7.26 (dd, 3J = 8.0, 3J = 8.0 Hz, 1 H), 7.03 (dt, 3J = 16.0, 3J = 8.0 Hz, 1 H), 6.43 (d, 3J = 16.0 Hz, 1 H), 6.24 (dd, 3J = 16.0, 3J = 7.5 Hz, 1 H), 5.89 (d, 3J = 16.0 Hz, 1 H), 4.22 (q, 3J = 7.0 Hz, 2 H), 4.04 (m, 1 H), 2.59 (m, 1 H), 2.47 (m, 2 H), 1.31 (t, 3J = 7.0 Hz, 3 H), 1.21 (d, 3J = 7.0 Hz, 3 H), 1.14 (s, 21 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 166.8, 146.4, 138.0, 132.2, 130.9, 128.9, 127.5, 126.5, 123.7, 75.9, 60.6, 43.3, 38.0, 18.7, 16.2, 14.7, 13.3 ppm. IR (film): = 3026 (w), 2943 (s), 2867 (s), 1723 (s), 1655 (m), 1600 (w), 1463 (s), 1367 (m), 1264 (m), 1169 (m), 1101 (s), 1045 (s), 882 (s), 747 (m) cm−1. HRMS (FAB): m/z calcd. C26H42O3NaSi [M + Na+] 453.2801, found 453.2783. LRMS (FAB): m/z (%) = 430.3 (10), 387.3 (39), 317.3 (33), 299.3 (95), 131.2 (65), 73.0 (75), 59.0 (100).
(2E,5S,6R,7E)-6-Methyl-8-phenyl-5-[(triisopropylsilanyl)oxy]octa2,7-dienonic Acid (21). From Ester 20:
A mixture of 20 (1.10 g, 2.55 mmol) and LiOH·H2O (320 mg, 7.66 mmol) in EtOH (20 mL) and H2O (20 mL) was vigorously stirred at room temperature for 2 h. After the reaction was quenched with saturated aqueous NH4Cl, the water layer was acidified with concentrated HCl (pH = 3) and extracted with DCM. The organic layer was dried with Na2SO4, concentrated in vacuo and chromatographically purified (25% EtOAc in hexane) to yield 21 (954 mg, 94%) as a white solid. From Aldehyde 19: A solution of 19 (160 mg, 0.42 mmol) and malonic acid (44.0 mg, 0.42 mmol) in Et3N (0.5 mL) and benzene (3 mL) was heated under reflux for 4 h. The reaction mixture was taken up with diethyl ether, washed with 20% aqueous HCl and subsequently dried with Na2SO4. Concentration in vacuo and chromatographic purification yielded 21 (60 mg, 33%) as a white solid. M.p. 88 °C. = +83.8 (c = 7.20, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.39 (d, 3J = 7.5 Hz, 2 H), 7.34 (dd, 3J = 8.0, 3J = 7.5 Hz, 2 H), 7.26 (dd, 3J = 8.0, 3J = 8.0 Hz, 1 H), 7.15 (dt, 3J = 16.0, 3J = 8.0 Hz, 1 H), 6.43 (d, 3J = 16.0 Hz, 1 H), 6.23 (dd, 3J = 16.0, 3J = 7.5 Hz, 1 H), 5.90 (d, 3J = 16.0 Hz, 1 H), 4.05 (m, 1 H), 2.57 (m, 1 H), 2.49 (m, 2 H), 1.20 (d, 3J = 7.0 Hz, 3 H), 1.14 (s, 21 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 172.2, 149.5, 137.9, 132.1, 131.0, 128.9, 127.5, 126.5, 123.0, 75.8, 43.4, 38.1, 18.6, 16.2, 13.2 ppm. IR (film): = 3025 (w), 2943 (s), 2866 (s), 1698 (s), 1652 (s), 1463 (m), 1420 (m), 1103 (s), 882 (s), 747 (m) cm−1. HRMS (FAB): m/z calcd. C24H38NaO3Si [M + Na+] 425.2488, found 425.2502. LRMS (FAB): m/z (%) = 447.2 (54), 425.2 (20) [M + Na+], 131.1 (34), 116.1 (38), 87.0 (50), 73.5 (84), 70.2 (100).
(R)-tert-Butyl 2-amino-3-(4-methoxyphenyl)propionate (23):
A mixture of 22 (1.00 g, 3.17 mmol), Me2SO4 (1.38 mL, 14.3 mmol), NaOH (960 mg, 23.8 mmol), EtOH (50 mL) and H2O (1 mL) was vigorously stirred under reflux for 3 h. After the reaction was quenched with saturated aqueous NH4Cl, the water layer was acidified with concentrated HCl (pH = 3) and extracted with diethyl ether. The organic layer was washed with brine, dried with Na2SO4 and concentrated in vacuo to yield (R)-2-{[(benzyloxy)-carbonyl]amino}−3-(4-methoxyphenyl)propionic acid (950 mg, 91%) as a white solid. M.p. 111–112 °C. = −37.8 (c = 1.66, CHCl3). 1H NMR (CDCl3, 500 MHz): δ = 7.38 (m, 5 H), 7.09 (d, 3J = 8.5 Hz, 2 H), 6.85 (d, 3J = 8.5 Hz, 2 H), 5.22 (d, 3J = 8.0 Hz, 1 H), 5.13 (d, 3J = 6.0 Hz, 2 H), 4.68 (dd, 3J = 8.5, 3J = 6.0 Hz, 1 H), 3.80 (s, 3 H), 3.17 (dd, 2J = 14.0, 3J 5.5 Hz, 1 H), 3.09 (dd, 2J = 14.0, 3J = 6.0 Hz, 1 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 176.6, 159.2, 156.3, 136.5, 130.8, 129.0, 128.7, 128.6, 127.7, 114.5, 67.6, 55.6, 55.1, 37.3 ppm. IR (film): = 3330 (br. s), 1715 (s), 1514 (s), 1248 (s), 1057 (m), 1036 (m), 738 (m) cm−1. HRMS (CI): m/z calcd. C18H20NO5 [M = H+] 330.1341, found 330.1351. LRMS (CI): m/z (%) = 347.2 (100) [M + NH4+], 330.2 (40) [M + H+], 286.2 (43), 196.1 (23). To a solution of (R)-2-{[(benzyloxy)carbonyl]amino}−3-(4-methoxyphenyl)propionic acid (1.27 g, 3.87 mmol) in THF (25 mL) were successively added iPr2NEt (674 μL, 3.87 mmol) and 2,4,6-trichlorobenzyl chloride (604 μL, 3.87 mmol). After 30 min of stirring, the solvent was evaporated and the residue was dissolved in toluene (20 mL). tBuOH (740 μL, 7.74 mmol) and DMAP (1.89 g, 15.5 mmol) were added and the mixture was stirred for a further 3 h. The reaction mixture was entirely transferred onto a column and chromatographically purified (25% EtOAc in hexane) to yield (R)-tert-butyl 2-{[(benzyloxy)carbonyl]amino}−3-(4-methoxyphenyl)propionate (946 mg, 64%) as a colorless oil. = −17.9 (c = 9.70, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.38 (m, 5 H), 7.09 (d, 3J = 8.5 Hz, 2 H), 6.83 (d, 3J = 8.5 Hz, 2 H), 5.26 (d, 3J = 8.0 Hz, 1 H), 5.13 (d, 3J = 6.0 Hz, 2 H), 4.52 (dd, 3J = 8.5, 3J 6.5 Hz, 1 H), 3.81 (s, 3 H), 3.06 (t, 3J = 5.0 Hz, 2 H), 1.44 (s, 9 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 171.1, 159.0, 156.0, 136.8, 130.9, 128.9, 128.9, 128.5, 128.4, 114.2, 82.6, 67.2, 55.7, 55.6, 37.9, 28.4 ppm. IR (film): = 3343 (br. s), 2977 (m), 2934 (w), 2835 (w), 1723 (s), 1613 (m), 1513 (s), 1368 (m), 1249 (s), 1155 (s), 1056 (m), 1038 (m), 740 (m) cm−1. HRMS (FAB): m/z calcd. C22H27NNaO5 [M + Na+] 408.1787, found 408.1796. LRMS (FAB): m/z (%) = 386.2 (14) [M H], 330.1 (81), 286.1 (95), 234.1 (82), 178.1 (28), 154.1 (34), 121.1 (79), 91.0 (100), 60.2 (50). A mixture of (R)-tertbutyl 2-{[(benzyloxy)carbonyl]amino}−3-(4-methoxyphenyl)propionate (640 mg, 1.66 mmol), 5% Pd/C (20 mg) and MeOH (20 mL) was stirred for 2 h under H2 and filtered through a pad of Celite®. Concentration of the filtrate in vacuo yielded 23 (406 mg, 95%) as a white solid. M.p. 176 °C (gas emission and formation of new solid). = +71.3 (c 0.08, CHCl3). 1H NMR (CDCl3, 500 MHz): δ = 7.14 (d, 3J = 8.5 Hz, 2 H), 6.86 (d, 3J = 8.5 Hz, 2 H), 3.80 (s, 3 H), 3.58 (dd, 3J = 7.5, 3J 5.5 Hz, 1 H), 3.00 (dd, 2J = 14.0, 3J = 5.5 Hz, 1 H), 2.81 (dd, 2J = 14.0, 3J = 8.0 Hz, 1 H), 1.68 (br. s, 2 H), 1.46 (s, 9 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 174.7, 158.8, 130.8, 129.8, 114.3, 81.6, 56.8, 55.7, 40.6, 28.4 ppm. HRMS (CI): m/z calcd. C14H22NO3 [M + H+] 252.1600, found 252.1598. LRMS (FAB): m/z = 252.3 (100) [M + H+], 196.1 (35).
(2R,2′E,5′S,6′R,7′E)-tert-Butyl 3-(4-Methoxyphenyl)-2-({6′-methyl-8′-phenyl-5′-[(triisopropylsilanyl)oxy]octa-2′,7′-dienoyl}amino)-propionate (24):
A solution of 21 (700 mg, 1.74 mmol), 23 (437 mg, 1.74 mmol), DMAP (105 mg, 0.87 mmol) and EDCI (334 mg, 1.74 mmol) was stirred at room temperature for 12 h. The reaction mixture was quenched with saturated aqueous NH4Cl and the organic phase was dried with Na2SO4. Concentration in vacuo and chromatographic purification of the residue (15% EtOAc in hexane) yielded 24 (931 mg, 83%) as a white solid. M.p. 6465 °C. = +29.9 (c = 0.87, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ = 7.347.26 (m, 4 H), 7.22 (dd, 3J = 8.0, 3J = 8.0 Hz, 1 H), 7.04 (d, 3J = 8.4 Hz, 2 H), 6.83 (dt, 3J = 16.0, 3J = 8.0 Hz, 1 H), 6.80 (d, 3J = 8.4 Hz, 2 H), 6.36 (d, 3J = 16.0 Hz, 1 H), 6.22 (dd, 3J = 16.0, 3J = 8.0 Hz, 1 H), 5.87 (d, 3J = 7.6 Hz, 1 H), 5.78 (d, 3J = 16.0 Hz, 1 H), 4.80 (ddd, 1 H, 3J = 5.6, 3J = 4.0, 3J = 1.6 Hz), 3.96 (m, 1 H), 3.77 (s, 3 H), 3.07 (d, 3J = 5.6 Hz, 1 H), 2.51 (qdd, 1 H, 3J = 7.2, 3J = 8.0, 3J = 7.3, 3J = 2.6 Hz), 2.40 (m, 2 H), 1.43 (s, 9 H), 1.15 (d, 3J = 6.8 Hz, 3 H), 1.09 (s, 21 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 171.2, 165.2, 159.0, 142.1, 138.1, 132.3, 131.0, 130.8, 128.9, 128.5, 127.4, 126.5, 125.7, 114.2, 82.8, 76.0, 55.6, 53.9, 42.9, 38.2, 37.5, 28.4, 18.7, 17.1, 13.3 ppm. IR (film): = 3299 (br. m), 2942 (s), 2866 (s), 1733 (s), 1671 (s), 1513 (s), 1463 (m), 1367 (s), 1249 (s), 1155 (s), 1109 (m), 1036 (s), 882 (m), 749 (m) cm−1. HRMS (FAB): m/z calcd. C38H57NNaO5Si [M + Na+] 658.3904, found 658.3912. LRMS (FAB): m/z (%) = 658.4 (82) [M + Na+], 602.4 (45), 176.1 (62), 122.1 (53), 116.1 (52), 88.0 (53), 60.9 (100).
(2R,2′E,5′S,6′R,7′E)-tert-Butyl 2-[(5′-Hydroxy-6′-methyl-8′-phenylocta-2′,7′-dienoyl)amino]-3-(4-methoxyphenyl)propionate (25):
A solution of 24 (440 mg, 0.69 mmol) and TBAF (690 μL, 0.69 mmol) in THF (30 mL) was stirred at room temperature for 6 h. The reaction mixture was concentration in vacuo, the residue was redissolved in DCM and filtered through a short pad of MgSO4. Evaporation of solvent yielded 25 (329 mg, 99%) as a highly viscous oil. = −13.3 (c = 0.15, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ = 7.37 (d, 3J = 7.4 Hz, 2 H), 7.31 (dd, 3J = 7.4, 3J = 7.4 Hz, 2 H), 7.25 (dd, 3J = 7.8, 3J = 7.8 Hz, 1 H), 7.06 (d, 3J = 8.4 Hz, 2 H), 6.85 (dt, 3J = 16.0, 3J = 8.0 Hz, 1 H), 6.80 (d, 3J = 8.4 Hz, 2 H), 6.48 (d, 3J = 16.0 Hz, 1 H), 6.12 (dd, 3J = 16.0, 3J = 8.0 Hz, 1 H), 5.91 (d, 3J = 7.2 Hz, 1 H), 5.82 (d, 3J = 16.0 Hz, 1 H), 4.80 (ddd, 1 H, 3J = 5.6, 3J = 4.0, 3J = 1.6 Hz), 3.77 (s, 3 H), 3.65 (m, 1 H), 3.08 (d, 3J = 6.6 Hz, 1 H), 2.462.33 (m, 3 H), 1.42 (s, 9 H), 1.14 (d, 3J = 6.8 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 171.1, 165.2, 159.0, 141.7, 137.5, 132.3, 131.4, 131.0, 129.0, 128.5, 127.8, 126.6, 126.3, 114.2, 82.8, 74.2, 55.6, 54.0, 43.6, 37.3, 37.5, 28.4, 17.3 ppm. IR (film): = 3340 (br. s), 2960 (m), 2925 (s), 2853 (s), 1730 (s), 1670 (s), 1635 (s), 1513 (s), 1457 (m), 1368 (s), 1249 (s), 1154 (s), 1036 (m), 751 (w) cm−1. HRMS (FAB): m/z calcd. C29H37NNaO5 [M + Na+] 502.2569, found 502.2558. LRMS (FAB): m/z (%) = 446.3 (36), 413.4 (25), 176.1 (100), 91.9 (30), 59.1 (29).
(S)-Benzyl 2-Hydroxy-4-methylpentanoate (27):
[45] To a solution of 26 (2.77 g, 21.0 mmol) in MeOH (40 mL) and H2O (8 mL) was added Cs2CO3 (3.42 g, 10.5 mmol). After stirring at room temperature for 30 min, the reaction mixture was concentrated to dryness and the residue was redissolved in DMF. The solution was cooled to 0 °C, benzyl bromide (2.5 mL, 20 mmol) was added and the resulting mixture was stirred at room temperature for 12 h. The reaction was quenched with saturated aqueous NH4Cl, extracted with EtOAc and the organic layer was dried with Na2SO4. Evaporation of solvent yielded 27 (4.32 g, quant.) as colorless oil. = −15.5 (c= 1.02, CHCl3) {ref.[45] = −15.2 (c = 2.96, CHCl3)}. 1H NMR (CDCl3, 500 MHz): δ = 7.40 (m, 5 H), 5.24 (s, 2 H), 4.27 (dd, 3J = 8.0, 3J = 5.0 Hz, 1 H), 2.62 (br. s, 1 H), 1.92 (m, 1 H), 1.60 (m, 2 H), 0.97 (d, 3J = 5.5 Hz, 3 H), 0.96 (d, 3J = 5.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 176.2, 135.6, 129.1, 129.0, 128.7, 69.6, 67.7, 43.8, 24.8, 23.7, 22.0 ppm.
(R)-Methyl 3-{[(tert-Butoxy)carbonyl]amino}−2-methylpropionate (29):
To a solution of 28 (2.55 g, 21.6 mmol) in DCM (50 mL) were added TsCl (4.12 g, 21.6 mmol), Et3N (3.01 mL, 21.6 mmol) and DMAP (1.32 g, 10.8 mmol) at 0 °C. After stirring at room temperature for 1 h, the reaction was quenched with saturated aqueous NH4Cl and the organic layer was dried with Na2SO4. Evaporation of the solvent furnished (R)-methyl 2-methyl-3-[(p-tolylsulfonyl)oxy]propionate (5.87 g, 99%) as a colorless oil. = −3.75 (c = 6.42, CHCl3). 1H NMR (CDCl3, 300 MHz): δ = 7.70 (d, 2 H), 7.28 (d, 2 H), 4.11 (dd, 3J = 9.3, 3J = 6.9 Hz, 1 H), 3.98 (dd, 3J = 9.6, 3J = 6.3 Hz, 1 H), 3.56 (s, 3 H), 2.73 (dq, 3J = 6.6, 3J = 6.0 Hz, 1 H), 2.37 (s, 3 H), 1.10 (d, 3J = 6.0 Hz, 3 H) ppm. 13C NMR (CDCl3, 75 MHz): δ = 173.0, 144.9, 132.7, 129.8, 127.9, 70.8, 52.0, 39.2, 21.6, 13.6 ppm. IR (film): = 2986 (m), 2954 (m), 1741 (s), 1598 (m), 1460 (m), 1363 (s), 1179 (s), 1097 (m), 977 (s), 818 (s) cm−1. HRMS (CI): m/z calcd. C12H20NO5S [M + NH4+] 290.1062, found 290.1055. LRMS (CI): m/z (%) = 290.2 (100) [M NH4]. A solution of (R)-methyl 2-methyl-3-[(p-tolylsulfonyl)oxy]propionate (710 mg, 2.61 mmol) and NaN3 (190 mg, 2.87 mmol) in DMSO (20 mL) was stirred at room temperature for 6 h. EtOAc (100 mL) and H2O (100 mL) were added, the organic layer was dried with Na2SO4 and concentrated in vacuo. The residue was dissolved in EtOAc (50 mL) and di-tert-butyl dicarbonate (0.60 mL, 2.61 mmol) and 10% Pd/C (25 mg) were added. The resulting mixture was stirred under H2 for 12 h and subsequently filtered through a pad of Celite©. Evaporation of the solvent and chromatographic purification (20% EtOAc in hexane) gave 29 (600 mg, quant.) as a colorless oil. = −17.6 (c = 2.74, CHCl3). 1H NMR (CDCl3, 300 MHz): δ = 4.90 (br. m, 1 H), 3.60 (s, 3 H), 3.19 (m, 2 H), 2.60 (dq, 3J = 6.9, 3J = 6.0 Hz, 1 H), 1.34 (s, 9 H), 1.08 (d, 3J = 7.2 Hz, 3 H) ppm. 13C NMR (CDCl3, 75 MHz): δ = 175.7, 156.9, 79.2, 51.8, 42.9, 40.0, 28.3, 14.7 ppm. IR (film): = 3381 (br. s), 2978 (s), 1716 (s), 1518 (s), 1367 (s), 1250 (s), 1175 (s) cm−1. HRMS (CI): m/z calcd. C10H20NO4 [M + H+] 218.1392, found 218.1385. LRMS (CI): m/z (%) 218.2 (100) [M + H+], 179.2 (40), 162.1 (81), 118.2 (27).
(R)-3-{[(tert-Butoxy)carbonyl]amino}−2-methylpropionic Acid (30):
A solution of 29 (2.62 g, 12.1 mmol) and LiOH·H2O (1.50 g, 36.3 mmol) in EtOH (50 mL) was stirred at room temperature for 30 min. The reaction mixture was acidified (pH = 4) with 10% aqueous citric acid and extracted with EtOAc. The organic layer was washed with brine, dried with Na2SO4 and concentrated in vacuo to furnish 30 (2.46 g, quant.) as a colorless oil. = −25.5 (c = 1.41, CHCl3). 1H NMR (CDCl3, 500 MHz): δ = 5.07 (br. m, 1 H), 3.38 (m, 1 H), 3.26 (m, 1 H), 2.70 (m, 1 H), 1.45 (s, 9 H), 1.22 (d, 3J = 7.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 181.4, 156.5, 81.5, 43.1, 40.4, 28.8, 15.0 ppm. IR (film): = 3380 (br. s), 2979 (s), 1708 (s), 1518 (s), 1368 (m), 1252 (m), 1170 (s) cm−1. HRMS (CI): m/z calcd. C9H18NO4 [M + H+] 204.1236, found 204.1239. LRMS (CI): m/z (%) = 204.2 (35) [M + H+], 165.2 (40), 148.2 (58), 104.1 (22).
(2S,2′R)-2-[(3′-{[(tert-Butoxy)carbonyl]amino}−2′-methylpropionyl)oxy]-4-methylpentanoic Acid (32):
A solution of 27 (2.50 g, 12.1 mmol), 30 (2.46 g, 12.1 mmol), DCC (2.50 g, 12.1 mmol) and DMAP (740 mg, 6.05 mmol) in DCM (50 mL) was stirred at room temperature for 12 h. The reaction was quenched with saturated aqueous NH4Cl and the organic layer was dried with Na2SO4. Evaporation of the solvent and chromatographic purification (10% EtOAc in hexane) afforded (2S,2R)-benzyl 2-[(3-{[(tertbutoxy)carbonyl]amino}−2-methylpropionyl)oxy]-4methylpentanoate (1.83 g, 91%) as colorless film. = −49.7 (c = 1.50, CHCl3). 1H NMR (CDCl3, 400 MHz): δ = 7.35 (m, 5 H), 5.21–5.10 (m, 4 H), 3.43 (ddd, 2J = 12.0, 3J = 6.5, 3J = 5.5 Hz, 1 H), 3.17 (ddd, 2J = 12.0, 3J = 6.0, 3J = 5.5 Hz, 1 H), 2.76 (m, 1 H), 1.821.60 (m, 3 H), 1.43 (s, 9 H), 1.17 (d, 3J = 7.5 Hz, 3 H), 0.94 (d, 3J = 6.4 Hz, 3 H), 0.91 (d, 3J = 6.4 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 175.2, 171.1, 156.4, 135.6, 129.0, 128.9, 128.7, 79.7, 71.3, 67.5, 43.5, 40.8, 39.9, 28.8, 25.1, 23.4, 21.9, 14.9 ppm. IR (film): = 3394 (br. s), 2962 (s), 1741 (s), 1718 (s), 1508 (s), 1456 (m), 1367 (m), 1252 (m), 1173 (s) cm−1. HRMS (FAB): m/z calcd. C21H32NNaO6 [M + Na+] 430.2206, found 430.2183. LRMS (FAB): m/z (%) = 352.1 (19), 308.1 (85), 130.1 (23), 90.9 (100), 60.2 (32). A mixture of (2S,2R)-benzyl 2-[(3-{[(tert-butoxy)carbonyl]amino}−2-methylpropionyl)oxy]-4-methylpentanoate (2.50 g, 6.13 mmol), 5% Pd/C (200 mg) and EtOAc (40 mL) was stirred for 5 h under H2 and then filtered through a pad of Celite®. Concentration of the filtrate in vacuo yielded 32 (1.84 g, 95%) as a white solid. M.p. 72 °C. = −47.9 (c = 4.70, CHCl3). 1H NMR (CDCl3, 500 MHz): δ = 5.20 (br. t, 3J = 5.5 Hz, 1 H), 5.14 (dd, 3J = 10.0, 3J = 3.5 Hz, 1 H), 3.42 (ddd, 2J = 12.0, 3J = 6.5, 3J = 5.5 Hz, 1 H), 3.26 (ddd, 2J = 12.0, 3J = 6.0, 3J = 5.5 Hz, 1 H), 2.76 (m, 1 H), 1.841.65 (m, 3 H), 1.45 (s, 9 H), 1.23 (d, 3J = 7.0 Hz, 3 H), 0.99 (d, 3J = 6.5 Hz, 3 H), 0.96 (d, 3J = 6.4 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 175.8, 175.2, 156.5, 79.9, 70.9, 43.5, 40.9, 39.9, 28.8, 25.1, 23.4, 21.9, 15.0 ppm. IR (film): = 3334 (br. s), 2962 (s), 1739 (s), 1727 (s), 1521 (s), 1458 (m), 1369 (m), 1253 (m), 1173 (s) cm−1. HRMS (FAB): m/z calcd. C15H31N2O6 [M + NH4+] 335.2182, found 335.2212. LRMS (FAB): m/z (%) = 318.3 (21) [M + H+], 279.2 (100), 263.2 (61), 218.2 (58), 104.1 (16).
(2S)-2-[(3′-{[(tert-Butoxy)carbonyl]amino}propionyl)oxy]-4-methylpentanoic Acid (33):
A solution of 27 (1.05 g, 5.11 mmol), 31[46] (966 mg, 5.11 mmol), DCC (1.05 g, 5.11 mmol) and DMAP (312 mg, 2.55 mmol) in DCM (50 mL) was stirred at room temperature for 12 h. The reaction was quenched with saturated aqueous NH4Cl and the organic layer was dried with Na2SO4. Evaporation of the solvent and chromatographic purification (30% EtOAc in hexane) afforded (2S,2R)-benzyl 2-[(3′-{[(tert-butoxy)carbonyl]amino}propionyl)oxy]-4-methylpentanoate (1.83 g, 91%) as white solid. M.p. 63 °C. =−27.8 (c = 0.94, CHCl3) {ref.[33] = −28.1 (c = 1.34, CHCl3)}. 1H NMR (CDCl3, 500 MHz): δ = 7.38 (m, 5 H), 5.23 (ABq, 2 H, J = 12.0 Hz, Δν = 12.0 Hz), 5.18 (br. s, 1 H), 5.13 (dd, 3J = 9.5, 3J 4.0 Hz, 1 H), 3.44 (br. m, 2 H), 2.62 (t, 3J = 6.0 Hz, 2 H), 1.841.70 (m, 2 H), 1.67 (m, 1 H), 1.47 (s, 9 H), 0.97 (d, 3J = 5.5 Hz, 3 H), 0.93 (d, 3J = 6.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 176.1, 171.0, 156.4, 135.6, 129.0, 128.9, 128.6, 79.8, 71.6, 67.5, 40.0, 36.7, 35.0, 28.8, 25.0, 23.4, 22.0 ppm. IR (film): ν˜ = 3399 (br. m), 2962 (s), 2873 (w), 1743 (s), 1716 (s), 1509 (m), 1367 (m), 1250 (s), 1170 (s), 1078 (m), 749 (m) cm−1. HRMS (CI): m/z calcd. C21H32NO6 [M + H+] 394.2230, found 394.2219. A mixture of (2S,2R)-benzyl 2-[(3-{[(tert-butoxy)carbonyl]amino}propionyl)oxy]-4-methylpentanoate (1.50 g, 3.81 mmol), 10% Pd/C (50 mg) and EtOAc (50 mL) was stirred for 1 h under H2 and then filtered through a pad of Celite©. Concentration of the filtrate in vacuo yielded 33 (1.05 mg, 97%) as a white solid. M.p. 58 °C. = −18.3 (c = 1.55, CHCl3). 1H NMR (CDCl3, 500 MHz): δ 5.20 (br. s, 1 H), 5.13 (dd, 3J 9.0, 3J 4.5 Hz, 1 H), 3.47 (br. m, 2 H), 2.64 (br. m, 2 H), 1.851.75 (m, 2 H), 1.73 (m, 1 H), 1.46 (s, 9 H), 0.99 (d, 3J 6.0 Hz, 3 H), 0.96 (d, 3J 6.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 175.6, 172.5, 156.4, 80.0, 71.2, 39.9, 36.7, 35.0, 28.5, 25.1, 23.4, 21.9 ppm. IR (film): = 2961 (w), 2874 (w), 1741 (s), 1722 (s), 1521 (m), 1368 (s), 1251 (s), 1170 (s), 1076 (m) cm−1. HRMS (CI): m/z calcd. C14H25NNaO6 [M + Na+] 326.1580, found 326.1589.
(2S,2R,1S,2R,1R)-1-(3-{1-[(tert-Butoxy)carbonyl]2-p-methoxyphenylethylcarbamoyl}allyl)-2-methyl-4-phenylbut-3-enyl 2-[(3-{[(tert-Butoxy)carbonyl]amino}−2-methylpropionyl)oxy]-4-methylpentanoate (34):
To a solution of 32 (794 mg, 2.50 mmol) in THF (10 mL) were successively added iPr2NEt (436 mL, 2.50 mmol) and 2,4,6-trichlorobenzyl chloride (391 mL, 2.50 mmol). After 2 h of stirring at room temperature, the solvent was evaporated and the residue was dissolved in benzene (20 mL). 25 (400 mg, 0.83 mmol) and DMAP (306 mg, 2.50 mmol) were added and the mixture was stirred at room temperature for a further 1 h. The reaction mixture was quenched with saturated aqueous NH4Cl and extracted with diethyl ether. The organic layer was dried with Na2SO4, concentrated in vacuo and chromatographically purified (30% EtOAc in hexane) to yield 34 (549 mg, 84%) as a white solid. M.p. 184 °C. = −26.9 (c = 0.26, CH2Cl2). 1H NMR (CDCl3, 500 MHz): δ = 7.377.30 (m, 4 H), 7.25 (dd, 3J = 8.0, 3J = 7.7 Hz, 1 H), 7.09 (d, 3J = 8.5 Hz, 2 H), 6.83 (m, 3 H), 6.43 (d, 3J = 16.0 Hz, 1 H), 6.32 (d, 3J = 6.0 Hz, 1 H), 6.04 (dd, 3J = 16.0, 3J 8.5 Hz, 1 H), 5.91 (d, 3J = 16.0 Hz, 1 H), 5.25 (t, 3J = 4.5 Hz, 1 H), 5.06 (dd, 3J = 5.5, 3J = 4.5 Hz, 1 H), 4.96 (dd, 3J = 10.0, 3J = 5.0 Hz, 1 H), 4.82 (ddd, 1 H, 3J = 7.5, 3J = 4.0, 3J = 1.6 Hz), 3.79 (s, 3 H), 3.47 (m, 2 H), 3.09 (d, 3J = 6.5 Hz, 1 H), 2.77 (m, 1 H), 2.64 (m, 1 H), 2.56 (m, 2 H), 1.751.68 (m, 2 H), 1.58 (m, 1 H), 1.46 (s, 9 H), 1.43 (s, 9 H), 1.22 (d, 3J = 6.0 Hz, 3 H), 1.13 (d, 3J = 6.5 Hz, 3 H), 0.88 (d, 3J = 6.5 Hz, 3 H), 0.85 (d, 3J = 6.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 175.4, 171.2, 170.8, 165.2, 158.9, 156.5, 138.9, 137.3, 132.1, 131.0, 130.6, 129.0, 128.7, 127.8, 126.6, 126.5, 114.1, 82.6, 79.5, 76.9, 71.6, 55.6, 54.2, 43.4, 41.4, 40.4, 39.9, 37.7, 33.9, 28.8, 28.4, 25.0, 23.3, 21.8, 17.1, 15.0 ppm. IR (film): = 3400 (br. s), 2964 (s), 2934 (s), 1737 (s), 1678 (m), 1641 (m), 1513 (s), 1367 (s), 1249 (s), 1175 (s) cm−1. HRMS (FAB): m/z calcd. C44H63N2O10 [M + H+] 779.4483, found 779.4480.
(2S,1′′S,2′′R,1′′R)-1′′-[3′′-({1′′′′-[(tert-Butoxy)carbonyl]-2′′′′-(p-methoxyphenyl)ethyl}carbamoyl)allyl]-2′′-methyl-4′′-phenylbut-3′′-enyl 2-[(3′-{[(tert-Butoxy)carbonyl]amino}propionyl)oxy]-4-methylpentanoate (35):
To a solution of 33 (38.0 mg, 125 μmol) in THF (3 mL) were successively added iPr2NEt (22 μL, 125 μmol) and 2,4,6-trichlorobenzyl chloride (20 μL, 125 μmol). After 2 h of stirring at room temperature, the solvent was evaporated and the residue was dissolved in benzene (6 mL). 25 (30.0 mg, 62.6 μmol) and DMAP (15.0 mg, 125 μmol) were added and the mixture was stirred at room temperature for a further 1 h. The reaction mixture was quenched with saturated aqueous NH4Cl and extracted with diethyl ether. The organic layer was dried with Na2SO4, concentrated in vacuo and chromatographically purified (35% EtOAc in hexane) to yield 35 (38.0 mg, 80%) as a white solid. M.p. 175 °C. = +7.9 (c = 0.38, CHCl3). 1H NMR (CDCl3, 500 MHz): δ 7.377.30 (m, 4 H), 7.24 (dd, 3J 7.8, 3J 7.8 Hz, 1 H), 7.09 (d, 3J 8.5 Hz, 2 H), 6.82 (m, 3 H), 6.43 (d, 3J = 16.0 Hz, 1 H), 6.38 (d, 3J = 6.4 Hz, 1 H), 6.04 (dd, 3J = 16.0, 3J = 8.5 Hz, 1 H), 5.91 (d, 3J = 16.0 Hz, 1 H), 5.29 (br. s, 1 H), 5.06 (dd, 3J = 5.5, 3J = 4.5 Hz, 1 H), 4.94 (dd, 3J = 10.0, 3J = 4.0 Hz, 1 H), 4.82 (ddd, 1 H, 3J = 5.6, 3J = 4.0, 3J = 1.6 Hz), 3.78 (s, 3 H), 3.47 (m, 2 H), 3.08 (d, 3J = 6.0 Hz, 1 H), 2.702.51 (m, 5 H), 1.761.68 (m, 2 H), 1.58 (m, 1 H), 1.45 (s, 9 H), 1.43 (s, 9 H), 1.13 (d, 3J = 6.5 Hz, 3 H), 0.88 (d, 3J = 6.5 Hz, 3 H), 0.86 (d, 3J = 6.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 172.7, 171.3, 170.8, 165.3, 159.0, 156.4, 139.0, 137.3, 132.1, 131.0, 130.6, 129.0, 128.7, 127.9, 126.6, 114.1, 82.6, 79.8, 76.9, 71.2, 55.6, 54.1, 41.4, 40.0, 37.7, 36.5, 34.9, 34.0, 28.8, 28.4, 25.0, 23.3, 21.8, 17.2 ppm. IR (film): = 3340 (br. s), 2964 (s), 2932 (s), 1739 (s), 1677 (m), 1641 (m), 1513 (s), 1367 (s), 1249 (s), 1167 (s) cm−1. HRMS (FAB): m/z calcd. C43H61N2O10 [M + H+] 765.4326, found 765.4341.
Cryptophycin D (4):
A mixture of 34 (288 mg, 0.37 mmol) and TFA (20 mL) was stirred at room temperature for 2 h. The reaction mixture was concentrated in vacuo and the residue was dissolved in THF (50 mL). To this solution were successively added iPr2NEt (225 μL, 1.29 mmol), 2,4,6-trichlorobenzyl chloride (58 μL, 0.37 mmol) and DMAP (90 mg, 0.37 mmol). After stirring at room temperature for 1 h, the solvent was evaporated and the residue was dissolved in benzene (20 mL) and stirred at room temperature for 12 h. The reaction mixture was quenched with saturated aqueous NH4Cl and extracted with diethyl ether. The organic layer was dried with Na2SO4, concentrated in vacuo and chromatographically purified (50% EtOAc in hexane) to yield 4 (169 mg, 76%) as a white solid. M.p. 186–189 °C. = +36.2 (c = 0.72, MeOH) {ref.[2] = +36.7 (c = 1.93, MeOH)}. 1H NMR (CDCl3, 500 MHz): δ = 7.367.31 (m, 4 H), 7.26 (dd, 3J = 7.0, 3J = 7.0 Hz, 1 H), 7.13 (d, 3J = 8.5 Hz, 2 H), 7.08 (dd, 3J = 6.0, 3J = 6.0 Hz, 1 H), 6.83 (d, 3J = 8.5 Hz, 2 H), 6.73 (ddd, 3J = 15.0, 3J = 10.0, 3J = 5.0 Hz, 1 H), 6.42 (d, 3J = 15.5 Hz, 1 H), 6.03 (dd, 3J = 15.5, 3J = 9.0 Hz, 1 H), 5.77 (d, 3J = 15.0 Hz, 1 H), 5.67 (d, 3J = 8.0 Hz, 1 H), 5.05 (ddd, 1 H, 3J = 8.5, 3J = 6.0, 3J = 1.0 Hz), 4.87 (dd, 3J = 10.0, 3J = 3.0 Hz, 1 H), 4.81 (dt, 3J = 7.5, 3J = 7.5 Hz, 1 H), 3.80 (s, 3 H), 3.42 (dd, 3J = 5.0, 3J = 5.0 Hz, 2 H), 3.16 (dd, 2J = 14.0, 3J = 5.0 Hz, 1 H), 3.10 (dd, 2J = 14.0, 3J = 7.5 Hz, 1 H), 2.70 (m, 1 H), 2.56 (m, 2 H), 2.40 (m, 1 H), 1.68 (m, 2 H), 1.36 (m, 1 H), 1.25 (d, 3J = 7.5 Hz, 3 H), 1.15 (d, 3J = 7.0 Hz, 3 H), 0.78 (d, 3J = 6.5 Hz, 3 H), 0.74 (d, 3J = 6.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 176.4, 171.6, 171.2, 165.7, 159.0, 141.9, 137.1, 132.2, 130.6, 130.5, 129.0, 128.9, 128.0, 126.6, 125.5, 114.5, 77.6, 72.0, 55.6, 54.3, 42.7, 41.2, 40.1, 38.5, 36.9, 35.8, 24.9, 23.1, 21.6, 17.8, 14.6 ppm. IR (CH2Cl2): = 3420 (m), 2963 (s), 2936 (m), 1746 (s), 1721 (s), 1680 (s), 1649 (s), 1513 (s), 1248 (s), 1179 (s) cm−1. HRMS (FAB): m/z calcd. C35H45N2O7 [M + H+] 605.3227, found 605.3231.
Deoxyarenastatin A (36):
A mixture of 35 (56.9 mg, 74.4 μmol) and TFA (10 mL) was stirred at room temperature for 2 h. The reaction was concentrated in vacuo and the residue was dissolved in THF (10 mL). To this solution were successively added iPr2NEt (12 μL, 74.4 μmol), 2,4,6-trichlorobenzyl chloride (12 μL, 74.4 μmol) and DMAP (36.0 mg, 298 μmol). After 1 h of stirring at room temperature, the solvent was evaporated and the residue was dissolved in benzene (10 mL) and stirred at room temperature for 1 h. The reaction was quenched with saturated aqueous NH4Cl and the mixture extracted with diethyl ether. The organic layer was dried with Na2SO4, concentrated in vacuo and chromatographically purified (65% EtOAc in hexane) to yield 36 (35.2 mg, 81%) as a white solid. M.p. 182188 °C. = +33.5 (c = 0.09, CH2Cl2) {ref.[2] = +34.0 (c = 1.36, CH2Cl2)}. 1H NMR (CDCl3, 400 MHz): δ = 7.367.26 (m, 4 H), 7.23 (dd, 3J = 7.8, 3J = 7.8 Hz, 1 H), 7.12 (d, 3J = 8.8 Hz, 2 H), 7.02 (dd, 3J = 4.5, 3J = 4.5 Hz, 1 H), 6.82 (d, 3J = 8.8 Hz, 2 H), 6.71 (ddd, 3J = 15.2, 3J = 10.4, 3J = 4.8 Hz, 1 H), 6.40 (d, 3J = 15.6 Hz, 1 H), 6.01 (dd, 3J = 15.6, 3J = 8.5 Hz, 1 H), 5.73 (d, 3J = 15.2 Hz, 1 H), 5.59 (d, 3J = 8.0 Hz, 1 H), 5.05 (ddd, 1 H, 3J = 9.6, 3J = 6.8, 3J = 1.3 Hz), 4.90 (dd, 3J = 9.6, 3J = 3.6 Hz, 1 H), 4.74 (dd, 3J = 7.2, 3J = 6.4 Hz, 1 H), 3.78 (s, 3 H), 3.55 (m, 1 H), 3.42 (m, 1 H), 3.14 (dd, 2J = 14.4, 3J = 6.0 Hz, 1 H), 3.05 (dd, 2J = 14.4, 3J = 7.6 Hz, 1 H), 2.54 (m, 4 H), 2.36 (dt, 2J = 14.0, 3J = 11.2 Hz, 1 H), 1.68 (m, 2 H), 1.40 (m, 1 H), 1.14 (d, 3J = 6.8 Hz, 3 H), 0.74 (d, 3J = 6.0 Hz, 3 H), 0.71 (d, 3J = 6.0 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 173.3, 171.2, 171.1, 165.9, 159.0, 141.9, 137.1, 132.2, 130.6, 130.5, 129.0, 128.9, 128.0, 126.6, 125.5, 114.5, 77.0, 72.0, 55.6, 54.5, 42.7, 40.1, 36.8, 35.6, 34.6, 32.9, 24.8, 23.0, 21.7, 17.7 ppm. IR (film): = 2957 (s), 2925 (s), 1741 (s), 1730 (s), 1678 (s), 1650 (s), 1513 (s), 1372 (m), 1247 (s), 1175 (s) cm−1. HRMS (FAB): m/z calcd. C34H43N2O7 [M + H+] 591.3070, found 591.3080. LRMS (LCQ): m/z (%) = 591.1 (22) [M + H+], 387.9 (100), 360.0 (35).
Cryptophycin B (2):
To a solution of 4 (6.0 mg, 9.92 μmol) in DCM (2 mL) was added dimethyldioxirane[47] (0.32 M in acetone, 310 μL, 99.2 μmol) at 30 °C. The resulting mixture was stirred at −30 °C to room temperature for 12 h and then concentrated in vacuo. Chromatographic purification [HPLC, YMC-PACK OD-AQ 5S 120Å 4.6 × 250 mm, MeOH/H2O, 3:1, 1 mL/min, room temp., tR(2) = 31.58 min] to yield 2 (5.4 mg, 87%, 3:1) as a white solid. = +20.6° (c = 0.24, MeOH) {ref.[2] = +20.4 (c = 0.54, MeOH)}. 1H NMR (CDCl3, 500 MHz): δ = 7.427.35 (m, 3 H), 7.287.24 (m, 2 H), 7.12 (d, 3J = 8.5 Hz, 2 H), 7.06 (dd, 3J = 5.9, 3J = 5.9 Hz, 1 H), 6.84 (d, 3J = 8.5 Hz, 2 H), 6.73 (ddd, 3J = 15.1, 3J = 10.1, 3J = 4.8 Hz, 1 H), 5.73 (d, 3J = 15.4 Hz, 1 H), 5.66 (d, 3J = 8.2 Hz, 1 H), 5.21 (ddd, 1 H, 3J = 9.5, 3J = 4.8, 3J = 1.6 Hz), 4.84 (dd, 3J = 9.8, 3J = 3.3 Hz, 1 H), 4.81 (dt, 3J = 7.2, 3J = 6.4 Hz, 1 H), 3.81 (s, 3 H), 3.71 (d, 3J = 1.7 Hz, 1 H), 3.44 (ddd, 2J = 13.6, 3J = 12.7, 3J = 5.5 Hz, 1 H), 3.39 (ddd, 2J = 13.6, 3J = 4.2, 3J = 4.1 Hz, 1 H), 3.15 (dd, 2J = 14.6, 3J = 5.6 Hz, 1 H), 3.10 (dd, 2J = 14.4, 3J = 6.9 Hz, 1 H), 2.94 (dd, 3J = 7.6, 3J = 1.8 Hz, 1 H), 2.71 (m, 1 H), 2.58 (dm, 1 H, 2J = 14.5 Hz), 2.40 (ddd, 1 H, 2J = 14.5, 3J = 10.8, 3J 10.5 Hz), 1.81 (m, 1 H), 1.73 (m, 2 H), 1.36 (m, 1 H), 1.25 (d, 3J = 7.3 Hz, 3 H), 1.17 (d, 3J = 6.9 Hz, 3 H), 0.88 (d, 3J = 6.5 Hz, 3 H), 0.86 (d, 3J = 6.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 125 MHz): δ = 176.4, 171.5, 171.1, 165.5, 159.0, 141.6, 137.1, 130.6, 129.1, 129.0, 128.7, 126.0, 125.5, 114.6, 76.7, 71.7, 63.5, 59.5, 55.6, 54.3, 41.2, 39.8, 38.5, 37.2, 35.7, 24.9, 23.3, 21.7, 14.7, 14.0 ppm. IR (DCM): = 3411 (m), 2962 (s), 2935 (m), 1743 (s), 1724 (s), 1682 (s), 1513 (s), 1247 (s), 1199 (s), 1179 (s) cm−1. HRMS (FAB): m/z calcd. C35H45N2O8 [M + H+] 621.3176, found 621.3160.
Arenastatin A (5):
To a solution of 36 (5.2 mg, 8.81 μmol) in DCM (2 mL) was added dimethyldioxirane[45] (0.23 M in acetone, 383 μL, 88.1 μmol) at −30 °C. The resulting mixture was stirred at −30 °C to room temperature for 12 h and then concentrated in vacuo. Chromatographic purification [HPLC, YMC-PACK OD-AQ 5S 120Å 4.6 × 250 mm, MeOH/H2O 3:1, 1 mL/min, room temp., tR(5) = 30.86 min] to yield 5 (4.0 mg, 75%, 3:1) as a colorless film. = +48.1 (c 0.09, CHCl3) {ref.[33] = +48.7 (c 0.87, CHCl3)[. 1H NMR (CDCl3, 500 MHz): δ = 7.437.24 (m, 5 H), 7.11 (d, 3J = 8.5 Hz, 2 H), 7.04 (t, 3J = 5.9 Hz, 1 H), 6.83 (d, 3J = 8.6 Hz, 2 H), 6.72 (ddd, 3J = 15.1, 3J = 10.2, 3J = 4.8 Hz, 1 H), 5.72 (d, 3J = 15.2 Hz, 1 H), 5.65 (d, 3J = 8.2 Hz, 1 H), 5.21 (ddd, 1 H, 3J = 9.4, 3J 4.8, 3J = 1.5 Hz), 4.90 (dd, 3J = 9.8, 3J = 3.4 Hz, 1 H), 4.75 (dt, 3J = 7.3, 3J = 6.3 Hz, 1 H), 3.78 (s, 3 H), 3.71 (d, 3J = 1.8 Hz, 1 H), 3.46 (ddd, 2J = 13.4, 3J = 12.6, 3J = 5.5 Hz, 1 H), 3.41 (ddd, 2J = 13.5, 3J = 4.2, 3J = 4.2 Hz, 1 H), 3.15 (dd, 2J = 14.6, 3J = 5.3 Hz, 1 H), 3.08 (dd, 2J = 14.5, 3J = 6.7 Hz, 1 H), 2.94 (dd, 3J = 7.6, 3J = 1.8 Hz, 1 H), 2.58 (m, 3 H), 2.39 (ddd, 1 H, 3J = 7.6, 3J = 7.5, 3J = 7.5 Hz), 1.751.69 (m, 3 H), 1.32 (m, 1 H), 1.15 (d, 3J = 6.9 Hz, 3 H), 0.84 (d, 3J = 6.5 Hz, 3 H), 0.83 (d, 3J = 6.5 Hz, 3 H) ppm. 13C NMR (CDCl3, 100 MHz): δ = 173.2, 171.1, 171.0, 165.8, 159.0, 141.5, 137.1, 130.6, 129.1, 128.9, 128.7, 126.0, 125.5, 114.5, 77.5, 71.7, 63.5, 59.5, 55.6, 54.5, 42.7, 40.1, 36.9, 35.6, 34.6, 32.9, 24.8, 23.1, 21.7, 14.0 ppm. IR (film): = 3410 (m), 2963 (s), 2932 (m), 1742 (s), 1726 (s), 1679 (s), 1513 (s), 1246 (s), 1200 (m), 1180 (s) cm−1. HRMS (FAB): m/z calcd. C34H43N2O8 [M + H+] 607.3019, found 607.3025.
Acknowledgments
Financial support by the National Institutes of Health is gratefully acknowledged.
References
- [1].Schwartz RE, Hirsch CF, Sesin DE, Flor JE, Chartrain M, Fromtling RE, Harris GH, Salvatore MJ, Liesch JM, Yudin K, J. Ind. Microbiol 1990, 51, 113. [Google Scholar]
- [2].Galakoti T, Ohtani I, Patterson GML, Moore RE, Corbett TH, Valeriote FA, Demchik L, J. Am. Chem. Soc 1994, 116, 4729. [Google Scholar]
- [3].Galakoti T, Ogino J, Heltzel CE, Husebo TL, Jensen CM, Larsen LK, Patterson GML, Moore RE, Mooberry SL, Corbett TH, Valeriote FA, J. Am. Chem. Soc 1995, 117, 12030. [Google Scholar]
- [4].Smith CD, Zhang X, Mooberry SL, Patterson GML, Moore RE, Cancer Res. 1994, 54, 3779. [PubMed] [Google Scholar]
- [5].Subbaraju GV, Galakoti T, Patterson GML, Moore RE, J. Nat. Prod 1997, 60, 302. [DOI] [PubMed] [Google Scholar]
- [6].Georg GI, Ali SM, Stella VJ, Waugh WN, Himes RH, Bioorg. Med. Chem. Lett 1998, 8, 1959. [DOI] [PubMed] [Google Scholar]
- [7].Kobayashi M, Kurosu M, Wang W, Fujii S, Kitagawa I, Chem. Pharm. Bull 1994, 42, 2196. [DOI] [PubMed] [Google Scholar]
- [8].Murakami N, Tamura S, Wang W, Takagi T, Kobayashi M, Tetrahedron 2001, 57, 4323. [Google Scholar]
- [9].Murakami N, Wang W, Tamura S, Kobayashi M, Bioorg. Med. Chem. Lett 2000, 10, 1823. [DOI] [PubMed] [Google Scholar]
- [10].Murakami N, Wang W, Ohyahu N, Ito T, Tamura S, Aoki S, Kobayashi M, Kitagawa I, Tetrahedron 2000, 56, 9121. [Google Scholar]
- [11].Morita K, Koiso Y, Hashimoto Y, Kobayashi M, Wang W, Ohyahu N, Iwasaki N, Biol. Pharm. Bull 1997, 20, 171. [DOI] [PubMed] [Google Scholar]
- [12].Mooberry SL, Taoka CR, Busquets L, Cancer Lett. 1996, 107, 53. [DOI] [PubMed] [Google Scholar]
- [13].Kerksiek K, Mejillano MR, Schwartz RE, Georg GI, Himes RH, FEBS Lett. 1995, 377, 59. [DOI] [PubMed] [Google Scholar]
- [14].Panda D, Himes RH, Moore RE, Wilson L, Jordan MA, Biochemistry 1997, 36, 12948. [DOI] [PubMed] [Google Scholar]
- [15].Muys J-M, Rej R, Nguyen D, Go B, Fortin S, Lavallée J-F, Bioorg. Med. Chem. Lett 1996, 6, 111. [Google Scholar]
- [16].Varie D-L, Shih C, Hay DA, Andis SL, Corbett TH, Gossett LS, Janisse SK, Martinelli MJ, Moher ED, Schultz RM, Toth JE, Bioorg. Med. Chem. Lett 1999, 9, 369. [DOI] [PubMed] [Google Scholar]
- [17].Menon K, Alvarez E, Forler P, Phares V, Amsrud T, Shih C, Al-Awar R, Teicher BA, Cancer Chemother. Pharmacol 2000, 46, 142. [DOI] [PubMed] [Google Scholar]
- [18].Patel VF, Andis SL, Kennedy JH, Ray JE, Schultz RM, J. Med. Chem 1999, 42, 2588. [DOI] [PubMed] [Google Scholar]
- [19].Norman BH, Hemscheidt T, Schultz RM, Andis SL, J. Org. Chem 1998, 63, 5288. [Google Scholar]
- [20].Wagner MM, Paul DC, Shih C, Jordan MA, Wilson L, Williams DC, Cancer Chemother. Pharmacol 1999, 43, 115. [DOI] [PubMed] [Google Scholar]
- [21].Kobayashi M, Kurosu M, Wang W, Kitagawa I, Chem. Pharm. Bull 1994, 42, 2394. [DOI] [PubMed] [Google Scholar]
- [22].Kobayashi M, Wang W, Ohyabu N, Kurosu M, Kitagawa I, Chem. Pharm. Bull 1995, 43, 1598. [DOI] [PubMed] [Google Scholar]
- [23].Barrow A, Hemscheidt T, Paik S, Liang J, Moore RE, Tius MA, J. Am. Chem. Soc 1995, 117, 2479. [Google Scholar]
- [24].Salamonczyk GM, Han K, Guo Z, Sih CJ, J. Org. Chem 1996, 61, 6893. [DOI] [PubMed] [Google Scholar]
- [25].Rej R, Nguyen D, Go B, Fortin S, Lavallée J-F, J. Org. Chem 1996, 61, 6289. [DOI] [PubMed] [Google Scholar]
- [26].Gardinier KM, Leahy JW, J. Org. Chem 1997, 62, 7098. [DOI] [PubMed] [Google Scholar]
- [27].Varie DL, Brennan J, Briggs B, Cronin JS, Hay DA, Rieck JA, Zmijewski MJ, Tetrahedron Lett. 1998, 39, 8405. [Google Scholar]
- [28].Dhokte UP, Khau VV, Hutchinson DR, Martinelli MJ, Tetrahedron Lett. 1998, 39, 8771. [Google Scholar]
- [29].White JD, Hong J, Robarge LA, Tetrahedron Lett. 1998, 39, 8779. [Google Scholar]
- [30].Eggen MJ, Georg GI, Bioorg. Med. Chem. Lett 1998, 8, 3177. [DOI] [PubMed] [Google Scholar]
- [31].Furuyama M, Shimizy I, Tetrahedron Asym. 1998, 9, 1351. [Google Scholar]
- [32].Liang J, Hoard DW, Khau VV, Martinelli MJ, Moher ED, Moore RE, Tius MA, J. Org. Chem 1999, 64, 1459. [DOI] [PubMed] [Google Scholar]
- [33].White JD, Hong J, Robarge LA, J. Org. Chem 1999, 64, 6206. [Google Scholar]
- [34].Ghosh AK, Bischoff A, Org. Lett 2000, 2, 1573. [DOI] [PubMed] [Google Scholar]
- [35].Eggen MJ, Mossman CJ, Buck SB, Nair SK, Bhat L, Ali SM, Reiff EA, Boge TC, Georg GI, J. Org. Chem 2000, 65, 7792. [DOI] [PubMed] [Google Scholar]
- [36].Liang J, Moher ED, Moore RE, Hoard DW, J. Org. Chem 2000, 65, 3143. [DOI] [PubMed] [Google Scholar]
- [37].Christopher JA, Kocienski PJ, Kuhl A, Bell R, Synlett 2000, 463. [Google Scholar]
- [38].Pousset C, Haddad M, Larchevêque M, Tetrahedron 2001, 57, 7163. [Google Scholar]
- [39].Eggen MJ, Nair SK, Georg GI, Org. Lett 2001, 3, 1813. [DOI] [PubMed] [Google Scholar]
- [40].Li L-H, Tius MA, Org. Lett 2002, 4, 1637. [DOI] [PubMed] [Google Scholar]
- [41].Ghosh AK, Fidanze S, Senanayake CH, Synthesis 1998, 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ghosh AK, Fidanze S, Onishi M, Hussain KA, Tetrahedron Lett. 1997, 38, 7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].von Kostanecki S, Lampe V, Ber. Dtsch. Chem. Ges 1904, 37, 773. [Google Scholar]
- [44].Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M, Bull. Chem. Soc. Jpn 1979, 52, 1989. [Google Scholar]
- [45].Degerbeck F, Fransson B, Grehn L, Ragnarsson U, J. Chem. Soc., Perkin Trans 1 1991, 11. [Google Scholar]
- [46].Keller O, Keller WE, Van Look G, Wersin G, Org. Synth. Coll. Vol 1990, 7, 70. [Google Scholar]
- [47].Pe´res-Encabo A, Perrio S, Slawin AMZ, Thomas S, Wierzchleyski AT, Williams DJ, J. Chem. Soc., Perkin Trans 1 1994, 629. [Google Scholar]
- [48].Tu Y, Wang Z-X, Shi Y, J. Am. Chem. Soc 1996, 118, 9806. [Google Scholar]
- [49].Wang Z-X, Tu Y, Frohn M, Shi Y, J. Org. Chem 1997, 62, 2328. [DOI] [PubMed] [Google Scholar]
- [50].Tu Y, Wang Z-X, Frohn M, He M, Yu H, Tang Y, Shi Y, J. Org. Chem 1998, 63, 8475. [Google Scholar]
- [51].Davis FA, Towson JC, Vashi DB, Reddy RT, McCauley JT, Harakal ME, Gosciniak DJ, J. Org. Chem 1990, 55, 1254. [Google Scholar]
- [52].Abramovitch RA, Smith EM, Humber M, Purtschert B, Srinivasan PC, Singer GM, J. Chem. Soc., Perkin Trans 1 1974, 2589. [Google Scholar]
- [53].Jacobsen EN, Zhang W, Muci AR, Ecker JR, Deng L, J. Am. Chem. Soc 1991, 113, 7036. [Google Scholar]
- [54].de Sousa SE, OBrien P, Pilgram CD, Roder D, Towers TD, Tetrahedron Lett. 1999, 40, 391. [Google Scholar]
- [55].Yang D, Wong M-K, Yip Y-C, J. Org. Chem 1995, 60, 3887. [Google Scholar]
- [56].Wang Z-X, Tu Y, Frohn M, Zhang J-R, Shi Y, J. Am. Chem. Soc 1997, 119, 11224, and references cited therein. [Google Scholar]