Abstract
Objective:
Lower socioeconomic status (SES) is related to poorer cognitive performance, but the neural underpinnings of this relation are not fully understood. This study examined whether SES-linked decrements in executive function were mediated by smaller dorsolateral prefrontal cortex (DLPFC) volumes. Given the literature demonstrating that SES-brain relations differ by race, we examined whether race moderated these mediations.
Methods:
Participants were 190 socioeconomically diverse, self-identified African American (AA) and White adults from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) SCAN study. Regional brain volumes were derived using T1-weighted MP-RAGE images. Adjusting for age and sex, moderated mediation analyses examined if the DLPFC mediated SES-executive function relations differently across racial groups. Executive function was measured using Trail Making Test part B (Trails B), Digit Span Backwards (DSB), and verbal fluency.
Results:
Moderated mediation demonstrated that DLPFC volume significantly mediated the association between SES and Trails B in Whites (lower confidence interval (CI) = 0.01; upper CI = 0.07), but not in AAs (lower CI = −0.05; upper CI = 0.01). No mediations were found for DSB or verbal fluency, although SES was related to all tests.
Conclusion:
The DLPFC may be important in the association of SES and mental flexibility for White, but not AA adults. It is possible that the well-replicated advantages of high SES among Whites do not readily translate, on average, to AAs. These findings highlight the importance of brain volume for cognitive functioning, while adding to the literature on sociodemographic health disparities.
Keywords: executive function, prefrontal cortex, socioeconomic status, brain volume, health disparities
It is well known that lower socioeconomic status (SES) is related to poorer cognitive function in both children (Hackman & Farah, 2009; Noble, Norman, & Farah, 2005) and adults (Mungas, Reed, Farias, & Decarli, 2009; Singh-Manoux, Richards, & Marmot, 2005). While lower levels of SES have been associated with poorer performance across most domains of cognitive functioning including language (Hart & Risely, 1995; Whitehurst, 1997), memory (Hermann & Guadagno, 1997; Noble, McCandliss, & Farah, 2007), and visuospatial ability (Farah et al., 2006; Levine, Duda, Avants, Wu, & Farah, 2005), executive function is thought to have one of the widest performance gaps across socioeconomic groups (Ardila, Rosselli, Matute, & Guajardo, 2005; Lipina, Martelli, Vuelta, Injoque-Ricle, & Colombo, 2005; Singh-Manoux et al., 2005; Turrell et al., 2002). One reason for this differential susceptibility may be due to the prolonged period of postnatal development of the frontal lobe and thus, executive function, making it particularly vulnerable to the influence of environmental factors for longer than most other neurocognitive domains (Noble et al., 2005). Executive function is a broad term used to describe a constellation of cognitive processes that support goal-oriented behavior, including attention, inhibition, set-shifting and mental flexibility, information generation, planning, working memory, rule acquisition, and self-monitoring (Baddeley & Hitch, 1974; Roberts & Pennington, 1996). As Miyake and colleagues (2000) noted, executive functions “modulate the operation of various cognitive subprocesses and thereby regulate the dynamics of human cognition” (p. 50), making them an important cognitive domain to examine in the context of SES.
The biological mechanisms responsible for the association between SES and executive function are poorly understood. One strong potential mechanism is SES-related reductions in brain volume. Contextual SES factors such as literacy, education, occupation, income, and health status appear to be related to structural brain development and atrophy (Brito & Noble, 2014; Farah et al., 2006; Fotenos, Mintun, Snyder, Morris, & Buckner, 2008; Lawson, Dude, Avants, Wu, & Farah, 2013; Raizada & Kishiyama, 2010; Raz et al., 2005; Staff et al., 2012; Taki et al., 2011; Tomalski et al., 2013). One potential pathway for these relations is stress. Those from lower SES homes report more stressful life events and are subject to more chronic stress (Baum et al., 1999), which in turn may over activate the neural stress response and lead to smaller brain volumes (Gianaros & Hackman, 2013; Noble, Houston, Kan, & Sowell, 2012). In addition to a stress-related pathway (Gianaros & Hackman, 2013; McEwen & Gianaros, 2010), lifelong exposure to low socioeconomic conditions may result in reductions in brain volume via multiple, interrelated pathways, including health behaviors (e.g., smoking, drinking, nutrition, physical activity), psychopathology, physical health, access to care and material resources, and other environmental conditions (e.g., toxic exposures).
Lower SES has been related to smaller volumes of several brain regions, including the hippocampus, amygdala, and the prefrontal cortex (PFC). Specifically, the dorsolateral PFC (DLPFC), a subdivision of the PFC involved in various executive function processes (Lezak, Howieson, Bigler, & Tranel, 2012), was found to be larger among children from high SES environments (Lawson et al., 2013; Noble et al., 2012). An important limitation of the available literature is that the majority of studies examining the relation of SES and brain volume have been conducted with children, with relatively few studies including adult participants. Individual neuroanatomical differences among children are largely related to variability in neurodevelopment, while these differences in adults may be attributable to neurodevelopment, atrophy to a matured adult brain, or both. These mechanistic differences have important implications for understanding SES-brain relations and suggest that more research is needed to delineate this phenomenon among adults. Nonetheless, one of the few available studies examining structural differences across sociodemographic groups in adults found a positive relation between level of education and volume of the middle frontal gyrus, an overlapping region of the DLPFC, in adult men (Mortby et al., 2014), suggesting that this region may also be sensitive to a lifetime of socioeconomic stressors in adults.
An extensive body of literature demonstrates that the DLPFC is highly related to components of executive function, such as working memory, set switching, and inhibitory control (Adólfsdóttir et al., 2014; Ruscheweyh et al., 2013; Yuan & Raz, 2014). Nonetheless, the primary executive subprocesses that are activated by the DLPFC are still equivocal. Structural and functional studies have consistently demonstrated that set-shifting and mental flexibility (as measured by Trail Making Test part B [Trails B]) are predominantly associated with the DLPFC (Ruscheweyh et al., 2013). Data manipulation and working memory (as measured by Digit Span Backwards [DSB]) have also been positively associated with DLPFC structure, but may be most strongly associated with the lateral temporal lobe (Ruscheweyh et al., 2013). Finally, studies of the neuroanatomical correlates of verbal regulation of behavior (as measured by verbal fluency [VF]) have yielded mixed results. Lezak and colleagues (2012) report on lesion studies that show impairment in phonemic- and semantic-based VF in patients with lesions in the DLPFC, but a meta-analysis by Yuan and Raz (2012) suggested that VF and the PFC may in fact have a weak relation (although the type of VF was not specified). While it seems that most studies examining PFC-cognition relations find that larger volumes relate to better cognitive performance, the particular neurocognitive tests that are most related to the DLPFC still need to be identified.
Based on the literature reviewed above, three limitations bear mention. First, given that much of the existing research has been done in children, more research is needed to further our understanding of the relation between SES and DLPFC volume in community-dwelling adults. Second, a more nuanced investigation of the relation between the DLPFC and several of the subcomponents of executive function is warranted. Finally, while an extensive body of literature exists looking at the respective relations of SES and cognition, SES and brain volume, and brain volume and cognition, a thorough analysis of all three variables together is lacking. It is evident that SES and cognition are strongly related, but the biological mechanisms of this relation are poorly understood. Furthermore, because it is known that SES affects brain volume (e.g., the DLPFC), and that brain volume effects cognitive ability, one might postulate that SES-linked cognitive correlates result, in part, from reductions in brain volumes.
When examining indicators of the SES-cognition relation it may be prudent to look at associations across racial groups. Glymour and Manly (2008) have stated that “with respect to research, profound differences in conditions across the life-course make black-white comparisons very difficult, and childhood exposures probably both influence and confound estimated exposures and cognitive outcomes” (p. 246). Relatedly, a study utilizing data from the Baltimore Memory Study found that differences in cognitive function across African Americans (AAs) and Whites were largely due to environmental factors, including contextual factors subsumed under the category of SES (Schwartz et al., 2004). Given the racial nonequivalence in social environment, which largely contributes to the methodological challenges outlined by Glymour and Manly (2008), it has been recommended to use race as a moderator as opposed to an adjustment variable (Williams et al., 1997).
Race may be a particularly important moderator to consider when examining SES-brain relations. Research suggests that poorer health outcomes among AAs compared to Whites is related to physiological “wear-and-tear” resulting from disproportionate levels of stress exposure and other environmental adversities, such as discrimination (Geronimus, 1992). Also, the “diminishing returns hypothesis” states that as levels of SES increase AAs do not exhibit the same health improvements as their White peers (Farmer & Ferraro, 2005). For example, using a portion of the present study’s sample, our lab recently found that while Whites benefited from their high socioeconomic positions with respect to global brain volume, AAs, on average, did not (Waldstein et al., 2017). Given these findings, it is possible that SES may relate differently to the DLPFC across racial groups.
In the present study, we sought to contribute further to this literature by examining whether the association of SES and executive function is mediated by DLPFC gray matter volume, adjusting for sex and age, in a sample of socioeconomically diverse, urban-dwelling adults. It was hypothesized that SES would relate to executive function, and that these relations would be significantly mediated by DLPFC volume. Further, we utilized a moderated mediation model to examine if the potential association between SES-DLPFC was moderated by race. Given the exploratory nature of examining race as a moderator, no hypotheses were put forth for mediational differences across racial groups.
Method
Participants
Participants were a subsample of adult AA and White participants in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. HANDLS is an ongoing, 20-year epidemiological study, focused on understanding age-related health disparities across a sociodemographically diverse group of individuals (Evans et al., 2010). The current participants were recruited from HANDLS to take part in HANDLS SCAN, an ancillary imaging study (Waldstein et al., 2017). HANDLS SCAN obtained magnetic resonance imaging (MRI) data from HANDLS participants that had completed their first or second complete follow-up visit. All participants provided both written informed consent and HIPAA authorization approved by the Institutional Review Boards (IRBs) at the University of Maryland, Baltimore and the University of Maryland, Baltimore County for HANDLS SCAN and by the IRB of the National Institute of Environmental Health Services, National Institutes of Health for HANDLS.
For the full protocol of HANDLS recruitment and data collection please see Evans and colleagues (2010). Briefly, the HANDLS sample is a fixed cohort of urban-dwelling adults living initially within 13 contiguous census segments in the city of Baltimore, MD. The census segments were pre-determined for their likelihood of yielding representative samples of participants who were AA and White, men and women, and with annual household incomes above and below 125% of the 2004 federal poverty guidelines (for more information regarding the 2004 Health and Human Services poverty guidelines, please visit https://aspe.hhs.gov/2004-hhs-poverty-guidelines). Data collection and informed consent occurred on the Mobile Medical Research Vehicles (MRVs) located within participants’ neighborhoods, where they completed medical history assessments, physical examinations, cognitive testing, as well as other evaluations irrelevant to the current study.
HANDLS study exclusions were (1) outside of the age range 30–64 years at baseline testing; (2) currently pregnant; (3) within six months of receiving chemotherapy, radiation, or biological treatments for cancer; (4) diagnosed with AIDS; (5) unable to provide informed consent due to mental incapacity resulting from drug or alcohol intoxication, severe developmental disability, or dementia; (6) unable to perform at least five of the following evaluations: medical history, physical examination, physical performance, cognitive testing, dietary recall, audio questionnaire, body composition, carotid Doppler, and pulse wave velocity assessment; and (7) without a verifiable address or valid government issued identification at time of consent. HANDLS SCAN participants had the following additional exclusions: history of dementia, stroke or transient ischemic attack; history of carotid endarterectomy; MRI contraindications (e.g., claustrophobia, indwelling ferromagnetic material); diagnosis of a terminal illness (e.g., metastatic cancer, end-stage liver or pulmonary diseases); HIV positive status; or other neurological disorder (e.g., multiple sclerosis, Parkinson’s disease).
HANDLS SCAN Procedure
Eligible HANDLS participants were invited to participate in HANDLS SCAN during their MRV visit. Those who expressed interest were contacted, given a MRI eligibility screener, and scheduled by a research coordinator. After providing informed consent, they were examined by a physician at the University of Maryland School of Medicine General Clinical Research Center for a brief medical evaluation to identify any acute medical problems since their last HANDLS visit, re-administer the MRI eligibility checklist, review current medications, assess whether there were any contraindications to the performance of HANDLS SCAN testing, and complete a brief physical function assessment. Participants underwent MRI acquisition in the Department of Diagnostic Radiology & Nuclear Medicine at the University of Maryland School of Medicine, and they received $50 remuneration.
The present sample included 190 participants who had complete imaging data (with no incidental clinical findings), sociodemographic data, and at least one cognitive measure used in this study. Analysis-specific samples varied due to missing cognitive data, ranging from 169–190 participants (see Table 2).
Table 2.
Path Coefficients (b) and Standard Errors (se) Representing Direct Effects of Socioeconomic Status (SES) on Executive Function and Conditional Indirect Effects of SES on Executive Function through the DLPFC
| Cognitive Outcome | Direct Effect | Conditional Indirect Effect by Race |
||
|---|---|---|---|---|
| Whites | African Americans | |||
| Trails B (n = 189) | b | 0.15** | 0.03† | −0.01 |
| se | 0.04 | 0.01 | 0.02 | |
| DSB (n = 169) | b | −0.96** | −0.09 | 0.03 |
| se | 0.37 | 0.11 | 0.08 | |
| VF (n = 190) | b | −2.06** | −0.13 | 0.05 |
| se | 0.75 | 0.30 | 0.18 | |
Note. Covariates for all models are age and sex. DLPFC = dorsolateral prefrontal cortex; VF = verbal fluency; DSB = Digit Span Backwards.
p<0.01
confidence intervals do not cross zero
Measures
Demographic variables.
The SES index was comprised of education and poverty status, with both variables dichotomized. Below median education (0 = ≥ 12 years; 1 = < 12 years) and income below 125% of the 2004 federal poverty line relative to family size and household income (0 = above the poverty line; 1 = below the poverty line) was considered low SES. A composite score of SES was computed as a dichotomous variable, with low SES defined as below median education and/or income below 125% of the poverty line. Participants that met neither of those criteria were labeled as high SES. The following demographic variables were also used: self-identified race (0 = White; 1 = AA), wherein participants were asked to select one category that described them best, either White, Black/African American, American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, or other. If someone did not identify as either African American or White they were beyond the scope of our sampling frame, and therefore not eligible for the study; biological sex (0 = female; 1 = male); and age (in years).
Magnetic Resonance Imaging.
Cranial magnetic resonance images were acquired using a Siemens Tim-Trio 3.0 Tesla scanner. Structural, volumetric data were obtained using T1-weighted MP-RAGE (TR/TE/TI = 2300/2.9/900 ms; FOV 25.6 cm), with 160 slices at 1.2 mm thickness in a sagittal plane. For comparison purposes, these images were reformatted into axial sections.
Image analysis.
Structural MRI scans were preprocessed using newly developed in-house techniques in the Section for Biomedical Image Analysis at the University of Pennsylvania. The first processing step involved a multi-atlas registration based method, requiring minimal manual correction (Doshi, Erus, Ou, Gaonkar, & Davatzikos, 2013), to remove extra-cranial material on the T1-weighted images (i.e., skull-stripping). Next, bias field correction was conducted and brain tissue was segmented into gray matter, white matter, and cerebrospinal fluid using the multiplicative intrinsic component optimization (MICO) method (Li, Gore, & Davatzikos, 2014).
Preprocessed images were segmented into a set of anatomical regions of interest (ROIs) using a new multi-atlas label fusion method, which has achieved state-of-the-art accuracy in segmentation of deep brain structures in an independent comparative evaluation (Doshi et al., 2016). Volumetric measurements for normal and abnormal (with lesion) tissue were calculated for each individual ROI, as well as for larger anatomical regions (e.g., overall PFC volume) by grouping ROIs. The ROI that was used for this study is the DLPFC. This neuroanatomical region was chosen due to its relevance to executive function (Newman et al., 2007; Ruscheweyh et al., 2013). Based on prior literature (Cox et al., 2013; Sanches et al., 2009), the DLPFC was constructed by summing the superior frontal gyrus and the middle frontal gyrus together. The DLPFC was not corrected for intracranial volume (ICV) for the following reasons: a) main differences in ICV are primarily due to sex and age (Whitwell, Crum, Watt, & Fox, 2001), which were covariates in all of our analyses, b) we were interested in the actual volumes of the DLPFC, as opposed to its volume relative to the entire brain, c) given that total brain volume is highly correlated with most ROIs, parsing out ICV would remove much of the DLPFC’s variance, and d) there is known to be bias in ICV estimation (Nordenskjöld et al., 2013).
Neuropsychological tests.
The following cognitive tests were selected to measure executive function ability.
Trails Making Test.
The Trail Making Test measures attention, sequencing, mental flexibility, visual search, and motor functioning (Spreen & Strauss, 1998). The test involves two parts: Trails A requires the interviewee to make connections between 25 encircled numbers randomly arranged on a page, in sequential order; Trails B requires them to connect 25 encircled numbers and letters in alternating order. Only Trails B was used in this study. The outcome variable is time in seconds; errors count only in increased performance time (Spreen & Strauss, 1998). The normative time cutoff of 300 seconds (Strauss, Sherman, & Spreen, 2006) was used.
Digit Span Backwards.
The Wechsler Adult Intelligence Scale-Revised (WAIS-R) DSB Test measures attention, concentration, and working memory (Lezak, Howieson, & Loring, 2004). Subjects are read a span of numbers at a rate of one digit per second, beginning with two digits. The interviewee is then required to repeat the digits in reverse order. After two trials of a specific digit length, the examiner moves to the next cluster of trials, which has an additional digit, until nine digits are reached. The test is discontinued when the subject fails both trials of a specific digit length. Each trial is worth a single point for a maximum of 14 points.
Verbal fluency.
For the purpose of this study, animal naming was used as the test of VF. Animal naming is part of the Boston Diagnostic Aphasia Examination as well as the Stanford-Binet test. The purpose of the test is to evaluate the spontaneous production of words of a given semantic category within one minute. The outcome variable is the total amount of admissible words.
Data analyses
Descriptive statistics.
Prior to any analyses, several assumptions were checked and intercorrelations were computed. Normality estimates for skewness and kurtosis were computed. Skewed variables were log-transformed to normalize their distribution. The mediator and outcome variables were also screened for outliers by converting them to z-scores and using an exclusion criteria of z > 3.29 or z < −3.29. Odds ratios (ORs) were computed to compare the present sample to the overall HANDLS sample with regards to relevant demographic factors.
Moderated mediation analyses.
Although path analysis does not assess causal relations between variables, the term “effect” is used here for consistency with its statistical use and the vocabulary of path analysis.
To evaluate if brain volume mediates the association of SES and executive function, three moderated mediation analyses were run. The SES composite served as the predictor (X), tests of executive function (i.e., Trails B, DSB, and VF) served as the respective outcome variables (Y), and DLPFC volume served as the mediator (M). Race served as the moderator between SES and the DLPFC (W; for conceptual model see Figure 1). Age and sex were used as covariates for all analyses. In this model, there were two effects of interest: (1) the direct effect of SES on executive function and (2) the indirect effect of SES on executive function through the DLPFC. This model also included the SES by race interaction, which specifies that the direct effect of SES on the DLPFC is conditional on race. Using this model, direct and conditional indirect effects were computed. Mediation analyses were run by the PROCESS macro (Hayes, 2013) in SPSS 22.0. Within PROCESS, model 7 (moderated mediation) was specified, and 5000 bootstrap samples were requested along with a 95% confidence interval (CI).
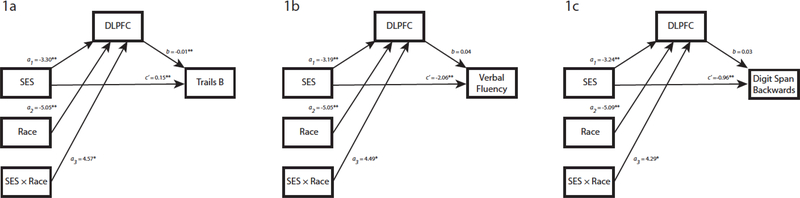
Figure 1.
Simplified path model used to assess the conditional (moderated by race) indirect effect of SES on executive function through the DLPFC. The path coefficients (a, b, c’) estimate the strength of effects using unstandardized coefficients, demonstrating the relative increase of the dependent variable given a 1-point increase in the independent variable; c’ is the direct effect of SES on executive function; conditional indirect effect of SES on executive function through DLPFC = (a1 + a3[Race])(b). Note that Trails B is log transformed and DLPFC volume is in cubic centimeters. *p < .05; **p < .01.
Results
Descriptives
The analysis sample included 190 participants, with 44% (n = 83) male and 58% (n = 111) White. On average, participants were middle-aged, with a mean of 52 years of age and 12 years of education. When education was converted into a dichotomous variable (≥ 12 years = high), 74% (n = 141) of the sample was in the “high” education group. 66% (n = 125) of the participants were above 125% of the 2004 federal poverty line relative to family size and household income. When computing the SES variable (“high” education and above poverty = “high” SES), 52% (n = 98) of the sample was in the high SES group. Out of those labeled “low SES” (n = 92), 43 had “high” education, but had an income below 125% of the 2004 federal poverty line, 27 had “low” education, but were above 125% of the 2004 federal poverty line, and 22 were characterized as having “low” education and were below the poverty line. Relative to the general HANDLS sample, the present sample was more likely to include Whites (OR = 2.08; lower CI = 1.55, higher CI = 2.78) and those of high SES (OR = 1.45; lower CI = 1.09, higher CI = 1.94), but did not significantly differ with regards to age (OR = 1.22; lower CI = .91, higher CI = 1.64) and sex (OR = 1.13; lower CI = .84, higher CI = 1.51). Sample characteristics for primary study variables are provided in Table 1.
Table 1.
Study Characteristics
| Mean | SD | Percent | Range | |
|---|---|---|---|---|
| Age (years) | 51.62 | 9.14 | 33–69 | |
| % Male | 43.7 | |||
| % White | 58.4 | |||
| Education (years) | 12.36 | 2.75 | 2–20 | |
| % High Education | 74.20 | |||
| % Above Poverty | 65.80 | |||
| % High SES | 51.60 | |||
| DLPFC (cc) | 55.59 | 7.12 | 40.55–85.77 | |
| Scores on Neuropsychological Tests | ||||
| Trails B (time) | 113.49 | 77.70 | 24–300 | |
| VF (total score) | 19.62 | 5.11 | 7–44 | |
| DSB (total score) | 5.84 | 2.33 | 1–13 |
Note. N = 190. High SES was defined as having more than 12 years of education and being above 125% of the 2004 federal poverty line. SD = standard deviation; SES = socioeconomic status; DLPFC = dorsolateral prefrontal cortex; cc = cubic centimeters; VF = verbal fluency; DSB = Digit Span Backwards.
Apart from Trails B, preliminary data screening suggested that there were no violations of assumptions of normality and linearity. Also, no outliers were identified and thus, no data points were removed. Trails B was log transformed because it had a non-normal distribution.
Mediational Analyses
Several path analyses were computed to examine the conditional mediating effect of DLPFC on the association of SES and three different tests of executive function: Trails B, DSB, and VF. All reported coefficients were unstandardized and expressed in units of the dependent variable at the time of analysis (e.g., a paths [Figure 1] are expressed in units of brain volume [i.e., cubic centimeters = cc]). To test the indirect effects of all mediations (by estimating if the indirect effect is different from zero), bootstrapping CIs were performed with 5,000 samples. Path coefficients and standard errors are outlined in Table 2.
Model 1 – Trails B.
Figure 1a shows the individual component paths for Model 1 with Trails B as the outcome variable. SES (a1: t(187) = −2.79, p < .01), race (a2: t(187) = −3.94, p < .01), and their interaction (a3: t(187) = 2.52, p < .05) were significantly associated with the mediating variable, DLPFC. When controlling for SES, DLPFC was significantly associated with Trails B (b: t(186) = −3.12, p < .01). The estimated direct effect of SES on Trails B was also significant (c’: t(186) = 4.05, p < .01). The lower and upper limits for the moderated mediation were −0.10 and −0.01, respectively. As the bootstrapped CI did not cross zero, moderated mediation was considered significant, suggesting that the model’s indirect effect was conditional on race. Across the moderator, the indirect effect was significant in Whites (lower CI = 0.01; upper CI = 0.07), but non-significant in AAs (lower CI = −0.05; upper CI = 0.01). Given that the direct path from SES to Trails B (c’) was also statistically significant, the effect of SES on Trails B in Whites was partially mediated by the DLPFC.
Model 2 – Digit Span Backwards.
Figure 1b shows the individual component paths for Model 2 with DSB as the outcome variable. SES (a1: t(167) = −2.53, p < .05), race (a2: t(167) = −3.76, p < .01), and their interaction (a3: t(167) = 2.22, p < .05) were significantly associated with the mediating variable, DLPFC. When controlling for SES, DLPFC was not associated with DSB (b: t(166) = 1.08, p = 0.28). The estimated direct effect of SES on DSB was significant (c’: t(166) = −2.61, p < .01). The lower and upper limits for the moderated mediation were −0.07 and 0.60, respectively. As the bootstrapped CI crossed zero, moderated mediation was not considered significant. Across the moderator, the indirect effect was non-significant in Whites (lower CI = −0.39; upper CI = 0.05) and AAs (lower CI = −0.04; upper CI = 0.31). This suggests that the effect of SES on DSB was not mediated by the DLPFC in Whites nor AAs.
Model 3 – Verbal Fluency.
Figure 1c shows the individual component paths for Model 3 with VF as the outcome variable. SES (a1: t(188) = −2.72, p < .01), race (a2: t(188) = −3.94, p < .01), and their interaction (a3: t(188) = 2.47, p < .05) were significantly associated with the mediating variable, DLPFC. When controlling for SES, DLPFC was not associated with VF (b: t(187) = 0.70, p = 0.49). The estimated direct effect of SES on VF was significant (c’: t(187) = −2.74, p < .01). The lower and upper limits for the moderated mediation were −0.44 and 1.44, respectively. As the bootstrapped CI crossed zero, moderated mediation was not considered significant. Across the moderator, the indirect effect was non-significant in Whites (lower CI = −1.02; upper CI = 0.30) and AAs (lower CI = −0.13; upper CI = 0.71). This suggests that the effect of SES on VF was not mediated by the DLPFC in Whites nor AAs.
Discussion
This study examined whether the relation between SES and executive function was mediated by DLPFC gray matter volume, adjusting for sex and age, in 190 socioeconomically diverse, community-dwelling AA and White adults. Overall, the findings revealed a significant moderated mediation with Trails B as the outcome variable. This finding demonstrated a significant SES-Trails B mediation by DLPFC in Whites, but not AAs. DLPFC volume did not mediate the relation between SES and DSB nor VF. Null mediations were due to non-significant relations between DLPFC and the cognitive tests, and not a result of SES-DLPFC relations, which were significant in all analyses.
Model 1 – Trails B
DLPFC volume significantly mediated the relation between SES and Trails B performance for White, but not AA adults. In this model, those in the lower SES group had poorer Trails B ability and smaller DLPFC volumes. Additionally, those with smaller DLPFC volume had poorer Trails B ability. Finally, the significant relation between SES and Trails B was partially mediated by DLPFC gray matter volume in Whites.
This finding is consistent with the strong relation previously noted between Trails B and the DLPFC. Trails B is known to measure working memory, sustained attention, and set-shifting/mental flexibility (Lezak et al., 2012), cognitive abilities that are dependent on the DLPFC (Lezak et al., 2012; Pardo, Fox, & Raichle, 1991; Sanchez-Cubillo et al., 2009). Also, Trails B performance has been directly linked to DLPFC structure and function (Moll et al., 2002; Newman et al., 2007; Stuss et al., 2001; Zakzanis et al., 2005). For example, Stuss and colleagues (2001) explored the relation between Trails B and specific regions within the frontal lobes, finding that patients with DLPFC lesions were most impaired on the task. Additionally, Trails B has been linked to SES, wherein individuals from lower SES homes display worse performance on this test as compared to those from higher SES homes (Lipina et al., 2005; Turrell et al., 2002). Considering Trails B’s strong reliance on the DLPFC, and the impact of SES on this test of executive function, our study’s findings are consistent with previous literature. One interpretation of these results is that higher SES may lead to better development and maintenance of DLPFC gray matter volume across the life span, which in turn supports enhanced executive function ability. While temporal associations cannot be evaluated in this cross-sectional study, this significant mediation suggests the important role that regional brain volume plays in the SES-cognition relation.
There are several pathways by which socioeconomic disparities can lead to volumetric differences in brain gray matter. Research suggests higher levels of stress exposure in low SES groups (Evans, 2004), and stress is known to have deleterious effects on prefrontal cortical structures as a result of an over-activated neural stress response (Arnsten et al., 2009; Liston et al., 2009; McEwen & Gianoros, 2010). Noble and colleagues (2012) propose that, in addition to stress, differences in the home linguistic environment across SES groups are associated with developmental brain differences. Future studies should examine the potential operative pathways through which SES impacts regional brain volumes, including stress, language, nutrition, substance use, psychological status, and biomedical risk factors and diseases.
Model 2 – Digit Span Backwards
Contrary to our hypotheses, DLPFC volume did not significantly mediate the relation between SES and DSB performance, attributable to a non-significant association between DLPFC and DSB. However, on average, those with lower SES performed worse on DSB. One explanation for the null mediation findings could be due to the nature of the task. Compared to Trails B, DSB allows for less variability in task performance. As shown in Table 1, the relative range for DSB is considerably smaller than for Trails B. Statistically, a restricted range of scores can make truly significant relationships harder to identify. It may also be the case that the distribution of scores for DSB in this sample of participants is lower than that of other populations (Kemtas & Allen, 2008; Ruscheweyh et al., 2013). For instance, in one study both younger (ages 18–30) and older (ages 65–78) adults performed better on DSB than the participants in this study (M(SD)=8.10(2.34) and 7.47(3.76), respectively, compared to 5.84(2.33) in this study; Kemtas & Allen, 2008). Average scores on Trails B in this study also appear modestly lower than in other (albeit, predominantly White) samples (e.g., Stuss et al., 2001; Tombaugh, 2004), although participants appeared to perform comparably on VF (Gladsjo et al., 1999; Tombaugh, Kozak, & Rees, 1999). The apparently poorer performance exhibited on two of three tests in our sample compared to others suggests that the present distribution may not be representative of the general population. Alternatively, it is possible that our sample is representative of the local population (i.e., Baltimore city), but that this population differs from those represented by other samples. This variability suggests that the relations seen in the prior literature may be not as readily applicable to HANDLS SCAN. More studies utilizing ethnically diverse samples are needed to determine the population-level distributions of these cognitive tests. Nonetheless, the present findings may imply that DSB is not fully sensitive to PFC-dependent and/or SES-influenced executive functioning ability.
Given that lack of mediation was due to the non-significant relation between DLPFC and DSB, the null results suggest that this neuroanatomical region may not be a primary brain correlate of this task. Executive function encompasses a wide-range of cognitive processes, some that may or may not predominantly rely on the DLPFC. One such ability involves attention, concentration, and focused behavior. This test, along with Digit Span Forward, requires both auditory attention and short-term retention capacity (Lezak et al., 2012). Regardless of true span ability, if the individual is inattentive or distracted he/she will miss pertinent information from the examiner and perform poorly. While functional imaging has shown that the DLPFC is indeed positively associated with DSB performance (Gerton et al., 2004), several other ROIs, including the lateral temporal lobe, may be even more critical for this test (Gerton et al., 2004; Ruscheweyh et al., 2013). Thus, it may be that other neuroanatomical areas are more likely to mediate the relation between SES and DSB.
Model 3 - Verbal Fluency
Also contrary to our hypotheses, DLPFC volume did not significantly mediate the relation between SES and VF performance due to a non-significant relation between DLPFC and category-based VF. However, in keeping with the proposed hypotheses, those with low SES performed worse on this task. One interpretation of the null mediation results may be related to the type of VF measure used. Traditionally, there are two types of VF tasks: letter-based and semantic-based categories. Semantic-based, such as the task used in this study, require individuals to generate words from a semantic category. This type of VF requires individuals to search through their semantic or conceptual memory and thus correlates with lateral and inferior temporal lobe regions thought to be involved in object perception, recognition, imagery, and naming (Gourovitch et al., 2000, Hirono et al., 2001; Kitbayashi et al., 2001; Mummery, Patterson, Hodges, & Wise, 1996). Individuals performing this task have the advantage of benefitting from the intrinsic organization of these categories. Alternatively, letter-based VF requires individuals to generate words that begin with a specific letter of the alphabet, requiring the use of phonemic and/or graphemic cues for word retrieval (Rascovsky et al., 2007). This type of VF necessitates the construction of novel categories, which may require a more effortful and strategic word search than that required for semantic VF. Thus, while both are considered tests of executive function and have been related to the DLPFC, letter-based fluency may rely more strongly on DLPFC functioning (Gourovitch et al., 2000; Mummery et al., 1996). Conversely, a review by Yuan and Raz (2014), suggested that VF may in fact have no link to PFC volume. Future research should examine if DLPFC volume mediates the relation between SES and letter-based VF, and if areas of the temporal lobe mediate the relation between SES and semantic-based VF.
Race-Moderated Mediations
This study examined the three primary mediations across self-identified AAs and Whites by utilizing a moderated mediation model. The reasons discussed above for the null findings related to DSB and VF can be generalized to both the AA and White subsamples in this study. The moderated mediation involving Trails B was significant in the White subsample, but not in the AA subsample (Figure 1a; Table 2). The significant main finding in this study illustrates that a higher level of SES is associated with larger DLPFC volume, which is positively related to executive function ability. While the SES-brain relation is likely bidirectional, these findings suggest that higher levels of education and income may be protective for brain volume and cognitive functioning. The differential findings across race suggest that this socioeconomic “protection” may only be present in Whites, not AAs.
Research has consistently demonstrated that equal levels (that is, in absolute numeric terms) of SES-related variables do not yield equal health outcomes. In fact, one striking statistic shows that within the same income levels, White males and females live 2–4 years longer than their AA counterparts (Williams, Mohammed, Leavell, & Collins, 2010). Related racial disparities have been found within educational achievement levels (Olshansky et al., 2012; Giovannucci, Liu, Platz, Stampfer, & Willett, 2007; Thomas, Thomas, Pearson, Klag, & Mead, 2007). These findings demonstrate an ostensible nonequivalence between AAs and Whites, such that AAs seem to have less “wealth” than Whites at all levels of income and that at equal levels of educational attainment, quality of education is less in AAs. Thus, sociodemographic variables may have differential validity across self-identified racial groups. An additional hypothesis for the racial disparities found in this study could be that of the “diminishing returns hypothesis” (Farmer & Ferraro, 2005) discussed earlier. The mechanisms for this have not been fully delineated, but one might presume that a lifetime of environmental stressors, such as discrimination, disproportionately experienced by AAs may contribute.
In addition to the race-moderated SES-brain relations examined in this study, the field may also benefit from complementary analyses examining race-moderated brain-cognition relations. The concept of cognitive reserve posits that certain life experiences, such as advantaged socioeconomic status, and perhaps racial privilege, are associated with a reduced risk of developing dementia and a slower rate of cognitive decline in normal aging, even when the brain is in a pathological state (Stern, 2009). In other words, the brain-cognition association is thought to vary across individuals with varying levels of reserve, and one might expect that a disproportionate amount of environmental stressors experienced by AAs might adversely impact their reserve. This question may be particularly relevant in an elderly sample with more brain pathology, unlike our current sample where reductions in brain volume are likely still within normal, rather than pathological, ranges of volumetric size.
Strengths and Limitations
Some limitations warrant discussion. For one, our measure of SES is limited. First, HANDLS does not have a full spectrum of SES based on specific annual income, partially because a number of participants had difficulty computing an annual income. Additionally, while continuous income can indeed be useful, it does not account for household size. Therefore, HANDLS investigators used poverty status (a specific income level adjusted for household size) as their primary SES stratification variable. Given our need to use poverty status (a dichotomized variable in its original form), it was most parsimonious to combine it with a dichotomized measure of education. Nonetheless, we acknowledge the statistical limitations associated with dichotomizing SES, and believe the field would benefit from complementary investigations utilizing continuous SES measures. Our SES measure also does not incorporate participants’ childhood SES, a variable that is especially important to consider when examining brain health (Staff et al., 2012). There are various other socioeconomic-related factors, such as intergenerational wealth, residential segregation, and racial migration that are particularly relevant when discussing socioeconomic racial disparities (Williams, 1996). Given these contextual factors, it is evident that income and education do not provide a comprehensive assessment of SES. Nonetheless, these variables are largely unexamined in the SES literature, including the present study.
Second, our study’s cross-sectional design prohibits identification of temporal relations between variables; this is important because the relation between socioeconomic status and brain volume is likely bidirectional. A third limitation is our characterization of the DLPFC. The DLPFC is not an anatomical structure, but rather a functional region, thus making it difficult to define using anatomically-defined ROIs. Therefore, the sum of the superior frontal gyrus and middle frontal gyrus (i.e., our index of the DLPFC) is likely to include anatomical subregions involved in cognitive functions extending beyond those historically attributed to the DLPFC, as well as to exclude anatomical structures that contribute to DLPFC function. Finally, while not adjusting for ICV has its benefits (see Methods), variability in DLPFC volume may reflect variability in overall brain size.
While several limitations exist, the unique methodological approach to the HANDLS study allows for utilization of a diverse sample of community-dwelling adults, with a strong emphasis on minority and disadvantaged groups. By utilizing this unique group of individuals, we were able to better understand complex interrelations among sociodemographic variables, regional brain volume, and cognitive function. This study utilized three distinct measures of executive function, resulting in a nuanced observation of some of the components of this cognitive domain, which is a complex, multi-faceted construct. Additionally, the study sample was comprised of adults, which in contrast to children, is an understudied population in research looking at the relations of SES and brain health. Finally, to our knowledge, this is the first study employing the mediation models presented here. While models applying these study variables can be constructed in several ways, this study proposed a novel representation of how SES, brain volume, and cognition interact with one another.
Summary and Conclusions
Consistent with the proposed hypotheses and prior literature, this study found that SES was related to smaller DLPFC volume and poorer executive function ability. This study also revealed novel findings suggesting that the relation between SES and Trails B was partially mediated by DLPFC gray matter volume in Whites, but not AAs. Mediation by DLPFC gray matter volume was not observed for VF or DSB. One plausible hypothesis for these discrepant findings is that other socioeconomically-relevant ROIs may be mediating these relations. These findings suggest that in adults, the DLPFC is one important influence on the association of SES and at least one measure of executive function in a subsample of the population. The differential findings across race suggest that compared to Whites, AAs may not be benefitting from their high SES with regards to DLPFC, which may, in part, be attributable to a disproportionate amount of environmental stressors experienced by this racial group. While the study’s findings should be generalized with caution, they are consistent with a large body of literature demonstrating the importance of brain health for proper cognitive functioning which, in turn, is likely to translate to better everyday (e.g., occupational, social) functioning. The results are also consistent with studies indicating that lower SES has a deleterious impact on the brain. Together, these findings improve our understanding of the interrelations of SES, brain health, and cognition, and add to the burgeoning empirical investigation of the racial health disparities that are pervasive in this country. This study offers an important advancement to socioeconomic health disparities research and paves the way for future studies in this area of inquiry.
Public Significance Statement:
Lower socioeconomic status (SES) has been associated with poorer executive function (EF) and smaller prefrontal cortex (PFC) volumes. The present findings indicate that, among Whites but not African Americans, smaller dorsolateral PFC volumes partially explain the association of low SES to lower levels of mental flexibility, but not working memory or verbal fluency. This suggests that differential brain mechanisms underlie SES–EF associations across EF subtypes and sociodemographic groups.
Acknowledgments
This research was supported by the National Institutes of Health Grants R01-AG034161 to Shari R. Waldstein and ZIA–AG000513 to Michele K. Evans and Alan B. Zonderman.
Contributor Information
Danielle Shaked, University of Maryland, Baltimore County and National Institute on Aging.
Leslie I. Katzel, University of Maryland School of Medicine and Baltimore VA Medical Center
Stephen L. Seliger, University of Maryland School of Medicine
Rao P. Gullapalli, University of Maryland School of Medicine
Christos Davatzikos, University of Pennsylvania.
Guray Erus, University of Pennsylvania.
Michele K. Evans, National Institute on Aging
Alan B. Zonderman, National Institute on Aging
Shari R. Waldstein, University of Maryland, Baltimore County and Baltimore VA Medical Center
References
- United States Department of Health and Human Services. (2004). 2004 HHS poverty guidelines. Retrieved from https://aspe.hhs.gov/2004-hhs-poverty-guidelines.
- Adólfsdóttir S, Haász J, Wehling E, Ystad M, Lundervold A, & Lundervold AJ (2014). Salient measures of inhibition and switching are associated with frontal lobe gray matter volume in healthy middle-aged and older adults. Neuropsychology, 28, 859. doi: 10.1037/neu0000082 [DOI] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Matute E, & Guajardo S (2005). The influence of the parents’ educational level on the development of executive functions. Developmental Neuropsychology, 28, 539–560. doi: 10.1207/s15326942dn2801_5 [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10, 410–422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, & Hitch G (1974). Working memory. Psychology of Learning and Motivation, 8, 47–89. [Google Scholar]
- Baum A, Garofalo JP, & Yali A (1999). Socioeconomic status and chronic stress: does stress account for SES effects on health? Annals of the New York Academy of Sciences, 896, 131–144. doi: 10.1111/j.1749-6632.1999.tb08111 [DOI] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8. doi: 10.3389/fnins.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SR, Ferguson KJ, Royle NA, Shenkin SD, MacPherson SE, MacLullich AM, ... Wardlaw JM (2014). A systematic review of brain frontal lobe parcellation techniques in magnetic resonance imaging. Brain Structure and Function, 219, 1–22. doi: 10.1007/s00429-013-0527-5 [DOI] [PubMed] [Google Scholar]
- Doshi J, Erus G, Ou Y, Gaonkar B, & Davatzikos C (2013). Multi-Atlas Skull-Stripping. Academic Radiology, 20, 1566–1576. doi: 10.1016/j.acra.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi J, Erus G, Ou Y, Resnick SM, Gur RC, Gur RE, ... Davatzikos C (2016). MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. NeuroImage, 127, 186–195. doi: 10.1016/j.neuroimage.2015.11.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW (2004). The environment of childhood poverty. American Psychologist, 59, 77. doi: 10.1037/0003-066X.59.2.77 [DOI] [PubMed] [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, & Zonderman AB (2010). Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & Disease, 20, 267–275. [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, ... Hurt H (2006). Childhood poverty: Specific associations with neurocognitive development. Brain Research, 1110, 166–174. doi: 10.1016/j.brainres.2006.06.072 [DOI] [PubMed] [Google Scholar]
- Farmer MM, & Ferraro KF (2005). Are racial disparities in health conditional on socioeconomic status? Social Science & Medicine, 60, 191–204. doi: 10.1016/j.socscimed.2004.04.026 [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, & Buckner RL (2008). Brain volume decline in aging: Evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Archives of Neurology, 65, 113–120. doi: 10.1001/archneurol.2007.27 [DOI] [PubMed] [Google Scholar]
- Geronimus AT (1992). The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethnicity & disease, 2, 207–221. [PubMed] [Google Scholar]
- Gerton BK, Brown TT, Meyer-Lindenberg A, Kohn P, Holt JL, Olsen RK, & Berman KF (2004). Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia, 42, 1781–1787. doi: 10.1016/j.neuropsychologia.2004.04.023 [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, & Hackman D (2013). Contributions of neuroscience to the study of socioeconomic health disparities. Psychosomatic Medicine, 75, 610–615. doi: 10.1097/PSY.0b013e3182a5f9c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Platz EA, Stampfer MJ, & Willett WC (2007). Risk factors for prostate cancer incidence and progression in the health professionals follow‐up study. International Journal of Cancer, 121, 1571–1578. doi: 10.1002/ijc.22788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, & Heaton RK (1999). Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment, 6, 147–178. [DOI] [PubMed] [Google Scholar]
- Glymour MM, & Manly JJ (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18, 223–254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, ... Berman KF (2000). A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology, 14, 353. doi: 10.1037/0894-4105.14.3.353 [DOI] [PubMed] [Google Scholar]
- Hart B, & Risley TR (1995). Meaningful differences in the everyday experience of young American children. Paul H Brookes Publishing. [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press. [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13, 65–73. doi: 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann D, & Guadagno MA (1997). Memory Performance and Socio‐Economic Status. Applied Cognitive Psychology, 11, 113–120. doi: 10.1002/(SICI)1099-0720. [DOI] [Google Scholar]
- Hirono N, Mori E, Ishii K, Imamura T, Tanimukai S, Kazui H, ... Sasaki M (2000). Neuronal substrates for semantic memory: A positron emission tomography study in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 12, 15–21. doi: 10.1159/000051231 [DOI] [PubMed] [Google Scholar]
- Kemtes KA, & Allen DN (2008). Presentation modality influences WAIS Digit Span performance in younger and older adults. Journal of Clinical and Experimental Neuropsychology, 30, 661–665. doi: 10.1080/13803390701641414 [DOI] [PubMed] [Google Scholar]
- Kitabayashi Y, Ueda H, Tsuchida H, Iizumi H, Narumoto J, Nakamura K, ... Fukui K(2001). Relationship between regional cerebral blood flow and verbal fluency in Alzheimer’s disease. Psychiatry and Clinical Neurosciences, 55, 459–463. doi: 10.1046/j.1440-1819.2001.00890.x [DOI] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, & Farah MJ (2013). Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science, 16, 641–652. doi: 10.1111/desc.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SC, Vasilyeva M, Lourenco SF, Newcombe NS, & Huttenlocher J (2005). Socioeconomic status modifies the sex difference in spatial skill. Psychological Science, 16, 841–845. doi: 10.1111/j.1467-9280.2005.01623. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, & Tranel D (2012). Neuropsychological assessment (5th ed.). New York: Oxford University Press. [Google Scholar]
- Lezak MD, Howieson DB, & Loring DW (2004). Neuropsychological assessment (4th ed.). New York: Oxford University Press. [Google Scholar]
- Li C, Gore JC, & Davatzikos C (2014). Multiplicative intrinsic component optimization (MICO) for MRI bias field estimation and tissue segmentation. Magnetic Resonance Imaging, 32, 913–923. doi: 10.1016/j.mri.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina SJ, Martelli MI, Vuelta BL, Injoque-Ricle I, & Colombo JA (2004). Poverty and executive performance in preschool pupils from Buenos Aires city (Republica Argentina). Interdisciplinaria, 21, 153–193 [Google Scholar]
- Liston C, McEwen BS, & Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences, 106, 912–917. doi: 10.1073/pnas.0807041106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. doi: 10.1111/j.1749-6632.2009.05331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. doi: 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Mortby ME, Burns R, Janke AJ, Sachdev PS, Anstey K, J., & Cherbuin, N. (2014). Relating education, brain structure, and cognition: The role of Cardiovascular Disease risk factors. BioMed Research International, 2014, 13. doi: 10.1155/2014/271487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, & Wise RJ (1996). Generating ‘tiger’ as an animal name or a word beginning with T: Differences in brain activation. Proceedings of the Royal Society B: Biological Sciences, 263, 989–995. doi: 10.1098/rspb.1996.0146 [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Farias ST, & DeCarli C (2009). Age and education effects on relationships of cognitive test scores with brain structure in demographically diverse older persons. Psychology and Aging, 24, 116. doi: 10.1037/a0013421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Oliveira-Souza RD, Moll FT, Bramati IE, & Andreiuolo PA (2002). The cerebral correlates of set-shifting: An fMRI study of the trail making test. Arquivos De Neuro-Psiquiatria, 60, 900–905. doi: 10.1590/S0004-282X2002000600002 [DOI] [PubMed] [Google Scholar]
- Newman LM, Trivedi MA, Bendlin BB, Ries ML, & Johnson SC (2007). The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging and Behavior, 1, 3–10. doi: 10.1007/s11682-007-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15, 516–527. doi: 10.1111/j.1467-7687.2012.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, & Farah MJ (2007). Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science, 10, 464–480. doi: 10.1111/j.1467-7687.2007.00600. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, & Farah MJ (2005). Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science, 8, 74–87. doi: 10.1111/j.1467-7687.2005.00394 [DOI] [PubMed] [Google Scholar]
- Nordenskjöld R, Malmberg F, Larsson EM, Simmons A, Brooks SJ, Lind L, ... & Kullberg J (2013). Intracranial volume estimated with commonly used methods could introduce bias in studies including brain volume measurements. Neuroimage, 83, 355–360. doi: 10.1016/j.neuroimage.2013.06.068 [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Antonucci T, Berkman L, Binstock RH, Boersch-Supan A, Cacioppo JT, ... Jackson J(2012). Differences in life expectancy due to race and educational differences are widening, and many may not catch up. Health Affairs, 31, 1803–1813. doi: 10.1377/hlthaff.2011.0746 [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, & Raichle ME (1991). Localization of a human system for sustained attention by positron emission tomography. Nature, 349, 61–64. doi: 10.1038/349061a0 [DOI] [PubMed] [Google Scholar]
- Raizada RD, & Kishiyama MM (2010). Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Frontiers in Human Neuroscience, 4. doi: 10.3389/neuro.09.003.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, & Galasko D (2007). Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology, 21, 20–30. doi: 10.1037/0894-4105.21.1.20 [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, ... Acker JD(2005). Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex, 15, 1676–1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Roberts RJ Jr, & Pennington BF (1996). An interactive framework for examining prefrontal cognitive processes. Developmental Neuropsychology, 12, 105–126. doi: 10.1080/87565649609540642 [DOI] [Google Scholar]
- Ruscheweyh R, Deppe M, Lohmann H, Wersching H, Korsukewitz C, Duning T, ... Knecht S (2013). Executive performance is related to regional gray matter volume in healthy older individuals. Human Brain Mapping, 34, 3333–3346. doi: 10.1002/hbm.22146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches M, Caetano S, Nicoletti M, Monkul ES, Chen HH, Hatch JP, ... Soares JC (2009). An MRI-based approach for the measurement of the dorsolateral prefrontal cortex in humans. Psychiatry Research: Neuroimaging, 173, 150–154. doi: 10.1016/j.pscychresns.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, & Barcelo F (2009). Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society, 15, 438–450 doi: 10.1017/S1355617709090626 [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Glass TA, Bolla KI, Stewart WF, Glass G, Rasmussen M, ... & Bandeen-Roche K (2004). Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environmental Health Perspectives, 112, 314. doi: 10.1289/ehp.6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Richards M, & Marmot M (2005). Socioeconomic position across the lifecourse: how does it relate to cognitive function in mid-life? Annals of Epidemiology, 15, 572–578. doi: 10.1016/j.annepidem.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Spreen O, and Strauss E (1998). A compendium of neuropsychological tests: Administration, norms, and commentary. New York: Oxford University Press. [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, & Whalley LJ (2012). Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Annals of Neurology, 71, 653–660. doi: 10.1002/ana.22631 [DOI] [PubMed] [Google Scholar]
- Stern Y (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, & Izukawa D (2001). The Trail Making Test: A study in focal lesion patients. Psychological assessment, 13, 230. doi: 10.1037/1040-3590.13.2.230 [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EM, & Spreen O (2006). A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press. [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, & Fukuda H (2011). Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS One, 6, e22734. doi: 10.1371/journal.pone.0022734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Thomas DJ, Pearson T, Klag M, & Mead L (1997). Cardiovascular disease in African American and white physicians: The Meharry cohort and Meharry-Hopkins cohort studies. Journal of Health Care for the Poor and Underserved, 8, 270–283. doi: 10.1353/hpu.2010.0526 [DOI] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff‐Smith A, & Kushnerenko E (2013). Socioeconomic status and functional brain development–associations in early infancy. Developmental Science, 16, 676–687. doi: 10.1111/desc.12079 [DOI] [PubMed] [Google Scholar]
- Tombaugh TN (2004). Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Ceuropsychology, 19, 203–214. doi: 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology, 14, 167–177. doi: 10.1016/S0887-6177(97)00095-4 [DOI] [PubMed] [Google Scholar]
- Turrell G, Lynch JW, Kaplan GA, Everson SA, Helkala EL, Kauhanen J, & Salonen JT (2002). Socioeconomic position across the lifecourse and cognitive function in late middle age. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57, S43–S51. doi: 10.1093/geronb/57.1.S43 [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Dore GA, Davatzikos C, Katzel LI, Gullapalli R, Seliger SL, ... Zonderman AB (2017). Differential associations of socioeconomic status with global brain volumes and white matter lesions in African American and White adults: The HANDLS SCAN study. Psychosomatic Medicine, 79, 327–335. doi: 10.1097/PSY.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehurst GJ (1997). Language processes in context: Language learning in children reared in poverty. Research on Communication and Language disorders: Contribution to Theories of Language Development, 233–266. [Google Scholar]
- Whitwell JL, Crum WR, Watt HC, & Fox NC (2001). Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. American Journal of Neuroradiology, 22, 1483–1489.. [PMC free article] [PubMed] [Google Scholar]
- Williams DR (1996). Race/ethnicity and socioeconomic status: measurement and methodological issues. International Journal of Health Services, 26, 483–505. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, & Collins C (2010). Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences, 1186, 69–101. doi: 10.1111/j.1749-6632.2009.05339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, & Raz N (2014). Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neuroscience & Biobehavioral Reviews, 42, 180–192. doi: 10.1016/j.neubiorev.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, & Graham SJ (2005). An fMRI study of the trail making test. Neuropsychologia, 43, 1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013 [DOI] [PubMed] [Google Scholar]