Abstract
Background
Invasive fractional flow reserve (FFR) is a standard tool for identifying ischemia-producing coronary stenosis. However, in clinical practice, over 70% of treatment decisions still rely on visual estimation of angiographic stenosis, which has limited accuracy (about 60%–65%) for the prediction of FFR < 0.80. One of the reasons for the visual–functional mismatch is that myocardial ischemia can be affected by the supplied myocardial size, which is not always evident by coronary angiography. The aims of this study were to develop an angiography-based machine learning (ML) algorithm for predicting the supplied myocardial volume for a stenosis, as measured using coronary computed tomography angiography (CCTA), and then to build an angiography-based classifier for the lesions with an FFR < 0.80 versus ≥ 0.80.
Methods and findings
A retrospective study was conducted using data from 1,132 stable and unstable angina patients with 1,132 intermediate lesions who underwent invasive coronary angiography, FFR, and CCTA at the Asan Medical Center, Seoul, Korea, between 1 May 2012 and 30 November 2015. The mean age was 63 ± 10 years, 76% were men, and 72% of the patients presented with stable angina. Of these, 932 patients (assessed before 31 January 2015) constituted the training set for the algorithm, and 200 patients (assessed after 1 February 2015) served as a test cohort to validate its diagnostic performance. Additionally, external validation with 79 patients from two centers (CHA University, Seongnam, Korea, and Ajou University, Suwon, Korea) was conducted. After automatic contour calibration using the caliber of guiding catheter, quantitative coronary angiography was performed using the edge-detection algorithms (CAAS-5, Pie-Medical). Clinical information was provided by the Asan BiomedicaL Research Environment (ABLE) system. The CCTA-based myocardial segmentation (CAMS)-derived myocardial volume supplied by each vessel (right coronary artery [RCA], left anterior descending [LAD], left circumflex [LCX]) and the myocardial volume subtended to a stenotic segment (CAMS-%Vsub) were measured for labeling. The ML for (1) predicting vessel territories (CAMS-%LAD, CAMS-%LCX, and CAMS-%RCA) and CAMS-%Vsub and (2) identifying the lesions with an FFR < 0.80 was constructed. Angiography-based ML, employing a light gradient boosting machine (GBM), showed mean absolute errors (MAEs) of 5.42%, 8.57%, and 4.54% for predicting CAMS-%LAD, CAMS-%LCX, and CAMS-%RCA, respectively. The percent myocardial volumes predicted by ML were used to predict the CAMS-%Vsub. With 5-fold cross validation, the MAEs between ML-predicted percent myocardial volume subtended to a stenotic segment (ML-%Vsub) and CAMS-%Vsub were minimized by the elastic net (6.26% ± 0.55% for LAD, 5.79% ± 0.68% for LCX, and 2.95% ± 0.14% for RCA lesions). Using all attributes (age, sex, involved vessel segment, and angiographic features affecting the myocardial territory and stenosis degree), the ML classifiers (L2 penalized logistic regression, support vector machine, and random forest) predicted an FFR < 0.80 with an accuracy of approximately 80% (area under the curve [AUC] = 0.84–0.87, 95% confidence intervals 0.71–0.94) in the test set, which was greater than that of diameter stenosis (DS) > 53% (66%, AUC = 0.71, 95% confidence intervals 0.65–0.78). The external validation showed 84% accuracy (AUC = 0.89, 95% confidence intervals 0.83–0.95). The retrospective design, single ethnicity, and the lack of clinical outcomes may limit this prediction model’s generalized application.
Conclusion
We found that angiography-based ML is useful to predict subtended myocardial territories and ischemia-producing lesions by mitigating the visual–functional mismatch between angiographic and FFR. Assessment of clinical utility requires further validation in a large, prospective cohort study.
Soo-Jin Kang and colleagues present a machine learning–based model for estimating the risk of ischemia resulting from a coronary stenosis. If prospectively validated, the tool may reduce the invasive nature of this diagnosis.
Author summary
Why was this study done?
Invasive fractional flow reserve (FFR, defined as the ratio of maximum flow in a diseased artery to the proximal normal maximum flow) has been a standard tool to detect ischemia-producing lesions with FFR < 0.80.
Although the current guidelines recommend the routine use of FFR for identifying ischemia-producing lesions, the majority of treatment decisions still rely on visual assessment of the degree of angiographic stenosis because of the time and expense associated with FFR-guided decision-making.
Conventional angiographic parameters cannot predict the presence of ischemia in cases in which this is affected by the size of subtended myocardium.
Integration and optimization of information about both myocardial territory and stenosis degree are expected to improve the performance of angiographic prediction of low FFR (FFR < 0.80).
What did the researchers do and find?
A retrospective study was conducted using data from 1,132 angina patients with 1,132 intermediate coronary lesions (932 in the training dataset and 200 in the internal test dataset), who underwent coronary angiography, coronary computed tomography angiography (CCTA), and FFR to evaluate the lesion morphology, subtended myocardial territories, and inducible ischemia, respectively.
The mean absolute errors between angiography-based machine learning (ML)-derived versus CCTA-derived subtended myocardial volume were 6.26% ± 0.55% for left anterior descending artery, 5.79% ± 0.68% for left circumflex artery, and 2.95% ± 0.14% for right coronary artery lesions.
Using all clinical and angiographic features, the ML models predicted an FFR < 0.80 with an overall accuracy of approximately 80% in the test set. In the external validation, the overall accuracy for predicting FFR < 0.80 was 84%.
What do these findings mean?
Angiography-based supervised ML is useful to predict subtended myocardial territories and to identify ischemia-producing lesions by mitigating the visual–functional mismatch between angiography and FFR.
The data-driven approach may support clinicians in identifying clinically relevant coronary lesions without FFR measurement and in making clinical decisions.
Performance and clinical utility require further validation in a large, prospective cohort study.
Introduction
Stratification of cardiovascular risk in patients with stable coronary artery disease is a key to identify high-risk patients who will benefit from percutaneous coronary intervention (PCI). The appropriateness of revascularization has been determined by the presence and extent of myocardial ischemia. A myocardial perfusion imaging study previously suggested that revascularization has a greater survival benefit in patients with a moderate to large degree of ischemic myocardium (≥10% of the total myocardium) [1]. Invasive fractional flow reserve (FFR, defined as the ratio of maximum flow in a diseased artery to the proximal normal maximum flow) has been a standard tool for lesion-specific hemodynamic assessment and treatment decision-making [2–4]. With abundant clinical evidence showing a significant reduction in major adverse cardiac events using FFR-guided PCI (versus angiography-guided PCI), current guidelines recommend FFR measurement when assessing intermediate coronary stenosis. However, in clinical practice, over 70% of treatment decisions still rely on a visual estimation of angiographic stenosis. This may be due to the prolonged procedure time and high short-term costs associated with FFR-guided diagnosis, as well as the need for adenosine-induced hyperemia and the fact that reimbursement systems do not favor this approach [5,6].
Although invasive coronary angiography and intravascular ultrasound (IVUS) are commonly utilized for evaluating coronary anatomy and optimizing PCI, the subjective nature of visual estimation limits the accurate estimation of stenosis severity [7]. In addition, the integration of morphologic and physiologic parameters and the identification of clinically relevant coronary lesions remain challenging [8–10]. In previous studies, the overall diagnostic accuracy of quantitative angiography for predicting FFR < 0.80 was shown to be only 60%–65% [10,11]. One of the reasons for the visual–functional mismatch is that myocardial ischemia is primarily determined by the variable size of the supplied myocardium at risk, as well as by the degree of stenosis [11]. Our previous data suggested that the application of coronary computed tomography angiography (CCTA)-based myocardial segmentation (CAMS)-derived percent myocardial volume subtended to a stenotic segment (CAMS-%Vsub) improves the diagnostic accuracy of angiographic indices used to identify ischemia-producing lesions [12,13]. Nonetheless, the necessity of concurrently performing noninvasive CCTA and invasive angiography limited the clinical utility of the mathematical model.
Machine learning (ML) techniques have emerged as highly effective computer algorithms for the identification of patterns in large datasets with a multitude of variables, facilitating the construction of models for data-driven prediction or classification [14–17]. The aims of this study were to develop an angiography-based supervised ML algorithm for predicting the CAMS-%Vsub and to build an angiography-based supervised ML model to classify lesions into those with an FFR < 0.80 and those ≥ 0.80.
Methods
Study population
Between 1 May 2012 and 31 January 2015, 5,378 consecutive patients with stable or unstable angina underwent invasive coronary angiography at the Asan Medical Center, Seoul, Korea. Preprocedural FFR and CCTA data for assessing an intermediate coronary lesion (defined as an angiographic stenosis diameter of 30%–80% on visual estimation) were available for 1,143 patients. Among them, 10 patients with tandem lesions, 10 with stented lesions, 17 with in-stent restenosis, 22 with chronic total occlusion, 10 with side branch evaluation, 145 with significant left main coronary artery stenosis, and 5 with scarred myocardium and regional wall motion abnormality on echocardiography were excluded. When FFR was measured in multiple lesions, the lesion with the lowest FFR value was selected. Following exclusions, 932 patients (932 lesions) were used for model training (the training sample). In addition, data from a nonoverlapping population of 200 stable and unstable angina patients (200 lesions) who underwent preprocedural angiography, IVUS, and FFR in a different phase (between 1 February 2015 and 30 November 2015) were used as a test sample to validate the diagnostic performance of the ML models for the prediction of FFR < 0.80 (Table 1). De-identified clinical information, including patient age and sex, was supported by the Asan BiomedicaL Research Environment (ABLE) system. All patients provided written informed consent for the procedures. The protocol of retrospective data analysis (1 January 2017 to approximately 30 November 2017) was approved by the institutional review board of the Asan Medical Center (S1 file), and a waiver for informed consent was granted. This study is reported as per the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines (S1 Checklist).
Table 1. Baseline clinical and angiographic characteristics in the training and test samples.
| Characteristics | Training sample | Test sample |
|---|---|---|
| Patient/lesion number | 932/932 | 200/200 |
| Age, years | 63.12 ± 9.81 | 63.86 ± 9.56 |
| Men | 700 (75%) | 158 (79%) |
| Diabetes mellitus | 289 (31%) | 59 (30%) |
| Hypertension | 592 (64%) | 138 (69%) |
| Current smoker | 387 (42%) | 86 (43%) |
| Hyperlipidemia | 597 (64%) | 134 (67%) |
| Unstable angina | 181 (19%) | 30 (15%) |
| Body mass index, kg/m2 | 24.97 ± 3.21 | 24.85 ± 3.09 |
| Body surface area, m2 | 1.73 ± 0.18 | 1.73 ± 0.17 |
| FFR at maximal hyperemia | 0.80 ± 0.11 | 0.80 ± 0.10 |
| Angiographic data | ||
| LAD artery lesion | 591 (63%) | 127 (64%) |
| LCX artery lesion | 117 (13%) | 24 (12%) |
| RCA lesion | 224 (24%) | 49 (24%) |
| DS, % | 53.90 ± 11.24 | 54.90 ± 9.84 |
| MLD, mm | 1.49 ± 0.44 | 1.52 ± 1.19 |
| Lesion length, mm | 17.51 ± 9.69 | 16.14 ± 8.28 |
| Proximal RLD, mm | 3.38 ± 0.56 | 3.34 ± 0.51 |
| Distal RLD, mm | 2.94 ± 0.55 | 2.89 ± 0.51 |
Data are shown as n (%) or mean ± standard deviation; all, p-values were >0.05 between training versus test samples.
Abbreviations: DS, diameter stenosis; FFR, fractional flow reserve; LAD, left anterior descending; LCX, left circumflex; MLD, minimal lumen diameter; RCA, right coronary artery; RLD, reference lumen diameter.
The external validation of the ML models was conducted in 79 angina patients (64 patients from CHA University, Seongnam, Korea, and 15 patients from Ajou University, Suwon, Korea) who underwent invasive coronary angiography and FFR to assess an intermediate coronary lesion.
Computed tomography imaging and CAMS analysis
Computed tomography imaging, including CCTA, was performed using first- or second-generation dual-source computed tomography (Definition or Definition Flash, Siemens, Germany). The CCTA data with the fewest motion artifacts and clearest demarcation of the coronary artery were transferred to customized software for CAMS analysis (A-View Cardiac, Asan Medical Center, Korea). After extracting the centerline of each coronary artery and the left ventricular myocardium on the computed tomographic images, the 3D Voronoi algorithm was used to assign the myocardial territories of the major epicardial coronary arteries, including the left anterior descending artery (LAD), left circumflex artery (LCX), and right coronary artery (RCA). In brief, the Voronoi algorithm is a mathematical algorithm that divides the area or space between predetermined points or lines according to the shortest distances from those points or lines [18–20]. The left ventricular myocardial volume was divided into three major epicardial coronary artery territories based on the shortest distance from the coronary artery. The CAMS-%RCA, CAMS-%LCX, and CAMS-%LAD were defined as the percentage ratios of the myocardial volumes supplied by the RCA, LCX, and LAD to the total left ventricular myocardial volume. CAMS-%Vsub was defined as the percentage ratio of the myocardial volume subtended to a stenotic coronary segment to the total left ventricular myocardial volume. Fig 1 shows an example of the CAMS analysis.
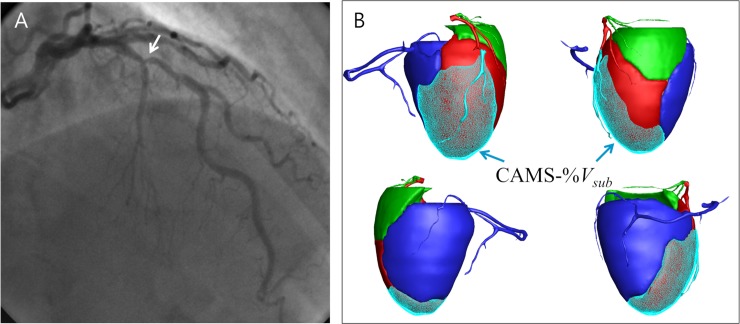
Fig 1.
(A) Angiography shows an intermediate stenosis (white arrow) of the mid LAD. (B) The CAMS-derived myocardial volume supplied by LAD was 38.5 cc (shown as red area), and the total left ventricular myocardial volume was 110 cc. The CAMS-%LAD was 35.0%. The myocardial volume subtended to the poststenotic segment was 29.0 cc (blue arrows), and the CAMS-%Vsub was 26.3%. The FFR was 0.82. CAMS, coronary computed tomography angiography–based myocardial segmentation; CAMS-%Vsub, CAMS-derived percent myocardial volume subtended to a stenotic segment; FFR, fractional flow reserve; LAD, left anterior descending.
Angiographic measurements
Quantitative coronary angiography was performed using standard techniques with automated edge-detection algorithms (CAAS-5, Pie-Medical, the Netherlands). After automatic contour calibration by using the known caliber of guiding catheter, angiographic diameter stenosis (DS), minimal lumen diameter (MLD), lesion length, and the proximal and distal reference lumen diameters (RLDs) were measured. Definitions of the angiographic features used for ML training are summarized in Table 2.
Table 2. Angiographic features used in the ML models.
| Features related to vessel territories | |
| DR #, mm | Maximal lumen diameter within the 10-mm segment from OS to proximal RCA |
| DL*, mm | Maximal lumen diameter within the 10-mm segment from OS to proximal LAD |
| DX*, mm | Maximal lumen diameter within the 10-mm segment from OS to proximal LCX |
| DLM*, mm | Maximal lumen diameter within left main coronary artery segment |
| Diminutive RCA | RCA ending prior to giving off the PDA and PL branch |
| Apex-LAD | LAD runs along the ventricular apex and curves towards the apico-inferior wall |
| Presence of RI | presence of ramus intermedius |
| Calculated %RCA† | estimated percent myocardial volume supplied by the RCA |
| Calculated %LAD† | estimated percent myocardial volume supplied by the LAD |
| Calculated %LCX† | estimated percent myocardial volume supplied by the LCX |
| Features related to myocardial volume subtended to a stenotic segment | |
| Distance to OS, mm | distance between the OS to the narrowest site |
| Proximal RLD, mm | proximal RLD |
| Distal RLD, mm | distal RLD |
| Averaged RLD, mm | average of proximal and distal RLDs |
| Proximal segment | disease involvement of proximal segment |
| Mid segment | disease involvement of mid segment |
| Distal segment | disease involvement of distal segment |
| D1‡, mm | diameter of the uppermost diagonal branch above the stenosis |
| D2‡, mm | diameter of the lower diagonal branch above the stenosis |
| S1‡, mm | diameter of the largest septal branch above the stenosis |
| D3‡, mm | diameter of the uppermost diagonal branch below the stenosis |
| D4‡, mm | diameter of the lower diagonal branch below the stenosis |
| S2‡, mm | diameter of the largest septal branch below the stenosis |
| D1 + D2, mm | sum of diagonal branch diameters above the stenosis |
| D1 + D2 + S1, mm | sum of all branch diameters above the stenosis |
| D3 + D4, mm | sum of diagonal branch diameters below the stenosis |
| D3 + D4 + S2, mm | sum of all branch diameters below the stenosis |
| D3‡, mm | diameter of the uppermost diagonal branch below the stenosis |
| D4‡, mm | diameter of the lower diagonal branch below the stenosis |
| First OM | the lesion located at the first OM |
| Second OM | the lesion located at the second OM |
| SB1, mm | diameter of the largest branch above the stenosis |
| SB2, mm | diameter of the uppermost branch below the stenosis |
| SB3, mm | diameter of the lower branch below the stenosis |
| SB2 + SB3, mm | sum of all branch diameters below the stenosis (SB2 and SB3) |
| Features related to lesion severity | |
| MLD | minimal lumen diameter |
| %DS | DS = (averaged RLD–MLD)/ averaged RLD × 100 |
| lesion length | length of stenosis |
# measured by using LAO view
*measured by using LAO caudal view
† calculated %RCA = 106.1 × DR / (DL + DX + DR)– 9.02; calculated %LCX = 140.9 × DX / (DL + DX + DR)– 18.24; calculated %LAD = 100 –calculated %RCA–calculated %LCX
‡ Only side branches with lumen diameter > 1.5 mm were included.
Abbreviations: DS, diameter stenosis; LAD, left anterior descending artery; LAO, left anterior oblique; LCX, left circumflex artery; ML, machine learning; OM, obtus marginalis; OS, ostium; PDA, posterior descending artery; PL, posterolateral; RCA, right coronary artery; RI, ramus intermedius; RLD, reference lumen diameter.
FFR measurement
FFR is defined as the ratio of the mean distal coronary pressure (Pd, measured with the pressure wire) to the mean aortic pressure (Pa, measured simultaneously with the guiding catheter) at maximum hyperemia. First, “Equalizing” was performed with the guidewire sensor positioned at the guiding catheter tip. A 0.014-inch FFR pressure guidewire (Radi, St. Jude Medical, Uppsala, Sweden) was then advanced distal to the stenosis. The FFR was measured at the maximum hyperemia induced by an intravenous infusion of adenosine administered through a central vein at 140 μg/kg/min increasing to 200 μg/kg/min, to enhance detection of hemodynamically relevant stenoses. Hyperemic pressure pullback recordings were performed. A stenosis was considered functionally significant when the FFR was <0.80 [3,4].
IVUS analysis
After intracoronary administration of 0.2 mg nitroglycerin, IVUS imaging was routinely performed using motorized transducer pullback (0.5 mm/s) and a commercial scanner (Boston Scientific Scimed, Minneapolis, MN, United States) with a rotating 40-MHz transducer within a 3.2-French imaging sheath. For the 630 patients for whom preprocedural IVUS data were available, the IVUS-derived minimum lumen area (IVUS-MLA) within a stenotic segment was obtained using computerized software (EchoPlaque 3.0, Indec Systems, Mountain View, CA, USA).
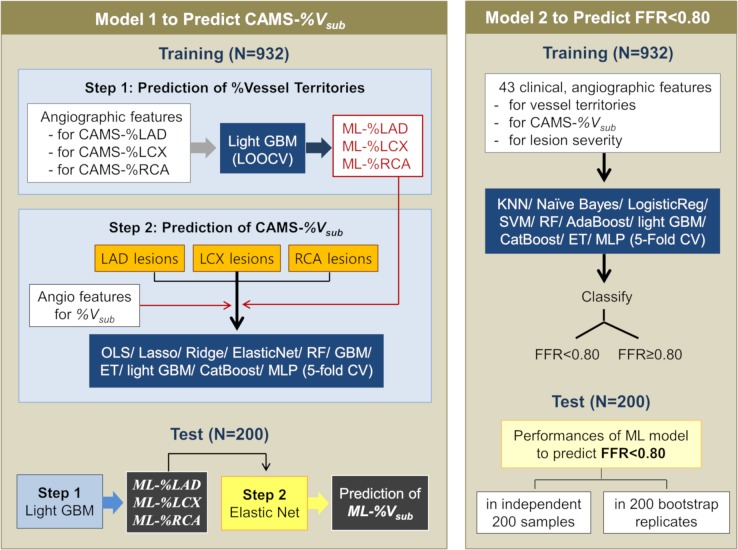
ML model for predicting CAMS-Vsub
The overall flow of the supervised ML models using the angiographic features is shown in Fig 2. A theoretical overview and summary of the ML algorithms and technical details are described in the supporting information (S1 Text).
Fig 2. Workflow for the ML.
CAMS-%RCA, CAMS-%LCX, and CAMS-%LAD are CCTA-measured percent ratios of the myocardial volumes supplied by the RCA, LCX, and LAD to the total left ventricular myocardial volume. ML-%RCA, ML-%LCX, and ML-%LAD are ML-predicted percent ratios of the myocardial volumes supplied by the RCA, LCX, and LAD to the total left ventricular myocardial volume. CAMS, CCTA-based myocardial segmentation; CAMS-%Vsub, CAMS-derived percent myocardial volume subtended to a stenotic segment; CCTA, coronary computer tomography angiography; CV, cross-validation; ET, extra tree; FFR, fractional flow reserve; GBM, gradient boosting machine; KNN, K-nearest neighbor; LAD, left anterior descending; LCX, left circumflex; LOOCV, leave-one-out cross-validation; ML, machine learning; ML-%Vsub, ML-predicted percent myocardial volume subtended to a stenotic segment; MLP, multilayer perceptron; OLS, ordinary least squares; RCA, right coronary artery; RF, random forest; SVM, support vector machine.
First, a light gradient boosting machine (GBM) with leave-one-out cross-validation was applied to predict the CAMS-derived percent myocardial volume supplied by each coronary artery (CAMS-%RCA, CAMS-%LCX, and CAMS-%LAD). The angiographic attributes affecting each vessel territory are summarized in Table 2. The variables estimated in our pilot data on the basis of the lumen diameters of LAD, RCA, and LCX (calculated %RCA, %LCX, and %LAD) were also included as attributes (see method in S1 Text). Then, the percent myocardial volumes supplied by each coronary artery (ML-%RCA, ML-%LCX, and ML-%LAD), as predicted by the algorithm, were used for the next step.
The second step was to build a model to predict the CAMS-%Vsub values. The ML algorithms evaluated were ordinary least squares (OLSs), ridge and lasso regressions, elastic net, random forests, extra trees, GBM, light GBM, CatBoost, and multilayer perceptrons. A 5-fold cross-validation scheme divided the training sample into five nonoverlapping partitions (method described in S1 Text). Each partition was rotated to be the validation set, with the remaining partitions being used as the training set (S1 Fig). As attributes, the ML-%RCA, ML-%LCX, and ML-%LAD were added to the angiographic features affecting the CAMS-%Vsub (Table 2). The mean absolute errors (MAEs) and mean squared errors (MSEs) were the metrics used to evaluate the performance of the models for predicting the CAMS-%Vsub values.
ML model for predicting FFR < 0.80
To develop the binary classifiers to separate the lesions with an FFR < 0.80 from those ≥0.80, 43 clinical and angiographic features including age, sex, involved segment (proximal LAD, mid LAD, distal LAD, proximal RCA, mid RCA, distal RCA, proximal LCX, distal LCX, first and second obtus marginalis), and the angiographic features affecting vessel territories, CAMS-%Vsub, and lesion severity were used and summarized in S2 Table. The evaluated algorithms were K-nearest neighbor, binary class L2 penalized logistic regression, support vector machine, random forest, extra tree, AdaBoost, light GBM, CatBoost, Gaussian naïve Bayes, and multilayer perceptron (S1 Text). The receiver operating curve (ROC), which was based on the relative performances considering the whole range of possible probability thresholds (from 0 to 1), has an area that ranges from 0.5 for classifiers without any prediction capability to 1 for perfectly classifying algorithms. Analyses based on precision-recall curves were also conducted. Using a 5-fold cross-validation scheme (S1 Fig), the accuracy was calculated by averaging the accuracies over the five tests performed in the multiple rounds of cross-validation. For a nonbiased assessment of the performance for identifying lesions with an FFR of <0.80, the classifiers that had been previously built on the training samples were applied to a completely independent test set of 200 lesions enrolled in the different phase.
In the training set, the algorithms were independently trained on the 200 train-validation random splits with a 3:1 ratio by bootstrap, and the average performances and 95% confidence intervals were calculated. In the 200 bootstrap replicates obtained by random sampling of 50 out of the 200 test samples, the average performance and bootstrap confidence intervals were also calculated.
Statistical analysis
The statistical analyses for evaluating patient and lesion characteristics at baseline were performed using SPSS (version 10.0, SPSS, Chicago, IL, USA). All values are expressed as means ± 1 standard deviation (continuous variables) or as counts and percentages (categorical variables). Continuous variables were compared using unpaired t tests; categorical variables were compared using χ2 statistics. A p-value < 0.05 was considered statistically significant. ROCs were analyzed using MedCalc Software (Mariakerke, Belgium) to assess the best cutoff for angiographic DS or IVUS-measured lumen area to predict FFR < 0.80 with maximal accuracy.
Results
Clinical and lesion characteristics
The clinical characteristics and angiographic data of the patients in the training and test sets are summarized in Table 1. The mean age was 63 ± 10 years, and 76% were men. The evaluated vessels were LAD in 63%. The overall CAMS-%RCA, CAMS-%LAD, and CAMS-%LCX were 27.2% ± 9.3%, 42.4% ± 7.2%, and 27.7% ± 9.6%, respectively. The CAMS-%Vsub was 31.1% ± 10.2%. FFR < 0.80 was shown in 41.6% of the lesions.
Angiographic prediction of vessel territory
By applying the light GBM to the training sample, the feature importance metrics for determining the CAMS-derived percent myocardial volume subtended to each coronary artery were determined, with the values being summarized in S1 Table. The estimated percent myocardial volumes (calculated %RCA, %LCX, and %LAD) based on the proximal vessel diameters were the most important features for predicting the CAMS-%RCA, CAMS-%LCX, and CAMS-%LAD, respectively. Additionally, the presence of ramus intermedius, a diminutive RCA, and an apical LAD curve affected the vessel territories. With the light GBM and leave-one-out cross-validation, the MAEs and MSEs were 5.42% and 7.10%, respectively, for predicting CAMS-%LAD, 8.57% and 11.28% for predicting CAMS-%LCX, and 4.54% and 6.51% for predicting CAMS-%RCA.
Angiographic prediction of percent myocardial volume subtended to stenotic segments
Table 3 summarizes the diagnostic performances of the various ML models used to predict CAMS-%Vsub. Among the models, the MAEs between the ML-predicted percent myocardial volume subtended to a stenotic segment (ML-%Vsub) and the CAMS-%Vsub were minimal with the use of the elastic net algorithm (6.26% ± 0.55% for LAD lesions, 5.79% ± 0.68% for LCX lesions, and 2.95% ± 0.14% for RCA lesions). When the elastic net algorithm was applied to all cases, the overall MAE was 5.39%. Table 4 shows the feature importance metrics by elastic net for the prediction of CAMS-%Vsub.
Table 3. Diagnostic performances of ML models for predicting the CAMS-%Vsub.
| Model | OLSs | Lasso | Ridge | Elastic net | Random forest | Extra tree | GBMs | Light GBMs | CatBoost | MLPs |
|---|---|---|---|---|---|---|---|---|---|---|
| LAD lesion | ||||||||||
| MAE, SD | 6.26, 0.56 | 6.26, 0.56 | 6.27, 0.55 | 6.26, 0.55 | 6.54, 0.42 | 6.65, 0.34 | 6.43, 0.51 | 6.63, 0.42 | 6.41, 0.32 | 6.29, 0.57 |
| MSE, SD | 7.89, 0.72 | 7.87, 0.72 | 7.88, 0.72 | 7.89, 0.69 | 8.17, 0.50 | 8.27, 0.41 | 8.07, 0.64 | 8.29, 0.50 | 8.14, 0.39 | 7.95, 0.69 |
| LCX lesion | ||||||||||
| MAE, SD | 5.77, 0.67 | 4.77, 0.70 | 5.84, 0.73 | 5.79, 0.68 | 6.69, 0.55 | 6.43, 0.59 | 6.31, 0.75 | 6.39, 0.36 | 6.28, 0.43 | 6.41, 0.62 |
| MSE, SD | 7.60, 0.92 | 7.62, 0.90 | 7.65, 0.98 | 7.66, 0.93 | 8.84, 0.86 | 8.51, 0.98 | 8.46, 1.01 | 8.53, 0.69 | 8.42, 0.92 | 8.52, 0.73 |
| RCA lesion | ||||||||||
| MAE, SD | 3.01, 0.16 | 2.96, 0.12 | 2.98, 0.16 | 2.95, 0.14 | 3.43, 0.44 | 3.47, 0.40 | 3.27, 0.43 | 3.45, 0.49 | 3.26, 0.28 | 4.26, 0.62 |
| MSE, SD | 4.23, 0.21 | 4.19, 0.18 | 4.20, 0.20 | 4.19, 0.19 | 4.62, 0.56 | 4.63, 0.57 | 4.51, 0.47 | 4.87, 0.49 | 4.41, 0.42 | 5.80, 0.43 |
Abbreviations: CAMS-%Vsub, coronary computed tomography angiography–based myocardial segmentation–derived percent myocardial volume subtended to a stenotic segment; GBM, gradient boosting machine; LAD, left anterior descending artery; LCX, left circumflex artery; MAE, mean absolute error; ML, machine learning; MLP, multilayer perceptron; MSE, mean squared error; OLS, ordinary least square; RCA, right coronary artery; SD, standard deviation.
Table 4. Ranked angiographic features for predicting CAMS-%Vsub and FFR < 0.80.
| Rank | Predictors of CAMS-%Vsub by Elastic Net* | Predictors of FFR by CatBoost# |
||
|---|---|---|---|---|
| LAD lesion | LCX lesion | RCA lesion | ||
| First | proximal LAD (3.07) | distal RLD (2.77) | ML-%RCA (2.04) | MLD (12.1%) |
| Second | distal RLD (1.15) | proximal LCX (2.75) | distal RLD (0.13) | %DS (6.1%) |
| Third | ML-%LAD (1.12) | SB2 + SB3 (2.05) | averaged RLD (0.12) | Age (5.9%) |
| Fourth | distal LAD (−1.06) | SB2 (0.75) | proximal RLD (0.10) |
DLM (5.4%) |
| Fifth | S1 (−1.04) | SB1 (0.68) | distance to OS (−0.02) | calculated %LCX (4.7%) |
| Sixth | averaged RLD (0.84) | averaged RLD (0.68) | proximal RCA (0.00) | lesion length (4.5%) |
| Seventh | D3 + D4 (0.82) | ML-%LCX (0.63) | mid RCA (0.00) | DRCA (4.4%) |
| Eighth | proximal RLD (0.52) | first OM (−0.59) | distal RCA (0.00) | calculated %LAD (4.3%) |
| Ninth | D3 (0.44) | SB3 (0.31) | distance to OS (4.3%) | |
| 10th | D2 (0.20) | distance to OS (0.00) | DLCX (4.3%) | |
| 11th | S2 (0.06) | proximal RLD (0.00) | DLAD (4.1%) | |
| 12th | distance to OS (0.05) | distal LCX (0.00) | proximal LAD (4.0%) | |
*coefficient (by Elastic Net)
#feature importance (by CatBoost)
Abbreviations: CAMS-%Vsub, coronary computed tomography angiography–based percent myocardial segmentation–derived myocardial volume subtended to a stenotic segment; DS, diameter stenosis; FFR, fractional flow reserve; LAD, left anterior descending artery; LCX, left circumflex artery; MLD, minimal lumen diameter; OM, obtus marginalis; OS, ostium; RCA, right coronary artery; RLD, reference lumen diameter.
Prediction of FFR < 0.80 in the training sample
To classify the lesions into those with an FFR < 0.80 versus ≥ 0.80, 43 clinical and angiographic features affecting vessel territories, CAMS-%Vsub, and lesion severity were used for ML (S2 Table). Based on the feature importance metrics by CatBoost, the top-12 features for determining the FFR were identified (Table 4). The ROC-based diagnostic performances of the ML algorithms are shown in Table 5, S3 Table, and Fig 3. In addition, the diagnostic performances based on the precision-recall curves are shown in S4 Table.
Table 5. Angiographic prediction of FFR < 0.80.
| Threshold of predictive score | AUC | Sensitivity | Specificity | PPV | NPV | Overall accuracy | |
|---|---|---|---|---|---|---|---|
| Prediction of FFR < 0.80 in the training sample (N = 932) | |||||||
| L2 penalized logistic regression* | 0.41 ± 0.03 (0.37–0.45) | 0.81 ± 0.04 (0.75–0.86) | 0.74 ± 0.05 (0.67–0.8) | 0.74 ± 0.04 (0.67–0.79) | 0.67 ± 0.05 (0.59–0.72) | 0.80 ± 0.04 (0.74–0.85) | 0.74 ± 0.04 (0.67–0.79) |
| Support vector machine* | 0.42 ± 0.01 (0.40–0.44) | 0.81 ± 0.04 (0.74–0.85) | 0.73 ± 0.05 (0.65–0.8) | 0.74 ± 0.04 (0.68–0.78) | 0.67 ± 0.05 (0.59–0.72) | 0.79 ± 0.04 (0.73–0.85) | 0.74 ± 0.04 (0.67–0.79) |
| Random forest* | 0.43 ± 0.02 (0.40–0.47) | 0.81 ± 0.05 (0.75–0.88) | 0.72 ± 0.04 (0.65–0.77) | 0.72 ± 0.05 (0.66–0.81) | 0.65 ± 0.06 (0.57–0.74) | 0.78 ± 0.04 (0.72–0.83) | 0.72 ± 0.05 (0.65–0.79) |
| AdaBoost* | 0.50 ± 0.00 (0.50–0.50) | 0.75 ± 0.05 (0.67–0.82) | 0.70 ± 0.03 (0.64–0.74) | 0.70 ± 0.04 (0.64–0.76) | 0.62 ± 0.04 (0.56–0.68) | 0.76 ± 0.03 (0.72–0.8) | 0.70 ± 0.04 (0.64–0.75) |
| CatBoost* | 0.37 ± 0.06 (0.27–0.45) | 0.78 ± 0.05 (0.73–0.86) | 0.71 ± 0.03 (0.67–0.76) | 0.72 ± 0.00 (0.65–0.78) | 0.64 ± 0.05 (0.58–0.71) | 0.77 ± 0.03 (0.73–0.82) | 0.71 ± 0.04 (0.66–0.77) |
| Prediction of FFR < 0.80 in the test sample (N = 200) | |||||||
| L2 penalized logistic regression | 0.41 | 0.86 | 0.79 | 0.81 | 0.78 | 0.82 | 0.80 |
| Support vector machine | 0.38 | 0.87 | 0.80 | 0.8 | 0.77 | 0.83 | 0.80 |
| Random forest | 0.44 | 0.84 | 0.78 | 0.81 | 0.77 | 0.81 | 0.80 |
| AdaBoost | 0.50 | 0.80 | 0.73 | 0.76 | 0.72 | 0.77 | 0.74 |
| CatBoost | 0.40 | 0.83 | 0.75 | 0.78 | 0.74 | 0.79 | 0.76 |
| External validation cohort (N = 79) | |||||||
| L2 penalized logistic regression | 0.33 | 0.91 | 0.84 | 0.8 | 0.66 | 0.91 | 0.81 |
| Support vector machine | 0.35 | 0.89 | 0.84 | 0.81 | 0.68 | 0.92 | 0.82 |
| Random forest | 0.37 | 0.89 | 0.84 | 0.81 | 0.68 | 0.92 | 0.82 |
| AdaBoost | 0.5 | 0.84 | 0.76 | 0.8 | 0.63 | 0.88 | 0.78 |
| CatBoost | 0.3 | 0.89 | 0.8 | 0.85 | 0.71 | 0.9 | 0.84 |
*average of 5-fold cross-validation results shown by mean ± standard deviation.
Abbreviations: AUC, area under the curve; FFR, fractional flow reserve; NPV, negative predictive value; PPV, positive predictive value.
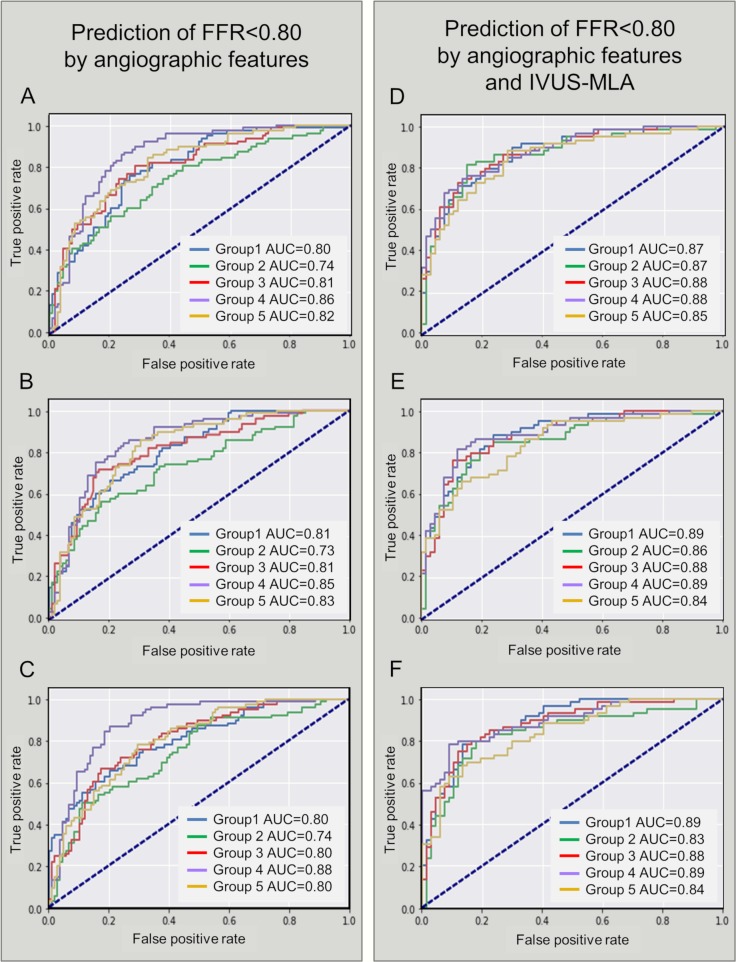
Fig 3. ROCs for predicting FFR < 0.80 in the training sample (N = 932).
(A) L2 penalized logistic regression using angiographic features. (B) Random forest using angiographic features. (C) CatBoost using angiographic features. (D) L2 penalized logistic regression using angiographic features and IVUS-MLA. (E) Random forest using angiographic features and IVUS-MLA. (F) CatBoost using angiographic features and IVUS-MLA. AUC, area under the curve; FFR, fractional flow reserve; IVUS-MLA, intravascular ultrasound–derived minimal lumen area; ROC, receiver operating curve.
In the subgroup that included the 630 patients with available preprocedural IVUS data, the IVUS-MLA was 2.77 ± 1.32 mm2. When the IVUS-MLA was added as an attribute, the classifiers using L2 penalized logistic regression, random forest, and support vector machine showed an overall accuracy of 78%–80% (area under the curve [AUC] = 0.87) to predict an FFR < 0.80 that is used as a hemodynamic index requiring revascularization (Fig 3 and S5 Table).
Performance in test sample
The test samples including the 200 lesions that were not utilized during the training showed no significant differences in clinical and lesion characteristics in comparison with the training sample (Table 1). In the identification of lesions with an FFR < 0.80, angiographic DS > 53% as the cutoff derived from an ROC analysis showed a sensitivity of 74%, a specificity of 61%, and an overall accuracy of 66% (AUC = 0.71). In addition, an IVUS-MLA < 2.34 mm2 had a sensitivity of 53%, a specificity of 79%, and an overall accuracy of 67% (AUC = 0.72).
Using clinical and angiographic features, the overall diagnostic accuracies of the ML classifiers (L2 penalized logistic regression, support vector machine, and random forest) in the test set were approximately 80% for predicting an FFR < 0.80 (AUC = 0.84–0.87, Table 5 and S6 Table). Table 6 summarizes the performances with bootstrap confidence intervals in the 200 bootstrap replicates for each of the training and test sets.
Table 6. Angiographic prediction of FFR < 0.80 in 200 bootstrap replicates.
| Threshold of predictive score | AUC | Sensitivity | Specificity | PPV | NPV | Overall accuracy | |
|---|---|---|---|---|---|---|---|
| 200 bootstrap replicates in the training set | |||||||
| L2 penalized logistic regression* | 0.42 ± 0.03 [0.37–0.48] | 0.81 ± 0.03 [0.76–0.86] | 0.74 ± 0.03 [0.69–0.78] | 0.74 ± 0.03 [0.68–0.79] | 0.67 ± 0.03 [0.61–0.72] | 0.80 ± 0.02 [0.76–0.84] | 0.74 ± 0.03 [0.69–0.79] |
| Support vector machine* | 0.42 ± 0.02 [0.37–0.46] | 0.80 ± 0.02 [0.76–0.85] | 0.73 ± 0.03 [0.68–0.78] | 0.73 ± 0.03 [0.68–0.79] | 0.66 ± 0.03 [0.61–0.73] | 0.79 ± 0.02 [0.75–0.84] | 0.73 ± 0.03 [0.69–0.79] |
| Random forest* | 0.43 ± 0.02 [0.4–0.47] | 0.81 ± 0.02 [0.76–0.85] | 0.73 ± 0.03 [0.67–0.8] | 0.73 ± 0.03 [0.67–0.78] | 0.66 ± 0.03 [0.59–0.72] | 0.79 ± 0.02 [0.74–0.84] | 0.73 ± 0.03 [0.67–0.79] |
| AdaBoost* | 0.50 ± 0.00 [0.50–0.50] | 0.75 ± 0.03 [0.7–0.8] | 0.69 ± 0.03 [0.64–0.74] | 0.69 ± 0.03 [0.64–0.74] | 0.62 ± 0.03 [0.56–0.67] | 0.76 ± 0.02 [0.72–0.80] | 0.69 ± 0.03 [0.64–0.74] |
| CatBoost* | 0.38 ± 0.06 [0.28–0.49] | 0.78 ± 0.03 [0.73–0.83] | 0.71 ± 0.03 [0.66–0.76] | 0.71 ± 0.03 [0.65–0.76] | 0.64 ± 0.03 [0.58–0.69] | 0.78 ± 0.02 [0.74–0.82] | 0.71 ± 0.03 [0.67–0.76] |
| 200 bootstrap replicates in the test set | |||||||
| L2 penalized logistic regression* | 0.47 ± 0.10 [0.27–0.65] | 0.83 ± 0.06 [0.71–0.94] | 0.75 ± 0.07 [0.65–0.87] | 0.76 ± 0.07 [0.63–0.89] | 0.73 ± 0.07 [0.61–0.86] | 0.78 ± 0.05 [0.68–0.88] | 0.76 ± 0.06 [0.66–0.86] |
| Support vector machine* | 0.47 ± 0.08 [0.34–0.61] | 0.85 ± 0.05 [0.76–0.94] | 0.78 ± 0.07 [0.65–0.87] | 0.78 ± 0.07 [0.67–0.93] | 0.76 ± 0.07 [0.63–0.9] | 0.81 ± 0.05 [0.70–0.90] | 0.78 ± 0.05 [0.68–0.88] |
| Random forest* | 0.46 ± 0.05 [0.38–0.56] | 0.81 ± 0.05 [0.71–0.91] | 0.74 ± 0.07 [0.61–0.87] | 0.74 ± 0.06 [0.63–0.85] | 0.71 ± 0.06 [0.6–0.83] | 0.77 ± 0.05 [0.67–0.88] | 0.74 ± 0.05 [0.64–0.84] |
| AdaBoost* | 0.50 ± 0.01 [0.48–0.52] | 0.77 ± 0.06 [0.65–0.88] | 0.71 ± 0.08 [0.52–0.87] | 0.71 ± 0.07 [0.56–0.81] | 0.68 ± 0.07 [0.54–0.8] | 0.74 ± 0.06 [0.62–0.87] | 0.71 ± 0.06 [0.58–0.82] |
| CatBoost* | 0.46 ± 0.17 [0.16–0.79] | 0.80 ± 0.05 [0.69–0.89] |
0.73 ± 0.07 [0.57–0.87] |
0.74 ± 0.07 [0.59–0.85] | 0.70 ± 0.06 [0.58–0.82] | 0.76 ± 0.06 [0.66–0.88] | 0.73 ± 0.06 [0.62–0.84] |
*average of 200 bootstrap replicates shown by mean ± standard deviation
[value] = bootstrap confidence intervals.
Abbreviations: AUC, area under the curve; FFR, fractional flow reserve; NPV, negative predictive value; PPV, positive predictive value.
By adding the IVUS-MLA, the classifiers using L2 penalized logistic regression and support vector machine achieved an overall accuracy of 78%–79% in the test set (AUC = 0.86–0.87, Fig 4 and S5 Table).
Fig 4. ROC analyses for predicting FFR < 0.80 in the test sample (N = 200).
(A) The ML models using angiographic features showed greater AUCs (0.83–0.86) than did the angiographic DS alone (AUC = 0.71). (B) The ML models using both angiographic features and IVUS-MLA showed larger AUC (0.82–0.87) than did the IVUS-MLA alone (AUC = 0.72). AUC, area under the curve; DS, diameter stenosis; FFR, fractional flow reserve; IVUS-MLA, intravascular ultrasound–derived minimal lumen area; ML, machine learning; ROC, receiver operating curve.
External validation
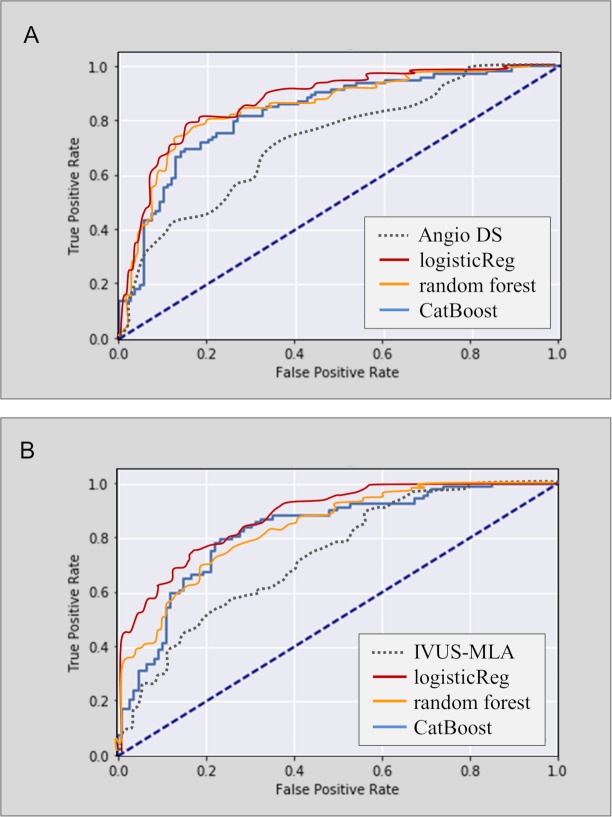
In the external validation cohort including 79 patients, the age was 59.6 ± 9.0 years, and 58 (73.4%) were men. An FFR < 0.8 was seen in 25 (31.6%) lesions. The angiographic DS and MLD were 48.3% ± 8.0% and 1.64 ± 0.39 mm, respectively. The performances of the ML models for the prediction of FFR < 0.8 were shown in Table 5.
Discussion
This study demonstrated that (1) angiography-based ML predicted the CAMS-%Vsub with an MAE of 6.26%, 5.79%, and 2.95% for LAD, LCX, and RCA lesions, respectively, and (2) for the identification of ischemia-producing lesions with a FFR < 0.80, the ML classifiers (L2 penalized logistic regression, support vector machine, and random forest) using the angiographic features showed an overall diagnostic accuracy of 80% (maximal AUC = 0.87), which was greater than that of angiographic DS criterion (66%, AUC = 0.71) or even that of the IVUS-MLA threshold (67%, AUC = 0.72).
Assessment of the myocardial mass at risk is of great importance because the presence and extent of ischemic myocardium determines the clinical relevance of revascularization [21–22]. A recent meta-analysis suggested that, in comparison with medical therapy, PCI significantly reduces mortality in patients with objective ischemia documented by functional tests [21]. Moreover, myocardial perfusion imaging suggests that revascularization has a greater survival benefit in patients with a moderate to large degree of ischemic myocardium [1]. These data have provided an insight into a higher-risk population that may benefit from an approach that incorporates ischemia-guided revascularization.
In daily practice, lesion-specific FFR is used to identify ischemia-producing lesions and to decide whether or not to treat it [2–4]. Although coronary angiography and IVUS have been commonly utilized to assess lesion severity, the diagnostic accuracy for predicting an FFR < 0.80 by angiographic DS or IVUS-MLA alone is <60%–70%, which restricts their clinical utility in treatment decision-making [8–10]. Similarly, the current study showed poor diagnostic accuracies for the detection of FFR < 0.80 using angiographic DS > 53% and IVUS-MLA < 2.34 mm2 based on the ROC analysis (66% and 67%, respectively). One of the reasons for the visual–functional mismatch is that myocardial ischemia is also determined by the variable size of the supplied myocardium, as well as the degree of stenosis [11].
In our previous study, the use of CAMS-Vsub improved the diagnostic performance of angiographic MLD and/or IVUS-MLA for the prediction of FFR < 0.80 [12,13]. Although a mathematical model using Vsub/MLD4 > 6.26 increased the accuracy to 82%, it could be applied only when the patient underwent noninvasive CCTA prior to catheterization. The current angiography-based ML model showed an overall MAE of 5.39% for predicting the CCTA-measured %Vsub. During the procedure, the angiographic prediction of the amount of supplied myocardium supports clinicians by confirming the clinical relevance of revascularization treatment in lesions with a large area of myocardium at risk and by precisely identifying the ischemia-producing lesions by reducing the discrepancy between anatomical and functional severity.
Several approaches for an FFR approximation of FFR using angiography-based models have recently been introduced [23–26]. A virtual functional assessment index and quantitative flow ratio based on computational fluid dynamics have shown the overall accuracies of 80%–86% in predicting an FFR < 0.80. Those approaches require a 3D reconstruction of at least two angiographic projections without foreshortening or overlapping vessels and the subsequent computational analyses. Using the clinical and 2D angiographic features affecting the subtended myocardial mass and degree of stenosis, our current ML classifiers predicted an FFR < 0.80 with an overall diagnostic accuracy of 80%. Therefore, the ML models not only reduce procedural expense by avoiding FFR testing but also provide information on the subtended myocardial territory that cannot be predicted by the FFR value. Ultimately, this data-driven approach extends the role of angiography in decision-making for the management of intermediate coronary stenosis.
Although traditional statistical methods validate the association between specific features and an endpoint, the development of a prediction model remains challenging, particularly in the setting of a nonlinear relationship between a factor and an outcome, interactions among variables, and the presence of many predictor variables. ML, an application of artificial intelligence, provides the ability to automatically learn a task without being explicitly programmed [14–17]. The algorithms attempt to balance two competing interests, “bias and variance,” which are summarized by loss functions to optimize a prediction model. Using angiographic features, both regression and decision tree models showed good performance in the prediction of CAMS-%Vsub, which led to greater detection of ischemia-producing lesions with reduced FFR.
The current study demonstrated the impact of the individual variables according to metrics (feature importance). For the prediction of CAMS-%Vsub, the important features were seen to be proximal segment involvement, RLD, ML-predicted territory of each vessel, the sum of the distal branch diameters, and the distance between the ostium and the narrowest site. Moreover, the key features for predicting FFR < 0.80 were MLD; %DS; age, proximal vessel size of LAD, LCX, and RCA; lesion length; distance between the ostium and MLD site; and the involvement of the proximal LAD, which suggested the importance of the impact of the angiographic determinant for stenosis degree and vascular territory on the FFR value. Although the rank in each algorithm is specific to the ML model, the approach may be hypothesis generating, suggesting which features are valuable for inclusion in future studies.
Limitations
This study may be subject to selection bias. As the analysis included the single ethnicity and excluded significant left main disease, side branch, and diffuse and tandem lesions, the model cannot be applied generally. Although the developed models were validated in the historical test set and the external validation cohort, the possibility of overfitting cannot be completely excluded. This model did not include the computational fluid dynamics for estimating the anatomical severity. A large prospective trial is required to validate whether the models allow clinicians to dispense with FFR measurement and therefore change the current clinical practice. Finally, prespecified angiographic features were used for ML; an image-based deep learning strategy using big data is worthy of investigation to achieve optimal diagnostic performance for clinical use.
Conclusion
Angiography-based ML models were useful for the prediction of CAMS-%Vsub and for improving the detection of ischemia-producing lesions. The data-driven approach may support clinicians in the identification of clinically relevant coronary lesions and in treatment decision-making.
Supporting information
TRIPOD, transparent reporting of a multivariable prediction model for individual prognosis or diagnosis.
(PDF)
CAMS, coronary computed tomography angiography–based myocardial segmentation; ML, machine learning.
(DOC)
CAMS, coronary computed tomography angiography–based myocardial segmentation.
(DOC)
FFR, fractional flow reserve; ML, machine learning.
(DOC)
FFR, fractional flow reserve.
(DOC)
FFR, fractional flow reserve.
(DOC)
FFR, fractional flow reserve; IVUS-MLA, intravascular ultrasound–derived minimum lumen area.
(DOC)
FFR, flow fractional reserve.
(DOC)
(TIF)
IRB, institutional review board.
(DOCX)
(ZIP)
Abbreviations
- ABLE
Asan BiomedicaL Research Environment
- AUC
area under the curve
- CAMS
CCTA-based myocardial segmentation
- CAMS-%Vsub
CAMS-derived percent myocardial volume subtended to a stenotic segment CCTA, coronary computed tomography angiography
- DS
diameter stenosis
- FFR
fractional flow reserve
- GBM
gradient boosting machine
- IVUS
intravascular ultrasound
- IVUS-MLA
IVUS-derived minimum lumen area
- KNN
K-nearest neighbor
- LAD
left anterior descending
- LAO
left anterior oblique
- LCX
left circumflex
- MAE
mean absolute error
- ML
machine learning
- MLD
minimal lumen diameter
- ML-%Vsub
ML-predicted percent myocardial volume subtended to a stenotic segment
- MSE
mean squared error
- NPV
negative predictive value
- OLS
ordinary least square
- Pa
aortic pressure
- PCI
percutaneous coronary intervention
- Pd
distal coronary pressure
- PDA
posterior descending artery
- PL
posterolateral
- PPV
positive predictive value
- RCA
right coronary artery
- RLD
reference lumen diameter
- ROC
receiver operating curve
- TRIPOD
transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
Data Availability
All relevant data are within the paper and its Supporting Information files (S1 Data)
Funding Statement
This study was funded by grants from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare Affairs, Republic of Korea (HI15C1790 and HI17C1080, S-JK); grants from the Ministry of Science and ICT (NRF-2017R1A2B4005886, S-JK); and grants from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (2017-0745, S-JK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. 10.1161/01.CIR.0000072790.23090.41 [DOI] [PubMed] [Google Scholar]
- 2.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. 10.1056/NEJM199606273342604 [DOI] [PubMed] [Google Scholar]
- 3.Tonino PA Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. 10.1016/j.jacc.2009.11.096 [DOI] [PubMed] [Google Scholar]
- 4.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, et al. ; FAME Study Investigators. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention FAME. N Engl J Med. 2009;360:213–224. 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. 10.1016/j.jacc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 7.White CW, Wright CB, Doty DB, Hiratza LF, Eastham CL, Harrison DG, et al. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis? N Engl J Med. 1984;310:819–824. 10.1056/NEJM198403293101304 [DOI] [PubMed] [Google Scholar]
- 8.Kang SJ, Ahn JM, Song H, Kim WJ, Lee JY, Park DW, et al. Usefulness of minimal luminal coronary area determined by intravascular ultrasound to predict functional significance in stable and unstable angina pectoris. Am J Cardiol. 2012;109:947–953. 10.1016/j.amjcard.2011.11.024 [DOI] [PubMed] [Google Scholar]
- 9.Ben-Dor I, Torguson R, Gaglia MA Jr, Gonzalez MA, Maluenda G, Bui AB, et al. Correlation between fractional flow reserve and intravascular ultrasound lumen area in intermediate coronary artery stenosis. EuroIntervention. 2011;7:225–233. 10.4244/EIJV7I2A37 [DOI] [PubMed] [Google Scholar]
- 10.Nascimento BR, de Sousa MR, Koo BK, Samady H, Bezerra HG, Ribeiro AL, et al. Diagnostic accuracy of intravascular ultrasound-derived minimal lumen area compared with fractional flow reserve—meta-analysis: pooled accuracy of IVUS luminal area versus FFR. Catheter Cardiovasc Interv. 2014;84:377–385. 10.1002/ccd.25047 [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Kang SJ, Ahn JM, Shim EB, Kim YT, Yun SC, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv. 2012;5:1029–1036. 10.1016/j.jcin.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 12.Kang SJ, Kweon J, Yang DH, Lee JG, Jung J, Kim N, et al. Mathematically Derived Criteria for Detecting Functionally Significant Stenoses Using Coronary Computed Tomographic Angiography-Based Myocardial Segmentation and Intravascular Ultrasound-Measured Minimal Lumen Area. Am J Cardiol. 2016;118:170–176. 10.1016/j.amjcard.2016.04.049 [DOI] [PubMed] [Google Scholar]
- 13.Kang SJ, Yang DH, Kweon J, Kim YH, Lee JG, Jung J, et al. Better Diagnosis of Functionally Significant Intermediate Sized Narrowings Using Intravascular Ultrasound-Minimal Lumen Area and Coronary Computed Tomographic Angiography-Based Myocardial Segmentation. Am J Cardiol. 2016;117:1282–1288. 10.1016/j.amjcard.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 14.Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J. 2017;38:1805–1814. 10.1093/eurheartj/ehw302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T. Artificial Intelligence in Precision Cardiovascular Medicine. J Am Coll Cardiol. 2017;69:2657–2664. 10.1016/j.jacc.2017.03.571 [DOI] [PubMed] [Google Scholar]
- 16.Henglin M, Stein G, Hushcha PV, Snoek J, Wiltschko AB, Cheng S. Machine Learning Approaches in Cardiovascular Imaging. Circ Cardiovasc Imaging. 2017. October;10 pii: e005614. 10.1161/CIRCIMAGING.117.005614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darcy AM, Louie AK, Roberts LW. Machine learning and the profession of medicine. JAMA. 2016;315:551–552. 10.1001/jama.2015.18421 [DOI] [PubMed] [Google Scholar]
- 18.Kurata A, Kono A, Sakamoto T, Kido T, Mochizuki T, Higashino H, et al. Quantification of the myocardial area at risk using coronary CT angiography and voronoi algorithm-based myocardial segmentation. Eur Radiol. 2015;25:49–57. 10.1007/s00330-014-3388-2 [DOI] [PubMed] [Google Scholar]
- 19.Sumitsuji S, Ide S, Siegrist PT, Salah Y, Yokoi K, Yoshida M, et al. Reproducibility and clinical potential of myocardial mass at risk calculated by a novel software utilizing cardiac computed tomography information. Cardiovasc Interv Ther. 2016;31:218–225. 10.1007/s12928-015-0370-0 [DOI] [PubMed] [Google Scholar]
- 20.Chung MS, Yang DH, Kim YH, Kang SJ, Jung J, Kim N, et al. Myocardial segmentation based on coronary anatomy using coronary computed tomography angiography: Development and validation in a pig model. Eur Radiol. 2017;27:4044–4053. 10.1007/s00330-017-4793-0 [DOI] [PubMed] [Google Scholar]
- 21.Gada H, Kirtane AJ, Kereiakes DJ, Bangalore S, Moses JW, Généreux P, et al. Meta-analysis of trials on mortality after percutaneous coronary intervention compared with medical therapy in patients with stable coronary heart disease and objective evidence of myocardial ischemia. Am J Cardiol. 2015;115:1194–1199. 10.1016/j.amjcard.2015.01.556 [DOI] [PubMed] [Google Scholar]
- 22.Anderson HV, Stokes MJ, Leon M, Abu-Halawa SA, Stuart Y, Kirkeeide RL. Coronary artery flow velocity is related to lumen area and regional left ventricular mass. Circulation. 2000;102:48–54. [DOI] [PubMed] [Google Scholar]
- 23.Papafaklis MI, Muramatsu T, Ishibashi Y, Lakkas LS, Nakatani S, Bourantas CV, et al. Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans: comparison with pressure wire—fractional flow reserve. EuroIntervention. 2014;10:574–583. 10.4244/EIJY14M07_01 [DOI] [PubMed] [Google Scholar]
- 24.Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M, et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc Interv. 2016;9:2024–2035. 10.1016/j.jcin.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 25.Xu B, Tu S, Qiao S, X, Chen Y, Yang J, et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J Am Coll Cardiol. 2017;70:3077–3087. 10.1016/j.jacc.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 26.Westra J, Tu S, Winther S, Nissen L, Vestergaard MB, Andersen BK, et al. Evaluation of Coronary Artery Stenosis by Quantitative Flow Ratio During Invasive Coronary Angiography: The WIFI II Study (Wire-Free Functional Imaging II). Circ Cardiovasc Imaging. 2018. March;11:e007107 10.1161/CIRCIMAGING.117.007107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TRIPOD, transparent reporting of a multivariable prediction model for individual prognosis or diagnosis.
(PDF)
CAMS, coronary computed tomography angiography–based myocardial segmentation; ML, machine learning.
(DOC)
CAMS, coronary computed tomography angiography–based myocardial segmentation.
(DOC)
FFR, fractional flow reserve; ML, machine learning.
(DOC)
FFR, fractional flow reserve.
(DOC)
FFR, fractional flow reserve.
(DOC)
FFR, fractional flow reserve; IVUS-MLA, intravascular ultrasound–derived minimum lumen area.
(DOC)
FFR, flow fractional reserve.
(DOC)
(TIF)
IRB, institutional review board.
(DOCX)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files (S1 Data)