Abstract
Vitiligo is a common, chronic skin disorder characterized by loss of epidermal melanocytes and progressive depigmentation. Vitiligo has complex immune, genetic, environmental, and biochemical etiology, but the exact molecular mechanisms of vitiligo development and progression, particularly those related to metabolic control, are poorly understood. Here we characterized the human vitiligo cell line PIG3V and the normal human melanocytes, HEM-l by RNA-sequencing (RNA-seq), targeted metabolomics, and shotgun lipidomics. Melanocyte-enriched miR-211, a known metabolic switch in non-pigmented melanoma cells, was severely downregulated in vitiligo cell line PIG3V and skin biopsies from vitiligo patients, while its predicted targets transcriptional co-activator PGC1-α (PPARGC1A), ribonucleotide reductase regulatory subunit M2 (RRM2), and serine-threonine protein kinase TAO1 (TAOK1) were reciprocally upregulated. miR-211 binds to PGC1-α 3’UTR locus and represses it. Although mitochondrial numbers were constant, mitochondrial complexes I, II, and IV and respiratory responses were defective in vitiligo cells. Nanoparticle-coated miR-211 partially augmented the oxygen consumption rate in PIG3V cells. The lower oxygen consumption rate, changes in lipid and metabolite profiles, and increased reactive oxygen species production observed in vitiligo cells appear to be partly due to abnormal regulation of miR-211 and its target genes. These genes represent potential biomarkers and therapeutic targets in human vitiligo.
Keywords: vitiligo, microRNA, mitochondria, metabolism
INTRODUCTION
Vitiligo is a common, chronic skin disorder characterized by loss of epidermal melanocytes and progressive depigmentation (Ezzedine et al., 2012, Le Poole et al., 1993). Vitiligo is classified into two main types: non-segmental (generalized) or segmental, affects approximately 0.5% of the global population (Ezzedine et al., 2012) and is often disfiguring and distressing to patients, who can suffer high levels of psychological morbidity (Silverberg and Silverberg, 2013).
The pathoetiology of vitiligo is multifactorial and has genetic, immunological, and environmental components. There is strong epidemiological and genetic evidence implicating an autoimmune pathogenesis in vitiligo and it is strongly associated with other immune disorders (Gey et al., 2013, Richmond et al., 2013, Sandoval-Cruz et al., 2011). Genome-wide analyses have revealed a number of autoimmune susceptibility loci in vitiligo including in tyrosinase (TYR), a crucial enzyme involved in melanin production (Jin et al., 2010, Spritz, 2010). Intrinsic cellular factors like oxidative stress and reactive oxygen species (ROS) also participate in vitiligogenesis (Jimbow et al., 2001); for example, NF-E2-related factor 2 (NRF2)- antioxidant responsive element (ARE) signaling is impaired in vitiliginous melanocytes rendering them vulnerable to oxidative stress (He et al., 2017, Jian et al., 2011, Jian et al., 2014). However, the exact molecular mechanisms underlying vitiligo pathogenesis remain unknown.
MicroRNAs (miRNAs) are ~22 nucleotide non-coding RNA molecules that usually bind to the 3’ untranslated region (3’UTR) of target messenger RNAs (mRNAs) to suppress gene expression (Bartel, 2004, Jonas and Izaurralde, 2015). miRNAs participate in various cellular processes including proliferation, signal transduction, metabolism, apoptosis, and immune responses (Kloosterman and Plasterk, 2006, Rebane and Akdis, 2013, Sonkoly EnikÖ et al., 2008). Dysregulated miRNA expression is associated with the pathogenesis of various inflammatory skin disorders such as psoriasis, atopic dermatitis, and allergic contact dermatitis (Sonkoly E et al., 2008), and there is some evidence implicating miRNAs in vitiligo. Gene expression microarray studies have revealed aberrant miRNA expression in the skin and serum of patients with vitiligo (Mansuri et al., 2014, Shi et al., 2014, Shi et al., 2013), with upregulation of miR-224-3p, miR-4712-3p, and miR-3940-5p in peripheral blood mononuclear cells (Wang et al., 2015), miR-25 upregulated in serum (Shi et al., 2016) and miR-99b, miR-125-b, miR-155, and miR-199a-3p upregulated in the skin (Šahmatova et al., 2016) of vitiligo patients. Little is known, however, about the exact role of miRNAs, their targets, and their mechanism of action in the pathophysiology of vitiligo.
We recently reported that miR-211 is highly expressed in human melanocytes and is severely reduced in non-pigmented melanoma cells (Mazar et al., 2010), acting as a metabolic switch and impacting growth, differentiation, and apoptosis pathways as observed in vitiligo (Wang et al., 2016). This prompted us to interrogate miR-211 expression and its downstream effects on metabolism in clinical samples and in vitro, hypothesizing that miR-211 contributes to the vitiligo phenotype. We show that miR-211 is lost in the vitiligo lesions and in the human vitiligo cell line PIG3V, while putative miR-211 target genes such as PPARGC1A, RRM2, and TAOK1 are highly upregulated. PIG3V cells fail to mount normal respiratory responses and it is not attributable to mitochondrial numbers alone, which is partially reversed by miR-211 overexpression. Finally, PIG3V cells show enhanced production of ROS and widespread alterations in metabolism that might explain the impaired respiratory function and oxidative imbalance.
RESULTS
Decreased miR-211 expression in vitiligo patient’s skin and vitiligo cell line PIG3V
Recent findings showing (i) abnormal miRNA expression in the skin and serum of patients with vitiligo (Mansuri et al., 2014, Shi et al., 2014, Shi et al., 2013); (ii) miR-211 positively controls pigmentation by targeting Transforming growth factor beta receptor 2 (TGFBR2), a negative regulator of melanogenic enzyme TYR and tyrosinase-related protein-1 (TYRP1) (Dai et al., 2015); (iii) our own recent findings that miR-211 acts as a metabolic switch in non-pigmented melanoma cells (Mazar et al., 2016), and (iv) miR-211-mediated reprogramming of primary fibroblasts triggers elevated expression of proinflammatory genes, cell proliferation, and migration leading to melanoma growth (Dror et al., 2016), prompted us to examine the expression and putative function of miR-211 and its downstream effectors in vitiligo pathogenesis. We reasoned that since miRNAs are known participants in the stress response and autoimmunity in vitiligo, the most highly differentially expressed genes in vitiligo lesions may regulate or be regulated by miRNAs and miR-211 would be represented in the top downregulated transcripts.
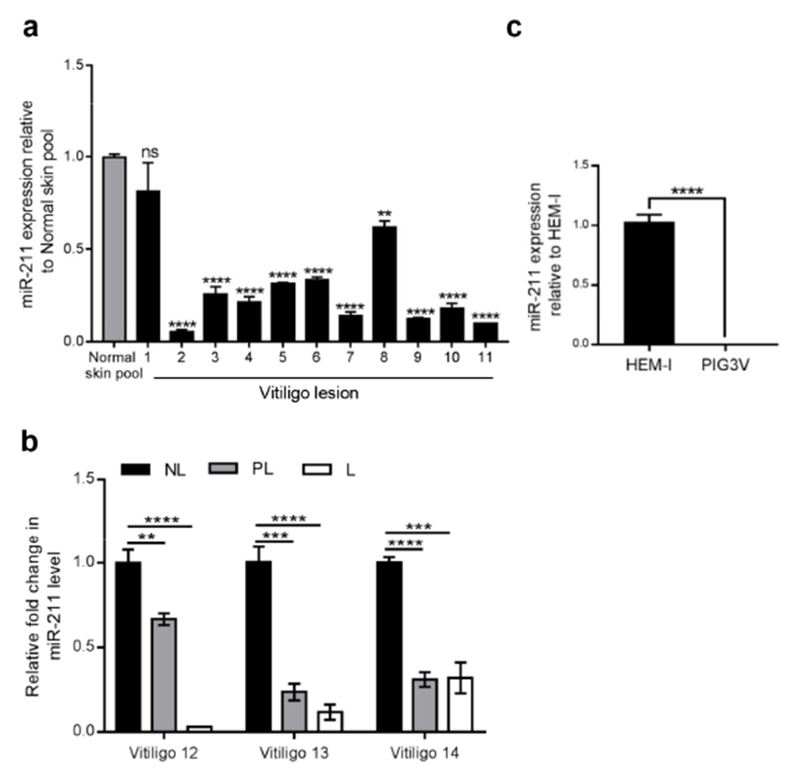
miR-211 expression was analyzed in vitiligo lesions (n=11) and healthy controls (normal skin pool from five different individuals) by qRT-PCR. miR-211 was significantly downregulated in 10 out of 11 of the biopsy samples taken from patients with vitiligo (p<0.0001) compared to normal skin (Figure 1a). Furthermore, analysis of non-lesional (NL), perilesional (PL), and lesional (L) tissues from three patients with vitiligo revealed a gradual loss of miR-211 expression from non lesional to lesional regions (Figure 1b). We went on to examine miR-211 expression in primary melanocytes (HEM-l) and immortalized peri-lesional melanocyte line from vitiligo (PIG3V) by qRT-PCR to examine whether the in vitro model replicated human disease. miR-211 expression was almost undetectable in PIG3V cells (Figure 1c). Thus, miR-211 is downregulated in vitiligo melanocytes and might participate in vitiligogenesis.
Figure 1. Differential miR-211 expression in vitiligo and normal melanocytes and human tissue samples.
(a) miR-211 expression in healthy skin (normal skin pool from 5 individuals) and vitiligo lesions (n=11) using qRT-PCR analysis. Graph shows fold change in miR-211 expression in each patient compared to normal skin pool.(b) miR-211 expression in non-lesional (NL), peri-lesional (PL), and lesional (L) regions from three patients with vitiligo Graph shows fold change in miR-211 expression in each patient compared to the corresponding NL region.(c) miR-211 expression in primary melanocytes (HEM-l) and vitiligo (PIG3V) cells. Graph shows fold change in miR-211 expression compared to HEM-l cells. Student’s t-test was performed to detect differences between the samples as indicated. P values :*< 0.05 ;**< 0.01; ***<0.001; ****<0.0001
Global transcriptomic changes in primary human melanocytes and vitiligo cells
To identify and characterize the genes and pathways participating in vitiligogenesis, we performed RNA-seq of HEM-l and PIG3V cells (Le Poole et al., 2000). Hierarchical clustering revealed that PIG3V cells showed a distinct expression pattern compared to primary melanocytes (Figure S1a). Listed are the top twenty upregulated and top twenty downregulated transcripts (p≤0.001, FDR<0.05; Tables S1a and 1b). The most significantly differentially expressed genes in pigmentation pathways (Table S2), cell cycle (Table S3), and immune responses (Table S4) are listed.
Transient receptor potential melastatin 1 (TRPM1), a calcium permeable cation channel expressed in melanocytes (Oancea et al., 2009) was observed in the top twenty downregulated transcripts in PIG3V cells compared to HEM-l cells (10-fold, Table S1b). The intronic sequence (6th intron) of TRPM1 is known to encode miR-211. In addition to absent miR-211 expression (Figure 1c), pigment production was almost absent in PIG3V vitiligo cells both visually (Figure S1b) and quantitatively (Figure S1c). Expression of major pigmentation pathway genes including KIT Proto-Oncogene Receptor Tyrosine Kinase (KIT), Melanogenesis Associated Transcription Factor (MITF), TYR, TYRP1, Dopachrome Tautomerase (DCT), premelanosome protein (PMEL), melanoma antigen recognized by T cells 1 (MART-1/MLANA), and Transient Receptor Potential Cation Channel Subfamily M Member 1 (TRPM1) was reduced in PIG3V cells compared to HEM-l cells (Figure S1d). TGFBR2, a known miR-211 target (Dai et al., 2015), was upregulated in PIG3V cells compared to HEM-l cells (Figure S1e). To exclude the possibility that immortalization and repeated passage in PIG3V cells was responsible for miR-211 expression and pigmentation, miR-211 and pigmentation pathway gene expression were examined in a pigment-producing immortalized melanocyte line PIG1 (Le Poole et al., 1997) (Figure S2). Similar results were seen between PIG1 and PIG3V cells for miR-211 expression (Figure S2a), melanin content (Figure S2b), and pigmentation pathway gene expression (Figure S2c).
To establish whether the transcriptional differences seen in HEM-l and PIG3V cells were representative of changes occurring in the lesions of patients with vitiligo, we overlaid our RNA-seq data with gene expression array data derived from biopsy samples taken from patients with vitiligo (GEO accession series GSE53148 (Rashighi et al., 2014)). In spite of the clinical samples originating from active vitiligo lesions and therefore containing infiltrating lymphocytes, highly up- or downregulated transcripts in the clinical samples were similar to those seen in HEML and PIG3V cells including melanocyte-specific genes such as MLANA and DCT (Table 1).
Table 1.
List of differentially expressed genes in PIG3V and vitiligo lesions of patients compared to primary melanocytes (HEM-l) and normal skin, respectively.
| PIG3V/HEM-1 | Vitiligo lesion / normal skin | ||||
|---|---|---|---|---|---|
| Gene | Gene Description | Log2FC | p-value | Log2FC | p-value |
| IL7R | interleukin-7 receptor | 15.2 | <0.0001 | 3.5 | <0.0001 |
| OAS1 | 2′-5′-oligoadenylate Synthetase | 12.8 | <0.0001 | 3.4 | 0.0057 |
| C3 | complement component 3 | 11.5 | <0.0001 | 2.6 | 0.0100 |
| FOSL1 | FOS-like Antigen 1 | 8.1 | <0.0001 | 2.8 | <0.0001 |
| RRM2 | ribonucleotide reductase regulatory subunit M2 | 7.7 | <0.0001 | 3.2 | 0.0014 |
| CCL5 | C-C motif chemokine ligand 5 | 4.3 | <0.0001 | 4.1 | 0.0007 |
| TAOK1 | TAO kinase 1 | 4.0 | <0.0001 | 3.0 | 0.0100 |
| IGFBP6 | insulin like growth factor binding protein 6 | 3.8 | <0.0001 | 2.6 | 0.0046 |
| HIST1H2AI | histone cluster 1, H2al | 3.6 | 0.0002 | 2.6 | 0.0038 |
| HIST1H4A | histone cluster 1, H4a | 1.6 | 0.0094 | 2.6 | 0.0026 |
| ZNF324B | zinc finger protein 324B | −1.0 | <0.0001 | −1.7 | 0.0073 |
| LYPLA2 | lysophospholipase II | −1.4 | <0.0001 | −2.2 | 0.0024 |
| ZNF500 | zinc finger protein 500 | −1.5 | 0.0002 | −2.1 | 0.0066 |
| PHLDA1 | (pleckstrin homology like domain family A member 1 | −1.8 | <0.0001 | −1.6 | 0.0047 |
| JOSD2 | Josephin domain containing 2 | −2.3 | <0.0001 | −2.7 | 0.0058 |
| GANC | glucosidase alpha, neutral C | −2.7 | 0.0094 | −2.6 | 0.0050 |
| SLC45A2 | solute carrier family 45 member 2 | −10.1 | <0.0001 | −3.0 | 0.0019 |
| MLANA | melan-A | −10.9 | <0.0001 | −4.0 | <0.0001 |
| BCAN | brevican | −11.1 | <0.0001 | −4.4 | <0.0001 |
PGC1-α is highly unregulated in vitiligo cells and is a direct miR-211 target
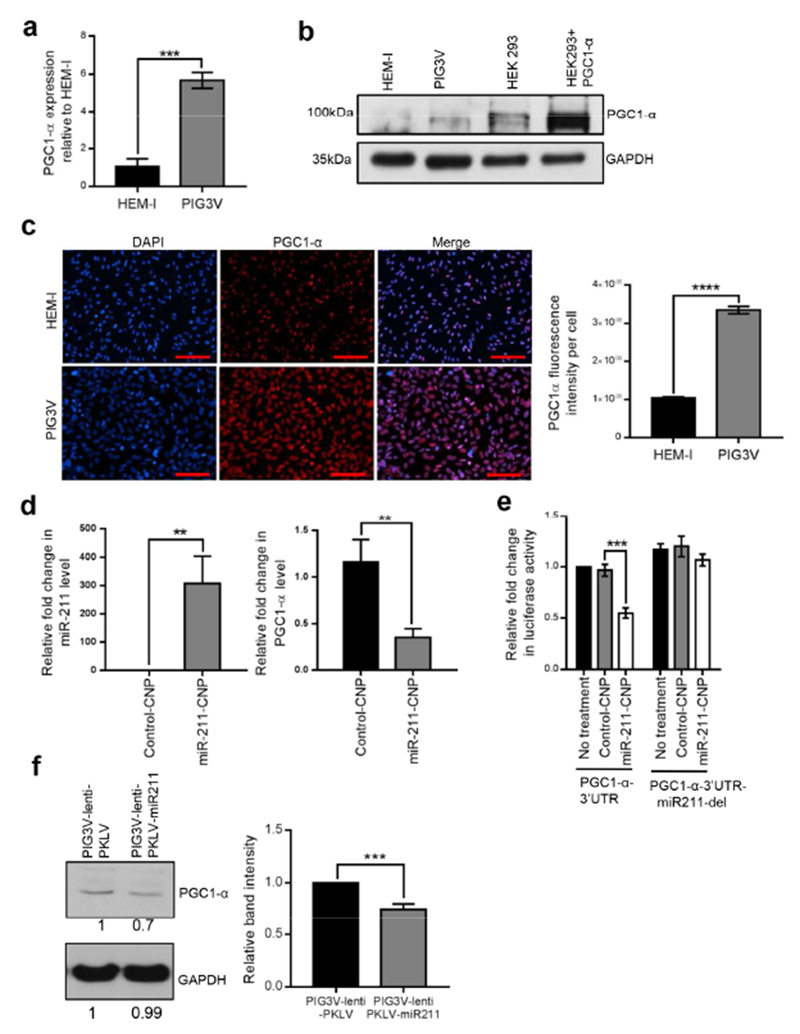
miRNAs are known to exert transcriptional and post-transcriptional control by binding to target transcripts (Bartel, 2004). Loss of miR-211 expression in vitiligo melanocytes prompted us to check in silico (TargetScan) for putative miR-211 targets that might contribute to vitiligo pathogenesis and progression. Of the targets identified, PPARGC1A/PGC1-α, a transcriptional co-activator that regulates energy metabolism by acting as a master regulator of mitochondrial biogenesis (Finck and Kelly, 2006) was a putative target and was 2.4-fold upregulated in PIG3V cells compared to HEM-l cells by RNA-seq (Table S1a). Further, confirmatory qRT-PCR (Figure 2a), western blotting (Figure 2b), and immunofluorescence (Figure 2c) showed significant upregulation of PGC1-α in PIG3V cells compared to HEM-l cells. The predicted miR-211 target-binding site is located within the 3′ UTR region of PPARGC1A/PGC1- α (Figure 4a). To directly confirm that PPARGC1A is a miR-211 target, we cloned the 3′ UTR region of the PPARGC1A transcript with and without the miR-211 seed sequence to a luciferase reporter plasmid (PGC1-α-3′UTR and PGC1-α-3′UTR-miR211-del) followed by transfection into HEK293 cells. There was no difference in reporter gene expression between PGC1-α-3′UTR and PGC1-α-3′UTR-miR211-del transfected cells (Figure S4b). However, when miR-211 was overexpressed, reporter activity was significantly reduced in PGC1-α-3′UTR transfected cells but not PGC1-α-3′UTR-miR211-del transfected cells (Figure S4b), confirming that miR-211 targets the 3’UTR region in PGC1-α.
Figure 2. Increased PGC1-α expression in vitiligo cells compared to normal melanocytes.
(a-b) HEM-l and PIG3V cells were analyzed for PGC1-α expression by qRT-PCR (a) and western blot analysis (b). (c) Immunofluorescent detection of PGC-1α (middle panel), nuclei (DAPI, left panel) and merged images (right panel) in PIG3V and HEM-l cells. 20× magnification, scale bar represents 100uM. Graph plot depicts relative fluorescence intensity of PGC1-α expression per cell. (d) PIG3V cells were treated with either miR-211-CNP or control CNP and analyzed for miR-211(left panel) and PGC1-α (right panel) expression 24 h post treatment and compared to untreated PIG3V cells. (e) PIG3V cells were transfected with luciferase expression vectors (pcDNA6-Luc) containing either PGC1-α-3’UTR or PGC1-α-3’UTR-miR211 del (miR-211 binding site deleted) sequences and treated with either miR211-CNP or control CNP at 2uM concentration 24 h post transfection. Graph shows relative fold change in luciferase activity 48hrs post transfection and compared to PIG3V cells transfected with PGC1-α-3’UTR alone. (f) Stable PIG3V lines were generated by infecting PIG3V cells with both control or miR-211 containing lentivirus and PGC1-α expression was analyzed by western blotting. Results shown are mean ± SDM and representative of at least three independent experiments. Student’s t-test was performed to detect significant differences. P values: **<0.01; ***<0.001; ****<0.0001
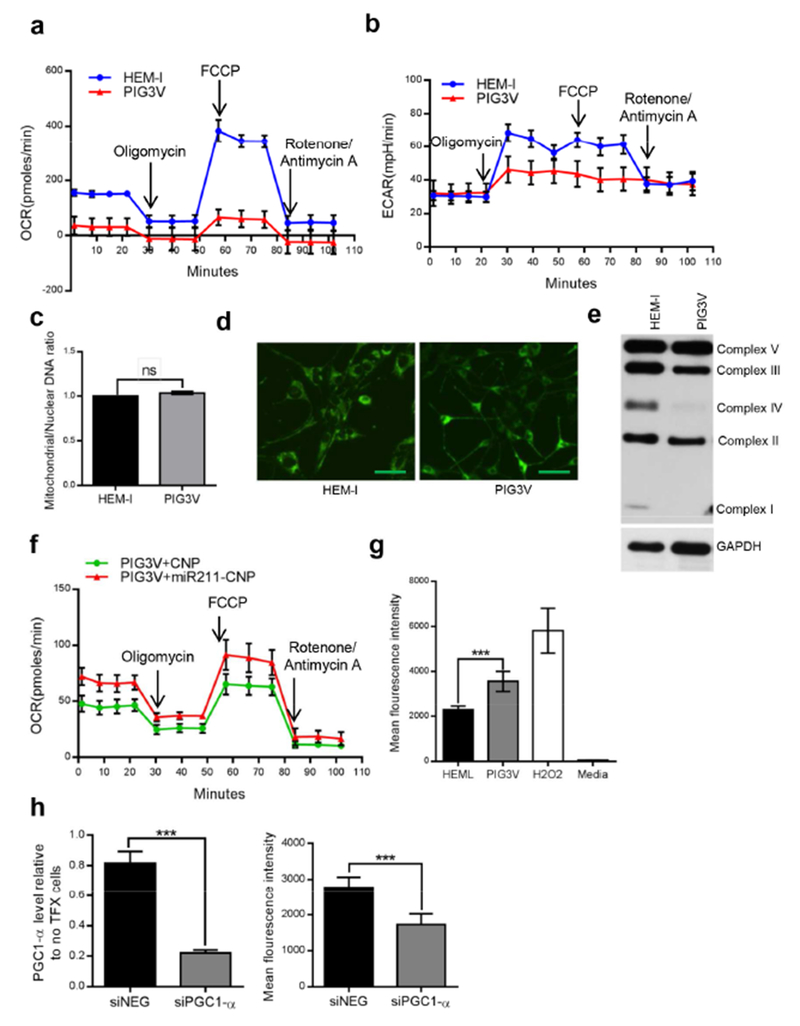
Figure 4. Reduced oxidative capacity in vitiligo melanocytes compared to normal melanocytes.
(a and b) Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were analyzed using the Seahorse XF analyzer in HEM-l and PIG3V cells. (c) Ratio of mitochondrial to nuclear DNA content in HEM-l and PIG3V cells. (d) Mitochondrial content in HEM-l and PIG3V cells was analyzed by staining the cells with MitoTracker® Green. 20× magnification, scale bar represents 25uM (e) Mitochodrial complex expression in HEM-l and PIG3V cells by western blotting. (f) OCR in PIG3V cells treated either with miR211-CNP or control CNP at 2uM concentration. (g) Intracellular ROS levels were measured in 40,000 HEM-l or PIG3V cells using the OxiSelect™ Intracellular ROS Assay Kit with hydrogen peroxide as positive control. (h) PIG3V cells were transfected with either scrambled siRNA (siNEG) or siRNA against PGC1-α and analyzed for PGC1-α expression and intracellular ROS levels. Results shown are mean ± SDM and representative of at least three independent experiments. P values: **<0.01; ***<0.001
Cerium oxide nanoparticles(CNP)s have recently been used therapeutically in pathologies associated with chronic oxidative stress and inflammation due to their regenerative antioxidant property (Das et al., 2013). Transient overexpression using miR-211-coated CNPs (miR-211-CNP) led to a dramatic increase (>500-fold) in miR-211 levels in PIG3V cells (Figure 2d, left panel) and reciprocal downregulation (>50%) of PGC1-α expression as expected (Figure 2d, right panel). Furthermore miR-211-CNP significantly reduced luciferase reporter activity in PIG3V cells transfected with PGC1-α-3′UTR but not PGC1-α-3′UTR-miR211-del plasmids (Figure 2e), further confirming that miR-211 targets PGC1-α in PIG3V cells. Finally, western blot analysis in PIG3V cells stably expressing miR-211 also revealed a significant decrease in PGC1-α protein levels (Figure 2f).
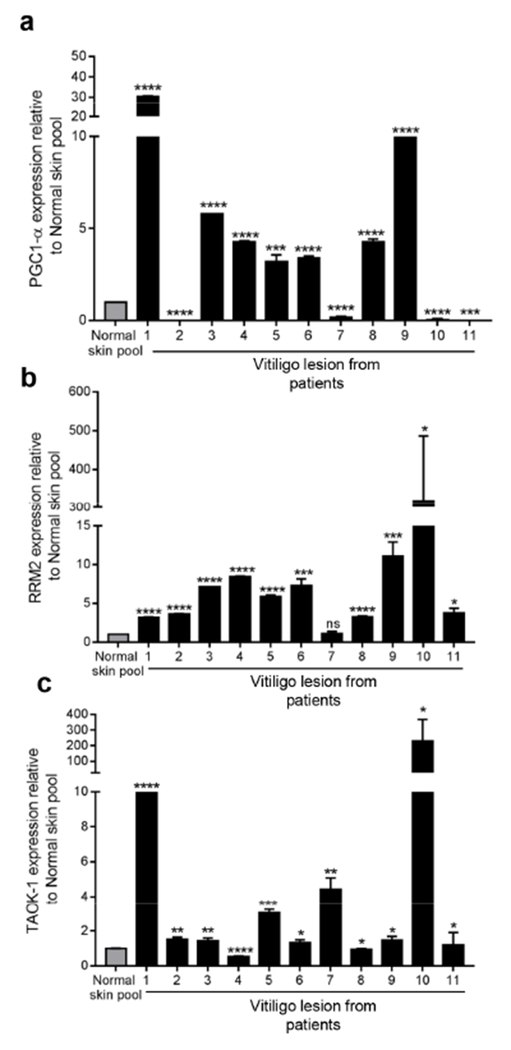
We further validated PGC1-α expression in vitiligo lesions (n=11) and healthy controls by qRT-PCR. Consistent with findings in PIG3V cells, PGC1-α expression was significantly (p<0.0001) upregulated in 7 of 11 patient samples compared to normal (Figure 3a). Furthermore, RRM2 and TAOK1, which also contain miR-211 binding sites in their 3’ UTR region, were upregulated in 90% (10/11) samples (Figure 3b and c). Reciprocal to miR-211 expression (Figure 1b), we observed a small but significant increase in PGC1-α and RRM2 but not TAOK1 expression in perilesional (PL) and lesional (L) areas compared to non-lesional (NL) areas in 2 of 3 patients (Figure S5a). Similar to PGC1-α, there was also a significant decrease in both RRM2 (20%) and TAOK1 (40%) gene expression in PIG3V cells transiently overexpressing miR-211, further supporting the hypothesis that miR-211 directly regulates their expression in vitiligo lesions (Figure S5b).
Figure 3. Expression of putative miR-211 target genes is increased in vitiligo lesions.
qPCR analysis of (a) PGC1-α, (b) RRM2, and (c) TAOK1 was analyzed in healthy skin (normal skin pool from 5 individuals) and vitiligo lesions (n=11) by qRT-PCR. Results shown are mean ± SDM. Student’s t-test was performed to detect differences in normal skin pool and individual patient samples. P values: *<0.05; **<0.01; ***<0.001; ****P<0.0001
Defective respiration and mitochondrial complexes in vitiligo cells
PGC1-α is the main member of a family of transcriptional co-activators central to metabolic control, particularly mitochondrial biogenesis, adaptive thermogenesis, and gluconeogenesis (Austin and St-Pierre, 2012, Handschin and Spiegelman, 2006). Reasoning that increased PGC1-α expression would increase the capacity of vitiligo cells to utilize oxygen, we first examined oxygen consumption rates (OCRs) using an extracellular metabolic flux analyzer. Surprisingly, the OCR of PIG3V cells was significantly lower (<50 pmol/min) than in HEM-l (150 pmol/min) cells (Figure 4a). Oligomycin, an ATP synthesis/electron transport inhibitor, reduced oxygen consumption in both cell types, suggesting that oxidative phosphorylation is active in these cell lines. However, when treated with trifluorocarbonylcyanide phenylhydrazone (FCCP), an uncoupler of electron transport and oxidative phosphorylation, the OCR increased to over 300 pmol/min in HEM-l cells compared to 90 pmols/min in PIG3V cells, suggesting that PIG3V vitiligo cells have little capacity to respond to respiratory stress. Furthermore, glycolysis, as measured by the extracellular acidification rate (ECAR), was also lower in PIG3V cells compared to HEM-l cells, suggesting that glycolysis does not substitute the energy requirements in these cells (Figure 4b) and that there is an intrinsic respiratory defect in vitiligo cells.
This prompted us to examine whether the respiratory defect seen in vitiligo cells could be explained by a decrease in mitochondrial number or occurred as a result of defects in the mitochondrial complexes forming the electron transport chain. The lower OCRs and dysfunctional maximal respiratory response was not attributable to mitochondrial numbers, which were similar in HEM-I and PIG3V cells (Figure 4c and d). However, oxidative phosphorylation (OXPHOS) complexes (mitochondrial complexes I–V in the inner mitochondrial membrane that generate ATP) were decreased in PIG3V cells (Figure 4e and Table S4), especially complexes I, II, and IV, compared to HEM-l cells. Further, treatment of PIG3V cells with miR-211-CNP resulted in a moderate but significant recovery in basal respiration rate (Figure 4f). PIG3V cells also showed increased PGC1-α mRNA and protein levels and decreased OCR and OXPHOS complexes compared to PIG1 cells (Figures S6a and b).Taken together, miR-211-mediated regulation of PGC1-α does not appear to result in mitochondrial biogenesis, but the respiratory stress response is impaired in PIG3V cells which might be attributable to mitochondrial complex dysregulation.
Higher reactive oxygen species (ROS) production in vitiligo cells
As well as regulating mitochondrial biogenesis, PGC1-α also impacts on the capacity of mitochondria to generate ROS either by upregulation of complex I and III subunits or alternatively via the detoxification of reactive oxygen species (St-Pierre et al., 2006, Valle et al., 2005). A host of studies have shown that ROS are overproduced in the vitiliginous lesions (Xie et al., 2016). As expected, intracellular ROS levels were significantly higher in PIG3V cells compared to HEM-l cells (Figure 4g). We hypothesized that overexpression of PGC1-α might be at least in part related to ROS overproduction in PIG3V cells. siRNA knockdown of PGC1-α (Figure 4h, left panel) significantly decreased intracellular ROS levels to baseline (HEM-l) (Figure 4h, right panel), suggesting that PGC1-α directly influences ROS levels in vitiligo cells.
Although PGC1-α expression is known to positively regulate the expression of ROS-detoxifying enzymes, its high expression in PIG3V cells was not related to higher expression of NRF2, the master transcriptional regulator of many phase II detoxification enzymes. NRF2 expression was markedly and significantly downregulated in PIG3V cells (Figure S7). Therefore, although the ROS-antioxidant balance is severely disrupted in PIG3V vitiligo cells and decreased PGC1-α partially reverses oxidative stress, it does not appear to exert its effects either via mitochondrial biogenesis or via ROS detoxification.
Vitiligo cells have unique lipid and metabolite profiles
Given that vitiligo cells showed defective respiratory activity and features of oxidative stress, we next performed global lipidomics and metabolomics profiling of vitiligo in PIG3V cells. We were keen to establish whether levels of important substrates were altered in vitiligo cells and might therefore contribute to the vitiligo phenotype in melanocytes.
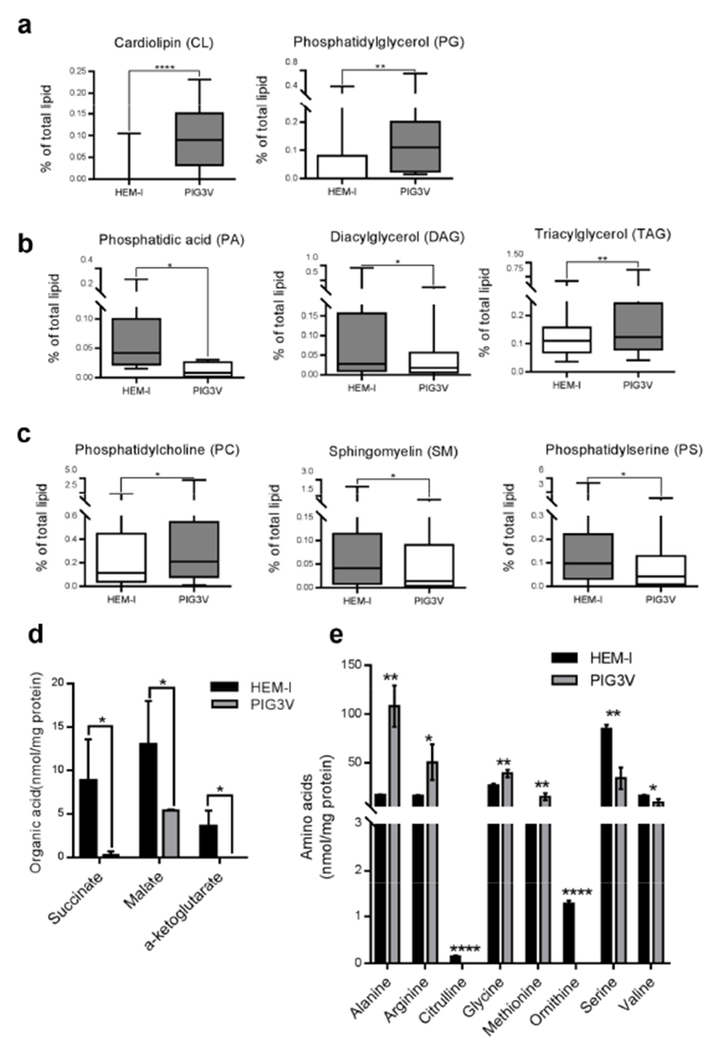
Lipidomic analysis revealed cardiolipin (CL) upregulation in PIG3V cells compared to HEM-l cells (Figure 5a, Table S6). Given that mitochondrial DNA levels were similar between these cells, it was more likely that ATP production in PIG3V cells was inefficient, perhaps due to the increased fatty acid biosynthesis utilizing Tricarboxylic acid (TCA) cycle-generated citric acid (see below) and loss of respiratory efficiency through uncoupling. The markedly higher phosphatidylglycerol (PG) levels in PIG3V cells compared to HEM-1 cells further supported the observation of increased CL biosynthesis in PIG3V cells, since PG is a key substrate for CL synthesis in mammalian systems (Figure 5a).
Figure 5. Lipidomics and metabolomics analysis of HEM-I and PIG3V cells.
(a-c) Graphs depict cumulative changes in fatty acid chains for different lipid groups. (a) Cardiolipin and phosphatidylglycerol. (b) Phosphatidic acid, diacylglycerol, and triacylglycerol. (c) Phosphatidylcholine, sphingomyelin, and phosphatidylserine. (d-e). Graphs depict significant changes in: (d) organic acids; (e) amino acids in HEM-1 and PIG3V cell lysates. P values: *<0.05; **<0.01; ***<0.001; ****<0.0001
Reduced phosphatidic acid (PA) and diacylglycerol (DAG) levels were, as expected, associated with increased triacylglycerol (TAG) levels since DAG is a substrate of TAG biosynthesis and is largely produced from PA (Coleman and Lee, 2004). Together with inefficient mitochondrial ATP production, these findings suggest increased de novo fatty acid synthesis and inappropriate energy storage in TAG in PIG3V cells for unknown reasons (Figure 5b), strongly supported by profoundly higher levels of 16:0, 16:1, 18:1, 20:1, and 20:2 fatty acyl chains in the TAG pool of PIG3V compared to HEM-l cells without significant changes in polyunsaturated fatty acids, which are exogenously supplied (Figure S8a). Phosphatidylcholine (PC), sphingomyelin (SM), and phosphatidylserine (PS) are major cellular membrane components and play important roles in maintaining normal cellular functions. For example, SM is a key component in lipid rafts (Lingwood and Simons, 2010), and PS is an important component of signal transduction and apoptosis (Tyurina et al., 2000). Marked changes in these lipids were observed in PIG3V compared to HEM-l cells. Specifically, there was two-fold higher PC but half the amount of SM and PS levels in PIG3V cells compared to HEM-1 cells (Figure 5c). Intriguingly, the most reduced SM species were N18:0 and N24:1 (Figure S8b), reduction of which indicates downregulation of ceramide synthase 1, further supported by the ceramide mass levels (N18:0 and N24:1 ceramide lower in PIG3V than HEM-1 cells and N16:0 ceramide the same; Figure S8c).
Finally, we performed targeted metabolomics in HEM-l and PIG3V cells and looked at the significant changes associated with amino acids and organic acids in HEM-I and PIG3V cells (Figure 5d and e). Significant decreases in succinate, malate, and alpha-ketoglutarate levels (Figure 5d) in PIG3V cells indicate multiple defects in mitochondrial TCA cycle intermediates. This corroborates our previous findings showing aberrant mitochondrial assembly and inefficient respiration in PIG3V cells compared to HEM-I cells (Figure 4a-e). Profiling of amino acids revealed significant increases in alanine, arginine, glycine, and methionine in PIG3V cells, while citrulline and ornithine levels were dramatically decreased (Figure 5e), and modest decreases in serine and valine levels were also observed. The turnover of respiratory substrates might be more important than mitochondrial numbers in energy utilization in vitiligo cells. Further analysis of lipid and metabolite profiles in miR211-CNP treated PIG3V cells could be helpful in understanding the role of miR-211 in vitiligo.
DISCUSSION
Here we report decreased levels of miR-211 in vitiliginous lesions and in PIG3V, an immortalized perilesional melanocyte cell line. Correspondingly, the putative miR-211 target genes PPARGC1A (PGC1-α), RRM2, and TAOK1 were upregulated in PIG3V cells and lesional biopsies. We show that miR-211 binds to and represses PGC1-α at the 3’UTR locus. PIG3V cells have abnormal respiratory responses and mitochondrial complex assembly, and reintroduction of miR-211 augmented oxygen consumption in affected melanocytes. This study represents a comprehensive lipidomics and metabolomics analysis of normal and vitiliginous melanocytes, and suggests that the turnover of respiratory substrates might be more important than mitochondrial numbers in energy utilization in vitiligo cells.
miR-211 is expressed in primary melanocytes where it influences various cellular processes including pigmentation. Its loss is implicated in melanomagenesis (Dror et al., 2016, Mazar et al., 2010, Mazar et al., 2016), where it alters metabolism (Mazar et al., 2016). Similarly, here we observed loss of miR-211 expression in vitiligo lesions. Although this loss may be attributable to the loss or negligible numbers of melanocytes in these lesions due to autoimmunity (Alikhan et al., 2011, Ezzedine et al.), it is unlikely that a single dominant pathway is responsible for melanocyte loss in vitiligo. Indeed, perilesional PIG3V cells also showed a significant decrease in miR-211 expression and pigmentation compared to both primary (HEM-l) melanocytes and the melanocyte line (PIG1), suggesting that miR-211 loss represents an intrinsic cellular defect in affected cells that contributes to vitiligo pathogenesis.
Although not obtained from the same patient, HEM-l and PIG3V cells proved to be a good in vitro model of clinical vitiligo. Transcriptomic analysis of HEM-l and PIG3V revealed similar changes in many of the immune, cell cycle, and pigmentation pathway genes shown to be differentially expressed in vitiliginous lesions (Dey-Rao and Sinha, 2016, Rashighi et al., 2014, Regazzetti et al., 2015). Our finding of highly significant downregulation of pigmentation genes downstream of MITF (a master regulator of melanogenesis, six-fold downregulated) A in PIG3V cells confirmed defects in melanin biosynthesis and pigmentation and provided confidence that the changes seen in vitro were representative of the clinical disease state.
Since miRNAs usually exert their effects by controlling the expression of target genes, understanding the role of miR-211 in vitiligogenesis requires an understanding of its downstream effects. Candidate genes such as PPARGC1A, RRM2, and TAOK1 were upregulated in vitiligo lesions and also had miR-211 binding sites in their 3’-UTR regions, making them putative miR-211 targets. Although PGC1-α is known to play a role in mitochondrial biogenesis, mitochondrial numbers, mitochondrial complex assembly proteins, and oxygen consumption rates were lower in PIG3V cells in our experiments. However, the inability of vitiligo melanocytes to mount normal respiratory responses reveals a different aspect of vitiligo pathophysiology, and this disordered metabolism might contribute to melanocyte fragility and subsequent loss in vitiligo. The decreased oxygen consumption rates may not be solely due to decreases in mitochondrial numbers but rather alterations in their composition and assembly and the way in which they utilize available substrates.
In summary, our results suggest that miR-211 may play a crucial role in regulating the pathophysiology of human vitiligo which is not only an immunological disorder but also a disease of abnormal cellular metabolism. miR-211 and its targets may play important roles in oxidative phosphorylation and mitochondrial energy metabolism in vitiligo and have therapeutic and biomarker potential. Ultimately, a thorough understanding of intracellular metabolism in vitiligo cells is required so that these pathways can be targeted for therapeutic purposes.
MATERIALS AND METHODS
Patient samples
This study received ethical approval from the Sanford Burnham Prebys Medical Discovery Institute IRB/SCRO Committee, and all participants signed written informed consent. miRNA expression analysis was conducted by qRT-PCR on 11 patients with vitiligo (7 males and 4 females; 25–70 years) attending the Associates in Dermatology outpatient clinics (Orlando, Florida). The diagnosis of vitiligo was based on history, examination, and pathological findings. Nonlesional (NL), perilesional (PL) and lesional (L) tissues were obtained from 3 patients with active non segmental vitiligo from Department of Dermatology and Pediatric Dermatology, National Reference Center for Rare Skin Disorders, Hôpital Saint-André, Bordeaux, France. RNA from human Adult Normal Tissue 5 Donor Pool: Skin (1 male and 4 females; 65-83 years; Catalogue # R1234218-P, BioChain, Newark, CA) was used as control. None of the control subjects had any history of chronic skin disease or vitiligo in their family. The samples collected from patients with vitiligo were either shave or punch skin biopsies taken from the lesional and perilesional areas and formalin fixed and paraffin embedded according to standard protocols.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Sanford Burnham Prebys Medical Discovery Institute Analytical Genomics core facility for deep-sequencing, Bioinformatics core for data analysis support, Drs. Jacob Brown and Julio Ayala for reagent support for OCR, and Ms. Debbie McFadden (SBP) for formatting the manuscript. This work was supported by National Institutes of Health grants CA165184, NCI 5P30CA030199 and Florida Department of Health, Bankhead-Coley Cancer Research Program 5BC08 to RJP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview: part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. Journal of the American Academy of Dermatology 2011;65:473–91. [DOI] [PubMed] [Google Scholar]
- Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci 2012;125:4963–71. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 2004;43:134–76. [DOI] [PubMed] [Google Scholar]
- Dai X, Rao C, Li H, Chen Y, Fan L, Geng H, et al. Regulation of pigmentation by microRNAs: MITF-dependent microRNA-211 targets TGF-β receptor 2. Pigment cell & melanoma research 2015;28:217–22. [DOI] [PubMed] [Google Scholar]
- Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S. Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine 2013;8:1483–508. [DOI] [PubMed] [Google Scholar]
- Dey-Rao R, Sinha AA. Interactome analysis of gene expression profile reveals potential novel key transcriptional regulators of skin pathology in vitiligo. Genes Immun 2016;17:30–45. [DOI] [PubMed] [Google Scholar]
- Dror S, Sander L, Schwartz H, Sheinboim D, Barzilai A, Dishon Y, et al. Melanoma miRNA trafficking controls tumour primary niche formation. Nat Cell Biol 2016;18:1006–17. [DOI] [PubMed] [Google Scholar]
- Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. The Lancet;386:74–84. [DOI] [PubMed] [Google Scholar]
- Ezzedine K, Lim H, Suzuki T, Katayama I, Hamzavi I, Lan C, et al. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment cell & melanoma research 2012;25:E1–E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. The Journal of clinical investigation 2006;116:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gey A, Diallo A, Seneschal J, Léauté-Labrèze C, Boralevi F, Jouary T, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. British Journal of Dermatology 2013;168:756–61. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006;27:728–35. [DOI] [PubMed] [Google Scholar]
- He Y, Li S, Zhang W, Dai W, Cui T, Wang G, et al. Dysregulated autophagy increased melanocyte sensitivity to H(2)O(2)-induced oxidative stress in vitiligo. Scientific Reports 2017;7:42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Z, Li K, Liu L, Zhang Y, Zhou Z, Li C, et al. Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. Journal of Investigative Dermatology 2011;131:1420–7. [DOI] [PubMed] [Google Scholar]
- Jian Z, Li K, Song P, Zhu G, Zhu L, Cui T, et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligo. Journal of Investigative Dermatology 2014;134:2221–30. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Chen H, Park JS, Thomas P. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. British Journal of Dermatology 2001;144:55–65. [DOI] [PubMed] [Google Scholar]
- Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. New England Journal of Medicine 2010;362:1686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nature Reviews Genetics 2015;16:421–33. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RHA. The Diverse Functions of MicroRNAs in Animal Development and Disease. Developmental Cell 2006;11:441–50. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, Boissy RE, Sarangarajan R, Chen J, Forristal JJ, Sheth P, et al. PIG3V, an immortalized human vitiligo melanocyte cell line, expresses dilated endoplasmic reticulum. In Vitro Cell Dev Biol Anim 2000;36:309–19. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, van den Berg FM, van den Wijngaard RMJGJ, Galloway DA, van Amstel PJ, Buffing AAM, et al. Generation of a human melanocyte cell line by introduction of HPV16 E6 and E7 genes. In Vitro Cellular & Developmental Biology - Animal 1997;33:42–9. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, van den Wijngaard RM, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. Journal of investigative dermatology 1993;100:816–22. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science 2010;327:46–50. [DOI] [PubMed] [Google Scholar]
- Mansuri M, Singh M, Dwivedi M, Laddha N, Marfatia Y, Begum R. MicroRNA profiling reveals differentially expressed microRNA signatures from the skin of patients with nonsegmental vitiligo. British Journal of Dermatology 2014;171:1263–7. [DOI] [PubMed] [Google Scholar]
- Mazar J, DeYoung K, Khaitan D, Meister E, Almodovar A, Goydos J, et al. The regulation of miRNA-211 expression and its role in melanoma cell invasiveness. PloS one 2010;5:e13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazar J, Qi F, Lee B, Marchica J, Govindarajan S, Shelley J, et al. MicroRNA 211 Functions as a Metabolic Switch in Human Melanoma Cells. Molecular and Cellular Biology 2016;36:1090–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Science signaling 2009;2:ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med 2014;6:223ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebane A, Akdis CA. MicroRNAs: Essential players in the regulation of inflammation. Journal of Allergy and Clinical Immunology 2013;132:15–26. [DOI] [PubMed] [Google Scholar]
- Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, et al. Transcriptional Analysis of Vitiligo Skin Reveals the Alteration of WNT Pathway: A Promising Target for Repigmenting Vitiligo Patients. J Invest Dermatol 2015;135:3105–14. [DOI] [PubMed] [Google Scholar]
- Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Current Opinion in Immunology 2013;25:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šahmatova L, Tankov S, Prans E, Aab A, Hermann H, Reemann P, et al. MicroRNA-155 is Dysregulated in the Skin of Patients with Vitiligo and Inhibits Melanogenesis-associated Genes in Melanocytes and Keratinocytes. Acta dermato-venereologica 2016. [DOI] [PubMed] [Google Scholar]
- Sandoval-Cruz M, García-Carrasco M, Sánchez-Porras R, Mendoza-Pinto C, Jiménez-Hernández M, Munguía-Realpozo P, et al. Immunopathogenesis of vitiligo. Autoimmunity Reviews 2011;10:762–5. [DOI] [PubMed] [Google Scholar]
- Shi Q, Zhang W, Guo S, Jian Z, Li S, Li K, et al. Oxidative stress-induced overexpression of miR-25: the mechanism underlying the degeneration of melanocytes in vitiligo. Cell death and differentiation 2016;23:496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-L, Weiland M, Lim HW, Mi Q-S, Zhou L. Serum miRNA expression profiles change in autoimmune vitiligo in mice. Experimental Dermatology 2014;23:140–2. [DOI] [PubMed] [Google Scholar]
- Shi YL, Weiland M, Li J, Hamzavi I, Henderson M, Huggins RH, et al. MicroRNA expression profiling identifies potential serum biomarkers for non-segmental vitiligo. Pigment cell & melanoma research 2013;26:418–21. [DOI] [PubMed] [Google Scholar]
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol 2013;149:159–64. [DOI] [PubMed] [Google Scholar]
- Sonkoly, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol: Elsevier; 2008. p. 131–40. [DOI] [PubMed] [Google Scholar]
- Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs: novel regulators in skin inflammation. Clinical and experimental dermatology 2008;33:312–5. [DOI] [PubMed] [Google Scholar]
- Spritz RA. Shared genetic relationships underlying generalized vitiligo and autoimmune thyroid disease. Thyroid 2010;20:745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006;127:397–408. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Shvedova AA, Kawai K, Tyurin VA, Kommineni C, Quinn PJ, et al. Phospholipid signaling in apoptosis: peroxidation and externalization of phosphatidylserine. Toxicology 2000;148:93–101. [DOI] [PubMed] [Google Scholar]
- Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-lalpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res 2005;66:562–73. [DOI] [PubMed] [Google Scholar]
- Wang P, Li Y, Nie H, Zhang X, Shao Q, Hou X, et al. The changes of gene expression profiling between segmental vitiligo, generalized vitiligo and healthy individual. J Dermatol Sci 2016;84:40–9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang K, Liang J, Yang H, Dang N, Yang X, et al. Differential expression analysis of miRNA in peripheral blood mononuclear cells of patients with non-segmental vitiligo. The Journal of dermatology 2015;42:193–7. [DOI] [PubMed] [Google Scholar]
- Xie H, Zhou F, Liu L, Zhu G, Li Q, Li C, et al. Vitiligo: How do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatol Sci 2016;81:3–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.