Abstract
Development of the human female reproductive is reviewed from the ambisexual stage to advanced development of the uterine tube, uterine corpus, uterine cervix and vagina at 22 weeks. Historically this topic has been under-represented in the literature, and for the most part is based upon hematoxylin and eosin stained sections. Recent immunohistochemical studies for PAX2 (reactive with Müllerian epithelium) and FOXA1 (reactive with urogenital sinus epithelium and its known pelvic derivatives) shed light on an age-old debate on the derivation of vaginal epithelium supporting the idea that human vaginal epithelium derives solely from urogenital sinus epithelium. Aside for the vagina, most of the female reproductive tract is derived from the Müllerian ducts, which fuse in the midline to form the uterovaginal canal, the precursor of uterine corpus and uterine cervix an important player in vaginal development as well. Epithelial and mesenchymal differentiation markers are described during human female reproductive tract development (keratins, homeobox proteins (HOXA11 and ISL1), steroid receptors (estrogen receptor alpha and progesterone receptor), transcription factors and signaling molecules (TP63 and RUNX1), which are expressed in a temporally and spatially dynamic fashion. The utility of xenografts and epithelial-mesenchymal tissue recombination studies are reviewed.
Keywords: Human Müllerian duct, Wolffian duct, urogenital sinus, uterovaginal canal, uterus, cervix, vagina
I. Introduction
The seminal paper on development of the human female reproductive tract appeared in 1933 (Koff, 1933) and is based upon gray scale histologic images and line drawings. Since then the collective trove on human female reproductive tract development has expanded to include several histologic and immunohistochemical studies (Bulmer, 1957; Cai, 2009; Forsberg, 1996; Fritsch et al., 2013; Fritsch et al., 2012; Hunter, 1930; Koff, 1933; Konishi et al., 1984; Mutter and Robboy, 2014; O’Rahilly, 1977; 1983; O’Rahilly and Muller, 1992; Reich and Fritsch, 2014; Sinisi et al., 2003; Sulak et al., 2007; Forsberg, 1973; Kurita et al., 2005a). We have recently added four papers to this literature that: (a) provide a modern insight on the derivation of human vaginal epithelium; (b) describe the changing pattern of epithelial differentiation markers and signaling pathways from 8 to 21 weeks; (c) explain how diethylstilbestrol (DES) affects human female reproductive tract development and expression of epithelial differentiation markers (Robboy et al., 2017; Cunha et al., 2017b; Cunha et al., 2018) and (d) examined the role of mesenchymal-epithelial interactions in differentiation of human reproductive tract epithelium and the expression of uterine epithelial estrogen receptor alpha and the progesterone receptor (Cunha et al., 2018).
The topic, development of the human female reproductive tract, is important for both academic and clinical reasons. Many congenital malformations of the female reproductive tract result from perturbation of normal morphogenetic mechanisms and their underlying molecular mechanisms. Although rare, many human female reproductive tract malformations result from abnormalities of Müllerian duct (MD) development (Table 1). Many occur spontaneously, while others are elicited by endocrine-disrupting substances, principally those having estrogenic activity. The best example of estrogeninduced malformation of the human female reproductive tract involves the administration of diethylstilbestrol (DES) to pregnant women, which was prescribed from the 1940s to 1971 when the Food and Drug Administration banned its use. Such treatment resulted in a broad spectrum of malformations of the uterine tubes, uterine corpus, cervix and vagina, which included T-shaped uterotubal junctions, malformed incompetent cervix, abnormally shaped endometrial cavity, vaginal adenosis (presence of glandular epithelium in the vagina where normally stratified squamous epithelium should reside) as well as clear cell adenocarcinoma of the vagina (Jefferies et al., 1984; Rennell, 1979; Stillman, 1982; Titus-Ernstoff et al., 2010; Herbst et al., 1971; Herbst et al., 1975; Robboy et al., 1977; Robboy et al., 1984a; Hoover et al., 2011). These observations in human are buttressed with a huge animal literature that preceded/confirmed the effects of exogenous estrogens on reproductive tract development, and have provided a molecular underpinning for the teratogenic effects of natural (17β-estradiol [Forsberg, 1972], 17α-estradiol [Forsberg and Kalland, 1981], estradiol benzoate [Plapinger and Bern, 1979]) and synthetic estrogens (diethylstilbestrol [DES], dienestrol [Forsberg and Kalland, 1981], clomiphene citrate [Gorwill et al., 1982, tamoxifen [Taguchi and Nishizuka, 1985], nafoxidine [Iguchi et al., 1986], coumestrol [Burroughs et al., 1990] and bisphenol A (BPA) [Newbold et al., 2009]) on urogenital development (Herbst and Bern, 1981; Bern and Talamantes, 1981; Bern et al., 1984; McLachlan et al., 1975; McLachlan, 1981; Newbold et al., 1983; Newbold and MaLachlan, 1985; Newbold, 1995; Newbold, 2004; 2008; McLachlan et al., 2001; McLachlan and Newbold, 1996; Kurita et al., 2004; Kurita, 2011; Laronda et al., 2012; Laronda et al., 2013). Environmental “endocrine disruptors” appear to adversely affect the health of wildlife and as well as humans (Colborn, 1995; 1994).
Table 1.
Malformations based upon abnormal Müllerian duct development
| Malformation | Possible cause(s) |
|---|---|
| Complete absence of the uterus and upper vagina |
Failure of MD development or failure of WD development. |
| Separate hemiuteri | Incomplete fusion of the MDs |
| Uterus didelphys | Incomplete fusion of the MDs |
| Uterus duplex | Incomplete fusion of the MDs |
| Uterus unicornis | Unilateral aplasia of a MD |
| Bilateral or unilateral doubling of the uterine tubes or uterus |
Duplication of MDs |
| Double vagina | Failure of MD fusion |
| Double cervix | Failure of MD fusion |
| Uterus septus | Failure of regression of the septum |
| Vagina with septum | Failure of regression of the septum |
| Absence of uterine tubes, uterus, cervix and upper vagina |
Complete androgen resistance in males due to Müllerian Inhibiting Substance |
| T-shaped uterotubal junction | Prenatal exposure to DES |
| Incompetent aplastic cervix | Prenatal exposure to DES |
| Abnormal uterine lumen | Prenatal exposure to DES |
| Vaginal adenosis | Prenatal exposure to DES |
Animal studies have been most useful in understanding normal and abnormal female reproductive tract development, in some case predicting human genital tract malformations even though there are significant differences in anatomy and molecular regulation among species. For example, progesterone receptor regulation in uterine epithelium differs substantially in the mouse versus human (Janne et al., 1975; Horwitz and McGuire, 1979; Kurita et al., 2000; Kurita et al., 2005b). In the mouse, uterine epithelial progesterone receptor (PGR) is strongly expressed following ovariectomy and is profoundly down regulated upon estrogen administration, an effect mediated indirectly via stromal estrogen receptor 1 (ESR1) (paracrine mechanism) (Kurita et al., 2000). In contrast, in humans PGR is regulated directly via ESR1 in the epithelium (Janne et al., 1975; Horwitz and McGuire, 1979; Kurita et al., 2005b; Cunha et al., 2018). For these reasons we felt it important to study human female reproductive tract development and so developed a xenograft model where observation and analysis of human development were possible ethically in a simulated in-vivo environment. The goals of this paper are (a) to provide detailed information on how to acquire human fetal female reproductive tracts for study, (b) to richly illustrate human female reproductive tract morphogenesis, (c) to review the ontogeny of epithelial and stromal differentiation markers, (d) to illustrate the utility of xenograft experiments designed to directly study the morphogenetic and molecular effects on human female reproductive tract development, and (e) to validate the role of mesenchymal-epithelial interactions in human female reproductive tract development.
II. Acquisition and isolation of human female reproductive tract
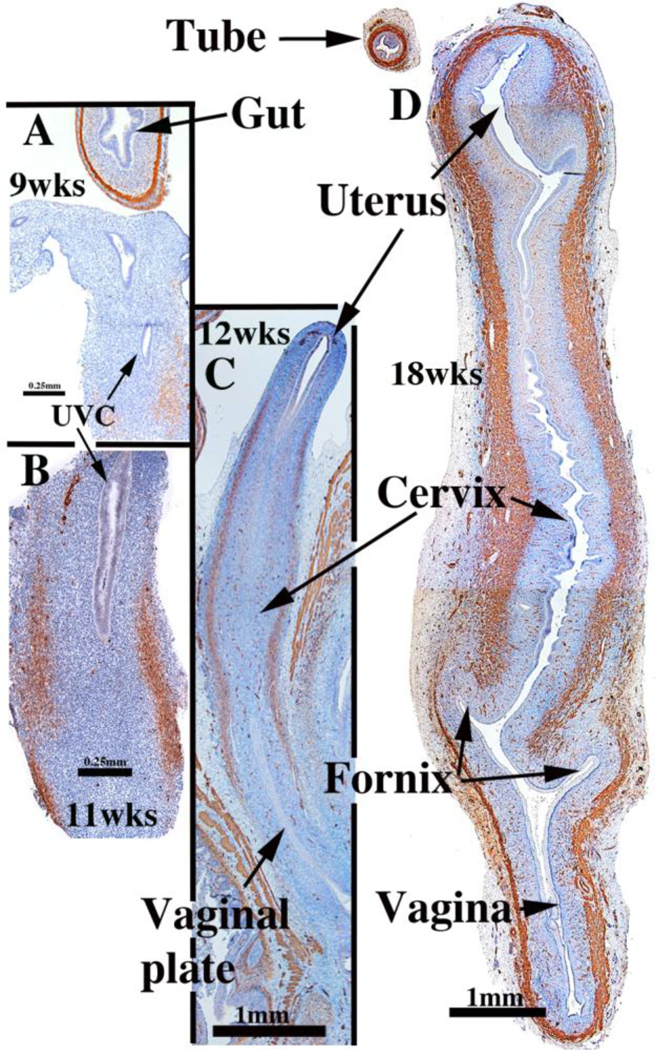
Collection of abortus specimens can only be achieved in locales where legal abortions are possible and where local laws permit investigation of human fetal organs/tissues. Institutional Committees on Human Research should be consulted to acquire authorization to carry out such research. The key proviso is collection of abortus material without patient identifiers. Given that current surgical procedures are disruptive, the initial challenge in human fetal organogenesis is finding the human female reproductive tract in the abortus specimen. Commonly, the bladder and the human female reproductive tract remain attached. Sometimes the bladder and female reproductive tract complex are attached to the proximal end of the free-floating umbilical cord. In other cases an intact pelvis is obtained from which the reproductive tract can be dissected. The key is to understand the developmental anatomy over the time frame of the youngest to the oldest specimens so that the gross morphology can be recognized in the disrupted specimen. Figure 1 is a montage of human female reproductive tracts from 9 to 22 weeks of gestation.
Figure 1.
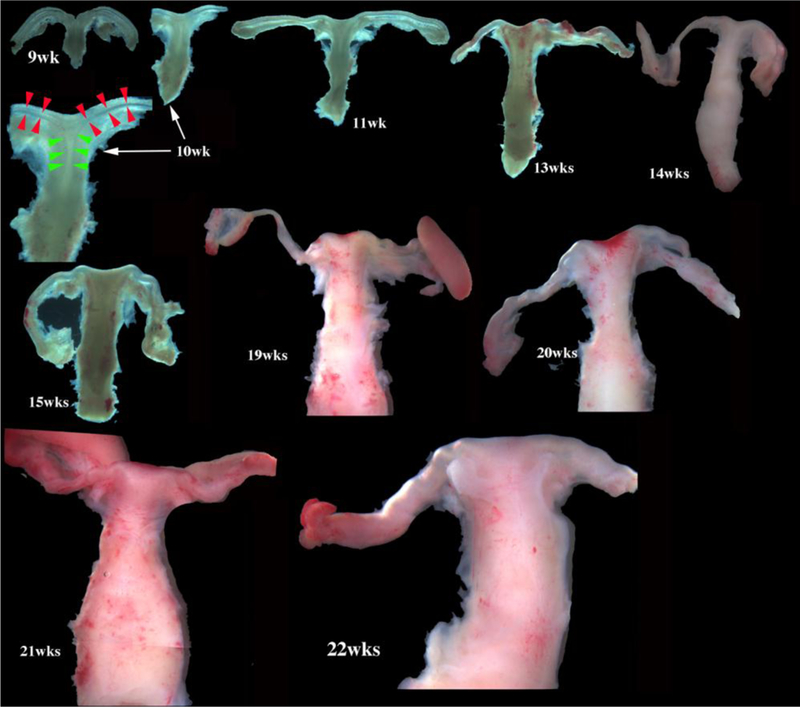
Wholemount photos of developing human fetal female internal genitalia staged by heel-toe measurements. Note (a) increase in size and morphological complexity with time, and (b) that it is impossible to distinguish the uterine corpus, cervix and vagina. Specimens photographed with transmitted light (9, 10, 11, 13 and 15 weeks) permit visualization of internal (epithelial) organization in regions not too thick. The 10-week specimen is shown at both low and high magnifications. Red arrowheads demarcate the epithelium defining the lumen of the uterine (Fallopian) tube. Green arrowheads define the epithelium lining the uterus. Relative sizes of specimens are not exact, but increase with age. From Robboy et al (2017) with permission.
Gestational age of disrupted surgical specimens was estimated by heel-toe length (Drey et al., 2005). Of import, accurate staging of abortus specimens is not precise, and specimen ages given in peer-reviewed papers are best approximations. As the Carnegie collection website states: “An embryo is assigned a Carnegie stage based on its external features. This staging system is not dependent on the chronological age or the size of the embryo. The stages are in a sense arbitrary levels of maturity based on multiple physical features. Embryos that might have different ages or sizes can be assigned the same Carnegie stage based on their external appearance because of the natural variation which occurs between individuals (Smith, 2016)”. In the not-so distant past, abortions involved vaginal delivery of intact embryos/fetuses from which crown-rump measurements were used as a measure of gestational age (Streeter, 1951). Currently, crown-rump measurements are rarely possible. Accordingly, heel-toe length is used to determine fetal maturity, which gives a rough estimate of fetal age.
III. Early Müllerian duct development
Most of the female reproductive tract develops from the MDs whose development is preceded by the Wolffian (mesonephric) duct (WD) growth within the paired urogenital ridges (Fig. 2). Following WD formation, the MDs arise as coelomic epithelial invaginations on the lateral surface of the paired urogenital ridges at 5 to 6 weeks of gestation (O’Rahilly, 1973). The coelomic invaginations later become the ostia of the uterine tubes. The paired MDs grow caudally within the urogenital ridges using the WDs as “guide wires.” Indeed, the WDs are requisite for caudal MD migration to the UGS (Gruenwald, 1941; Kobayashi et al., 2005). During the period of caudal MD migration, urogenital ridge mesenchyme separates the Müllerian and Wolffian ducts cranially (Fig. 3a). More caudally, MD and WD duct epithelia are separated only by their conjoined basement membranes without intervening mesenchyme (Fig. 3b). Even more caudally, the tip of the MD is in direct contact with WD epithelium without an intervening basement membrane (Fig. 3c). In mice Lhx1 is expressed in both the Wolffian and Müllerian ducts, and Wolffian duct-specific Lhx1-knockout elicits Wolffian duct degeneration and loss of the Müllerian ducts (Huang et al., 2014). Müllerian duct-specific knockout of Lhx1 blocks resulting in loss of the entire endometrium (luminal and glandular epithelium and stroma) (Huang et al., 2014). The track of the WDs and MDs in route to the UGS exhibits two gentle curves within the paired urogenital ridges that define vertical, horizontal, and vertical portions as seen in frontal view (Fig. 4). The point where the MDs initially contact the urogenital sinus, called the Müllerian tubercle, a controversial and poorly described entity. The urogenital sinus epithelium at the point of contact with the MDs subsequently proliferates to form the so-called sinovaginal or sinus bulbs (Fig. 5). The point of contact of the Müllerian ducts with the urogenital sinus is a critical step in female reproductive tract development. Failure of merging of the Müllerian ducts with the urogenital sinus can lead to lower vaginal agenesis. Lhfpl2 mutant mice exhibit such a phenotype (Zhao et al., 2016). During the 7th to 8th weeks, the caudal portions of the paired MDs lie between the two Wolffian ducts near the UGS. During the 8thweek, the bilateral Müllerian ducts fuse together, and temporarily a midline epithelial septum separates the lumina of the two adjacent Müllerian ducts (Fig. 5). This midline septum largely disappears in the 9 week resulting in formation of the midline uterovaginal canal (Fig. 5 & 6), lined throughout with an undifferentiated simple columnar Müllerian epithelium (Hunter, 1930; Koff, 1933; Robboy et al., 2017).
Figure 2.
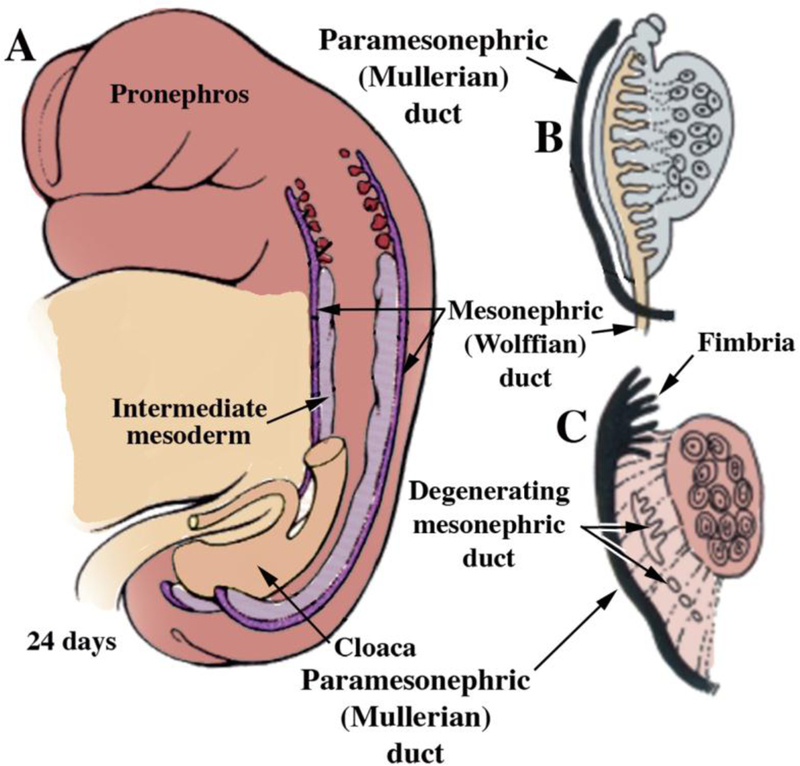
(A) Formation of the Wolffian (mesonephric) duct, which by 24 days has grown caudally to join the cloaca. At 5 to 6 weeks the paramesonephric (Müllerian) ducts appear as invaginations of the coelomic epithelium. At 7 weeks (B) the MDs have grown caudally towards the urogenital sinus. Subsequently (C, 8 weeks), the opening of the MDs into the coelomic cavity is fimbriated, and with further growth the MDs reach the UGS, while the Wolffian ducts degenerate. Modified from (Park, 2016) with permission.
Figure 3.
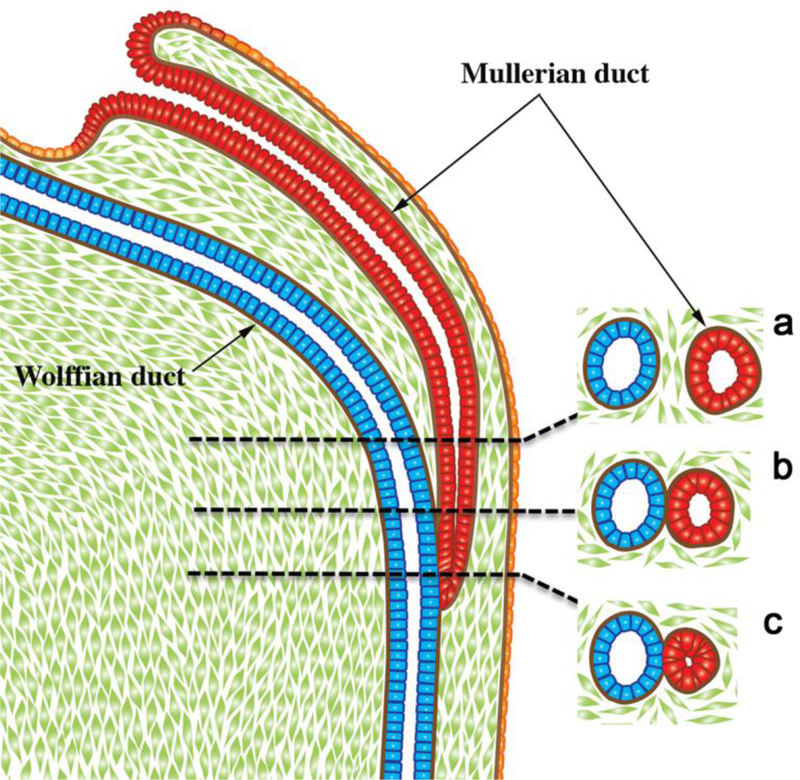
Diagrammatic representation of the caudal growth of the Müllerian duct using the Wolffian duct as a “guide wire”. Note in (a) mesenchyme intervening between the Müllerian and Wolffian ducts, (b) contact of the basement membranes of Müllerian and Wolffian ducts, (c) direct contact of the epithelia of the Müllerian and Wolffian ducts. From Robboy et al (2017) with permission.
Figure 4.
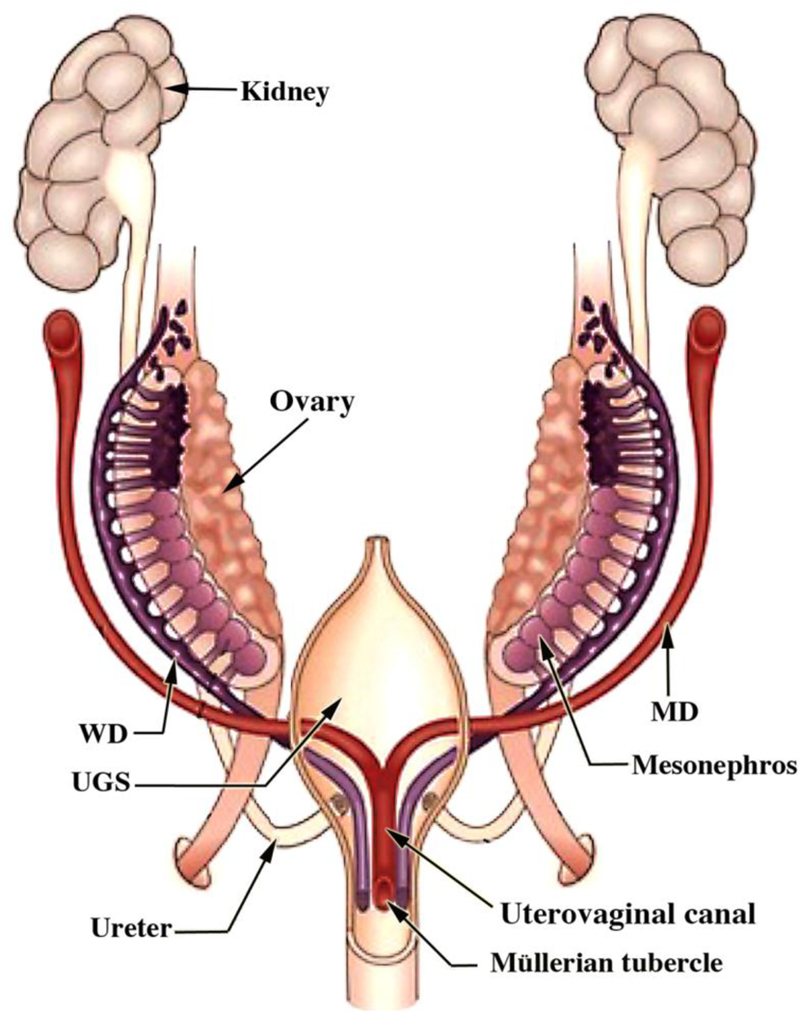
Diagram of developing human female internal genitalia in the indifferent, bisexual stage (~54 days of gestation, Carnegie Stage 22). The Müllerian derivatives are red and Wolffian derivatives are purple. Note the changing anatomical relationships between the Müllerian and Wolffian ducts. From Robboy et al (2017) with permission.
Figure 5.
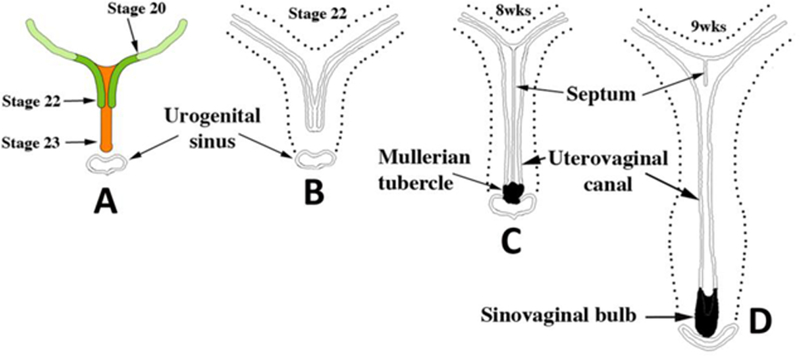
Early Müllerian duct growth and fusion to form the midline uterovaginal canal. Length of the uterovaginal canal increases with developmental age. In (A) the extent of MD caudal extension is depicted at Carnegie Stages 20 to 23 (50 to 56 days). (B–D) depict fusion of the right and left MDs to form the midline uterovaginal canal, formation of the septum and its subsequent disappearance. From Robboy et al (2017) with permission.
Figure 6.
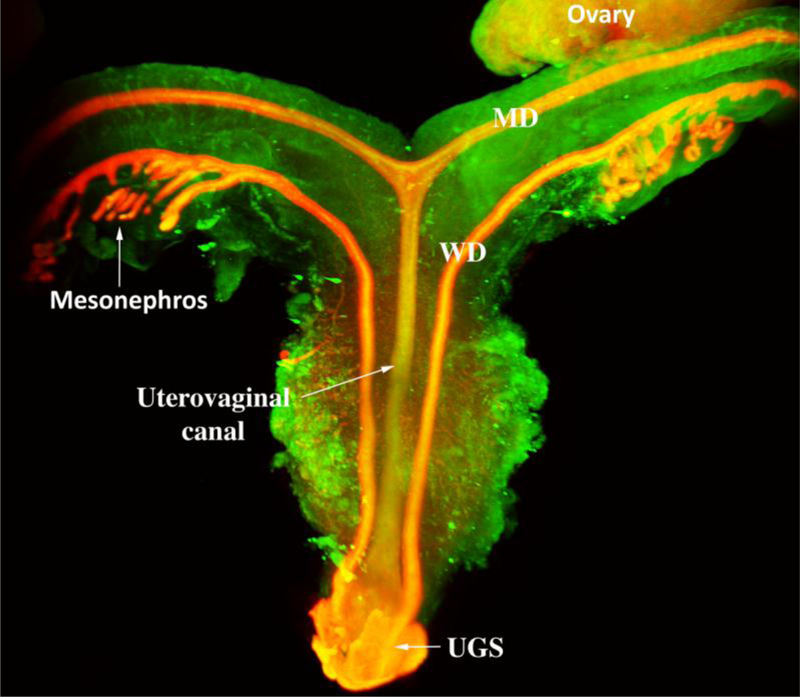
Light sheet microscopy™of a human female reproductive tract at 9.5 weeks stained with an antibody to E-cadherin. The mesonephros and Wolffian ducts (WD) are present. Cranially, the unfused MDs are destined to form the uterine tubes. Midline fusion of the MDs has created the uterovaginal canal that terminates caudally by joining the urogenital sinus (UGS).
The degree of midline MD fusion differs in humans versus mice. In humans, midline fusion is extensive, resulting in the midline uterovaginal canal and paired uterine tubes (Fallopian tubes) (Figs. 6 & 7). In mice most of the MDs remain (unfused), forming the paired oviducts and the large bilateral uterine horns. Only the caudal portions of the mouse MDs fuse to form the cervical canal and the so-called “Müllerian vagina” (Kurita, 2010). The degree of MD fusion (or non-fusion) as well as disappearance or retention of the midline septum within the uterovaginal canal is the substrate for multiple human congenital malformations (Table 1). Regression of the midline septum within the human uterovaginal canal is not understood, but perhaps bears some similarity to Müllerian duct regression in males, which is triggered by anti-Müllerian hormone (AMH). β-Catenin mediates AMH signaling for duct regression during male sexual differentiation (Kobayashi et al., 2011), and perhaps β-catenin mediates regression of the midline septum within the human uterovaginal canal.
Figure 7.
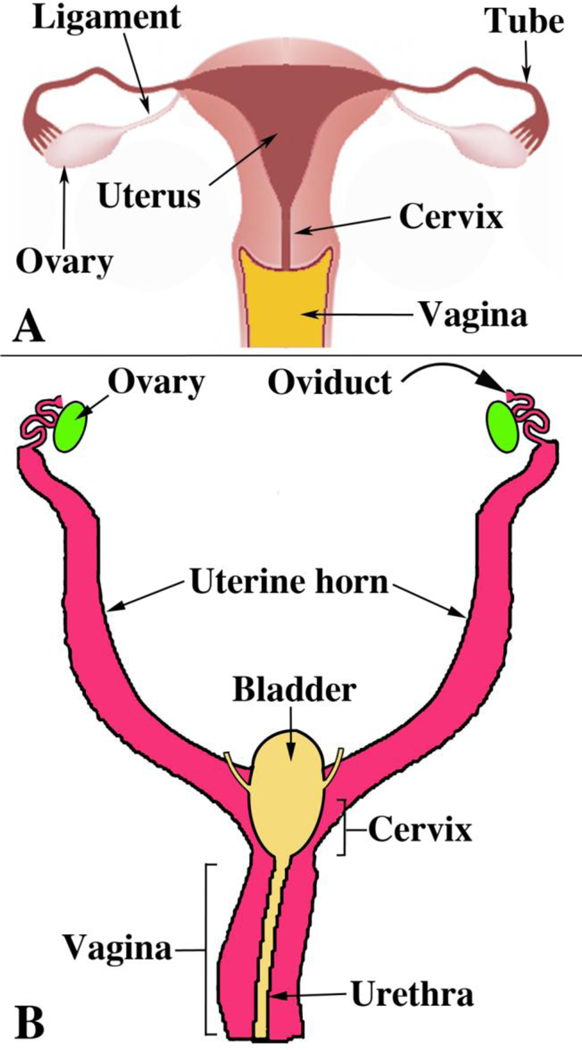
Diagrams of human (A) and mouse (B) female urogenital tracts emphasizing the marked difference in the degree of MD fusion. From Robboy et al (2017) with permission.
Development of the Müllerian ducts in mice and humans appears to be dependent upon the transcription factor, Lhx1 (Huang et al., 2014; Zhang et al., 2017)Mullerian duct-specific knockout of Lhx1 blocks results in loss of the entire endometrium and loss of the inner circular but not the outer longitudinal uterine muscle layer (Huang et al., 2014). Congenital absence of the uterus and vagina in humans is associated with a novel missense mutation in LHX1 (Zhang et al., 2017).
IV. Organogenesis of the human female reproductive tract
A. Uterine Tube
The uterine tubes, eponymically called the Fallopian tubes, consist of 4 parts: (a) the funnel-shaped infundibulum with its finger-like projections (fimbria) (Fig. 2C), (b) the ampulla where fertilization typically occurs, (c) the isthmus, immediately lateral to the uterus, and (d) the intramural (or interstitial) portion within the uterine wall terminating in the uterotubal junction (Pauerstein et al., 1974). The uterine tubes develop from the paired cranial portions of the MDs retained after the caudal segments fuse to form the midline uterovaginal canal (Figs. 6–7) (Robboy et al., 2017). The fimbriae of the uterine tube develop from the irregular ostia of the MDs into the abdominal cavity (Fig. 2C). The epithelium lining the fetal uterine tube is a single layer of columnar cells. As development proceeds two changes occur: (a) Mucosal folds (plicae) form, most prominently in the infundibulum and ampulla, and by 14 weeks a distinct gradient of mucosal plication is evident (Robboy et al., 2017). (b) The mesenchyme surrounding the epithelial tube differentiates into an inner stromal layer in contact with the epithelium and outer circularly oriented smooth muscle layer. In adulthood tubal epithelium contains ciliated cells, secretory cells, and intercalary or “peg” cells (with long slender dark nuclei compressed between adjoining cells).
Epithelial cells of the human fetal uterine tube express multiple differentiation markers (Table 2). Keratins 7, 8 and 19 are expressed from the earliest stages examined (9 weeks) and were maintained thereafter. Androgen receptor and ESR1 were first detected in tubal epithelium at 14 weeks. The progesterone receptor remained undetected in tubal epithelium from 8 to 21 weeks, but was induced by DES in tubal epithelium in xenografts (Cunha et al., 2017a).
Table 2.
Epithelial differentiation markers in human fetal uterine tube
| KRT6 | KRT7 | KRT8 | KRT10 | KRT14 | KRT19 | TP63 | RUNX1 | AR | ESR1 | PR |
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | + | − | − | + | − | − | + | + | −* |
Epithelial PR is induced in xenografts by DES, but is otherwise absent from 8 to 21 weeks. From (Cunha et al., 2017b).
B. Uterus corpus
The uterine corpus develops from the cranial portion of the midline uterovaginal canal (Koff, 1933; Robboy et al., 2017). The molecular mechanism by which the MDs fuse is poorly understood. However, based upon the human malformation, bicornuate uterus (Campbell, 1952; Davies and Walpole, 1949), it appears that MD fusion begins caudally and progresses cranially. The overall shape of the adult uterotubal junction and the lumen of the uterine corpus are unique. Wholemount specimens photographed with transmitted light reveal epithelial contours within the developing uterovaginal canal (Fig. 8). The basic delta shape of the cranial portion of the uterovaginal canal (uterine corpus anlage) is recognizable by weeks 9–10 (Fig. 6), which subsequently expands and broadens (Robboy et al., 2017) (Fig. 8). Accordingly, transverse sections exhibit marked lateral expansion of the uterovaginal canal cranially and less so caudally (Fig. 8). The overall morphogenetic process surely involves growth in overall size and gradual shape change of the uterine lumen, which will continue to evolve until the final adult morphology is achieved. The uterine corpus remains grossly undeveloped at parturition, undergoing considerable growth postnatally (Cooke et al., 2013). Functional and anatomical maturity is achieved at menarche.
Figure 8.
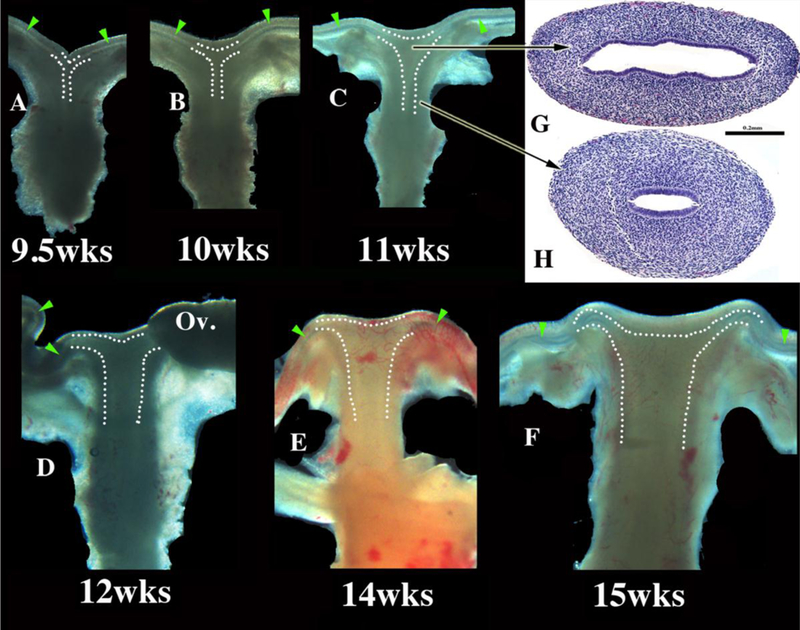
Wholemount images of human fetal female reproductive tracts (A–F) photographed with transmitted light, and H&E stained transverse sections of the uterovaginal canal (G–H). Dotted lines indicate the contours of the uterotubal junction and the uterine cavity. Green arrowheads indicate the epithelium of the uterine tubes. Note the changing shape of the uterine lumen. The cranial portion is laterally expanded (G) and narrow caudally (H). From Robboy et al (2017) with permission.
At all stages examined up to 21 weeks the boundary between the uterine corpus and cervix cannot be discerned with certainty in gross specimens (Figs. 1 & 8) or histologically. Glands form within the developing uterine corpus and uterine cervix beginning about 14 to 15 weeks (Robboy et al., 2017). The rudimentary glands are shallow outpouchings lined by a simple columnar epithelium. Glands are initially found a considerable distance caudal to the uterine fundus, suggesting a regionality in gland formation. Indeed, the first glands to form are probably cervical in nature. By 21 weeks uterine and cervical glands are elongated and branched within the stroma (Robboy et al., 2017).
Epithelial cells of the human fetal uterine corpus express various differentiation markers (Table 3). Like the presumptive uterine (Fallopian) tubes, the epithelium of the cranial aspect of the uterovaginal canal (uterine precursor) expresses keratins 7, 8 19 and Runx1 from the earliest stages examined (9 weeks), and these markers are maintained thereafter. ESR1 was first detected in the uterine corpus at 16 weeks in a small subset of epithelial cells, while the surrounding stromal cells are ESR1-positive. At 21 weeks, the epithelium of the uterine corpus contains a mixture of ESR1-reactive and ESR1unreactive epithelial cells with the former predominant (Cunha et al., 2017b). The progesterone receptor was undetectable in epithelium of the uterine corpus throughout the 9- to 21-week period of study.
Table 3.
Epithelial differentiation markers in human fetal uterine corpus
Epithelial ESR1 is undetectable at 14 weeks, sparsely expressed at 16 weeks and predominant at 21 weeks. Xenografts of 13-week uterine corpus (before expression of ESR1) when treated with DES exhibit prominent uterine epithelial ESR1 immunoreactivity.
Epithelial PGR is induced in xenografts by DES. From (Cunha et al., 2017b).
Investing and also deep to the glands of the presumptive endometrium is a stromal layer (endometrial stroma) composed of loose mesenchymal cells, best identified by their lack of smooth muscle α-actin reactivity (ACTA2). CD10, a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma, was not used in this study.
Myometrial smooth muscle, whose development has been described recently (Robboy et al., 2017; Fritsch et al., 2013), is the most abundant tissue in the uterus. Faint ACTA2 immunoreactivity, indicative of myometrial differentiation, was initially observed in the outer mesenchymal wall of the uterovaginal canal at 8 to 9 weeks of gestation (Fig. 9A). By the 11 to 12 weeks ACTA2 was detected most prominently in the middle 2/4ths of the uterovaginal canal corresponding to the region of the presumptive cervix and possibly upper vagina (Fig. 9B–C). At this time the mesenchymal wall of the uterine fundus and inferior vagina showed negligible ACTA2 immunostaining. By the 18th week, all organs of the female reproductive tract displayed strong ACTA2 reactivity within their fibromuscular walls (Fig. 9D), especially in the cervix, which is relatively advanced in development compared to the other female reproductive tract organs, thus confirming a previous study (Fritsch et al., 2013).
Figure 9.
α-Actin immunoreactivity in human female reproductive tracts at (A) 9, (B) 11, (C) 12, and (D) 18 weeks. Faint α-actin immunoreactivity first appears focally in mesenchyme of the 9-week uterovaginal canal, increasing in intensity by the 11 weeksBy 12 weeks α-actin immunoreactivity is strong in the middle 2/4ths of the developing reproductive tract, and by 18 weeks, is strongly expressed throughout the developing female reproductive tract. From Robboy et al (2017) with permission.
C. Uterine cervix
The cervix develops from the middle 2/4ths of the uterovaginal canal. Initially, the boundaries between the cervix and the uterine corpus as well as the between the cervix and vagina are indistinct. Koff asserts that the cervix can be “recognized as a spindleshaped thickening or condensation of the mesenchyme of the genital cord” in 8 to 10week fetuses (Koff, 1933), a subtlety which we find impossible to discern (Figs. 1, 8, 10). Once vaginal fornices become recognizable at roughly 18 weeks (Fig. 10F), the boundary between the exocervix and the vagina becomes obvious. For this reason, in “early stages” of cervical organogenesis, the cervical domain can only be inferred based upon cranialcaudal position within the uterovaginal canal, subtle changes of the endocervical contour in cross-section, and subtle histodifferentiation of the epithelium.
Figure 10.
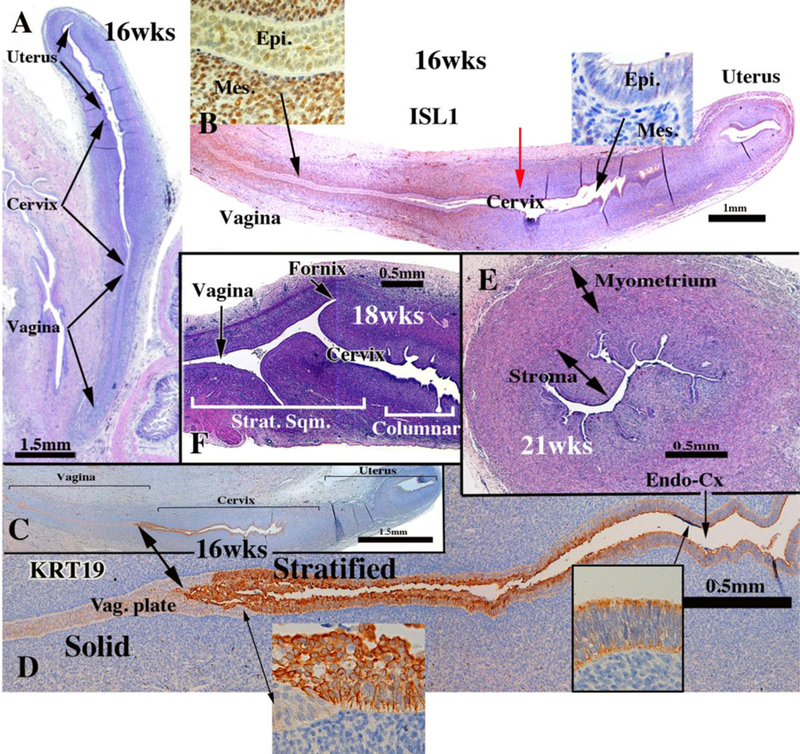
Cervical development. (A–D) are sagittal sections of the female reproductive tract of a 16 week fetus (A=H&E, B=ISL1 immunostain, C-D=keratin 19 immunostain). Boundaries between the vagina, cervix and uterus are nebulous up to 18 weeks, when vaginal fornices become apparent (F). (B) ISL1 immunostaining is strong in vaginal stroma, absent in uterine stroma, with a sharp fall off in staining intensity at the mid-point of the uterovaginal canal (red arrow in B). Keratin 19 immunostaining (C–D) may also be indicative of vaginal-exocervical boundary. Cervical glands are prominent at 21 weeks of gestation (E). Epi. = Epithelium, Mes. = mesenchyme, Strat. Sqm. = Stratified squamous, Vag. Plate = vaginal plate, Endo-Cx = endocervix. From Robboy et al (2017) with permission.
Initially, simple columnar Müllerian epithelium, indistinguishable from area to area, lines the uterovaginal canal throughout (Figs. 8 G & H). Epithelium of the fetal cervix at 21 weeks, the oldest we examined, was of two types. Caudally, the epithelium was stratified squamous near the external os and on the exocervix. Cranially cervical epithelium is simple columnar with associated glands (Fig. 10E & F). At this time, there is no evidence of mucin expression. During development, the “columnar epithelium lining the caudal portion of the uterovaginal canal becomes stratified” (Koff, 1933) (Figs. 10D). At about 11 weeks “the conversion of columnar to stratified epithelium (within the uterovaginal canal) continues cranially until the transition between the two types becomes abrupt, a point which marks the junction of cervical and vaginal epithelium in the cervical canal” (Koff, 1933). We disagree slightly with Koff’s interpretatiom. In our opinion the zone of epithelial stratification within the uterovaginal canal respresents differentiating vaginal epithelium as wells as stratified squamous cervical epithelium (Figs. 10C–F).
The transcription factor, ISL1, may define the boundaries between the endo- and exocervix, uterine corpus and vagina because it is is enriched in cervicovaginal mesenchyme, but not uterine mesenchyme (Robboy et al., 2017). ISL1 immunostaining is prominent in vaginal and exocervical mesenchyme (Fig. 10B), but absent in the wall of the endocervix and uterine corpus. The steep reduction in ISL1 staining (Red arrow in Figure 10B) may denote the future endocervical-exocervical boundary. Immunohistochemical observations show that the domains of ISL1 in cervicovaginal mesenchyme and HOXA11 in uterine mesenchyme have minimal overlap (Cunha et al., 2017b).
HOX genes specify the developmental fate of individual regions of the female reproductive tract. HOXA9 specifies the uterine tube, HOXA10 and HOXA11 specify the uterus and HOXA13 specifies vaginal development (Du and Taylor, 2015). HOXA11 expression is enhanced in human uterine mesenchyme (Cunha et al., 2017b), which in mice is the inducer of uterine epithelial differentiation (Cunha, 1976). Whether inductivity of human uterine mesenchyme is dependent on expression of HOXA11 remains to be determined, likewise for inductivity of human vaginal mesenchyme in relation to expression of ISL1.
The simple columnar epithelium of the uterovaginal canal remains as such during development of the uterine tube, uterine corpus and endocervix as discussed above, and glands of simple columnar epithelium are well developed within the cervix at 21 weeks of gestation (Fig. 10E). More caudally, the simple columnar epithelium of the uterovaginal canal differentiates into a stratified squamous epithelium in the vagina and exocervix, a process preceded by conversion of the uterovaginal canal into the solid vaginal plate. To follow this process of epithelial differentiation in the vagina and exocervix, we employed immunohistochemistry using a panel of relevant keratin antibodies known to be reactivity to either simple columnar, stratified squamous (or both) epithelia (Moll et al., 1982; Moll et al., 2008).
KRT19 expression is particularly dynamic, changing dramatically in certain areas. KRT19 immunostaining was seen at 9 weeks in simple columnar epithelium of the Müllerian duct and uterovaginal canal and in the solid vaginal plate at 12 weeks of gestation (Cunha et al., 2017b). At this stage, the KRT19 and the PAX2 immunostaining (indicative of Müllerian epithelium) are congruent (Cunha et al., 2017b; Robboy et al., 2017). At 14 weeks and 16 weeks, the solid vaginal plate was KRT19-negative (Fig. 10C–D and insets) (Cunha et al., 2017b), and a sharp boundary was revealed with KRT19-reactive stratified epithelium associated with the lumen, as well as KRT19positive simple columnar epithelium that extends cranially to the epithelium of the uterine corpus (Fig. 10C–D and insets) (Cunha et al., 2017b). There are several possible interpretations. The absence of KRT19 staining in the solid vaginal plate versus the intense KRT19 staining in the stratified epithelium lining the lumen immediately cranial to the vaginal plate may reflect the UGE/MDE boundary. The KRT19-negative solid vaginal plate is FOXa1-positive indicative of endodermal urogenital sinus epithelium, whereas the KRT19-positive epithelium extending uninterrupted to the uterine corpus is PAX2-positive indicative of Müllerian epithelium (Cunha et al., 2017b). This zone of positive/negative KRT19 reactivity is likely to be one of constant flux until the final distribution of Müllerian and urogenital sinus epithelium is achieved.
The epithelium lining the uterine cervix eventually differentiates into stratified squamous cells in the exocervix, but remains simple columnar with associated glands in the endocervix (Fig. 10E & F). Accordingly, epithelial differentiation in the uterine cervix exhibits patterns of differentiation markers shared by both the uterine corpus and vagina depending on cranial-caudal position (Table 4). Keratins 7, 8 and 19 were detected in the cervical region of the uterovaginal canal from as early as 9 weeks, which is consistent with the presence of simple columnar epithelium. Expression of these keratins was maintained in the endocervical epithelium to 21 weeks. Keratin 14 is a protein normally expressed in basal cells of stratified epithelia, including in mature vaginal epithelium (Moll et al., 1982; Moll et al., 2008). Mature exocervical and vaginal epithelium seen in our 21 weeks specimen, exhibited KRT14 reactivity in basal cells as expected (Cunha et al., 2017b). In the developing human female reproductive tract KRT14 expression is clearly dynamic, temporally regulated and not strictly related to epithelial stratification. This is illustrated in the 18-week specimen in which the vaginal fornices, exocervix and endocervix are readily identified. The stratified squamous epithelia of the vagina and exocervix were KRT14-negative with the exception of a patch of KRT14-positivity in epithelium of the caudal-most segment of the vagina, yet earlier the solid vaginal plate is KRT14-positive (Cunha et al., 2017b) (Table 4). In cervicovaginal epithelia of mice keratin14 is expressed early in postnatal development, and keratin 14 has been shown to be down stream of p63 and Runx1 (Laronda et al., 2013). The organotypic expression pattern of TP63 and RUNX1 in the developing human female reproductive tract (Cunha et al., 2017b) departs substantially from that seen in the mouse (Kurita and Cunha, 2001; Laronda et al., 2013).
Table 4.
Epithelial differentiation markers in human fetal uterine cervix
Stratified epithelium was KRT6-reactive, while simple columnar epithelium in the (presumed) endocervix) was KRT6-negative.
KRT14 reactivity was absent from the putative endocervix at 18 and 21 weeks, but was observed in areas of stratified squamous epithelium considered to be exocervix.
Epithelial ESR1 was generally negative until reactivity appeared ≥ 21 weeks.
DES induced epithelial PGR- and ESR1-reactivity in xenografts. From (Cunha et al., 2017b).
At 16 and 18 weeks the cervical epithelia (exocervix and endocervix) were uniformly ESR1-negative, whereas at 21 weeks ESR1 was strongly reactive in the presumptive epithelium of the exocervix, but patchy in epithelium of the endocervix. Thus, while ESR1 expression exhibits regionality of expression, its relevance to exocervical and endocervical boundaries is uncertain. In xenografts, DES strongly induced ESR1 reactivity in the “cervical region” (Cunha et al., 2017a). The progesterone receptor was not detected in the epithelium and stroma of the uterine cervix from 8 to 21 weeks.
D. Vagina
The origin of vaginal epithelium has been debated ever since Johannes Müller described the duct that bears his name (Müllerian duct) (Müller 1830). Vaginal epithelium has been proposed to receive contributions from epithelia of the urogenital sinus, Müllerian and Wolffian ducts alone or in combination (Koff, 1933; O’Rahilly, 1977; Forsberg, 1978; Bulmer, 1957). Historically, Koff’s publication in 1933 and Bulmer’s in 1957 were the two most seminal works examining the origin of the human vaginal epithelium (Bulmer, 1957; Koff, 1933). Both investigators understood that Müllerian and urogenital sinus epithelia play major roles. Koff asserted that the Müllerian epithelium formed most of the vaginal epithelium, assigning a minor contribution from urogenital sinus epithelium (UGE) to vaginal epithelium. Bulmer stated that UGE upgrowth “forms the whole of (the vaginal)epithelial lining” (Bulmer, 1957). For both studies the interpretations were based upon hematoxylin and eosin (H&E) stained sections without further verification.
To explore this issue, we carried out immunohistochemical studies of PAX2 and FOXA1 expression (Robboy et al., 2017). PAX2 is a protein expressed in Müllerian epithelium (Kurita, 2010), while FOXA1 is a protein expressed in endodermal urogenital sinus epithelium and its derivatives (Diez-Roux et al., 2011; Robboy et al., 2017; Besnard et al., 2004). The anatomical distribution of these two differentiation markers is dynamic during development of the female reproductive tract (See Fig. 15 for summary). At 11 to 12-weeks the cranial aspect of the uterovaginal canal has a lumen lined with a simple columnar epithelium (Figs. 8G–H), while the caudal aspect of the uterovaginal canal becomes occluded forming the solid vaginal plate abutting the introital epithelium. At 12 weeks the epithelia of the uterovaginal canal and almost all of the vaginal plate was PAX2-reactive (Figs. 11A–B & 15). The most caudal portion of the vaginal plate was PAX2-negative and FOXA1-positive (Figs. 11B & C & 15). Epithelia of the bladder and urethra were FOXA1-positive as expected (Fig. 11D). During weeks 14 to 16 the solid vaginal plate increased in extent, and at 16 weeks constituted about 1/3 of the developing female reproductive tract’s total length (Figs. 12 A & D & 15). At 16 weeks the solid vaginal plate consisted of FOXA1-reactive epithelial cells (of UGS origin) (Figs. 12C & D &15), while more cranially the stratified epithelium associated with a lumen was PAX2-reactive (Fig. 12A & B). Clearly, from 12 to 16 weeks the domain of UGS-derived FOXA1-positive epithelial cells had expanded cranially (see figure 15).When the vaginal fornices became defined at 18 weeks of gestation (Figs. 10F & 13A & D–E, & 15), epithelia of the uterine tube, uterine corpus, uterine cervix, and the upper (cranial) portion of the vagina remained PAX2- and KRT19-reactive (and thus Müllerian in origin), while the lower 2/3rds of the vagina was lined with FOXA1-reactive epithelial cells (Figs. 13C–D & 15), thus suggesting a substantial contribution of UGE to developing vaginal epithelium as proposed previously (Bulmer, 1957). At 18 weeks of gestation the vaginal vault and the exocervix were lined by a stratified squamous epithelium (Fig. 10F), which was PAX2-reactive (Fig. 15). This is consistent with the idea that simple columnar Müllerian epithelium of the caudal portion of the utero vaginal canal can transform directly into stratified squamous epithelium independent of UGE as is the case during development of mouse vaginal epithelium (Kurita and Cunha, 2001).
Figure 15.
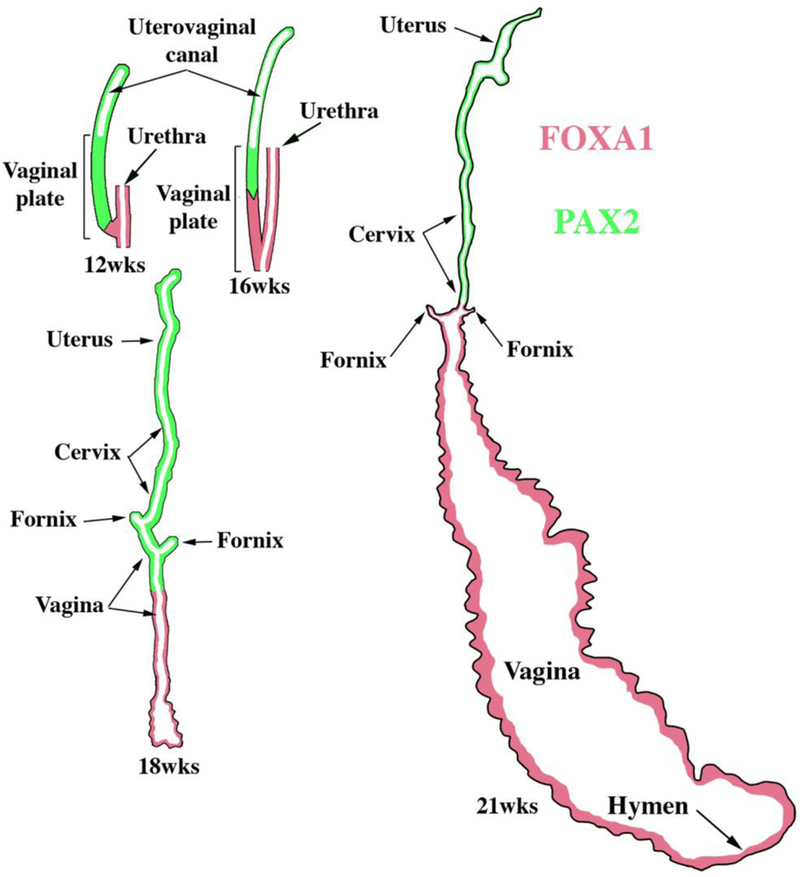
Summary of the changing patterns of PAX2 epithelial expression (green) indicative of Müllerian epithelium and FOXA1 epithelial expression (red) indicative of endodermal urogenital sinus epithelium over the time frame of 12 to 21 weeks.
Figure 11.
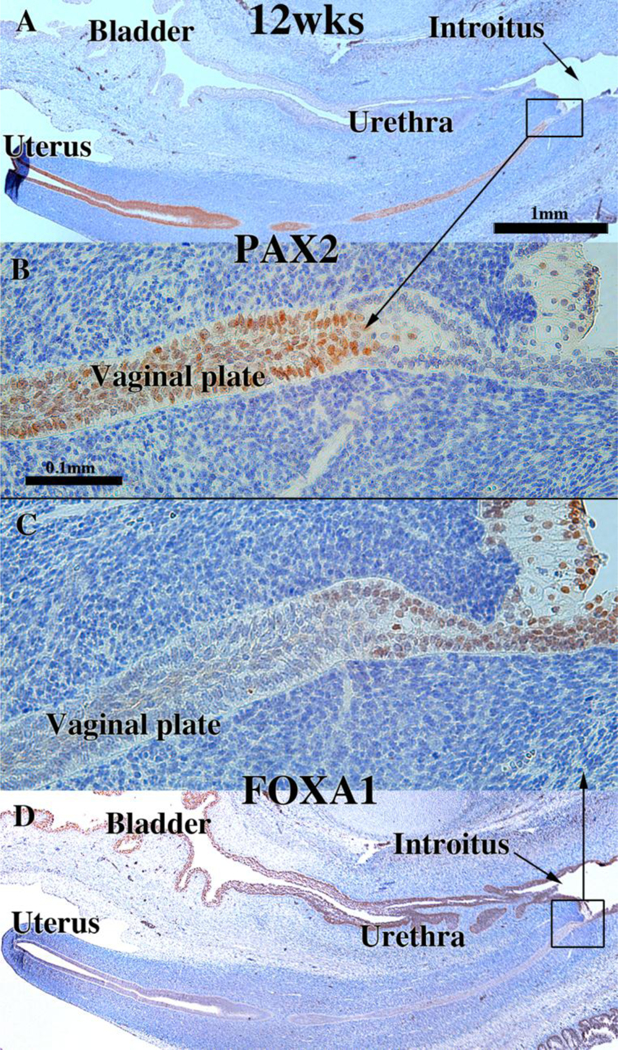
Sagittal sections of a 12-week human female fetal reproductive tract immunostained with PAX2 (A–B) and FOXA1 (C–D). PAX2-reactive epithelial cells extend to near the junction with urethral epithelium (A–B). FOXA1-reactive epithelial cells extend only a short distance into the solid vaginal plate (C–D). Scale bar in A also refers to D. Scale bar in D also refers to C. From Robboy et al (2017) with permission.
Figure 12.
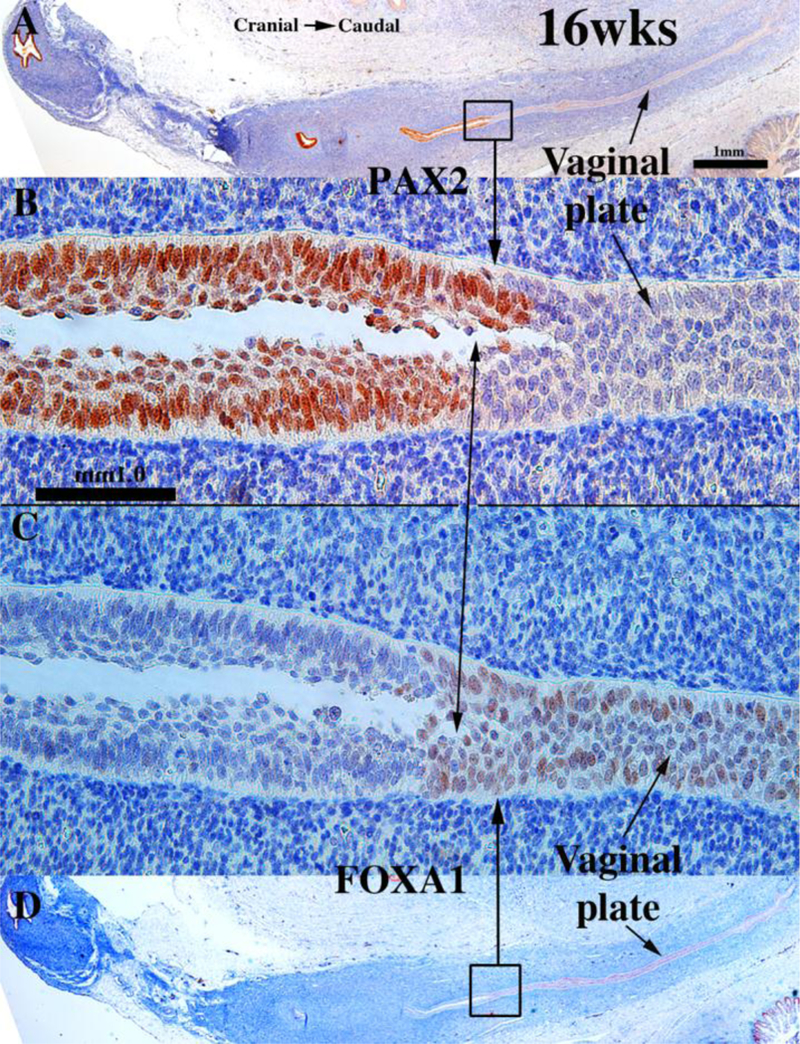
Sagittal sections of a 16-week human female fetal reproductive tract immunostained with PAX2 (A–B) and FOXA1 (C–D). FOXA1-reactive epithelial cells form all of the solid vaginal plate (C–D). PAX2-reactive epithelial cells constitute a epithelium lining the lumen of the uterovaginal canal (A–B). Scale bar in A also refers to D. Scale bar in D also refers to C. From Robboy et al (2017) with permission.
Figure 13.
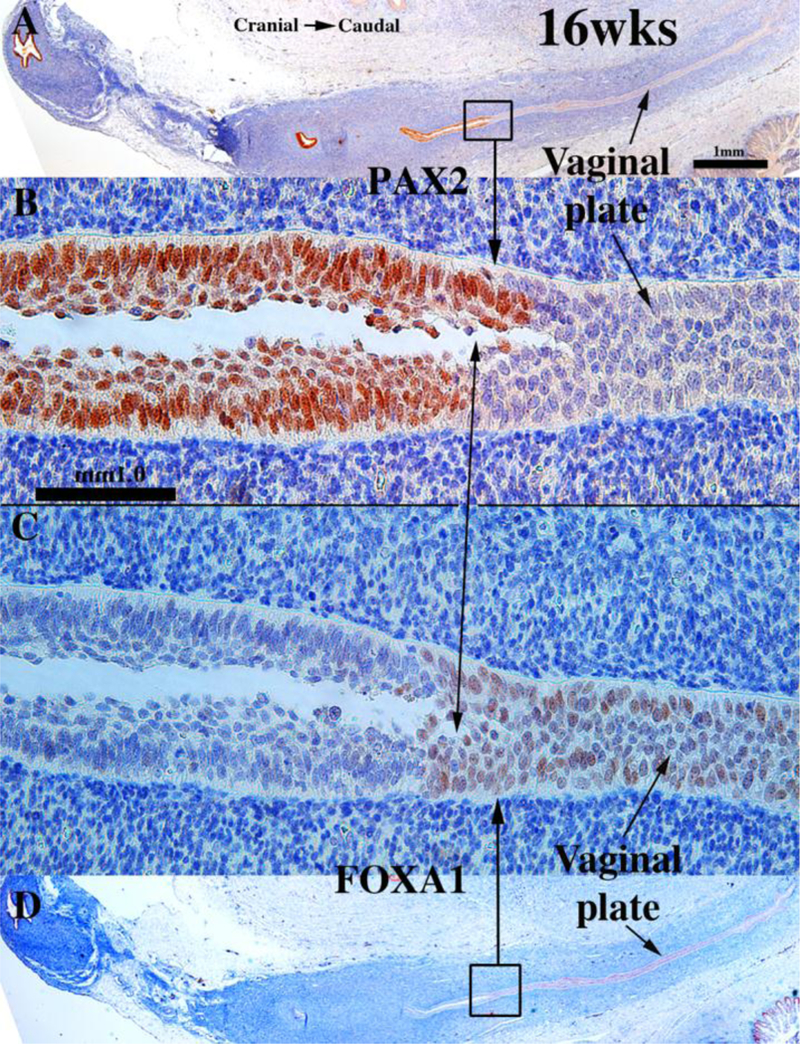
Sagittal sections of an 18-week human female fetal reproductive tract immunostained with PAX2 (A–B) and FOXA1 (C–D). PAX2-reactive epithelial cells line the lumen of the female reproductive tract from the uterine tube to the cranial aspect of the vagina (A–B). PAX2 staining is present, but weak in vaginal epithelium (A–B). FOXA1-reactive epithelial cells line the lower (caudal) vagina (C–D). Note abrupt fall off in KRT19 staining in the lower vagina (arrowheads). Scale bar in A also refers to D. Scale bar in D also refers to C. (E) KRT19 mirrors PAX2 immunostaining. From Robboy et al (2017) with permission.
At 21 weeks vaginal epithelium exhibited estrogenic stimulation throughout and was many cell layers thick, presumably due to elevated endogenous estrogen (Oakey, 1970). The vaginal fornices which were evident at 18 weeks (Fig 10F), were “filled in” (Fig. 14B) by epithelial proliferation. Epithelial FOXA1 staining was now observed from the introitus to the cervix, while epithelial PAX2 immunostaining remained confined to the endocervix (Figs. 14C–D & 15) and the uterine body, suggesting the replacement of Müllerian epithelium by urogenital sinus epithelium in the vagina. The abrupt change in FOXA1/PAX2 staining occurred at the same point in adjacent sections (Fig. 14C, E, F). The dynamic changes in the distribution of PAX2-reactive Müllerian epithelium and FOXA1-reactive UGE is summarized in figure 15. These data support Bulmer’s proposal that the entire vaginal epithelium derives from urogenital sinus epithelium (Bulmer, 1957). While our observations support displacement of Müllerian epithelium by UGE upgrowth, the molecular mechanistic scenario in human vaginal development requires further research. Also, since the oldest specimen analyzed in our study came from a 21week fetus, further changes in the relative contribution MDE versus UGE to human vaginal epithelium may occur at later stages. How these changes in the expression pattern of these markers relate to DES-induced adenosis is discussed below.
Figure 14.
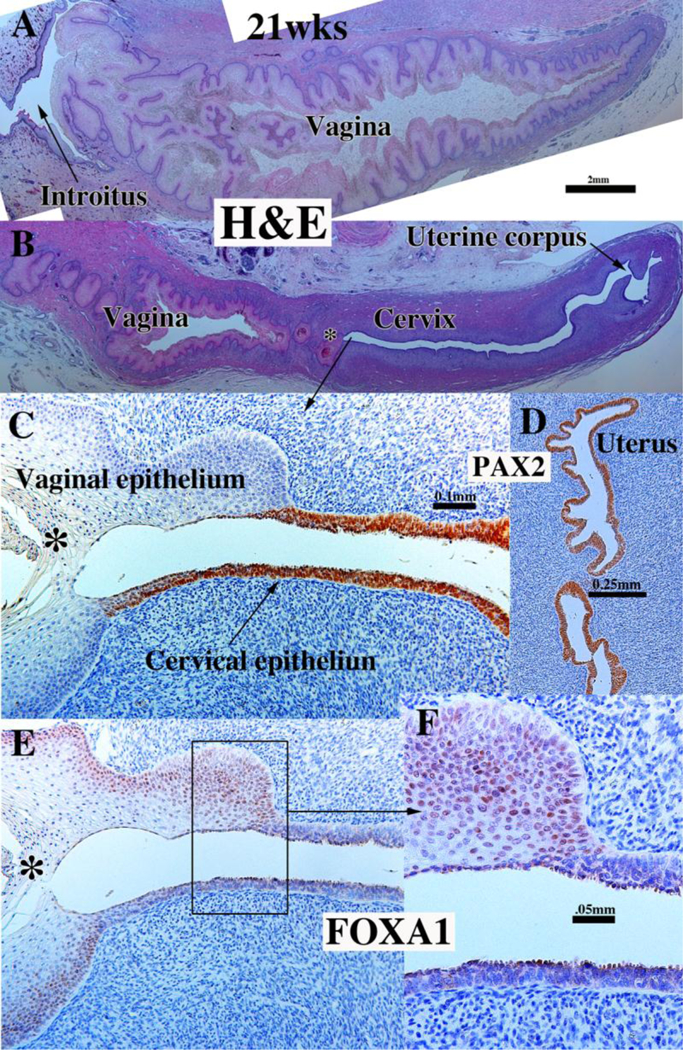
Sagittal sections of a 21-week female reproductive tract. Section (A) depicts the lower portion of the vagina (introitus to upper vagina). Section (B) depicts the upper portion of the specimen (vagina, cervix and uterine corpus) (both (A & B) are H&E stain. The vaginal epithelium is many layers thick due to estrogenic stimulation (A–B). Sections (C, E & F) are adjacent sections. In (B, C & E) note the abrupt transition in epithelial differentiation at the vaginal/cervical border and the change in PAX2/FOXA1 staining (indicative of a MD/UGE boundary). The vaginal epithelium is PAX2-negative (C), while epithelium of the uterine corpus (D) is strongly PAX2-reactive. FOXA1 immunostaining was seen uniformly throughout the entire vagina, and FOXA1 immunostaining abruptly stopped at the vaginal/cervical border (E–F). Asterisks in (B, C and E) are at the vaginal exocervical boundary. From Robboy et al (2017) with permission.
Epithelial cells of the human fetal vagina express a variety of differentiation markers whose expression changes during development (Table 5). Keratin 6 was not detected in the simple columnar epithelial cells of the uterovaginal canal. However, KRT6 was prominently expressed in epithelium of the solid vaginal plate epithelium at 12 weeks of gestation and thereafter, a pattern consistent with its known expression in basal and suprabasal cells of stratified epithelia (Moll et al., 1982; Moll et al., 2008). Keratin 19 was expressed in simple columnar epithelium of the uterovaginal canal at 9 weeks, and at 12 to 16 weeks was expressed the simple columnar epithelia of the uterine corpus, the cervix and stratified epithelium immediately cranial to the solid vaginal plate, which itself was KRT19-negative (Fig. 10C–D) (Cunha et al., 2017b). At 18 and 21 weeks, KRT19 was detected in epithelium of the upper vagina, but not in epithelium of the lower vagina (Fig. 13E, arrowheads). Keratin 14 was first detected in patches within the vaginal plate at 14 weeks and more broadly in the vaginal plate at 16 weeks. At 21 weeks KRT14 was expressed in basal and supra-basal cells of stratified vaginal epithelium (Cunha et al., 2017b) (Table 5). ESR1 was first detected in isolated patches in epithelium of the vaginal plate at 14 weeks. At 16 weeks ESR1 was uniformly expressed within the solid vaginal plate, and at 21 weeks the thick estrogen-stimulated vaginal epithelium expressed ESR1 strongly in basal and suprabasal epithelial cells (Cunha et al., 2017b) (Table 5). Runx1 was detected the uterovaginal canal at 9 weeks, in the solid vaginal plate at 12 weeks and was maintained thereafter during differentiation of the vaginal epithelium (Cunha et al., 2017b). TP63 was initially detected in the solid vaginal plate at 12 weeks and was maintained thereafter during differentiation of the vaginal epithelium. At 21 weeks TP63 was uniformly expressed in basal cells of vaginal epithelium, and KRT10 was detected in the thick estrogen-stimulated vaginal epithelium for the first time at 21 weeks (Cunha et al., 2017b). The progesterone receptor was not detected in vaginal epithelium from 8 to 21 weeks, but was induced by DES in vaginal epithelium in xenografts (Cunha et al., 2017a) (Table 5).
Table 5.
Epithelial differentiation markers in human fetal vagina
KRT10 was only detected in the 21-week specimen showing intense estrogenic stimulation.
Epithelial PGR is induced in xenografts by DES. From (Cunha et al., 2017b).
V. Xenograft studies.
A. Xenografts of human fetal female reproductive tracts.
Xenograft studies provide the unique opportunity to study human female reproductive tract development, estrogen-induced malformations and their molecular mechanisms. The underpinning of this approach to organogenesis/pathogenesis within the human derives from our studies published in 1982 in which human fetal female reproductive tracts were grown in athymic mouse hosts treated with DES and other hormonally active agents (Cunha et al., 1987b; Cunha et al., 1987a; Robboy et al., 1982a; Taguchi et al., 1983). Recently, we have (a) revisited our xenograft model of human female fetal reproductive tract development, (b) provided new data to validate normal development in xenografts grown in untreated hosts and (c) explored the effects of DES exposure on morphogenesis, differentiation and molecular expression within human female fetal reproductive tracts (Cunha et al., 2017a; Cunha et al., 2018).
The methodology begins with the abortus specimen and isolation of the fetal reproductive tract. For “young” specimens (< 14 weeks), the entire human reproductive tract can be grafted under the renal capsule, which is the preferred graft site (Cunha and Baskin, 2016). Older specimens can be split into right and left halves that in turn can be cut into cranial-caudal segments. This is particularly useful when contralateral control and Rx-treated groups are necessary. The endocrine status of the host mice can be precisely controlled via ovariectomy and exogenous hormone treatment. Xenografts can be grown for various periods, which in all cases should be >1 week to avoid effects that may be due to the “healing in” process. We typically grow grafts for 2 to 4 weeks.
Based upon several published studies (Cunha et al., 1987b; Cunha et al., 1987a; Robboy et al., 1982a; Taguchi et al., 1983; Cunha et al., 2017a), human fetal female reproductive tracts grown in untreated hosts develop normally and express the normal complement of epithelial and mesenchymal differentiation markers (Cunha et al., 1987b; Cunha et al., 1987a; Robboy et al., 1982a; Taguchi et al., 1983; Cunha et al., 2017a). Our most recent study (Cunha et al., 2017a) has extended our previous work by exploring the effect that DES exerts on epithelial differentiation marker expression. Accordingly, xenografts were grown in ovariectomized mice that were either untreated or treated with a 20mg subcutaneous DES pellet. DES stimulated endometrial and cervical gland formation (Fig. 16E) and increased plication (folding) of tubal epithelium (Fig. 16C).
Figure 16.
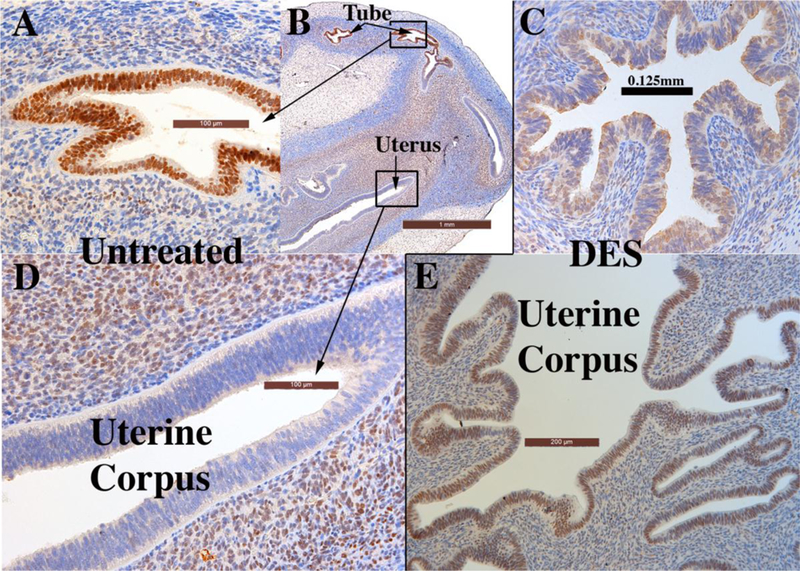
Xenografts of twin 13-week human female reproductive tracts grown for 4 weeks in untreated ovariectomized (A, B & D) and ovariectomized DES-treated athymic mice (C & E). Note in (A–B) uniform strong ESR1 immunostaining of the tubal epithelium in the untreated ovariectomized host, and (C) the mixture of weakly ESR1positive and ESR1-negative tubal epithelial cells in the DES-treated specimen. Sections (B & D) depict the uterine corpus of a xenograft grown in an untreated ovariectomized host. The epithelium of the uterine corpus is ESR1-negative, while the uterine mesenchyme is ESR1-reactive. Section (E) depicts the uterine corpus of the twin 13week specimen grown in a DES-treated ovariectomized host. Note the strong uniform expression of ESR1 in the uterine epithelium. Note also the substantial DES-induced plication of the uterine tube (compare B & C), and the profound DES-induced uterine gland formation (compare (B & E).
DES also affected ESR1 expression in tubal and uterine epithelium. Control specimens (non-grafted human uterine tubes (Cunha et al., 2017b) and tubal grafts grown in untreated ovariectomized hosts) disclosed uniform epithelial ESR1-reactivity (Fig. 16A & B). Grafts of uterine tube treated with DES exhibited weak ESR1-reactivity at best with many epithelial cells being ESR1-negative (Fig. 16C). Uterine epithelium at 16 weeks and younger is ESR1-negative and such ESR1-negativity is maintained in xenografts to untreated ovariectomized hosts (Figs. 16B & D). DES treatment induced ESR1 in the uterine epithelium (Fig. 16E).
Use of xenografts confirmed our previous report (Robboy et al., 1982a) of DESinduced vaginal adenosis. Vaginal adenosis is the presence of simple columnar epithelium on the surface or as submucosal glands in the vagina (Robboy et al., 1982b). Adenosis was never observed in vaginal grafts grown in untreated hosts (Cunha et al.,1987b; Cunha et al., 1987a; Robboy et al., 1982a; Taguchi et al., 1983; Cunha et al., 2017a). In contrast, xenografts in one DES-treated specimen from a previous study (Robboy et al., 1982a) and in two specimens in our recent study (Cunha et al., 2017a) disclosed the presence of adenosis (Fig. 17). The histology of the adenosis was of the embryonic (immature) form, and not that seen as the more differentiated tuboendometrial or mucinous forms. While DES elicited formation of a thickened and glycogenated squamous epithelium as anticipated, simple columnar epithelium (adenosis) was also present, and both epithelial types strongly expressed ESR1 (Fig. 17A). PGR, not present at the time of grafting, was also expressed in both the adenotic and the mature vaginal epithelium in response to DES (Fig. 17B). The DES-stimulated hyperplastic vaginal epithelium expressed keratins 6 (not illustrated) and 14 (Fig. 17C), TP63 (Fig. 17D), and RUNX1 (Fig. 17F), while the adenotic glandular vaginal epithelium expressed keratins 7 (Fig. 17E) and 8 (not illustrated). DES-induced adenosis was PAX2-reactive and FOXA1-negative, whereas the mature stratified vaginal epithelium was PAX2-negative and FOXA1-reactive (Figs. 17G–H), confirming that DES-induced adenosis is occurring in residual Müllerian epithelium. Table 6 summarizes the differences in marker expression in the mature stratified squamous epithelium versus adenosis.
Figure 17.
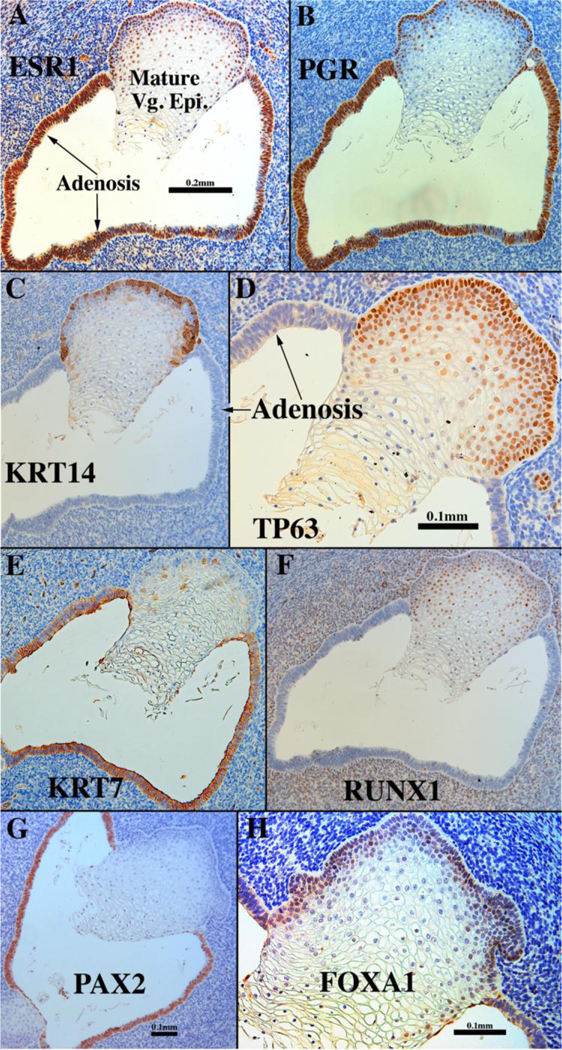
Sections of a 13-week vaginal specimen grown for 1 month in a DES-treated host via a 20mg subcutaneous pellet and immune stained as indicated. Mature stratified squamous vaginal epithelium and simple columnar adenotic epithelium exhibit remarkably different marker profiles (see Table 6).
Table 6.
Differences in differentiation marker expression in mature stratified squamous vaginal epithelium versus adenosis in xenografts grown in ovariectomized DES-treated hosts
| Differentiation markers |
Mature stratified squamous epithelium | Adenosis |
|---|---|---|
| ESR1 | Present | Present |
| PGR | Present | Present |
| KRT14 | Present | Absent |
| TP63 | Present | Absent |
| KRT7 | Absent | Present |
| RUNX1 | Present | Absent |
| PAX1 | Absent | Present |
| FOXA1 | Present | Absent |
From (Cunha et al., 2017a).
B. Xenografts of tissue recombinants composed of mouse vaginal mesenchyme and human fetal uterine tubal epithelium.
Studies in mice have shown that differentiation of Müllerian epithelium is induced and specified by cues from the mesenchyme (Cunha, 1976; Kurita et al., 2001; Kurita, 2011). Accordingly, vaginal mesenchyme instructively induces uterine epithelium to undergo vaginal epithelial differentiation (VgM+UtEvaginal differentiation), and uterine mesenchyme instructively induces vaginal epithelium to undergo uterine epithelial differentiation (UtM+VgEuterine differentiation). To determine whether mesenchymal induction plays a role in human Müllerian epithelial differentiation, tissue recombinants were prepared with 3-day postnatal mouse vaginal mesenchyme and human uterine tubal epithelium (mVgM+hTubE) from 12- to 13-week fetuses and grown under the renal capsule of ovariectomized female athymic mice. The mouse hosts were treated with DES (via subcutaneous pellet) to promote stratified squamous vaginal differentiation. In response to DES grafts of human fetal vagina differentiate a thick glycogenated stratified epithelium (Fig. 17), while grafts of human fetal uterine tube remain simple columnar (Fig. 16C) (Cunha et al., 2017a).
Epithelial differentiation markers (androgen receptor [AR], KRT6, TP63 and RUNX1) differ considerably in the human tubal versus human vaginal epithelium (Table 7) (Cunha et al., 2017a). In mVgM+hTubE recombinants grown in DES-treated female athymic mouse hosts, the mouse vaginal mesenchyme elicited a shift in epithelial histodifferentiation and differentiation marker expression from uterine tubal epithelial differentiation to vaginal epithelial differentiation. The shift in epithelial differentiation elicited by vaginal mesenchyme was incomplete as some tubal epithelial markers (KRT14 and KRT19) remained unchanged in mVgM+hTubE recombinants (Table 7), suggesting that mouse vaginal mesenchyme was able to elicit only a subset of vaginal differentiation markers in the tubal epithelium (Cunha et al., 2018). This partial shift in epithelial differentiation may be due to the age of the epithelium.
Table 7.
Epithelial differentiation of human uterine tube, human vagina and mVgM+hTubE tissue recombinants.
| Feature | Uterine tube | Vagina | mVgM+hTubE |
|---|---|---|---|
| Simple columnar epithelium | Yes | No | No |
| Stratified squamous epithelium | No | Yes | Yes |
| KRT6 | No | Yes | Yes |
| TP63 | No | Yes | Yes |
| Androgen receptor | Yes | No | No |
| RUNX1 | No | Yes | Yes |
| KRT14 | No | Yes | No |
| KRT19 | Yes | No | Yes |
Uterine tube epithelial features are shaded red, vaginal epithelial features are shaded green. From (Cunha et al., 2018)
C. Use of tissue recombinants to explore direct versus paracrine regulation of ESR1 and PGR in human fetal uterine epithelium.
ESR1 is first expressed in mesenchymal cells of the human uterine corpus at about 14 weeks, but rarely in uterine epithelium before the 21st gestational week, when the endogenous serum estrogen levels are elevated (Oakey, 1970). This suggests that uterine epithelial ESR1 may be estrogen induced, an idea confirmed in human fetal uterine xenografts in which epithelial ESR1 was broadly induced by DES (Fig. 16) (Cunha et al., 2018). PGR is undetectable in human fetal reproductive tracts from 9 to 21 weeks, but is also induced throughout the human fetal reproductive tracts in DES-treated xenografts (Cunha et al., 2018). Thus, there are two potential mechanisms of DES induction of uterine epithelial ESR1 and PGR: (a) DES may induce uterine epithelial ESR1 and PGR directly via epithelial ESR1 whose expression is below the sensitivity of immunohistochemistry. (b) Alternatively, DES may induce uterine epithelial ESR1 and PGR indirectly via mesenchymal ESR1 (paracrine mechanism). To distinguish between these two scenarios, we prepared tissue recombinants composed of epithelium of the human corpus (hUtE) and uterine mesenchyme from either Esr1-positive wild-type mice or Esr1-negative Esr1KO mice (wt UtM+hUtE and Esr1KO UtM+hUtE) (Cunha et al., 2018). All tissue recombinants were grown for 4 weeks in ovariectomized hosts treated with a subcutaneous 20mg DES pellet. As expected, wt UtM+hUtE tissue recombinants consistently (6/6) contained an ESR1- and PGR-reactive human uterine epithelium (Figs. 19A & B). Esr1KO UtM+hUtE tissue recombinants also (5/5) contained an ESR1- and PGR-reactive human uterine epithelium even though the surrounding stromal cells were ESR1-negative (Figs. 19C & D) (Cunha et al., 2018), thus confirming that DES acts directly on the epithelium.
Figure 19.
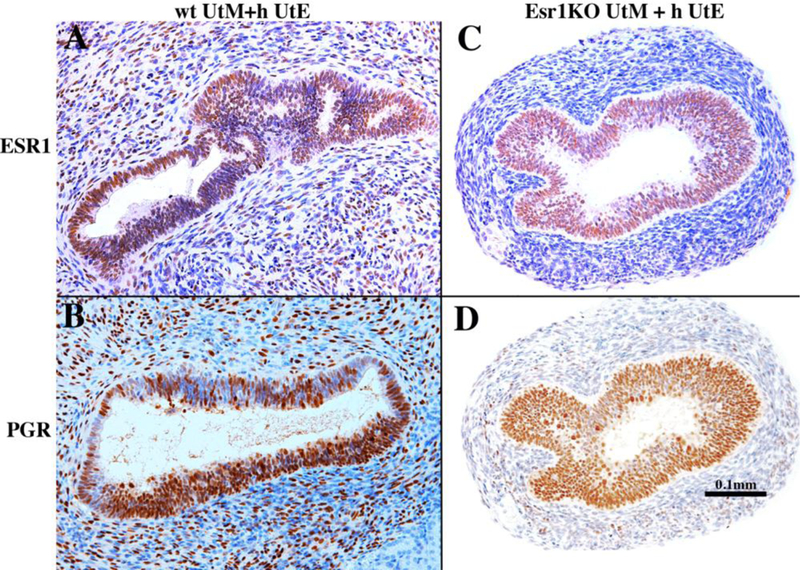
Tissue recombinants composed of neonatal mouse wild-type uterine mesenchyme plus human fetal uterine tube epithelium (wt UtM+hUtE) (A &B) and αERKO uterine mesenchyme plus human uterine tube epithelium Esr1KO UtM+ h UtE) (C & D) grown in DES-treated hosts and immune stained for ESR1 (A & C) and PGR (B & D). DES induced ESR1 and PGR even when the mesenchyme was genetically devoid of ESR1. Sections (C) and (D) are adjacent sections stained for ESR1 (C) and PGR (D). From Cunha et al (2018) with permission.
VI. Discussion
The tacit, but usually unproven, assumption inherent in animal models is that they reflect the underlying morphogenetic and molecular mechanisms of human biology. This approach is generally useful, even though substantial differences exist between human and animal anatomy, development and pathology. Accordingly, it is critical to understand both the similarities and differences between human and laboratory animal development. This, of course, necessitates a modern approach to human development in which the full technical palate is utilized. Sadly, most of our knowledge of human female reproductive tract development rests upon ancient studies based primarily upon H&E stained sections. This paper on human female reproductive tract development chronicles recent studies that shed new light on this topic and provide practical information facilitating future exploration.
The early papers on development of the human female reproductive tract by Koff in 1933 and Bulmer in 1957 (Koff, 1933; Bulmer, 1957) have been augmented by immunohistochemical studies of Fritsch et al covering the period of 8 to 34 weeks of gestation (Fritsch et al., 2012; Fritsch et al., 2013) and our studies covering the period of 9 to 21 weeks (Robboy et al., 2017; Cunha et al., 2017b). Taken together, these immunohistochemical studies show that several differentiation markers are present throughout this entire period, while others have a unique temporal expression profile. Note the substantial concurrence between our and Fritsch’s studies (Table 8) (Fritsch et al., 2012; Fritsch et al., 2013; Robboy et al., 2017; Cunha et al., 2017b).
Table 8.
Proteins detected by immunohistochemistry in epithelia*** of the human fetal female reproductive tract
| Protein | Fritsch* | Cunha Group** | Other groups |
|---|---|---|---|
| E-cadherin | YES | ND | |
| Vimentin | YES | ND | |
| TP63 | YES | YES | |
| Laminin | YES | ND | |
| Ki67 | YES | YES | |
| KRT19 | YES | YES | |
| KRT17 | YES | ND | |
| KRT14 | YES | YES | |
| KRT13 | YES | ND | |
| KRT10 | ND | YES | |
| KRT8 | YES | YES | |
| KRT7 | ND | YES | |
| KRT6 | ND | YES | |
| PAX2 | YES | YES | |
| BCL-2 | YES | ND | |
| FOXA1 | ND | YES | |
| HOXA13 | YES | ND | |
| BMP4 | YES | ND | |
| RUNX1 | ND | YES | |
| ESR1 | ND | YES | YES# |
| PGR | ND | NO | |
| AR | ND | YES | |
| ACTA2 | YES | YES | |
| HOXA11 | ND | YES | |
| ISL1 | ND | YES | |
| Uroplakin | ND | YES |
See text and references for detail, tissue expression and temporal changes.
ND=not done
The derivation of human vaginal epithelium has been debated for decades with Koff (1933) asserting that vaginal epithelium is mostly derived from Müllerian epithelium with a minor contribution ascribed to UGE, while Bulmer (1957) concluded the opposite, namely that virtually all of the human vaginal epithelial lining derives from UGS. Reich and Fritsch (2014) assert that squamous epithelia of the exocervix and the upper vagina are of Müllerian origin, thus supporting Koff’s interpretation. Unfortunately, these previous studies lacked markers indicative of both Müllerian and urogenital sinus epithelia. Our work, using PAX2 (a Müllerian epithelial marker) and FOXA1 (an endodermal UGE marker), supports Bulmer’s interpretation by showing that UGE of the vaginal plate grows cranially to completely replace the Müllerian epithelium to the level of the cervical os. The replacement of Müllerian epithelium by UGE appears to be perturbed by exposure to DES, which generates PAX2-positive adenotic epithelium in the vagina, an epithelium that appears to be retained columnar Müllerian epithelium. Our studies, showing that the lining epithelium of the human vagina is of urogenital sinus origin, nonetheless emphasize the need to further decipher this complicated process.
The human vaginal plate is a fascinating dynamic structure not having a mouse counterpart. The human vaginal plate first appears at about 11 weeks of gestation through occlusion of the Müllerian-derived uterovaginal canal, originally lined with a simple columnar epithelium. At this age, the vaginal is plate is almost entirely PAX2 reactive, indicative of its Müllerian origin. As gestation progresses during the next 4 weeks, the vaginal plate lengthens, and an increasing percentage of the vaginal plate shows reactivity with FOXA2, indicative of UGS origin. Ultimately, the vaginal plate “disappears” due to its canalization Concurrently, the cranial PAX2 (Müllerian) reactive area shrinks in extent. What triggers this change remains unknown. One potential explanation is differential growth of Müllerian versus urogenital sinus epithelia, with UGE out-competing Müllerian epithelium. Another possibility may involve differential adhesion of basal epithelial cells of Müllerian versus urogenital sinus origin to the epithelial basement membrane mediated via hemidesmosomes (Cunha et al., 1978).
The vaginal plate was initially considered to be uniform throughout, due to its homogeneous microscopic appearance in H&E histology. Clearly the squamous epithelial cells of the vaginal plate (and its derivative, vaginal epithelium) are not uniform, based upon immunohistochemical observations of keratins 7, 14 and 19, RUNX1 and ESR1 (Cunha et al., 2017b). Also, the vaginal plate is described as being a solid structure throughout. Yet it in some areas it appears to have a central lumen and with solid laterals “wings” (Koff, 1933; Robboy et al., 2017). Given the difficulty of obtaining human specimens, all of our specimens were sectioned sagittally or transversely. A series of coronal sections would be most helpful. Three-dimensional graphics allowing for specimen rotation would also be useful. Indeed, modern imaging techniques such as optical projection tomography, thick section confocal microscopy or light-sheet microscopy™ can be used to more fully understand development of the human vaginal plate.
The undifferentiated simple columnar Müllerian epithelium lining the embryonic uterovaginal canal differentiates and matures in the adult as one of three basic forms: a tuboendometrial form with various degrees of ciliation in the uterine (Fallopian) tube, endometrium, and as adenosis in the vagina. The second basic form, depicted by mucinous columnar epithelial cells, typifies the endocervical epithelium, which in the adult when found on the exocervical is called “ectropion”and when extending into the vagina is called adenosis. Finally, in the vagina and exocervix a stratified squamous epithelium forms. These various forms of epithelial differentiation may be induced and specified by mesenchymal-epithelial interactions in both normal and abnormal development of the human female reproductive tract.
Mesenchymal-epithelial interactions are known to play a central role in differentiation of the murine female reproductive tract. Mouse vaginal mesenchyme is capable of inducing certain aspects of vaginal differentiation in human fetal uterine tube epithelium. The cellular target of mesenchymal induction within the epithelium may be relatively rare epithelial stem cells, or may be a large subset of the fetal epithelium. It is perhaps worth noting that organs of the female reproductive tract (especially the uterus) undergo dramatic morphogenetic and growth changes during reproductive cycles due in part to the presence of stem cells (Maruyama, 2015).
Mesenchymal-epithelial interactions between rodent and human tissues are so highly conserved that rodent mesenchymal inducers can reprogram differentiation of human fetal and adult epithelia (Aboseif et al., 1999; Cunha et al., 1983). This approach can be extended by combining mutant mouse mesenchyme plus human fetal epithelium or human mesenchyme plus mutant mouse epithelium in experiments designed to explore molecular mechanisms of human organogenesis as illustrated in our Esr1KOUtM+hUtE studies. This approach was used to verify that estrogenic up-regulation of both ESR1 and PGR in the human fetal uterine epithelium is mediated directly via epithelial ESR1 (Cunha et al., 2018).
An important finding from our prior work is that differentiation of myometrial smooth muscle from uterine mesenchyme in mice depends upon a signal(s) from uterine epithelium (Cunha et al., 1992). Likewise, urinary bladder epithelium induces detrusor smooth muscle differentiation (Baskin et al., 1996; Baskin et al., 2001). In the bladder, this effect of epithelium (smooth muscle induction) appears to be non-specific as a range of epithelia can induce smooth muscle differentiation in bladder mesenchyme (DiSandro et al., 1998), and in the bladder this effect appears to be mediated by sonic hedgehog (Cao et al., 2010). Whether similar developmental mechanisms of myometrial differentiation are at play in differentiation of human myometrium could be examined in tissue recombination studies.
Another fascinating area to explore is why and where does the clear cell adenocarcinoma develop in DES daughters. One thought proffered is that DES acts as a carcinogen, directly affecting the epithelium to transform into a cancer (Saeed et al., 2009). Alternatively, DES may act more like a teratogen via the developing vaginal stroma (Cunha et al., 1977; Kurita, 2011; Robboy, 1983). The process may involve inhibition of upgrowth of the UGE to replace the native columnar Müllerian epithelium. The resultant retention of simple columnar Müllerian epithelium in the vagina is called adenosis.Of import, cancers in patients consistently occur at a specific location, namely the junction where metaplastic squamous epithelium (the “healing” manifestation of mucinous adenotic epithelium) abuts glycogenated squamous epithelium (Robboy et al.,1982b), presumably representing UGE. This hypothesis can be assessed with PAX2 and FOXA2 immunohistochemistry, if tumor specimens are available that were serially blocked when removed and locations noted. Our earlier work showed that all clear cell adenocarcinomas arise in beds of tuboendometrial epithelium, and where at least a single focus of atypical tuboendometrial epithelium was present, suggesting a transition from a teratogenic origin of tuboendometrial adenosis, through atypicality, to eventual cancer (Robboy et al., 1984b)
The main finding of our studies are listed below:
PAX2 and FOXa1 immunostaining support Bulmer’s proposals that human vaginal epithelium derives solely from urogenital sinus epithelium.
That portion of Müllerian ducts and uterovaginal canal lined by simple columnar epithelium (endocervix, uterine corpus and uterine tube) expresses keratins 7, 8 and 19.
That portion of the female reproductive tract undergoing stratified squamous differentiation (exocervix and vagina) expresses keratins 6, 14 and 10 in an agedependent fashion.
TP63 and RUNX1 are expressed in vaginal epithelium prior to keratin 14, as these two transcription factors are known to be upstream from keratin 14.
HOXA11 is expressed in uterine mesenchyme and ISL1 is expressed in vaginal mesenchyme.
The ontogeny of estrogen receptor alpha (ESR1), progesterone receptor and the androgen receptor provides the mechanistic underpinning for the teratogenicity of estrogens, progestins and androgens on female reproductive tract development.
Normal morphogenesis and normal patterns of differentiation marker expression occur in xenografts of human female reproductive tracts grown in untreated hosts.
DES treatment of human female reproductive tract xenografts: (a) stimulated endometrial/cervical glands formation, (b) increased plication (folding) of tubal epithelium, (c) elicited stratified squamous maturation of vaginal epithelium and (d) elicited vaginal adenosis.
DES treatment of xenografts also induced ESR1 in epithelia of the uterine corpus, cervix and globally induced PGR in most cells of the developing human female reproductive tract.
DES treatment of xenografts induced vaginal adenosis was associated with altered expression of epithelial marker proteins.
DES perturbed smooth muscle patterning in xenografts of the uterine tube.
Mouse vaginal mesenchyme can induce human tubal epithelium to undergo partial conversion to a vaginal phenotype, thus demonstrating the importance of mesenchymal-epithelial interactions in development of the human female reproductive tract.
DES-induction of uterine epithelial progesterone receptor (PGR) and estrogen receptor 1 (ESR1) is mediated directly via epithelial ESR1 and not via a paracrine mechanism.
Investigation of human female reproductive tract development can be extended through application of the full palate of modern biological techniques including modern imaging techniques, xenografting, single cell RNA-seq, tissue recombinant and crisprcas9 technologies. Many carcinogenetic processes recapitulate to some extent developmental processes, and thus modern studies of human organogenesis and epithelial differentiation may provide important insights into human pathogenesis.
Figure 18.
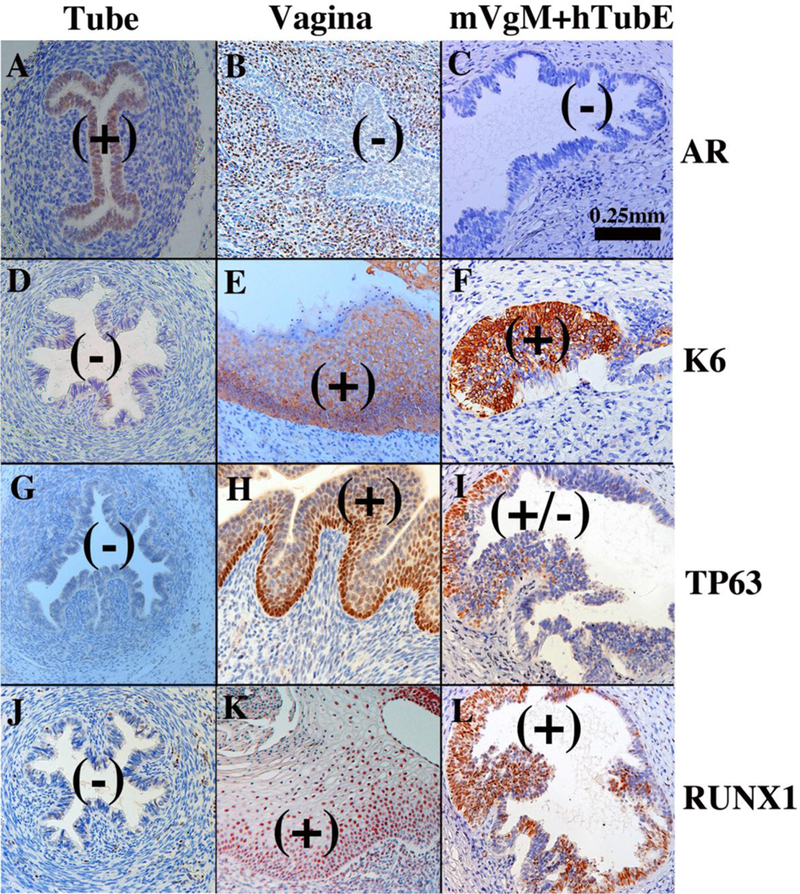
Tissue recombinants composed of neonatal mouse vaginal mesenchyme plus 13-week human fetal uterine tube epithelium (mVgM+hTubE) grown for 4 weeks in DES-treated hosts and immune stained for various vaginal epithelial markers as indicated. Human uterine tube (A, D, G, J) and vagina (B, E, H, K) at 16 to 18 weeks of gestation serve as controls. Note induction of KRT6, TP63 and RUNX1 and down regulation of AR in epithelium of the mVgM+hTubE recombinants, indicative of an effect of mouse vaginal mesenchyme on expression of differentiation markers in human tubal epithelium. (+) and (−) indicate epithelial marker expression. From Cunha et al (2018) with permission.
Acknowledgements
This study was supported by NIH grant DK058105 to Dr. Baskin and R01 CA154358 to Dr. Kurita. The authors appreciate the superb technical expertise of Mei Cao.
Abbreviations:
- H&E
hematoxylin & eosin
- UGS
urogenital sinus
- UGE
urogenital sinus epithelium
- MD
Müllerian duct
- MDE
Müllerian duct epithelium
- WD
Wolffian duct
- DES
diethylstilbestrol
- KRT
keratin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboseif S, El-Sakka A, Young P, and Cunha G (1999) Mesenchymal reprogramming of adult human epithelial differentiation. Differentiation; research in biological diversity 65:113–118. [DOI] [PubMed] [Google Scholar]
- Baskin L, DiSandro M, Li Y, Li W, Hayward S, and Cunha G (2001) Mesenchymal-epithelial interactions in bladder smooth muscle development: effects of the local tissue environment. The Journal of urology 165:1283–1288. [PubMed] [Google Scholar]
- Baskin LS, Hayward SW, Young P, and Cunha GR (1996) Ontogeny of the rat bladder: Smooth muscle and epithelial differentiation. Acta anatomica 155:104112. [DOI] [PubMed] [Google Scholar]
- Bern HA, Mills KT, Ostrander PI, Schoenrock B, Graveline B, and Plapinger L (1984) Cervicovaginal abnormalities in BALB/c mice treated neonatally with sex hormones. Teratology 30:267–274. [DOI] [PubMed] [Google Scholar]
- Bern HA, and Talamantes FJ (1981) Neonatal mouse models and their relation to disease in the humal female In: Herbst A, and Bern HA (eds) Developmental Effects of Diethylstilbestrol (DES) in Pregnancy Thieme Stratton Inc., New York, pp. 129–147. [Google Scholar]
- Besnard V, Wert SE, Hull WM, and Whitsett JA (2004) Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene expression patterns : GEP 5:193–208. [DOI] [PubMed] [Google Scholar]
- Bulmer D (1957) The development of the human vagina. J. Anat 91:490–509. [PMC free article] [PubMed] [Google Scholar]
- Cai Y (2009) Revisiting old vaginal topics: conversion of the Mullerian vagina and origin of the “sinus” vagina. The International journal of developmental biology 53:925–934. [DOI] [PubMed] [Google Scholar]
- Campbell JG (1952) Bicornuate uterus. Can Med Assoc J 67:355–356. [PMC free article] [PubMed] [Google Scholar]
- Cao M, Tasian G, Wang MH, Liu B, Cunha G, and Baskin L (2010) Urotheliumderived Sonic hedgehog promotes mesenchymal proliferation and induces bladder smooth muscle differentiation. Differentiation; research in biological diversity 79:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T (1994) The wildlife/human connection: modernizing risk decisions. Environmental health perspectives 102 Suppl 12:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T (1995) Environmental estrogens: health implications for humans and wildlife. Environmental health perspectives 103 Suppl 7:135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Spencer TE, Bartol FF, and Hayashi K (2013) Uterine glands: development, function and experimental model systems. Mol Hum Reprod 19:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR (1976) Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J. Exp. Zool 196:361–370. [DOI] [PubMed] [Google Scholar]
- Cunha GR, and Baskin L (2016) Use of sub-renal capsule transplantation in developmental biology. Differentiation; research in biological diversity 91:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Battle E, Young P, Brody J, Donjacour A, Hayashi N, and Kinbara H (1992) Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol 1:76–83. [PubMed] [Google Scholar]
- Cunha GR, Kurita T, Cao M, Shen J, Cooke PS, Robboy S, and Baskin L (2018) Tissue interactions and estrogenic response during human female fetal reproductive tract development. Differentiation; research in biological diversity 101:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Kurita T, Cao M, Shen J, Robboy SJ, and Baskin L (2017a) Response of xenografts of developing human female reproductive tracts to the synthetic estrogen, diethylstilbestrol. Differentiation; research in biological diversity 98:35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Lee AK, and Lung B (1978) Electron microscopic observations of vaginal development in untreated and neonatally estrogenized Balb/c Crgl mice. Am J Anat 152:343–381. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B, and Kato K (1977) Role of the epithelial-stromal interaction during the development and expression of ovary-independent vaginal hyperplasia. Develop. Biol 56:52–67. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Sekkingstad M, and Meloy BA (1983) Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit, and human tissues. Differentiation; research in biological diversity 24:174–180. [DOI] [PubMed] [Google Scholar]
- Cunha GR, T K, Cao M, Shen J, Robboy S, and L B (2017b) Molecular mechanisms of development of the human fetal female reproductive tract. Differentiaiton 97:54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Namikawa R, Nishizuka Y, and Robboy SJ (1987a) Teratogenic effects of Clomid, tamoxifen, and diethylstilbestrol on the developing human female genital tract. Human Pathol 18:1132–1143. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Sugimura Y, Lawrence DW, Mahmood F, and Robboy SJ (1987b) Absence of teratogenic effects of progesterone on the developing gential tract of the human female fetus. Human Pathol 19:777–783. [DOI] [PubMed] [Google Scholar]
- Davies WM, and Walpole GR (1949) Bicornuate uterus. Med J Aust 2:166. [PubMed] [Google Scholar]
- Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, Lin-Marq N, Koch M, Bilio M, Cantiello I, Verde R, De Masi C, Bianchi SA, Cicchini J, Perroud E, Mehmeti S, Dagand E, Schrinner S, Nurnberger A, Schmidt K, Metz K, Zwingmann C, Brieske N, Springer C, Hernandez AM, Herzog S, Grabbe F, Sieverding C, Fischer B, Schrader K, Brockmeyer M, Dettmer S, Helbig C, Alunni V, Battaini MA, Mura C, Henrichsen CN, Garcia-Lopez R, Echevarria D, Puelles E, Garcia-Calero E, Kruse S, Uhr M, Kauck C, Feng G, Milyaev N, Ong CK, Kumar L, Lam M, Semple CA, Gyenesei A, Mundlos S, Radelof U, Lehrach H, Sarmientos P, Reymond A, Davidson DR, Dolle P, Antonarakis SE, Yaspo ML, Martinez S, Baldock RA, Eichele G, and Ballabio A (2011) A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol 9:e1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSandro MJ, Li Y, Baskin LS, Hayward S, and Cunha G (1998) Mesenchymalepithelial interactions in bladder smooth muscle development: epithelial specificity. The Journal of urology 160:1040–1046; discussion 1079. [DOI] [PubMed] [Google Scholar]
- Drey EA, Kang MS, McFarland W, and Darney PD (2005) Improving the accuracy of fetal foot length to confirm gestational duration. Obstetrics and gynecology 105:773–778. [DOI] [PubMed] [Google Scholar]
- Du H, and Taylor HS (2015) The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb Perspect Med 6:a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg JG (1973) Cervicovaginal epithelium: its origin and development. American journal of obstetrics and gynecology 115:1025–1043. [DOI] [PubMed] [Google Scholar]
- Forsberg JG (1978) Development of the human vaginal epithelium. In: Hafez ESE, and Evans TN (eds) The Human Vagina Elsevier, No. Holland, New York, pp. 3–20. [Google Scholar]
- Forsberg JG (1996) A morphologist’s approach to the vagina. Acta Obstet Gynecol Scand Suppl 163:3–10. [PubMed] [Google Scholar]
- Fritsch H, Hoermann R, Bitsche M, Pechriggl E, and Reich O (2013) Development of epithelial and mesenchymal regionalization of the human fetal utero-vaginal anlagen. Journal of anatomy 222:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch H, Richter E, and Adam N (2012) Molecular characteristics and alterations during early development of the human vagina. Journal of anatomy 220:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatstein IZ, and Yeh J (1995) Ontogeny of the estrogen receptor in the human fetal uterus. The Journal of clinical endocrinology and metabolism 80:958–964. [DOI] [PubMed] [Google Scholar]
- Gray SW, and Skandalakis JE (1972) Embryology for Surgeons W.B. Saunders Co., Philadelphia. [Google Scholar]
- Gruenwald P (1941) The relation of the growing Mullerian duct to the Wolffian duct and its importance for the genesis of malformations. Anatomical Rec 81:1–19. [Google Scholar]
- Herbst A, and Bern H (1981) Developmental effects of DES in pregnancy Thieme Stratton, New York. [Google Scholar]
- Herbst AL, Scully RE, and Robboy SJ (1975) Vaginal adenosis and other diethylstilbestrol-related abnormalities. Clin Obstet Gynecol 18:185–194. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, and Poskanzer DC (1971) Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. New Eng. J. Med 284:878–881. [DOI] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, Karlan BY, Kaufman R, Noller KL, Palmer JR, Robboy SJ, Saal RC, Strohsnitter W, Titus-Ernstoff L, and Troisi R (2011) Adverse health outcomes in women exposed in utero to diethylstilbestrol. The New England journal of medicine 365:1304–1314. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, and McGuire WL (1979) Estrogen control of progesterone receptor induction in human breast cancer: role of nuclear estrogen receptor. Adv Exp Med Biol 117:95–110. [DOI] [PubMed] [Google Scholar]
- Huang C-C, Orvis GD, Kwan KM, and Behringer RR(2014)Lhx1 is required in Mullerian duct epithelium for uterine development. Developmental biology 389:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RH (1930) Observations on the development of the human female genital tract. Contr. Embryol. Carneg. Inst 22:91–108. [Google Scholar]
- Janne O, Kontula K, Luukkainen T, and Vihko R (1975) Oestrogen-induced progesterone receptor in human uterus. Journal of steroid biochemistry 6:501–509. [DOI] [PubMed] [Google Scholar]
- Jefferies JA, Robboy SJ, O’Brien PC, Bergstralh EJ, Labarthe DR, Barnes AB, Noller KL, Hatab PA, Kaufman RH, and Townsend DE (1984) Structural anomalies of the cervix and vagina in women enrolled in the Diethylstilbestrol Adenosis (DESAD) Project. American journal of obstetrics and gynecology 148:59–66. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, and Behringer RR (2005) Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132:2809–2823. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, and Behringer RR (2011) beta-Catenin is essential for Mullerian duct regression during male sexual differentiation. Development 138:1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff AK (1933) Development of the vagina in the human fetus. Contrib. Embryol. Carnegie Inst. Wash 24:59–90. [PubMed] [Google Scholar]
- Konishi I, Fujii S, Okamura H, and Mori T (1984) Development of smooth muscle in the human fetal uterus: an ultrastructural study. J. Anat 139:239–252. [PMC free article] [PubMed] [Google Scholar]
- Kurita T (2010) Developmental origin of vaginal epithelium. Differentiation; research in biological diversity 80:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T (2011) Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation; research in biological diversity 82:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, and Cunha GR (2001) Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Developmental biology 240:194–211. [DOI] [PubMed] [Google Scholar]
- Kurita T, and Cunha GR (2001) Roles of p63 in differentiation of Mullerian duct epithelial cells. Ann N Y Acad Sci 948:9–12. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA, and Medina RT (2005a) Differential expression of p63 isoforms in female reproductive organs. Mechanisms of development 122:1043–1055. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, and Cunha GR (2000) Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biology of reproduction 62:821–830. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, and Gold LI (2005b) The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation; research in biological diversity 73:313–322. [DOI] [PubMed] [Google Scholar]
- Kurita T, Mills AA, and Cunha GR (2004) Roles of p63 in the diethylstilbestrolinduced cervicovaginal adenosis. Development 131:1639–1649. [DOI] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Butler LM, and Kurita T (2012) The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation; research in biological diversity 84:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Ishi K, Serna VA, Butler LM, Mills AA, Orvis GD, Behringer RR, Deng C, Sinha S, and Kurita T (2013) Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Mullerian duct epithelium. Developmental biology 381:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T (2015) Stem Cells in the Uterus: Past, Present and Future. Seminars in reproductive medicine 33:315–316. [DOI] [PubMed] [Google Scholar]
- McLachlan J (1981) Rodent models for perinatal exposure to diethylstilbestrol and their relation to human disease in the male In: Herbst A, and Bern HA (eds) Developmental Effects of Diethylstilbestrol (DES) in Pregnancy Thieme Stratton Inc., New York, pp. 148–157. [Google Scholar]
- McLachlan JA, and Newbold RR (1996) Cellular and molecular mechanisms of cancers of the uterus in animals. Prog Clin Biol Res 394:175–182. [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, and Bullock BC (1975) Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science 190:991–992. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Burow ME, and Li SF (2001) From malformations to molecular mechanisms in the male: three decades of research on endocrine disrupters. APMIS 109:263–272. [DOI] [PubMed] [Google Scholar]
- Moll R, Divo M, and Langbein L (2008) The human keratins: biology and pathology. Histochem Cell Biol 129:705–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, and Krepler R (1982) The catalog of human cytokeratin polypeptides: patterns of expression of specific cytokeratins in normal epithelia, tumors, and cultured cells. Cell 31:11–24. [DOI] [PubMed] [Google Scholar]
- Moore KL, and Persaud TVN (2003) The Developing Human Saunders, Philadelphia. [Google Scholar]
- Müller , J. (1830) Bildungsgeschichte der Genitalien Arnz, Dusseldorf. [Google Scholar]
- Mutter GL, and Robboy SJ (2014) Embryology In: Mutter GS, and Prat J (eds) Pathology of the Female Reproductive Tract Churchill Livingston, London, pp. 1–17. [Google Scholar]
- Newbold R (1995) Cellular and molecular effects of developmental exposure to diethylstilbestrol: Implications for other environmental estrogens. Environ. Health Perspect 103:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR (2004) Lessons learned from perinatal exposure to diethylstilbestrol. Toxicology and applied pharmacology 199:142–150. [DOI] [PubMed] [Google Scholar]
- Newbold RR (2008) Prenatal exposure to diethylstilbestrol (DES). Fertility and sterility 89:e55–56. [DOI] [PubMed] [Google Scholar]
- Newbold RR, and MaLachlan JA (1985) Diethylstilbestrol-associated defects in murine genital tract development In: McLachlan JA (ed) Estrogens in the Environment II Elsevier, New York, pp. 288–318. [Google Scholar]
- Newbold RR, Tyrey S, Haney AF, and McLachlan JA (1983) Developmentally arrested oviduct: a structural and functional defect in mice following prenatal exposure to diethystilbestrol. Teratology 27:417–426. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R (1973) The embryology and anatomy of the uterus In: Norris HJ, Hertig AT, and Abell MR (eds) The Uterus Williams and Wilkins Co., Baltimore, pp. 17–39. [Google Scholar]
- O’Rahilly R (1977) Prenatal human development. In: Wynn RM (ed) Biology of the Uterus Plenum Press, New York, pp. 35–57. [Google Scholar]
- O’Rahilly R (1983) The timing and sequence of events in the development of the human reproductive system during the embryonic period proper. Anatomy and embryology 166:247–261. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, and Muller F (1992) The Reproductive System In: O’Rahilly R, and Muller F (eds) Human Embryology and Teratology Wiley-Liss, New York, pp. 207–224. [Google Scholar]
- Oakey RE (1970) The progressive increase in estrogen production in human pregnancy: an appraisal of the factors responsible. Vitam Horm 28:1–36. [DOI] [PubMed] [Google Scholar]
- Park JM (2016) Embryology of the Genitourinary In: Wein AJ, Kavoussi LR, Partin AW, and Peters CA (eds) Campbell-Walsh Urology Elsevier, Amsterdam, pp. 3121–3148. [Google Scholar]
- Pauerstein CJ, Hodgson BJ, and Kramen MA (1974) The anatomy and physiology of the oviduct. Obstet Gynecol Annu 3:137–201. [PubMed] [Google Scholar]
- Reed CE, and Fenton SE (2013) Exposure to Diethylstilbestrol during Sensitive Life Stages: A legacy of heritable health effects. Birth defects research. Part C, Embryo today : reviews 99:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich O, and Fritsch H (2014) The developmental origin of cervical and vaginal epithelium and their clinical consequences: a systematic review. J Low Genit Tract Dis 18:358–360. [DOI] [PubMed] [Google Scholar]
- Rennell CL (1979) T-shaped uterus in diethylstilbestrol (DES) exposure. AJR. American journal of roentgenology 132:979–980. [DOI] [PubMed] [Google Scholar]
- Robboy SJ (1983) A hypothetic mechanism of diethylstilbestrol(DES)-induced anomalies in exposed progeny. Hum Pathol 14:831–833. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Kurita T, Baskin L, and Cunha GR (2017) New insights insights into human female reproductive tract development. Differentiation; research in biological diversity 97:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robboy SJ, Noller KL, R.H. K, Barnes AB, Townsend D, Gunderson JH, Kurland L, and Nash S (1984a) An atlas of findings in the human female after intrauterine exposure to diethylstilbestrol. DHEW publication 84–2344.
- Robboy SJ, Scully RE, Welch WR, and Herbst AL (1977) Intrauterine diethylstilbestrol exposure and its consequences: pathologic characteristics of vaginal adenosis, clear cell adenocarcinoma, and related lesions. Arch Pathol Lab Med 101:1–5. [PubMed] [Google Scholar]
- Robboy SJ, Taguchi O, and Cunha GR (1982a) Normal development of the human female reproductive tract and alterations resulting from experimental exposure to diethylstilbestrol. Human Pathol 13:190–198. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Welch WR, Young RH, Truslow GY, Herbst AL, and Scully RE (1982b) Topographic relation of cervical ectropion and vaginal adenosis to clear cell adenocarcinoma. Obstetrics and gynecology 60:546–551. [PubMed] [Google Scholar]
- Robboy SJ, Young RH, Welch WR, Truslow GY, Prat J, Herbst AL, and Scully RE (1984b) Atypical vaginal adenosis and cervical ectropion. Association with clear cell adenocarcinoma in diethylstilbestrol-exposed offspring. Cancer 54:869–875. [DOI] [PubMed] [Google Scholar]
- Sadler TW (2004) Langman’s Medical Embryology Lippincott Williams & Wilkins, New York. [Google Scholar]
- Saeed M, Rogan E, and Cavalieri E (2009) Mechanism of metabolic activation and DNA adduct formation by the human carcinogen diethylstilbestrol: the defining link to natural estrogens. International journal of cancer . Journal international du cancer 124:1276–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinisi AA, Pasquali D, Notaro A, and Bellastella A (2003) Sexual differentiation. Journal of endocrinological investigation 26:23–28. [PubMed] [Google Scholar]
- Smith B (2016) The Multi-Dimensional Human Embryo University of Michigan, Ann Arbor, MI. [Google Scholar]
- Stillman RJ (1982) In utero exposure to diethylstilbestrol: adverse effects on the reproductive tract and reproductive performance and male and female offspring. American journal of obstetrics and gynecology 142:905–921. [DOI] [PubMed] [Google Scholar]
- Streeter GL (1951) Developmental horizons in human embryos. Age groups XI to XXIII Carnegie Institution of Washington, Washington DC. [Google Scholar]
- Sulak O, Cosar F, Malas MA, Cankara N, Cetin E, and Tagil SM (2007) Anatomical development of the fetal uterus. Early Hum Dev 83:395–401. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, and Robboy SJ (1983) Experimental study of the effect of diethylstilbestrol on the development of the human female reproductive tract. International J. Biological Res. in Pregnancy 4:56–70. [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K, and Hoover RN (2010) Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES). International journal of andrology 33:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhou X, Liu L, Zhu Y, Liu C, Pan H, Xing Q, Wang J, Wang X, Zhang X, Cao Y, and Wang B (2017) Identification and functional analysis of a novel LHX1 mutation associated with congenital absence of the uterus and vagina. Oncotarget 8:8785–8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhou J, Li R, Dudley EA, and Ye X (2016) Novel function of LHFPL2 in female and male distal reproductive tract development. Scientific reports 6:23037. [DOI] [PMC free article] [PubMed] [Google Scholar]


