Abstract
Purpose of review
Prostate cancer bone metastasis is the lethal progression of the disease. The disease frequently presents with osteoblastic lesions in bone. The tumor-induced bone can cause complications that significantly hamper the quality of life of patients. A better understanding of how prostate cancer induces aberrant bone formation and how the aberrant bone affects the progression and treatment of the disease may improve the therapies for this disease.
Recent findings
Prostate cancer-induced bone was shown to enhance tumor growth and confer therapeutic resistance in bone metastasis. Clinically, Radium-223, an alpha emitter that selectively targets bone, was shown to improve overall survival in patients, supporting a role of tumor-induced bone in prostate cancer progression in bone. Recently, it was discovered that PCa-induced aberrant bone formation is due, in part, from tumor-associated endothelial cells that were converted into osteoblasts through endothelial-to-osteoblast (EC-to-OSB) conversion by tumor-secreted BMP4.
Summary
The unique bone-forming phenotype of prostate cancer bone metastasis plays a role in prostate cancer progression in bone and therapy resistance. Therapies that incorporate targeting the tumor-induced osteoblasts or EC-to-OSB conversion mechanism may reduce tumor-induced bone formation and improve therapy outcomes.
Keywords: Bone metastasis, prostate cancer, tumor-induced bone, osteocrines, EC-to-OSB conversion
Introduction
Prostate cancer (PCa) when progressed often develops metastasis in bone. Bone metastasis occurs in approximately 70% of men with advanced prostate cancer (1). Bone marrow is a favorite fertile soil into which prostate tumors tend to colonize and proliferate (2, 3). Colonization of prostate tumor cells in bone is frequently associated with tumor-induced bone lesions. Tumor-induced bone lesions generally arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts induced by PCa cells. While bone metastasis from breast and other cancers frequently induce osteolytic or the bone-lysing lesions, PCa uniquely induces bone formation. The osteoblastic bone-forming lesions of PCa bone metastasis could be detected by plain radiograph, bone scan, bone biopsy, and increased levels of serum alkaline phosphatase. Histology of bone lesions shows that tumor cells are surrounded by irregular woven bone (4). The woven bone found in the bone metastases is structurally weak and prone to fracture. The frailty of the tumor-induced bone is likely due to a heterogeneous mixture of both osteopenic and osteodense lesions (4). The osteoblastic bone lesions of PCa frequently contain an increased number of activated osteoblasts in the tumor-induced bone (5). These observations suggest a close interaction between prostate tumor cells and osteoblasts.
Tumor-induced bone enhances PCa progression in bone
Osteoblasts have been shown to contribute to prostate tumor growth in bone. Zhang et al. (6) showed that physical contact of osteoblasts with tumor cells promoted proliferation of prostate tumor cells in vitro. In addition, Li et al. (7) reported that osteoblasts could stimulate PCa cell growth in co-culture models. In the in vivo setting, Gleave et al. (8) co-inoculated athymic mice with human PCa cells LNCaP with human bone fibroblasts and found that bone fibroblasts were able to induce LNCaP tumor formation in vivo. Furthermore, Sung et al. (9) showed that co-injection of bone stromal cells and human PCa cells enhanced prostate tumor growth in mice.
MDA-PCa-118b (PCa-118b) is a patient-derived xenograft (PDX) generated from osteoblastic bone lesions (10). Li et al. (10) demonstrated that mice treated with neutralizing antibody against FGF9, one of the factors secreted by MDA-PCa-118b, reduced tumor-induced bone formation and also resulted in smaller tumors. Lee et al. (11) identified that MDA-PCa- 118b cells also secrete BMP4 together with other factors. They found that blocking the BMP receptor activation in osteoblasts with LDN-193189, a BMP receptor inhibitor, reduced the growth of PCa-118b tumors in mice (11). These findings provide support that tumor-induced osteoblasts/bone increase prostate tumor growth in bone.
How tumor-induced osteoblasts/bone support tumor progression in bone is still unclear. Sung et al. (9) showed that co-cultures of human PCa cells with bone marrow stromal cells in three-dimensional culture stimulated stromal cell expression of extracellular matrix proteins versican and tenascin and chemokine ligands CXCL5 and CXCL16. Ozdemir et al. (12) investigated the transcriptome changes that occurred in the stroma compartment of bones xenografted with human C4–2B or VCaP PCa cells and identified a set of transcripts, including PTN, EPHA3 and FSCN1, which were upregulated in mouse stromal cells. Whether these stromal secreted factors provide a support for PCa cells in bone remains to be examined.
Taken together, tumor-induced osteogenesis promotes tumor growth. Hence, interfering with PCa-induced bone-formation should be integrated into the therapy for PCa bone metastasis. The bone-targeting radionucleolide, radium-223 (Ra223), has been shown to improve overall patient survival in the treatment of PCa bone metastasis (13), providing the support for a role of tumor-induced bone in PCa progression in the clinical setting.
Tumor-induced bone confers de novo therapeutic resistance in metastatic prostate cancer
Although several targeted therapeutic treatments have improved the survival of patients with bone metastasis (14–16), resistance to the targeted therapy develops and significantly reduces the survival of PCa patients (17). Cabozantinib, an oral multikinase inhibitor with potent activity against Met and VEGFR2 (18–22), was used to treat PCa bone metastasis in a phase II clinical trial (18, 20). One of the striking observations was that cabozantinib treatment led to a decrease in the bone scan and alkaline phosphatase activity (18). However, cabozantinib failed in phase III trial for PCa bone metastasis (19). This is possibly due to the toxicity from high doses of cabozantinib that led to treatment cessation and tumor recurrence (19). The tumor-induced bone was shown to provide one of the resistance mechanisms to therapies. Studies by Lee et al. (23) showed that tumor cells resistant to cabozantinib treatment resided in a niche adjacent to tumor-induced bone. They further showed that osteoblasts present in the tumor-induced bone secreted osteocrines, many of which are integrin ligands that activate integrin signaling in PCa cells. Because integrin activation plays a key role in PCa cell survival, and integrin activation has been associated with resistance to chemotherapy, radiography and targeted treatments (24–27), osteocrine-induced integrin activation may increase cell survival. Indeed, Lee et al. (23) found that resistant tumor cells expressed high levels of pFAK and pTalin, mediators of integrin signaling, and inhibition of FAK activity with FAK inhibitors, PF562271 or VS-6063, reduced the survival of prostate tumor cells after cabozantinib treatment. Thus, prostate tumor-induced bone provides one of the de novo therapy resistance mechanisms (Figure 1).
Figure 1. Therapy resistance from prostate tumor-induced bone.
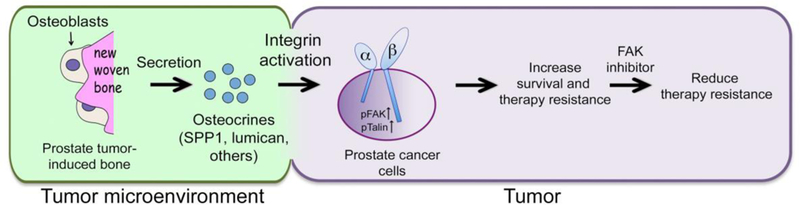
PCa cells secrete factors that increase new bone formation. Osteoblasts in the new bone produce factors (osteocrines). Some of the osteocrines activate integrin signaling in PCa cells, resulting in phosphorylation of FAK-Y397 and Talin-S425, which leads to therapy resistance. Inhibition of FAK activity decreases therapy resistance. This figure was reprinted from Lee et al with permission from American Association of Cancer Research (23).
Osteoblastic factors secreted from metastatic prostate cancer cells
PCa cells may secrete factors to increase osteoblast activity. Using osteogenic PCa cells, a number of proteins secreted by tumor cells have been identified as candidate factors that promote osteoblastic activity. These candidates include BMPs (11, 23, 28–30), TGFβ (31), and Endothelin-1 (ET-1) (32). Indeed, the PCa-118b PDX that exhibited strong bone forming activity (10, 11), secreted factors that belong to the BMP/TGFβ and FGF family of proteins to affect osteoblast proliferation and differentiation (10, 11,31, 33). Lee et al. (11) showed that the BMP4 secreted by PCa-118b cells acted as a paracrine factor to stimulate osteoblast differentiation, resulting in tumor growth. Their studies also found that PCal 18b tumor secreted high levels of TGFβ2, which can activate TGFβ signaling in osteoblasts and endothelial cells. Since TGFβ2 is known to promote epithelial-to-mesenchyme transition (EMT), TGFβ2 secreted from PCa-118b cells may function as an autocrine factor leading to EMT of tumor cells as well as a paracrine factor that modulates the properties of tumor-associated fibroblasts, osteoblasts and endothelial cells. Li and colleagues (10) showed that FGF9 secreted by PCa-118b induced osteoblast proliferation and promoted the osteoblastic phenotype of PCa-118 tumor. PCa-118b cells also express FGF19 and FGF3, both of which exerted autocrine as well as paracrine effects on tumor cells and stromal cells (31).
Besides BMP/TGFβ and FGF family proteins, PCa-118b cells are found to secrete CXCL1. Because PCa-118b does not express CXCR2, the receptor for CXCL1, CXCL1 likely exerts a paracrine effect in the metastatic tumor microenvironment in bone. Lee et al. (31) showed that CXCL1 stimulated Erkl/2 phosphorylation in osteoblasts, suggesting a role in osteoblast proliferation and/or differentiation. Together, these observations suggest that PCa cells secrete many factors that may enhance osteoblast proliferation and/or differentiation to contribute to metastatic PCa progression in bone.
Osteoblastic factors in extracellular vesicles from metastatic prostate cancer cells
Tumor cells can also release extracellular vesicles (EVs), which are circular membrane- enclosed particles with sizes from 30 nm to 10 μm (34), to communicate with cells in their microenvironment. Exosomes, the smallest EVs of 30–150 nm particles released from the endosomal compartment of most living cells, are the best-characterized EVs. Exosomes carry a variety of cytoplasmic proteins, nucleic acids and lipids, which reflect both the identity and internal condition of the cell of origin. Exosomes have been shown to mediate intercellular communication through the exchange of intracellular information between cells that leads to physiological and/or pathological changes in the recipient cells. Cancer cell-derived exosomes have been shown to facilitate communication between the cancer cells and the microenvironment to support tumor growth (35–37). In PCa, Hashimoto et al. (38) identified a cluster of eight miRNAs in the exosomes released by several human PCa cell lines that induce osteosclerotic lesions. In particular, exosomal miR-940 could induce the osteogenic differentiation of mesenchymal stem cells by targeting ARHGAP1 and FAM134A. This study demonstrated that cancer-secreted miRNAs, transferred via exosomes, are capable of modifying the tumor microenvironment and stimulating bone-formation. Hence, exosomes also play a role in the intercellular communication between PCa cells and osteoblasts.
EC-to-OSB conversion as a novel mechanism of prostate tumor-induced osteogenesis
Because osteoblast progenitors exist in the bone marrow, it has been generally assumed that the PCa-induced bone results from the activation of nearby, existing osteoblast precursors (39). However, histological analyses of osteoblastic metastases of PCa by Roudier et al., (4) revealed that early new bone formation did not occur from the adjacent bone surface, but rather in the tumor stroma (4). They found that the stroma contains spindle-shape cells that produced osteoid directly in the vascularized connective tissue within the tumor, suggesting that PCa- induced new bone may be derived from distinct stromal components. In the osteogenic PDX PCa-118b, PCa-118b not only can generate bone formation when implanted into mouse femur but also can induce ectopic bone formation when injected subcutaneously (10, 11). Because osteoblasts are not normally present in the subcutaneous site, these observations together with those from Roudier et al. (4) suggest that osteoblasts in PCa bone metastasis may be derived from cell types other than osteoblasts.
We recently demonstrated that endothelial cells are one of the sources of tumor- associated osteoblasts in PCa-118b tumor. We found that PCa-induced osteoblasts in the PCa- 118b PDX as well as in human PCa bone metastasis specimens co-expressed the osteoblast (OSB) marker osteocalcin and the endothelial cell (EC) marker Tie2 (40). We named these tumor-associated intermediate cell type as EC-OSB hybrid cells. These observations suggest that PCa tumor-induced osteoblasts may be derived from Tie2-expressing endothelial cells, which underwent an intermediate cell type conversion before becoming mature osteocalcin-expressing osteoblasts. Following treatment by BMP4, the tumor-derived endothelial cell line 2H11 was induced to express bone-specific markers, including osterix and osteocalcin, supporting that BMP4 induces EC-to-OSB conversion in vitro (40). Furthermore, when we expressed BMP4 in the non-osteogenic PCa cells C4–2b, C4–2b-BMP4 cells were found to stimulate ectopic bone formation when inoculated subcutaneously. In an in vivo lineage tracing study, in which genetically-engineered mice (Tie2Cre/RosatdTomato) expressing red fluorescence protein in Tie2- lineage endothelial cells were used for tumor inoculation, we found that injection of BMP4- expressing PCa cells (TRAMP-BMP4) in the mouse femurs led to increased bone formation. The osteoblasts rimming the tumor-induced bone were found to express red fluorescence protein (40), consistent with the notion that osteoblasts in bone metastases are derived from a Tie2- expressing endothelial precursor. The role of EC-to-OSB conversion in PCa-induced osteogenesis was further addressed by using a trigenic mouse (Tie2cre/Osxflox/flox/B6scid/scid) with deletion of osterix (Osx), a transcription factor that controls the development of osteoblast lineage, in the endothelial cells (40). The tumor-induced ectopic bone formation was significantly decreased when compared to control mice without endothelial-specific deletion of osterix (40). The tumor size was also decreased (23), consistent with previous observations that PCa induced bone formation influences tumor growth. Taken together, these data support that tumor-induced osteoblasts in the PCa bone lesions are, at least in part, derived from tumor- associated endothelial cells through EC-to-OSB transition (Figure 2).
Figure 2. Tumor-induced osteoblasts in the PCa bone lesions are derived from tumor- associated endothelial cells through EC-to-OSB transition.
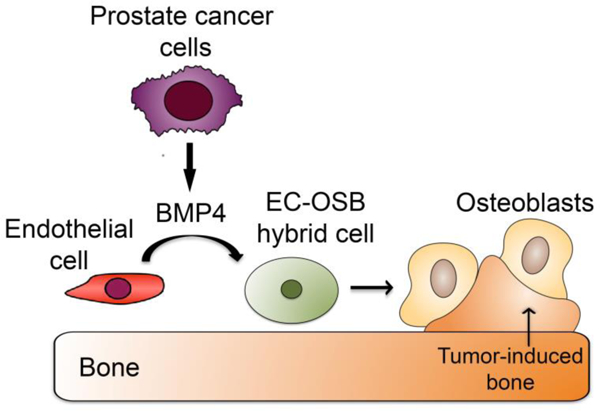
PCa cells secrete factors, e.g. BMP4, which induce endothelial cells to transition into osteoblasts. The EC-to-OSB conversion is one of the mechanisms for the characteristic osteoblastic bone lesion of PCa. This figure was reprinted from Lin et al with permission from Developmental Cell (40).
Osteolytic components in prostate cancer bone metastasis
While PCa skeletal metastases have an overall bone-forming phenotype, the clinical presentation of PCa bone metastasis suggests that this disease also carries an osteolytic component (41, 42). Because osteoclast and osteoblast activities are coupled during bone remolding, it is likely that there is an interplay between bone destruction and bone formation in prostate tumors (43). It has been shown that patients with PCa bone metastasis show elevated markers of osteoclast activity, including pyridinoline-crossed-linked peptides and deoxypyridinoline-crossed-linked peptides (44–46), and high levels of the bone resorption marker N-telopeptide of type I collagen in their urine (47). Because androgen depletion therapies frequently lead to osteoporosis, the elevated markers of osteoclast activity seen in patients with PCa bone metastasis may be in part due to hormone blockade therapy. Histomorphometric quantification of bone biopsy showed that the eroded surfaces within metastases were greater but the presence of abnormal woven bone gave an overall appearance of sclerosis (48). Thus, PCa bone metastasis showed disturbance of bone formation and resorption within metastases. However, a randomized, double-blind study comparing denosumab versus zoledronic acid for the treatment of bone metastases in men with castration-resistant prostate cancer (49) suggest that interfering with the osteolytic components of prostate cancer bone metastasis was not sufficient to alter the biology of the osteoblastic bone metastasis of prostate cancer. Thus, targeting the osteoblastic bone lesions that play a role in PCa progression in bone may be a more promising therapeutic approach.
Strategies to treat tumor-induced osteoblastic bone lesions
Currently, bone metastases remain incurable and therapies are mainly for the prevention of skeletal-related events as well as pain management. Samarium-153 and strontium-89 have been previously used to reduce bone pain in patients with bone metastasis (50–52). Rad-223, an alpha-emitter, was the first bone-targeting radionuclide successfully used to prolong life for a median of a few months when administered as a single agent (13). The improvement of Rad-223 in the treatment of PCa bone metastasis further supports a role of tumor-induced bone in PCa progression. Because Rad-223 does not cause marrow toxicity (13, 53, 54), its combination with chemotherapy or other agents that target tumors are expected to improve therapy outcomes for bone metastasis.
Because EC-to-OSB conversion has been identified as one of the mechanisms that confer the osteoblastic phenotype in PCa bone metastasis, therapies that block EC-to-OSB conversion will likely constitute a promising strategy for reducing tumor-induced bone. Identification of the mechanisms underlying such a cell type switch will be critical for developing strategies to interfere with this conversion during bone metastasis.
Conclusions
The tumor-induced bone by metastatic PCa provides a unique tumor microenvironment that not only supports tumor growth but also causes resistance to therapies. Continued efforts in the discovery of cellular and molecular determinants in the bone microenvironment, together with a focus on combinatorial therapeutics, are fundamental to improving therapies for bone metastasis.
Acknowledgements
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
This work was supported by grants from the NIH R01CA174798, NIH 5P50CA140388, NIH P30CA16672 Core grant to M.D. Anderson Cancer Center; Cancer Prevention Research Institute of Texas CPRIT RP150179; and Sister Institute Network Fund at U. Texas M.D. Anderson Cancer Center.
Footnotes
Compliance with Ethical Guidelines Conflict of Interest
Song-Chang Lin, Li-Yuan Yu-Lee, and Sue-Hwa Lin declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
••Of major importance
- 1.Hensel J, Thalmann GN. Biology of Bone Metastases in Prostate Cancer. Urology. 2016;92:6–13. [DOI] [PubMed] [Google Scholar]
- 2.Olechnowicz SW, Edwards CM. Contributions of the host microenvironment to cancer- induced bone disease. Cancer Res. 2014;74(6): 1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buenrostro D, Mulcrone PL, Owens P, Sterling JA. The Bone Microenvironment: a Fertile Soil for Tumor Growth. Curr Osteoporos Rep. 2016; 14(4): 151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roudier MP, Morrissey C, True LD, Higano CS, Vessella RL, Ott SM. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008; 180(3): 1154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roodman GD. Mechanisms of bone metastasis. Discov Med. 2004;4(22): 144–8. [PubMed] [Google Scholar]
- 6.Zhang S, Wang J, Bilen MA, Lin SH, Stupp SI, Satcher RL. Modulation of prostate cancer cell gene expression by cell-to-cell contact with bone marrow stromal cells or osteoblasts. Clin Exp Metastasis. 2009;26(8):993–1004. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Sikes RA, Malaeb BS, Yeung F, Law A, Graham SE, et al. Osteoblasts can stimulate prostate cancer growth and transcriptionally down-regulate PSA expression in cell line models. Urol Oncol. 2011;29(6):802–8. [DOI] [PubMed] [Google Scholar]
- 8.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51(14):3753–61. [PubMed] [Google Scholar]
- 9.Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, et al. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res. 2008;68(23):9996–10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li ZG, Mathew P, Yang J, Starbuck MW, Zurita AJ, Liu J, et al. Androgen receptor negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J Clin Invest. 2008;118(8):2697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YC, Cheng CJ, Bilen MA, Lu JF, Satcher RL, Yu-Lee LY, et al. BMP4 Promotes Prostate Tumor Growth in Bone through Osteogenesis. Cancer Res. 2011;71(15):5194–203.•This study demonstrates that osteogenesis is necessary for prostate tumor growth in bone.
- 12.Ozdemir BC, Hensel J, Secondini C, Wetterwald A, Schwaninger R, Fleischmann A, et al. The molecular signature of the stroma response in prostate cancer-induced osteoblastic bone metastasis highlights expansion of hematopoietic and prostate epithelial stem cell niches. PLoS One. 2014;9(12):el 14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23.•First clinical study that demonstrates that targeting tumor-induced aberrant bone by radium-223 improved overall survival in men with castration-resistant prostate cancer and bone metastases.
- 14.Dayyani F, Gallick GE, Logothetis CJ, Com PG. Novel therapies for metastatic castrate- resistant prostate cancer. JNatl Cancer Inst. 2011;103(22):1665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krzeszinski JY, Wan Y. New therapeutic targets for cancer bone metastasis. Trends Pharmacol Sci. 2015;36(6):360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thoreson GR, Gayed BA, Chung PH, Raj GV. Emerging therapies in castration resistant prostate cancer. Can J Urol. 2014;21(2 Supp 1):98–105. [PubMed] [Google Scholar]
- 17.Ramos P, Bentires-Alj M. Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene. 2015;34(28):3617–26. [DOI] [PubMed] [Google Scholar]
- 18.Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Com PG, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith M, De Bono J, Sternberg C, Le Moulec S, Oudard S, De Giorgi U, et al. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J Clin Oncol. 2016;34(25):3005–13. [DOI] [PubMed] [Google Scholar]
- 20.Lee RJ, Saylor PJ, Michaelson MD, Rothenberg SM, Smas ME, Miyamoto DT, et al. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res. 2013; 19(11):3088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai J, Zhang H, Karatsinides A, Keller JM, Kozloff KM, Aftab DT, et al. Cabozantinib inhibits prostate cancer growth and prevents tumor-induced bone lesions. Clin Cancer Res. 2014;20(3):617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C, Whang YM, Campbell P, Mulcrone PL, Elefteriou F, Cho SW, et al. Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Lett. 2018;414:205–13. [DOI] [PubMed] [Google Scholar]
- 23.Lee YC, Lin SC, Yu G, Cheng CJ, Liu B, Liu HC, et al. Identification of Bone-Derived Factors Conferring De Novo Therapeutic Resistance in Metastatic Prostate Cancer. Cancer Res. 2015;75(22):4949–59.•This study shows that paracrine factors secreted from tumor-induced bone can confer resistance to therapies for prostate cancer bone metastasis.
- 24.Sakamoto S, McCann RO, Dhir R, Kyprianou N. Talinl promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 2010;70(5): 1885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwakwa KA, Sterling JA. Integrin alphavbeta3 Signaling in Tumor-Induced Bone Disease. Cancers (Basel). 2017;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YC, Jin JK, Cheng CJ, Huang CF, Song JH, Huang M, et al. Targeting constitutively activated betal integrins inhibits prostate cancer metastasis. Mol Cancer Res. 2013; 11(4):405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahangiri A, Aghi MK, Carbonell WS. betal integrin: Critical path to anti angiogenic therapy resistance and beyond. Cancer Res. 2014;74(l):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brubaker KD, Corey E, Brown LG, Vessella RL. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem. 2004;91(l):151–60. [DOI] [PubMed] [Google Scholar]
- 29.van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-beta. Cell Tissue Res. 2012;347(1): 177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005;65(18): 8274–85. [DOI] [PubMed] [Google Scholar]
- 31.Lee YC, Gajdosik MS, Josic D, Clifton JG, Logothetis C, Yu-Lee LY, et al. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating crosstalk of cancer and stromal cells within the tumor microenvironment. Mol Cell Proteomics. 2015;14(3):471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97(3 Suppl):779–84. [DOI] [PubMed] [Google Scholar]
- 33.Marie PJ, Debiais F, Hay E. Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling. Histol Histopathol. 2002;17(3):877–85. [DOI] [PubMed] [Google Scholar]
- 34.Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–4. [DOI] [PubMed] [Google Scholar]
- 35.Hood JL, Pan H, Lanza GM, Wickline SA, Consortium for Translational Research in Advanced I, Nanomedicine. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89(11): 1317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greening DW, Gopal SK, Mathias RA, Liu L, Sheng J, Zhu HJ, et al. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Semin Cell Dev Biol. 2015;40:60–71. [DOI] [PubMed] [Google Scholar]
- 37.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184(1):28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashimoto K, Ochi H, Sunamura S, Kosaka N, Mabuchi Y, Fukuda T, et al. Cancer- secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc Natl Acad Sci USA. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5(l):21–8. [DOI] [PubMed] [Google Scholar]
- 40.Lin SC, Lee YC, Yu G, Cheng CJ, Zhou X, Chu K, et al. Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer. Developmental cell. 2017;41(5):467–80 e3.•This study demonstrates that one of the mechanisms that leads to osteoblastic bone metastasis is through tumor-induced endothelial-to osteoblast conversion.
- 41.Keller ET. The role of osteoclastic activity in prostate cancer skeletal metastases. Drugs Today (Bare). 2002;38(2):91–102. [DOI] [PubMed] [Google Scholar]
- 42.Sottnik JL, Keller ET. Understanding and targeting osteoclastic activity in prostate cancer bone metastases. Curr Mol Med. 2013;13(4):626–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortiz A, Lin SH. Osteolytic and osteoblastic bone metastases: two extremes of the same spectrum? Recent Results Cancer Res. 2012;192:225–33. [DOI] [PubMed] [Google Scholar]
- 44.Hoskin PJ, Stratford MR, Folkes LK, Regan J, Yamold JR. Effect of local radiotherapy for bone pain on urinary markers of osteoclast activity. Lancet. 2000;355(9213): 1428–9. [DOI] [PubMed] [Google Scholar]
- 45.Sano M, Kushida K, Takahashi M, Ohishi T, Kawana K, Okada M, et al. Urinary pyridinoline and deoxypyridinoline in prostate carcinoma patients with bone metastasis. Br J Cancer. 1994;70(4):701–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeuchi S, Arai K, Saitoh H, Yoshida K, Miura M. Urinary pyridinoline and deoxypyridinoline as potential markers of bone metastasis in patients with prostate cancer. J Urol. 1996; 156(5): 1691–5. [PubMed] [Google Scholar]
- 47.Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97(l):59–69. [DOI] [PubMed] [Google Scholar]
- 48.Clarke NW, McClure J, George NJ. Morphometric evidence for bone resorption and replacement in prostate cancer. Br J Urol. 1991;68(l):74–80. [DOI] [PubMed] [Google Scholar]
- 49.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris MJ, Pandit-Taskar N, Carrasquillo J, Divgi CR, Slovin S, Kelly WK, et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27(15):2436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu SM, Kim J, Pagliaro LC, Vakar-Lopez F, Wong FC, Wen S, et al. Therapy tolerance in selected patients with androgen-independent prostate cancer following strontium-89 combined with chemotherapy. J Clin Oncol. 2005;23(31):7904–10. [DOI] [PubMed] [Google Scholar]
- 52.Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol. 2005;6(6):392–400. [DOI] [PubMed] [Google Scholar]
- 53.Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. The Lancet Oncology. 2014;15(7):738–46. [DOI] [PubMed] [Google Scholar]
- 54.Vapiwala N, Glatstein E. Fighting prostate cancer with radium-223--not your Madame’s isotope. N Engl J Med. 2013;369(3):276–8. [DOI] [PubMed] [Google Scholar]


