Abstract
Since many types of cancers overexpress EGFR, this surface receptor has been used as a target for therapy or diagnosis of malignant disease. Uptake kinetics of EGFR-targeted fluorescent Affibody (ABY-029) were studied with a view toward optimizing efficacy of tumor detection in a glioma as a function of both delivered dose and concurrent administration of unlabeled cetuximab (an EGFR antagonist). U251 glioma cells were inoculated in brain of nude rats and the fluorescence from each brain was analyzed after administration of ABY-029. Although cetuximab was able to systematically block ABY-029 binding to EGFR in a dose-dependent manner in cell culture, no influence on the tumor-to-normal brain contrast was seen when unlabeled cetuximab was administered prior to ABY-029. Ex vivo imaging of ABY-029 fluorescence showed increasing values of the tumor-to-normal brain ratio with an increasing injected dose. A saturation value was obtained at a dose of 245 µg/kg which represents a 10-fold increase over a ‘microdose’ value. According to FDA, the microdose of protein products is considered ≤ 30 nanomoles due to its difference in molecular weight as compared to synthetic drugs. This observation indicates that glioma detection will be optimal if the ABY-029 dose exceeds the ‘microdose’ value.
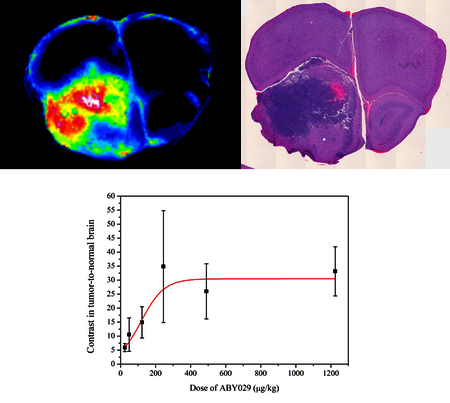
We have examined how the ABY-029 dose injected in rats affects the contrast seen in brain tumors. The results showed a linear dose-response relationship, followed by an apparent saturation in the contrast, occurring above 10× ABY-029 microdose administration. these experiments carried out in preclinical tumor models, taken together with toxicological studies, are important to guide the success of its phase 0 clinical trials.
INTRODUCTION
Fluorescence guided surgery (FGS) is an emerging approach to improve tumor resection through fluorescent labels that provide the surgeon with some level of molecular specificity of the tissue during resection. Pilot human studies have been performed for ovarian cancer (1), head and neck cancer (2), pancreatic cancer (3), colorectal cancer (4), and glioblastoma (5–7). Several studies have reported the use of antibodies conjugated to a near-infrared (NIR) fluorescence imaging probe to detect cancer specific cell-surface receptors, such as epidermal growth factor receptor (EGFR), folate receptor-α (FR-α), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) (1,2,8–10). Recent work with Affibody molecules has shown the advantage of increased tissue penetration into positive margins o ftumor, due to the molecules being smaller proteins (8 kDa molecular weight) than antibodies (155 kDa molecular weight) (11), but still retain reasonable affinity for the target receptor (12). Furthermore, Affibody molecules have no observed immune response effect in vivo, which is known to be an issue with some antibodies. An additional benefit of surface receptor targeting is that early phase clinical trials studies can be utilized in a Phase 0 design, provided that only a microdose of the compound is used. The aim of microdosing studies is to estimate key pharmacokinetic parameters of new chemical entities (13). However, the design of these trials needs to be done in a manner to maximizes contrast, i. e., to improve the differentiation of tumor and normal tissues, which can be challenging with the non-linear kinetics of uptake, binding and clearance, and it is known that lower doses can lead to lower contrast.
In previous work using ABY-029 (an anti-EGFR Affibody molecule conjugated to the near-infrared IRDye800CW®) as a fluorescent marker to detect EGFR positive (EGFR+) tumors, an increase in contrast of tumor-to-normal brain tissue was observed in a nearly linear dose-dependent manner (14). This same molecule has now entered into a Phase 0 clinical trial for glioma imaging in neurosurgery (clinical trial #NCT02901925), where doses are designed at 1×, 3× and 6× microdose levels. Microdose was defined for ABY-029 as 24.5 µg/kg in rats, which corresponds to the analogous FDA definition of 30 nmol in a 60 kg human, as calculated by the molecular weight and total body surface area difference between humans and rats. The purpose of Phase 0 trials is to confirm the molecule activity and its value for imaging, while FDA guidance is designed to prevent any undesired pharmacodynamic effects by restricting the utilized level in humans to a microdose. However, in practice, it is feasible to request a Phase 0 design study from FDA eIND, which allows doses slightly above the microdose level to be used, provided that the safety profile is consistent with the goals of non-pharmacologic effects and is 100× lower than the no observed adverse effect level (NOAEL), as defined by the pre-clinical toxicity study. Thus, it is critical to design a Phase 0 study with the maximal allowable dose if the contrast is known to increase with injected dose, and also critical to establish the NOAEL level which allows the higher doses to be used.
The origin of the study posed here is related to the fact that the contrast observed in the tissue is affected by a range of physiological parameters, with the injected concentration in the blood being one potential primary factor, especially at the lowest concentrations. This is illustrated in Fig. 1, as a function of both temporal plasma kinetics (a) and binding dye concentration (b). Given the saturable nature of biologics that target receptors, shown in Fig. 1(b), there is likely an optimum delivered dose beyond with the contrast does not increase. Yet at the lowest concentrations, there are likely sub-optimal contrast levels. In the pre-clinical glioma studies, high fluorescence from ABY-029 was seen in the tumor up to 48 h after administration (14), and in toxicity studies the NOAEL level was found to be at least 1,000× microdose (24.5 mg/kg) in rats (15).
Figure 1.

Expected trends over time are shown for the plasma concentration (a). The plasma concentration is what supplies concentrations by convective diffusion, which can bind to receptors based upon Michaelis-Menten kinetics (b). These combine to contribute to the resulting tumor and normal tissue temporal kinetics observed in measurements (c), and the ratio of these result in the dynamics of the tumor/normal tissue contrast (d).
Cetuximab (CTX), an EGFR antagonist, is one of the most intensively studied monoclonal antibodies that has been used for EGFR targeted cancer diagnostics and therapy, including advanced stage of colorectal cancer, lung cancer, head and neck cancer, and glioblastoma. Due to EGFR being also expressed by a few normal tissues, such as liver, skin, and submaxillary salivary gland, pre-doses of unlabeled cetuximab as a receptor ‘cold dose’ have been used in FGS of patients with head and neck squamous cell carcinoma to improve the contrast tumor-to-normal tissue (16).
The primary aim of this work was to determine the administration dose that maximizes contrast in glioma tumor-to-normal brain while still staying consistent with the intentions of a microdose study. This was designed as a pre-clinical fluorescent imaging study of orthotopic EGFR+ glioma tumors, with results relevant to an ongoing clinical trial with the same agent for human neurosurgery (#NCT02901925). A secondary aim was to evaluate if pre-doses of unlabeled cetuximab administered prior to the injection of ABY-029 would have any effect on contrast in tumor-to-normal brain.
MATERIALS AND METHODS
Glioma cell line.
The human glioblastoma cell line U251 (obtained from Dr. Mark Israel, Norris Cotton Cancer Center, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA), with moderate level of EGFR expression (4.4 × 105 receptors/cell) (17), was used in all studies. Culture was performed in DMEM medium, supplemented with 10% fetal bovine serum and 100 IU/mL penicillin-streptomycin. The cells were grown at 37 °C and 5% CO2 in a humid environment and sub-cultivated at 80–90% of confluence.
ABY-029 labeled affibody molecules.
Pre-good manufacturing practice (pre-GMP) conditions were used to produce ABY-029, and they have been described in detail elsewhere (15). ABY-029 (7914.95 g/mol) is composed of the 58-amino acide synthetic peptide, Z03115, an anti-EGFR Affibody molecule developed by Affibody (Sweden). Z03115-Cys was synthesized by Bachem AG (Switzerland) and conjugated to IRDye800CW maleimide (LI-COR Biosciences, Inc.) by the Vector Production Facility at the University of Alabama Birmingham (UAB, Birmingham, AL). ABY-029 for this study was used diluted from the 1000× human equivalent stock (4,900 µg/ml) provided by UAB.
Orthotopic U251 implantation in rats.
Female nude rats (6–8-week old) were randomly separated into experimental groups. The animals were inoculated with orthotopic implantations of the U251 cell line (1 × 106 cells in 5 μl phosphate-buffered saline) at a 3-mm depth into the left cerebral hemisphere and 3 mm in front to the bregma, guided by a stereotaxic frame (Stoelting Co, Wood Dale, IL, USA). Implantations were performed with animals under isoflurane anesthesia (2% at 1 L/min oxygen). An incision was made in the scalp, with the brain accessed by a 1-mm rotary drill to create a burr hole. The cells were injected over a 5-min period, and the needle was slowly retracted from the brain. Bone wax (Ethicon, Inc., Piscataway, NJ, USA) was used to close the hole in the skull and the incision in the scalp was closed using 5–0 sterile non-absorbable suture material (Ethicon, Inc., Piscataway, NJ, USA). Animals were sacrificed prior to the time of brain harvest.
All animal procedures were conducted in accordance with institutional Public Health Service (PHS) and Office of Laboratory Animal Welfare (OLAW) guidelines, and they were approved by the Dartmouth College Institutional Animal Care and Use Committee (IACUC).
Ex vivo brain tissue fluorescent imaging.
Two weeks post-tumor cell inoculation, the rats received a non-fluorescent diet (Purified Mouse Diet, MP Biomedicals, LLC, Illkirch, France) to reduce auto-fluorescence from chlorophyll contained in regular chow. Four to five weeks post-tumor inoculation, the rats in each group received by simple tail vein injection either one of 6 different doses of ABY-029 (24.5, 49, 122.5, 245, 490, or 1225 μg/kg) or preload doses of non-fluorescent cetuximab, prior to administration of the ABY-029 (1:1, 1:2.5 or 1:10 of ABY-029:cetuximab, mol:mol) at different time points (1 h or 24 h). At 1 h post ABY-029 injection, the animals were euthanized by cervical dislocation; the brain was removed and sectioned into thick slices. Three to four U251 inoculated rats were used for each group, and 3–8 slices from each animal were used for analysis.
Fluorescence images from the sequential brain sections were acquired by scanning the 2-mm thick slices with the Odyssey Infrared Imaging System (LI-COR Biosciences) using the 800-nm channel, at 21-μm lateral spatial resolution. Brain tissue slices were analyzed in a single scan, and completed within minutes of brain removal.
For fluorescence image contrast analysis, the relative difference between the fluorescence intensity in the tumor and normal brain tissue was calculated according to the equation:
where F is the fluorescence quantified in ImageJ® software (18,19), from each slice of brain tumor (FT), average of several slices of normal brain from each rat and the background value outside the brain slice from each rat (FB). The regions for analysis of both tumor and normal brain were guided by and confirmed with the hematoxylin and eosin (H&E) images of the tissue developed from formalin fixed sections after scanning. The saturating contrast in tumor-to-normal brain (L) and the midpoint of the curve (x0) were calculated by nonlinear regression fitting of the data to the sigmoidal logistic function,
Competitive EGFR binding between ABY-029 and cetuximab in vitro.
To verify the level of ABY-029 EGFR-binding activity, competitive binding assay was completed with cetuximab as a higher affinity probe in vitro with flow cytometry assay. A total of 1 × 105 U251 cells were used in each batch (precooled for 15 min at 4 °C and washed once with serum-free medium) and were incubated for 1 h, during slow movement, at 4 °C with either ABY-029, non-fluorescent cetuximab or a mixture of both. Each tube contained 1 mL of protein diluted in complete culture medium. After incubation, the culture medium was withdrawn, and the cells were washed with PBS. The cells were then resuspended in 200 µL of PBS and the fluorescence was measured by flow cytometry (MACSQuant® Analyzer 10, Miltenyi Biotec). Data analysis was performed using the FlowJo® software program.
Statistical analysis.
Shapiro-Wilk’s test was used to evaluate the normality of the data distribution. For the analysis of normally distributed groups, ANOVA with either post-hoc Tukey Test (cell experiments) or Dunnett’s Multiple Comparison Test (pre-doses of unlabeled cetuximab prior to ABY029 injection) was performed. A non-parametric Kruskal-Wallis with Dunn’s Multiple Comparison Test was applied to compare groups with non-normal distribution.
RESULTS
Affibody ABY-029 binds to EGFR in a saturable manner
The influence of ABY-029 dose on the contrast in tumor-to-normal brain was evaluated by injecting different doses of ABY-029 (10×, 20×, and 50× the microdose of 24.5 µg/kg, which is the rat equivalent microdose adjusted per unit of human surface area) and comparing with the previous results obtained for 1×, 2×, and 5× the microdose (14) (Fig. 2). Although the results show no statistical difference (P > 0.05, Kruskal-Wallis, Dunn’s Multiple Comparison Test) in the range of ABY-029 doses studied in the present work, due to the binding receptor saturation, the contrast in tumor-to-normal brain is significantly higher than 1×, 2×, and 5× the microdose. The value observed at the saturating contrast in tumor-to-normal brain was 30.5 ± 3.1 and the dose necessary to obtain half of the maximum contrast was 117 ± 25 µg/kg (about 5× the microdose), as extracted from the fitting results obtained from the sigmoid logistic function shown in Fig. 2.
Figure 2.

The observed ABY-029 contrast in tumor-to-normal brain is shown versus administered dose, where each dose of ABY-029 was sampled from multiple animals and tissue slices as shown by the standard deviation indicated by error bars. A logistic function fitting was completed to estimate the observed saturation level of the data at higher concentrations (Adjusted R2 = 0.93). One outlier in the dose of 245 µg/kg was removed which was 3× standard deviations outside the other data points.
A representative example of the spatial pattern of ABY-029 fluorescence within the brain tumor is shown in Fig. 3. As expected, ABY-029 distribution was heterogeneous, which is associated to the heterogeneity of the blood vessels pattern and EGFR density.
Figure 3.
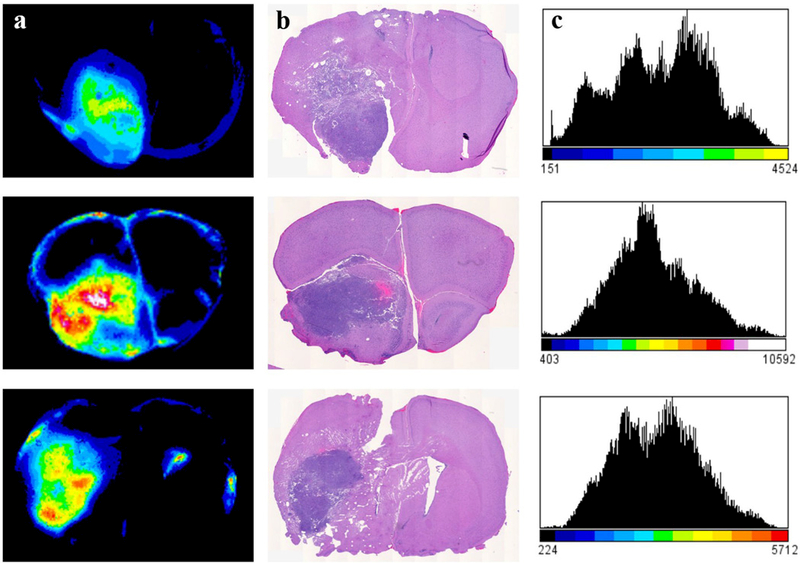
Tissue slices and histology comparisons show (a) ABY-029 fluorescence in the brain slices, with corresponding H&E histology slice (b) showing the co-registration between ABY-029 fluorescence and tumor, and (c) the histogram of fluorescence range heterogeneity from ABY-029 in the tumor region. Doses of ABY-029 from top to bottom: 245 µg/kg, 490 µg/kg, and 1225 µg/kg, respectively.
Binding of ABY-029 to EGFR was blocked by cetuximab in vitro
In order to determine if cetuximab antibody would block the ABY-029 binding to EGFR, cell experiments were performed in the U251 line. As seen in Fig. 4, cetuximab competes for EGFR binding with ABY-029 in a concentration-dependent manner. Cetuximab was able to decrease ABY-029 fluorescence by 1.74× (ABY-029:CTX 20:1, molar ratio), and the remaining Affibody fluorescence was ~2% when a 20-fold excess of unlabeled cetuximab was added.
Figure 4.
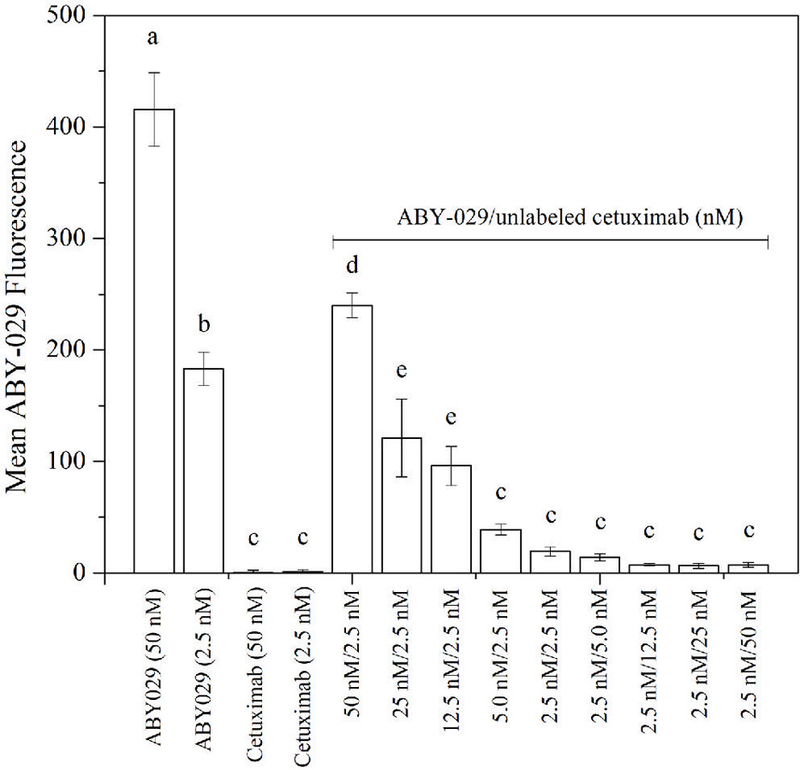
Inhibition of ABY-029 binding to EGFR by unlabeled cetuximab was investigated, with bars showing the incubation conditions. Cetuximab bars show the autofluorescence. Shared letters mean no statistical difference (ANOVA, Tukey’s Multiple Comparison Test).
Injection of unlabeled cetuximab in brain tumor-bearing nude rats prior to ABY-029 administration has no influence on the contrast in tumor-to-normal brain
In order to evaluate if the administration of unlabeled cetuximab could change the contrast in tumor-to-normal brain, different cetuximab concentrations were administered either 1 or 24 h before the injection of ABY-029 microdose. The results are presented in Fig. 5, and show no statistical significance when unlabeled cetuximab was pre-administered as compared to ABY-029 alone (as described in our previous publication, as well as in Fig. 2) (14) (P > 0.05, ANOVA, Dunnett’s Multiple Comparison Test).
Figure 5.
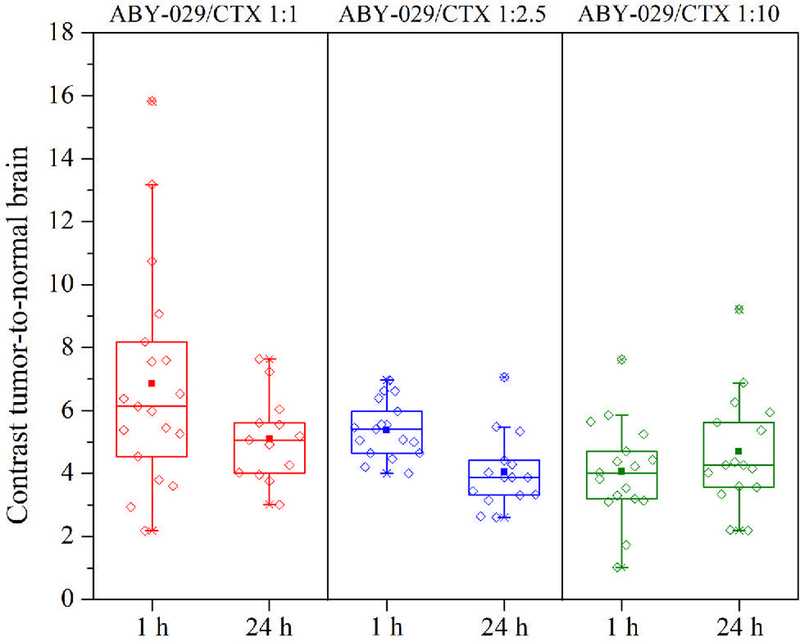
Effect of pre-injection of unlabeled cetuximab on contrast tumor-to-normal brain, where the ratio of ABY-029 to cetuximab was varied, as denoted by the three columns, 1:1, 1:2.5 and 1:10, with the assay done at both 1 h and 24 h.
DISCUSSION
It is now possible to identify cellular surface antigens that are overexpressed, mutated, or selectively expressed by tumor cells, compared to normal tissues (20), and to exploit these biomarkers as targets to treat diseases, using therapeutic levels of antibodies which can block or suppress cell signaling. These adjuvant therapies have been widely implemented for the treatment of different types of cancer, with cetuximab treatment for EGFR overexpression being a major therapeutic strategy for EGFR+ cancers. The use of ABY-029 has been directed as a diagnostic signal, to guide surgery with fluorescence in the tissue, whose intensity is a reporter of the phenotypic expression of the receptor. In this study we investigated whether the fluorescence contrast from ABY-029 varied as a function of the injected dose, and secondarily if this would be influenced by the pre-administration of cetuximab, as used in some cold dose studies in nuclear medicine.
Based on a previous observation that there was an enhancement of contrast in tumor-to-normal brain as a result of ABY-029 modest dose escalation (14), in this work we further increased the ABY-029 dose levels to include 10×, 20×, and 50× the microdose level. A linear dose-response relationship was observed until 10× the ABY-029 microdose, which may be correlated to EGFR saturation. It is also possible that the increase in contrast is related to background fluorescence signals. However, no matter the origin there was an apparent contrast increase with increasing administered dose. These experiments, carried out in preclinical tumor models, taken together with toxicological studies (15), are important to guide the success of the phase 0 clinical trials.
Cetuximab inhibits in vitro Affibody binding, due to its affinity to EGFR being over an order of magnitude higher than that of ABY-029 Affibody (KD = 0.1 nM vs. KD = 2.8 nM, respectively). It is worth pointing out that, despite cetuximab having high specificity to EGFR, it did not have any impact in contrast in tumor-to-normal brain when injected either 1 or 24 h before ABY-029 injection. A new insight drawn from our study is that unlabeled cetuximab, since it has more affinity to EGFR than the Affibody molecule, could increase the contrast in tumor-to-normal brain by binding to “sink” EGFR present in the skin and liver. Providing a non-fluorescent ‘cold-dose’ of cetuximab to bind to biological “sinks” of EGFR has been previously demonstrated in fluorescence guided surgery of head and neck cancers, and may therefore also apply to brain cancers (16). However, this hypothesis could not be tested in this work because cetuximab is a human antibody which does not bind to rat EGFR. Future studies with either humanized EGFR in pre-clinical models or in human studies might be useful to examine the value of this concept with ABY-029.
CONCLUSION
In vivo studies using ABY-029 for tumor guidance in surgery need to be designed to appreciate that the uptake increases with the increase in dose up to a point, and that the binding to in vivo EGFR is saturable above injected doses just over the microdose level. Maximal tumor to normal tissue contrast appears to require more than a microdose to achieve maximal contrast, with suggested target doses nearer 10× the microdose level.
ACKNOWLEDGEMENTS:
This work has been supported by NIH research grant R01 CA167413. ALRS acknowledges funding from CAPES—Proc n° BEX 1376/14-4 (Brazil). Flow cytometry analysis were carried out in DartLab, the Immune Monitoring and Flow Cytometry Shared Resource, supported by a National Cancer Institute Cancer Center Support Grant to the Norris Cotton Cancer Center (P30CA023108-37) and an Immunology COBRE Grant (P30GM103415-15) from the National Institute of General Medical Sciences.
REFERENCES
- 1.van Dam GM, Themelis G, Crane LMA, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJG, van der Zee AGJ, Bart J, Low PS, Ntziachristos V (2011) Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med 17, 1315–1319 [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal EL, Warram JM, De Boer E, Chung TK, Korb ML, Brandwein-Gensler M, Strong TV, Schmalbach CE, Morlandt AB, Agarwal G, Hartman YE, Carroll WR, Richman JS, Clemons LK, Nabell LM, Zinn KR (2015) Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin. Cancer Res 21, 3658–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metildi CA, Kaushal S, Pu M, Messer KA, Luiken GA, Moossa AR, Hoffman RM, Bouvet M (2014) Fluorescence-guided surgery with a fluorophore-conjugated antibody to carcinoembryonic antigen (CEA), that highlights the tumor, improves surgical resection and increases survival in orthotopic mouse models of human pancreatic cancer. Ann. Surg. Oncol 21, 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushal S, McElroy MK, Luiken GA, Talamini MA, Moossa AR, Hoffman RM, Bouvet M (2008) Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J. Gastrointest. Surg 12, 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7, 392–401. [DOI] [PubMed] [Google Scholar]
- 6.Roberts DW, Valdés PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD (2011) Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between δ-aminolevulinic acid–induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J. Neurosurg 114, 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Rey-Dios R, Roberts DW, Valdés PA, Cohen-Gadol AA (2014) Intraoperative fluorescence-guided resection of high-grade gliomas: A comparison of the present techniques and evolution of future strategies. World Neurosurg 82, 175–185. [DOI] [PubMed] [Google Scholar]
- 8.McElroy M, Kaushal S, Luiken GA, Talamini MA, Moossa AR, Hoffman RM (2008) Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J. Surg 32, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metildi CA, Kaushal S, Luiken GA, Talamini MA, Hoffman RM, Bouvet M (2014) Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in an orthotopic nude mouse model. J. Surg. Oncol 109, 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maawy AA, Hiroshima Y, Zhang Y, Luiken GA, Hoffman RM, Bouvet M (2014) Polyethylene glycol (PEG) linked to near infrared (NIR) dyes conjugated to chimeric anti-carcinoembryonic antigen (CEA) antibody enhances imaging of liver metastases in a nude-mouse model of human colon cancer. PLoS One 9, e97965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sexton K, Tichauer K, Samkoe KS, Gunn J, Hoopes PJ, Pogue BW (2013) Fluorescent Affibody Peptide Penetration in Glioma Margin Is Superior to Full Antibody. PLoS One 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lofblom J, Feldwisch J, Tolmachev V, Carlsson J, Stahl S, Frejd FY (2010) Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett 584, 2670–2680 [DOI] [PubMed] [Google Scholar]
- 13.Tewari T, Mukherjee S (2010) Microdosing: concept, application and relevance. Perspect Clin. Res 1, 61–63. [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza ALR, Marra K, Gunn J, Samkoe KS, Hoopes PJ, Feldwisch J, Paulsen KD, Pogue BW (2017) Fluorescent Affibody Molecule Administered In Vivo at a Microdose Level Labels EGFR Expressing Glioma Tumor Regions. Mol. Imaging Biol 19, 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samkoe KS, Gunn JR, Marra K, Hull SM, Moodie KL, Feldwisch J, Strong TV, Draney DR, Hoopes PJ, Roberts DW, Paulsen K, Pogue BW (2017) Toxicity and pharmacokinetic profile for single-dose injection of ABY-029: A fluorescent anti-EGFR synthetic affibody molecule for human use. Mol. Imaging Biol 19, 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore LS, Rosenthal EL, de Boer E, Prince AC, Patel N, Richman JM. Morlandt AB, Carroll WR, Zinn KR, Warram JM (2017) Effects of an unlabeled loading dose on tumor-specific uptake of a fluorescently labeled antibody for optical surgical navigation. Mol. Imaging Biol 19, 610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tichauer KM, Samkoe KS, Sexton KJ, Hextrum SK, Yang HH, Klubben WS, Gunn JR, Hasan T, Pogue BW (2012) In vivo quantification of tumor receptor binding potential with dual-reporter molecular imaging. Mol. Imaging Biol 14, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW (2015) The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev 82, 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott AM, Wolchok JD, Old LJ (2012) Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287. [DOI] [PubMed] [Google Scholar]


