Abstract
Hypertrophic cardiomyopathy (HCM) is the second most prevalent form of cardiomyopathy in children. The etiology of the HCM is heterogeneous, so is the age of onset of symptoms. The HCM associated with metabolic disorders and genetic syndromes presents early in childhood. There are very few case reports of early-onset infantile HCM secondary to the PRKAG2 gene. Here, we report a case of HCM in a neonate diagnosed prenatally and eventually diagnosed with a missense mutation in the PRKAG2 gene.
Keywords: Infantile Hypertrophic Cardiomyopathy, PRKAG2 gene mutation, worse outcome
Introduction
Hypertrophic cardiomyopathy (HCM), with a reported incidence of 0.24 to 0.47 per 100,000 children, is one of the major causes of sudden cardiac death in young people. 1 2 It is characterized by abnormal thickening of the heart muscle, especially of the left ventricle and the interventricular septum. HCM is inherited as an autosomal dominant disorder with idiopathic HCM contributing to approximately 70% of cases in infancy. 3 The other etiologies for HCM in infancy include metabolic disorders (∼15%), malformation syndromes (∼15%), and neuromuscular disorders (0.9%). 3 The mutations in the PRKAG2 genes, an inborn error of metabolism affecting gamma subunit of AMP-activated protein kinase, are known to be associated with HCM. 4 The loss or gain of function mutation of these gene leads to excessive deposition of the glycogen in skeletal muscles and cardiac myocytes. There are very few cases of fatal HCM reported in patients with c.1592G > A (p.Arg531Gln) mutation in the PRKAG2 gene. 5 Here, we report a case of HCM diagnosed prenatally; genetic testing postnatally showed a pathogenic variant, c.1592G > A (p.Arg531Gln) in the PRKAG2 gene.
Case Report
IC was a premature infant delivered at 36 weeks in light of fetal hydrops secondary to severe HCM. The pregnancy was complicated by gestational diabetes mellitus (GDM) requiring treatment with glyburide. A fetal echocardiogram done at 28 weeks of gestation in view of GDM and pericardial effusion on a routine prenatal ultrasound was significant for severe form of HCM with involvement of both ventricles, the interventricular septum and the right atrial wall ( Fig. 1 ). He required positive pressure ventilation with nasal continuous positive airway pressure support postdelivery secondary to pulmonary edema caused by severe diastolic dysfunction related to the HCM. A12-lead electrocardiogram (ECG) performed postnataly demonstrated biventricular hypertrophy, a short PR interval raising suspicion for storage disorder ( Fig. 2 ). Serial postnatal echocardiograms demonstrated hyperdynamic ventricular function (shortening fraction of 49%), diastolic dysfunction, decrease end-diastolic left ventricular volumes ( z -score of –6), and dynamic left ventricular outflow tract obstruction (peak instantaneous systolic gradient of 58–88 mm Hg) with near complete obliteration of the left ventricular cavity in end systole ( Fig. 3 ). In view of the echocardiographic findings, the infant was started on β-blockers and diuretics. His diuretic regimen was titrated every day to reflect his clinical and overall volume status. Acid α-glucosidase activity was normal at 61.1 nm L/mL/hour and no acid alpha-glucosidase mutation was found, ruling out Pompe disease. Other metabolic work up performed postdelivery was negative for storage disorders. A HCM gene panel done in light of negative metabolic screen was significant for pathogenic variant in the PRKAG2 gene, c.1592G > A (p.Arg531Gln). As the excessive deposition of the glycogen in this disorder is limited to cardiac myocytes, heart transplant evaluation was initiated. Around 4 weeks of age, the infant's over all hemodynamic status worsened requiring mechanical invasive ventilation. Despite aggressive medical management, the infant developed progressively worsening respiratory failure, pulmonary edema, and hypotension. In spite of our aggressive resuscitative efforts, he suffered a cardiac arrest and eventually died at around 7 weeks of age. The histological examination of the heart at autopsy with periodic acid–Schiff stain showed accumulation of glycogen in the cardiac myocytes ( Fig. 4 ). The cardiac evaluation of three siblings done post the diagnosis of HCM in this neonate demonstrated normal muscle wall thickness and functional parameters.
Fig. 1.
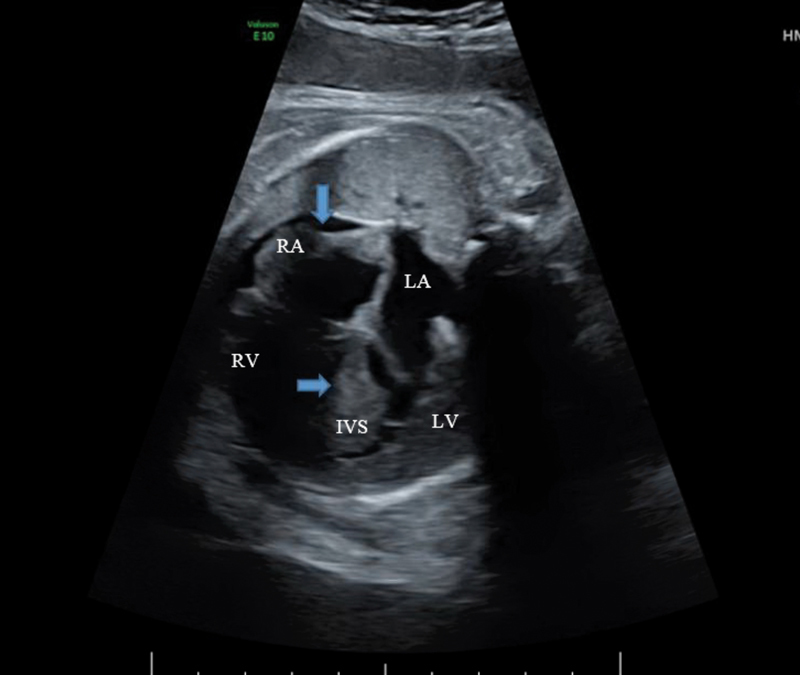
Fetal echocardiogram demonstrating severe biventricular hypertrophy and asymmetric septal hypertrophy demonstrated by
 . The right atrial wall is also noted to be hypertrophied indicated by
. The right atrial wall is also noted to be hypertrophied indicated by
 . A small pericardial effusion is noted along the right ventricular side. IVS, interventricular septum; LA, left atrium; LV, left ventricle; RA, right ventricle; RV, right ventricle.
. A small pericardial effusion is noted along the right ventricular side. IVS, interventricular septum; LA, left atrium; LV, left ventricle; RA, right ventricle; RV, right ventricle.
Fig. 2.
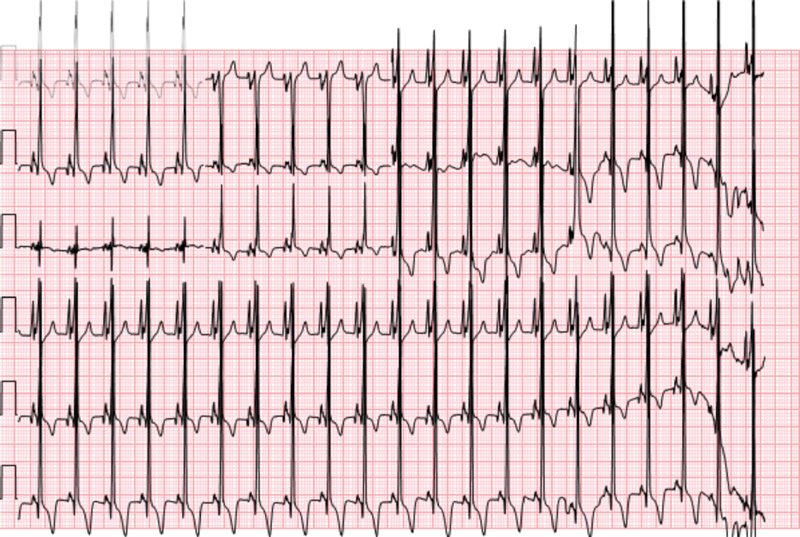
A 12-lead electrocardiogram demonstrating short PR interval, biatrial enlargement, and biventricular hypertrophy.
Fig. 3.
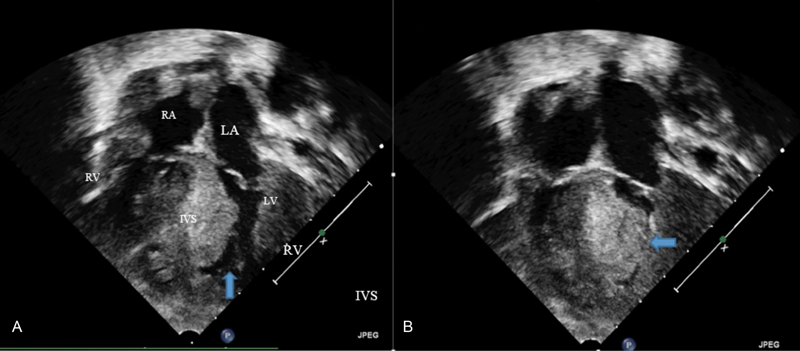
A postnatal echocardiogram showing severe HCM. (
A
) An end-diastolic still frame showing severe hypertrophy and a small end-diastolic ventricular cavity as marked by
 (
B
) An end-systolic still frame showing complete obliteration of the ventricular cavity in systole as shown by
(
B
) An end-systolic still frame showing complete obliteration of the ventricular cavity in systole as shown by
 . IVS, interventricular septum; LA, left atrium; LV, left ventricle; LV, left ventricle; RA, right atrium.
. IVS, interventricular septum; LA, left atrium; LV, left ventricle; LV, left ventricle; RA, right atrium.
Fig. 4.
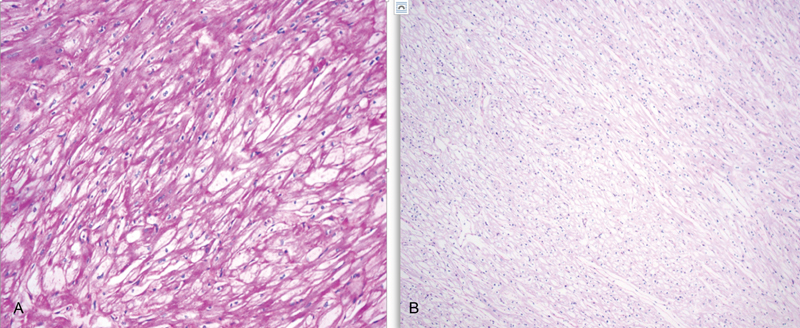
Periodic acid–Schiff (PAS) stain followed by the treatment with diastage enzyme of cardiac myocytes. ( A ) Excessive deposition of PAS-positive substrate in the cardiac myocytes. ( B ) Loss of stain following the treatment with diastase enzyme (enzyme that dissolves glycogen) indicates that PAS-positive substrate in the slide A is glycogen.
Discussion
Hypertrophic cardiomyopathy is the second most common form of pediatric cardiomyopathy following dilated cardiomyopathy. Even though the etiology of the HCM is heterogeneous, it can broadly be classified into familial, inborn errors of metabolism, malformation syndromes, neuromuscular disorders, and idiopathic disease. 3 Patients with familial HCM are most often thought to have sarcomeric gene defects and are classified as idiopathic HCM if there is no identifiable gene defect in these patients.
PRKAG2 encodes for gamma 2 regulatory subunit of AMP-activated protein kinase enzyme. Wolf–Parkinson–White syndrome 6 and cardiac hypertrophy are known to be associated with mutation of this gene. Although there is well-known association between this gene mutation and HCM, there are few reported cases of infantile onset of HCM in patients with PRKAG2 gene mutation ( Table 1 ). The mutation of PRKAG2 gene leads to non-lysosomal glycogen accumulation in cardiac myocyte leading to their hypertrophy. HCM in patients with mutation of PRKAG2 gene typically presents in teenage to adulthood with a very few reported cases of early onset. The wide variation in the age of presentation is thought to be due to severity of loss or gain of function associated with the mutation. 5 Yang et al reported a pVal335Leu mutation of PRKG2 gene affecting five members of a Chinese family. 7 Over three generations, six members of the family tested positive for the genotype and five of them exhibited the phenotype either ventricular hypertrophy or pre-excitation or both.
Table 1. Gene mutations and outcomes in previously reported cases of infantile hypertrophic cardiomyopathy due to PRKAG2 gene.
| Author name | Journal, year of publication | Age at diagnosis | Gene mutation | Outcome |
|---|---|---|---|---|
| Burwinkel et al 5 | American Journal of Human Genetics, 2005 | Neonate | Heterozygous p. Arg531Gln mutation of PRKAG2 gene | Death (21 days of life) |
| Kelly et al 9 | Pediatric Cardiology,2009 | 6 months | Heterozygous p. Glu506Gln mutation of PRKAG2 gene | Underwent surgical septal myectomy |
| Regalado et al 10 | Pediatric Cardiology, 1999 | Fetus ∼31 weeks of gestation | Heterozygous p. Arg531Gln mutation of PRKAG2 gene | Death (75 days of life) |
| DOL 10 | Heterozygous p. Arg531Gln mutation of PRKAG2 gene | Death (60 days of life) | ||
| Akman et al 11 | Pediatric Research, 2007 | 10 weeks of age | Heterozygous p. Arg384Thr mutation of PRKAG2 gene | Death (5 months of age) |
| Torok et al 12 | Journal of Inherited Metabolic Disease, 2017 | At birth | Heterozygous p. Lys475Glu mutation of PRKAG2 gene | Asymptomatic at 5 years |
| 5 weeks | Heterozygous p. Arg531Gln mutation of PRKAG2 gene | Death (4 months of age) | ||
| 2 months | Heterozygous p. Gly100Ser mutation of PRKAG2 gene | Worsening muscle weakness, asymptomatic from cardiac standpoint at 7 years of age | ||
| Present case | Fetus ∼28 weeks of gestation | Heterozygous p. Arg531Gln mutation of PRKAG2 gene | Death (7 weeks of age) |
Abbreviation: DOL, days of life.
Hypertrophic cardiomyopathy secondary to PRKAG2 gene is typically diagnosed in adolescence to fourth and fifth decade of life with a mean age of 30 years at diagnosis. 8 The commonly reported gene mutations are p.Arg302Gly (57%) and p.Asn488Ile (21%). Short PR interval, a common electrocardiographic manifestation, is seen in approximately 68% of the patients. The incidence of supraventricular tachycardia is approximately 38% in patients with PRKAG2 gene mutation. Also, the sudden cardiac death was reported in approximately 9% of the patients with this gene mutation. There are no clear data on the pathophysiological process leading to sudden cardiac death and is thought to be secondary to ventricular arrhythmia and abrupt onset of heart block.
Onset HCM due to PRKAG2 gene in the first year of life is rare and is associated with poor outcomes. Upon review of the literature, we found five articles with a total of eight patients reported to have early onset of HCM secondary to mutations in PRKAG2 gene (Table 1). Kelly et al reported a case of severe HCM in a 6-month-old infant with novel mutation of PRKAG2 gene with replacement of glutamate by glutamine at p. Glu506Gln position. 9 The reported patient underwent surgical myomectomy to decrease the dynamic outflow tract obstruction. Pathological evaluation of the biopsy in this patient surprisingly demonstrated normal amount of glycogen with mild increase in interstitial connective tissue. Of the other family members evaluated, the infant's father and sister were noted to be positive for the same mutation. In 1999, Regalado et al reported two cases of fatal neonatal HCM secondary to PRKAG2 gene mutation. 10 In addition to these two patients, Burwinkel et al reported another patient with severe form of fatal HCM in neonatal period. All these patients were positive for the same heterogeneous missense mutation of PRKAG2 gene, p. Arg531Gln. 5 They had symptoms of cardiorespiratory compromise in the immediate newborn period. One of these patients was diagnosed with HCM prenatally on a fetal echocardiogram. Unfortunately, all three patients had progressively worsening heart failure and expired at 75, 34, and 21days of life, respectively. In addition to severe HCM, all of them exhibited pre-excitation on the ECG. Of note, all these three patients had de novo mutations as these mutations cause fatal HCM invariably leading to infantile death and therefore cannot be passed over to the next generation. 5 Akman et al reported another case of fatal HCM in an infant secondary to a novel heterozygous mutation, p.Arg384Thr gene mutation of the PRKAG2 gene. 11 This infant was diagnosed with HCM at the age of 10 weeks with ECG showing severe hypertrophy of the interventricular septum and atrial walls. This infant died at the age of 5 months while awaiting heart transplantation. Torok et al reported three cases of infantile HCM initially suspected to have Pompe's disease and were eventually diagnosed with PRKAG2 gene mutation. 12 Non-lysosomal cardiac glycogenosis is considered to be genetically heterogeneous because of the wide variability in clinical presentation and genetic mutations in affected patients.
There is an overlap in the clinical features among the patients with PRKAG2 gene mutation and Pompe's disease. As reported above, some of the patients with PRKAG2 gene mutation are misdiagnosed to have Pompe's disease and treated with enzyme replacement therapy. Lack of clinical improvement or unusual clinical features/course for Pompe's disease prompts further evaluation, eventually leading to the diagnosis of PRKAG2 gene mutation. 12
It is noted of all the mutations of PRKAG2 gene reported to date, p. Arg531Gln mutation has least affinity to the regulatory nucleotides of AMP protein but has enhanced basal activity and phosphorylation of the α subunit. These unusual biochemical characteristics of the AMP protein kinase enzyme might be responsible for severe phenotypic variations associated with this mutation. 5 This variant of the PRKAG2 gene has not been reported in population database. It has been described in association with severely affected individuals where it has occurred as a de novo mutation. Our patient, described in this case report, also had a p. Arg531Gln mutation, a missense mutation where the amino acid arginine is replaced by glutamine at codon 531 of the PRKAG2 gene. Similar to reported cases, our patient also had severe and early-onset HCM. The hypertrophy of the heart was noted as early as 28 weeks of gestation, while the previously reported earliest hypertrophy of the heart was at 31 weeks of gestation.
Conclusion
PRKAG2 gene mutation-induced HCM is heterogeneous in age of presentation and severity. Typically diagnosed in adulthood with relatively benign course, early onset of HCM is associated with poor prognosis. The mutations leading to early-onset HCM are different from the mutations leading to HCM in adolescence to adulthood. To our knowledge, five mutations (p. Arg531Gln, p. Glu506Gln, p. Arg384Thr, p. Lys475Glu, and p. Gly100Ser) in this gene have been reported to be associated with early onset of HCM and poor prognosis. Of all these, p. Arg531Gln mutation is associated with very severe form of the disease leading to fatal neonatal HCM.
Funding Statement
Funding None.
Conflict of Interest None.
Ethical Standards
Not required.
Authors' Contributions
Dr. Gorla and Dr. Raja drafted the initial manuscript.
Dr. Garg reviewed and revised the manuscript.
Dr. Barbouth reviewed and revised the manuscript.
Dr. Rusconi critically reviewed and revised the manuscript.
All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
References
- 1.Lipshultz S E, Sleeper L A, Towbin J A et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348(17):1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 2.Nugent A W, Daubeney P E, Chondros P et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348(17):1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 3.Colan S D, Lipshultz S E, Lowe A M et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115(06):773–781. doi: 10.1161/CIRCULATIONAHA.106.621185. [DOI] [PubMed] [Google Scholar]
- 4.Blair E, Redwood C, Ashrafian H et al. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10(11):1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 5.Burwinkel B, Scott J W, Bührer C et al. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76(06):1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayrak F, Komurcu-Bayrak E, Mutlu B, Kahveci G, Basaran Y, Erginel-Unaltuna N. Ventricular pre-excitation and cardiac hypertrophy mimicking hypertrophic cardiomyopathy in a Turkish family with a novel PRKAG2 mutation. Eur J Heart Fail. 2006;8(07):712–715. doi: 10.1016/j.ejheart.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Yang K Q, Lu C X, Zhang Y et al. A novel PRKAG2 mutation in a Chinese family with cardiac hypertrophy and ventricular pre-excitation. Sci Rep. 2017;7(01):2407. doi: 10.1038/s41598-017-02455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porto A G, Brun F, Severini G M et al. Clinical spectrum of PRKAG2 syndrome. Circ Arrhythm Electrophysiol. 2016;9(01):e003121. doi: 10.1161/CIRCEP.115.003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly B P, Russell M W, Hennessy J R, Ensing G J. Severe hypertrophic cardiomyopathy in an infant with a novel PRKAG2 gene mutation: potential differences between infantile and adult onset presentation. Pediatr Cardiol. 2009;30(08):1176–1179. doi: 10.1007/s00246-009-9521-3. [DOI] [PubMed] [Google Scholar]
- 10.Regalado J J, Rodriguez M M, Ferrer P L. Infantile hypertrophic cardiomyopathy of glycogenosis type IX: isolated cardiac phosphorylase kinase deficiency. Pediatr Cardiol. 1999;20(04):304–307. doi: 10.1007/s002469900471. [DOI] [PubMed] [Google Scholar]
- 11.Akman H O, Sampayo J N, Ross F A et al. Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma2-subunit of AMP-activated protein kinase. Pediatr Res. 2007;62(04):499–504. doi: 10.1203/PDR.0b013e3181462b86. [DOI] [PubMed] [Google Scholar]
- 12.Torok R D, Austin S L, Phornphutkul C et al. PRKAG2 mutations presenting in infancy. J Inherit Metab Dis. 2017;40(06):823–830. doi: 10.1007/s10545-017-0072-0. [DOI] [PubMed] [Google Scholar]


