Abstract
Background
For many childhood cancers, survival is lower in non-Hispanic blacks and Hispanics compared to non-Hispanic whites, which may be attributed to underlying socioeconomic factors. However, prior childhood cancer survival studies have not formally tested for mediation by socioeconomic status (SES). We applied mediation methods to quantify the role of SES in racial/ethnic differences in childhood cancer survival.
Methods
We used population-based cancer survival data from the Surveillance, Epidemiology, and End Results 18 database for black, white, and Hispanic children, ages 0-19 years, diagnosed 2000-2011 (N=31,866). We estimated black-white and Hispanic-white mortality hazard ratios (HR) and 95% confidence intervals (CI), adjusted for age, sex, and stage at diagnosis. We used the inverse odds weighting (IOW) method to test for mediation by SES, measured with a validated census tract composite index.
Results
Whites had a significant survival advantage over blacks and Hispanics for several childhood cancers. SES significantly mediated the race/ethnicity-survival association for acute lymphoblastic leukemia, acute myeloid leukemia, neuroblastoma, and non-Hodgkin lymphoma; SES reduced the original association between race/ethnicity and survival by 44% ((log hazard ratio total effect – log hazard ratio direct effect)/log hazard ratio total effect), 28%, 49%, and 34% respectively for blacks vs. whites, and by 31%, 73%, 48%, and 28% respectively for Hispanics vs. whites.
Conclusions
SES significantly mediates racial/ethnic childhood cancer survival disparities for several cancers. However, the proportion of the total race/ethnicity-survival association explained by SES varied between black-white and Hispanic-white comparisons for some cancers, suggesting that mediation by other factors differs across groups.
Keywords: socioeconomic status, childhood cancer, cancer survival, racial and ethnic disparities, mediation
Introduction
Despite improvements over the last four decades in cancer survival among the US pediatric population, marked racial and ethnic disparities persist.1 Compared to non-Hispanic white (white) children, non-Hispanic black (black) and Hispanic children experience lower survival from many cancers including leukemias,2, 3 lymphomas,4, 5 central nervous system (CNS) tumors,6 and extracranial solid tumors.7–9 The underlying causes of racial/ethnic survival differences are not well understood, and may vary by cancer type. As outlined in Figure 1, both biological and socioeconomic pathways have been proposed in the literature.10, 11 Underlying genetic variations associated with ancestry may lead to differences in tumor biology and pharmacogenetics for some childhood cancers.10 However, race/ethnicity is a socially constructed taxonomy that is not synonymous with ancestry.12 Race/ethnicity is highly correlated with socioeconomic status (SES), especially in the United States where embedded institutionalized racism continues to place racial and ethnic minorities at high risk of low SES.13 Given emerging evidence of a positive association between SES and survival from some childhood cancers,11 racial/ethnic survival disparities may also be explained by socioeconomic differences.
Figure 1.
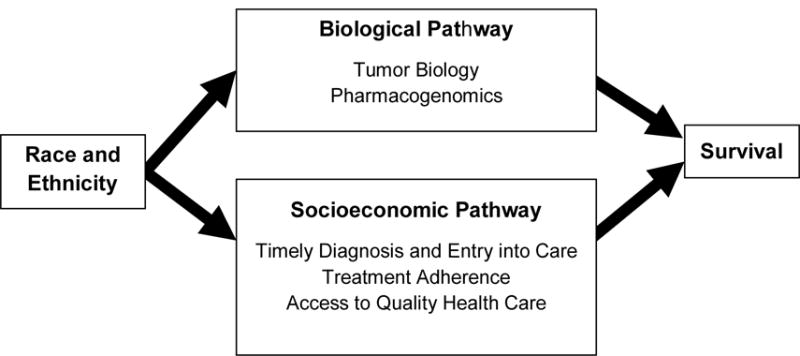
Proposed mediating pathways between race/ethnicity and childhood cancer survival
Quantifying the relative role of SES in explaining racial/ethnic survival disparities will help inform practice and intervention efforts. If SES accounts for racial/ethnic survival differences, then interventions addressing social and economic barriers to treatment and care are warranted. However, if SES does not fully account for survival differences by race/ethnicity, then other social factors (e.g. immigration), and biological mechanisms (e.g. tumor biology), must be considered. To date, formal mediation methods have not been employed to disentangle race/ethnic disparities in childhood cancer survival. Therefore, we conducted a mediation analysis using population-based data, representative of the US pediatric cancer population, to measure the role of SES in racial and ethnic childhood cancer survival disparities. We assessed survival from several childhood cancers to determine if mediation by SES differs across cancer type.
Methods
Study Population
We obtained population-based cancer registry data from the Surveillance, Epidemiology, and End Results (SEER) 18 database, excluding the Alaska Native Tumor Registry. We restricted to black, Hispanic, and white cases, ages 0 to 19 years, with microscopically confirmed first primary malignancies. Race was assigned in SEER through medical record abstraction. Hispanic ethnicity was assigned in SEER based on self/guardian-report of Spanish origin in the medical record, or by a computer algorithm that searches surnames and maiden names to determine Spanish origin. Individuals of Spanish origin were categorized as Hispanic regardless of racial background.14 SES data were available in SEER for diagnostic years 2000 to 2012. Therefore, we restricted our sample to cases diagnosed 2000-2011, followed through December 31, 2012, to allow for at least one year of follow-up. We excluded 45 cases with in situ tumors, 707 cases with missing/zero months of follow-up, and 725 cases missing SES data. We assessed cancers with ≥200 cases for each racial/ethnic group, classified using the International Classification of Childhood Cancer, third edition.15 Our final analytic sample consisted of 31,866 cases.
Measures
Overall Survival
Overall Survival was calculated in SEER as months from date of cancer diagnosis to date of death from any cause, or censored at date of last contact.
Socioeconomic Status
Socioeconomic Status was measured at the neighborhood level, based on residential address at date of cancer diagnosis, using a validated census tract composite index.16 As described in prior literature,17 the index was constructed through factor analysis of nationwide 2000 decennial census data and 2005-2009 American Community Survey (ACS) data.16 Seven indicators of neighborhood SES, previously specified by Yost et al. (2001), were included in the index: proportion employed in working-class occupations, proportion aged 16+ unemployed, education index,18 median household income, proportion below 200% poverty level, median rent, and median house value.19 Addresses were geocoded to census tracts, 2000 geographic boundaries. 2000 census values were assigned to cases diagnosed 2000-2003; 2005-2009 ACS values were assigned to cases diagnosed 2004-2011.16 The index is available in SEER as a five-level variable categorized into quintiles (Q1=lowest SES; Q5=highest SES).
Covariates
We controlled for diagnostic age group (<1, 1-4, 5-9, 10-14, 15-19 years), sex, and stage at diagnosis (SEER Summary Stage 2000 (1998+): localized, regional, distal, unknown/unstaged).20
Statistical Analysis
For each cancer type, we estimated black-white and Hispanic-white mortality hazard ratios (total effects) from multivariable Cox proportional hazards regression models. No substantial violations of the proportional hazards assumption were identified. For cancers with a statistically significant total effect, we used the inverse odds weighting (IOW) method to test for mediation by SES.21, 22 IOW analyses were conducted separately for black-white and Hispanic-white comparisons to account for the possibility that SES may mediate differently by race/ethnicity, although sensitivity analyses using multinomial models of all three racial/ethnic groups documented comparable results. IOW is a semiparametric weight-based approach that overcomes many limitations of traditional parametric mediation methods.23 For example, IOW is appropriate for any functional form (rather than just linear models), can test multiple mediators simultaneously (as opposed to testing them one by one), and is valid even in the presence of exposure-mediator interactions.24
Applying the IOW method, we estimated the (natural)25 direct effect of race/ethnicity on survival by fitting a weighted multivariable Cox proportional hazards model. Weighting by the inverse odds of exposure creates a pseudo-population in which the exposure and mediator are independent, thus estimating the race/ethnicity-survival association (direct effect) that remains after accounting for the pathway through SES.25 To obtain the IOW weights, we first estimated the odds of exposure (i.e. race/ethnicity) for each subject from a multivariable logistic regression model specifying SES and covariates. We then took the inverse of the predicted odds to create the IOW weight for whites; non-whites were assigned a weight of one. The non-white racial/ethnic group was selected as the reference to minimize extreme weighting values. Next, we estimated the (natural)25 indirect effect of race on survival operating through SES by subtracting the direct (log hazard ratio (β)) from the total effect, and bootstrapping to obtain standard errors (500 replications). A significant indirect effect provides statistical evidence of mediation. To quantify the magnitude of mediation by SES, we calculated the percent reduction from the total to the direct effect ((βtotal − βdirect)/βtotal). Statistical significance was determined as p<0.05 for a 2-sided hypothesis test. Analyses were performed using Stata 14.2 (Stata Corporation, College Station, Texas).26
Secondary Analyses
The tract-level SES index likely captures an array of socioeconomic factors contributing to survival. One such factor may be health insurance status. To empirically test this, we compared indirect effects of mediation by tract SES index and also by individual-level health insurance status (private versus otherwise), testing each of these mediators separately and simultaneously. This analysis was confined to cancers with a significant tract indirect SES effect in the primary analysis and to cases diagnosed 2007-2011, when health insurance data were available in SEER. We also explored whether we inadvertently overly adjusted IOW models by including stage at diagnosis as a covariate. Stage at diagnosis could theoretically operate as a downstream mediator of the SES-survival association if, for example, SES influences diagnostic timing.10 We tested logistic models of SES predicting tumor stage (local versus otherwise; distal versus otherwise), and we compared SES indirect effect estimates from IOW models unadjusted and adjusted for stage at diagnosis.
Results
Descriptive characteristics by cancer type are provided in Table 1. All-cause mortality from 2000 to 2012 varied across cancers, ranging from 5.2% among Hodgkin lymphoma (HL) cases to 33.8% among acute myeloid leukemia (AML) cases. Mean age at diagnosis varied across cancers, ranging from 2.5 years (SD=3.3) among neuroblastoma cases to 14.9 years (SD=3.8) among HL cases. There was a higher proportion of males compared to females for all cancers except Wilms tumor (53.4% female). The distribution of tumor stage varied across cancers (stage does not apply to leukemias). For example, only 1.9% of astrocytoma cases were classified as distal stage at diagnosis compared to 48.9% of neuroblastoma cases. The distribution of cases across SES categories was consistent across cancers. Sample characteristics by race/ethnicity are available in the supplemental materials (Table S2).
Table 1.
Characteristics of childhood cancer cases, ages 0-19 years, diagnosed 2000-2011, SEER 18 registries
| Race/Ethnicity N |
Survival Months | All-Cause Mortality | Age at Diagnosis | Female % |
Stage at Diagnosis % |
Tract-Level SES Indexa % |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Type | N | W | B | H | Mean (SD) | % | Mean (SD) | L | R | D | U | Q1 | Q2 | Q3 | Q4 | Q5 | |
| Acute Lymphoblastic Leukemia | 8,492 | 4,357 | 634 | 3,501 | 70.2 (42.6) | 12.9 | 6.9 (5.3) | 43.1 | n/a | n/a | n/a | n/a | 23.2 | 21.4 | 19.3 | 17.9 | 18.3 |
| Acute Myeloid Leukemia | 1,832 | 965 | 253 | 614 | 54.6 (43.8) | 33.8 | 8.9 (6.7) | 48.0 | n/a | n/a | n/a | n/a | 25.4 | 20.5 | 18.6 | 18.7 | 16.8 |
| Neuroblastoma | 1,901 | 1,214 | 264 | 423 | 63.5 (43.0) | 21.6 | 2.5 (3.3) | 47.6 | 20.8 | 25.3 | 48.9 | 5.0 | 20.5 | 19.7 | 21.0 | 18.6 | 20.3 |
| Non-Hodgkin Lymphoma | 2,065 | 1,169 | 343 | 553 | 68.1 (44.2) | 15.5 | 12.6 (5.0) | 37.1 | 30.9 | 19.6 | 44.4 | 5.2 | 22.4 | 19.1 | 19.3 | 19.4 | 19.9 |
| Hodgkin Lymphoma | 3,078 | 1,947 | 384 | 747 | 76.8 (41.5) | 5.2 | 14.9 (3.8) | 46.6 | 14.5 | 48.3 | 34.3 | 2.9 | 20.0 | 20.2 | 18.2 | 19.7 | 21.9 |
| Astrocytoma | 3,195 | 2,080 | 360 | 755 | 69.2 (45.4) | 17.2 | 9.1 (5.6) | 47.9 | 82.7 | 11.5 | 1.9 | 3.8 | 19.2 | 19.2 | 19.5 | 20.3 | 21.9 |
| Non-Astrocytoma CNS Tumors | 2,827 | 1,718 | 326 | 783 | 60.7 (45.1) | 31.2 | 7.5 (5.8) | 42.1 | 69.7 | 13.3 | 12.8 | 4.2 | 21.1 | 19.1 | 19.6 | 19.7 | 20.4 |
| Non-Rhabdomyosarcoma Soft Tissue Sarcomas | 1,784 | 974 | 296 | 514 | 65.3 (44.9) | 22.9 | 11.9 (5.9) | 46.6 | 56.6 | 22.8 | 15.3 | 5.4 | 20.0 | 21.5 | 19.9 | 19.0 | 19.7 |
| Rhabdomyosarcoma | 1,202 | 656 | 208 | 338 | 59.0 (43.1) | 32.6 | 7.9 (5.7) | 42.9 | 31.9 | 34.1 | 29.7 | 4.3 | 20.7 | 22.0 | 18.1 | 19.1 | 20.1 |
| Wilms Tumor | 1,430 | 789 | 254 | 387 | 71.0 (43.2) | 8.3 | 3.4 (3.0) | 53.4 | 42.4 | 29.7 | 24.8 | 3.2 | 22.5 | 21.1 | 19.7 | 18.1 | 18.6 |
| Osteosarcoma | 1,247 | 619 | 217 | 411 | 60.8 (42.1) | 32.6 | 13.2 (3.7) | 45.1 | 32.3 | 44.0 | 20.6 | 3.1 | 21.3 | 22.5 | 20.2 | 18.3 | 17.6 |
| Germ Cell Tumors | 2,813 | 1,549 | 231 | 1,033 | 72.7 (43.5) | 7.5 | 13.8 (6.0) | 35.4 | 55.5 | 22.7 | 18.2 | 3.6 | 20.9 | 20.2 | 19.8 | 19.1 | 19.9 |
Abbreviations: B, non-Hispanic black; D, distal; H, Hispanic; L, localized; N, sample size; Q, quintile; R, regional; SD, standard deviation; SES, socioeconomic status; U, unknown/unstaged; W, non-Hispanic white
Higher quintiles represent higher SES (i.e. Q1=lowest SES quintile; Q5=highest SES quintile)
We compare all-cause mortality between black versus white and Hispanic versus white cases (total effects) in Table 2. Compared to whites, black cases had a statistically significant higher hazard of death for all cancers except Wilms tumor, osteosarcoma, and germ cell tumors (GCT). Across the 9 cancers with significant racial disparities in mortality, black compared to white children exhibited from 38% (neuroblastoma) to 95% (astrocytoma) higher risk of mortality (p<0.05). Compared to whites, Hispanic cases had a statistically significant, or marginally significant (AML and non-rhabdomyosarcoma soft tissue sarcomas (NRSTS)), higher hazard of death for all cancers except HL, non-astrocytoma CNS tumors, rhabdomyosarcoma, and osteosarcoma. Among the 6 cancers exhibiting significant ethnic disparities in mortality, Hispanic children, compared to their white counterparts, exhibited from 31% (neuroblastoma) to 65% (non-Hodgkin lymphoma (NHL)) higher risk of mortality (p<0.05).
Table 2.
Comparison of all-cause mortality by race/ethnicity among childhood cancer cases, ages 0 to 19 years, diagnosed 2000-2011, SEER 18 registries
| Black versus White | Hispanic versus White | |||||
|---|---|---|---|---|---|---|
| Cancer Type | Mortality HRa | 95% CI | P | Mortality HRa | 95% CI | P |
| Acute Lymphoblastic Leukemia | 1.43 | 1.15, 1.77 | <0.01 | 1.63 | 1.44, 1.85 | <0.001 |
| Acute Myeloid Leukemia | 1.68 | 1.35, 2.08 | <0.001 | 1.19 | 0.99, 1.42 | 0.06 |
| Neuroblastoma | 1.38 | 1.07, 1.78 | 0.02 | 1.31 | 1.03, 1.66 | 0.03 |
| Non-Hodgkin Lymphoma | 1.53 | 1.15, 2.05 | <0.01 | 1.65 | 1.28, 2.13 | <0.001 |
| Hodgkin Lymphoma | 1.66 | 1.09, 2.53 | 0.02 | 1.11 | 0.76, 1.64 | 0.59 |
| Astrocytoma | 1.95 | 1.55, 2.46 | <0.001 | 1.34 | 1.10, 1.64 | <0.01 |
| Non-Astrocytoma CNS Tumors | 1.53 | 1.26, 1.86 | <0.001 | 1.07 | 0.92, 1.25 | 0.36 |
| Non-Rhabdomyosarcoma Soft Tissue Sarcomas | 1.40 | 1.08, 1.82 | 0.01 | 1.22 | 0.98, 1.53 | 0.08 |
| Rhabdomyosarcoma | 1.44 | 1.10, 1.88 | 0.01 | 1.11 | 0.88, 1.41 | 0.37 |
| Wilms Tumor | 0.96 | 0.57, 1.62 | 0.88 | 1.60 | 1.06, 2.39 | 0.02 |
| Osteosarcoma | 0.88 | 0.67, 1.16 | 0.37 | 0.99 | 0.79, 1.23 | 0.91 |
| Germ Cell Tumors | 0.98 | 0.57, 1.69 | 0.94 | 1.63 | 1.23, 2.18 | <0.01 |
Abbreviations: CI, confidence interval; HR, hazard ratio
Adjusted for age at diagnosis, sex, and stage at diagnosis (stage not applicable for leukemias)
In Table 3, we present IOW results for mediation by SES of the racial (black-white) disparity in all-cause mortality among childhood cancer cases. SES was determined to be a significant mediator of the race-survival association if the indirect effect of race on survival operating through SES was statistically significant. SES significantly mediated the black-white survival disparity for ALL (indirect effect HR (iHR)=1.17; CI=1.07, 1.28; p<0.01; 44% reduction from the total to the direct effect of the racial disparity in mortality), AML (iHR=1.15; CI=1.03, 1.29; p=0.01; 28% reduction), and neuroblastoma (iHR=1.17; CI=1.03, 1.33; p=0.02; 49% reduction). SES was a marginally significant mediator of the black-white survival disparity for NHL (iHR=1.16; CI=0.97, 1.37; p=0.10; 34% reduction). First-leg mediation results are available in the supplemental materials (Table S1).
Table 3.
Mediation by SES of racial (black versus white) survival disparities among childhood cancer cases, ages 0 to 19 years, SEER 18 registries, 2000-2011 diagnoses
| Total Effect of race on survival through all mediating pathways | Direct Effect of race on survival after blocking the SES pathway | Indirect Effect of race on survival operating through the SES pathway | Percent Reduction from total to direct effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Type | Mortality HRa | 95% CI | P | Mortality HRa | 95% CI | P | Mortality HRa | 95% CI | P | %b |
| Acute Lymphoblastic Leukemia | 1.43 | 1.15, 1.77 | <0.01 | 1.22 | 0.96, 1.54 | 0.10 | 1.17 | 1.07, 1.28 | <0.01 | 44 |
| Acute Myeloid Leukemia | 1.68 | 1.36, 2.07 | <0.001 | 1.45 | 1.15, 1.84 | <0.01 | 1.15 | 1.03, 1.29 | 0.01 | 28 |
| Neuroblastoma | 1.38 | 1.08, 1.75 | 0.01 | 1.18 | 0.91, 1.52 | 0.22 | 1.17 | 1.03, 1.33 | 0.02 | 49 |
| Non-Hodgkin Lymphoma | 1.53 | 1.14,2.07 | 0.01 | 1.33 | 0.94, 1.88 | 0.11 | 1.16 | 0.97, 1.37 | 0.10 | 34 |
| Hodgkin Lymphoma | 1.66 | 1.06, 2.60 | 0.03 | 1.50 | 0.87, 2.58 | 0.15 | 1.11 | 0.83, 1.48 | 0.50 | 20 |
| Astrocytoma | 1.95 | 1.57, 2.43 | <0.001 | 1.80 | 1.42, 2.30 | <0.001 | 1.08 | 0.98, 1.20 | 0.12 | 12 |
| Non-Astrocytoma CNS Tumors | 1.53 | 1.25, 1.88 | <0.001 | 1.41 | 1.11, 1.78 | <0.01 | 1.09 | 0.97, 1.22 | 0.14 | 20 |
| Non-Rhabdomyosarcoma Soft Tissue Sarcomas | 1.40 | 1.06, 1.84 | 0.02 | 1.34 | 0.96, 1.87 | 0.08 | 1.04 | 0.87, 1.26 | 0.65 | 13 |
| Rhabdomyosarcoma | 1.44 | 1.10, 1.88 | 0.01 | 1.33 | 0.98, 1.81 | 0.07 | 1.08 | 0.93, 1.25 | 0.31 | 21 |
Abbreviations: CI, confidence interval; HR, hazard ratio; SES, socioeconomic status
Adjusted for age at diagnosis, sex, and stage at diagnosis (stage not applicable for leukemias)
Percent reduction from the total to the direct effect ((βtotal − βdirect)/βtotal)
In Table 4, we present IOW results testing mediation by SES of the ethnic (Hispanic-white) disparity in all-cause mortality. SES significantly mediated the ethnic mortality disparity for ALL (iHR=1.16; CI=1.08,1.26; p<0.001; 31% reduction from the total to the direct effect of the ethnic disparity in mortality), AML (iHR=1.13; CI=1.03, 1.25; p=0.01; 73% reduction), neuroblastoma (iHR=1.14; CI=1.03, 1.26; p=0.01; 48% reduction), and NHL (iHR=1.15; CI=1.01, 1.31; p=0.04; 28% reduction). Notably, SES significantly mediated both the racial and ethnic disparity in survival for the same four cancers.
Table 4.
Mediation by SES of ethnic (Hispanic versus white) survival disparities among childhood cancer cases, ages 0 to 19 years, SEER 18 registries, 2000-2011 diagnoses
| Total Effect of ethnicity on survival through all mediating pathways | Direct Effect of ethnicity on survival after blocking the SES pathway | Indirect Effect of ethnicity on survival operating through the SES pathway | Percent Reduction from total to direct effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Type | Mortality HRa | 95% CI | P | Mortality HRa | 95% CI | P | Mortality HRa | 95% CI | P | %b |
| Acute Lymphoblastic Leukemia | 1.63 | 1.43, 1.86 | <0.001 | 1.40 | 1.21, 1.63 | <0.001 | 1.16 | 1.08, 1.26 | <0.001 | 31 |
| Acute Myeloid Leukemia | 1.19 | 0.99, 1.43 | 0.071 | 1.05 | 0.85, 1.29 | 0.66 | 1.13 | 1.03, 1.25 | 0.011 | 73 |
| Neuroblastoma | 1.31 | 1.04, 1.65 | 0.021 | 1.15 | 0.89, 1.49 | 0.27 | 1.14 | 1.03, 1.26 | 0.011 | 48 |
| Non-Hodgkin Lymphoma | 1.65 | 1.29, 2.12 | <0.001 | 1.44 | 1.08, 1.92 | 0.013 | 1.15 | 1.01, 1.31 | 0.04 | 28 |
| Astrocytoma | 1.34 | 1.10, 1.64 | <0.01 | 1.26 | 1.01, 1.56 | 0.041 | 1.07 | 0.98, 1.16 | 0.12 | 23 |
| Non-Rhabdomyosarcoma Soft Tissue Sarcomas | 1.22 | 0.96, 1.55 | 0.10 | 1.13 | 0.87, 1.46 | 0.38 | 1.08 | 0.95, 1.24 | 0.25 | 41 |
| Wilms Tumor | 1.60 | 1.04, 2.45 | 0.032 | 1.57 | 0.95, 2.52 | 0.061 | 1.02 | 0.83, 1.24 | 0.883 | 3 |
| Germ Cell Tumor | 1.63 | 1.19, 2.24 | <0.01 | 1.70 | 1.19, 2.42 | <0.01 | 0.96 | 0.81, 1.15 | 0.685 | -8 |
Abbreviations: CI, confidence interval; HR, hazard ratio; SES, socioeconomic status
Adjusted for age at diagnosis, sex, and stage at diagnosis (stage not applicable for leukemias)
Percent reduction from the total to the direct effect ((βtotal − βdirect)/βtotal)
Secondary Analyses
Except for NHL, the mediating effect of tract-level SES was greater than the mediating effect of health insurance status among black-white and Hispanic-white comparisons (supplemental materials, Table S3). For example, the indirect effect of tract SES on the black-white mortality disparity for ALL was 1.22 (CI=1.01, 1.48; p=0.04; 44% reduction), while the indirect effect of health insurance was 1.09 (CI=0.94, 1.27; p=0.24; 19% reduction). Among cancers with significant SES indirect effects, SES was not associated with stage at diagnosis (supplemental materials, Table S4). Exclusion of stage at diagnosis from IOW models did not lead to notably stronger indirect SES effects (supplemental materials, Tables S5–S6).
Discussion
This is the first study to use formal mediation methods to unpack childhood cancer survival disparities by race/ethnicity, which generated several findings. First, we replicated results from prior studies that whites have a significant survival advantage over blacks and Hispanics for several childhood cancers including leukemias,2, 3 lymphomas,4, 5 CNS tumors,6 neuroblastoma,7 and NRSTS.9 In no instance was survival among whites significantly worse than that of either black or Hispanic children. Racial and ethnic survival differences were not uniform across cancers, and some variability between black-white and Hispanic-white comparisons was observed. Second, we demonstrated that SES contributes to racial/ethnic survival disparities (e,g. ALL, AML, neuroblastoma, and NHL), and mediation conclusions were consistent between the black-white and Hispanic-white mediation models for these four cancers. However, SES did not fully account for racial/ethnic survival disparities (indicated by some direct effects that remained significant after testing the SES pathway); this finding aligns with prior research suggesting that race captures more than just SES, including racism and differential treatment, which we did not have the data to test in this study.27 Finally, secondary findings suggest that the association between tract SES and childhood cancer survival is not explained by differential access to health insurance, since both SES measures contributed independently to mediate the disparity; nor is it explained by tumor stage at diagnosis.
Among childhood cancers with significant mediation by SES (ALL, AML, neuroblastoma, and NHL), indirect HRs fell within a narrow range (1.13 to 1.17) for both black-white and Hispanic-white comparisons. This suggests that the association between SES and survival is not modified by, and may be shared across, race/ethnicity. Conversely, the proportion of the overall survival disparity explained by SES (i.e. % reduction) did vary by race/ethnicity for some cancers. For example, among AML cases, SES explained only 28% of the black-white survival disparity compared to 73% of the Hispanic-white disparity. This may indicate that mediation by other factors, not captured by the SES index, differs across racial/ethnic groups for some cancers. Such factors may include differences in tumor biology, pharmacogenomics, or other social factors, such as health care quality. For example, prior evidence suggests that, among AML cases, a significantly lower proportion of black children have matched family donors available compared to white and Hispanic children.28
The downstream mechanisms through which SES influences childhood cancer survival are not fully understood. Prior literature suggests that the strong association between SES and ALL survival may be explained by differences in treatment adherence.10 Adherence to the prolonged maintenance phase required for treatment of ALL may be difficult for low SES families due to social and economic constraints.10 This is supported by prior evidence of lower treatment adherence among children with ALL living in a single-mother household compared to a two-parent household.29 Research on SES and survival is less developed for other types of childhood cancer. Secondary findings from this study suggest that factors beyond health insurance status and stage at diagnosis contribute to the SES-survival association, at least for some childhood cancers. Thus, additional studies are needed to further unpack the association between SES and childhood cancer survival.
Limitations
We relied on an area-based variable as our primary measure of SES given the lack of individual-level SES measures in SEER data; moreover we selected an SES index in order to operationalize the SES construct over a meaningful period of time. Though this improves upon many prior population-based cancer studies that lacked any measures of SES or relied on county-level measures, tract-level SES is still a proxy for individual-level SES in this study because we could not comprehensively control for SES at the individual level.30, 31 Because the tract-level SES index was only available in SEER for years 2000-2012, sample size and follow-up time were limited. This prevented us from testing more homogenized cancer and racial/ethnic subgroups or stratifying by age. Additional research is thus needed for other smaller populations of racial and ethnic groups not considered in this analysis due to the rarity of childhood cancer that limited power. We also lacked geographic variables to explore potential spatial variations in survival. Further, the lack of clinical data in SEER limited our ability to account for diagnostic, therapeutic, and biological factors, such as cytogenetic or molecular features. Finally, there is the potential for differential loss to follow-up by race and SES.
Conclusion
Through the application of formal mediation methods, we demonstrated that SES significantly contributes to racial and ethnic survival disparities for several childhood cancers including ALL, AML, neuroblastoma, and NHL. Thus, for these cancers in particular, racial/ethnic survival disparities could theoretically be addressed through initiatives that reduce social and economic barriers to effective care. Such efforts may include expanded health insurance coverage, improved patient care coordination, increased health literacy, and supplementation of transportation and childcare costs during treatment. However, because SES did not fully account for survival disparities, we cannot rule out the potential role of other mediating pathways, such as tumor biology, pharmacogenomics, health care quality, or other social factors. A multipronged intervention approach that both addresses socioeconomic barriers to care and invests in personalized treatment regimens may ultimately be needed to fully eliminate childhood cancer survival disparities.
Supplementary Material
Significant Conclusions.
Socioeconomic status mediates the association between race/ethnicity and childhood cancer survival, though to varying degrees across cancers. The proportion of the total effect accounted for by socioeconomic status varied by race/ethnicity for some cancers.
Acknowledgments
Funding – This work was supported by the National Institutes of Health Translational Pediatric Cancer Epidemiology Training Grant (T32CA099936).
Footnotes
Conflicts of Interest – The authors have no conflicts of interest to disclose.
Author Contributions – All authors contributed to the conceptualization and methodology of this manuscript. R Kehm conducted the data curation and formal analysis. R Kehm wrote the original manuscript draft, and all authors reviewed and edited the manuscript.
References
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. p. 9. Based on the November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- 2.Bhatia S. Influence of race and socioeconomic status on outcome of children treated for childhood acute lymphoblastic leukemia. Current opinion in pediatrics. 2004;16:9–14. doi: 10.1097/00008480-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hossain MJ, Xie L, Caywood EH. Prognostic factors of childhood and adolescent acute myeloid leukemia (AML) survival: Evidence from four decades of US population data. Cancer epidemiology. 2015;39:720–726. doi: 10.1016/j.canep.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grubb W, Neboori H, Diaz A, Li H, Kwon D, Panoff J. Racial and ethnic disparities in the pediatric Hodgkin lymphoma population. Pediatric blood & cancer. 2016;63:428–435. doi: 10.1002/pbc.25802. [DOI] [PubMed] [Google Scholar]
- 5.Kent EE, Breen N, Lewis DR, de Moor JS, Smith AW, Seibel NL. US trends in survival disparities among adolescents and young adults with non-Hodgkin lymphoma. Cancer Causes & Control. 2015;26:1153–1162. doi: 10.1007/s10552-015-0609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin MT, Hamilton E, Zebda D, et al. Health disparities and impact on outcomes in children with primary central nervous system solid tumors. Journal of Neurosurgery: Pediatrics. 2016;18:585–593. doi: 10.3171/2016.5.PEDS15704. [DOI] [PubMed] [Google Scholar]
- 7.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children’s Oncology Group study. Journal of Clinical Oncology. 2010;29:76–82. doi: 10.1200/JCO.2010.29.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson KA, Aplenc R, Bagatell R. Survival by race among children with extracranial solid tumors in the United States between 1985 and 2005. Pediatric blood & cancer. 2011;56:425–431. doi: 10.1002/pbc.22825. [DOI] [PubMed] [Google Scholar]
- 9.Waxweiler TV, Rusthoven CG, Proper MS, et al. Non-rhabdomyosarcoma soft tissue sarcomas in children: a surveillance, epidemiology, and end results analysis validating cog risk stratifications. International Journal of Radiation Oncology* Biology* Physics. 2015;92:339–348. doi: 10.1016/j.ijrobp.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatric blood & cancer. 2011;56:994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Wilejto M, Pole JD, Guttmann A, Sung L. Low socioeconomic status is associated with worse survival in children with cancer: a systematic review. PLoS One. 2014;9:e89482. doi: 10.1371/journal.pone.0089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorde LB, Wooding SP. Genetic variation, classification and ‘race’. Nature genetics. 2004;36:S28–S33. doi: 10.1038/ng1435. [DOI] [PubMed] [Google Scholar]
- 13.Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. American journal of public health. 2000;90:1212. doi: 10.2105/ajph.90.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Division of Cancer Prevention and Control, Centers for Disease Control and Prevention. Interpreting race and ethnicity in cancer data. United States Cancer Statistics. 2016 [Google Scholar]
- 15.Steliarova‐Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer. Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 16.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes & Control. 2014;25:81–92. doi: 10.1007/s10552-013-0310-1. [DOI] [PubMed] [Google Scholar]
- 17.Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. Journal of the National Cancer Institute Monographs. 2014;2014:236–243. doi: 10.1093/jncimonographs/lgu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Deapen D, Bernstein L. Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States) Cancer Causes and Control. 1998;9:369–380. doi: 10.1023/a:1008811432436. [DOI] [PubMed] [Google Scholar]
- 19.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes & Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 20.Young J, Roffers S, Ries L, Fritz A, Hurlbut A. SEER summary staging manual 2000: codes and coding instructions. 2001 [cited 2012 Dec 20]. Availabe from: http://seer.cancer.gov/tools/ssm/intro.pdf.
- 21.Tchetgen Tchetgen EJ, Shpitser I. Semiparametric estimation of models for natural direct and indirect effects. Berkeley; CAbepress; 2011. (Harvard University Biostatistics Working Paper Series Working Paper 129). [Google Scholar]
- 22.Tchetgen Tchetgen EJ. Inverse odds ratio‐weighted estimation for causal mediation analysis. Statistics in medicine. 2013;32:4567–4580. doi: 10.1002/sim.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51:1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. American Journal of Epidemiology. 2015;181:349–356. doi: 10.1093/aje/kwu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearl J. Direct and indirect effects Proceedings of the seventeenth conference on uncertainty in artificial intelligence. Morgan Kaufmann Publishers Inc; 2001. pp. 411–420. [Google Scholar]
- 26.StataCorp L. College Station TX, USA: 2011. [Google Scholar]
- 27.Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Affairs. 2005;24:343–352. doi: 10.1377/hlthaff.24.2.343. [DOI] [PubMed] [Google Scholar]
- 28.Aplenc R, Alonzo TA, Gerbing RB, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children9s Oncology Group. Blood. 2006;108:74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. Journal of Clinical Oncology. 2012;30:2094–2101. doi: 10.1200/JCO.2011.38.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American journal of public health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. American Journal of Epidemiology. 1998;148:475–486. doi: 10.1093/oxfordjournals.aje.a009673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


