Abstract
The human penis and clitoris develop from the ambisexual genital tubercle. To compare and contrast the development of human penis and clitoris, we used macroscopic photography, optical projection tomography, light sheet microscopy, scanning electron microscopy, histology and immunohistochemistry. The human genital tubercle differentiates into a penis under the influence of androgens forming a tubular urethra that develops by canalization of the urethral plate to form a wide diamondshaped urethral groove (opening zipper) whose edges (urethral folds) fuse in the midline (closing zipper). In contrast, in females, without the influence of androgens, the vestibular plate (homologue of the urethral plate) undergoes canalization to form a wide vestibular groove whose edges (vestibular folds) remain unfused, ultimately forming the labia minora defining the vaginal vestibule. The neurovascular anatomy is similar in both the developing human penis and clitoris and is the key to successful surgical reconstructions.
Keywords: Development, Human, Penis, Clitoris, Canilization and Fusion
Introduction
Male and female external genitalia play an essential role in human reproduction, and disorders of structure and function of male and female external genitalia can have profound deleterious effects on fertility, urinary continence and renal function. Proper function of male and female external genitalia requires precise anatomical organization of penile and clitoral erectile bodies, the penile urethra, as well as precise somatic, sympathetic and parasympathetic innervation of the penis, the clitoris and the vulva. The exquisite anatomical organization of male and female external genitalia emerge during embryonic and fetal development, and the developmental biology of external genitalia is critical for understanding common malformations of the penis and clitoris and for surgical repair of congenital malformations of the external genitalia (Baskin, 2017a; Baskin, 2017b). This singular fact has led to a sizable literature on animal models (principally mouse) of development of male and female external genitalia and more specifically on hypospadias (Cunha et al., 2015; Liu Ge, 2018; Rodriguez et al., 2011; Weiss et al., 2012; Mahawong et al., 2014; Sinclair et al., 2016a). While we have contributed substantially to the mouse hypospadias literature, in recent years we have recognized that the mouse is not the ideal model for normal human penile development and hypospadias for a host of reasons (Cunha et al., 2015; Liu Ge, 2018; Sinclair et al., 2016a). This realization has emerged through detailed studies of human penile (and clitoral) development, and until recently the literature on development of human male and female external genitalia has been inadequate and based for the most part on simple anatomical studies. To address this deficit, we have recently devoted considerable effort in investigating development of human male and female external genitalia in comparison with development of external genitalia in mice and rats using a broad range of modern techniques (Li et al., 2015; Overland et al., 2016; Cunha et al., 2015; Liu Ge, 2018; Shen et al., 2016; Sinclair et al., 2016a). Such a developmental approach is critical for understanding normal morphogenetic mechanisms in human external genitalia, has provided insights into the mechanism of human hypospadias and has facilitated surgical correction of both penile and clitoral malformations (Baskin et al., 1998; Baskin et al., 1999; Baskin, 2017b).
Sexual dimorphism of external genitalia in humans is particularly profound in humans as size and morphology of the penis and clitoris are strikingly different even though both structures develop from the remarkably similar ambisexual genital tubercle, which is capable of either penile or clitoral development irrespective of genotype. Androgens are the key hormonal factor eliciting penile development in normal males (Shen et al., 2018a; Shen et al., 2016) (Wilson et al., 1981). However, female patients with congenital adrenal hyperplasia, an autosomal recessive disorder, characterized by impaired cortisol synthesis, produce androgens in utero and thereby undergo varying degrees of virilization of the external genitalia, which in the most severe cases can result in development of normal penile morphology (Speiser et al., 2010). In the absence of androgens (as in normal females) or due to impaired androgen action (due to defects and or absence of the androgen receptor), the genital tubercle of a genetic male will develop clitoral morphology (Wilson et al., 2011). Thus, the genital tubercle has the bipotential to differentiate into either a penis or clitoris depending on androgen action or the lack thereof independent of genetic sex (Baskin, 2017b).
Both the human penis and clitoris have analogous corporal bodies containing sinusoidal erectile tissue surrounded by a thick tunica albuginea (Baskin et al., 1999; Breza et al., 1989; Clemente, 1985). Distally, both organs have an analogous glans penis and glans clitoris, respectively. The major difference between the male and female is the lack of tubular urethra within the clitoris (Li et al., 2015; Overland et al., 2016).
An understanding of human penile urethral development has evolved over time. In 1954, Glenister proposed that surface ectoderm grows into the glans penis contacting the endodermal urethral plate at the junction of the penile body and the glans, the socalled ectodermal intrusion theory of penile urethral development within the glans (Glenister, 1954). The ectodermal ingrowth was proposed to account for the stratified squamous lining of the fossa navicularis. Our studies based on cytokeratin immunostaining of serial sections of human fetal penile specimens show that the urethral plate is an extension of the endodermal urogenital sinus, extending from the bladder to just proximal to the tip of the glans penis (Kurzrock et al., 1999a). Foxa1 immunostaining further supports an endodermal origin of the human penile urethra. Foxa1 is a marker of endodermal lineage cells (Diez-Roux et al., 2011; Robboy et al., 2017a; Besnard et al., 2004), and Foxa1 immunostaining is observed within the urethral epithelium in the penile shaft as well as in the glans (Liu et al., 2018a). Thus, in humans the epithelium of the entire urethra appears to be of endodermal origin (Kurzrock et al., 1999a; Liu et al., 2018a). This appears not to be the case in mice in which the developmental mechanism of penile urethral development differs substantially from that of human (Liu Ge, 2018; Cunha et al., 2015; Seifert et al., 2008; Hynes and Fraher, 2004; Li et al., 2015; Overland et al., 2016). Accordingly, the distal portion of the mouse urethra appears to have a substantial ectodermal contribution based upon the observation that the distal portion of the mouse urethra forms via fusion of epithelium of the preputial urethral groove which has the histologic and immunohistochemical signature of epidermis, thus suggesting an ectodermal derivation (Liu Ge, 2018).
At least two mechanisms are necessary for normal human urethral development within the penile shaft: (a) canalization of the urethral plate to form an open urethral groove and (b) fusion of the urethral folds. Canalization of the urethral plate takes place between 8 to 16 weeks of gestation to form an open diamond-shaped groove along the ventral aspect of the penile shaft (Li et al., 2015). In the penile shaft the edges of the urethral groove (urethral folds) fuse to form the urethra, while in females the analogous vestibular groove remains open (Overland et al., 2016). The fusion process appears to be more complex than two smooth epithelial surfaces touching and fusing as occurs in the palate (Shen et al., 2016) and neural tube (Ogura and Sasakura, 2016). Within the glans, penile urethral development occurs via an entirely different morphogenetic mechanism than that in the penile shaft, and involves direct canalization of the urethral plate without formation of an open urethral groove (Liu et al., 2018a).
The neuro and vascular anatomy of the penis and clitoris has been extensively studied (Altemus and Hutchins, 1991; Baskin, 1999) (Glenister, 1954; Kurzrock et al., 1999a; van der Werff, 2002; van der Werff et al., 2000) and is critical for understanding the mechanism of erectile function (Lue et al., 1984). Anatomical studies of penile innervation has also allowed for strategic design of penile straightening procedures for ventral curvature associated with hypospadias and congenital penile curvature without hypospadias (Baskin et al., 2000; Baskin et al., 1998; Baskin et al., 1996). Along the penile shaft, tightly arranged nerve bundles course distally at the 11 and 1 o’clock positions along the external surface of the corporal bodies and in turn send delicate branches ventrally on the external surface of the tunica albuginea to the junction of the corporal body and the urethral spongiosum. Thus, the 12:00 o’clock position is a nervefree zone amendable to placement of dorsal plication sutures to ameliorate mild to moderate degrees of penile curvature (Baskin et al., 1998). Also, the tunica albuginea is thickest at the 12:00 position, which facilitates the anchoring of plication sutures (Baskin et al., 1998).
An understanding of penile development and anatomy has also been critical for understanding and correcting the common congenital anomaly, hypospadias. Hypospadias occurs in ~ 1:250 newborn males (Baskin, 2017a). Hypospadias can be defined as (a) an ectopic location of the urethral meatus with abnormal development of the urethral spongiosum. (b) incomplete development of the prepuce (dorsal hooded foreskin) and (c) ventral skin deficiency/penile curvature. The most common location of the ectopic urethral meatus is at the junction between the penile shaft and glans penis at the coronal sulcus (Baskin, 2017a), the site of junction between two disparate mechanisms of urethral formation (Liu Ge, 2018; Liu et al., 2018a; Li et al., 2015). This is consistent with the different mechanisms of urethral formation between the shaft of the penis (fusion) and the glans (canalization) (Liu Ge, 2018; Liu et al., 2018a; Li et al., 2015). The anatomy of the hypospadiac penis is exactly like a normal penis except for the abnormalities in the development of the urethral meatus, corpus spongiosum, overlying skin and absence of a ventral prepuce (Baskin et al., 1998). The abortive elements of the urethra and urethral spongiosum is consistent with hypospadias being an arrest in normal development and not a deformation anomaly (Baskin et al., 1998).
In the overwhelming majority of cases the etiology of hypospadias remains unknown. A reasonable hypothesis is that hypospadias is caused by genetic susceptibility and maternal exposure to endocrine disruptors (Baskin et al., 2001). Rarely, a known genetic defect is present to explain hypospadias. For example, a defect in the enzyme 5α-reductase type 2 (that converts testosterone to dihydrotestosterone) leads to severe hypospadias. 5α-reductase type 2 is expressed at the site of urethral fold fusion along the penile shaft and appears necessary for the normal urethral development (Kim et al., 2002). However, severe hypospadias is a consistently found in patients with a normal 5α-reductase gene.
Recently we have applied an integrated multi-technical approach to obtain a detailed description of human penile and clitoral development using state of the art imaging techniques including optical projection tomography, lightsheet fluorescence microscopy, and scanning electron microscopy along with gross wholemounts, histology and immunohistochemistry (IHC) (Li et al., 2015; Overland et al., 2016; Shen et al., 2018a; Shen et al., 2016; Isaacson et al., 2018b; Shen et al., 2018b). Herein, we review literature of the distant past and our most recent studies on human penile and clitoral development and in addition provide new data on development of human fetal male and female external genitalia from the indifferent stage through the fully developed penis and clitoris. Our working hypothesis is that understanding of normal genital development will lead to a better understanding of abnormal development thereby facilitating better preventive and reconstructive strategies for patients with congenital anomalies of the external genitalia.
Methods
First and second-trimester human fetal specimens were collected free of patient identifiers after elective termination of pregnancy (Committee on Human Research approval at the University of California at San Francisco, IRB# 12–08813). Gender was determined by inspection with a dissecting microscope for Wolffian (mesonephric) and Müllerian (paramesonephric) ductal morphology. For the youngest specimens at the indifferent stage (8 to 10 weeks), PCR of the SRY- chromosomal sequences was used to determine sex (Cui et al., 1994) (Li et al., 2015). Heel-toe length was used as a determinant of gestational age (Drey et al., 2005; Mercer et al., 1987; Mhaskar et al., 1989). Human fetal specimens were then examined by optical projection tomography (Li et al., 2015), lightsheet fluorescence microscopy (Isaacson et al., 2018a), and scanning electron microscopy (Shen et al., 2016) as previously described. Transverse and sagittal serial paraffin sections of human fetal specimens were prepared and stained with hematoxylin and eosin or immunostained using antibodies for cytokeratins (KRT) 6, 7, 10 and 14, androgen receptor, Foxa1, Mafb, Runx1/2/3, Notch, the proliferation marker Ki67, and the apoptotic marker caspase 3 (Li et al., 2015; Overland et al., 2016). Immunostaining was detected using horseradish-peroxidase-based Vectastain kits (Vector Laboratories, Burlingame, CA) or by immunofluorescence. Negative controls deleted the primary antibody (Robboy et al., 2017b).
Results
Anatomic studies
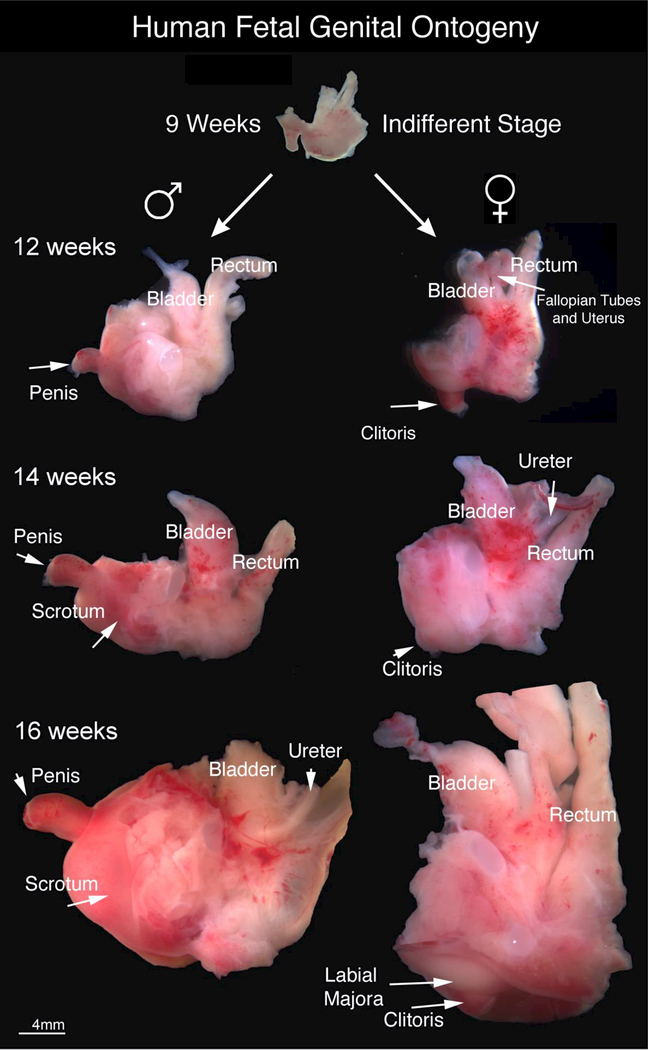
This study is based upon 80 human fetal specimens from ~6.5 weeks of gestation (heel toe length 3.5 mm) to 24 weeks of gestation (heel-toe length 57 mm) taken from previous and new investigations. Gross ontogeny of the male and female human fetal pelvis from 9 weeks of gestation (end of the indifferent stage) to 16 weeks of gestation is shown in Figure 1. Note the divergent development after 9 weeks of gestation especially in respect to the orientation of the external genitalia with the penis clearly projecting at ~90-degree angle from the body and the clitoris recessed close to the body wall. At the 12-week time point the penis and clitoris are similar in size whereas at later stages the penis is clearly larger (see also (Shen et al., 2018a)). Note the bladder and rectum appear similar in size and location in both sexes.
Figure 1.
Gross Human Fetal Pelvic Ontogeny: Gross ontogeny of the human fetal pelvis at 9 weeks of gestation (end of indifferent stage), 12 weeks, 14 weeks and 16 weeks of gestation. Note the divergent development after 9 weeks of gestation especially in respect to the orientation of the external genitalia with the penis clearly visible at ~ 90-degree angle from the body and the clitoris recessed close to the body wall.
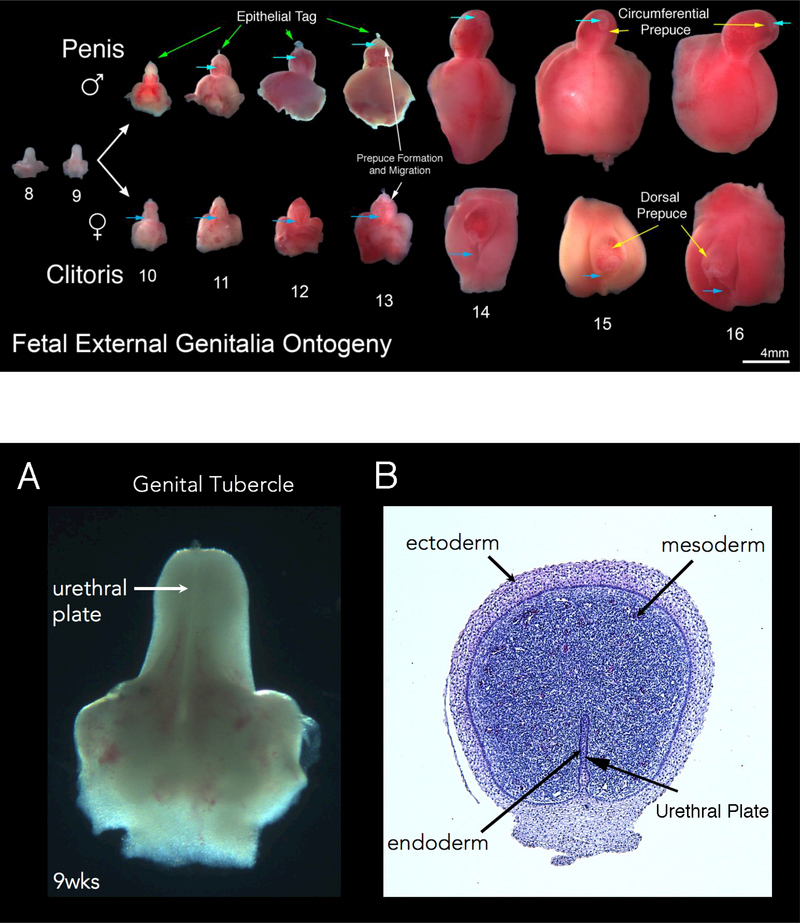
Figure 2 shows representative examples of human fetal male (top row) and female (bottom row) external genitalia from 8 to 16 weeks of gestation. Note the morphologic differences between the male and female after the indifferent stage (8–9 weeks of gestation). In males the urethra forms within the penile shaft and glans. In contrast, in females the urethra opens to the exterior at the “base” of the clitoris. Note progression of the urethral meatus in the male specimens from proximal to distal (light blue arrows) (Figure 2) from the penoscrotal junction to the distal tip of the glans. In contrast, in females the urethral meatus remains fixed in a proximal position (blue arrows). Also note that the clitoris remains close to the body wall, compared to the penis which extends outward ~90 degrees from the body wall (also seen in Figure 1). At 9 and 12 weeks of gestation sizes of the external genitalia are similar in male and female specimens (Figure 2), consistent with an androgen-independent growth/developmental mechanism. Both developing male and female external genitalia have epithelial tags that are visible (Figure 2, green arrows) near the tip of the glans from 10–13 weeks of gestation. These epithelial tags subsequently disappear after ~13 weeks of gestation. At ~13 weeks of gestation the dorsal prepuce begins to envelope the glans in both the male and female specimens. The prepuce becomes circumferential in males by 14–15 weeks of gestation, in contrast to the female where the ventral aspect of the prepuce does not fuse (Figure 2, yellow arrows). In males the prepuce is completely formed by ~16 weeks of gestation, covering the glans (Figure 2). Additional information on preputial development can be seen in Liu et al (Liu et al., 2018a).
Figure 2.
Fetal External Genitalia Ontogeny: Representative ventral views of external genitalia of the human males (top row) and females (bottom row) (8–16 weeks of gestation). Note the morphologic differences between male and female specimens after the indifferent stage (8–9 weeks of gestation) with formation of the penile urethra within the shaft due to urethral fold fusion in the penis and lack of urethral (vestibular) fold fusion in female specimens (light blue arrows depict the location of the urethra meatus in both the male and female specimens). Note the divergent evolution of the male and female prepuce (yellow arrows) with complete circumferential formation of the prepuce at 14–16 weeks of gestation in the male and a resulting dorsal prepuce in the female. The epithelial tag is seen in both male (green arrows) and female (clearly visible without arrows)) specimens from 10–13 weeks of gestation disappearing after this time point.
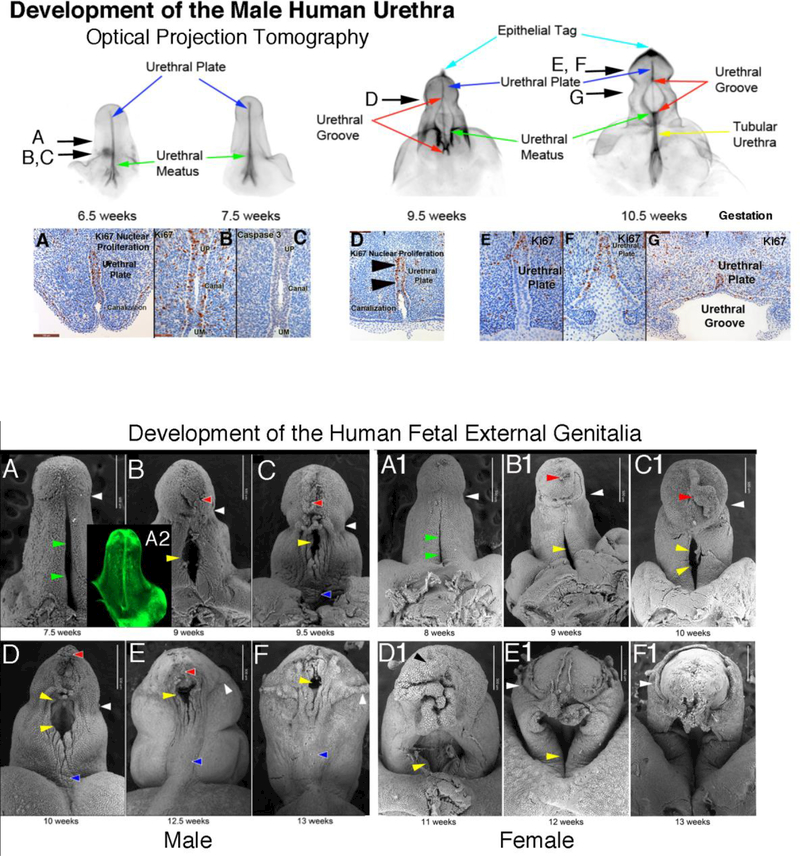
Figure 3 shows a 9-week human male genital tubercle/future penis. Note the mid-line urethral plate in the gross specimen (A). In the corresponding histologic cross section (B), the three embryonic layers of external genitalia development are labeled: ectoderm (future skin and prepuce), mesoderm (erectile tissue and stroma) and endoderm (urethral plate/future urethra). The fetal clitoris of the same age has an identical histologic appearance (not illustrated).
Figure 3:
Human male genital tubercle/ future human penis at 9 weeks of gestation. Note the urethral plate in the gross specimen (A). In the corresponding histologic cross section (B), the three embryonic layers of external genitalia development are labeled.
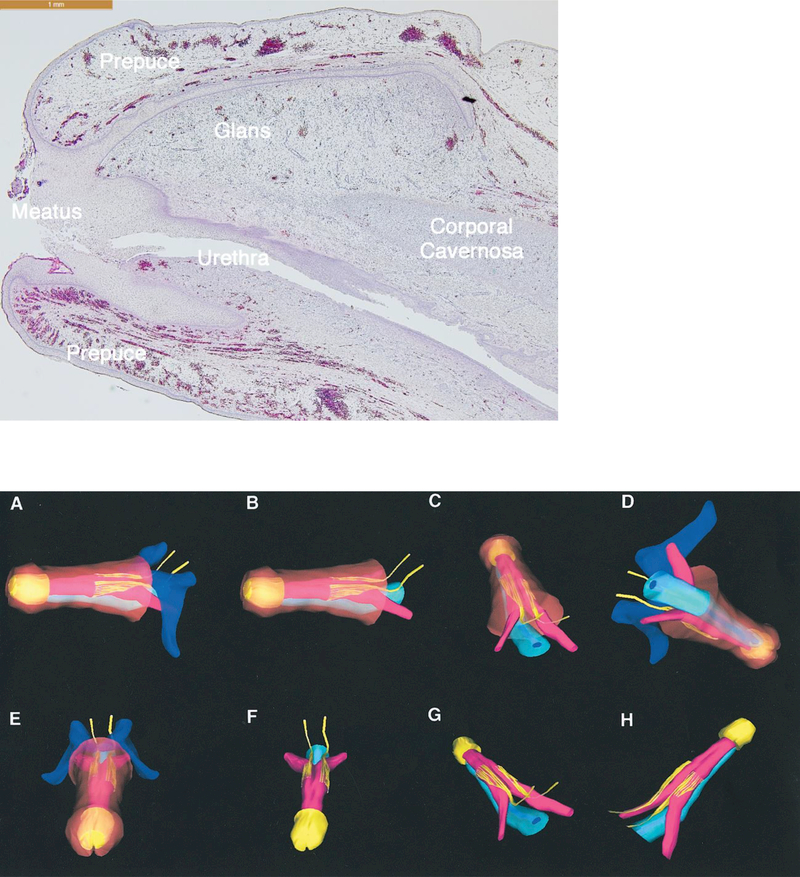
Figure 4 shows optical projection tomographic images of male urethral development from 6.5 to 10.5 weeks fetal age. Note the urethral plate (blue arrow) that ends distally within the glans, and the progression of the urethral meatus (green arrows) from the level of the scrotal folds at 6.5 weeks to the proximal penile shaft at 10.5 weeks. The wide-open urethral groove (red arrows) is best seen from 9.5 to 10.5 weeks with clear progression of proximal to distal fusion of the edges of the urethral folds to form the tubular urethra (yellow arrow). The epithelial tag, which is of unknown significance, is marked by the light blue arrow. Corresponding serial immunohistochemical sections localizing the proliferation marker Ki67 are labeled with arrows in the OPT specimens Figure 4 A-G, with the exception of C which illustrates an absence of staining for the apoptotic marker caspase 3. As noted, canalization of the urethral plate is visible in histologic sections Figure 4 A-D.
Figure 4:
Optical projection tomography of male urethral development from 6.5 to 10.5 weeks fetal age. Note the urethral plate (blue arrow) that ends within the glans. The wide-open urethral groove (red arrows) is best seen from 9.5 to 10.5 weeks with clear progression of proximal to distal fusion of the edges of the urethral groove to form the tubular urethra (yellow arrow). The epithelial tag is marked by the light blue arrow. The proliferation marker Ki67 are labeled with arrows in the OPT specimens A-G, with the exception of C which illustrates no staining for the apoptotic marker caspase 3. Canalization of the urethral plate is visible in histologic section A-D.
Reproduced from Li, Y., A. Sinclair, M. Cao, et al., Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the double zipper hypothesis. J Urol, 2015. 193(4): p. 1353–59, with permission.
Scanning electron microscopy has been particularly useful in revealing the ontogeny of the developing human fetal penis and clitoris from 7.5 weeks to 13 weeks of gestation. Figure 5 provides ventral views of the developing penis (A-F) and clitoris (A1-F1). Note the junction of the glans with the body of the penis and clitoris (white arrowheads) and the advancing prepuce beginning to cover the glans (12 weeks gestation) in Figure 5E and F. Note the progressive closure of the diamond-shaped urethral groove within the penile shaft from proximal to distal (Figure 5 B-F, yellow arrows). The distal epithelial tag is visible from 9–12.5 weeks (red arrowheads), and penile raphe is indicated with blue arrowheads. The green arrows in Figure 5 A denote the urethral plate which is not open at 7.5 to 8 weeks based on histologic analysis (see Figure 3 & 4).
Figure 5:
Scanning electron microscopy ontogeny of the developing human fetal penis (A-F) from 7.5 −13 weeks and developing human clitoris (A1-F1) 8–13 weeks of gestation. White arrowheads indicate the junction of the penile and clitoral shaft with the glans (ages 7.5–11 weeks A-D & A1-D1). At 12 weeks of gestation in both males and females note advancing of the prepuce over the glans (white arrowheads) (E, E1, F & F1). The red arrowheads denote the epithelial tag. The blue arrowheads indicate the median penile raphe. Yellow arrowheads indicate the open urethral groove in males and vestibular groove in females. The green arrows in A & A1 denote the urethral plate in males and vestibular plate in females which is not open based on histologic analysis (see figure 3) and lightsheet microscopy staining with E-cadherin (A2). Modified from Shen, J., M. Overland, A. Sinclair, et al., Complex epithelial remodeling underlie the fusion event in early fetal development of the human penile urethra. Differentiation, 2016. 92(4): p. 169–182, with permission.
Morphology of the glandular urethra is depicted in a sagittal section of an 18weeks gestation human fetal penis (Figure 6). Please refer to the accompanying paper Formation of the Glans Penis for more details (Liu et al., 2018b).
Figure 6:
Sagittal section of an 18-weeks gestation human fetal glans penis. Note that the penile epidermis meets the distal aspect of the urethral epithelium at the meatus.
The 3-D neuroanatomy of the completely formed human fetal penis is shown in Figure 7 (Akman et al., 2001). Note the respective dorsal nerves fanning out at the hilum of the penis from the 11:00 position to the junction with the urethral spongiosum. The relationship of the nerves to the corporal and crural bodies, spongiosum, penile skin, glans and symphysis pubis is well visualized. Note the proximity of the dorsal nerve of the penis to the pubic arch and urethra. As the dorsal nerve transverses under the pubic bone, its course is located near the pubic bones, slightly lateral to the urethra and medial to the crural bodies. Note the nerve bundles at the origin of the crural bodies where they attach to the inferior pubic rami.
Figure 7.
3-D Neuroanatomy of the fully formed fetal penis at 17.5 weeks of gestation. A to H, multiple views of 3-D reconstruction of human fetal penis dorsal nerve neuroanatomy with respective layers removed for selective visualization. Dark blue areas represent pubic arch and symphysis. Yellow areas represent nerves and glans.Light blue areas represent urethra. Pink areas represent corporal bodies. Brown areas represent penile skin. (Used with permission Akman, Y et al., Penile anatomy under the pubic arch: reconstructive implications. J Urol, 2001. 166: p. 229.) with permission.
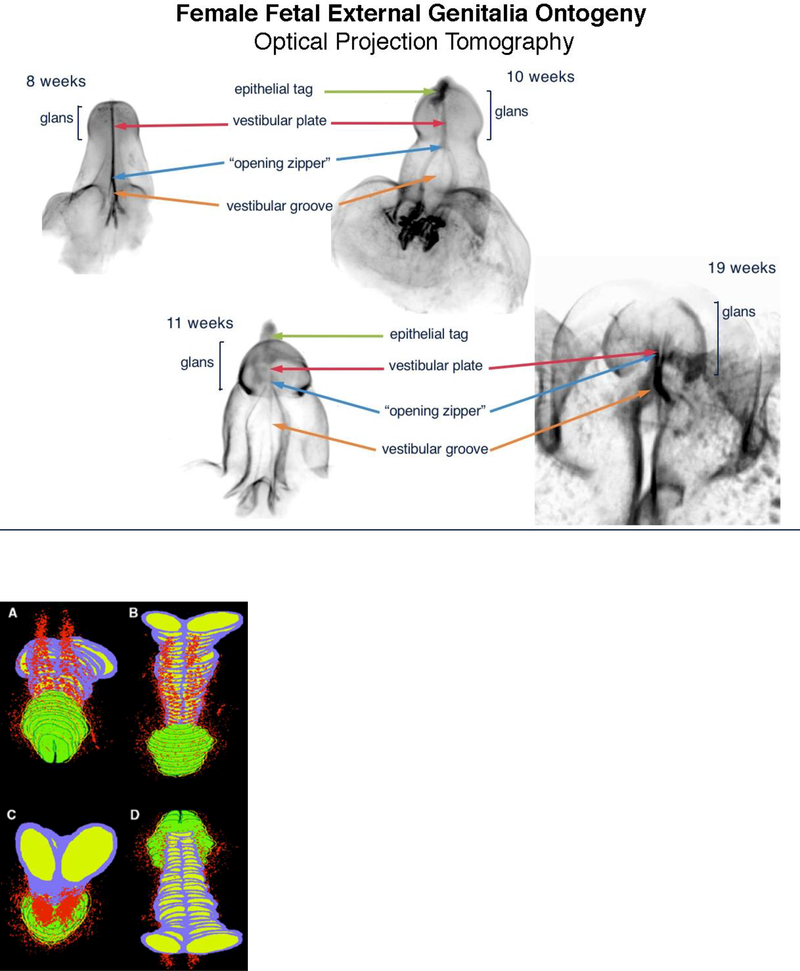
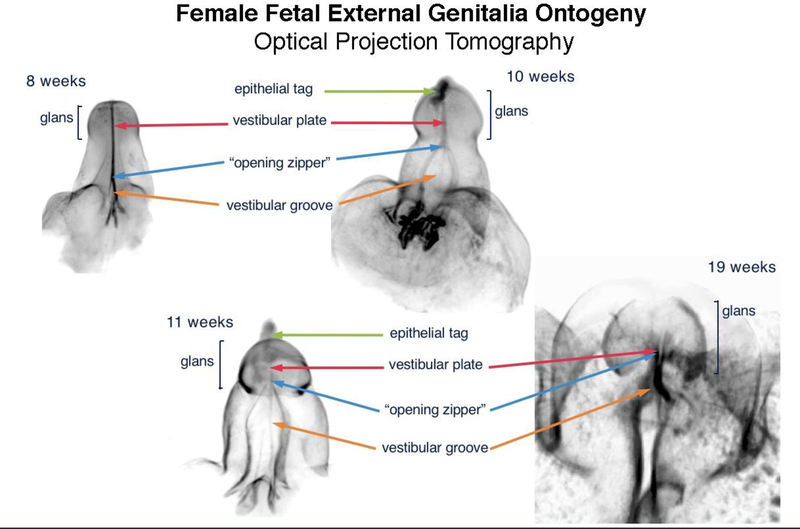
Figure 8 shows the ontogeny of human clitoris from 8 to 19 weeks of gestation. Note the opening zipper (canalization of the vestibular plate (homologue of the urethral plate) which results is a wide-open vestibular groove. In contrast to the male (Figure 4), the vestibular groove (urethral groove in males) lacks a closing zipper (fusion process) resulting in retention of a persistently open vestibular groove. Note the analogous male structures in the female: the epithelial tag, vestibular plate, opening zipper and vestibular groove.
Figure 8.
Optical Projection Tomography: Fetal ontogeny of human clitoris from 8 to 19 weeks of fetal age. Note the opening zipper, which facilitates vestibular plate opening to form vestibular groove, and lack of closing zipper (seen in the male) with the vestibular groove remaining open. Epithelial tag (green arrows), vestibular plate (red arrows) opening zipper (blue arrows) and vestibular groove (orange arrows).
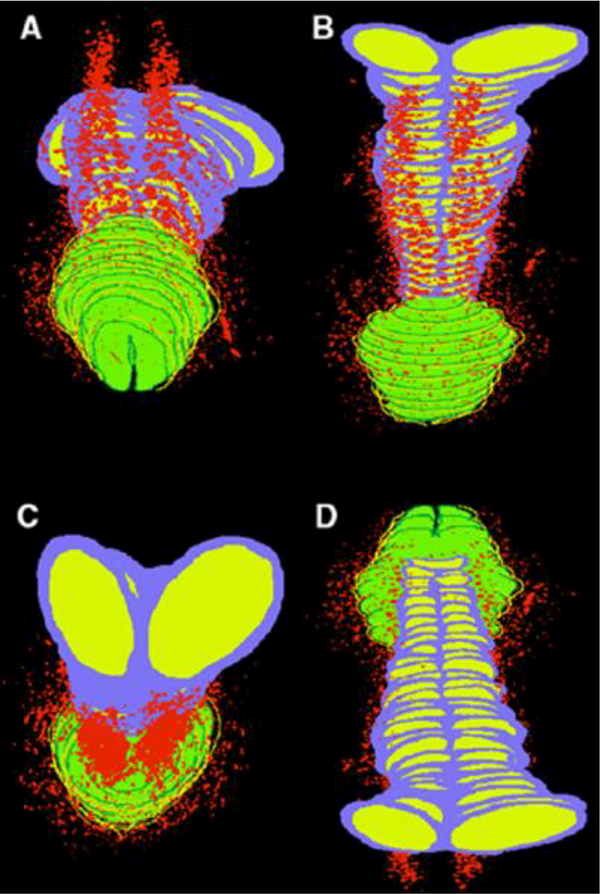
Figure 9 shows a computer generated 3-D reconstruction of a completely developed normal human fetal clitoris at 24 weeks of gestation. The dorsal clitoral nerves are seen in red splaying out ventral-laterally in analogous fashion to the male counterpart (Figure 7). Note the paucity of nerves on the ventral aspect of the clitoris (9D).
Figure 9.
Computer generated 3-D reconstruction of normal human fetal clitoris at 24 weeks of gestation. Red areas represent nerve pathway with paucity of nerves at bottom and top midline of clitori., Purple= tunica of corporal bodies, yellow= interior of corporal bodies, green= glans clitoris, and dark green and yellow= clitoral hood. A, dorsal front view. B, dorsal back view. C, back ventral view. D, ventral view. (Used with permission Baskin, L.S., et al., Anatomical studies of the human clitoris. J Urol, 1999. 162(3 Pt 2): p. 1017.) with permission.
Immunohistochemical studies
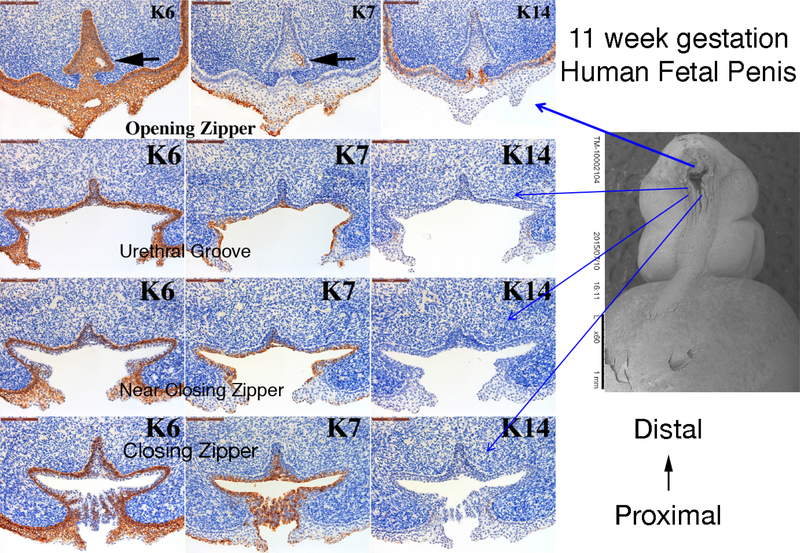
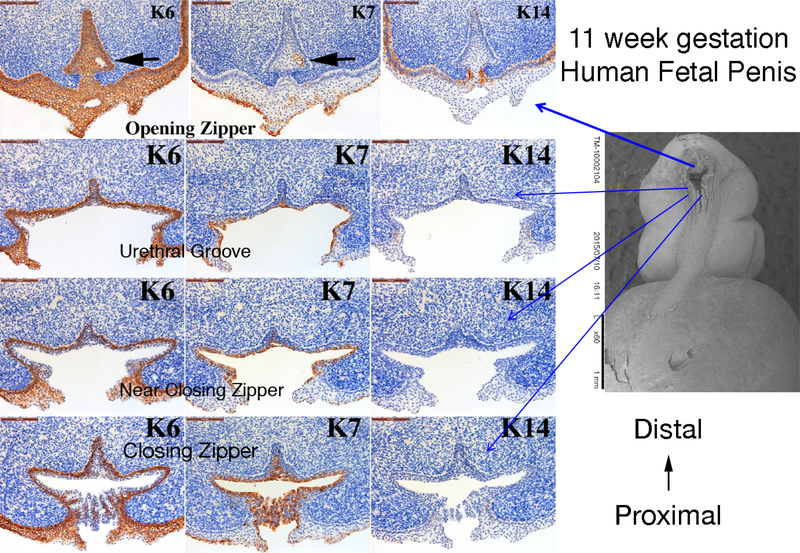
Figure 10 shows cytokeratin (KRT) IHC of an 11-week human fetal penis at four cross sectional regions of urethral development: 1) Opening Zipper – Canalization, 2) Open Urethral Groove, 3) Just distal to the Closing Zipper and 4) Closing Zipper – Fusion. The corresponding SEM image shows the approximate location of each cross section. Note the differential expression patterns of KRT6, KRT7 and KRT14 at each position of urethral development. For example, KRT6 is expressed in the basal layer of the epithelium at the closing zipper and in the urethral groove. At the opening zipper KRT6 is expressed throughout the urethral plate and ventral skin. In contrast, KRT7 is expressed in the apical layer of the opening urethral groove and urethral plate and in the area of the canalization process in the opening zipper (black arrow). KRT14 is only expressed in basal epidermal cells.
Figure 10.
Cytokeratin (K) 6, 7 and 14 expression in an 11-week human fetal penis. Representative sections from the opening zipper, urethral groove, near the closing zipper and the closing zipper. Approximate section location is depicted by the blue arrows in the scanning electron microscopic image. Note the canalization (black arrows) in the opening zipper. Note the K6 localization to basal epithelial cells and K7 apical epithelial cells, and the complex arrangement of epithelial cells fusing in the closing zipper.
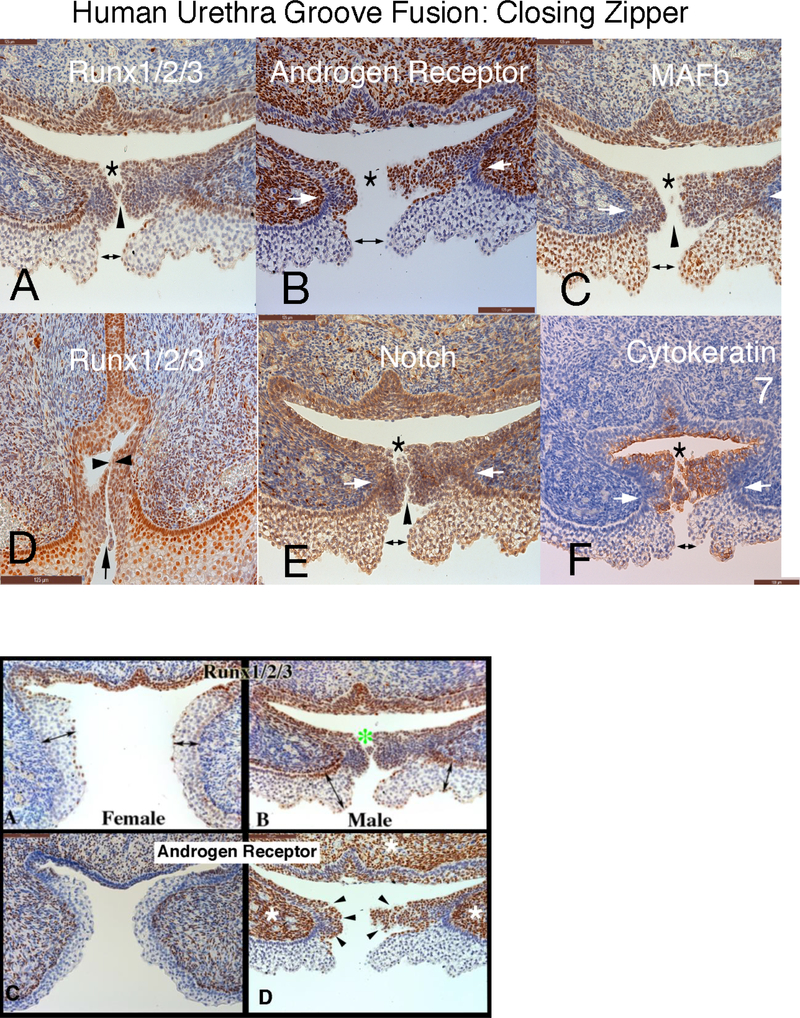
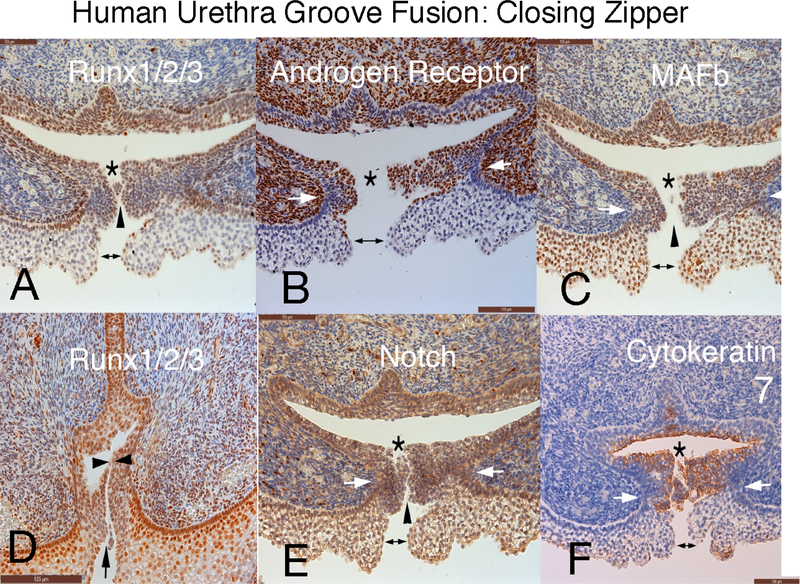
Figure 11 depicts the closing zipper (fusion of the urethral folds in an 11-week human fetal penis immunostained for RUNX 1/2/3, androgen receptor, MAFb, Notch and cytokeratin 7. Note that in all the panels formation of the tubular urethra involves three fusion events: (a) ectodermal fusion to complete the ventral penile skin (doubleheaded arrow); (b) right-left mesenchymal fusion (mesenchymal confluence) ventral to the forming penile urethra (white arrows) and (c) endodermal fusion to form the urethral tube (*). During the fusion process, multiple epithelial processes come into close apposition without initially fusing based upon the observation of clear channels visible between epithelial cells (Figure 11, black arrowheads).
Figure 11.
Human fetal penis at 11 weeks of gestation: Closing zipper - fusion of the urethral folds. Immunohistochemical localization of: A) Runx 1/2/3. B) Androgen Receptor. C) MAFb. D) Runx 1/2/3. E) Notch. and F) Cytokeratin 7. This figure illustrates that formation of the penile urethra occurs as a result of 3 separate fusion events of all three germ layers. Note that in all the panels the cells that ultimately undergo fusion to form the tubular urethra appear to fuse in three areas. 1) ectoderm (double headed arrows) 2) mesenchymal confluence (white arrows) and 3) endoderm (*). Furthermore, epithelial cells appear to have a “delayed cellular fusion”, with clear spaces visible between epithelial cells (black arrowheads).
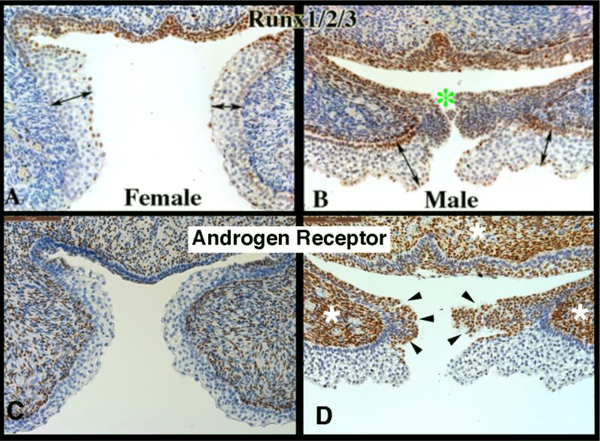
The fundamental difference between development of human male and female external genitalia is fusion of the urethral folds to form the penile urethra in males and the absence of this fusion process in females. Differences in protein expression were seen in the male and female external genitalia at the site in males where fusion processes take place versus the comparable area in females where fusion processes are not occurring (Figure 12). In the developing penis RUNX1/2/3-positive epithelial cells are seen in the (Figure 12-B) in the floor of the urethral groove and at the point of epithelial fusion (green asterisk). The corresponding epithelium during clitoral development (vestibular groove) has a paucity of RUNX1/2/3-negative (Figure 12A). Also note the differential expression of the androgen receptor at the corresponding epithelial fusion site. The male specimen has prominent androgen receptor expression in the mesenchyme (Figure 12D, white asterisk) and along the epithelial surfaces destined to fuse in the midline (black arrowheads) in contrast to the female (Figure 12C). Note the differential locations of the maturing epidermis in the developing penis versus clitoris (Figures 12A & B double-headed arrows).
Figure 12.
Runx1/2/3 and androgen receptor Immunohistochemistry of 11-week female and male human fetal clitoris and penis at the level of the closing zipper. RUNX1/2/3-positive epithelial cells are seen in males (B) in the floor of the urethral groove and at the point of epithelial fusion (green asterisk). The corresponding epithelium during female (A) external genitalia development has a paucity of RUNX1/2/3 expression. Note the different location of the maturing epidermis (A & B, double-headed arrows). Note the increased amount of androgen receptor expression in the male (D) in both the epithelium (black arrowheads) and mesenchyme compared to the female (C) (white asterisk).
The table summarizes differential morphologic events between male and female external genitalia development.
Discussion
Gross features of development of human male and female external genitalia.
Photographs of fresh human fetal pelvises and dissected male and female external genitalia are presented as a guide to future investigators interested in pursuing development of human internal and external genitalia (Isaacson et al., 2018b; Shen et al., 2018a)). To validate the tacit (but largely unproven) assumption that animal models are representative of human development, requires detailed information on human development, a topic under-represented in the literature. The gross images of penile and clitoral development emphasize two interesting points: (a) The ambisexual genital tubercle of male and female fetuses is virtually the same size at 6 to ~ 12 weeks. (b) Subsequently, a substantial size differential becomes apparent. The morphogenetic and molecular mechanisms, which account for the dramatic size differential between the human penis and clitoris, remains to be determined.
Development of the Male External Genitalia
At 8–9 weeks of gestation, the external genitalia consist of an indifferent genital tubercle. Sexual differentiation of the external genitalia is dependent upon prior differentiation of the gonads. Sex is determined by the SRY gene on the short arm of the Y chromosome (Larney et al., 2014; Ohnesorg et al., 2014). SRY triggers the testis development cascade which are largely autosomal and x-linked in some cases. The gene products of the SRY genetic cascade direct testicular development (Windley and Wilhelm, 2015). Additional genetic information required for development of male and female accessory sexual structures are located on the X chromosome and on the autosomes (Blaschko et al., 2012).
At ~8–9 weeks of gestation, Leydig cells differentiate in the testis and begin to secrete testosterone (Dufau, 1988; Blaschko et al., 2012). Testosterone or the more potent androgen, dihydrotestosterone (DHT) masculinize the genital tubercle, Wolffian duct and urogenital sinus (UGS). Metabolic conversion of testosterone to DHT is mediated by type 2 5α−reductase, expressed within the UGS and the genital tubercle, and is required for normal penile and prostatic development (Wilson et al., 1993; Wilson, 2001; Blaschko et al., 2012). Thus, initiation of androgen production by the fetal testes precedes initiation of sex differentiation of the external genitalia. Androgens signal their morphogenetic effects via AR, which are expressed in tissues of the developing male and female external genitalia during sex differentiation. Based on clinical observations, genetic females with congenital adrenal hyperplasia can form a normal appearing penis consistent with androgen receptor expression in both the male and female developing human genital tubercle (Speiser et al., 2010; Baskin, 2017b). Mouse studies have also shown expression of the androgen receptor in the developing mouse genital tubercle and the ability of the female mouse fetus to from a male phallus under the influence of exogeneous prenatal androgen exposure (Agras et al., 2006; Yucel et al., 2003) (Rodriguez et al., 2012) (Blaschko et al., 2013).
Masculinization of the external genitalia takes place under the influence of androgens. One of the first signs of masculinization is an increase in the distance between the anus and the genital structures (known as anogenital distance), followed by elongation of the genital tubercle and formation of a urethral plate. The urethral plate is derived from endoderm (confirmed by Foxa1 immunostaining (Bernardo and Keri, 2012) (Besnard et al., 2004; Diez-Roux et al., 2011; Robboy et al., 2017a)) and extends from the “pelvic urethra” into the future glans penis terminating just proximal to the tip of the future glans (Shen et al., 2018b; Liu et al., 2018a). The genital tubercle, future penis and clitoris contain tissues derived from all 3 germ layers. Ectoderm gives rise to the skin of the phallus and clitoris as well as the prepuce (Liu et al., 2018a). Mesoderm gives rise to the corporal bodies/erectile tissue and connective tissue stroma of both the penis and clitoris. Endoderm gives rise to the penile urethra (Kurzrock et al., 1999b). In males the urethral plate canalizes from proximal to distal to form a wide diamondshaped urethral groove within the penile shaft (opening zipper), a process that appears not to be mediated by apoptosis as caspase 3 was not detected in the canalizing urethral plate (Li et al., 2015). In contrast, epithelial proliferation (documented by the expression of Ki67) is abundant in the canalizing urethral plate and in the floor of the urethral groove. Such epithelial proliferation may be responsible for lateral expansion of the urethral groove.
An important point to recognize is that initially canalization of the urethral plate extends distally only to the coronal sulcus and thus the urethral groove does not extend into the glans. The coronal sulcus defining the glans from the shaft of the penis is evident at 9–10 weeks (Figure 5). The coronal sulcus is an important landmark as the mechanism of penile urethral development is vastly different in the penile shaft versus the glans (Liu et al., 2018a) (Liu et al., 2018a). As noted, within the penile shaft the urethra forms by canalization of the urethral plate to form a wide-open urethral plate whose edges fuse to form the urethra (Table). In contrast, within the glans the urethral lumen is formed by limited canalization that does not involve formation of an open urethral plate (Liu et al., 2018a). It is worth noting that these two disparate morphogenetic mechanisms of penile urethral development occur in both human and mouse, but in different proximal/distal regions of the developing penis. Direct canalization of the urethral plate without formation of an open urethral groove occurs distally in humans within the glans, whereas in mice the comparable canalization of the urethral plate occurs within the proximal portion of the penis. Fusions events are also common to mouse and human penile development. However, in mice fusion events account for the development of the distal portion of the penile urethra, whereas in humans fusion events are involved in formation of the urethra within the penile shaft (Liu et al., 2018a; Liu Ge, 2018).
Table.
Differential Morphologic Events between male and female urethral and external genitalia development.
| Male | Female | |
|---|---|---|
| Canalization | + | + |
| Epithelial Fusion | + | - |
| Mesenchymal Confluence | + | - |
| Penile Raphe | + | - |
The actual fusion of the urethral folds during human penile urethral formation within the shaft is a surprisingly complex process. As the medial edges of the urethral folds approach each other, longitudinal intertwining ridges form that initially come into close association without fusing as clear channels between the forming urethral lumen and the exterior are evident initially, but later close (Figures 5 and 11) (Shen et al., 2016). Thus, the process of urethral fold fusion is substantially different from palatal shelf fusion (Li et al., 2017) and the neural fold fusion (Lawson and England, 1998) in which smooth-edged epithelial surfaces fuse. As penile urethral development progresses, the complex epithelial edges of the urethral folds fuse in the midline to form the median penile raphe (closing zipper). However, due to the complex intertwining of epithelial processes at the edges of the urethral folds, the penile raphe frequently diverges from the midline. From a broader perspective the fusion process during formation of the human penile urethra (within the shaft) consist of three separate fusion events: (a) Epidermal fusion completes the ventral penile skin. (b) Endodermal fusion forms the penile urethra. (c) Finally, upon removal of the midline epithelial seam, rightleft mesenchymal confluence is established ventral to the forming urethra.
Immunohistochemical studies reveal interesting male-female differences in protein expression within the developing penis and clitoris. Keratins are structural proteins within epithelium. Many keratins have been described encoded by different genes, and different keratins are expressed in different types of epithelia (simple columnar, stratified, cornified versus non-cornified) which may in part be reflective of germ layer of origin (Moll et al., 1982; Moll et al., 2008). Keratin 7 appears to be particularly interesting in penile development as it is expressed in the solid endodermal urethral plate, in the floor of the urethral groove and in the fusing edges of the urethral folds, a pattern mimicked by that of RUNX1/2/3 and the androgen receptor. Thus, these proteins may play a significant role in formation of the penile urethra within the penile shaft in which epithelial fusion processes are involved. Keratin 6 is expressed in basal epithelial cells of the epidermis, the preputial lamina, the urethral folds and urethral groove. Keratin 14 is also expressed in basal epithelial cells, but only in the epidermis of the developing penis. The presence of androgen receptor in penile mesenchyme at the site of incipient mesenchymal confluence (right-left mesenchymal fusion) further supports a morphogenetic role of androgen action in development of the penile urethra. It is noteworthy that male versus female differences in protein expression were observed particularly when comparing the site of urethral fold fusion in males with the comparable region (non-fusion) in females. RUNX1/2/3, keratin 7 and the androgen receptor are strategically expressed at the site of urethral fold fusion in males, but not in females. The differential expression of epidermal maturation marked by keratin 10 (not illustrated) expression at these male and female sites is also noteworthy.
Anatomical and immunohistochemical studies support the idea that in humans epithelium of the entire urethra is of endodermal urogenital sinus origin (Kurzrock et al., 1999a) with the caveat that the urethral meatus represents an interface between ectodermal epidermis and endodermal epithelium of the urethra. As a cautionary note comments concerning the origin/derivation of urethral epithelium based upon data from the developing penis require validation with adult specimens. There are reports of germ layer derivation of penile urethral epithelium in the mouse that miss this important mark (Seifert et al., 2008). The entire human male urethra, including the glandular urethra, is formed by growth of the urethral plate into the genital tubercle and fusion of the urethral folds along the penile shaft. Additional evidence for endodermal derivation urethral epithelium is the fact that FOXA1 stains the entire developing urethra (see Figure 3 in an accompanying paper (Liu et al., 2018a)). This is in contrast to the outdated ectodermal extrusion theory of Glenister which hypothesized that the glandular urethra forms by skin (ectodermal intrusion) growing into the glans and meeting the endodermally derived urethra at the junction of the penile shaft and glans (Glenister, 1954).
The origin of the nerve supply of the penis can be traced to week 5 of gestation, when neural crest cells emerge to form the peripheral and autonomic nervous systems. The somatic innervation of the penis comes principally from spinal nerves S2 to S4 by way of the pudendal nerve (Lue et al., 1984). After passing over the ischial spine, under the sacrotuberous ligament and hence through pudendal canal, the pudendal nerve passes through the transverse perineal muscle to course onto the dorsum of the penis as the dorsal nerve of the penis (Figure 7) (Breza et al., 1989). The autonomic innervation of the penis arises from the vesical and prostatic plexi, which are composed of sympathetic nerves from L1 and L2 and parasympathetic nerves from S2 to S4. Parasympathetic innervation of the penis is involved in erection, while sympathetic innervation is involved in ejaculation (Lue et al., 1984). The cavernous nerve leaves the pelvis between the transverse perineal muscles and the pubic arch to supply each corpus cavernosum (Paick et al., 1993).
The dorsal nerve bundles traverse distally in the penis at the 11 and 1 o’clock positions and extend fine branches around the corporal body to the junction with the corpus spongiosum. The nerve free zone at the 12:00 position (Figure 7)(Baskin et al., 1998) is strategic for placing sutures for the correction of mild to moderate penile curvature associated with and without hypospadias.
Development of the Female External Genitalia
The clitoris forms in an analogous fashion to the penis from the genital tubercle except for lack of formation of the urethra within the clitoris (Overland et al., 2016) (Table). During clitoral development, fusion of the vestibular folds (closing zipper) does not take place resulting in an open vestibular groove (Overland et al., 2016) that will form the vaginal vestibule. Similar to the glans penis, the clitoris forms a cap (the glans clitoris) at the distal end of the narrowed corporal body. Large bundles of nerves course along the corporal bodies with the highest density on the dorsal aspect (Figure 9) in analogous fashion to that of the penis (Baskin et al., 1999). Nerves are not present at the 12 o’clock position, but fine nerve branches extend completely around the tunica in a fashion similar to that of the fetal penis. Like in the male, innervation of the glans occurs via multiple perforating branches that enter at the dorsal junction of the corporal body and the glans. The lowest density of nerves in the glans is on the ventral aspect in juxtaposition to the glans septum (Baskin et al., 1999).
Canalization (opening zipper) of the vestibular plate (homologue of the urethral plate) occurs in females and thus is presumably androgen-independent. Initial growth of the genital tubercle is virtually identical in males and females (Figure 1 and 2), raising the distinct possibility that initial development and growth are androgen-independent in male and female genital tubercles. Sexual differences in size and morphology of the penis and clitoris have been correctly attributed to androgen action (Wilson et al., 1995).
However, this does not exclude the possibility that certain aspects of penile development are not in fact androgen-independent as the examples above illustrate. Inferences from other species also suggest androgen-independent events in development of the external genitalia. In the spotted hyena male and female phalluses are approximately the same size in adulthood (Cunha et al., 2014), and penile and clitoral growth (length) has been shown to be androgen-independent in spotted hyenas (Glickman et al., 1992; Glickman et al., 1998). The prepuce of several species of moles is of similar size in both males and females (Sinclair et al., 2016b). In these examples various aspect of male external genitalia are only slightly larger than their female counterparts, raising the possibility that certain aspects of development/growth of male external genitalia are androgen-independent.
In summary the human genital tubercle differentiates into either a penis under the influence of androgens with a tubular urethra within the shaft that develops by canalization of the urethral plate and subsequent fusion of the urethral folds. In contrast, the clitoris undergoes vestibular plate canalization without subsequent fusion (Table). The fusion process occurs along the penile shaft and is a complex interaction between a three germ layers. The neurovascular anatomy is similar between both structures. Formation of the penile urethra within the glans occurs via direct limited canalization of the urethral plate without formation of an open urethral groove. Evidence now favors the idea that the entire urethral epithelium is derived from endoderm in humans. The dramatic size difference between the adult human penis and clitoris remains to be explained in molecular mechanistic terms.
Acknowledgments
Supported by NIH grant K12DK083021
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Agras K, Willingham E, Liu B, and Baskin LS (2006) Ontogeny of androgen receptor and disruption of its mRNA expression by exogenous estrogens during morphogenesis of the genital tubercle. J Urol 176:1883–1888. [DOI] [PubMed] [Google Scholar]
- Akman Y, Liu W, Li YW, and Baskin LS (2001) Penile anatomy under the pubic arch: reconstructive implications. J Urol 166:225–230. [PubMed] [Google Scholar]
- Altemus AR, and Hutchins GM (1991) Development of the human anterior urethra. J Urol 146:1085–1093. [DOI] [PubMed] [Google Scholar]
- Baskin L (2017a) What Is Hypospadias? Clin Pediatr (Phila) 56:409–418. [DOI] [PubMed] [Google Scholar]
- Baskin L, Duckett J, and Lue T (1996) Penile Curvature. Urology 48:347–356. [DOI] [PubMed] [Google Scholar]
- Baskin LS (1999) Fetal genital anatomy reconstructive implications. J Urol 162:527–529. [PubMed] [Google Scholar]
- Baskin LS (2017b) Restoring normal anatomy in female patients with atypical genitalia. Semin Perinatol 41:227–231. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Erol A, Li YW, and Cunha GR (1998) Anatomical studies of hypospadias. J Urol 160:1108–1115; discussion 1137. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Erol A, Li YW, and Liu WH (2000) Anatomy of the neurovascular bundle: is safe mobilization possible? J Urol 164:977–980. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Erol A, Li YW, Liu WH, Kurzrock E, and Cunha GR (1999) Anatomical studies of the human clitoris. J Urol 162:1015–1020. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Himes K, and Colborn T (2001) Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect 109:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo GM, and Keri RA (2012) FOXA1: a transcription factor with parallel functions in development and cancer. Biosci Rep 32:113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, and Whitsett JA (2004) Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 5:193–208. [DOI] [PubMed] [Google Scholar]
- Blaschko SD, Cunha GR, and Baskin LS (2012) Molecular mechanisms of external genitalia development. Differentiation 84:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko SD, Mahawong P, Ferretti M, Cunha TJ, Sinclair A, Wang H, Schlomer BJ, Risbridger G, Baskin LS, and Cunha GR (2013) Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethylstilbestrol-treated mice. Anatomical record 296:1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breza J, Aboseif SR, Orvis BR, Lue TF, and Tanagho EA (1989) Detailed anatomy of penile neurovascular structures: surgical significance. J Urol 141:437–443. [DOI] [PubMed] [Google Scholar]
- Clemente CD (ed) (1985) Gray’s Anatomy. Lea and Febiger, Philadelphia. [Google Scholar]
- Cui KH, Warnes GM, Jeffrey R, and Matthews CD (1994) Sex determination of preimplantation embryos by human testis-determining-gene amplification. Lancet 343:79–82. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Risbridger G, Wang H, Place NJ, Grumbach M, Cunha TJ, Weldele M, Conley AJ, Barcellos D, Agarwal S, Bhargava A, Drea C, Siiteri PK, Coscia EM, McPhaul MJ, Hammond GL, Baskin LS, and Glickman SE (2014) Development of the External Genitalia: Perspectives from the Spotted Hyena (Crocuta crocuta). Differentiation 87:4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Sinclair A, Risbridger G, Hutson J, and Baskin LS (2015) Current understanding of hypospadias: relevance of animal models. Nat Rev Urol 12:271–280. [DOI] [PubMed] [Google Scholar]
- Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, Lin-Marq N, Koch M, Bilio M, Cantiello I, Verde R, De Masi C, Bianchi SA, Cicchini J, Perroud E, Mehmeti S, Dagand E, Schrinner S, Nurnberger A, Schmidt K, Metz K, Zwingmann C, Brieske N, Springer C, Hernandez AM, Herzog S, Grabbe F, Sieverding C, Fischer B, Schrader K, Brockmeyer M, Dettmer S, Helbig C, Alunni V, Battaini MA, Mura C, Henrichsen CN, Garcia-Lopez R, Echevarria D, Puelles E, Garcia-Calero E, Kruse S, Uhr M, Kauck C, Feng G, Milyaev N, Ong CK, Kumar L, Lam M, Semple CA, Gyenesei A, Mundlos S, Radelof U, Lehrach H, Sarmientos P, Reymond A, Davidson DR, Dolle P, Antonarakis SE, Yaspo ML, Martinez S, Baldock RA, Eichele G, and Ballabio A (2011) A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol 9:e1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drey EA, Kang MS, McFarland W, and Darney PD (2005) Improving the accuracy of fetal foot length to confirm gestational duration. Obstet Gynecol 105:773–778. [DOI] [PubMed] [Google Scholar]
- Dufau ML (1988) Endocrine regulation and communicating functions of the Leydig cell. Annu Rev Physiol 50:483–508. [DOI] [PubMed] [Google Scholar]
- Glenister T (1954) The origin and fate of the urethral plate in man. J Anat 288:413–418. [PMC free article] [PubMed] [Google Scholar]
- Glickman S, Frank L, Pavgi S, and Licht P (1992) Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta). 1. Infancy to sexual maturity. J Reprod Fert. 95:451–462. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Coscia EM, Frank LG, Licht P, Weldele ML, and Drea CM (1998) Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 3. Effects of juvenile gonadectomy. Journal of reproduction and fertility 113:129–135. [DOI] [PubMed] [Google Scholar]
- Hynes PJ, and Fraher JP (2004) The development of the male genitourinary system: III. The formation of the spongiose and glandar urethra. British journal of plastic surgery 57:203–214. [DOI] [PubMed] [Google Scholar]
- Isaacson D, Shen J, McCreedy D, Calvert M, McDevitt T, Cunha G, and Baskin L (2018a) Lightsheet fluorescence microscopy of branching human fetal kidney. Kidney Int 93:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson D, Shen J, Overland M, Li Y, Sinclair A, Cao M, McCreedy D, Calvert M, McDevitt T, Cunha GR, and Baskin L (2018b) Three-Dimensional Imaging of the Developing Human Fetal Urogenital-Genital Tract: Indifferent Stage to Male and Female Differentiation. Differentiaiton this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Liu W, Cunha GR, Russell DW, Huang H, Shapiro E, and Baskin LS (2002) Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res 307:145–153. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, and Cunha GR (1999a) Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation 64:115–122. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, Li Y, and Cunha GR (1999b) Epithelial-mesenchymal interactions in development of the mouse fetal genital tubercle. Cells Tissues Organs 164:125–130. [DOI] [PubMed] [Google Scholar]
- Larney C, Bailey TL, and Koopman P (2014) Switching on sex: transcriptional regulation of the testis-determining gene Sry. Development 141:2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson A, and England MA (1998) Neural fold fusion in the cranial region of the chick embryo. Developmental dynamics: an official publication of the American Association of Anatomists 212:473–481. [DOI] [PubMed] [Google Scholar]
- Li C, Lan Y, and Jiang R (2017) Molecular and Cellular Mechanisms of Palate Development. J Dent Res 96:1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sinclair A, Cao M, Shen J, Choudhry S, Botta S, Cunha G, and Baskin L (2015) Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the double zipper hypothesis. J Urol 193:1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Ge XL, Joel Shen, Adriane Sinclair, Baskin Laurence S, Cunha Gerald R (2018) Contrasting mechanisms of penile urethral formation in mouse and human. Differentiaiton 101. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu G, Shen J, Isaacson D, Sinclair A, Cao M, Liaw A, Cunha GR, and Baskin LS (2018a) Human Glans and Preputial Development. Differentiaiton this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shen J, Sinclair A, Liu G, Cao M, Cunha J, and Baskin L (2018b) Human Urethral Development in the Glans Requires Canalization, Mesenchymal Confluence and Resorption of Excess Urethral Plate Epithelial Cells Differentiaiton.
- Lue TF, Zeineh SJ, Schmidt RA, and Tanagho EA (1984) Neuroanatomy of penile erection: its relevance to iatrogenic impotence. J Urol 131:273–280. [DOI] [PubMed] [Google Scholar]
- Mahawong P, Sinclair A, Li Y, Schlomer B, Rodriguez E Jr., Ferretti MM, Liu B, Baskin LS, and Cunha GR (2014) Comparative effects of neonatal diethylstilbestrol on external genitalia development in adult males of two mouse strains with differential estrogen sensitivity. Differentiation 88:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer BM, Sklar S, Shariatmadar A, Gillieson MS, and D’Alton ME (1987) Fetal foot length as a predictor of gestational age. Am J Obstet Gynecol 156:350–355. [DOI] [PubMed] [Google Scholar]
- Mhaskar R, Agarwal N, Takkar D, Buckshee K, Anandalakshmi, and Deorari A (1989) Fetal foot length--a new parameter for assessment of gestational age. Int J Gynaecol Obstet 29:35–38. [DOI] [PubMed] [Google Scholar]
- Moll R, Divo M, and Langbein L (2008) The human keratins: biology and pathology. Histochem Cell Biol 129:705–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, and Krepler R (1982) The catalog of human cytokeratin polypeptides: patterns of expression of specific cytokeratins in normal epithelia, tumors, and cultured cells. Cell 31:11–24. [DOI] [PubMed] [Google Scholar]
- Ogura Y, and Sasakura Y (2016) Switching the rate and pattern of cell division for neural tube closure. Neurogenesis (Austin) 3:e1235938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnesorg T, Vilain E, and Sinclair AH (2014) The genetics of disorders of sex development in humans. Sex Dev 8:262–272. [DOI] [PubMed] [Google Scholar]
- Overland M, Li Y, Cao M, Shen J, Yue X, Botta S, Sinclair A, Cunha G, and Baskin L (2016) Canalization of the Vestibular Plate in the Absence of Urethral Fusion Characterizes Development of the Human Clitoris: The Single Zipper Hypothesis. J Urol 195:1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paick J, Donatucci C, and Lue T (1993) Anatomy of cavernous nerves distal to prostate: microdissection study in adult male cadavers. Urology 42:145–149. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Kurita T, Baskin L, and Cunha GR (2017a) New insights insights into human female reproductive tract development. Differentiation 97:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robboy SJ, Kurita T, Baskin L, and Cunha GR (2017b) New insights into human female reproductive tract development. Differentiation 97:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E Jr., Weiss DA, Ferretti M, Wang H, Menshenia J, Risbridger G, Handelsman D, Cunha G, and Baskin L (2012) Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differentiation 84:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E Jr., Weiss DA, Yang JH, Menshenina J, Ferretti M, Cunha TJ, Barcellos D, Chan LY, Risbridger G, Cunha GR, and Baskin LS (2011) New insights on the morphology of adult mouse penis. Biol Reprod 85:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, and Cohn MJ (2008) Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol 318:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Cunha G, Sinclair A, Cao M, Isaacson D, and Baskin L (2018a) Macroscopic Whole-Mounts of the Developing Human Fetal Urogenital-Genital Tract: Indifferent Stage to Male and Female Differentiation. Differentiation in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Cunha GR, Sinclair A, Cao M, Liu G, Isaacson D, and Baskin L (2018b) Immuohistochemical Expression Analysis of the Human Fetal Lower Urogenital Tract. Differentiaiton in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Overland M, Sinclair A, Cao M, Yue X, Cunha G, and Baskin L (2016) Complex epithelial remodeling underlie the fusion event in early fetal development of the human penile urethra. Differentiation 92:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AW, Cao M, Shen J, Cooke P, Risbridger G, Baskin L, and Cunha GR (2016a) Mouse hypospadias: A critical examination and definition. Differentiation 92:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AW, Glickman S, Catania K, Shinohara A, Baskin L, and Cunha GR (2016b) Comparative morphology of the penis and clitoris in four species of moles (Talpidae). J. Exptl. Zool. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, MeyerBahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC, and Endocrine S (2010) Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 95:4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff JF (2002) Embryology of the male anterior urethra. Plastic and reconstructive surgery 110:367; author reply 367–368. [DOI] [PubMed] [Google Scholar]
- van der Werff JF, Nievelstein RA, Brands E, Luijsterburg AJ, and Vermeij-Keers C (2000) Normal development of the male anterior urethra. Teratology 61:172–183. [DOI] [PubMed] [Google Scholar]
- Weiss DA, Rodriguez E Jr., Cunha T, Menshenina J, Barcellos D, Chan LY, Risbridger G, Baskin L, and Cunha G (2012) Morphology of the external genitalia of the adult male and female mice as an endpoint of sex differentiation. Molecular and cellular endocrinology 354:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J, George F, and Griffin J (1981) The hormonal control of sexual development. Science 211:1278–1284. [DOI] [PubMed] [Google Scholar]
- Wilson JD (2001) The role of 5alpha-reduction in steroid hormone physiology. Reprod Fertil Dev 13:673–678. [DOI] [PubMed] [Google Scholar]
- Wilson JD, George FW, and Renfree MB (1995) The endocrine role in mammalian sexual differentiation. Recent Prog Horm Res 50:349–364. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Griffin JE, and Russell DW (1993) Steroid 5 alpha-reductase 2 deficiency. Endocr Rev 14:577–593. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Arnhym A, Champeau A, Ebbers M, Coakley F, and Baskin L (2011) Complete androgen insensitivity syndrome: an anatomic evaluation and sexual function questionnaire pilot study. J Pediatr Urol 7:416–421. [DOI] [PubMed] [Google Scholar]
- Windley SP, and Wilhelm D (2015) Signaling Pathways Involved in Mammalian Sex Determination and Gonad Development. Sex Dev 9:297–315. [DOI] [PubMed] [Google Scholar]
- Yucel S, Cavalcanti AG, Wang Z, and Baskin LS (2003) The impact of prenatal androgens on vaginal and urogenital sinus development in the female mouse. J Urol 170:1432–1436. [DOI] [PubMed] [Google Scholar]