Abstract
Multiple endocrine neoplasia (MEN) has not been reported in baboons but this condition is well described in humans. An internal database was searched for all cases of concurrent endocrine hyperplasia and neoplasia in baboons. Twenty-four baboons (Papio spp.) with concurrent endocrine hyperplasia and neoplasia were identified. Twenty-one baboons had lesions in two endocrine organs, two baboons had lesions in three organs, and one baboon had lesions in four organs. Ten baboons aligned with the MEN1 classification; 14 baboons did not match any current human MEN classification. We report 24 cases of MEN-like syndrome in baboons. MEN1-like lesions accounted for nearly half (41 %) of the affected animals. Genetic analysis of baboons with MEN-like syndrome could further elucidate the mechanisms of MEN and support the use of baboons as animal models for human MEN.
Keywords: Pituitary Neoplasms, Pancreatic Neoplasms, Adrenal Gland Neoplasms, Thyroid Neoplasms, Primate
Introduction
Endocrine neoplasia has been minimally discussed in baboons with only a handful of reports found in the literature [1–5]. Three large surveys infer the incidence of endocrine neoplasia in the baboon. A review of 4619 baboon necropsies identified 97 endocrine neoplasms (2.1% incidence) [4]. A review of neoplasms in approximately 4000 captive baboons identified 363 spontaneous neoplasms, including 40 endocrine neoplasms (11% of neoplasms; 1% incidence) [2]. Another review of all reported spontaneous neoplasms of baboons reported that endocrine neoplasia accounted for 7% of 204 neoplasms [3]. Based on these studies, the incidence of endocrine neoplasia in baboons appears to be around 1-2%, and the endocrine neoplasms account for around 7-10% of all neoplasms. Additional reports of proliferative endocrine lesions include 8 cases of adrenal gland cortical hyperplasia [6] and a primary hepatic neuroendocrine carcinoma [1].
Multiple endocrine neoplasia (MEN) describes a group of genetic disorders involving the simultaneous development of proliferative lesions (hyperplasia, benign neoplasms, and/or malignant neoplasms), in two or more endocrine organs [5, 7–13]. In humans, MEN has been divided into four major subtypes: MEN1, MEN2A (also referred to as MEN2), MEN2B (also referred to as MEN3), and MEN4 (or MENX in rats) [7–11, 13, 14]. Each subtype is associated with a unique genetic mutation and has a characteristic set of lesions [8, 11, 13–15]. Few reports of MEN and MEN-like disease exist in veterinary literature, and, there is no published report of MEN-like syndrome in baboons. We present a retrospective case series of MEN-like findings in baboons, with comparison to the human condition.
Materials and Methods
Animals
At the Southwest National Primate Research Center, Texas Biomedical Research Institute, the approximate average baboon population during the period of this review was 3,300 animals. The colony was established in the late 1950’s and is self-sustaining, although baboons are occasionally added to the colony for genetic diversity and special projects. Baboons were housed in an open-top 6-acre metal and concrete corral with dirt floors, gang cages with concrete floors, and in individual metal cages if special handling is required (i.e., for medical care). The commercial monkey chows fed were supplemented with an enrichment fare of grains, fruits, and vegetables; water was provided ad libitum. The baboons were screened every 6 months for Mycobacterium tuberculosis by intradermal intrapalpebral tuberculin skin testing. All animal care and procedures were approved by the Texas Biomedical Research Institute Animal Care and Use Committee. The Texas Biomedical Research Institute is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Pathology
A necropsy was performed on all baboons that died or were euthanized at the Southwest National Primate Research Center. Tissues were collected for histologic evaluation as required for diagnosis, fixed in 10% neutral buffered formalin, processed conventionally, embedded in paraffin, cut at 4 μM and stained with hematoxylin and eosin or other stains as needed for diagnosis. When indicated, individual tissues were frozen in liquid nitrogen and stored at −80°C, fixed in 2% glutaraldehyde for electron microscopy, placed in normal saline or transport medium for cytogenetic evaluation, cultured for bacteria and viruses, or frozen in Optimal Cutting Temperature Compound (Tissue-Tek®) compound for frozen sectioning. Further evaluation using immunohistochemistry was performed as required. The necropsy and histologic evaluation were performed by board certified veterinary pathologists. Conventional nomenclature was used for all lesions, and results were stored in an internal database (apath). Microscopic findings that were equivocal or otherwise challenging were reviewed by three to five other board-certified veterinary pathologists. If deemed necessary, cases were referred to the Joint Pathology Center (Silver Spring, MD) or other individual pathologists with expertise in the field.
Records Review
To be included in this study, the baboons had to have benign or malignant neoplasms or hyperplasia in two or more endocrine organs. A computer search of the pathology database identified baboons that met these criteria in any of the following endocrine organs: endocrine pancreas (islets of Langerhans), pituitary gland, thyroid gland, parathyroid gland, and adrenal gland. All original or computerized medical records, gross necropsy reports, histopathology reports and related laboratory results were retrieved and diagnoses were verified.
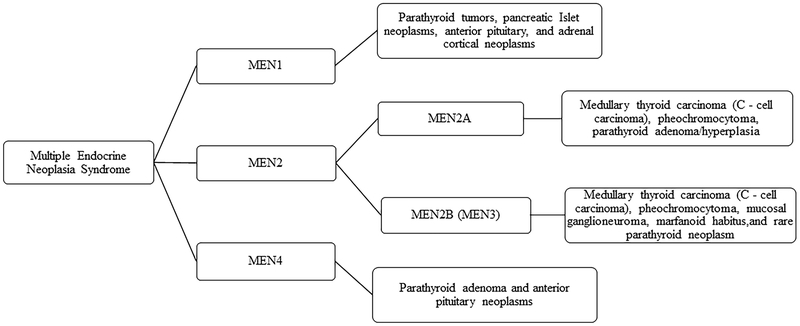
Some variation exists in the literature when describing the characteristic organ and lesion involvement of MEN in humans [7, 8, 10, 13–15]. For this study, MEN classifications were based on the scheme presented in Figure 1, which is consistent with recent literature in humans [14, 16].
Figure 1.
Schematic representation of classification of Multiple Endocrine Neoplasia Syndrome in humans.
Literature searches were performed using the Texas Biomedical Northup Library Collections of print books, ebooks, journals, conference proceedings, institutional repositories, as well as online databases including Pubmed, Scopus, Web of Science, and PrimateLit. The online veterinary professional resource VIN (Veterinary Information Network) was also utilized. Keyword searches included: neoplasia nonhuman primate, endocrine neoplasia nonhuman primate, multiple endocrine neoplasia nonhuman primate, multiple endocrine neoplasia, multiple endocrine neoplasia vet med, and endocrine neoplasia vet med.
Results
A total of 8769 baboon necropsies were performed during this period (1995 to 2016). Twenty-four baboons (F=16; M=8) ranging from 16 to 33 years of age (23.04 +/− 4.22) were identified with concurrent endocrine hyperplasia and neoplasia in two or more organs. Table 1 lists the affected baboons, the 38 endocrine neoplasms, and 14 hyperplastic endocrine lesions identified, and the category of MEN those lesions are most consistent with, where applicable.
Table 1.
Summary of demographics and MEN like lesions in baboons. Cases 1 through 10 align with MEN1 classification in humans.
| Case no. | Sex | Age (years) | Pituitary Gland | Pancreas | Thyroid Gland | Adrenal Medulla | Adrenal Cortex | Parathyroid Gland |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 20 | Adenoma | Adenoma | ||||
| 2 | F | 25 | Adenoma | Adenoma | ||||
| 3 | F | 26 | Adenoma | Adenoma | ||||
| 4 | F | 28 | Adenoma | Adenoma | ||||
| 5 | F | 33 | Adenoma | Adenoma | Pheochromocytoma | Adenoma | ||
| 6 | F | 25 | Adenoma | Hyperplasia | ||||
| 7 | M | 24 | Adenoma | Adenoma | ||||
| 8 | F | 24 | Hyperplasia | Adenoma | ||||
| 9 | M | 25 | Hyperplasia | Adenoma | ||||
| 10 | M | 16 | Hyperplasia | Hyperplasia | Adenoma | |||
| 11 | F | 24 | Adenoma | Adenoma | ||||
| 12 | F | 30 | Adenoma | Adenoma | ||||
| 13 | M | 18 | Adenoma | Adenoma | ||||
| 14 | M | 21 | Adenoma | Adenoma | ||||
| 15 | F | 20 | Adenoma | Carcinoma | ||||
| 16 | F | 25 | Adenoma | Carcinoma | ||||
| 17 | F | 24 | Adenoma | Hyperplasia | ||||
| 18 | M | 16 | Adenoma | Hyperplasia | ||||
| 19 | M | 18 | Adenoma | Hyperplasia | ||||
| 20 | F | 23 | Hyperplasia | Hyperplasia | ||||
| 21 | F | 18 | Adenoma | Hyperplasia | ||||
| 22 | F | 22 | Hyperplasia | Pheochromocytoma | ||||
| 23 | F | 26 | Adenoma | Pheochromocytoma | ||||
| 24 | M | 22 | Adenoma | Hyperplasia | Hyperplasia |
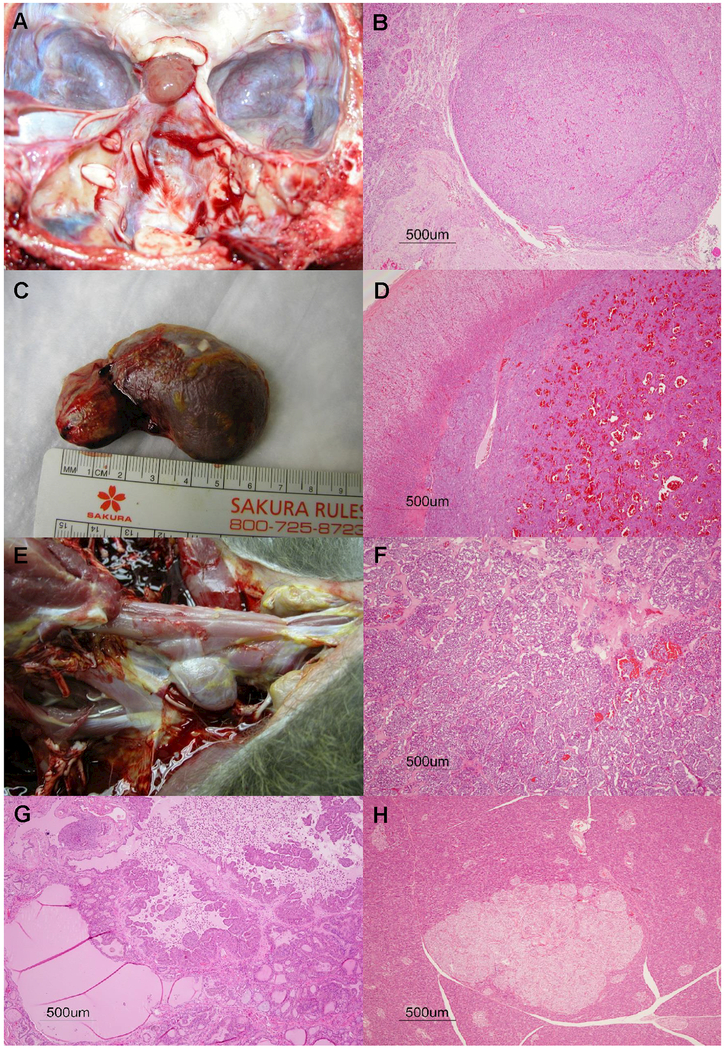
Twenty-one baboons had lesions in two endocrine organs, two baboons had lesions in three organs, and one baboon had lesions in four organs. Of the 38 neoplasms, 33 were adenomas with 18 in the pituitary gland, 7 in the pancreas, 5 in the thyroid gland, 2 in adrenal gland, and 1 in parathyroid gland. There were five malignant neoplasms; three pheochromocytomas, and two thyroid follicular carcinomas. The pituitary gland had the most proliferative lesions (n = 23), followed by the thyroid gland (n = 13), adrenal gland (n = 8), pancreas (n = 7), and parathyroid gland (n = 1). Figure 2 (A-H) shows a representative panel of images for some of the endocrine neoplasms described in this manuscript.
Figure 2.
Representative panel of images for some of the endocrine neoplasms reported in this manuscript. Pituitary gland adenoma, gross image for Case 2 (A), histopathological lesion of pituitary gland adenoma for Case 19 (B). Pheochromocytoma gross image for Case 5 (C), and histopathological presentation for pheochromocytoma in Case 22 (D). Gross image of thyroid gland adenocarcinoma, Case 15 (E). Histopathology of thyroid adenocarcinoma in Case 16 (F), and thyroid adenoma in Case 13 (G). Photomicrograph of islet cell adenoma in pancreas for Case 5.
Ten cases (7 females, 3 males) were considered consistent with MEN1 classification with lesions primarily involving the anterior pituitary (10 of 10; hyperplasia and adenoma), pancreas (7 of 10; islet cell adenoma), adrenal gland (4 of 10; hyperplasia, adenoma or pheochromocytoma), or parathyroid gland (1 of 10; adenoma). Nine females and five males were diagnosed with proliferative lesions that do not appear to fit any of the current human MEN classifications. Eleven of these had concurrent proliferative lesions involving the anterior pituitary and thyroid follicular epithelium; two had concurrent proliferative lesions involving the anterior pituitary and adrenal medulla, one had proliferative lesions in thyroid follicular epithelium and adrenal medulla, and one had concurrent proliferative lesions in anterior pituitary, thyroid follicular epithelium, and adrenal medulla.
Discussion
MEN is divided in to 4 subgroups: MEN1, MEN2A, MEN2B, MEN4 [7, 13–15]. Though clear variation exists between the subtypes, all are inherited in an autosomal dominant pattern with moderate to high penetrance [7–10, 13, 14]. MEN1 results from mutations of the MEN1 gene locus which encodes the tumor suppressor protein MENIN [7, 8, 14, 15]. Tumors typically develop in the parathyroid gland, pancreatic islet cells, pituitary gland, and occasionally adrenal cortex [7–9, 14, 15]. Both MEN2A and MEN2B are associated with mutations in the RET proto-oncogene [8, 9, 14]. The common feature among MEN2 variants is the development of medullary thyroid carcinoma which is a neoplasm of C cells [14]. MEN2A also includes tumors in the parathyroid glands and pheochromocytomas [7, 9, 15]. MEN2B (also referred to as MEN3) is further characterized by pheochromocytomas, mucosal and digestive ganglioneuromas, and marfanoid habitus [13, 14]. MEN4 (MENX) is the newest subtype of MEN and was discovered spontaneously in a rat colony approximately a decade ago [8–10, 13–15]. Unlike other forms of MEN, MENX in rats was inherited in an autosomal recessive pattern [8, 10, 14, 15]. MEN4 arises from mutations in the CDKN1B gene which is an important regulator of cell cycle progression and encodes for putative tumor suppressor protein p27 [8–10, 13, 14]. MEN4 is much less common than MEN1 and MEN2 in humans, although the low reported frequency could relate to historic disease classification, as the cases now described as MEN4 were previously categorized as part of MEN1 [14]. While autosomal dominant inheritance has been suspected in human cases of MEN4, it is not yet definitively supported with the limited number of emerging cases and investigations [8, 14, 15]. Tumor development occurs in the parathyroid gland and anterior pituitary gland, with sporadic occurrence of pheochromocytomas [7, 8, 10, 15].
Ten cases in the present study (7 females, 3 males) were consistent with MEN1 classification with lesions involving parathyroid gland, pancreas, anterior pituitary gland, or adrenal gland [14]. MEN2, which for the scope of this paper was not sub-divided in to the human subgroups of MEN2A and MEN2B, is characterized by medullary thyroid gland carcinoma or hyperplasia of parafollicular cells (C-cells) in early lesions, pheochromocytoma, and neoplasms or hyperplasia in the parathyroid gland [14]. Two cases involved similar distribution of lesions seen in human cases of MEN2: a 26 year old female baboon with pheochromocytoma and thyroid follicular adenoma, and a 22 year old male baboon with thyroid follicular hyperplasia and adrenal medullary hyperplasia, however, both these cases did not fit the MEN2 definition because they lack the proliferative lesions in C –cells [17] . None of the cases described here were consistent with the MEN4 subtype of parathyroid gland and anterior pituitary gland involvement [14]. Lastly, five male and nine female baboons were diagnosed with proliferative lesions involving two or more endocrine tissues, but did not appear to fit any of the current human MEN classifications.
MEN is not limited to humans. Clinical cases of MEN have been documented in small and large animals, and animal models have been effectively utilized in research to study these disorders. Since the early 1990’s, lines of MEN mouse and rat models have been generated and characterized, significantly contributing to our understanding of the important biochemical, physiological, and pathological processes of cancer onset and spread in intact living organisms [13]. In veterinary medicine, for the lack of confirmatory testing for genetic mutations, these cases have often been called as MEN-like syndromes, and few have been diagnosed or suspected in a variety of species including horses [18, 19], cats [20, 21], bulls [22–24], ferrets [25, 26], dogs [27–30], and non-human primates [17, 31].
A 10-year retrospective analyses of 72 horses with endocrine neoplasia described the coexistence of hyperplasia and neoplasia of the thyroid and adrenal glands, similar to MEN syndrome of humans [18]. A 2006 study of 2 cats with MEN1 like syndrome analyzed the MENIN gene in cats, reporting that it shared 93% homology with the human MENIN gene and 98% homology in their respective protein sequences [21]. The authors concluded that the similarities observed at the clinical and pathologic level and the high homology of the protein sequence suggest a similar role of the MENIN gene in cats and humans [21]. It has been suggested that the incidence of MEN like syndromes among cats, dogs, and horses may be considerably higher than reported and that MEN like syndromes are underdiagnosed by veterinarians because of a failure to recognize the signs clinically [20]. In ferrets, the presentation of tumors in different endocrine organs has a close resemblance to MEN like syndrome reported in humans and other domestic animals [25]. Importantly, the MEN1 and RET genes in ferrets have been sequenced. The ferret MEN1 gene had 97.7% and 99.4% homology with the human and dog MEN1 genes, respectively; the ferret RET gene had 97.6% and 100% homology to human and dog RET genes [25]. Colgin et al reported a case of pheochromocytoma in an 18 year old rhesus macaque with concurrent parafollicular (C-cell) adenoma and hyperplasia, this spectrum of lesions is consistent with a human MEN2 phenotype [17].
Together, these reports strengthen the likelihood of numerous species being affected by some form of genetically based MEN-like syndromes, much like that in humans. Our study suggests that MEN1-like syndrome does exist in captive baboons. Since, genetic testing was not performed on any of our cases, we prefer to use the term MEN-like syndrome to classical MEN categories as described in humans. MEN2 and MEN4 were not detected in our review of cases and perhaps are specific to human gene mutations or phenotypic presentation. The next steps in investigating MEN-like syndrome in baboons would be genetic analysis and comparison to those strongly associated mutations seen in the human condition. If similarities continue to appear, baboons may serve as an excellent animal model for MEN in humans.
Acknowledgements
The authors wish to thank Renee Escalona, Tony Perez, Jesse Martinez, and Sarah Pennington for their anatomic pathology support, and the clinical research and support staff. This investigation used resources which were supported by the Southwest National Primate Research Center grant P51 RR013986 from the National Center for Research Resources, National Institutes of Health and which are currently supported by the Office of Research Infrastructure Programs through P51 OD011133. This investigation was conducted in facilities constructed with support from the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number 1 C06 RR016228.
REFERENCES
- 1.Aloisio F, Dick EJ, Hubbard GB: Primary hepatic neuroendocrine carcinoma in a baboon (Papio sp.). J Med Primatol 2009; 38:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cianciolo RE, Butler SD, Eggers JS, Dick EJ, Leland MM, de la Garza M, Brasky KM, Cummins LB, Hubbard GB: Spontaneous neoplasia in the baboon (Papio spp.). J Med Primatol 2007; 36:61–79. [DOI] [PubMed] [Google Scholar]
- 3.Cianciolo RE, Hubbard GB: A review of spontaneous neoplasia in baboons (Papio spp.). J Med Primatol 2005; 34:51–66. [DOI] [PubMed] [Google Scholar]
- 4.Guardado-Mendoza R, Dick EJ, Jimenez-Ceja LM, Davalli A, Chavez AO, Folli F, Hubbard GB: Spontaneous pathology of the baboon endocrine system. J Med Primatol 2009; 38:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owston MA, LaRue MK, Dick EJ, Ambrus A, Porter BF: Pancreatic neuroendocrine tumors in twelve baboons (Papio spp.). J Med Primatol 2016; 45:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skelton-Stroud PN, Ishmael J: Adrenal lesions in the baboon (Papio spp). Vet Pathol 1985; 22:141–146. [DOI] [PubMed] [Google Scholar]
- 7.Grajo JR, Paspulati RM, Sahani DV, Kambadakone A: Multiple Endocrine Neoplasia Syndromes: A Comprehensive Imaging Review. Radiol Clin North Am 2016; 54:441–451. [DOI] [PubMed] [Google Scholar]
- 8.Lee M, Pellegata NS: Multiple endocrine neoplasia syndromes associated with mutation of p27. J Endocrinol Invest 2013; 36:781–787. [DOI] [PubMed] [Google Scholar]
- 9.Pasquali D, Di Matteo FM, Renzullo A, Accardo G, Esposito D, Barbato F, Colantuoni V, Circelli L, Conzo G: Multiple endocrine neoplasia, the old and the new: a mini review. G Chir 2012; 33:370–373. [PubMed] [Google Scholar]
- 10.Pellegata NS: MENX and MEN4. Clinics (Sao Paulo) 2012; 67 Suppl 1:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sizemore GW: Multiple Endocrine Neoplasia In: Principles and Practice of Endocrinology and Metabolism. Becker KL (ed): Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 12.Walls GV : Multiple endocrine neoplasia (MEN) syndromes. Semin Pediatr Surg 2014; 23:96–101. [DOI] [PubMed] [Google Scholar]
- 13.Wiedemann T, Pellegata NS: Animal models of multiple endocrine neoplasia. Mol Cell Endocrinol 2016; 421:49–59. [DOI] [PubMed] [Google Scholar]
- 14.Pacheco MC: Multiple Endocrine Neoplasia: A Genetically Diverse Group of Familial Tumor Syndromes. J Pediatr Genet 2016; 5:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakker RV: Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol Cell Endocrinol 2014; 386:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinom [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colgin LM, Schwahn DJ, Casti a. Thyroid 2015; 25:567–610.25810047 [Google Scholar]; llo-Alcala F, Kiupel M, Lewis AD: Pheochromocytoma in Old World Primates (Macaca mulatta and Chlorocebus aethiops). Vet Pathol 2016; 53:1259–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Cock HE, MacLachlan NJ: Simultaneous occurrence of multiple neoplasms and hyperplasias in the adrenal and thyroid gland of the horse resembling multiple endocrine neoplasia syndrome: case report and retrospective identification of additional cases. Vet Pathol 1999; 36:633–636. [DOI] [PubMed] [Google Scholar]
- 19.Luethy D, Habecker P, Murphy B, Nolen-Walston R: Clinical and Pathological Features of Pheochromocytoma in the Horse: A Multi-Center Retrospective Study of 37 Cases (2007–2014). J Vet Intern Med 2016; 30:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimer SB, Pelosi A, Frank JD, Steficek BA, Kiupel M, Hauptman JG: Multiple endocrine neoplasia type I in a cat. J Am Vet Med Assoc 2005; 227:101–104, 186. [DOI] [PubMed] [Google Scholar]
- 21.Roccabianca P, Rondena M, Paltrinieri S, Pocacqua V, Scarpa P, Faverzani S, Scanziani E, Caniatti M: Multiple endocrine neoplasia type-I-like syndrome in two cats. Vet Pathol 2006; 43:345–352. [DOI] [PubMed] [Google Scholar]
- 22.Capen CC, Black HE: Animal model of human disease. Medullary thyroid carcinoma, multiple endocrine neoplasia, Sipple’s syndrome. Animal model: ultimobranchial thyroid neoplasm in the bull. Am J Pathol 1974; 74:377–380. [PMC free article] [PubMed] [Google Scholar]
- 23.Seimiya YM, Takahashi M, Furukawa T, Mizutani K, Kimura K, Haritani M: An aged bull with concurrent thyroid C cell carcinoma, adrenal pheochromocytoma and pituitary chromophobe adenoma. J Vet Med Sci 2009; 71:225–228. [DOI] [PubMed] [Google Scholar]
- 24.Sponenberg DP, McEntee K: Pheochromocytomas and ultimobranchial (C-cell) neoplasms in the bull: evidence of autosomal dominant inheritance in the Guernsey breed. Vet Pathol 1983; 20:396–400. [DOI] [PubMed] [Google Scholar]
- 25.Bakthavatchalu V, Muthupalani S, Marini RP, Fox JG: Endocrinopathy and Aging in Ferrets. Vet Pathol 2016; 53:349–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox JG, Dangler CA, Snyder SB, Richard MJ, Thilsted JP: C-cell carcinoma (medullary thyroid carcinoma) associated with multiple endocrine neoplasms in a ferret (Mustela putorius). Vet Pathol 2000; 37:278–282. [DOI] [PubMed] [Google Scholar]
- 27.Kiupel M, Mueller PB, Ramos Vara J, Irizarry A, Lin TL: Multiple endocrine neoplasia in a dog. J Comp Pathol 2000; 123:210–217. [DOI] [PubMed] [Google Scholar]
- 28.Peterson ME, Randolph JF, Zaki FA, Heath H: Multiple endocrine neoplasia in a dog. J Am Vet Med Assoc 1982; 180:1476–1478. [PubMed] [Google Scholar]
- 29.Thuróczy J, van Sluijs FJ, Kooistra HS, Voorhout G, Mol JA, van der Linde-Sipman JS, Rijnberk A: Multiple endocrine neoplasias in a dog: corticotrophic tumour, bilateral adrenocortical tumours, and pheochromocytoma. Vet Q 1998; 20:56–61. [DOI] [PubMed] [Google Scholar]
- 30.Walker MC, Jones BR, Guildford WG, Burbidge HM, Alley MR: Multiple endocrine neoplasia type 1 in a crossbred dog. J Small Anim Pract 2000; 41:67–70. [DOI] [PubMed] [Google Scholar]
- 31.Miller AD: Chapter 6 - Neoplasia and Proliferative Disorders of Nonhuman Primates In: Nonhuman Primates in Biomedical Research (Second Edition). Abee CR, Mansfield K, Tardif S & Morris T (eds). Boston: Academic Press; 2012: 325–356. [Google Scholar]