Abstract
Background
Macaques are outstanding animal models for the development of new contraceptives. In women, progestin-only contraceptives often fail to block ovulation and are believed to act by altering cervix physiology. Herein, we assessed oviductal glycoprotein 1 (OVGP1) in the macaque cervix as a marker for progestogen action.
Materials
Rhesus macaques were treated with estradiol (E2), E2 plus progesterone (P), and E2 plus levonorgestrel, a contraceptive progestin. Samples consisted of archived blocks of mid-cervix mucosa (epithelium and lamina propria) and fresh epithelial cells collected non-invasively by cytobrush. OVGP1 was assayed by quantitative real-time PCR and localized by immunocytochemistry.
Results
OVGP1 transcript was maximal after E2 and reduced after treatment with E2 + P (P<0.05). Levonorgestrel also reduced OVGP1 expression (P<0.05). OVGP1-specific staining localized to epithelial cells and transcript was quantifiable in cytobrush collected samples.
Conclusions
OVGP1 expression in cytobrush samples of macaque cervix provides a noninvasive indicator of contraceptive progestin action.
Keywords: Oviductal glycoprotein, cervix, progestogen, hormonal regulation, cytobrush
1 Introduction
Estrogen-free contraceptives benefit women with contraindications for estrogen therapy 1. The most common estrogen-free hormonal methods are the progestin-only contraceptives that act by either suppressing ovulation2 or by altering cervical mucus secretion 3. Unfortunately, some women also display unwanted side effects of progestin therapy, such as breakthrough bleeding 4, and weight gain 5. Identifying novel completely hormone free methods that specifically target cervical mucus secretion would benefit women who cannot use hormonal methods.
Nonhuman primates, especially macaques, are excellent animal models for the studies of reproductive tract physiology and for the development of new contraceptives in advance of clinical trials in women 6,7. In women, the effect of hormones on the cervix has been traditionally evaluated by Insler score of cervical mucus 8,9. Insler scoring examines such aspects as ferning, spinnbarkeit (stretchability), the volume of an aspirate, cellularity, and perceived viscosity. The Insler scoring system combines quantitative values with subjective values to produce a total, semi-quantitative score 10. Insler score was initially developed for identifying the fertile period of the human menstrual cycle, in practice, the effect of contraceptives on cervical mucus is difficult to assess accurately 10. Although it is possible to replicate cervical mucus scoring in the macaque 11,12, the volume of mucus in the presence of progestins is often close to the limit of measurement in the luteal phase of the menstrual cycle. Therefore, there are currently no reliable markers for identifying the action of contraceptive progestins in the macaque.
In a preliminary study, we carried out a GeneChip™ Genome Array analysis of cervix RNA from cycling rhesus macaques 13. Oviductal glycoprotein 1 (OVGP1; MUC9) was identified as transcript expressed in the cervix arrays. OVGP1 is a carbohydrate-rich glycoprotein secreted by oviductal epithelial cells that has sperm binding properties 14,15. Early studies of the macaque and baboon oviduct report that estrogen stimulates and progestogens suppress OVGP1 16. OVGP1 is expressed in the cervix of rabbits17 and primates 13, but hormonal regulation is undocumented. We hypothesized that OVGP1 could be hormonally regulated in the cervix, and provide a marker for progestogen action. To test this hypothesis we characterized hormonal regulation and localization of OVGP1 in the cervix of hormone-treated macaques. Our goal was to develop a molecular marker of progestogen action in cervix epithelial cells of macaques.
2 Materials and Methods
2.1 Humane care guidelines
The Oregon National Primate Research Center (ONPRC), Division of Comparative Medicine provided animal care and husbandry for these studies. All animal procedures were reviewed and approved by the ONPRC Animal Care and Use Committee. All work was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
2.2 Treatments & Samples
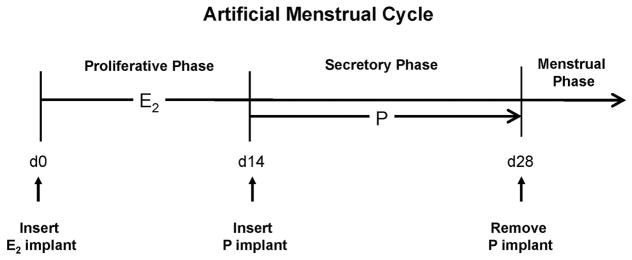
To conserve animal resources, we first analyzed archived samples of rhesus macaque (Macaca mulatta) cervix. The samples were obtained at necropsy from adult oophorectomized animals treated sequentially with subcutaneous Silastic capsules 18 that released either estradiol (E2) or progesterone (P) to create controlled artificial menstrual cycles (Figure 1). The capsules produced normal proliferative phase levels of E2 (80–100 pg/ml) and secretory phase levels of P (5–7 ng/ml) as previously reported 19,20. Treatments and time points are shown in Table 1. Similar samples were also obtained from ovariectomized animals treated with E2 plus levonorgestrel (LNG) releasing implants for 28 days. The LNG implants (5 cm long by 0.335 cm i.d. and 0.465 cm o.d.) produced 0.36 ± 0.1 ng LNG/ml in plasma and provided a clinically relevant example of animals treated with a contraceptive progestin.
Figure 1.
Diagram showing hormone treatments for the archived samples. On artificial cycle day 0, ovariectomized animals receive a subcutaneous capsule releasing E2 and then after 14 days of E2 priming; the animals received a similar capsule releasing P. Removal of the P implant (leaving E2 in place) stimulated menstruation to complete the menstrual cycle. When animals received E2 + LNG, the LNG implant was inserted instead of P and kept in place for 28 days (not shown).
Table 1.
Collection time points for archived samples.
| Treatment | Description | Sample Size |
|---|---|---|
| Premenstrual | Collected the end of the cycle before P implant removal | n = 10 |
| Menstrual Phase | Day 3 after P implant removal, active menstruation | n = 4 |
| Proliferative Phase | Treatment with E2 alone for 14 days | n=10 |
| Secretory Day 1 | 1 day after P insertion | n = 4 |
| Secretory Day 3 | 3 days after P insertion | n = 5 |
| Secretory Day 7 | 7 days after P insertion | n= 5 |
| Levonorgestrel (LNG) | 28 days of E2 plus LNG capsule | n=4 |
The archived samples consisted of frozen and paraffin-embedded blocks of mid-cervical mucosa (luminal epithelium, glands and lamina propria) collected from the mid cervix at or near the cervical colliculus (Figure 2). Frozen blocks were used for protein and RNA isolation, and cryoblocks frozen in OCT were used for immunohistochemistry (IHC) of steroid receptors. Paraffin blocks were used for IHC of OVGP1. Representative blocks of fixed and frozen oviductal ampulla were used as positive control tissue.
Figure 2.
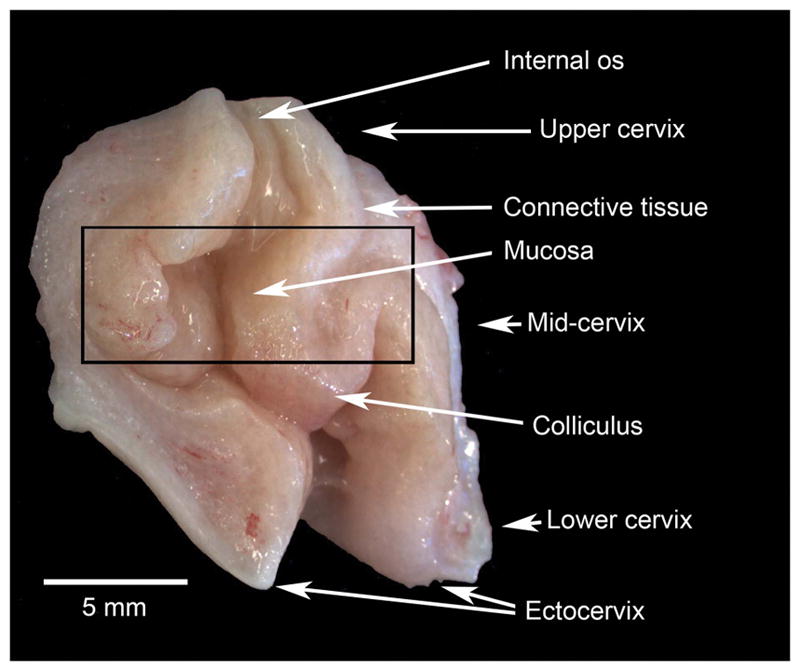
Macro photograph showing a rhesus macaque cervix that has been cut along the longitudinal axis. The photograph shows the striking colliculus 34, which provides an excellent anatomical reference for tissue collection for excluding the squamous epithelium of the ectocervix. A black rectangle shows the portion of the cervix that provided the archived samples in this work.
In addition to the archived samples, fresh samples of cervix epithelial cells were obtained by cytobrush from macaques in the proliferative and secretory phases of the artificial cycle (n=4). For cytobrush samples, proliferative phase (cycle day 8–10) E2 levels were 97 ± 17pg/ml) and secretory phase (cycle day 17–23) E2 and P levels were 85 ± 11 pg E2, and 5.6 ± 6.1 ng P/ml, respectively. Briefly, the animals were sedated with Ketamine (10 mg/kg) and placed in ventral recumbency and the cervix was viewed with the aid of a speculum. A sterile Cytobrush Plus (Cooper Surgical; Trumbull, CT, USA) was inserted into the external cervical os approximately 1 cm (to the cervical coniculus; Figure 2), and gently turned one full circle. The brush was removed from the animal and immediately submerged into 1ml of Trizol (InVitrogen, Carlsbad, CA, USA) for RNA extraction. A representative sample of cells collected by cytobrush in the follicular phase was transferred to SuperFrost Plus slides (Fisher Scientific, Waltham, MA, USA cat#12-550-15) slides. The slides were then either immediately stained with hematoxylin cover slipped with glycerin and photographed; or fixed in with 4% paraformaldehyde for 10 minutes at room temperature and subjected to IHC.
2.3 RNA analysis
Samples for RNA isolation were frozen in liquid nitrogen and stored at −80°C. The samples were thawed in Trizol and homogenized with a Polytron (Brinkman Instruments, Westbury, NY, USA) and then total RNA was purified with DNase digestion on Qiagen Quick columns (Qiagen, Valencia, CA, USA). RNA concentration was determined on a Nanodrop Spectrophotometer (Model ND-1000; Thermo Scientific, Wilmington, DE, USA) and RNA integrity was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Total RNA (1 μg) was reverse transcribed into cDNA, in the presence of random hexamer primers (Promega Corporation, Madison, WI, USA) using an Omniscript RT kit (Qiagen; Valencia, CA, USA). Quantitative real-time PCR (RT-qPCR) reactions were carried out on a 7500 Fast Real-time PCR System (ABI; Applied Biosystems, Carlsbad, CA, USA). Real-time PCR reactions were performed in duplicate on 2 μl of diluted (1:2) cDNA and compared against a 5-point standard curve of pooled oviductal RNA 21. Table 2 shows PCR primers and hydrolysis probes. Target genes expression was referenced to Ribosomal S10 RNA expression 21 and averaged to give a single level of expression.
Table 2.
PCR Primer and Probe sequences
| Name | Accession # | Forward Primer | Reverse Primer | Hydrolysis Probe |
|---|---|---|---|---|
| OVGP1 | NM_002557.3 | CGTACCTTTCGCCTCCTCAA | TTGGTGTACTTCCCTGGAGAT | CCTCTAAGAATGGGTTGCAGGCCA |
| ESR1 | XM_015137107.1 | GGTGCCAGGCTTTGTGGAT | CGCCAGACGAGACCAATCATC | TTGACCCTCCATGATCAGGTCCAC |
| PGR | NM_001278457.1 | CACCACGACGATGGATTTCAC | AGTGCGGGCTGCCAATAAGG | CACGTGCCCATCCTGCCTCTCAAT |
| S10 | NM_001193579.1 | AATGTGCCCAACCTTCATGTC | TCCAGGCAAACTGTTCCTTCA | TGAAGGCCATGCAGTCTCTCAAGTCCC |
RT-qPCR data were examined for statistical outliers using Grubbs’ test 22 and then and evaluated Levene’s test for equality of variance. Data sets were transformed (log 10 + 1) to correct for hetereogeneity and then analyzed by Analysis of Variance followed by Fishers LSD test for comparison of means 23. Untransformed mean (± standard errors; SE) were plotted using ORIGIN Lab (Version 9; Northhampton, MA) Software.
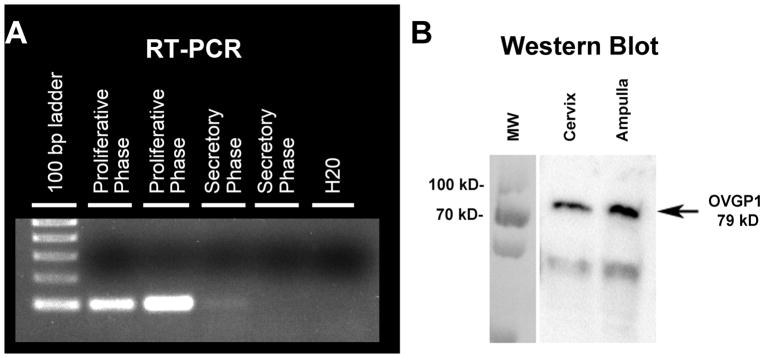
Conventional RT-PCR was conducted on a subset of samples to validate RT-qPCR reactions (Figure 3A). Total RNA was reverse transcribed into cDNA as described above. 1 ug of cDNA was amplified using a HotStar Taq PCR (Qiagen, Valencia, CA, USA) kit with 3 step cycling for 30 cycles. For each cycle denaturation was 0.75 min@94 C; annealing was 0.75 min @ 68C; extension was 1 min at 72C. PCR products were visualized after fractionation on 1% agarose gels in the presence of ethidium bromide.
Figure 3.
Blots showing OVGP1 analysis by conventional PCR and Western Blot with ab 11850. (A) RT PCR analysis demonstrating probe specificity, and Western blot (B) showing antibody specificity.
2.4 Western blot
Anti-OGP1 (Abcam cat #118590, Cambridge, MA, USA) antibody specificity was demonstrated by Western blot (Figure 3B). Macaque tissue total protein was extracted from frozen cervix tissue blocks using 1× RIPA buffer (50 mM Tris-HCl pH7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS) containing 1× Complete Protease Inhibitor Cocktail (Roche, Basel, Switzerland). Fifty μg of total protein in 1× Laemmli sample buffer was heated at 85°C for 5 minutes and then cooled to room temperature before being separated on a 10% SDS-PAGE gel. The protein was transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). The membrane with immobilized protein was blocked with 5% non-fat dry milk (NFDM) and TBS-T buffer for one hour and then blotted with a rabbit anti-OVGP1 antibody (ab118590) at a 1:500 dilution. Goat anti-rabbit biotinylated BA-2000 (Vector Laboratories) secondary was used at a 1:9,000 dilution in 5% NFDM/TBST overnight. Following a 5 minute incubation with enhanced chemiluminescence (ECL) Western blotting substrate (Millipore), the signal was visualized on a Fluorochem M system (Proteinsimple, San Jose, CA, USA). The size of the detected protein band was determined by the PageRuler™ Plus Prestained Protein Ladder (Thermo Scientific, Wilmington, DE, USA) separated on the same gel.
2.4 Immunohistochemistry
IHC for OVGP1 was conducted on (6 μm) paraffin sections using a standardized technique 21 at room temperature unless otherwise noted. The sections were mounted on SuperFrost Plus slides and allowed 3 days for full adherence to the slides. The slides were deparaffinized in xylene and rehydrated. Antigen retrieval was carried out by heating sections in citrate buffer (BioGenex, Fremont, CA, USA) in a pressure cooker at 15 psi for 10 minutes. Sections were treated with 3% hydrogen peroxide in methanol for 30 min and then treated with species-specific normal serum (ABC-kit, Vector Laboratories Inc, Burlingame, CA, USA) for 1 hr at room temperature and then with the primary antibody overnight at 4C. Anti-OVGP1 antibody (Abcam, Cambridge, MA, USA, cat #118590) was diluted from stock in 0.01M phosphate buffered solution in a variety of dilutions: 0.5 μg, 0.67 μg, 1 μg, 1.3 μg, 2 μg, 4 μg, and 10 μg. A concentration of 2 μg was determined to provide the best signal to background ratio and was used for subsequent staining of cervix tissue. We also stained sections with Abcam Antibody cat #74544 (4 μg/ml), validated by Wright et al., 24 to show specific staining in macaque oviduct and ovarian surface epithelium.
IHC for ESR1 and PGR was conducted on 7 um cryosections as previously described 18. Target specific antibodies were anti-ESR1 (estrogen receptor 1) Neomarkers cat#Ms-354-P), and an anti-progesterone receptor IgG that detects both isoforms of PGR (anti-PGR; Neomarkers, Freemont, CA, USA, cat#Ms-298-P). Antibody concentrations for ESR1 and PGR have been published previously 18.
After overnight incubation, sections were washed, and secondary biotinylated antibodies were added and incubated (30 minutes) at RT. After rinsing with PBS containing BRIJ, the sections were incubated in avidin-biotin-peroxidase reagent solution, (Vector Laboratories Inc, Burlingame, CA, USA). Color was developed with 0.025% 3,3′-diaminobenzidine, (Dojindo Inc, Richmond, VA, USA) darkened with 0.026% osmium tetroxide for 1 minute and then counterstained with Meyer’s hematoxylin. After dehydration with ethanol and clearing with xylene, the slides were mounted with Permount. Methodological controls included no primary antibody, replacement of primary antibody with nonspecific normal serum, and replacement of primary antibody with an irrelevant antibody (anti-Br(d)U; Abcam Cat #ab1893).
Digital images were taken using a Zeiss Axiolmager A.I microscope (Carl Zeiss, Inc., Oberkochen, Germany) and a Leica DFC 480 camera (Leica, Wetzler, Germany).
3 Results
3.1 OVGP1 Transcript expression
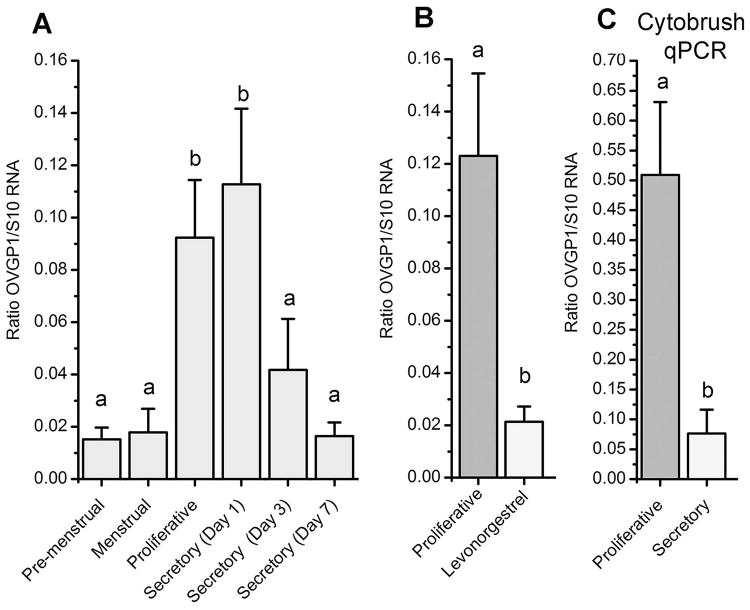
Conventional RT-PCR revealed a single specific band in proliferative phase samples but nearly absent in secretory phase samples (Figure 3A). Figure 4 shows levels of OVGP1 transcript detected by RT-qPCR. Based on RT-qPCR OVGP1 was present in all samples analyzed. The cyclic patterns revealed that OVGP1 transcript was minimal in the premenstrual and menstrual phase of the cycle. Levels increased significantly (P<0.05) during the proliferative phase and maximal on day 1 of the secretory phase. After that, levels OVGP1transcript decreased to baseline by day 3 of P treatment and remained low throughout the secretory phase.
Figure 4.
Mean (±SE) levels of OVGP1 transcript assayed by gene array with RMA normalization (A) or by real-time PCR (B). Samples for array analysis (A) were from artificially-cycled animals only, samples for RT-qPCR (B) included treatment with LNG. Cytobrush collect cervical cells (C) were analyzed by RT-qPCR. Bars with different superscripts are significantly different (P<0.05).
Figure 4B shows a comparison of treatment with E2 alone or with E2 plus LNG for 28 days. Treatment with LNG significantly reduced OVGP1 transcript compared to E2 alone (P<0.05) similar to the late secretory phase. RT-qPCR also revealed OVGP1 transcript in cervix epithelial cells collected by cytobrush (Figure 4C). Levels were elevated in the proliferative phase and significantly suppressed in the secretory phase (P<0.05).
3.2 OVGP1 Immunostaining
Figure 3B shows Western Blot staining for ab 116737. As expected from the literature 25,26 blotting revealed two specific glycoprotein bands at approximately 79 and 66kDa indicating antibody specificity for the antibody we used preferentially for IHC. Immunostaining (see below) was also conducted with antibody ab74544. Ab 74544 is reported by to detect a larger 150 kDA form. However, Western blot of macaque tissues (both oviduct and cervix) produced blots with 6–8 multiple bands between 180 and 66 kDA (not shown).
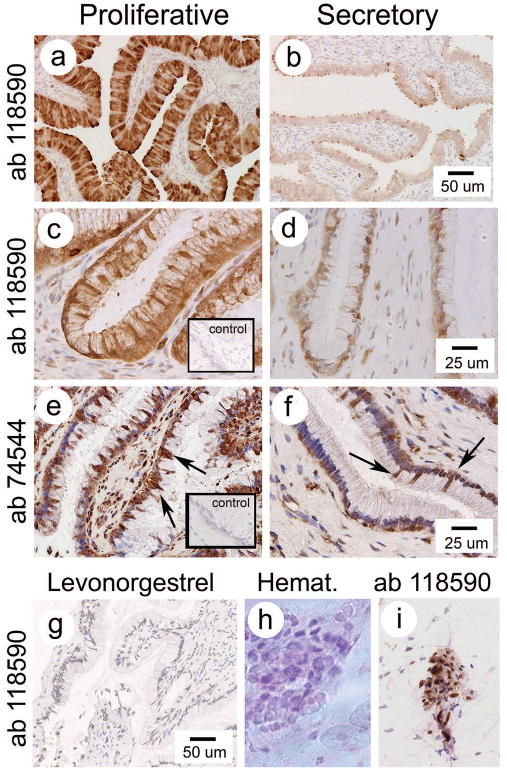
Figure 5 shows IHC staining for OVGP1 with ab 119590 in the oviductal ampulla (positive control; a, b) and cervix (c,d). The specific staining for OGVP1 with ab 119590 in the epithelium of the cervix was strikingly stronger in samples from the proliferative phase of the cycle (Figure 5c). No specific staining was detected in the cervix stroma. Minimal staining, approaching background was detected in the cervix during the secretory phase (Figure 5b). Only hematoxylin background staining was observed after treatment with LNG (Figure 5g). Figure 5e and 5f show staining for ab 74544. Ab 74544 also showed stronger staining in the cervix epithelium from the proliferative phase than secretory phase, but also show unexpected staining in stromal and endothelial cells. As expected, samples of ampulla (Figure 5c) revealed strong staining for OVGP1 with ab 119590, which was decreased in the secretory phase (Figure 5d). Oviductal ampulla revealed strong epithelial staining as reported by Wright 24 (not shown).
Figure 5.
Photographs showing IHC for OVGP1. Staining with ab 118590 is shown for both oviduct (positive control; a,b) and cervix (c–d; inset shows negative ab control). Ab 118590 staining was reduced in the secretory phase compared to the proliferative phase and completely lost after treatment with levonorgestrel (g). Staining with ab 74544 was also stronger in the proliferative phase (e; more strongly positive cells [arrows]) than secretory phase (f). Inset shows negative ab control). Cells collected by cytobrush are shown in (h) and (I).
Figure 5h shows an image of [fresh mount] cervical epithelial cells stained with hematoxylin. Of these cells, the majority (>80%) stained positive for OVGP antibody ab 119590 and cytokeratin (not shown). The remaining cells were OVGP and cytokeratin negative.
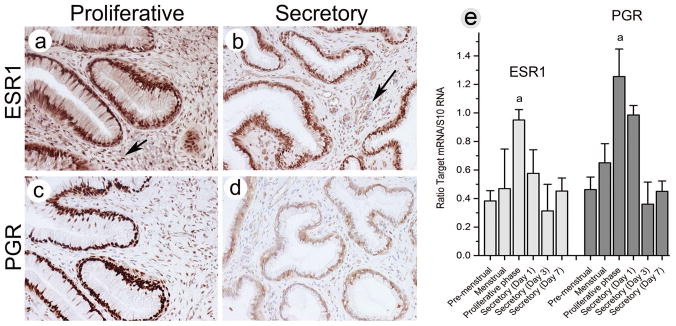
3.3 Steroid receptor expression and immunostaining
Figure 6 shows IHC staining for cervix ESR1 (a,b) and PGR (c,d) along with transcript expression in RNA samples from the artificial cycle (e). Strong nuclear staining for ESR1 was localized to both the epithelial and stromal compartments in both the proliferative and secretory phases of the cycle. ESR1 staining was reduced notably in the stroma in the secretory phase. In contrast, PGR staining (both isoforms) was strong in the nuclei of the glands and stroma during the proliferative phase and strikingly reduced in both glands and stroma in the secretory phase. Therefore, staining intensity for PGR in the epithelium paralleled the staining for OVGP1 detected by both ab 119590, and ab 74544. Receptor expression was supported by levels of transcript expression for both ESR1 and PGR which was significantly increased in the proliferative phase than at any other time point in the cycle.
Figure 6.
Immunostaining for ESR1(a,b) and PGR (c,d). RT-qPCR analysis of ESR1 and PGR mRNA expression show significant upregulation of both ESR1 and PGR in the proliferative phase of the cycle (e).
4 Discussion
OVGP1 is an estrogen-dependent mucin that is secreted by the fallopian tube of women, primates and non-primate species 14,27,28. OVGP1 protein has also isolated from the mammalian cervix 17 (e.g. MUC9). Early studies suggested that OVGP1 expression was restricted to the oviductal epithelium, and focused primarily on a potential role for OVGP1 on gamete interactions 29. More recently, studies report that that OVGP1 is also expressed by ovarian epithelium 30 and some reproductive tract carcinomas 30–32. We report that OVGP1 is expressed by epithelial cells of the macaque cervix and strongly in the proliferative phase and down-regulated in the secretory phase of the artificial menstrual cycle. Overall, OVGP1 levels parallel other estrogen-regulated target including PGR, and are increased at points in the cycle when cervical mucus secretion is reported to be high11,12.
In this study we assessed two antibodies for OVGP1 (ab1185090 and ab74544), which in theory, detect the multiple moieties with differing glycosylation. The presence of multiple bands with ab74544 could represent a nonspecific reaction, or simply detect variable glycosylation. However, in both cases, specific staining was observed in the epithelial cells supporting secretion of the glycoprotein into the cervix lumen. It appears that multiple forms of OVGP1 are secreted into the cervix and regulated by steroid hormones. Comparison of antibody staining, is not a sufficiently robust measure to characterize the proportion of OVGP1 expression based on glycosylation. Further study is needed to identify the physiological role of the various glycosylated forms.
Rhesus macaques are excellent animal models for these studies because the macaque reproductive tract responds to estrogen and progestogen stimulation similarly to women. There are few reliable tests to assess cervix function in nonhuman primates. In women, mucus quality and secretion are measured by Insler score. The Insler score was developed in the 1970’s to evaluate infertility, and to date has never been validated for the ability to predict contraceptive efficacy. Instead, correlation to contraceptive efficacy is hypothesized. Similarly, in vitro and in vivo sperm penetration testing serves as proxies for sperm-mucus interaction, but is highly variable, subjective, difficult to execute and most importantly, unvalidated in nonhuman primates. The strength of our artificial cycle nonhuman primate model is that the cycles are driven solely by changes in the exposure to E2 and P. In this model, we can clearly test the effects of controlled levels of E2 and P and we demonstrate that OVGP1 expression not only increases during estrogen treatment but is suppressed by P in the presence of continued exposure to E2. Therefore, downregulation of OVGP1 in the secretory phase is due specifically to the action of progestogen. This is physiologically relevant because the primate corpus luteum produces significant levels of estrogen during the natural menstrual cycle. The significance of this outcome is underscored by the observation that treatment with E2 plus the contraceptive progestin, LNG, also displayed reduced levels of OVGP1 transcript compared to E2 treatment alone. Therefore, we propose that OVGP1 should provide a realistic molecular marker of progestin action on cervix mucin production.
Progestin downregulation of OVGP1 is further supported by effect of LNG. LNG appears to result in a greater suppression of OVGP1 than P in the artificial cycle. LNG is primarily a progesterone receptor agonist, with weak androgen action33. There substantial overlap in the transcriptional response to progestogens and androgens in cells that express both receptors. It is also likely that the prolonged 28 day LNG further reduced OVGP1 compared to the 14 day secretory phase.
Unlike other compartments of the female reproductive tract, the cervical epithelium is easily accessible by biopsy or cervical brush. We have demonstrated that RNA can be isolated from these cells and assayed by RT-qPCR for OVGP1 to produce a rapid, sensitive assay for contraceptive progestin action. It is well documented that OVGP1 is secreted into the oviductal fluid and it is likely that it is also secreted into cervical mucus. OVGP1 is reported to have interactions with sperm as well as zona pellucida, sperm health before fertilization 14. However, the physiological role for OVGP1 in the cervix is unknown. Assessment of the concentrations of OVGP1 in cervical mucus as well as the extent of glycosylation and the role of OVGP1 in gamete passage is outside the scope of this work. Future studies are needed to titrate an array of progestins for contraceptive action and regulation of OVGP1.
5 Conclusion
In conclusion, we propose that cervix epithelium collected by cytobrush and assayed for OVGP1 expression by RT-qPCR can provide a rapid and very sensitive method for identifying the effect of progestogen-based contraceptives on the cervix in preclinical experiments in cycling monkeys and clinical trials.
Acknowledgments
The authors thank the Division of Comparative Medicine at the Oregon National Primate Research Center; for assistance with animal studies; Shan Yao for assistance with Western blot, Fangzhou Luo for immunohistochemistry and Corinne Wilcox for help with RT-qPCR analysis. This work was supported by NIH grants U54 HD055744; NIH P51OD011092.
References
- 1.Tepper NK, Whiteman MK, Marchbanks PA, James AH, Curtis KM. Progestin-only contraception and thromboembolism: A systematic review. Contraception. 2016;94(6):678–700. doi: 10.1016/j.contraception.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Dusterberg B. Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide. Contraception. 2011;84(6):549–557. doi: 10.1016/j.contraception.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Lewis RA, Taylor D, Natavio MF, Melamed A, Felix J, Mishell D., Jr Effects of the levonorgestrel-releasing intrauterine system on cervical mucus quality and sperm penetrability. Contraception. 2010;82(6):491–496. doi: 10.1016/j.contraception.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Critchley H. Endometrial effects of progestogens. Gynaecology Forum. 2003;8(3):6–10. [Google Scholar]
- 5.Luukkainen T, Pakarinen P, Toivonen J. Progestin-releasing intrauterine systems. SeminReprodMed. 2001;19(4):355–363. doi: 10.1055/s-2001-18643. [DOI] [PubMed] [Google Scholar]
- 6.Slayden OD. Cyclic remodeling of the nonhuman primate endometrium: a model for understanding endometrial receptivity. SeminReprodMed. 2014;32(5):385–391. doi: 10.1055/s-0034-1376357. [DOI] [PubMed] [Google Scholar]
- 7.Slayden OD. Translational In Vivo Models for Women’s Health: The Nonhuman Primate Endometrium--A Predictive Model for Assessing Steroid Receptor Modulators. Handb Exp Pharmacol. 2016;232:191–202. doi: 10.1007/164_2015_22. [DOI] [PubMed] [Google Scholar]
- 8.Insler V. The evaluation and treatment of cervical mucus diseases leading to infertility. Adv Exp Med Biol. 1977;89:477–488. doi: 10.1007/978-1-4613-4172-7_32. [DOI] [PubMed] [Google Scholar]
- 9.Insler V, Glezerman M, Bernstein D. Diagnosis and treatment of the cervical factor of infertility. Reproduccion. 1981;5(4):295–300. [PubMed] [Google Scholar]
- 10.Leader A, Wiseman D, Taylor PJ. The prediction of ovulation: a comparison of the basal body temperature graph, cervical mucus score, and real-time pelvic ultrasonography. Fertil Steril. 1985;43(3):385–388. doi: 10.1016/s0015-0282(16)48436-0. [DOI] [PubMed] [Google Scholar]
- 11.Kanagawa H, Hafez ES, Mori J, Kurosawa T, Kothari L. Cyclic changes in cervical mucus and LH levels in the bonnet macaque (Macaca radiata) Folia Primatol (Basel) 1973;19(2):208–217. doi: 10.1159/000155539. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari AN, Hafez ES, Syner F. Detection of ovulation by color changes in cervical mucus of crab-eating macaque (Macaca fascicularis) Int J Fertil. 1972;17(4):169–177. [PubMed] [Google Scholar]
- 13.Bond KR, Slayden OD. Pathways regulating the nonhuman primate cervix. Fertility and Sterility. 2015;104(3):e150. [Google Scholar]
- 14.O’Day-Bowman MB, Mavrogianis PA, Reuter LM, Johnson DE, Fazleabas AT, Verhage HG. Association of oviduct-specific glycoproteins with human and baboon (Papio anubis) ovarian oocytes and enhancement of human sperm binding to human hemizonae following in vitro incubation. BiolReprod. 1996;54(1):60–69. doi: 10.1095/biolreprod54.1.60. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Zhao Y, Yang X, Kan FW. Recombinant hamster oviductin is biologically active and exerts positive effects on sperm functions and sperm-oocyte binding. PLoS One. 2015;10(4):e0123003. doi: 10.1371/journal.pone.0123003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhage HG, Fazleabas AT, Mavrogianis PA, et al. The baboon oviduct: characteristics of an oestradiol-dependent oviduct-specific glycoprotein. HumReprodUpdate. 1997;3(6):541–552. doi: 10.1093/humupd/3.6.541. [DOI] [PubMed] [Google Scholar]
- 17.Hendrix E, Hewetson A, Mansharamani M, Chilton BS. Oviductin (Muc9) is expressed in rabbit endocervix. Endocrinology. 2001;142(5):2151. doi: 10.1210/endo.142.5.8285. [DOI] [PubMed] [Google Scholar]
- 18.Slayden OD, Brenner RM. Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol. 2004;67(5):393–409. doi: 10.1679/aohc.67.393. [DOI] [PubMed] [Google Scholar]
- 19.Slayden OD, Brenner RM. A critical period of progesterone withdrawal precedes menstruation in macaques. Reprod Biol Endocrinol. 2006;4(Suppl 1):S6. doi: 10.1186/1477-7827-4-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slayden OD, Brenner RM. Role of progesterone in the structural and biochemical remodeling of the primate endometrium. Ernst Schering Res Found Workshop. 2005;(52):89–118. doi: 10.1007/3-540-27147-3_5. [DOI] [PubMed] [Google Scholar]
- 21.Almeida-Francia CC, Keator CS, Mah K, Holden L, Hergert C, Slayden OD. Localization and hormonal regulation of endometrial matrix metalloproteinase-26 in the rhesus macaque. Hum Reprod. 2012;27(6):1723–1734. doi: 10.1093/humrep/des086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokal RR, Rohlf FJ. Introduction to Biostatistics. Vol. 2. New York: W.H. Freeman and Compnay; 1987. [Google Scholar]
- 23.Petersen RG. Design and Analysis of Experiments. Vol. 66. New York: Marcel Dekker, Inc; 1985. [Google Scholar]
- 24.Wright JW, Pejovic T, Jurevic L, Bishop CV, Hobbs T, Stouffer RL. Ovarian surface epitheliectomy in the non-human primate: continued cyclic ovarian function and limited epithelial replacement. Hum Reprod. 2011;26(6):1422–1430. doi: 10.1093/humrep/der061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Prasad S, Gupta HP, Singhal S, Gupta AK, Kumar A. Isolation and characterization of oviduct-specific glycoproteins from ampulla and isthmus parts of cyclic and acyclic buffalo for studying differential microenvironment. Appl Biochem Biotechnol. 2012;166(7):1814–1830. doi: 10.1007/s12010-012-9599-6. [DOI] [PubMed] [Google Scholar]
- 26.Saccary L, She YM, Oko R, Kan FW. Hamster oviductin regulates tyrosine phosphorylation of sperm proteins during in vitro capacitation. Biol Reprod. 2013;89(2):38. doi: 10.1095/biolreprod.113.109314. [DOI] [PubMed] [Google Scholar]
- 27.Martus NS, Verhage HG, Mavrogianis PA, Thibodeaux JK. Enhancement of bovine oocyte fertilization in vitro with a bovine oviductal specific glycoprotein. JReprodFertil. 1998;113(2):323–329. doi: 10.1530/jrf.0.1130323. [DOI] [PubMed] [Google Scholar]
- 28.Fazleabas AT, Verhage HG. Synthesis and release of polypeptides by the baboon (Papio anubis) uterine endometrium in culture. BiolReprod. 1987;37(4):979–988. doi: 10.1095/biolreprod37.4.979. [DOI] [PubMed] [Google Scholar]
- 29.Buhi WC. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction. 2002;123(3):355–362. doi: 10.1530/rep.0.1230355. [DOI] [PubMed] [Google Scholar]
- 30.Woo MM, Gilks CB, Verhage HG, Longacre TA, Leung PC, Auersperg N. Oviductal glycoprotein, a new differentiation-based indicator present in early ovarian epithelial neoplasia and cortical inclusion cysts. GynecolOncol. 2004;93(2):315–319. doi: 10.1016/j.ygyno.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Joshi A, Iaconis L, et al. Oviduct-specific glycoprotein is a molecular marker for invasion in endometrial tumorigenesis identified using a relevant mouse model. IntJCancer. 2009;124(6):1349–1357. doi: 10.1002/ijc.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo MM, Alkushi A, Verhage HG, et al. Gain of OGP, an estrogen-regulated oviduct-specific glycoprotein, is associated with the development of endometrial hyperplasia and endometrial cancer. ClinCancer Res. 2004;10(23):7958–7964. doi: 10.1158/1078-0432.CCR-04-1261. [DOI] [PubMed] [Google Scholar]
- 33.Burton KA, Henderson TA, Hillier SG, et al. Local levonorgestrel regulation of androgen receptor and 17beta-hydroxysteroid dehydrogenase type 2 expression in human endometrium. HumReprod. 2003;18(12):2610–2617. doi: 10.1093/humrep/deg510. [DOI] [PubMed] [Google Scholar]
- 34.Hafez ESE, Jaszczak S. Comparative anatomy and histrology of the cervix uteri in nonhuman primates. Primates. 1972;13(3):297–316. [Google Scholar]