Abstract
Curcumin has well-established anti-cancer properties in vitro, however its therapeutic potential has been hindered by its poor bioavailability. Lipocurc™ is a proprietary liposome-encapsulated curcumin formulation that enables intravenous delivery and has been shown to reach its highest concentration within lung tissue. The goal of this study was to characterize the anti-cancer and anti-angiogenic activity of Lipocurc™ in vitro, in addition to evaluating Lipocurc™ infusions in dogs with naturally occurring cancer. We therefore evaluated the effect of Lipocurc™, relative to free curcumin, on the viability of canine osteosarcoma, melanoma and mammary carcinoma cell lines, as well as the ability of Lipocurc™ to inhibit endothelial cell viability, migration and tube formation. We also undertook a pilot clinical trial consisting of four weekly 8-hour Lipocurc™ infusions in 10 cancer-bearing dogs. Tumor cell proliferation was inhibited by curcumin at concentrations exceeding those achievable in the lung tissue of dogs. Similarly, equivalent high concentrations of Lipocurc™ and curcumin also inhibited endothelial cell viability, migration and tube formation. Four out of six dogs completing planned infusions of Lipocurc™ experienced stable disease, however, no radiographic responses were detected.
Keywords: Curcumin, liposomes, dogs, metastasis, lung
Introduction
Primary and metastatic neoplasia within the lung is a common cause of cancer-related mortality in pet dogs. Therapeutic options for these cancers remain limited when the extent of disease precludes surgical excision, and current chemotherapeutic protocols generally offer unimpressive response rates in this species. Studies of dogs with metastatic OSA treated with a variety of different therapeutic protocols including chemotherapy and/or radiation therapy yielded median survival times between 61 and 95 days.1–4 Response rates to chemotherapeutics in dogs with pulmonary metastases from solid tumors are typically below 30%.2,4–6 These studies illustrate the need for development of therapeutic agents that can enhance the activity, without increasing toxicity, of established therapeutic protocols.
Curcumin is a phytochemical isolated from the rhizome of turmeric (Curcuma longa), and has been used in traditional medicine in India and China for hundreds of years.7 Recently, it has drawn interest as a potential anticancer agent due to its suppressive and chemosensitizing activity in vitro against OSA, as well as colon, breast, bladder, lung, pancreatic and prostate carcinomas, amongst others.8–26 Curcumin’s anti-tumor effects on cancer cells in vitro have included proapoptotic activity,10–14 inhibition of cell proliferation,15,16,27,28 and inhibition of angiogenesis.19,20 Furthermore, curcumin may also exert a therapeutic effect via its broad anti-inflammatory activity mediated by modulation of IL1β, IL6, and TNFα.29 In vivo anticancer activity has also been illustrated in rodent models of OSA, and breast, ovarian and pancreatic carcinomas.12,18,20,26,30 Various formulations of curcumin have been evaluated in Phase I and II clinical trials for several human cancer types and are consistently well tolerated, although randomized controlled trials are needed to elucidate the added clinical benefit of curcumin.31
A major limitation of curcumin as a potential therapeutic agent however, is low bioavailability owing to a low aqueous solubility, suboptimal tissue absorption, and rapid metabolism and elimination.32 Less than 1% of curcumin administered orally will be absorbed, and subsequent glucuronidation and sulfation by gastrointestinal mucosa followed by reductive metabolism and conjugation in the liver ensures that minimal amounts of unchanged curcumin reach systemic circulation.33 These properties have significantly hindered the application of curcumin in the clinical setting. While most studies report a concentration of curcumin at which cancer cell growth is inhibited by 50% in vitro (IC50) in the low micromolar range (e.g. 10 – 20 μM), oral dosing of curcumin at doses up to 12 grams results in plasma and tissue levels in the low nanomolar range.8,11,15,20,32 As a result, several different formulations of curcumin and modified schedules of curcumin administration have been developed in an attempt to increase bioavailability and tissue exposure to the active components. Lipocurc™ (SignPath Pharma Inc.) is a proprietary liposomal curcumin preparation that enables intravenous drug delivery, avoiding the problem of poor bioavailability associated with oral administration. Preclinical studies in beagle dogs revealed that while a short infusion of 20 mg/kg resulted in hemolysis, infusions of 10 mg/kg over 2 hours were non-toxic.34 Pharmacokinetic studies in beagle dogs revealed that Lipocurc™ reaches concentrations in lung tissue that are 24-fold higher than plasma. Following a dose of 10 mg/kg administered over 8 hours, mean curcumin concentrations of 317.93 ng/g (0.86 μM) were measured in the lungs of 4 dogs.35
The aim of this study was to evaluate the in vitro activity of Lipocurc™ against canine cancer cell lines, and perform a pilot phase II study evaluating prolonged Lipocurc™ infusions for the treatment of canine metastatic cancers. Herein we demonstrate the ability of Lipocurc™, at high concentrations, to inhibit canine cancer cell proliferation, as well as endothelial cell migration and tube formation in vitro. In addition, we report the feasibility and clinical outcome of Lipocurc™ infusions in 10 dogs with primary or metastatic pulmonary neoplasia.
Methods
Cell lines and reagents
Authenticated canine OSA (cOSA) cell lines (Abrams and D17)36,37 were provided as gifts from Colorado State University, while human OSA (hOSA) cell lines (U2OS and MG63)38 were purchased from the MD Anderson Characterized Cell Line Core Facility and thus were authenticated and determined to be free of mycoplasma. All other cell lines were obtained directly from the laboratories that they were derived. Dr. Curtis Bird at Auburn University provided two canine mammary carcinoma cell lines (CMT12Q2 and CMT27),39 and two canine melanoma cell lines (UCDK9M3 and UCDK9M5)40 were provided by Dr. Michael Kent at the University of California, Davis. The mouse lung endothelial cell line (LungEC) was a kind gift from Dr. Isaiah J. Fidler and Dr. Robert Langley at MD Anderson.41 Expansion of LungECs in culture was performed at 33°C. LungECs were cultured at 37°C for a minimum of 24 hours before being used in an experiment, in order to minimize SV40 activity.41,42 All cell lines were tested in-house and determined to be free of mycoplasma.
Curcumin (Merck kGaA, cat# 8203540002) was solubilized in DMSO at a concentration of 25 mg/ml. GMP-grade Lipocurc™ was kindly supplied by the manufacturer SignPath Pharma Inc. at a concentration of 6.9 mg of curcumin per ml of solution. All cell lines were cultured in complete media (CM), consisting of DMEM supplemented with 10% fetal bovine serum and 1× penicillin/streptomycin, all from Life Technologies (Invitrogen).
Cell proliferation
All OSA cell lines were plated at 500 cells/well in a 96 well plate. The remaining cell lines were plated at various numbers determined experimentally to be optimal for their doubling time; CMT12Q2 at 700 cells/well, CMT27 at 3000 cells/well, UCDK9M3 at 1000 cells/well, UCDK9M5 at 500 cells/well, and LungECs at 5000 cells/well. Each cell line was plated in triplicate and allowed to rest overnight in CM. The following day cells were treated with DMSO (vehicle), curcumin or Lipocurc™, at concentrations between 100 and 12000 ng/ml based on previous publications.8,9,43 Additional controls included cells plated with CM only (untreated), or CM alone without cells to detect background absorbance. Cell viability was determined after 72 hours via MTS assay for all cell lines. Briefly, cell supernatants were removed and replaced with CellTiter 96®Aqueous One Solution reagent (Promega) diluted in CM at a concentration of 20 ul per 100 ul of CM. Plates were incubated at 37°C for 2 hours, and absorbance was measured at 490 nm on a Spectramax 190 spectrophotometer (Molecular Devices, Sunnyvale, CA). Percent survival was determined relative to untreated cells. MTS experiments were repeated a minimum of two times with similar results.
Statistical analysis was performed using GraphPad Prism software (version 7.0a, GraphPad Software Inc.). IC50 values were calculated using nonlinear regression modeling with normalized response values, and statistical significance was determined using a Welch’s T test or an ANOVA followed by a Tukey’s multiple comparisons test for comparison of 2, and greater than 2 groups, respectively.
Migration
Effects on cell migration were evaluated by performing a modified cell culture wound closure assay.44 LungEC cells were seeded into 12 well plates at 50,000 cells/well and allowed to grow to 90% confluence (72 hours). CM was then changed to DMEM with 1% FBS and cells were treated with vehicle (DMSO), curcumin or Lipocurc™ for 24 hours at a concentration of 1000, 4000, 10,000 and 20,000 ng/ml. After the 24-hr treatment, a 200 μL pipette tip was used to make a scratch (gap) in each well. For each well, scratch areas with gaps of similar size were marked and photographed under a light microscope and measured using ImageJ Software.45 Gaps were measured at 0, 5, 9, and 24 hours. The average initial gap distance (time 0), as measured by the distance between the 2 cell fronts, was 801 (vehicle), 737 (curcumin), and 782 (Lipocurc) pixels (arbitrary units). Percent wound closure was calculated using the following equation, where GD is the gap distance for each time point Tx:
Three wells were used for each treatment group and the average percent wound closure of the 3 wells was reported. Significance was determined by one-way ANOVA and Tukey’s multiple comparison’s test using GraphPad Prism software.
Tube formation
LungEC cells were cultured as described above. 115 μL of cold Matrigel solution (10 mg/mL, Corning #356234) was added to 12 wells of a 48 well plate on ice, which was then transferred to 37°C for 40 min to solidify.
1 × 105 LungEC cells in 100 μL of CM was added to each well, followed by 100 μL of vehicle (DMSO), curcumin, or Lipocurc™ (all dissolved in CM). The plate was mixed with gentle shaking. Drug concentrations used were 4000, 10,000, and 20,000 ng/mL and images were recorded 4 hours post treatment.
Pilot clinical study
Approval by the Clinical Trials Review Board (CTRB) and Institutional Animal Care and Use Committee (IACUC) was granted for a clinical study evaluating Lipocurc™ administration to client-owned dogs. Dogs with pulmonary neoplasia presenting to the William R. Pritchard Veterinary Medical Teaching Hospital at the University of California- Davis were considered for enrollment. To test for preliminary efficacy, a Simon minimax design was used in which the goal was to enroll at least 9 dogs.46,47 To be considered for enrollment, dogs had to have a cytologic or histopathologic diagnosis of malignant neoplasia within the lungs, or alternatively, confirmation of a primary malignant tumor along with thoracic radiographs consistent with metastatic disease. At least 1 target lesion on thoracic radiographs was required to be 2 cm or greater, in order to apply Response Evaluation Criteria in Solid Tumors (RECIST v 1.0) criteria, and relapsed/recurrent disease was allowed.48 The remaining inclusion criteria stipulated that the dog had to be older than 1 year, have a performance status of either 0 or 1 on Day 0 according to the modified Eastern Cooperative Oncology Group (ECOG) performance scheme,49 and signed informed consent be provided by the owner. Dogs were excluded if they were treated with chemotherapy or radiation therapy within 2 weeks prior to study enrollment, had any significant comorbidities, received a diagnosis of hemangiosarcoma, or had a history of immune mediated hemolytic anemia. Concurrent chemotherapy or radiation therapy was not allowed. Corticosteroids and NSAIDs were allowed if the dog had been on this therapy for at least 2 weeks prior to enrolling the trial and had no evidence of gastrointestinal bleeding.
Prior to enrolment, a physical examination was performed by a veterinarian from the oncology service, and baseline thoracic radiographs and bloodwork, including a complete blood count (CBC), serum biochemistry and urinalysis, were performed. Baseline evaluation was performed within 2 weeks of the first treatment. Liposomal curcumin was administered at a dose of 10mg/kg in an infusion delivered over 8 hours. The total dose was delivered diluted in 5% dextrose and administered at a fluid rate of 2 mls/kg/hr. Four total doses were planned on a weekly schedule. Toxicity was evaluated on a weekly basis during treatment until 1 week after the 4th dose, using the common terminology criteria for adverse events established by the Veterinary Cooperative Oncology Group (VCOG-CTCAE v 1.1).50 Weekly physical examinations and CBCs were performed, in addition to quality-of-life (QOL) assessment performed on a visual analog scale by the owner. A serum biochemistry panel and urinalysis were repeated at the final visit, 1 week after the 4th dose, in dogs completing the trial. Response was evaluated with repeat thoracic radiographs 28 days after the first infusion. Radiographs were reviewed by a board-certified veterinary radiologist (EJ) using RECIST criteria to determine response.48
Results
In vitro activity of Lipocurc™ against canine cancer cell lines
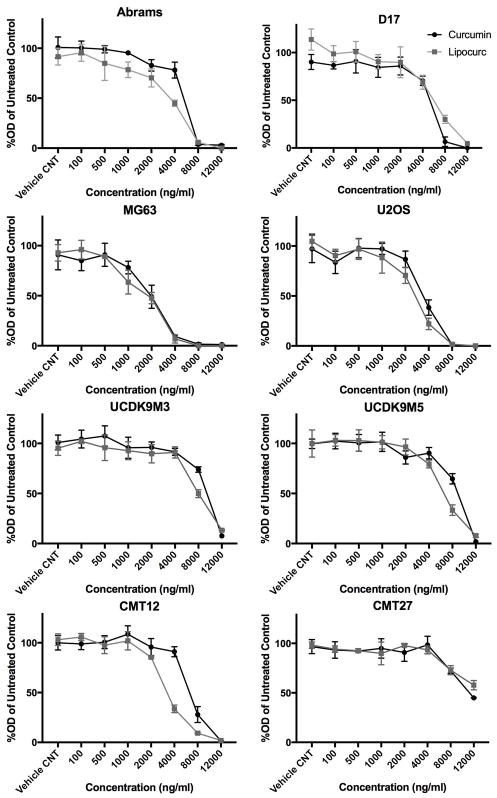
We evaluated the inhibitory effects of Lipocurc™ on several cancer cell lines including OSA (Abrams, D17, U2OS, MG62), mammary carcinoma (CMT12Q2, CMT27), and melanoma (UCDK9M3, UCDK9M5), and compared these findings to natural curcumin. Proliferation assays revealed a concentration dependent decrease in cell viability for all cell lines at concentrations above 2,000 ng/mL (Figure 1). IC50 values for each cell line treated with either curcumin or Lipocurc™ are represented in Table 1. As expected, hOSA cell lines were more sensitive to the effects of curcumin compared to cOSA cell lines (p < 0.003).9 Similarly, the D17 cell line was significantly less sensitive to Lipocurc™ than both MG63 and U2OS cell lines (p < 0.0001), while Abrams was only significantly less sensitive to Lipocurc™ than MG63 (p = 0.0025).
Figure 1.
Concentration dependent effects of curcumin and Lipocurc on cancer cell viability. cOSA (Abrams, D17), hOSA (MG63, U2OS), canine mammary carcinoma (CMT12, CMT27), and canine melanoma (UCDK9M3, UCDK9M5) cell lines were exposed to increasing concentrations of curcumin (black) or Lipocurc (grey). Viability was assessed via an MTS assay after 72 hours of drug exposure. Values represent the proportion of viable cells remaining compared to untreated controls. Error bars represent standard deviation. Results are representative of 2 separate experiments.
Table 1.
IC50 values of curcumin and Lipocurc for each cancer cell line.
| Cell line | IC50 of Curcumin (ng/ml) | IC50 of Lipocurc (ng/ml) |
|---|---|---|
| Abrams | 4939 | 2888 |
| D17 | 4744 | 5347 |
| MG63 | 1816 | 1529 |
| U2OS | 3457 | 2606 |
| UCDK9M3 | 9020 | 7797 |
| UCDK9M5 | 8454 | 6230 |
| CMT12 | 6531 | 3335 |
| CMT27 | 11187 | 14430 |
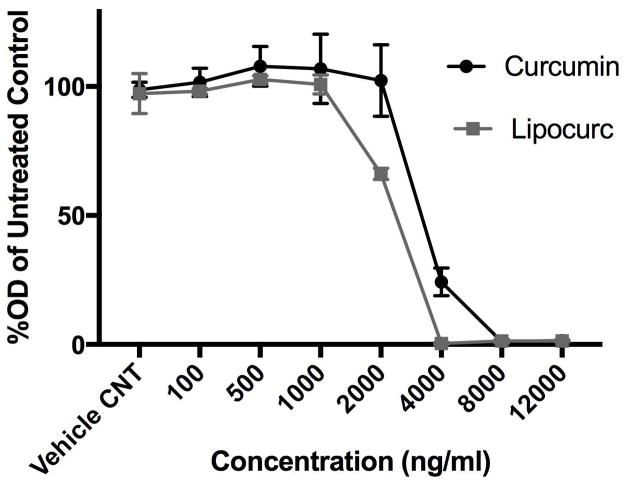
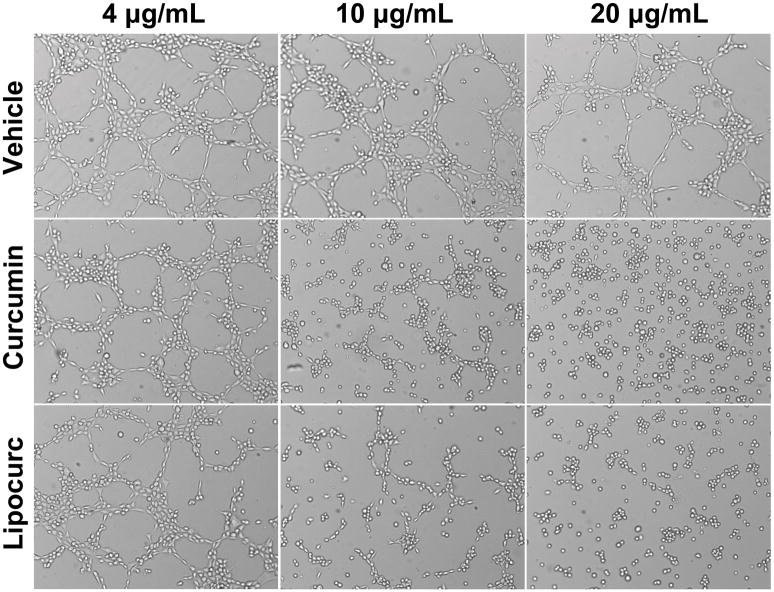
Given previous reports describing an anti-angiogenic effect of curcumin, we then evaluated the effect of Lipocurc™ exposure on LungEC viability, cell migration, and tube formation. The IC50 values of curcumin and Lipocurc™ on LungEC cells were 3786 ng/ml and 2137 ng/ml respectively (Figure 2A). Furthermore, we also observed decreased migration of LungEC with exposure to either curcumin or Lipocurc™ at a minimum concentration of 4000 ng/ml, at 5 and 9 hours using a scratch assay protocol (Figure 2B). Finally, tube formation by LungECs was also inhibited with exposure of LungECs to curcumin or Lipocurc™ (Figure 2C).
Figure 2.
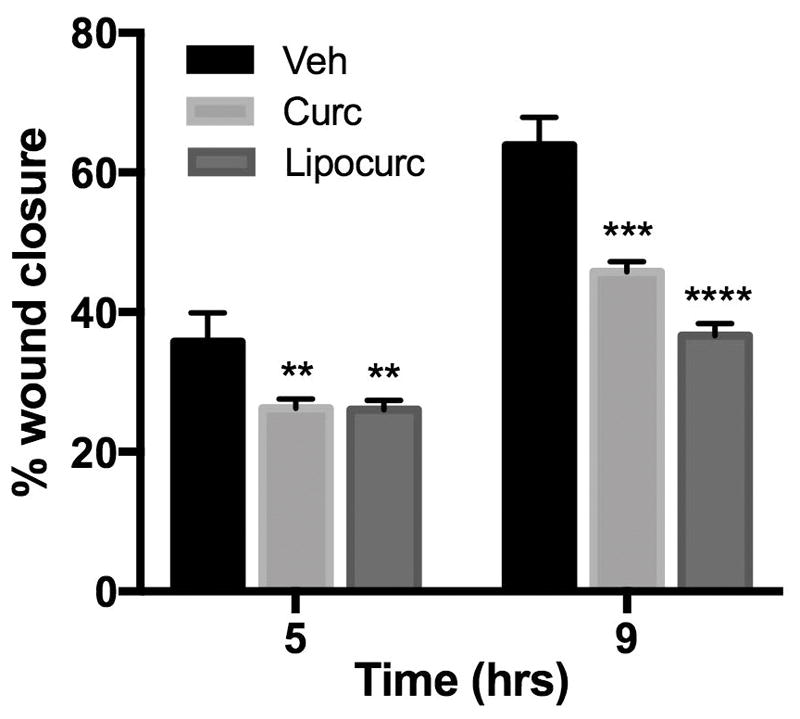
Figure 2A – Concentration dependent effects of curcumin and Lipocurc on LungEC proliferation assessed with an MTS assay after 72 hours of drug exposure. Values represent the proportion of viable cells remaining compared to untreated controls. Error bars represent standard deviation. Results are representative of 2 separate experiments.
Figure 2B – Curcumin and Lipocurc inhibit LungEC migration at a concentration of 4000 ng/ml after 5 and 9 hours. Error bars represent the standard deviation. Results are representative of 2 separate experiments. **= P≤0.01; ***= P≤0.001; ****= P≤0.0001
Figure 2C – Concentration dependent effects of curcumin and Lipocurc on LungEC tube formation. Results are representative of 2 separate experiments.
In vivo safety and efficacy of Lipocurc™ in dogs with pulmonary neoplasia
A total of 11 patients were enrolled. Patient characteristics are outlined in Table 2. The mean age of the enrolled dogs was 9.27 years (range: 5 – 13 years) and the mean weight was 25.7 kg (range: 8 – 42.3 kg). Six dogs had previously received cancer treatment, including 4 dogs that received prior surgery and chemotherapy, 1 dog that underwent surgery only, and 1 dog that received chemotherapy only. In 7 dogs, the diagnosis of pulmonary metastatic disease was made based on characteristic radiographic findings and the cytologic or histologic diagnosis of a primary malignancy. These primary cancers included 2 mammary tumors, appendicular OSA, chest wall sarcoma, thyroid carcinoma, oral malignant melanoma, and an eccrine carcinoma of the digit. In addition, 1 dog underwent a fine needle aspirate (FNA) and cytology of their lung nodules, which was consistent with metastasis from a primary high-grade perianal peripheral nerve sheath tumor (PNST). The remaining 3 dogs received a diagnosis of primary pulmonary carcinoma, either by needle core biopsy (1 dog), or via FNA and cytology (2 dogs).
Table 2.
Characteristics of dogs enrolled in the Lipocurc clinical study.
| ID | Age | Sex | Breed | Diagnosis at enrollment | No. doses | Toxicity | Response at 28 days |
|---|---|---|---|---|---|---|---|
| 1 | 7 | FS | Belgian Malinois | Presumed metastasis from appendicular OSA | 2 | Grade 1 normocytic, hypochromic, non-regenerative anemia 7 days after 2nd dose. | N/A |
| 2 | 5 | MC | Mixed breed | Presumed metastasis from chest wall sarcoma | 1 | None reported (decreasing HCT) | N/A |
| 3 | 5 | MC | Golden retriever | Cytology consistent with metastasis from perianal high grade PNST | 4 | None reported (decreased HCT) | Stable (1 lesion) |
| 4 | 13 | FS | Border Collie | Primary pulmonary carcinoma | 4 | Grade 2 AST elevation | Stable (2 lesions) |
| 5 | 10 | FS | Wirehaired fox terrier | Cytology consistent with primary pulmonary carcinoma | 0 | N/A | N/A |
| 6 | 12 | MC | Labrador | Presumed metastasis from a historical primary thyroid carcinomaa | 4 | None, static mild non-regenerative anemia | Stable (2 lesions) |
| 7 | 12 | MC | English Cocker | Presumed metastasis from oral malignant melanoma | 4 | Grade 1 regenerative anemia after last dose | Progressive (2 lesions) |
| 8 | 9 | FS | Mixed breed | Presumed metastasis from eccrine carcinoma on digit | 1 | Died at home the day after infusion following an acute progression of respiratory signs. | N/A |
| 9 | 10 | FS | Ridgeback | Cytology consistent with primary pulmonary carcinoma | 4 | Grade 2 vomiting | Stable (1 lesion) |
| 10 | 8 | F | American Cocker Spaniel | Presumed metastasis from multiple mammary tumors | 4 | Intermittent grade 1 regenerative anemia during treatment. Grade 2 allergic reaction during 4th infusion. |
Lung nodule not detected at recheck.b |
| 11 | 11 | FS | Golden Retriever | Presumed metastasis from mammary carcinoma | 1 | Became progressively weaker after infusion. Euthanized 2 days post treatment. | N/A |
FS- Female spayed; MC- Male castrated; F- Female
Identified as a bronchiolar adenoma upon histologic interpretation at necropsy.
The pulmonary lesion detected at baseline may have been superimposition of normal structures upon review. No change in the size of the primary mammary tumors was observed throughout the study period.
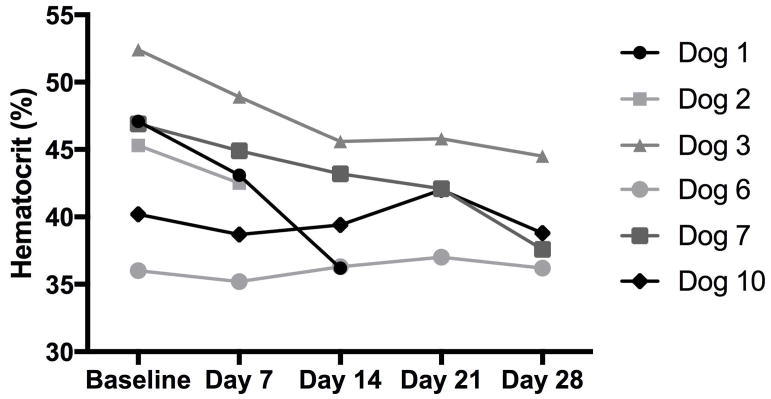
Ten dogs were available for evaluation of toxicity (all but dog 5). Amongst these 10 dogs, 3 dogs developed a grade 1 anemia during or 1 week after completion of the course of treatment (dog 1, 7 and 10). In 2 of these 3 dogs, the anemia was mildly regenerative as evident by a reticulocyte count elevation. In the remaining dog, the anemia was characterized as normocytic/hypochromic, with a reticulocyte count at the upper limits of normal (62,100/ul; reference range 7,000 – 65,000/ul). An additional dog was noted to have a mild anemia at baseline, and continued to be mildly anemic throughout treatment (dog 6). An additional 2 dogs were observed to have decreasing hematocrits (HCT) throughout treatment, although they were never overtly anemic (dog 2 and 3). Serial assessments of HCT in these 6 dogs with documented anemia or decreases in their HCT during treatment are displayed in Figure 3. Changes in serum biochemical values were only evaluable in the 6 dogs that completed all 4 planned doses of Lipocurc™. One of these 6 dogs had a mild aspartate transaminase (AST) elevation prior to any Lipocurc™ treatments, and developed a grade 2 AST elevation after completion of all 4 doses (dog 4). No other serum biochemical or urinalysis abnormalities were detected. Another dog experienced Grade 2 vomiting, during the first treatment and continued for several days after administration (dog 9). The owner of this dog had reported one episode of vomiting the day prior to the first infusion. This dog received premedication with maropitant and light sedation with acepromazine prior to subsequent treatments and did not experience any vomiting during or immediately following the remaining 3 treatments. Intense pruritus was observed during the fourth Lipocurc™ infusion in one dog, and was classified as a grade 2 allergic reaction (dog 10). Diphenhydramine was administered to this dog and her reaction was not observed to progress despite continuation of the infusion at the same rate. One dog died at home the day after the first treatment, following what the owner described as a rapid worsening in her breathing effort (dog 8). Finally, another dog appeared to experience persistent and progressive lethargy and weakness after receiving sedation for baseline thoracic radiographs that corresponded with her first treatment with Lipocurc™ (dog 11). She was euthanized 2 days after her initial treatment and necropsy revealed diffuse metastatic disease to her lungs, brain, liver and spleen.
Figure 3.
HCT of the 6 dogs with anemia or decreased HCT throughout treatment.
In total, 29 Lipocurc™ infusions were administered. Only 6 dogs received all 4 planned doses of Lipocurc™. The reasons for early withdrawal from the study of the remaining 4 dogs included progressive disease in 2 dogs (dog 1 and 2), death (dog 8), and euthanasia due to decreased quality of life (dog 11). One of the 6 dogs receiving the complete course of Lipocurc™ had progressive disease at 28 days (dog 7), and 4 dogs had stable disease at 28 days (dogs 3, 4, 6 and 9). One of these 4 dogs was enrolled with the presumptive diagnosis of metastatic thyroid carcinoma, however at necropsy this lung nodule was identified as a primary bronchiolar adenoma. This dog was euthanized 512 days after completion of the study due to acute respiratory distress after multiple bouts of aspiration pneumonia. The remaining dog experiencing stable disease at the end of the study period was enrolled with a diagnosis of primary pulmonary carcinoma. This dog received multiple doses of vinorelbine at another clinic and maintained static disease for 10 months prior to enrollment in this study. Finally, 1 of these 6 dogs had no evidence of their original lung nodule after the full course of Lipocurc™ (dog 10). On review of the thoracic radiographs performed at baseline it was considered possible that the original lesion represented fortuitous superimposition of normal structures, rather than a definitive soft tissue lung nodule. This dog had multiple mammary tumors measuring up to 1.5 cm that were diagnosed cytologically and therefore were not confirmed to be malignant.
Discussion
While the in vitro anti-cancer activity of curcumin has been studied for decades, low bioavailability and rapid clearance from the body has limited its utility in cancer-bearing patients. Liposomal curcumin however, appears to reach its highest concentrations in the lungs, and achieves peak concentrations closer to those reported to have effects on tumor cells in vitro. In this study, we identified similar effects of Lipocurc™ compared to curcumin, on inhibiting cell viability of several canine and human cancer cell lines, and inhibition occurred at concentrations equivalent to those reported previously.9 Furthermore, at high concentrations, we observed that both drugs inhibited endothelial cell viability, migration, and tube formation. Concentrations eliciting cancer and endothelial cell cytotoxicity, as well as inhibition of endothelial cell migration and tube formation, remained well above that achievable in lung tissues in prior canine studies.35 Finally, we performed a small clinical study to evaluate the potential efficacy of 8-hr Lipocurc™ infusions in dogs with pulmonary neoplasia. While several patients experienced stable disease, no overt responses were seen according to RECIST criteria. Two patient deaths occurred during the study for which any contributions of Lipocurc™ could not be completely ruled out.
We show that the anti-cancer effects of Lipocurc™ in vitro are at least equivalent to that of curcumin on canine cancer cell lines. We observed inhibition of cell viability from concentrations as low as 1000 ng/ml (MG63), or as high as 8000 ng/ml (CMT27). Our findings concur with Fossey et al. in that we detected a greater proportion of viable cOSA cells at the 4000 ng/ml concentration compared to hOSA cells, which is roughly equivalent to the 10 μM concentration used in this prior study.9 We also noted in vitro activity of curcumin and Lipocurc™ on Lung ECs, including concentration dependent decreases in cell viability, decreased migration and decreased tube formation. The in vitro and in vivo anti-angiogenic effects of curcumin have been described in several tumor models, including pancreatic, colorectal and lung carcinoma cell lines.51–53
Prior pharmacokinetic studies in beagles revealed Lipocurc™ achieves its highest peak concentration of 317.93 ng/g (+/− 101.28 ng/g) within lung tissue after an 8-hour infusion.35 Our in vitro results suggest that overt effects on cell proliferation were not apparent at these concentrations. However, while the plasma half-life of Lipocurc™ remains short, the half-life of Lipocurc™ within the lungs is unknown.34,35
Our pilot clinical study demonstrated that the application of an 8-hour Lipocurc™ infusion for the treatment of pulmonary neoplasia in client-owned dogs is generally well tolerated, however two dogs with advanced stage disease died during the study and therefore toxicity cannot be completely ruled out in these two patients. We noted the development of anemia in 3 dogs either during or shortly after completing the treatment course (regenerative or borderline regenerative), and an additional 2 dogs exhibited decreasing HCT throughout treatment. Initial pre-clinical studies in beagles revealed Lipocurc™ boluses could cause acute hemolysis upon administration.54 This side effect was abrogated with prolongation of the infusion time and a dose reduction. In addition, curcumin has chemical properties consistent with an iron chelator, and was found to decrease the HCT of mice and rats treated for several months.55,56 Therefore, it is possible that the anemia observed in our study could have been related to Lipocurc™ administration, although this finding is also common in dogs with end stage metastatic cancer.
In addition, one dog was reported to have grade II vomiting during and after their first infusion, but not after subsequent infusions prior to which antiemetics and anti-anxiety medications were given. The owner reported that this dog did have one episode of vomiting the day prior to the first infusion, and therefore it is possible that this may have been unrelated to, or exacerbated by the first infusion. In human clinical trials, mild gastrointestinal upset, including nausea, diarrhea, and abdominal distension, have been observed in people receiving oral curcumin at doses of 0.45 – 12 g/day.57,58 Another dog experienced what appeared to be a mild allergic reaction characterized by pruritus during the final Lipocurc™ infusion. Treatment with diphenhydramine enabled completion of the Lipocurc™ treatment without altering the rate of infusion. To the authors’ knowledge, allergic reactions to curcumin are quite rare, and a potential hypersensitivity reaction to other components of the liposomal formulation should be considered. One dog died at home the day after their first treatment, presumably due to complications from progressive pulmonary metastatic disease given the respiratory difficulty described by the owner. However, given that Lipocurc™ accumulates in lung tissue, toxicity from the infusion cannot be ruled out as no necropsy was performed. In addition, another dog was euthanized 2 days after her first treatment due to persistent and progressive lethargy following sedation for thoracic radiographs at her Day 0 visit. At necropsy, she was found to have metastatic disease within her brain, which could have contributed to her neurological decline, and other than her extensive metastatic disease no other significant abnormalities were detected. Lipocurc-related toxicity cannot be ruled out in these 4 dogs.
The anti-cancer effects of curcumin have been evaluated in human clinical trials for a broad range of different pre-cancerous and cancerous lesions at doses from 0.1 – 8 g orally. For example, a phase II clinical trial evaluating the activity of 8 g/day of curcumin given orally in patients with advanced pancreatic cancer, despite peak plasma curcumin levels of only 22 – 41 ng/ml, 2 out of 25 patients experienced biological activity from the treatment.59 In our study, only 6 dogs received all 4 doses of the Lipocurc™ course, of which 4 experienced stable disease. It should be noted that the treatment protocol and time to evaluation (28 days) in this study was quite short. This time point was chosen because many dogs with pulmonary metastases progress rapidly. It is possible that this 28-day study endpoint may have underestimated tumor responses occurring after more prolonged treatment. Furthermore, the RECIST criteria used in our study did not account or allow for possible transient disease progression, or psuedoprogression, as can be seen prior to sustained responses in some successful immunotherapies. An additional dog’s pulmonary nodule could not be seen on recheck radiographs after 4 weeks of treatment, although radiographic review suggested that fortuitous superimposition of anatomical structures may have led to the false appearance of a nodule on the baseline radiographs.
In summary, our results indicate that Lipocurc™ has in vitro inhibitory effects on the viability of canine cancer cell lines at concentrations known to be higher than what is achievable in canine lung tissue. At high concentrations, we also identified inhibitory effects on cell proliferation and angiogenesis in vitro. Finally, we demonstrated the administration of weekly 8-hour Lipocurc™ infusions at 10 mg/kg was feasible in cancer-bearing dogs, and observed the infusions to be well tolerated. No dogs experienced conclusive responses using this dosing scheme. This treatment schedule was previously shown to be safe in purpose-bred dogs, and appeared well tolerated by most in this small cohort of cancer-bearing dogs. However, the possible contribution of Lipocurc™ infusion to the death of 2 dogs during the trial cannot be ruled out, and further dose-finding studies in cancer-bearing pet dogs are likely indicated before proceeding with any additional clinical studies. Future studies of Lipocurc™ in dogs could also be considered in combination with other therapeutic modalities such as radiation or chemotherapy.
Acknowledgments
Lipocurc was provided as a gift from SignPath Pharma Inc.
Funding sources: Funded by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis. http://www.vetmed.ucdavis.edu/CCAH/. SW is partially supported by the American Kennel Club Canine Health Foundation Clinician-Scientist Fellowship Program. RR was supported by NCRR K01RR031272 (Office of Research Infrastructure Programs K01OD011111).
Footnotes
Conflict of Interest:
The authors have no conflicts of interest in regards to the work published in this manuscript.
References
- 1.Boston SE, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985–2004) J Am Vet Med Assoc. 2006;228(12):1905–1908. doi: 10.2460/javma.228.12.1905. [DOI] [PubMed] [Google Scholar]
- 2.Batschinski K, Dervisis NG, Kitchell BE. Evaluation of ifosfamide salvage therapy for metastatic canine osteosarcoma. Vet Comp Oncol. 2014;12(4):249–257. doi: 10.1111/j.1476-5829.2012.00355.x. [DOI] [PubMed] [Google Scholar]
- 3.Ogilvie GK, Straw RC, Jameson VJ, et al. Evaluation of single-agent chemotherapy for treatment of clinically evident osteosarcoma metastases in dogs: 45 cases (1987–1991) J Am Vet Med Assoc. 1993;202(2):304–306. [PubMed] [Google Scholar]
- 4.Laver T, London CA, Vail DM, Biller BJ, Coy J, Thamm DH. Prospective evaluation of toceranib phosphate in metastatic canine osteosarcoma. Vet Comp Oncol. 2018;16(1):E23–E29. doi: 10.1111/vco.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirier VJ, Burgess KE, Adams WM, Vail DM. Toxicity, dosage, and efficacy of vinorelbine (Navelbine) in dogs with spontaneous neoplasia. J Vet Intern Med. 2004;18(4):536–539. doi: 10.1892/0891-6640(2004)18<536:tdaeov>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Wouda RM, Miller ME, Chon E, Stein TJ. Clinical effects of vinorelbine administration in the management of various malignant tumor types in dogs: 58 cases (1997–2012) J Am Vet Med Assoc. 2015;246(11):1230–1237. doi: 10.2460/javma.246.11.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devassy JG, Nwachukwu ID, Jones PJ. Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev. 2015;73(3):155–165. doi: 10.1093/nutrit/nuu064. [DOI] [PubMed] [Google Scholar]
- 8.Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest New Drugs. 2008;26(4):289–297. doi: 10.1007/s10637-007-9099-7. [DOI] [PubMed] [Google Scholar]
- 9.Fossey SL, Bear MD, Lin J, et al. The novel curcumin analog FLLL32 decreases STAT3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer. 2011;11:112. doi: 10.1186/1471-2407-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 11.Watson JL, Hill R, Yaffe PB, et al. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010;297(1):1–8. doi: 10.1016/j.canlet.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Tian B, Wang Z, Zhao Y, et al. Effects of curcumin on bladder cancer cells and development of urothelial tumors in a rat bladder carcinogenesis model. Cancer Lett. 2008;264(2):299–308. doi: 10.1016/j.canlet.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Yang CL, Ma YG, Xue YX, Liu YY, Xie H, Qiu GR. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012;31(2):139–150. doi: 10.1089/dna.2011.1300. [DOI] [PubMed] [Google Scholar]
- 14.Woo JH, Kim YH, Choi YJ, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24(7):1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Loo WT, Sze SC, Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine. 2009;16(10):916–922. doi: 10.1016/j.phymed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Chung IK. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Cancer Lett. 2010;290(1):76–86. doi: 10.1016/j.canlet.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Hassan ZK, Daghestani MH. Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac J Cancer Prev. 2012;13(7):3259–3264. doi: 10.7314/apjcp.2012.13.7.3259. [DOI] [PubMed] [Google Scholar]
- 18.Bachmeier B, Nerlich AG, Iancu CM, et al. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2007;19(1–4):137–152. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- 19.El-Azab M, Hishe H, Moustafa Y, El-Awady el S. Anti-angiogenic effect of resveratrol or curcumin in Ehrlich ascites carcinoma-bearing mice. Eur J Pharmacol. 2011;652(1–3):7–14. doi: 10.1016/j.ejphar.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104(6):1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 21.Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta. 1994;1224(2):255–263. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 22.Kunwar A, Barik A, Sandur SK, Indira Priyadarsini K. Differential antioxidant/pro-oxidant activity of dimethoxycurcumin, a synthetic analogue of curcumin. Free Radic Res. 2011;45(8):959–965. doi: 10.3109/10715762.2011.571681. [DOI] [PubMed] [Google Scholar]
- 23.Holy J. Curcumin inhibits cell motility and alters microfilament organization and function in prostate cancer cells. Cell Motil Cytoskeleton. 2004;58(4):253–268. doi: 10.1002/cm.20012. [DOI] [PubMed] [Google Scholar]
- 24.Kim HI, Huang H, Cheepala S, Huang S, Chung J. Curcumin inhibition of integrin (alpha6beta4)-dependent breast cancer cell motility and invasion. Cancer Prev Res (Phila) 2008;1(5):385–391. doi: 10.1158/1940-6207.CAPR-08-0087. [DOI] [PubMed] [Google Scholar]
- 25.Kim SR, Park HJ, Bae YH, et al. Curcumin down-regulates visfatin expression and inhibits breast cancer cell invasion. Endocrinology. 2012;153(2):554–563. doi: 10.1210/en.2011-1413. [DOI] [PubMed] [Google Scholar]
- 26.Si M, Zhao J, Li X, Tian JG, Li YG, Li JM. Reversion effects of curcumin on multidrug resistance of MNNG/HOS human osteosarcoma cells in vitro and in vivo through regulation of P-glycoprotein. Chin Med J (Engl) 2013;126(21):4116–4123. [PubMed] [Google Scholar]
- 27.Wang Z, Zhang K, Zhu Y, Wang D, Shao Y, Zhang J. Curcumin inhibits hypoxia-induced proliferation and invasion of MG-63 osteosarcoma cells via downregulating Notch1. Mol Med Rep. 2017;15(4):1747–1752. doi: 10.3892/mmr.2017.6159. [DOI] [PubMed] [Google Scholar]
- 28.Chakravarti N, Myers JN, Aggarwal BB. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane) Int J Cancer. 2006;119(6):1268–1275. doi: 10.1002/ijc.21967. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, He ZM, Wang FL, et al. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur J Pharmacol. 2016;772:33–42. doi: 10.1016/j.ejphar.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 30.Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13(11):3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 31.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. The AAPS journal. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 33.Helson L. Curcumin (diferuloylmethane) delivery methods: a review. Biofactors. 2013;39(1):21–26. doi: 10.1002/biof.1080. [DOI] [PubMed] [Google Scholar]
- 34.Helson L, Bolger G, Majeed M, Vcelar B, Pucaj K, Matabudul D. Infusion pharmacokinetics of Lipocurc (liposomal curcumin) and its metabolite tetrahydrocurcumin in Beagle dogs. Anticancer Res. 2012;32(10):4365–4370. [PubMed] [Google Scholar]
- 35.Matabudul D, Pucaj K, Bolger G, Vcelar B, Majeed M, Helson L. Tissue distribution of (Lipocurc) liposomal curcumin and tetrahydrocurcumin following two- and eight-hour infusions in Beagle dogs. Anticancer Res. 2012;32(10):4359–4364. [PubMed] [Google Scholar]
- 36.Fowles JS, Dailey DD, Gustafson DL, Thamm DH, Duval DL. The Flint Animal Cancer Center (FACC) Canine Tumour Cell Line Panel: a resource for veterinary drug discovery, comparative oncology and translational medicine. Veterinary and comparative oncology. 2017;15(2):481–492. doi: 10.1111/vco.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.York D, Withers SS, Watson KD, Seo KW, Rebhun RB. Enrofloxacin enhances the effects of chemotherapy in canine osteosarcoma cells with mutant and wild-type p53. Vet Comp Oncol. 2017;15(3):1087–1100. doi: 10.1111/vco.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tirino V, Desiderio V, d’Aquino R, et al. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PloS one. 2008;3(10):e3469. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal P, Sandey M, DeInnocentes P, Bird RC. Tumor suppressor gene p16/INK4A/CDKN2A-dependent regulation into and out of the cell cycle in a spontaneous canine model of breast cancer. Journal of cellular biochemistry. 2013;114(6):1355–1363. doi: 10.1002/jcb.24476. [DOI] [PubMed] [Google Scholar]
- 40.Aina OH, Maeda Y, Harrison M, et al. Canine malignant melanoma alpha-3 integrin binding peptides. Vet Immunol Immunopathol. 2011;143(1–2):11–19. doi: 10.1016/j.vetimm.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63(11):2971–2976. [PubMed] [Google Scholar]
- 42.Rebhun RB, Langley RR, Yokoi K, Fan D, Gershenwald JE, Fidler IJ. Targeting receptor tyrosine kinase on lymphatic endothelial cells for the therapy of colon cancer lymph node metastasis. Neoplasia. 2006;8(9):747–757. doi: 10.1593/neo.06322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DS, Lee MK, Kim JH. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009;29(12):5039–5044. [PubMed] [Google Scholar]
- 44.Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp. 2014;(88):e51046. doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vail DM. Cancer clinical trials: development and implementation. Vet Clin North Am Small Anim Pract. 2007;37(6):1033–1057v. doi: 10.1016/j.cvsm.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2015;13(3):176–183. doi: 10.1111/vco.12032. [DOI] [PubMed] [Google Scholar]
- 49.Morges MA, Burton JH, Saba CF, Vail DM, Burgess KE, Thamm DH. Phase II evaluation of VDC-1101 in canine cutaneous T-cell lymphoma. J Vet Intern Med. 2014;28(5):1569–1574. doi: 10.1111/jvim.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veterinary cooperative oncology group. common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1. 1. Vet Comp Oncol. 2016;14(4):417–446. doi: 10.1111/vco.283. [DOI] [PubMed] [Google Scholar]
- 51.Rajitha B, Nagaraju GP, Shaib WL, et al. Novel synthetic curcumin analogs as potent antiangiogenic agents in colorectal cancer. Mol Carcinog. 2017;56(1):288–299. doi: 10.1002/mc.22492. [DOI] [PubMed] [Google Scholar]
- 52.Nagaraju GP, Zhu S, Ko JE, et al. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett. 2015;357(2):557–565. doi: 10.1016/j.canlet.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Jiao D, Wang J, Lu W, et al. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol Ther Oncolytics. 2016;3:16018. doi: 10.1038/mto.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Intravenous infusion of curcumin and a calcium channel blocker. 2011. [Google Scholar]
- 55.Jiao Y, Wilkinson Jt, Di X, et al. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113(2):462–469. doi: 10.1182/blood-2008-05-155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Toxicology P. NTP Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79%-85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1993;427:1–275. [PubMed] [Google Scholar]
- 57.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 58.Carroll RE, Benya RV, Turgeon DK, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4(3):354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]