Abstract
Background
Intensive care unit (ICU) sleep disturbance is severe and potentially related to abnormal light and sound exposure.
Objectives
To assess the prevalence of measures of light and sound disturbance in ICU patient rooms, and whether these could be modified by a sleep-promotion intervention.
Methods
This observational study with a before and after design for a quality improvement initiative surveyed environmental factors in ICU rooms at 01:00 08:00, and12:00. Surveys assessed light usage, television usage, window shade position, and room door/curtain position. Factors were compared before and after an ICU sleeppromotion intervention.
Results
990 (pre-intervention) and 819 (post-intervention) occupied rooms were surveyed. Pre-intervention, the prevalence of night-time factors included: bright lights on (21%), television on (46%), and room door open (94%). Post-intervention, more rooms had all lights off at night (41% v 50%, p=0.04), and fewer rooms had open door curtains (57% v 42%, p=0.001) and window shades (78% v 62%, p=0.002).
Conclusions
Disruptive environmental factors are common in the ICU. Some factors improve with sleep-promotion interventions.
Keywords: sleep disturbance, circadian rhythm, intensive care unit, environmental survey, quality improvement, light
Introduction
Sleep disruption during intensive care unit (ICU) admission is severe. Patients commonly endorse that their sleep quality in the ICU is significantly worse than at home.1–3 Studies using polysomnography (PSG) demonstrate that ICU patients experience diminished total sleep time, sleep fragmentation, decreased rapid-eye movement sleep, and increased daytime sleep.4–6 Sleep deprivation has been linked with poor pulmonary outcomes,7,8 abnormal immune function and inflammation,9 and dysregulated endocrine function.10,11 Furthermore, it is hypothesized that sleep disruption contributes to ICU delirium, which is associated with longer hospital stays, prolonged cognitive impairment, and increased mortality.4,12–14 Sleep disruption occurring during critical illness may even persist after discharge requiring months to normalize.15–17 Thus, it is essential to identify and target underlying causes of ICU sleep loss to help optimize outcomes of critical illness.
Sleep disruption in ICU patients likely derives from a combination of chronic sleep dysfunction, acute critical illness, medical interventions, pain, anxiety, and the ICU environment itself. The ICU environment has high sound levels, frequent care interactions, and abnormal light patterns, all of which disturb sleep.18 With respect to sound, patients and staff commonly describe noise as one of the most disruptive factors to sleep in the ICU,1–3,19,20 and studies have documented that background noise levels in the ICU are often significantly higher than those recommended by the U.S.
Environmental Protection Agency for hospitals.2,21–24 A study combining PSG with sound and video recording in ICU rooms found that 20% of arousals/awakenings in ICU patients were identifiably the result of noise peaks25. There is also a high frequency of night-time patient care interactions occurring in the ICU, which can increase sound and light and oftentimes directly wake patients or cause them anxiety or pain. Tamburri et al retrospectively assessed the medical records of 50 critically ill patients across 4 ICUs, and found that patients experienced an average of 42.6 night-time care interactions between 19:00 and 07:00, with only 6% of the 147 patient-nights assessed having a 2hour period without interruption.26 Notably, routine daily baths were performed between 2AM and 5AM in 61% of nights.26 In another prospective study of 200 patients across 5 ICU types, nurses reported a mean of up to 12 nocturnal care interactions at night, with SICU and MICU nurses stating that 21% and 20% of their nighttime interactions with patients, respectively, could have been safely omitted27. Though light data is more limited, Elliot et al 2014 recorded ICU patient room light levels over 24-hour periods and found that daytime illuminance levels were too dim to promote proper circadian function (median 74 lux).2 A similarly low average light intensity (79.7 lux) during daytime hours was observed in Gehlbach et al 2012 in rooms of sedated mechanically ventilated patients.28
Given the importance of light, sound, and night-time care interactions to sleep, clinical practice guidelines recommend that ICUs optimize light and other environmental factors to best promote patient sleep cycles and limit the risk of delirium.13 Nonetheless, there exists a significant gap between provider awareness of these issues and actual daily clinical practice. In an international survey of 1,223 critical care providers, 75% rated their patient’s sleep as “poor” or worse, and a similar majority of respondents felt that poor ICU sleep was likely associated with worse outcomes such as delirium, longer length of stay, and prolonged mechanical ventilation.29 Only 32% of respondents, however, reported that a sleep-promoting protocol was in place in their ICU.
Our objective was to determine how commonly our medical ICU (MICU) staff optimized the patient environment for sleep before and after a quality improvement sleep promotion intervention. To assess this, we used a survey of environmental factors influencing light, sound and other potential disturbance in patient rooms. We particularly focused on measuring simple patterns of behavior that affect environmental light exposure and sound disturbance. These include the number and types of lights kept on in patient rooms, how often the window shade is kept open, the presence of television light and sound, and how often the door and door curtains are kept open. We performed environmental surveys both before and after a unit-wide sleep promotion intervention. Overall, we hypothesize that there are highly prevalent, modifiable patterns of behaviors that could be leveraged to improve patient sleep and circadian rhythmicity in the ICU.
Methods
Study Site and Subjects
This was an observational study with a before and after design for a quality improvement initiative conducted in the MICU of a 1,500-bed academic tertiary hospital in the northeast United States. Patients are most commonly admitted for acute respiratory failure and sepsis. The unit is rectangular with 38 private patient rooms located on two adjacent floors. Each room has 3 solid walls, a clear sliding glass door with a curtain leading into the hallway, and a single exterior window. Rooms are equipped with 5 to 6 lights of varying intensity including night lights, general room lighting, and procedure lighting, as well as a television and window shade. Rooms were surveyed by direct observation from just outside the room by investigators (MA, CP, FM, MK). During a survey test period which included full surveys of more than fifty rooms, there was perfect agreement between investigators for all variables. Room surveys were conducted in two periods—August to November 2016 before, and April to May 2017 after our quality improvement intervention. This study was judged to be consistent with quality improvement and exempted from IRB oversight.
Survey Tool
Utilizing the Qualtrics (www.qualtrics.com) survey tool, investigators tallied factors at three specified times: morning (08:00), noon (12:00) and night (01:00). Surveys included observation of all patient-occupied rooms and within one hour of the designated times. Each room survey included the number and identity of lights on, whether the television picture and television sound was on, the window shade level, whether the room door was open, whether the door curtain was open, and if the patient was mechanically ventilated. No identifying patient data was collected.
Non-pharmacologic Sleep Promotion Intervention
From February to April 2017, our MICU staff implemented a non-pharmacologic, multidisciplinary, clustered care sleep promotion project, termed “Naptime.” The focus of this intervention was to promote a period of consolidated rest between 00:00 to 04:00. This protocol was developed in collaboration with a wide group of MICU stakeholders, including physicians, nurses, pharmacists, patient care assistants, and respiratory therapists. The protocol was piloted and revised with individual patients prior to unitwide implementation. The details of this protocol are published elsewhere.30 Briefly, the goal was 1) to conduct routine care before 00:00 or after 04:00 and 2) to reduce in-room activity, noise, and light between 00:00 and 04:00. The protocol emphasized rescheduling of routine medications, labs, imaging, vital signs, invasive monitoring, and skin/wound care outside of the rest period. Bedside care that had to be delivered during the 00:00 to 04:00 rest period was clustered to keep the number of room entrances to a minimum. Relevant to our survey, the Naptime protocol includes suggestions to turn off lights and televisions and to close window shades, room curtains and room doors during the rest period. The protocol also suggests opening window shades and keeping the room light during the daytime.
Statistical Analysis
Measures of room disturbance are reported as simple proportions, based on the number of rooms demonstrating a given component of disturbance at the indicated time of day. Chi-squared analysis was used to compare proportions of disturbance measures between pre- and post-intervention periods and to determine any effects of the Naptime intervention on disturbance factors. Using ventilator status as a crude measure of illness severity, the pre- and post-intervention changes in disturbance measures were stratified by mechanical ventilation to assess for confounding or effect modification.
For these analyses, the “window shade open” disturbance was defined as the shade being at least 50% open. The “door open” disturbance was defined as the room door being open enough to allow someone to walk unimpeded into the room. The “door curtain open” disturbance was defined as the room door curtain being more than halfway open. The “bright lights on” disturbance was defined as a room having either the procedure light or headboard light on—the two objectively brightest lights in the room.
Results
Room Characteristics
There were 990 instances of room observation during the pre-intervention period, and 819 instances of room observation during the post-intervention period. In the preintervention period, 414 observations were made in the morning, 341 at noon, and 235 at night. In the post-intervention period, 257 observations were made in the morning, 308 at noon, and 254 at night. 42% of pre-intervention and 46% of post-intervention observed rooms included patients who were mechanically ventilated; this was not a statistically significant difference.
Disturbance Factors Related to Room Lighting
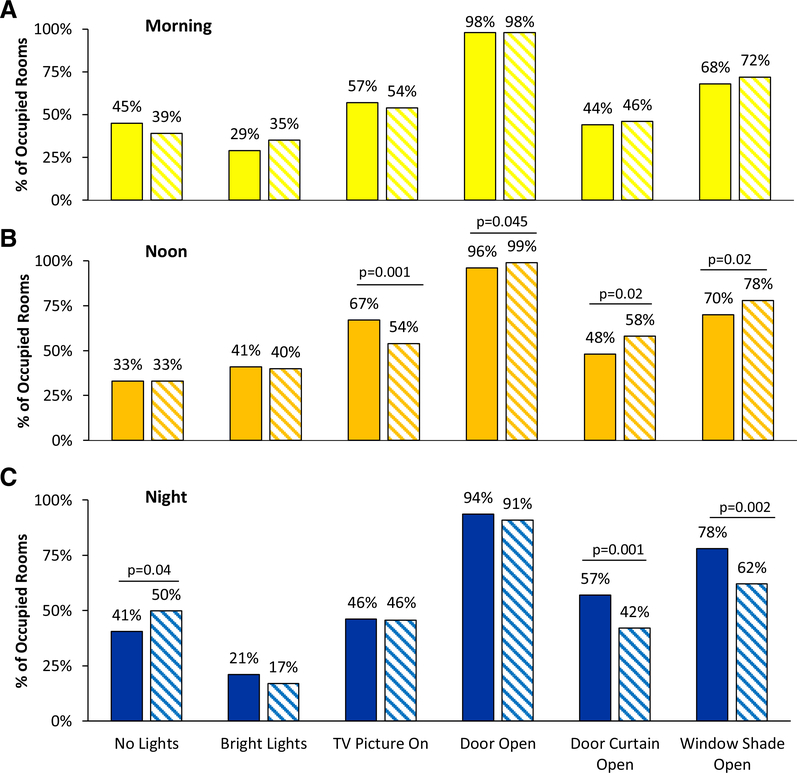
Disturbance factors observed in patient rooms at three different times of day are shown in Table 1 and Figure 1 (both pre- and post-intervention). With respect to room lighting, rooms were observed to have “no lights on” 45% (morning), 33% (noon) and 41% (night) of the time in the pre-intervention period. After the intervention, there was a statistically significant increase in nighttime rooms with “no lights on” (41% vs 50%, p=0.04) (Figure 1). Meanwhile, the brightest lights (either the headboard light or procedure light) were turned “on” in the pre-intervention period 29% (morning), 41% (noon) and 21% (night) of the time. These proportions did not significantly change post-intervention.
Table 1.
Disturbance environmental factors before and after intervention.
| Pre-implementation | Morning (n=414) | Noon (n=341) | Night (n=235) |
| No lights on | 45% | 33% | 41% |
| Bright lights on | 29% | 41% | 21% |
| Television picture on | 57% | 67% | 46% |
| Television sound on | 16% | 26% | 33% |
| Door open | 98% | 96% | 94% |
| Door curtain open | 44% | 48% | 57% |
| Window shade open | 68% | 70% | 78% |
| Post-implementation | Morning (n=257) | Noon (n=308) | Night (n=254) |
| No lights on | 39% | 33% | 50%* |
| Bright lights on | 35% | 40% | 17% |
| Television picture on | 54% | 54%** | 46% |
| Television sound on | 42%** | 44%** | 32% |
| Door open | 98% | 99%* | 91% |
| Door curtain open | 46% | 58%* | 42%** |
| Window shade open | 72% | 78%* | 62%** |
p<0.05 for difference in pre vs. post-intervention at the same time-point
p<0.005 for difference in pre vs. post-intervention at the same time-point
Figure 1.
Disturbance factors before and after the sleep-promotion intervention. Solid bars are pre-intervention; hatched bars are post-intervention. “No lights” indicates that no artificial lights were on in the patient’s room. “Bright lights” indicates use of either the headboard or procedure lights, which are the brightest lights in patient rooms. “Door open” indicates that the room door was open enough to allow someone to walk unimpeded into the room. “Door curtain open” indicates that a room had its curtain more than halfway open. “Window shade open” indicates that a room had its shade at least halfway open.
The television picture was found to be “on” in 57%, 67%, and 46% of rooms at morning, noon, and night respectively in the pre-intervention period. The postintervention noon time-period showed a statistically significant decrease in the number of rooms with television picture “on” (67% vs. 54%, p=0.001).
Window shades were “open” in 68% of rooms in the morning, 70% of rooms at noontime, and 78% of rooms at night before the intervention. Post-intervention, there was a statistically significant increase in the proportion of rooms with window shade “open” at noon-time (70% vs. 78%, p=0.02), while night-time rooms had significantly fewer window shades “open” (78% vs. 62%, p=0.002). There was no change in the proportion of rooms with window shade “open” at the morning time-point (68% vs. 72%, p=0.26).
Factors Related to Sound and Other Disturbance
The room door was found to be “open” in 98% (morning), 96% (noon), and 94% (night) of observations pre-intervention, compared to 98%, 99%, and 91% in the postintervention period. Post-intervention, only the noon time-period showed a significant increase in the proportion of rooms with door kept “open” (p=0.045). The door curtain was “open” in the pre-intervention period at rates of 44% (morning), 48% (noon), and 57% (night). By contrast, the door curtain was “open” in the post-intervention period at rates of 46% (morning), 58% (noon), and 42% (night). The post-intervention noon timeperiod showed a significant increase in the proportion of rooms with door curtains “open” (48% vs. 58%, p=0.02), while the night time-period had significantly fewer rooms with door curtains kept “open” (57% vs. 42%, p=0.001).
Mechanical Ventilation as an Effect Modifier
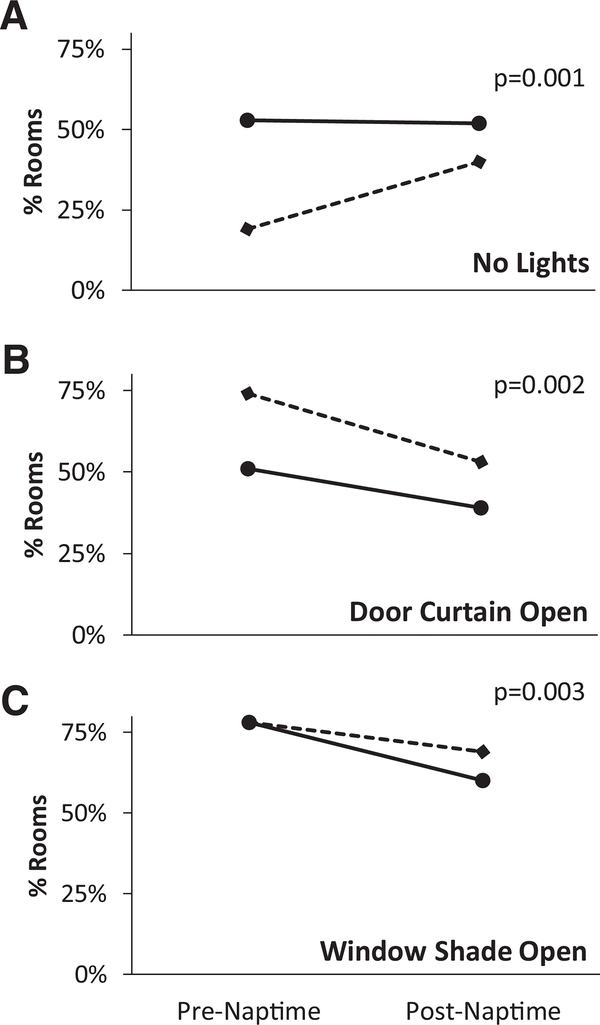
Utilizing mechanical ventilation as a crude estimate of illness severity, we assessed whether illness severity could account for changes in the three environmental factors that showed differences between pre- and post-intervention periods at night— “no lights on,” “door curtain open”, and “window shade open” (Figure 2, Supplementary Table S1 and S2).
Figure 2.
Mechanical ventilation as an influence on changes in night-time disturbance factors. Solid lines indicate non-mechanically ventilated patients; dashed lines indicate mechanically ventilated patients.
In the pre-intervention period, rooms with mechanically ventilated patients were significantly more likely than non-ventilated rooms to have lights on at night (19% of ventilated rooms with no lights on vs. 53% for non-ventilated rooms, p<0.001). This difference in artificial lighting was no longer seen post-intervention (40% vs 52%, p=0.09) (Figure 2A). In other words, mechanically ventilated rooms showed a statistically significant increase in the proportion of rooms with no lights on (19% preintervention and 40% post-intervention, p=0.001), whereas non-ventilated patient rooms did not show a statistically significant change (p=0.82).
With regards to door curtains, mechanically ventilated patients showed a significant decrease in the proportion of night-time rooms with door curtains open (74% pre-intervention vs. 53% post-intervention, p=0.002) (Figure 2B). Non-ventilated patients also showed a decrease in rooms with door curtain open; however, the p-value is marginal (51% pre-intervention vs. 39% post-intervention, p=0.06). For window shade open, on the other hand, only non-ventilated patient rooms showed a statistically significant change after the intervention (78% pre-intervention vs. 60% post-intervention p=0.003) (Figure 2C).
Discussion
Overall, this study observed a high prevalence of environmental disturbance factors. At baseline, during the nighttime observation period, rooms were frequently illuminated with their brightest lights, doors and door curtains were open, and television picture and sound were on. Greater than half the time rooms had some artificial lighting turned on. We also frequently observed daytime behaviors which conflict with proper circadian entrainment by limiting bright light exposure. During the morning and noon time-points, about one-third of rooms had their window shades closed and nearly onethird (noon) to one-half (morning) of rooms had no lights in use. These data suggest that there is a significant opportunity to improve the MICU environment via simple interventions which have the potential to improve patient sleep and circadian rhythms.
After implementation of a unit-wide sleep-promoting protocol in our MICU, we observed several improvements. Rooms were significantly more likely to have all lights off, door curtains closed, and window shades closed at night. We also observed some post-intervention improvement in noon-time lighting factors such as an increased opening of door curtains and window shades, although morning-time observations showed no identifiable changes. Taken together, these data suggest that a multidisciplinary intervention focused on environmental control and clustered care could indeed modify behavior to improve specific environmental parameters. Interestingly, mechanical ventilation status was found to be an effect modifier for room lighting at night. Mechanically-ventilated patient rooms showed a significant improvement in having lights turned off post-intervention, whereas non-mechanically ventilated rooms were not affected by the intervention. One hypothesis is that mechanically ventilated patients are less able to communicate and express a preference regarding their room environment, and thus at baseline are more likely to be inadvertently subjected to lighting conditions that conflict with their preference. Additionally, these patients may have greater illness severity, prompting staff to prefer “lights on” for closer monitoring. It seems plausible that non-ventilated patients, who can communicate, can express a preference over light conditions pre-intervention and thus there was relatively little change made by the intervention. Mechanically ventilated patients may, therefore, represent a particularly vulnerable ICU population for which unit interventions can improve environmental disturbances.
It is unclear to what degree patient preferences influenced environmental disturbance factors observed in our study. A qualitative study of ICU sleep disturbance found that patients and staff had contrasting beliefs. Staff commonly believed that inroom provider interruption, noise, and excessive light were the major environmental factors disrupting sleep, while patients and surrogates described emotions and anxiety as the major deterrents.20 In fact, patients commonly described noise and in-room disturbance as expected aspects of a MICU stay, and that they felt reassured by being closely monitored.20 This is despite multiple studies suggesting that that subjective sleep quality in the ICU is poor and that a variety of environmental factors, including high noise levels, light exposure, and patient care interactions, are playing a role.1–3,16,24–26 These different needs are a potential area for staff and patient education. Patients could be given information regarding the importance of a quiet and dark environment for sleep, allowed to use dim lighting options, and be reassured that there is continuous remote monitoring outside of their room.
Overall, there exists a substantial gap between provider awareness that sleep in the ICU is important to recovery and the low rate of individual or unit-wide sleep promoting practices implemented.20,29 Quality improvement interventions and new care bundles in the ICU can be complicated to implement; they require iteration and integration across multiple stakeholders in an already high-stakes environment.30 Barriers to ICU protocol implementation that have been identified include patient-related barriers, provider-related barriers, protocol-related barriers (such as cumbersome protocol or lack of defined role and responsibilities), and ICU contextual barriers (such as staff turnover and low prioritization of the protocol relative to patient needs).31 Increased workload and safety concerns are common issues with implementing change in the high-acuity ICU environment.32 Our focus herein was to demonstrate that there are simple, safe, modifiable behaviors that can be leveraged to improve the environment for sleep and circadian biology in critically ill patients.
There are several limitations of this study. First, no patient data other than mechanical ventilation was collected, limiting our ability to test for effect modification based on other patient-specific factors. For example, we did not have data on time or date of ICU admission for each patient; patients earlier in their admission may experience more in-room activity necessitating room lights kept on and doors open. Similarly, we did not collect any patient-specific subjective data, and so cannot determine to what extent environmental factors are related to patient preference. Second, several of our measures are potentially subjective with regards to data collection (degree of door curtain and window shade closure), and it is furthermore unclear to what extent these factors would affect patient sleep—nonetheless, we report them here as indicators of provider “intent” towards optimizing the patient environment. Investigators did have perfect agreement on environmental factors during a survey pilot (described above). Finally, we only collected one set of data points approximately one month after the implementation of the sleep promotion intervention, and thus cannot make inferences about the lasting effects of this intervention on behavior, or account for changes in staffing and education of providers over time.
Conclusions
Non-circadian light, sound, and patient care patterns are highly prevalent in the ICU. During the daytime periods (morning and noon, when bright light is needed), less than half of rooms had the brightest lights on, and nearly one-third had the window shade closed. Rooms at night commonly demonstrated factors such as bright lights kept on, televisions on, window shades open, room doors open, and room curtains open. After a sleep-promoting quality improvement intervention, there was a significant nighttime improvement in some measures of disturbance, including lights turned off, door curtains closed, and window shades closed. Future work is needed to optimize ICU workflow and to reduce environmental factors that disturb patient sleep and circadian rhythmicity.
Supplementary Material
Acknowledgements:
We would like to thank the nurses and other Yale New Haven Hospital MICU staff for their participation in this study and their engagement in our MICU sleep-promotion intervention.
Funding:
MTA was supported by the Yale University School of Medicine Medical Research Fellowship, the William U. Gardner Memorial Student Research Fellowship, and the National Institutes of Health-NHLBI Medical Student Research Fellowship. Research reported in this publication was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health under Award Number T35HL007649.
MAP was supported by funds from the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
MPK was supported by CTSA grant number KL2 TR000140 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations
- ICU
intensive care unit
- MICU
medical intensive care unit
- PSG
polysomnography
Footnotes
Declarations of interest/financial disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. American journal of respiratory and critical care medicine. 1999;159(4 Pt 1):1155–1162. [DOI] [PubMed] [Google Scholar]
- 2.Elliott R, Rai T, McKinley S. Factors affecting sleep in the critically ill: an observational study. J Crit Care. 2014;29(5):859–863. [DOI] [PubMed] [Google Scholar]
- 3.Little A, Ethier C, Ayas N, Thanachayanont T, Jiang D, Mehta S. A patient survey of sleep quality in the Intensive Care Unit. Minerva anestesiologica. 2012;78(4):406–414. [PubMed] [Google Scholar]
- 4.Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma. 2007;63(6):1210–1214. [DOI] [PubMed] [Google Scholar]
- 6.Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43(5):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):477–485. [DOI] [PubMed] [Google Scholar]
- 8.Chen HI, Tang YR. Sleep loss impairs inspiratory muscle endurance. The American review of respiratory disease. 1989;140(4):907–909. [DOI] [PubMed] [Google Scholar]
- 9.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16(2):137–149. [DOI] [PubMed] [Google Scholar]
- 10.Schussler P, Uhr M, Ising M, et al. Nocturnal ghrelin, ACTH, GH and cortisol secretion after sleep deprivation in humans. Psychoneuroendocrinology. 2006;31(8):915–923. [DOI] [PubMed] [Google Scholar]
- 11.Schmid SM, Hallschmid M, Jauch-Chara K, Bandorf N, Born J, Schultes B. Sleep loss alters basal metabolic hormone secretion and modulates the dynamic counterregulatory response to hypoglycemia. The Journal of clinical endocrinology and metabolism. 2007;92(8):3044–3051. [DOI] [PubMed] [Google Scholar]
- 12.Oldham MA, Lee HB, Desan PH. Circadian Rhythm Disruption in the Critically Ill: An Opportunity for Improving Outcomes. Crit Care Med. 2016;44(1):207–217. [DOI] [PubMed] [Google Scholar]
- 13.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. [DOI] [PubMed] [Google Scholar]
- 14.Trompeo AC, Vidi Y, Locane MD, et al. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva anestesiologica. 2011;77(6):604–612. [PubMed] [Google Scholar]
- 15.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep patterns in patients with acute coronary syndromes. Sleep Med. 2010;11(2):149–153. [DOI] [PubMed] [Google Scholar]
- 16.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep disordered breathing in patients with acute coronary syndromes. J Clin Sleep Med. 2012;8(1):21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman MT, Knauert MP, Pisani MA. Sleep Disturbance after Hospitalization and Critical Illness: A Systematic Review. Ann Am Thorac Soc. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knauert MP, Haspel JA, Pisani MA. Sleep Loss and Circadian Rhythm Disruption in the Intensive Care Unit. Clin Chest Med. 2015;36(3):419–429. [DOI] [PubMed] [Google Scholar]
- 19.Hilton BA. Quantity and quality of patients’ sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs. 1976;1(6):453–468. [DOI] [PubMed] [Google Scholar]
- 20.Ding Q, Redeker NS, Pisani MA, Yaggi HK, Knauert MP. Factors Influencing Patients’ Sleep in the Intensive Care Unit: Perceptions of Patients and Clinical Staff. Am J Crit Care. 2017;26(4):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer TJ, Eveloff SE, Bauer MS, Schwartz WA, Hill NS, Millman RP. Adverse environmental conditions in the respiratory and medical ICU settings. Chest. 1994;105(4):1211–1216. [DOI] [PubMed] [Google Scholar]
- 22.Redding JS, Hargest TS, Minsky SH. How noisy is intensive care? Crit Care Med. 1977;5(6):275–276. [DOI] [PubMed] [Google Scholar]
- 23.Knauert M, Jeon S, Murphy TE, Yaggi HK, Pisani MA, Redeker NS. Comparing average levels and peak occurrence of overnight sound in the medical intensive care unit on A-weighted and C-weighted decibel scales. J Crit Care. 2016;36:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topf M, Bookman M, Arand D. Effects of critical care unit noise on the subjective quality of sleep. J Adv Nurs. 1996;24(3):545–551. [DOI] [PubMed] [Google Scholar]
- 25.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167(5):708–715. [DOI] [PubMed] [Google Scholar]
- 26.Tamburri LM, DiBrienza R, Zozula R, Redeker NS. Nocturnal care interactions with patients in critical care units. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2004;13(2):102–112; quiz 114–105. [PubMed] [Google Scholar]
- 27.Le A, Friese RS, Hsu CH, Wynne JL, Rhee P, O’Keeffe T. Sleep disruptions and nocturnal nursing interactions in the intensive care unit. J Surg Res. 2012;177(2):310–314. [DOI] [PubMed] [Google Scholar]
- 28.Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):11051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamdar BB, Knauert MP, Jones SF, et al. Perceptions and Practices Regarding Sleep in the Intensive Care Unit. A Survey of 1,223 Critical Care Providers. Ann Am Thorac Soc. 2016;13(8):1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knauert MP, Redeker NS, Yaggi HK, Bennick M, Pisani MA. Creating Naptime: An Overnight, Nonpharmacologic Intensive Care Unit Sleep Promotion Protocol. Journal of Patient Experience. 2017;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa DK, White M, Ginier E, et al. Identifying barriers to delivering the ABCDE bundle to minimize adverse outcomes for mechanically ventilated patients: A systematic review. Chest. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eakin MN, Ugbah L, Arnautovic T, Parker AM, Needham DM. Implementing and sustaining an early rehabilitation program in a medical intensive care unit: A qualitative analysis. Journal of critical care. 2015;30(4):698–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.