Summary
Background
Adverse skin reactions to skin care products have been increasing in recent years. However, these reactions have not been characterized well to date.
Objective
To describe symptoms, clinical signs and frequency of adverse cutaneous reactions to skin care products on the face in males vs. females of various ages.
Patients and Methods
All outpatients diagnosed with adverse cutaneous reactions to skin care products on the face examined by dermatologists at Dermatology Hospital of Southern Medical University between November 1, 2016 and October 31, 2017, employing a questionnaire and interviewed by a dermatologist, were eligible. The association of adverse cutaneous reactions with age and gender was analyzed.
Results
A total of 433 outpatients, accounting for 0.12% of total outpatients, were assessed. Of these, 223 patients, including 204 females and 19 males, aged 4 to 75 years old, were eventually diagnosed with adverse reactions to skin care products on the face. Eighty-two per cent of patients experienced pruritus, while 80% showed erythema, and 48% visible swelling. The incidences of both xerosis and edema correlated positively with age, while acne-like lesions were negatively associated with age, but not with gender.
Conclusions
Our results indicate that pruritus, xerosis and erythema are common adverse cutaneous reactions to facial skin care products. These reactions vary with age, but not gender. Vigorous safety testing should precede marketing of skin care products.
Keywords: Skin care products, Inflammation, Adverse reactions, Age, Gender
1. Introduction
The use of skin care products is becoming more popular in China because of increased attention to beauty and health, as well as improvements in economic conditions. Meanwhile, the incidence of adverse cutaneous reactions to skin care products is also increasing, particularly over the last five years (1, 2). Moreover, recent studies indicate that even branded skin care products can cause severe adverse cutaneous reactions (3). While eczematous reactions account for 90% of these adverse reactions (4), a link between skin care products and an increased risk of breast cancer has been observed (5). The types of adverse cutaneous reactions to skin care products vary with the products. For example, certain skin care products can cause stinging and erythema within 30 min after topical application (6), while long-term use of anti-aging products that contain retinol and ascorbic acid can induce facial xerosis (7). Use of skin whitening formulations, such as hydroquinone-containing products, can induce acne-like lesions, eczema and irritant contact dermatitis (8–10). Moreover, hydroquinone–containing products can induce ochronosis mostly in patients with dark skin (11–13). Likewise, induction of burning and erythema by other depigmenting agents, such as azelaic acid and kojic acid, has also been documented (14,15). Even skin care product-induced toxic epidermal necrolysis has been reported (16). Finally, recent studies have demonstrated that certain infant skin care products can compromise epidermal functions, including epidermal permeability barrier, stratum corneum hydration and stratum corneum pH in murine skin (17), while potentially inducing contact dermatitis in humans (18).
Notably, evidence on adverse reactions to skin care products is from various case reports. Accordingly, the clinical characteristics of adverse cutaneous reactions to skin care products, particularly on the face, have not yet been systematically characterized. In addition, it is unknown whether adverse cutaneous reactions to skin care products vary with age and gender. In the present study, we characterize the clinical and demographic features of 223 Chinese patients with adverse skin facial cosmetics.
2. Patients and Methods
All outpatients presenting with skin disorders were evaluated by dermatologists at the Department of Aesthetics, Dermatology Hospital of Southern Medical University, Guangzhou, China between November 1, 2016 and October 31, 2017. The diagnosis of adverse cutaneous reactions to skin care (cosmetic) products was made according to the national “General Guideline: Diagnostic Criteria and Principles of Management of Skin Diseases Induced by Cosmetics”, established by State Health Department, China (GB17149.1-7-1997). Patients with above diagnosis were further interviewed and examined in person by dermatologists, according a standardized questionnaire. GraphPad Prism 4 software was used for all statistical analyses. Two-sided Chi-square test was used to determine significance. Data are expressed as mean ± standard error of the mean (SEM).
3. Results
A total of 367,679 outpatients attended the clinics of the Dermatology Hospital of Southern Medical University during the one-year study period. Over 0.1% of these outpatients (n=433) were diagnosed with adverse cutaneous reactions to skin care and/or cosmetic products. Out of these 433 patients, 223 (51%) presented with adverse cutaneous reactions to skin care products on the face (Online supplemental Fig. 1). The demographic data of these 223 patients are detailed in Table 1.
Table 1.
Demographics of Subjects
| Gender | N | Age Range (yr) | Mean ± SEM |
|---|---|---|---|
| Females | 204 | 4~75 | 31.21 ± 0.76 |
| Males | 19 | 18~65 | 36.05 ± 3.38 |
| Total | 223 | 4~75 | 31.62 ± 0.75 |
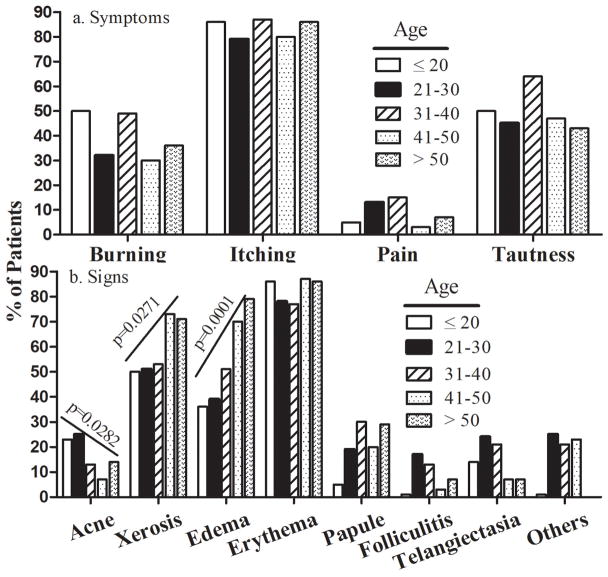
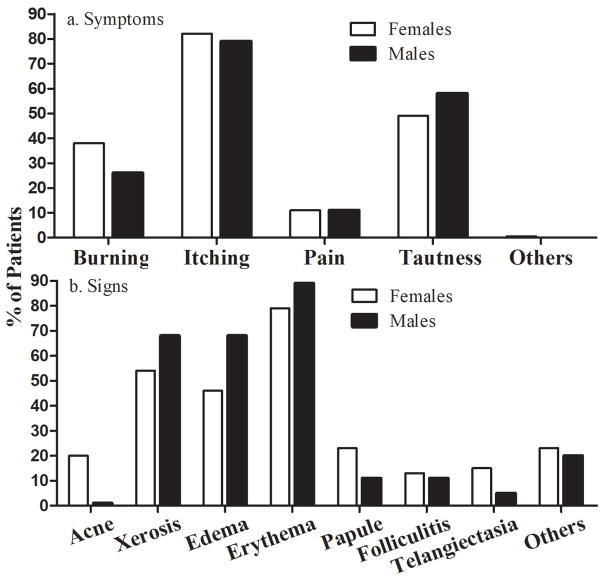
Most patients (82%) experienced pruritus (Table 2). Approximately 50% of patients suffered from a feeling of tautness. Neither age nor gender were associated with cutaneous symptoms induced by skin care products (Tables 2 and 3, Fig. 2a and 3a).
Table 2.
Clinical Symptoms Are not Age-Dependent
| Age | Burning | Itching | Pain | Tautness |
|---|---|---|---|---|
| ≤20 (n=22) | 11 (50%) | 19 (86%) | 1(5%) | 11 (50%) |
| 21–30 (n=110) | 35 (32%) | 87 (79%) | 14 (13%) | 49 (45%) |
| 31–40 (n=47) | 23 (49%) | 41 (87%) | 7 (15%) | 30 (64%) |
| 41–50 (n=30) | 9 (30%) | 24 (80%) | 1 (3%) | 14 (47%) |
| >50 (n=14) | 5 (36%) | 12 (86%) | 1 (7%) | 6 (43%) |
| Overall 4–75 (N=223) | 83 (37%) | 183 (82%) | 24 (11%) | 110(49%) |
| P values | NS | NS | NS | NS |
Chi-square test
Table 3.
Clinical signs and symptoms do not vary with gender
| Gender | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Signs | Acne | Dry | Edema | Erythema | Papule | Folliculitis | Telangiectasia | Others | Average number of signs/subject |
|
|
|||||||||
| Females (n=204) | 40 (20%) | 111 (54%) | 94 (46%) | 162 (79%) | 44 (23%) | 27 (13%) | 30 (15%) | 44 (23%) | 2.16 ± 0.06 |
| Males (n=19) | 3 (1%) | 13 (68%) | 13 (68%) | 17 (89%) | 2 (11%) | 2 (11%) | 1 (5%) | 4 (20%) | 2.26 ± 0.18 |
| P values | NS | NS | 0.0623 | NS | NS | NS | NS | NS | NS |
| Symptoms | Burning | Itching | Pain | Tautness | Others | Average number of symptoms/subject | |||
|
|
|||||||||
| Females (n=204) | 78 (38%) | 168 (82%) | 22 (11%) | 99 (49%) | 1 (0.5%) | 2.35 ± 0.05 | |||
| Males (n=19) | 5 (26%) | 15 (79%) | 2 (11%) | 11 (58%) | 0 | 2.42 ± 0.21 | |||
| P values | NS | NS | NS | NS | NS | NS |
Chi-square test
Figure 2.
Correlation of Clinical Signs and Patients’ Symptoms with Age. (A) Correlation of patients’ symptoms with Age. (B) Correlation of clinical signs with Age. Number of subjects in each group is detailed in the Tables, and significances are indicated in the Figure. Two-sided Chi-square test was used to determine heterogeneity between groups. Chi-square test was used to analyze the trend.
Figure 3.
Correlation of Clinical Signs and Patients’ Symptoms with Age. (A) Comparison of patients’ symptoms between females and males. (B) Comparison of clinical signs between females and males. Two-sided Chi-square test was used to determine heterogeneity between groups. Number of subjects in each group is detailed in the Tables.
Concerning clinical signs, 179 patients (80%) showed erythema and 48% edema (Table 4). Other clinical features included vesicles, plaque formation and both hyper- and hypopigmentation, together accounting for ≈20%. Occurrence both of edema and xerosis was associated with age (P=.0001), while acne-like lesion were more common in young patients (P=.028). Papules tended to increase with age (P=0.087), while gender was not associated (Fig. 3b).
Table 4.
Clinical signs vary with age
| Age | Acne-like | Xerosis | Edema | Erythema | Papule | Folliculitis | Telangiectasia | Others |
|---|---|---|---|---|---|---|---|---|
| ≤20 (n=22) | 5 (23%) | 11 (50%) | 8 (36%) | 19 (86%) | 1 (5%) | 2 (1%) | 3 (14%) | 2 (1%) |
| 21–30 (n=110) | 28 (25%) | 56 (51%) | 43 (39%) | 86 (78%) | 21 (19%) | 19 (17%) | 15 (24%) | 28 (25%) |
| 31–40 (n=47) | 6 (13%) | 25 (53%) | 24 (51%) | 36 (77%) | 14 (30%) | 6 (13%) | 10 (21%) | 10 (21%) |
| 41–50 (n=30) | 2 (7%) | 22 (73%) | 21 (70%) | 26 (87%) | 6 (20%) | 1 (3%) | 2 (7%) | 7 (23%) |
| >50 (n=14) | 2 (14%) | 10 (71%) | 11 (79%) | 12 (86%) | 4 (29%) | 1 (7%) | 1 (7%) | 0 |
| Overall 4–75 (N=223) | 43 (19%) | 124 (56%) | 107 (48%) | 179 (80%) | 46 (21%) | 29 (13%) | 31 (14%) | 45 (20%) |
| P values | NS | NS | .003 | NS | NS | NS | NS | NS |
| P for trend | .028 | .027 | .0001 | .087 |
Chi-square test
Over 40% of patients (95 cases) had ≥ 3 different types of symptoms, and 45 subjects (20%) developed ≥ 3 different types of lesions. Analysis showed subjects’ age correlated with neither the number of different symptoms nor different types of lesions. In contrast, the number of different types of patients’ symptoms correlated positively with the number of different types of lesions (r2=0.023, P=.024). Hence, patients with a higher number of different types of lesions also reported more symptoms. With regard to the causative product brands, 53% of the products were domestic brands, while 46% were overseas brands.
4. Discussion
Although all skin care products must pass safety tests before being deployed in the market, incidences of adverse reactions appear to be increasing recently (1) and may have been underestimated (19). In certain regions, the incidence can be as high as 14% (20). Although we cannot specify the incidence of adverse cutaneous reactions to skin care products in the general population of Guangzhou city, China, the 0.1 % frequency among all outpatients in a general dermatology clinic is high enough to point attention to the safety of skin care products. Impaired skin condition or certain co-existent dermatoses, such as atopic dermatitis, could contribute to the development of adverse reactions (21, 22), but ingredients in skin care products appear primarily responsible for induction of these reactions. Although we did not further investigate patients by patch testing to identify the responsible ingredients, certain substances, such as stearic acid, ceteareth 20, PEG-40 castor oil and PEG-100 stearate, can induce inflammation and development of clinical signs and symptoms, particularly at higher concentrations (23–26). Also some naturally derived ingredients, such as balsams, or aloe and cucumber, may also cause adverse cutaneous reactions (27, 28). Evidently, preservatives are also on the list of potentially harmful ingredients in skin care products (29–32) which are now considered an import case of contact dermatitis (19). This evidence, taken together, suggests that skin care products are not necessarily safe, and special caution should be taken when using them, particularly in individuals with compromised skin conditions, such as atopic, aged, lightly pigmented or glucocorticoid-treated skin.
In the present study, we show that over 80% of affected patients experienced itching, and 56% had xerosis. Both erythema and edema, signs of contact dermatitis, were the major clinical features of harmful reactions induced by skin care products. We could not differentiate between allergic or irritant reactions, but these could account for equal portions of dermatitis (33). Consistent with previous findings (20), we also observed that a substantial portion of patients had acne (19%) and telangiectasia (14%), which likely due to long term usage of products containing steroids because certain common skin care products contain steroids (34–37), which can cause acne (38). It appeared that more females had acne than males, but the difference was not significant, possibly due to small number of male subjects. Interestingly, our results demonstrate that age, but not gender, is associated with the clinical signs of adverse cutaneous reactions to skin care products. It is no surprise that more patients over 40 years of age developed xerosis because sebum content, which regulates stratum corneum hydration levels, on the face begins to decline at ≈40 years of age (39–41). Moreover, aged epidermis displays a reduction in expression levels of filaggrin (42), which is degraded into urocanic acid, a moisturizer in the skin. Furthermore, reduced sweat gland activity and amino acid content in the stratum corneum can also contribute to dryness in the aged (43,44). Thus, these alterations in cutaneous function can make skin more vulnerable to develop xerosis if challenged by topical agents. The increased occurrence of edema in older patients is likely due to age-dependent increased vascular permeability (45, 46). Nevertheless, the present study demonstrates that age is a determinant of clinical signs of adverse cutaneous reactions to skin care products. It is worthwhile noting that the proportion of adverse cutaneous reactions induced by domestic branded skin care products was not greater than that induced by overseas branded although the latter costs more, suggesting pricing is not associated with safety.
In summary, the present study demonstrates that the prevalence of adverse cutaneous reactions to skin care products on the face is relatively high, and that age is a contributor to the risk of these reactions. More effective and vigorous safety testing in both normal and diseased skin should be undertaken before skin care products are marketed to the general public.
Supplementary Material
Flowchart of Patient Enrollment
Acknowledgments
This work was supported in part by the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the National Institutes of Health (2R01AR061106-06A1), administered by the Northern California Institute for Research and Education, with additional resources provided by The Veterans Affairs Medical Center, San Francisco, California. This content is solely the responsibility of the authors and does not necessarily represent the official views of either the National Institutes of Health or the Department of Veterans Affairs.
References
- 1.Kwa M, Welty LJ, Xu S. Adverse Events Reported to the US Food and Drug Administration for Cosmetics and Personal Care Products. JAMA Intern Med. 2017;177:1202–1204. doi: 10.1001/jamainternmed.2017.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornell E, Kwa M, Paller AS, Xu S. Adverse events reported to the Food and Drug Administration from 2004 to 2016 for cosmetics and personal care products marketed to newborns and infants. Pediatr Dermatol. 2018;35:225–229. doi: 10.1111/pde.13419. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Hu L, Elias PM, Man MQ. Skin care products can aggravate epidermal function: studies in a murine model suggest a pathogenic role in sensitive skin. Contact Dermatitis. 2018;78:151–158. doi: 10.1111/cod.12909. [DOI] [PubMed] [Google Scholar]
- 4.Berne B, Boström A, Grahnén AF, Tammela M. Adverse effects of cosmetics and toiletries reported to the Swedish Medical Products Agency 1989–1994. Contact Dermatitis. 1996;34:359–62. doi: 10.1111/j.1600-0536.1996.tb02223.x. [DOI] [PubMed] [Google Scholar]
- 5.Taylor KW, Troester MA, Herring AH, et al. Associations between Personal Care Product Use Patterns and Breast Cancer Risk among White and Black Women in the Sister Study. Environ Health Perspect. 2018;126:027011. doi: 10.1289/EHP1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draelos ZD. Differences in Cutaneous Irritation of Five Commonly Used Topical Products. J Drugs Dermatol. 2016;15:870–3. [PubMed] [Google Scholar]
- 7.Herndon JH, Jr, Jiang LI, Kononov T, Fox T. An Open Label Clinical Trial to Evaluate the Efficacy and Tolerance of a Retinol and Vitamin C Facial Regimen in Women with Mild-to-Moderate Hyperpigmentation and Photodamaged Facial Skin. J Drugs Dermatol. 2016;15:476–82. [PubMed] [Google Scholar]
- 8.Kombaté K, Mouhari-Toure A, Saka B, et al. Acne and skin bleaching in Lomé, Togo. Int J Dermatol. 2012;51:S27–9. 30–2. doi: 10.1111/j.1365-4632.2012.05560.x. [DOI] [PubMed] [Google Scholar]
- 9.Prignano F, Ortonne JP, Buggiani G, Lotti T. Therapeutical approaches in melasma. Dermatol Clin. 2007;25:337–42. viii. doi: 10.1016/j.det.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Mahé A, Ly F, Aymard G, Dangou JM. Skin diseases associated with the cosmetic use of bleaching products in women from Dakar, Senegal. Br J Dermatol. 2003;148:493–500. doi: 10.1046/j.1365-2133.2003.05161.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagler A, Hale CS, Meehan SA, Leger M. Exogenous ochronosis. Dermatol Online J. 2014:20. pii: 13030/qt0v91k51s. [PubMed] [Google Scholar]
- 12.Ribas J, Schettini AP, de Cavalcante MS. Exogenous ochronosis hydroquinone induced: a report of four cases. An Bras Dermatol. 2010;85:699–703. doi: 10.1590/s0365-05962010000500017. [DOI] [PubMed] [Google Scholar]
- 13.Martins VM, Sousa AR, de Portela NC, et al. Exogenous ochronosis: case report and literature review. An Bras Dermatol. 2012;87:633–6. doi: 10.1590/s0365-05962012000400021. [DOI] [PubMed] [Google Scholar]
- 14.Lynde CB, Kraft JN, Lynde CW. Topical treatments for melasma and postinflammatory hyperpigmentation. Skin Therapy Lett. 2006;11:1–6. [PubMed] [Google Scholar]
- 15.Nakagawa M, Kawai K, Kawai K. Contact allergy to kojic acid in skin care products. Contact Dermatitis. 1995;32:9–13. doi: 10.1111/j.1600-0536.1995.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 16.Kokcam I. Toxic epidermal necrolysis probably due to cosmetic cream: a case report. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:39–42. [PubMed] [Google Scholar]
- 17.Man MQ, Sun R, Man G, et al. Commonly Employed African Neonatal Skin Care Products Compromise Epidermal Function in Mice. Pediatr Dermatol. 2016;33:493–500. doi: 10.1111/pde.12901. [DOI] [PubMed] [Google Scholar]
- 18.Galzote C, Thomas M, Sachdev M. Assessment of Irritation and Sensitization Potential of Eight Baby Skin Care Products. J Drugs Dermatol. 2016;15:1244–1248. [PubMed] [Google Scholar]
- 19.Lindberg M, Tammela M, Boström A, et al. Are adverse skin reactions to cosmetics underestimated in the clinical assessment of contact dermatitis? A prospective study among 1075 patients attending Swedish patch test clinics. Acta Derm Venereol. 2004;84:291–5. doi: 10.1080/00015550410025921. [DOI] [PubMed] [Google Scholar]
- 20.Bilal AI, Tilahun Z, Osman ED, et al. Cosmetics Use-Related Adverse Events and Determinants among Jigjiga Town Residents, Eastern Ethiopia. Dermatol Ther (Heidelb) 2017;7:143–153. doi: 10.1007/s13555-016-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misery L, Boussetta S, Nocera T, et al. Sensitive skin in Europe. J Eur Acad Dermatol Venereol. 2009;23:376–81. doi: 10.1111/j.1468-3083.2008.03037.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu F, Yan S, Wu M, et al. Self-declared sensitive skin in China: a community-based study in three top metropolises. J Eur Acad Dermatol Venereol. 2013;27:370–5. doi: 10.1111/j.1468-3083.2012.04648.x. [DOI] [PubMed] [Google Scholar]
- 23.Miao H, Chen L, Hao L, et al. Stearic acid induces proinflammatory cytokine production partly through activation of lactate-HIF1α pathway in chondrocytes. Sci Rep. 2015;5:13092. doi: 10.1038/srep13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson EK, Hill AA, Hasty AH. Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1687–95. doi: 10.1161/ATVBAHA.112.250142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.http://www.healthinsurancequotes.org/7-most-harmful-body-pollutants-in-your-beauty-products/ obtained on 04/06/2018
- 26.http://chemicaloftheday.squarespace.com/todays-chemical/2009/8/3/peg-40-castor-oil.html (obtained on 04/06/2018)
- 27.Antignac E, Nohynek GJ, Re T, et al. Safety of botanical ingredients in personal care products/cosmetics. Food Chem Toxicol. 2011;49:324–41. doi: 10.1016/j.fct.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Hasan T, Rantanen T, Alanko K, et al. Patch test reactions to cosmetic allergens in 1995–1997 and 2000–2002 in Finland--a multicentre study. Contact Dermatitis. 2005;53:40–5. doi: 10.1111/j.0105-1873.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 29.Nohynek GJ, Antignac E, Re T, Toutain H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010;243:239–59. doi: 10.1016/j.taap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Towle KM, Drechsel DA, Warshaw EM, et al. A Quantitative Risk Assessment of the Skin Sensitization Induction Potential of the Kathon CG Preservative in Rinse-off and Leave-on Personal Care and Cosmetic Products. Dermatitis. 2018 Mar 22; doi: 10.1097/DER.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 31.Ezendam J, Bokkers BGH, Bil W, Delmaar JE. Skin sensitisation quantitative risk assessment (QRA) based on aggregate dermal exposure to methylisothiazolinone in personal care and household cleaning products. Food Chem Toxicol. 2018;112:242–250. doi: 10.1016/j.fct.2017.12.054. [DOI] [PubMed] [Google Scholar]
- 32.Timm-Knudson VL, Johnson JS, Ortiz KJ, Yiannias JA. Allergic contact dermatitis to preservatives. Dermatol Nurs. 2006;18:130–6. [PubMed] [Google Scholar]
- 33.Berne B, Tammela M, Färm G, et al. Can the reporting of adverse skin reactions to cosmetics be improved? A prospective clinical study using a structured protocol. Contact Dermatitis. 2008;58:223–7. doi: 10.1111/j.1600-0536.2007.01309.x. [DOI] [PubMed] [Google Scholar]
- 34.http://www.cirs-reach.com/news-and-articles/Result-Summary-of-Random-Sample-Check-for-Cosmetics-on-the-Market-2017.html (obtained on April 7, 2018)
- 35.Goldstein R. http://theconversation.com/time-for-a-reality-check-on-skin-lightening-creams-7770 (obtained on April 7, 2018)
- 36.https://www.forthepeople.com/class-action-lawyers/mario-badescu-skin-cream/ (obtained on April 7, 2018)
- 37.https://www.gov.uk/drug-safety-update/herbal-product-intensive-body-lotion-with-aloe-vera-contains-steroids (obtained on April 7, 2018)
- 38.Liu KJ, Antaya RJ. Midchildhood acne associated with inhaled corticosteroids: report of two cases and review of the literature. Pediatr Dermatol. 2014;31:712–5. doi: 10.1111/pde.12108. [DOI] [PubMed] [Google Scholar]
- 39.Man MQ, Xin SJ, Song SP, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22:190–9. doi: 10.1159/000231524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fluhr JW, Mao-Qiang M, Brown BE, et al. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol. 2003;120:728–37. doi: 10.1046/j.1523-1747.2003.12134.x. [DOI] [PubMed] [Google Scholar]
- 41.Choi EH, Man MQ, Wang F, et al. Is endogenous glycerol a determinant of stratum corneum hydration in humans? J Invest Dermatol. 2005;125:288–93. doi: 10.1111/j.0022-202X.2005.23799.x. [DOI] [PubMed] [Google Scholar]
- 42.Rinnerthaler M, Duschl J, Steinbacher P. Age-related changes in the composition of the cornified envelope in human skin. Exp Dermatol. 2013;22:329–35. doi: 10.1111/exd.12135. [DOI] [PubMed] [Google Scholar]
- 43.Stapleton JM, Fujii N, McGinn R, McDonald K, Kenny GP. Age-related differences in postsynaptic increases in sweating and skin flow post exercise. Physiol Rep. 2014;2 doi: 10.14814/phy2.12078. pii: e12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horii I, Nakayama Y, Obata M, Tagami H. stratum corneum hydration and amino acid content in xerotic skin. Br J Dermatol. 1989;121:587–92. doi: 10.1111/j.1365-2133.1989.tb08190.x. [DOI] [PubMed] [Google Scholar]
- 45.Oakley R, Tharakan B. Vascular hyperpermeability and aging. Aging Dis. 2014;5:114–25. doi: 10.14336/AD.2014.0500114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen M, Pace AJ, Koller BH. Age-induced reprogramming of mast cell degranulation. J Immunol. 2005;175:5701–7. doi: 10.4049/jimmunol.175.9.5701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of Patient Enrollment