Abstract
Purpose
The overuse, misuse, and underuse of antimicrobial agents often lead to the spread of antibiotic-resistant microorganisms. The aim of our study was to describe the pattern of antibiotic prescriptions for acute respiratory tract infections (RTIs) among the adult population and the factors associated with antibiotic prescribing.
Patients and methods
The study involved patients who visited a general practitioner with suspected acute RTI. Patients with diagnosis of acute sinusitis, acute pharyngitis, acute bronchitis, and influenza were included in the study. We evaluated the presence of an indication for antibiotic therapy for selected diseases according to international guidelines. The appropriateness of any prescribed molecule was also evaluated.
Results
A total of 1,979 cases of acute RTIs were included: 1,196 (60.4%) pharyngitis, 359 (18.2%) bronchitis, 234 (11.8%) influenza, and 190 (9.6%) sinusitis. An antibiotic prescription was given in 67.3% of the consultations and was not indicated by the guidelines in 66.5% of the total RTIs. Macrolides were the most frequently prescribed antibiotics accounting for 32.5% of all those prescribed, followed by amoxicillin with clavulanic acid (31.1%) and fluoroquinolones (14.2%). The highest overprescription was associated with pharyngitis (65.9%) and the lowest with influenza (4.9%). A throat swab was performed only in 11 of all the patients with a diagnosis of acute pharyngitis.
Conclusion
The present study showed a very high frequency of nonevidence-based prescription of antibiotics at the primary care level. Future improvement programs should focus on development of evidence-based guidelines, access to postgraduate training, and better availability of diagnostic tools.
Keywords: antibiotic prescribing, appropriateness, general practitioner, primary care, respiratory tract infections, Italy
Introduction
Between 2000 and 2010, worldwide use of antibiotic drugs by humans increased by 36%,1 and its pattern has shifted toward newer broad spectrum and last-resort antibiotics, including fluoroquinolones, carbapenems, and polymixins.2
It has been demonstrated that antibiotic use is a significant and modifiable driver of antibiotic resistance.3,4 Inappropriate prescribing and over-the-counter sales of antibiotics without a prescription have resulted in a massive increase in antimicrobial use.5 The overuse of antimicrobial agents often leads to the adaptation of microorganisms, so that resistant strains may become the predominant organisms in the community, healthcare settings, or the environment. Analogously, the misuse and subtherapeutic dosing of antibiotics have also led to resistance.6
The magnitude of the problem is of concern. A recent approximation showed that worldwide 700,000 people die of resistant infections every year.7 The European Centre for Disease Prevention and Control estimates that in the United States, more than 2 million people are made ill every year with antibiotic-resistant infections, resulting in at least 23,000 deaths.8
Since a vast majority of antibiotics are prescribed in primary care and the RTIs are the most common reason for antibiotic treatment in this setting,9 primary care represents a key area to investigate with the aim to identify strategic actions to mitigate, prevent, and control antibiotic resistance. In primary care, electronic decision support system to improve physicians antimicrobial prescribing was established. Moreover, Scientific Societies and work groups have provided a general direction in clinical practice developing guidelines for appropriate antibiotic use for acute infections, such as RTIs.10–13 The observation that some antimicrobials are prescribed much more in winter suggests that they are being used to treat colds and flu, for which they have no benefit. In fact, general practitioner (GP) visits for RTIs commonly result in an antibiotic prescription.14–16 Given the predominantly viral nature of these conditions, we hypothesized that a significant proportion of the prescription is unnecessary. Therefore, we aimed to describe the pattern of antibiotic prescribing for RTIs among patients of >18 years of age who were consulting their GPs and the factors associated with inappropriate antibiotic prescription.
Patients and methods
Our study involved adult patients aged >18 years, who visited their GP with suspected RTI from January 2015 to February 2017.17
The target population consisted of the GPs practicing within the Local Health Unit (LHU) of a city in Southern Italy that provided health care within the Regional Health Service. The GPs are contractors of LHUs and responsible for the provision of primary care and the access to other health services. Indeed, in Italy, every citizen can choose freely a GP who is registered in a list of the LHU, and the maximum number of citizen registered for each GP is 1,500.18 We randomly selected from the lists provided by the LHU seven GPs who used electronic medical records and practicing both in the center and in the outskirts of the city, for an overall sample of ~10,000 patients. Before starting the study, a meeting with the randomly selected GPs was arranged to present the study and to explain that participation in the survey was voluntary and confidential, and written informed consent and collaboration were obtained.
Each physician was asked to complete a questionnaire about personal demographic information, date of graduation, eventual postgraduate medical specialization earned in addition to the certificate in general medicine, and number of years in practice. Patients’ data were collected from electronic medical records through searching specific ICD Diagnosis Code (ICD-9). The following diagnosis were included: acute sinusitis (ICD-9: 461), acute pharyngitis (ICD-9: 462), acute bronchitis (ICD-9: 466), and influenza (ICD-9: 487), whereas follow-up visits for the selected conditions were excluded. Data, extracted from the patients’ electronic medical records, included information about demographics (sex and age); date of visit; diagnosis (ICD-9 code); antibiotic prescription (antibiotic type and class) any laboratory tests, for example, throat swab culture, rapid streptococcal test, C-reactive protein (date of prescription, results); and eventually any comorbidity that could modify the antibiotic prescription (such as chronic bronchitis or COPD). We decided to not consider the duration of the antibiotic therapy, since we could only assume the length based on the number of prescribed packages of antibiotics during the first visit.
We evaluated the presence of an indication of antibiotic therapy for selected diseases according to international guidelines.10–13 In particular, antibiotic treatment is indicated:
for acute sinusitis in presence of moderate symptoms that do not improve after 10 days or worsen after 5–7 days or in presence of severe symptoms;
for acute pharyngitis in presence of a positive rapid group A streptococcal antigen detection or throat culture;
for acute bacterial bronchitis, if the patients have a history of chronic bronchitis and COPD, especially if they are smokers; and
for influenza in presence of clinical evidence of overinfection or concomitant bacterial infection.
We considered an “overprescription” when an antibiotic was prescribed without an indication, and an “underprescription” when the treatment was actually indicated but was not prescribed. The appropriateness of antibiotic prescription was also evaluated, being judged “appropriate” if there was an indication and the chosen molecule was in accordance with the guidelines.
The study protocol was ratified by the Institutional Ethical Committee (“Mater Domini” Hospital of Catanzaro, Italy) (27 April 2017).
Statistical analysis
Data were summarized using frequencies and percentages for categorical data and means and SDs for continuous data. The outcome of interest was the antibiotic prescription among subjects diagnosed with a selected condition that had not any indication for an antibiotic prescription at the first GP visit. Since the study had a cluster-sampling design, data were set up and analyzed taking into account the survey design function for handling the cluster-sampling effect. Pearson’s chi-squared tests (for categorical variables) and Student’s t-test (for continuous variables) were employed to examine the unadjusted associations between the outcome variable and other potential determinants. The same analyses were then performed adjusting for the cluster-sampling effect of patients among the GPs. A P-value <0.05 was considered statistically significant.
Statistical analysis was performed using STATA software program, version 14 (Stata Corporation College Station, TX, USA).19
Results
Of the seven GPs who were approached, five agreed to participate, for an overall sample of ~7,125 patients with an effective response rate of 71.4%. The selected five GPs were four males and one female, with an average of 32.4 years in general practice, and with a mean practice size of 1,425 (range 1,199–1,500) registered patients in the study period. All GPs are members of a professional network, but practice separately (three of them in the center, the other two in the outskirts of the city).
No diagnostic and treatment algorithms that can aid clinical decision were in place at the time of the study period.
The majority of patients were females (56.6%) and the mean age was 50 years (±19.9). A total of 1,979 cases of acute RTIs were included in the study; of these, 1,196 (60.4%) were cases of pharyngitis, 359 (18.2%) of acute bronchitis, 234 (11.8%) of influenza, and 190 (9.6%) of sinusitis.
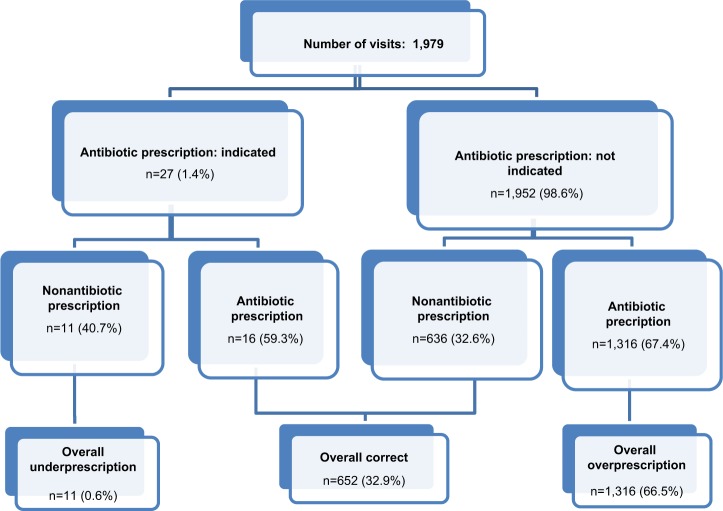
The pattern of antibiotic prescriptions is shown in Figure 1. A prescription was given in 1,332 (67.3%) consultations for acute RTI, in 73.5% of acute bronchitis, 72.5% of pharyngitis, 71.6% of sinusitis and 27.8% of influenza cases. Prescription of an antibiotic was not indicated by the guidelines in 66.5% of the selected RTIs (overprescription). Prescription pattern analyzed separately for specific RTIs showed that the highest overprescription was associated with pharyngitis (65.9%) and the lowest with influenza (4.9%). A throat swab culture was performed only in 11 (0.9%) of all the patients with a diagnosis of acute pharyngitis and was always prescribed simultaneously to the antibiotic prescription. Therefore, an empiric therapy was started before the culture result was available. No point-of-care tests, such as the rapid streptococcal test or C-reactive protein, were available in the GPs ambulatory. In accordance with the guidelines, an antibiotic prescription was actually indicated in 27 (7.5%) of all acute bronchitis episodes (18.2%), since these patients had a history of chronic bronchitis and COPD, and 16 (59.3%) of them were prescribed with an antibiotic. In 11 (40.7%) cases, although a prescription was indicated, no antibiotic treatment was prescribed.
Figure 1.
Antibiotic prescriptions analyzed according to indication for specific RTIs.
Abbreviation: RTI, respiratory tract infection.
In Table 1, the number and the type of antibiotics prescribed by GPs according to RTIs were reported. Macrolides were the most frequently prescribed antibiotics accounting for 32.5% of all prescription, followed by amoxicillin with clavulanic acid (31.1%) and fluoroquinolones (14.2%). The pattern of antibiotic use differed according to specific RTIs. In acute bronchitis, fluoroquinolones were the most frequently used antibiotics (53.1%), followed by cephalosporins of the first, second, and third generation (20.4%) and amoxicillin with clavulanic acid (13.6%). In the 16 episodes of acute bronchitis when an antibiotic was indicated and prescribed, the chosen molecules were appropriate in 10 cases. In four and six cases, a first-line agent (amoxicillin) and a second-line agent (clarithromycin or azithromycin) were prescribed, respectively. In the remaining cases, levofloxacin and cefixime were inappropriately prescribed. Leading antibiotics prescribed to patients with episodes of acute pharyngitis were macrolides (43.8%) and amoxicillin with clavulanic acid (36.1%). With regard to acute sinusitis, the most commonly chosen antibiotics were phenicols (36.1%), amoxicillin with clavulanic acid (20.6%), and macrolides (16.9%). Similarly, for influenza, the most frequently prescribed antibiotics were amoxicillin with clavulanic acid (56.9%), fluoroquinolones (13.8%), and amoxicillin (12.3%).
Table 1.
Number and type of antibiotics prescribed by the GPs according to RTIs
| Pattern of antibiotic prescription | Total n=1,979 (%) | Pharyngitis n=1,196 (%) | Acute bronchitis n=359 (%) | Influenza n=234 (%) | Sinusitis n=190 (%) |
|---|---|---|---|---|---|
| Episodes with antibiotic prescribinga | 1,332 (67.3) | 867 (72.5) | 264 (73.5) | 65 (27.8) | 136 (71.6) |
| Amoxicillin + clavulanic acid | 414 (31.1) | 313 (36.1) | 36 (13.6) | 37 (56.9) | 28 (20.6) |
| Cephalosporins | 106 (7.9) | 43 (4.9) | 54 (20.4) | 2 (3.1) | 7 (5.1) |
| Phenicols | 84 (6.3) | 35 (4.1) | 0 (0.0) | 0 (0.0) | 49 (36.1) |
| Fluoroquinolones | 189 (14.2) | 20 (2.3) | 140 (53.1) | 9 (13.8) | 20 (14.7) |
| Fusafungine | 21 (1.6) | 14 (1.6) | 0 (0.0) | 2 (3.1) | 5 (3.7) |
| Macrolides | 433 (32.5) | 380 (43.8) | 23 (8.7) | 7 (10.8) | 23 (16.9) |
| Penicillins | 51 (3.8) | 38 (4.4) | 1 (0.4) | 8 (12.3) | 4 (2.9) |
| Sulfamide + diaminopyrimidine | 34 (2.6) | 24 (2.8) | 10 (3.8) | 0 (0.0) | 0 (0.0) |
| Episodes with no antibiotic prescribing | 647 (32.7) | 329 (27.5) | 95 (26.5) | 169 (72.2) | 54 (28.4) |
Notes:
The number of antibiotics prescribed adds up to more than the number of patients because some patients received more than one. Values in bold report the recommended antibiotics for each diagnosis.
Abbreviations: GPs, general practitioners; RTIs, respiratory tract infections.
Results of the bivariate analysis, unadjusted and adjusted for the cluster-sampling effect, showed that no statistically significant difference between the antibiotic prescription and the independent covariates was found (data not shown).
Discussion
To the best of our knowledge, the present study is the first to describe the pattern of antibiotic prescriptions in patients with diagnosed RTI in the South Italian context. This is of particular importance because most RTIs are viral in nature.15,16
The present study showed a very high frequency of nonevidence-based prescription of antibiotics at the primary care level. We estimated that 66.5% of patients with RTIs were prescribed antibiotics without any indication. This is in line with a previous study conducted among Italian GPs which reported that a quite high number of physicians do not use the results of randomized controlled trials (38.7%) or meta-analysis (55.5%) to make a decision in their clinical practice.20 Such overprescription of antibiotics is particularly alarming given that there is mounting evidence to suggest that there is more harm than benefit in treating such infections with antibiotics.21 The high rate of antibiotic prescription documented in this study is not surprising, since Italy is a country where large amounts of antibiotics are used,22 also for prophylaxis purposes23 but it may be of particular concern given that recent data reported that Italy has one of the fastest growing rates of antibiotic resistance.24 However, this phenomenon is not confined to Italy alone; similar studies from other parts of Europe25 and from the United States26 have also shown that there is substantial antibiotic overprescription.
We documented that the GPs included in the study gave an antibiotic prescription without using a diagnostic test, traditional or rapid, performing a so-called empirical diagnosis, and they always used the result of the culture performed later to confirm or change their therapy. A traditional diagnostic test, such as bacterial culture, requires 36 hours or more to confirm the type of infection and the drugs to which a particular bacterial strain is susceptible, and therefore it is a sort of last resort tool to confirm a diagnosis. It is also plausible that the GPs did not prescribe the culture since it was paid by the patient. In Italy, much more effort should be put into providing the point-of-care tests, such as a Strep-A test and C-reactive protein test, to help GPs to assess the probability of the bacterial origin of symptoms. Moreover, to assess the severity of underlying conditions, pulse oximetry represents a practical noninvasive tool in patients with suspected COPD acute exacerbation since ≥50% of them involve bacteria,27 and current guidelines28 recommend antibiotic therapy for patient with more severe symptoms. Having rapid, low-cost, and readily available diagnostic tests is an essential part of the solution to the enormous unnecessary antibiotic use.
Studies in North America and Europe have also reported that GPs are generally aware of, and concerned about, the broad issue of antimicrobial resistance. However, they frequently do not perceive it as a problem,29,30 since antimicrobial resistance is considered more as a community public health issue, whereas the GPs’ priority is the health of the individual patient. Several studies have indicated that other issues such as consequences for the future physician–patient relationship are more of a concern for GPs prescribing antibiotics than antibiotic resistance.31,32 On the other hand, as reported in previous studies, within the context of the healthcare system in Southern Italy, the GP could be consulted mainly for drug prescription,33 with the more educated patients preferring private specialists.34 With this in mind, the competency of all healthcare professionals should be guaranteed by continuous training development activities on appropriate antimicrobial use, and antimicrobial stewardship should be included in all specialty training curricula for clinical specialties.
But patients also need to be educated about this, particularly in the adult age group, where parents could represent a target category for educative programs on safe self-medication practices. In Italy, antibiotics can be purchased without a medical prescription, and it has been shown that parents tend to have a positive attitude toward self-care, also for their children, with a high prevalence of use of nonprescription medicines.35 Indeed, a survey carried out in seven EU member states on the use of antibiotics without a prescription showed that, among Italian patients who had used antibiotics, 8.5% purchased them without a prescription.36 Moreover, as reported in a previous study conducted in the same area, the adult population that used Internet and social media to search for antibiotic-related information self-reported that they have practiced self-medication with antibiotics.37
Macrolides were the most commonly prescribed class of antibiotic (32.5%), contrary to the antibiotic-prescribing guidelines that establish standards of care and suggest beta-lactams (amoxicillin and penicillin V) as the recommended first-line therapy. In particular, antibiotics prescribed to patients with an acute pharyngitis were never a first-line antibiotic with a reliable activity against group A beta-hemolytic streptococcal infection, the only indication for antibiotic therapy for sore throat cases. Regarding sinusitis, a recommended first-line therapy (amoxicillin/clavulanate) was used only in 20.6% of the episodes. Moreover, as demonstrated in a study using a large pharmaceutical database to compare the effectiveness of first-line and second-line antibiotics for the treatment of acute uncomplicated sinusitis, there was no significant difference in success rates for patients treated with first-line or second-line antibiotics.38 It should be emphasized that, in Italy and in other countries, a barrier to the use of penicillin V could be the discontinuation of marketing of this antibiotic, mainly for economic reasons, as demonstrated by a survey performed in 38 countries aimed at assessing the availability of potentially useful older antibiotics.39 Therefore, antimicrobial policies to promote better use of these drugs should be implemented in primary care as well as in the hospital setting where 30%–50% of antibiotics prescribed are unnecessary40 and up to the 90% of last-line antibiotics are inappropriately prescribed.41
Strengths and limitations
Our findings are subject to some limitations. The results are based on data from five GPs. It is not clear whether our findings would generalize to clinicians in the whole country or in other countries where the medical culture may differ. Although we cannot exclude that our results pertain only to our area, it is reasonable to suppose that an analogous antibiotic prescription pattern may be referred to the Southern part of our country. Second, we acknowledge that we cannot explore the presence of comorbidity and socioeconomic characteristics of the patients as possible predictors of antibiotic prescribing, but such analyses typically only explain a small proportion of the variation between practices.42 Moreover, considering the retrospective nature of the study, the medical records were not specifically designed for the study; therefore, it was not possible to estimate information concerning detailed diagnostic categories and severity of illness, which might have accounted for the prescription of antibiotics in some cases, but we have reason to believe that the absence of an indication could have been overestimated in very few cases of complicated bronchitis. On the other hand, the recruitment process ensured we obtained views from practicing GPs, and the retrospective design yielded a realistic snapshot of everyday practice, since GPs did not modify their prescribing habit, thereby minimizing the risk of observation bias.
Conclusion
Even with these potential limitations, this study provides important information to recognize and tackle this alarming problem. Single, simple solutions are therefore unlikely to change prescribing habits at the primary care level. It has been shown that training in communication skills to provide understandable arguments to explain nonprescribing and to educate the patient on the proper use of antimicrobials might help rationalizing antibiotic use for RTIs. Similarly, limiting the availability of antimicrobials without prescription and selective use of testing might help GPs to more properly manage the patients with RTIs.43 Future improvement programs should focus on development of evidence-based guidelines, access to postgraduate training, and better availability of diagnostic tools. Future research should take into account prescribing behavior among pediatric patients.
Acknowledgments
We are very grateful to the members of the Collaborative Working Group who agreed to collect data for this study: Giacinto Nanci, Vincenzo Capilupi, Annibale Battaglia, Carmelo Luciano Rossi, and Antonietta Greco.
Publishing expenses were partly provided by the Department of Health Sciences, University of Catanzaro “Magna Graecia”, Catanzaro, Italy.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. AB and MP conceived and designed the study, were responsible for the data analysis and interpretation, wrote the article, and are guarantors for the study. RP participated in the conception and design of the study, collected the data, and contributed to the data interpretation.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 2.Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 3.Hillier S, Roberts Z, Dunstan F, Butler C, Howard A, Palmer S. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case-control study. J Antimicrob Chemother. 2007;60(1):92–99. doi: 10.1093/jac/dkm141. [DOI] [PubMed] [Google Scholar]
- 4.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan VK. Off-label abuse of antibiotics by bacteria. Gut Microbes. 2014;5:3–4. doi: 10.4161/gmic.28027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill J. The Review on Antimicrobial Resistance. London: HM Government/Wellcome Trust; 2016. Tackling drug-resistant infections globally: final report and recommendations. [Google Scholar]
- 8.European Centre for Disease Prevention and Control/European Medicine Agency The bacterial challenge: time to react. A call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. Technical Report. 2009. [Accessed December 22, 2017]. Available from: https://ecdc.europa.eu/en/publications-data/ecdcemea-joint-technical-report-bacterial-challenge-time-react.
- 9.Petersen I, Hayward AC, on behalf of the SACAR Surveillance Subgroup Antibacterial prescribing in primary care. J Antimicrob Chemoter. 2007;60(Suppl 1):i43–i47. doi: 10.1093/jac/dkm156. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Antibiotic prescribing and use in doctor’s offices. Adult Treatment Recommendations. [Accessed December 22, 2017]. Available from: https://www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html.
- 11.Harris AM, Hicks LA, Qaseem A, High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425–434. doi: 10.7326/M15-1840. [DOI] [PubMed] [Google Scholar]
- 12.Wong DM, Blumberg DA, Lowe LG. Guidelines for the use of antibiotics in acute upper respiratory tract infections. Am Fam Physician. 2006;74:956–966. [PubMed] [Google Scholar]
- 13.Harper SA, Bradley JS, Englund JA, et al. Expert Panel of the Infectious Diseases Society of America Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(8):1003–1032. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–1061. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278(11):901–904. [PubMed] [Google Scholar]
- 16.Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med. 2014;12:96. doi: 10.1186/1741-7015-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legge 8 marzo 1975, N.39. Attribuzione della maggiore età ai citta-dini che hanno compiuto il diciottesimo anno e modificazione di altre norme relative alla capacità di agire e al diritto di elettorato [Italian Law No. 39 of March 8, 1975. The Recognition of the Legal Age of Majority for Citizens Having Reached 18 Years Old and Amendment of Other Provisions relating to the Capacity to Act and the Right to Vote. Gazzetta Ufficiale della Repubblica Italiana. 1975 Mar 10; N 67. Italian. [Google Scholar]
- 18.Legge 23 dicembre 1978, N.833. Istituzione del Servizio Sanitario Nazionale [Italian Law No. 833 of December 23, 1978. National Health Service Institution] Gazzetta Ufficiale della Repubblica Italiana. 1978 Dec 28; N 360. Italian. [Google Scholar]
- 19.Stata Corporation . Stata reference manual. Release 14. College Station, TX: 2009. [Google Scholar]
- 20.De Vito C, Nobile CG, Furnari G, et al. Physicians’ knowledge, attitudes and professional use of RCTs and meta-analyses: a cross-sectional survey. Eur J Public Health. 2009;19(3):297–302. doi: 10.1093/eurpub/ckn134. [DOI] [PubMed] [Google Scholar]
- 21.Butler CC, Francis N. Commentary: controversies in NICE guidance on antibiotic prescribing for self limiting respiratory tract infections in primary care. BMJ. 2008;337:a656. doi: 10.1136/bmj.a656. [DOI] [PubMed] [Google Scholar]
- 22.European Centre for Disease Prevention and Control Summary of the latest data on antibiotic consumption in the European Union ESAC-Net surveillance data. Nov, 2017. [Accessed 22 January 2018]. Available from: https://ecdc.europa.eu/en/publications-data/summary-latest-data-antibiotic-consumption-eu-2017.
- 23.Giordano M, Squillace L, Pavia M. Appropriateness of surgical antibiotic prophylaxis in pediatric patients in Italy. Infect Control Hosp Epidemiol. 2017;38(7):823–831. doi: 10.1017/ice.2017.79. [DOI] [PubMed] [Google Scholar]
- 24.European Centre for Disease Prevention and Control Summary of the latest data on antibiotic resistance in the European Union EARS-Net surveillance data. Nov, 2017. [Accessed 22 January 2018]. Available from: https://ecdc.europa.eu/en/publications-data/summary-latest-data-antibiotic-resistance-european-union.
- 25.Lusini G, Lapi F, Sara B, et al. Antibiotic prescribing in paediatric populations: a comparison between Viareggio, Italy and Funen, Denmark. Eur J Public Health. 2009;19(4):434–438. doi: 10.1093/eurpub/ckp040. [DOI] [PubMed] [Google Scholar]
- 26.Nash DR, Harman J, Wald ER, Kelleher KJ. Antibiotic prescribing by primary care physicians for children with upper respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(11):1114–1119. doi: 10.1001/archpedi.156.11.1114. [DOI] [PubMed] [Google Scholar]
- 27.Wilson R, Sethi S, Anzueto A, Miravitlles M. Antibiotics for treatment and prevention of exacerbations of chronic obstructive pulmonary disease. J Infect. 2013;67(6):497–515. doi: 10.1016/j.jinf.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Khan JH, Lababidi HM, Al-Moamary MS, et al. The Saudi guidelines for the diagnosis and management of COPD. Ann Thorac Med. 2014;9(2):55–76. doi: 10.4103/1817-1737.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson SA, Wood F, Butler CC. General practitioners’ perceptions of antimicrobial resistance: a qualitative study. J Antimicrob Chemother. 2007;59(2):292–296. doi: 10.1093/jac/dkl467. [DOI] [PubMed] [Google Scholar]
- 30.Wood F, Simpson S, Butler CC. Socially responsible antibiotic choices in primary care: a qualitative study of GPs’ decisions to prescribe broad-spectrum and fluoroquinolone antibiotics. Fam Pract. 2007;24(5):427–434. doi: 10.1093/fampra/cmm040. [DOI] [PubMed] [Google Scholar]
- 31.Tonkin-Crine S, Yardley L, Little P. Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. J Antimicrob Chemother. 2011;66(10):2215–2223. doi: 10.1093/jac/dkr279. [DOI] [PubMed] [Google Scholar]
- 32.Lucas PJ, Cabral C, Hay AD, Horwood J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care. 2015;33(1):11–20. doi: 10.3109/02813432.2015.1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pileggi C, Carbone V, Pavia M, Angelillo IF. Patients’ perceptions and related behaviours on role of primary care physician in Italy. Eur J Public Health. 2004;14(3):258–260. doi: 10.1093/eurpub/14.3.258. [DOI] [PubMed] [Google Scholar]
- 34.Manuti B, Rizza P, Pileggi C, Bianco A, Pavia M. Assessment of perceived health status among primary care patients in Southern Italy: findings from a cross-sectional survey. Health Qual Life Outcomes. 2013;11:93. doi: 10.1186/1477-7525-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pileggi C, Mascaro V, Bianco A, Pavia M. Over-the-counter drugs and complementary medications use among children in Southern Italy. Biomed Res Int. 2015;2015:413912–413918. doi: 10.1155/2015/413912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paget J, Lescure D, Versporten A, Goossens H, Schellevis F, Van Dijk L. Antimicrobial Resistance and Causes of Non-Prudent Use of Antibiotics in Human Medicine in the EU. Brussels: European Commission; 2017. The use of antibiotics without a prescription in seven EU Member States; pp. 78–103. [Google Scholar]
- 37.Zucco R, Lavano F, Anfosso R, Bianco A, Pileggi C, Pavia M. Internet and social media use for antibiotic-related information seeking: findings from a survey among adult population in Italy. Int J Med Inform. 2018;111:131–139. doi: 10.1016/j.ijmedinf.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Piccirillo JF, Mager DE, Frisse ME, Brophy RH, Goggin A. Impact of first-line vs second-line antibiotics for the treatment of acute uncomplicated sinusitis. JAMA. 2001;286(15):1849–1856. doi: 10.1001/jama.286.15.1849. [DOI] [PubMed] [Google Scholar]
- 39.Pulcini C, Bush K, Craig WA, et al. ESCMID Study Group for Antibiotic Policies Forgotten antibiotics: an inventory in Europe, the United States, Canada, and Australia. Clin Infect Dis. 2012;54(2):268–274. doi: 10.1093/cid/cir838. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prenention Antibiotic prescribing and use in hospitals and long-term care. [Accessed June 28, 2018]. Available from: https://www.cdc.gov/antibiotic-use/healthcare/
- 41.Bianco A, Rizza P, Scaramuzza G, Pavia M. Appropriateness of glycopeptide use in a hospital in Italy. Int J Antimicrob Agents. 2006;27(2):113–119. doi: 10.1016/j.ijantimicag.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Boggon R, Hubbard R, Smeeth L, et al. Variability of antibiotic prescribing in patients with chronic obstructive pulmonary disease exacerbations: a cohort study. BMC Pulm Med. 2013;13:32. doi: 10.1186/1471-2466-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cockburn J, Pit S. Prescribing behaviour in clinical practice: patients’ expectations and doctors’ perceptions of patients’ expectations—a questionnaire study. BMJ. 1997;315(7107):520–523. doi: 10.1136/bmj.315.7107.520. [DOI] [PMC free article] [PubMed] [Google Scholar]