Abstract
We present a detailed review of fetal development of the male and female human urogenital tract from 8–22 weeks gestation at the macroscopic and morphometric levels. Human fetal specimens were sexed based on macroscopic identification of fetal testes or ovaries, Wolffian or Müllerian structures and the presence of the SRY gene in the specimens at or near the indifferent stage (8–9 weeks). Specimens were photographed using a dissecting microscope with transmitted and reflected light. Morphometric measurements were taken of each urogenital organ.
During this time period, development of the male and female urogenital tracts proceeded from the indifferent stage to differentiated organs. The kidneys, ureters, bladder developed identically, irrespective of sex with the same physical dimensions and morphologic appearance. The penis, prostate and testis developed in males and the clitoris, uterus and ovary in females. Androgen-dependent growth certainly influenced size and morphology of the penile urethra and prostate, however, androgenindependent growth also accounted for substantial growth in the fetal urogenital tract including the clitoris.
Introduction
An understanding of normal development of the male and female human urogenital tract lays the foundation to understanding congenital malformations and abnormal development. Congenital abnormalities of the human urogenital tract are relatively common, with clinical implications running the spectrum from no clinical consequences, to those requiring medical intervention or surgical reconstruction to those with lifethreatening consequences. The most common congenital anomalies of the urinary tract and their incidences are listed in Table 1 (Wein et al., 2016).
Table 1:
Incidence and Treatment of Congenital Anomalies of the Urinary Tract
| Congenital Anomaly | Incidence | Treatment |
|---|---|---|
| Hypospadias | (1/250) | Observation for Mild Surgical Reconstruction of Severe Cases (Standard and Severe Hypospadias (Baskin, 2017) |
| Vesicoureteral Reflux | (1/500–1000) | Observation for Mild Surgical Reconstruction of Severe Reflux (Grades IV and V) |
| Uretero-pelvic Junction | (1/1000–2000) | Observation for Mild Surgical Reconstruction for Obstruction |
| Ectopic or Horse Shoe | (1/500–2000) | Observation |
| Kidneys | ||
| Multicystic Dysplastic | (1/2,400–4800 | Observation |
| Kidneys or Renal Agenesis | ||
| Ectopic Ureter/Ureterocele | (1/5000) | Surgical Reconstruction |
| Posterior Urethral Valves | (1/8000) | Surgical Reconstruction |
| Bladder and Cloacal | (rare) | Surgical Reconstruction |
| Exstrophy | ||
| Imperforate Anus | (rare) | Surgical Reconstruction |
| Urogenital Sinus | (rare) | Surgical Reconstruction |
| Abnormalities | ||
| Müllerian Abnormalities | (rare) | Surgical Reconstruction |
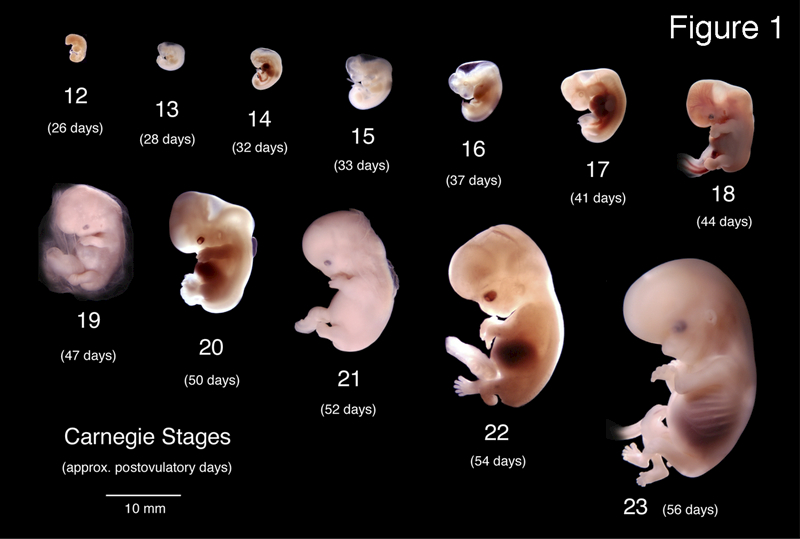
The development of the urogenital tract is initiated in the first trimester and is mostly complete by the end of the second trimester. The Carnegie collection of embryos depicts human development from approximately 3 to 8 weeks’ gestation from the time of fertilization (Figure 1) (Smith, 2016; Mall and Meyer, 1921). Herein, we present wholemount macroscopic imaging of the human fetal urogenital tract organs from the indifferent stage (~8 weeks, the last time point of the Carnegie collection) to approximately 22 weeks of gestation where differentiation between the male and female specimens is clearly apparent.
Figure 1.
Wholemount photos of developing human embryos from the Carnegie collection. Note increasing size and morphological complexity with developmental stage. Image from Dr. Brad Smith, University of Michigan (http://embryo.soad.umich.edu/carnStages/carnStages.html) NIH award N01-HD-6–3257 P/G F003637, with permission.
2. Material and Methods:
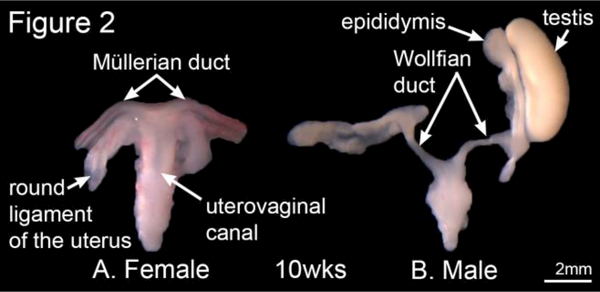
Human first and second-trimester fetal specimens were collected free of patient identifiers after elective termination of pregnancy (Committee on Human Research approval at the University of California at San Francisco, IRB# 12–08813). Gender was determined by inspection with a dissecting microscope of the specimens for Wolffian (mesonephric) and Müllerian (paramesonephric) duct morphology (Figure 2). For the youngest specimens, PCR of the SRY- chromosomal sequences was used to determine sex (Cui et al., 1994) (Li et al., 2015). Heel-toe length was used to estimate gestational age (Drey et al., 2005; Mercer et al., 1987; Mhaskar et al., 1989).
Figure 2.
Wholemount photos of female and male human fetal reproductive tracts from 10-week female (A) and 10-week male (B) fetuses. Note the subtle differences between the sexes in morphology of the Wolffian and Mullerian ducts (uterine tubes). Morphology of male and female specimens is distinctive and diagnostic of sex, which was verified by PCR for the SRY gene.
After sex identification and dissection, specimens were photographed with a dissecting microscope using reflected and transmitted light. Ontogenies were assembled for human fetal gonads, kidneys, prostates, bladders, uteri and both male and female external genitalia (Figures 3A–8A). Measurements were taken for each specimen type using a calibrated millimeter bar on the dissecting microscope. At each time point from the indifferent stage to 22 weeks of gestation, three separate specimens of the same age were measured to document morphometrics of each organ. (In a minority of cases two or rarely one measurement was used). The location of each measurement taken is illustrated in the Figures. Maximum length and width were measured for the testes, ovaries, kidneys, bladders, and external genitalia as illustrated in Figures 3B, 4B, 5B and 8B, respectively. Since the prostate and uterus do not have gross morphologic features that distinguish where the bladder neck starts in males or where the cervix meets the vagina in females, we elected to only document a maximum width (Figure 6B and7B). Since it was rare to obtain specimens with intact ureters connected to both the kidney and the bladder, two ureteral width measurements were obtained: one near the kidney and one near the bladder as illustrated in Figure 4C. The morphometric data were presented in graph form with calculated means and standard deviations.
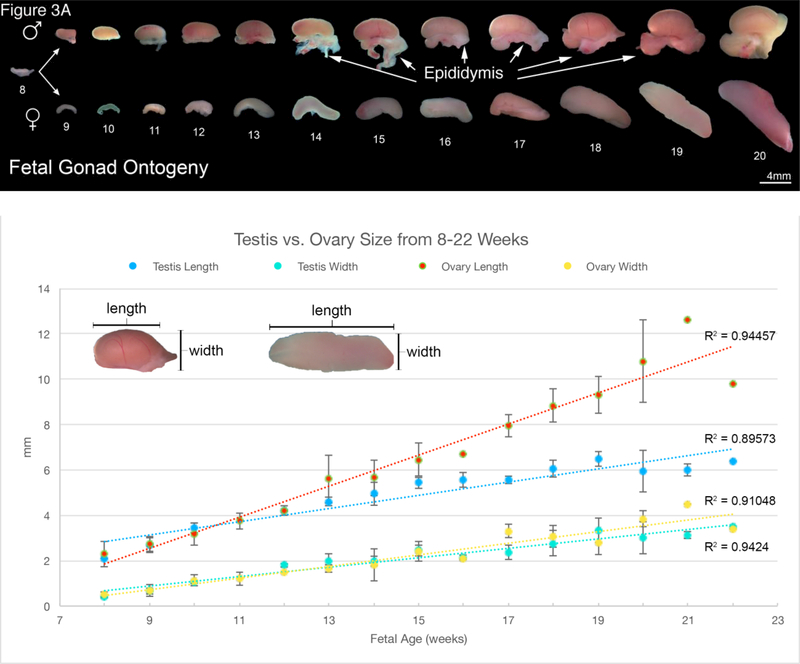
Figure 3.
A. Wholemount photos of human fetal gonadal ontogeny. Testis ontogeny is on the top row and ovarian ontogeny on the bottom row. The testis has a more spheroid shape in contrast to the oblong shape of the ovary. Note the epididymis in many of the testicular specimens. B. Morphometrics (in millimeters) of the human testis and ovary during fetal development (weeks).
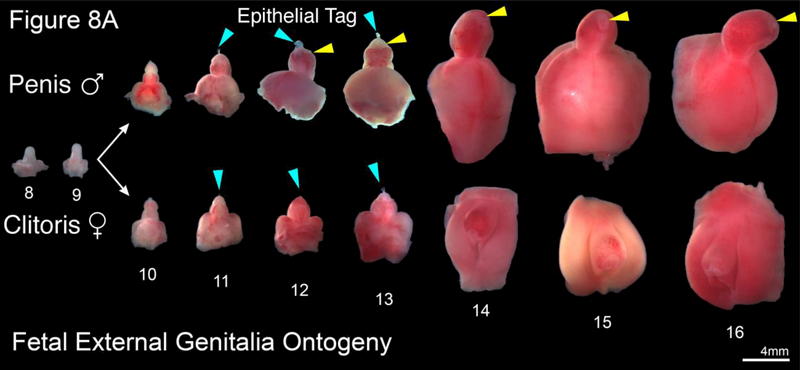
Figure 8.
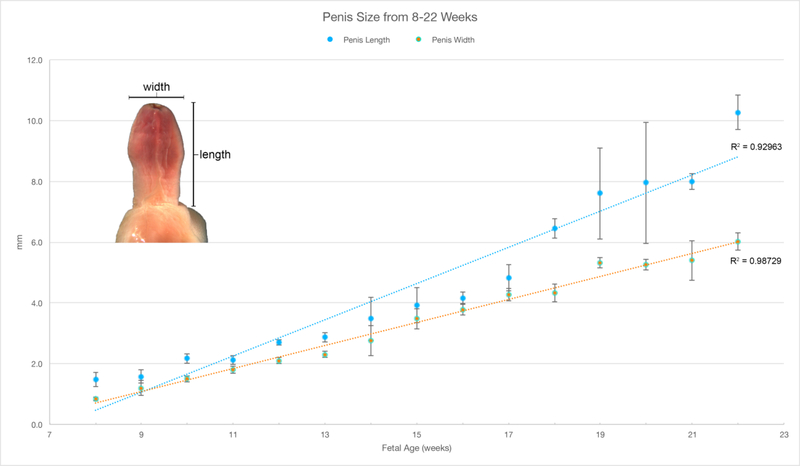
A. Representative examples of human male (top row) and female (bottom row) external genitalia (8–16 weeks gestation). Note the morphologic differences between the male and female after the indifferent stage (8–9 weeks gestation) with lack of urethral fold fusion in the female specimens. Blue arrowheads indicate the epithelial tag in both male and female specimens. Yellow arrowheads illustrate the prepuce in the male which starts developing at 12 days gestation. B. Morphometrics (in millimeters) of the human penis during fetal development (weeks).
Figure 4.
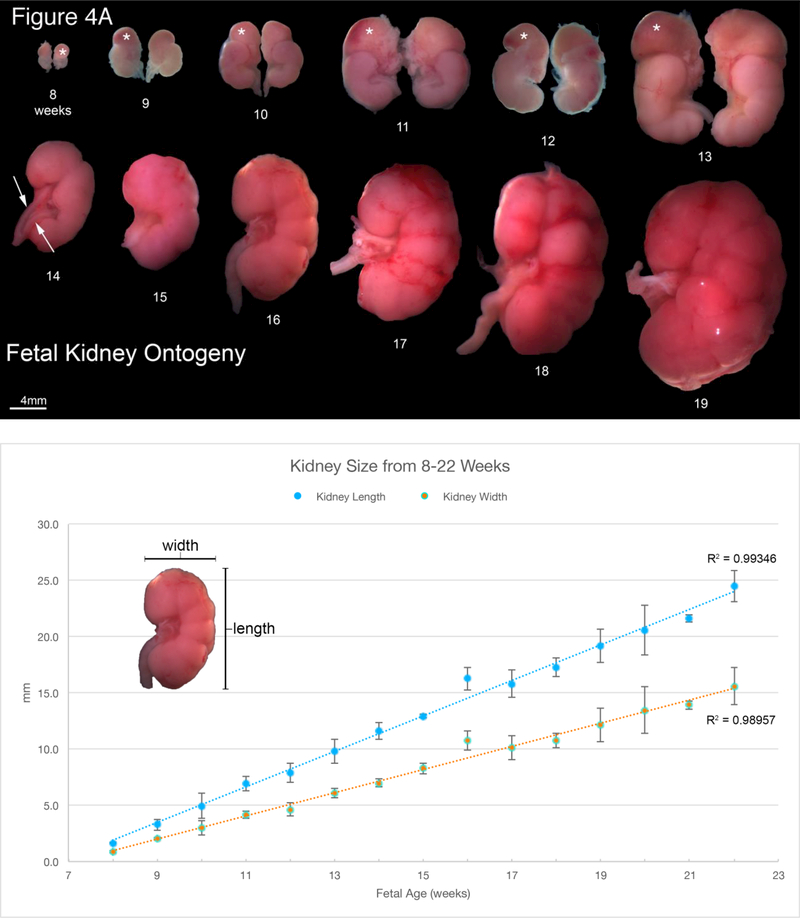
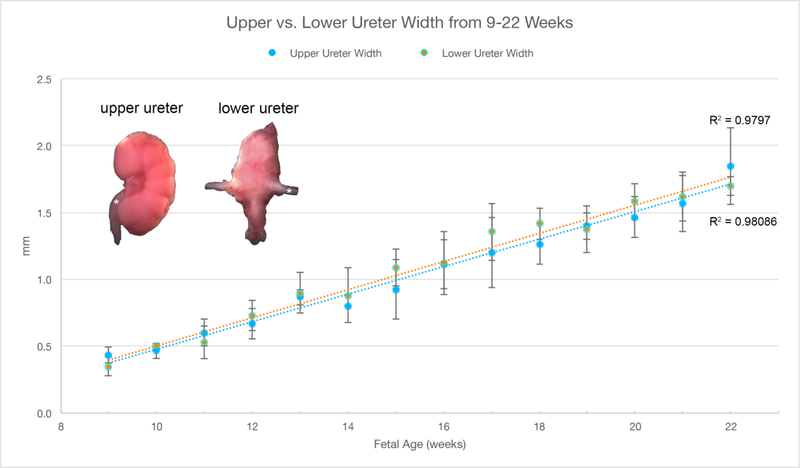
A. Wholemount photos of human fetal kidneys (8–19 weeks of gestation). Note the progressive increase in renal size over time and well visualized fetal lobulations (starting at 12 weeks of gestation). Adrenal glands (*) can be seen capping the upper pole of the fetal kidney in the 8–13 week specimens. Bifid ureter (arrows) is seen in the 14-week specimen. B. Morphometrics (in millimeters) of the human kidney during fetal development (weeks). Values from male and female kidneys are combined. C.Morphometrics (in millimeters) of the human ureter during fetal development (weeks). Values from male and female ureters are combined.
Figure 5.
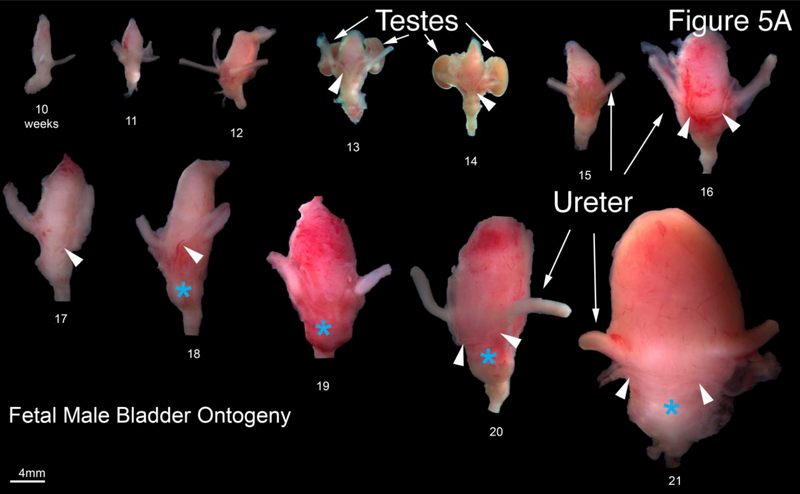
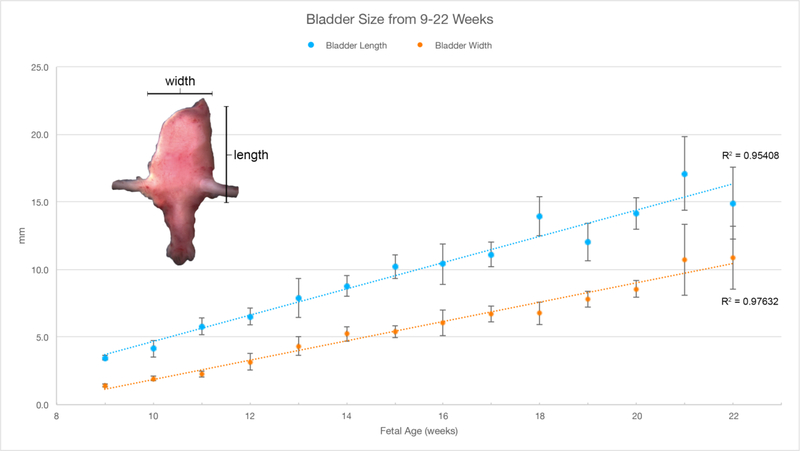
A. Wholemount photos of human fetal male bladders and prostates (10–21 weeks of gestation). Note the presence of the vas deferens (arrowheads) medial to the ureters on the posterior aspect of the majority of specimens. The fetal testes can be seen in the 13- and 14-week specimens. The fetal prostate (blue asterisks) is seen as a bulge of tissue just caudal to the bladder neck which increases in size over time. B. Morphometrics (in millimeters) of the human male and female bladders during fetal development (weeks). Values from male and female bladders are combined.
Figure 6.
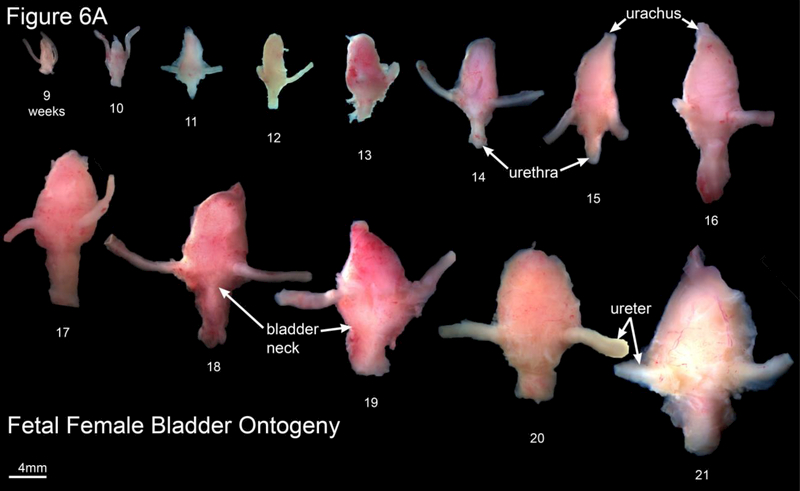
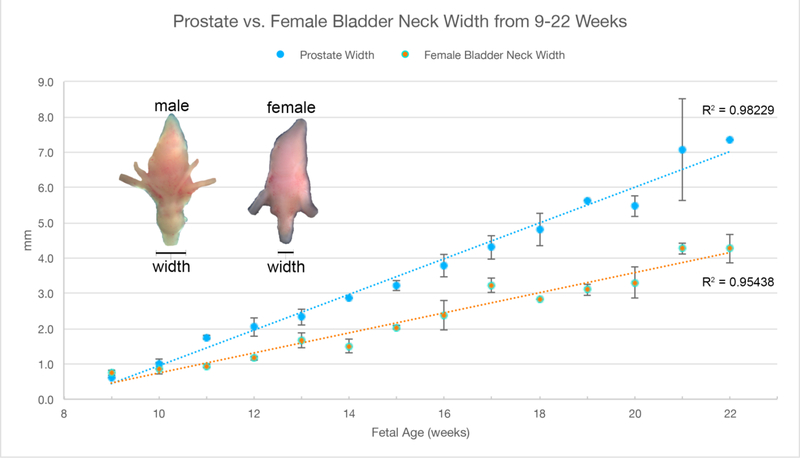
A. Wholemount photos of human female fetal bladders (9–21 weeks of gestation). Compare to the male ontogeny (Fig. 5A) and note the absence of the prostate and vas deferens. Male and female bladder size is similar at each gestational age as indicated in figure 5B. B. Morphometrics (in millimeters) of the human fetal prostate and female bladder neck during fetal development (weeks).
Figure 7.
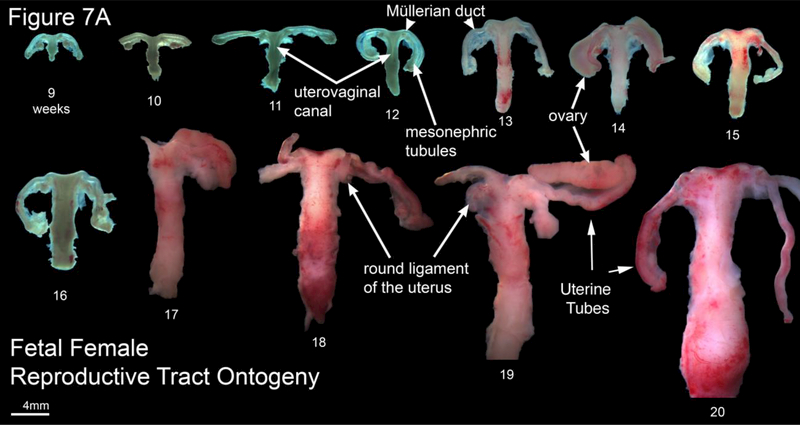
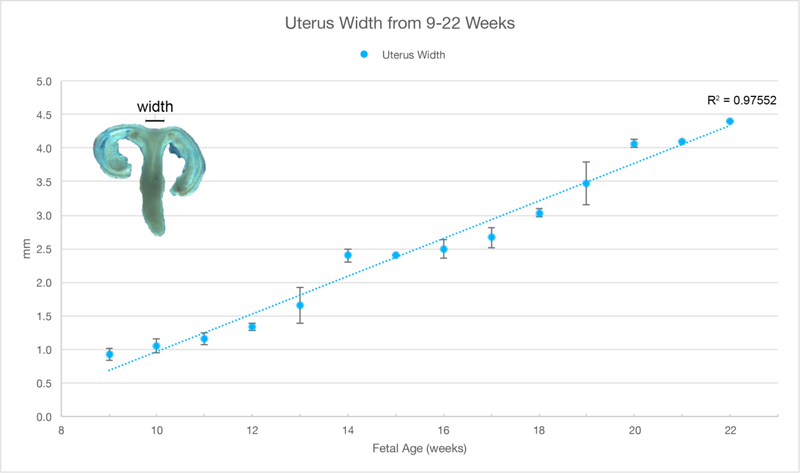
A. Wholemount photos of developing human fetal female internal genitalia 9–20 weeks of gestation. Note the increase in size and morphological complexity with time, and the absence of landmarks distinguishing the uterine corpus, cervix and vagina. The Mullerian duct, utero-vaginal canal, round ligament of the uterus, mesonephric tubules and uterine tube are labeled. Specimens photographed with transmitted light (9, 10–13 and 16 weeks) permit visualization of internal (epithelial) organization in translucent regions (Note the epithelium defining the lumen of the Mullerian duct and the epithelium of the cranial aspect of the uterovaginal canal). B. Morphometrics (in millimeters) of human fetal uterine width at the uterotubal junction during fetal development (weeks).
Results
Differentiation between the male and female reproductive tracts is first seen in the wholemount specimens at 10-weeks of gestation (Figure 2). In the male specimen the Wolffian duct is clearly visible and will subsequently develop into the epididymis, the vas deferens and the seminal vesicles (Figure 2B) (Aumuller and Riva, 1992; Moore et al., 2016a). In females, the cranial portions of the Müllerian ducts (future uterine or Fallopian tubes) are well visualized, while the caudal portions of the Müllerian ducts have fused in the midline to form the uterovaginal canal (Figure 2A), which subsequently develops into the uterine corpus and the uterine cervix and plays a role in the development of the vagina (Cunha et al., 2018a; Robboy et al., 2017).
Note the differences between the sexes in morphology of the Wolffian and Mullerian ducts (Figure 2). In males, the mesonephric tubules develop into the efferent ducts and in females will be represented by the vestigial paraoophoron and epoophoron. During development, the testis assumes a spheroid shape, while the ovary remains oblong (Figure 3A). Morphometrics of testicular and ovarian development are shown in Figure 3B. Note over time that length of the ovary becomes greater than the length of the testis in age-matched specimens. In contrast, the width of both the male and female gonads remains equivalent over time.
Representative wholemount examples of human fetal kidneys from 8–19 weeks’ gestation are seen in Figure 4A. Note the progression of kidney growth over time. The length and width of the fetal kidneys increased over time with no difference based on sex (Figure 4B). Also note the presence of fetal lobulations beginning at 12 weeks of gestation (Moore et al., 2016b). In many of the specimens a delicate ureter can be seen exiting the renal pelvis. Note the bifid ureter in 14-week specimen with a separate ureter to each of the upper and lower poles (Tanagho, 1981) (Figure 4A, arrows). Ureteral width was similar in males and females whether measured close to the bladder or near the kidney increasing from just below 0.5 mm to ~1.75 mm during the time period studied (Figure 4C).
At 8 weeks’ gestation the adrenal gland caps the upper pole of the kidney and is about the same size as the fetal kidney. Over time the kidney grows proportionally larger than the adrenal gland seen in the ontogeny series from 8–13 weeks gestation (Figure 4A).
Representative wholemount examples of human male fetal bladders and prostates from 10–21 weeks’ gestation are seen in Figure 5A. The base of the bladder is defined by the site of insertion of the ureters, which is best seen on posterior views. Also visualized on the posterior aspect is the insertion of the vas deferens into the posterior superior aspect of the prostate (Figure 5A, arrowheads). Note the path of the vas deferens as it enters the prostate along the inferolateral aspect of the bladder medial to the ureters. Below the prostate are segments of the urethra. A close look at the uretero-vesical junction shows the insertion of both ureters in the posterior/inferior aspect of the bladder at the trigone (Figure 5A arrowheads). Comparison of bladder length and width between males and females did not show any differences (Figure 5B). For additional information on the prostate see Cunha et al (Cunha, 2018)
Wholemount examples of female bladder ontogeny are seen in Figure 6A. While male and female bladder size is virtually identical (Figure 5B), the urethra immediately distal to the bladder neck differs significantly in size due to prostatic development in males. The width of the urethra at the female bladder neck is smaller than age-matched prostates (Figure 6B).
Wholemount photos of the ontogeny of human Müllerian structures or fetal female internal genitalia are seen in Figure 7A, which illustrates the midline uterovaginal canal and the bilateral Müllerian ducts destined to form the uterine tubes. Note the increase in size and morphological complexity over time. There are no obvious landmarks within the uterovaginal canal (midline fused Müllerian ducts) that distinguish the uterine body, uterine cervix and vagina. Measurement of uterine width near the uterotubal junction over time illustrates fetal growth of the organ (Figure 7B). Specimens photographed with transmitted light (9 −13 weeks) permit visualization of internal (epithelial) organization of Müllerian structures in both the uterine tube and uterine body. For further information see Cunha et al (Cunha et al., 2018a).
The male and female human fetal external genitalia start out at the indifferent stage (8–9 weeks’ gestation) and grow differentially into a penis or clitoris, which are fully formed by 17–18 weeks gestation (wholemount ontogeny to 16-weeks gestation) (Figure 8A). Note the formation of a tubular urethra that exits on the terminal aspect of the glans and formation of a symmetric circumferential prepuce (yellow arrowheads). The epithelial tag at the tip of the glans penis and clitoris at 9 weeks’ gestation is consistently seen until 13 weeks’ gestation when it is no longer present (Baskin et al., 2018). The size of the penis increases over time, becoming ~ 5 times longer between 8 weeks’ and 22 weeks’ gestation (Figure 8B).
The female human fetal external genitalia also start out at the indifferent stage and are initially indistinguishable from the male external genitalia at 8–9 weeks’ gestation (Figure 8A). However, in females the urethra does not form in the clitoral body or glans. Unlike the male, the female prepuce does not fully cover the glans clitoris and remains dorsal to form the dorsal-hooded foreskin of the clitoris. The clitoris also remains closer to the body wall and maintains its initial curvature. As in the male, female specimens have an epithelial tag on the glans clitoris that disappears after 13 weeks of gestation (Baskin et al., 2018). The size of the clitoris increases substantially over time presumably reflecting androgen-independent growth (Figure 8A). Precise measurements are difficult to make, as the proximal end of the clitoris is difficult/impossible to define as compared to the distinct penoscrotal junction in males. For further information see Baskin et al. (Baskin et al., 2018).
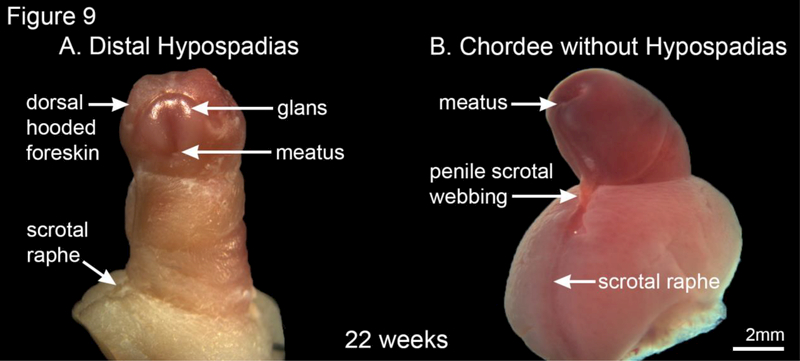
In our series of human fetal external genitalia, we found a 22-week gestation human fetal penis with classic distal hypospadias (Figure 9A). Note the ectopic urethral meatus and lack of ventral prepuce with the expected dorsal-hooded foreskin in this specimen. We also found a 22-week specimen (Figure 9B) with chordee (penile curvature) and penoscrotal webbing without hypospadias.
Figure 9.
A 22-week gestation human fetal penis with classic distal hypospadias (A) and a 22-week human fetal specimen with chordee and penoscrotal webbing without hypospadias (B).
Discussion
The image from the Carnegie collection of embryos illustrates the stages of human embryonic development from approximately 26 days post conception to 56 days (Smith, 2016; Mall and Meyer, 1921). As noted in the original manuscript the dating of each Carnegie stage is an estimate. What is reliable is the progression of staging based on increasing embryonic size and morphological complexity. This is also true for series presented in our study, which is based upon the best estimate of fetal age from heel-toe measurements (Drey et al., 2005; Mercer et al., 1987). Heel-toe measurements are the only reliable method of staging disrupted abortus specimens. As in the Carnegie series, we have assembled our ontogenic series based upon age (as estimated from heel-toe measurements) and increasing size and morphogenetic complexity from the youngest and smallest (~ 8 weeks gestation) to oldest and largest (~ 21 weeks gestation).
The present series of human fetal specimens complements the Carnegie embryo collection with macroscopic wholemounts of the genitourinary tract starting at the last Carnegie stage 23 (56 days) and augments some earlier observations (England, 1983). Our wholemount genitourinary ontogeny extends to 21–22 weeks fetal gestation (16 weeks in the external genitalia) when the complete formation of these fetal organs has taken place and should be a valuable guide to the gross morphology of developing human urogenital organs.
The indifferent stage of genital development ends after approximately 8–9 weeks of gestation when wholemount analysis reveals the first macroscopic signs of divergent male and female differential development. This is best seen in Figure 2 where the presence or absence of testosterone and Müllerian inhibiting substance synthesized in the fetal testis initiates male and female differentiation (Behringer et al., 1994; Jost, 1971; 1972). In males, prostatic buds appear at ~10 weeks of gestation (Cunha et al., 2018b), the Wolffian ducts are stabilized and the Müllerian ducts regress. In contrast, in females the Wolffian ducts regress and the Müllerian ducts fuse in the midline to form the uterovaginal canal that subsequently forms the uterine body, uterine cervix and participates in vaginal development (Robboy et al., 2017) (Cunha et al., 2018a). The cranial aspects of the Müllerian ducts from the Fallopian tubest. In the uterine body, the uterine septum normally regresses or uterus didelphys will develop.
The gonads develop within the genital ridge, and at the indifferent stage at eight weeks they are macroscopically indistinguishable in males and females (Figure 3A). The testes then form a spheroidal structure, while the ovaries remain oblong in shape. Morphometrics show that the length of the ovary at each gestational age studied is greater than that of the testis (Figure 3B).
The kidneys develop in an identical fashion regardless of sex (Figure 4). As noted, fetal lobulations are first seen at 12–13 weeks gestation and become more prominent as development progresses. Fetal lobulation remains visible through the time period studied (19 weeks), but subsequently disappears. The length and width of the kidney increase over time (Figure 4B). The 14-week gestation kidney shows a bifid ureter, a variation of normal development in which the upper and lower poles have separate ureters that connect into a single ureter, in this case at the lower pole of the kidney (Moore et al., 2016b). In humans, multiple variations of normal ureteral development have been described including bifid ureters as seen here as well as complete ureteral duplication (Mackie et al., 1975).
The ureters and bladder in males and females also develop in a similar fashion (Figure 5 and 6) with similar morphometrics (Figure 4C and 5B). In males, the prostate develops from the urethra below the bladder (Figure 5A) and becomes distinctly larger in size than the comparable area in females (Figure 6A). Also, note that the ureterovesical junction inserts into the trigone of the bladder in exactly the same fashion in both males and females during development. For further information see Liaw et al (Liaw et al., 2018).
Müllerian structures degenerate in the male specimens as expected, secondary to the secretion of Müllerian Inhibiting Substance from the testis (Behringer et al., 1994). In female specimens, which lack testosterone, the Wolffian ducts are resorbed (Blaschko et al., 2012), while Müllerian structures are well-preserved with progressive growth over time resulting in development of the uterine body, uterine cervix and Fallopian tubes (Figure 7A). The lower end of the uterovaginal canal (fused Müllerian ducts) participates in vaginal development even though vaginal epithelium appears to be derived solely from urogenital sinus epithelium (Cunha et al., 2018a; Robboy et al., 2017). Note that in the wholemount specimens, it is impossible to differentiate the uterine body from the cervix and vagina. As expected, the size of the uterine body increases over time (Figure 7B).
Development of male and female external genitalia diverges after the indifferent stage with clear morphologic differences seen in wholemounts at 10 weeks of gestation (Figure 8A). Note in males the urethra continues to form via canalization of the urethral plate and subsequent fusion of the urethral folds with the meatus on the terminal aspect of the glans along with formation of a circumferential prepuce (Li et al., 2015; Shen et al., 2016; Baskin et al., 2018). In contrast, the female vestibular plate (homologue of the urethral plate in males) undergoes canalization without fusion of the vestibular folds (Overland et al., 2016). During the course of development, the clitoris remains closer to the body wall in contrast to the penis which extends outward at an approximately 90-degree angle (Baskin et al., 2018). Interestingly, the size of the clitoris is similar to that of the penis in the first and second trimester (Figure 8A). This implies that an androgenindependent phase of growth of external genitalia associated with the canalization of the urethral/vestibular plates seen in both the male and female specimens. In males, an androgen-dependent phase accounts for formation of the male urethra and an increase in penile size/length as compared to the clitoris (Li et al., 2015; Overland et al., 2016; Shen et al., 2016). Note the subsequent five-fold increase in penile size from 8 weeks’ gestation to 22 weeks (Figure 8B).
As stated, the most common anomaly of the human fetal male urinary tract is hypospadias (Table 1) (Baskin, 2017). In our series of approximately 500 human fetal external genitalia, one 22-week gestation human fetal penis had classic distal hypospadias (Figure 9). Note the ectopic urethral meatus and lack of ventral prepuce with the presence of a dorsal-hooded foreskin. We also found 22-week specimen (Figure 9B) demonstrating chordee (penile curvature) and penoscrotal webbing without hypospadias. This is consistent with the incidence of hypospadias of approximately 1/250 (Table 1)(Baskin, 2017).
In conclusion, development of the male and female urogenital tracts proceeds from the indifferent stage to differentiated organs over the 14-week time period studied. The kidneys, ureters, bladder developed identically, irrespective of sex, with the same physical dimensions and morphologic appearance. The penis, prostate and testis (and associated structures) develop in males, and the clitoris, uterus (and associated structures) and ovary develop in females. Androgen-dependent growth certainly influences the size and morphology of male urogenital organs (penile urethra and prostate in males), however, androgen-independent growth also accounts for substantial growth in the fetal clitoris.
Acknowledgments
Supported by NIH grant K12DK083021
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Aumuller G, and Riva A (1992) Morphology and functions of the human seminal vesicle. Andrologia 24:183–196. [DOI] [PubMed] [Google Scholar]
- Baskin L (2017) What Is Hypospadias? Clin Pediatr (Phila) 56:409–418. [DOI] [PubMed] [Google Scholar]
- Baskin L, Shen J, Sinclair A, Cao M, Liu X, Liu G, Isaacson D, Overland M, Li Y, and Cunha J (2018) Development of the Human Penis and Clitoris. Differentiaiton this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, and Cate RL (1994) Mullerian-inhibiting substance function during mammalian sexual development. Cell 79:415–425. [DOI] [PubMed] [Google Scholar]
- Blaschko SD, Cunha GR, and Baskin LS (2012) Molecular mechanisms of external genitalia development. Differentiation 84:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui KH, Warnes GM, Jeffrey R, and Matthews CD (1994) Sex determination of preimplantation embryos by human testis-determining-gene amplification. Lancet 343:79–82. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Robboy SJ, Kurita T, Isaacson D, Shen J, Cao M, and Baskin LS (2018a) Development of the human female reproductive tract. Differentiation; research in biological diversity (This issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Vezina C, Isaacson D, Ricke WA, Timms B, and Baskin LS (2018b) Development of the human prostate and seminal vesicle. Differentiation; research in biological diversity (This issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GV,C; Isaacson D; Ricke W; Timms B; and Baskin LS; (2018) Development of the human prostate and seminal vesicle.. Differentiation this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drey EA, Kang MS, McFarland W, and Darney PD (2005) Improving the accuracy of fetal foot length to confirm gestational duration. Obstet Gynecol 105:773–778. [DOI] [PubMed] [Google Scholar]
- England MA (1983) Color atlas of life before birth: Normal fetal development. Year Book Medical Publishers, Chicago. [Google Scholar]
- Jost A (1971) Use of androgen antagonists and antiandrogens in studies on sex differentiation. Gynecol Invest 2:180–201. [DOI] [PubMed] [Google Scholar]
- Jost A (1972) A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med J 130:38–53. [PubMed] [Google Scholar]
- Li Y, Sinclair A, Cao M, Shen J, Choudhry S, Botta S, Cunha G, and Baskin L (2015) Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the double zipper hypothesis. The Journal of urology 193:1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw A, Cunha GR, Shen J, Cao M, Liu G, Sinclair A, and Baskin LS (2018) Embryological Development of the Bladder and Ureterovesical Junction. Differentiation; research in biological diversity (This issue). [DOI] [PubMed] [Google Scholar]
- Mackie GG, Awang H, and Stephens FD (1975) The ureteric orifice: the embryologic key to radiologic status of duplex kidneys. J Pediatr Surg 10:473–481. [DOI] [PubMed] [Google Scholar]
- Mall F, and Meyer A (1921) Studies on abortuses: a survey of pathologic ova in the Carnegie Embryological Collection.. Contribution Embryology 12: 1–364.:1–364. [Google Scholar]
- Mercer BM, Sklar S, Shariatmadar A, Gillieson MS, and D’Alton ME (1987) Fetal foot length as a predictor of gestational age. Am J Obstet Gynecol 156:350–355. [DOI] [PubMed] [Google Scholar]
- Mhaskar R, Agarwal N, Takkar D, Buckshee K, Anandalakshmi, and Deorari A (1989) Fetal foot length--a new parameter for assessment of gestational age. Int J Gynaecol Obstet 29:35–38. [DOI] [PubMed] [Google Scholar]
- Moore K, Persaud T, and Torchia M (2016a) The Urogenital System. The Developing Human Clinically Oriented Embryology, pp. 241–282. [Google Scholar]
- Moore K, Persaud TVN, and Torchia M (2016b) The Developing Human 10th Edition Clinically Oriented Embryology. saunders. [Google Scholar]
- Overland M, Li Y, Cao M, Shen J, Yue X, Botta S, Sinclair A, Cunha G, and Baskin L (2016) Canalization of the Vestibular Plate in the Absence of Urethral Fusion Characterizes Development of the Human Clitoris: The Single Zipper Hypothesis. The Journal of urology 195:1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robboy SJ, Kurita T, Baskin L, and Cunha GR (2017) New insights into human female reproductive tract development. Differentiation 97:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Overland M, Sinclair A, Cao M, Yue X, Cunha G, and Baskin L (2016) Complex epithelial remodeling underlie the fusion event in early fetal development of the human penile urethra. Differentiation 92:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B (2016) The Multi-Dimensional Human Embryo on line University of Michigan, Ann Arbor, MI. [Google Scholar]
- Tanagho E (1981) Dvelopment of Ureter In: Bergman H (ed) The Ureter. Spinger Verlag. [Google Scholar]
- Wein AJ, Louis R Kavoussi, Partin AW, and Peters CA (2016) Campbells and Walsh. Elsevier. [Google Scholar]