Abstract
Background:
Low cancer clinical trial (CCT) enrollment may contribute to survival disparities affecting adolescents and young adults (AYAs, 15–39 years). This study evaluated whether differences in CCT availability related to treatment site could explain this low CCT enrollment.
Methods:
This prospective, observational cohort study was conducted at an academic children’s hospital and its affiliated but geographically separated adult cancer hospital within a National Cancer Institute-designated comprehensive cancer center. For consecutive, newly-diagnosed AYA patients, it was determined whether an appropriate CCT existed nationally, was available at the treatment site, and was utilized for enrollment. Proportions of AYAs in these categories were compared between sites using the χ2test.
Results:
One hundred fifty-two consecutive AYA patients were included from the children’s hospital (n=68, aged 15–20 years) and adult cancer hospital (n=84, aged 18–39 years). Although there was no difference in CCT existence for individual AYA patients by site (children’s hospital 36/68 [52.9%] versus adult cancer hospital 45/84 [53.6%], p=0.938), CCT availability was significantly lower at the adult cancer hospital (14/84 [16.7%] versus 30/68 [44.1%], p<0.001). The proportion of AYAs enrolled was low at both sites (8/68 [11.8%] versus 6/84 [7.1%], p=0.327). Fewer existing CCTs were available at the adult cancer hospital (4/27 [14.8%] versus 8/14 [57.1%], respectively), and those were directed toward solid tumors and new agents.
Conclusions:
Efforts to improve low CCT enrollment among AYAs should be differentiated by treatment site. In the adult setting, these should be aimed at improving CCT availability by overcoming site-level barriers to opening existing CCTs.
Keywords: Pediatric Oncology, Adolescent, Clinical Trial as Topic, Clinical Oncology, Multicenter Studies as Topic, AYA, Young Adult
Precis Statement:
In this prospective study comparing adolescents/young adults (AYAs, 15–39 years) treated at either a children’s hospital or its affiliated adult cancer hospital, we discovered that despite similar existence of cancer clinical trials (CCTs) nationally, significantly fewer AYAs at the adult site had an available CCT. The major implication of this study is that efforts to improve low CCT enrollment among AYAs must be differentiated by site, and in the adult setting should be aimed at overcoming institution-level barriers to opening existing CCTs.
INTRODUCTION
Lagging survival improvement compared with both younger and older patients is prominent among the many challenges facing adolescents and young adults (AYAs, aged 15–39 years) with cancer, as identified by the United States (US) National Cancer Institute (NCI). 1–3 The cause of this lower survival improvement is likely multifactorial, but low participation of AYAs in NCI-sponsored cancer clinical trials (CCTs) is considered a key contributor.4 Low CCT enrollment also prevents AYAs from gaining access to promising investigational therapies, providing biospecimens essential for basic and translational research, and offering their unique perspective in studies of supportive care, quality of life, and other non-survival endpoints.5
There are multiple reports of low CCT enrollment for AYAs in the literature. Compared with approximately 40–60% of children < 15 years of age, only 10–20% of early AYAs (15–21 years old) and less than 10% of older AYAs participate in CCTs.1,4,6–9 At our own institution, 15% of early AYAs treated at our children’s hospital were enrolled onto clinical trials compared with < 7% of older AYAs treated at our adult cancer hospital.8 Reasons for lower CCT enrollment of AYAs are not well understood. Factors associated with low CCT enrollment include suboptimal insurance, low socioeconomic status, distance to the cancer center, older age, type of cancer specialist, treatment at non-academic institutions, and clinicians who do not participate in NCI-sponsored CCTs.7,10,11 Limited availability of CCTs for AYAs is another potential factor often speculated to be of major importance.7,9,12,13 In theory, a lack of CCTs would be decisive in that without an available trial enrollment is impossible.5
However, we recently conducted a prospective, observational cohort study of CCT availability and enrollment among early AYAs within our academic children’s hospital. The prospective, case-level methodology we used allowed real-time ascertainment of individual patient eligibility in relation to CCTs confirmed open at our institution. Unexpectedly, we discovered that although CCT enrollment was significantly lower for early AYAs compared with children,14 CCT availability was no different. Our findings suggested that, in the setting of a children’s hospital, provider and patient/family-level barriers involving priorities, knowledge, beliefs and attitudes were more critical than CCT availability.
Yet CCT enrollment barriers may not be the same for early AYAs and older AYAs due to differences in life-stage and the type of treating institution. Additionally, it is commonly hypothesized, though without compelling data, that a major cause of low enrollment is that CCT availability is lower for older than for younger AYAs.7,15 To explore these potential differences, we conducted this prospective study comparing front-line therapeutic CCT national existence, institutional availability, and utilization for enrollment among AYAs diagnosed at either the academic children’s hospital or the adult cancer hospital within our NCI-designated comprehensive cancer center. The primary study aim was to compare the proportions of AYAs at each location for whom an appropriate CCT existed, was available locally, and was utilized for enrollment. Our overall hypothesis was that all three components would be significantly lower among AYAs at the adult cancer hospital.
METHODS
Study Design, Setting and Case Ascertainment
This was a prospective, observational cohort study conducted at two sites that are part of the University of Southern California (USC) Norris Comprehensive Cancer Center: Children’s Hospital Los Angles (CHLA, an academic children’s hospital) and the Norris Cancer Hospital (NCH, an adult cancer hospital). Though affiliated, these two sites are clinically and administratively distinct, located on separate campuses about 6 miles apart. In general, AYAs 15–21 years of age are treated at CHLA, while AYAs aged 18–39 years are treated at NCH. The two sites have a relationship as members of an over-arching AYA program, but a coordinated CCT enrollment mechanism for AYAs has not been implemented as yet. Finally, the institutions utilize separate Institutional Review Boards (IRBs).
To confirm the feasibility of our methods, we first conducted a pilot study of ten patients at CHLA and ten patients at NCH, who are included in this cohort. Case ascertainment was site-specific to allow for concurrent, prospective identification of newly-diagnosed patients. At CHLA, all consecutive pathology reports from patients aged 15–21 years were screened in real-time by collaborating pediatric pathology fellows (HT, JS) and transmitted to the principal investigator (SMT) with name, medical record number, and final pathology diagnosis. Pathology reports were reviewed by SMT and disregarded if they represented pathology-only consultations for patients not receiving cancer care at CHLA, or pathology specimens from second surgeries for patients already included in this study. The remaining pathology reports were used to identify unique patients 15–21 years old with first diagnosis of cancer who had cancer treatment initiated at CHLA. At NCH, Cancer Registry staff identified newly-diagnosed patients 15–39 years of age and transmitted their names, medical record numbers and cancer diagnosis to SMT every two weeks. SMT reviewed the associated medical records to identify unique patients aged 15–39 with a first diagnosis of cancer and cancer treatment initiated at NCH. At both sites, patients with relapsed cancer, subsequent malignant neoplasm, or history of already starting cancer treatment elsewhere were excluded.
For each eligible patient, pertinent demographic (age, sex, and race/ethnicity) and disease-related (cancer diagnosis, stage, grade, risk group and relevant genomics) information was abstracted from the medical record. Patients were classified by treatment site. Both the CHLA and USC IRBs approved this study with a waiver for informed consent, as only anonymous data were collected and there was no patient contact.
Determination of CCT Existence, Availability, and Enrollment Status
At both sites, the following methods were applied for each eligible patient. For purposes of this study, “clinical trial existence” was operationalized as a CCT appropriate for the patient’s age, diagnosis, and stage/risk-group registered nationally on ClinicalTrials.gov and listed as open and recruiting. “Clinical trial availability” was operationalized as an existing CCT that was IRB-approved, activated and open to enrollment at the site when that patient was diagnosed. “Clinical trial enrollment” was operationalized as the patient being successfully entered onto the CCT according to standard procedures of the respective clinical trials support office.
For each patient, existence of a CCT was determined by SMT within two weeks of receiving the diagnosis by searching ClinicalTrials.gov for a relevant CCT. The two-week timeframe was established because trial sponsors are required to post updates on ClinicalTrials.gov within four weeks of changes in enrolling status. The diagnosis was entered into the search term area with studies limited to those “open and recruiting,” “interventional,” and available in the US. If a trial was so identified, patient-specific clinical information (e.g., histology, stage, grade, risk group, genomic status, and other characteristics) was employed to determine the patient’s specific eligibility for that trial. Those confirmed as eligible were thus classified as having an existing CCT. Trial-specific data available on ClinicalTrials.gov were recorded including the National Clinical Trial identification number, phase, categorical type (e.g., National Clinical Trials Network [NCTN] group, other national collaborative group, multi-center collaboration, industry, or institutional), the specific sponsor, and the date the trial opened. Patients with an existing CCT were then assessed for availability of and enrollment upon the CCT. Chart review was reassessed weekly until treatment was initiated to capture any CCTs that may have been activated after diagnosis but prior to therapy initiation allowing for “just-in-time” activation of CCTs.
For patients who had more than one applicable trial listed on ClinicalTrials.gov but none available at their treatment site, only one trial was recorded as existing using the following hierarchy: NCTN, other national collaborative group, multi-center collaboration, industry-sponsored, or institutional.
Statistical Analysis
Descriptive statistics were used to characterize the sample. Differences in demographics, CCT existence, CCT availability, and CCT enrollment among AYAs treated at CHLA versus at NCH were evaluated using the χ2 test of proportions with a p-value of < 0.05 defined as significant. SPSS statistical software was used for all analyses16.
RESULTS
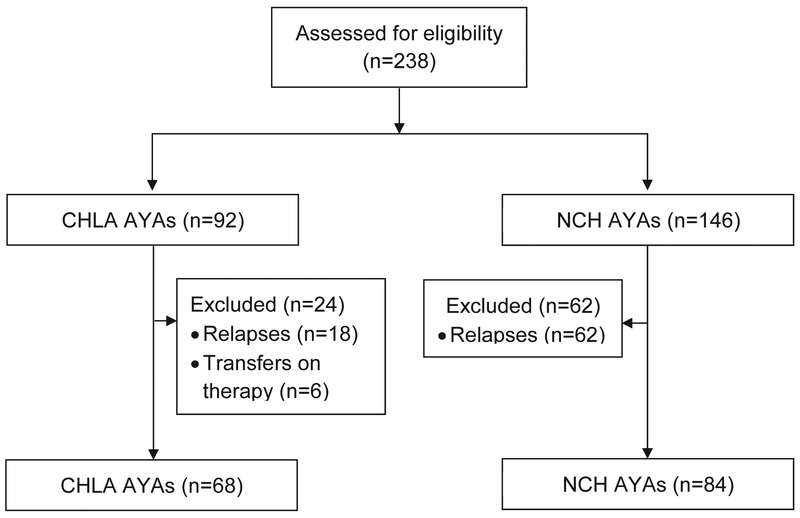
Between November 1, 2015 and December 1, 2016, a total of 238 unique AYA patients were screened for eligibility (CHLA = 92; NCH=146). Of these, 86 were excluded for having relapsed disease or starting treatment prior to referral (CHLA = 24, NCH = 62; Figure 1). There were no patients with SMN.
Figure 1:
CONSORT diagram of the current study comparing cancer clinical trial availability for adolescent and young adult (AYA) patients treated at Children’s Hospital Los Angeles (CHLA) or the adult-focused Norris Cancer Hospital (NCH).
Patient Characteristics
A total of 152 AYAs were included in this study (CHLA = 68, NCH = 84; Table 1). The median ages of AYAs at CHLA and NCH were 17 years (range, 15–20) and 31 years (range, 18–39), respectively. There were 22 AYAs in the age bracket of 18–21 years who could have been treated at either site (CHLA = 18, NCH = 4). Distribution by sex and race/ethnicity was similar across groups. Whereas leukemia was the most common diagnosis at CHLA, solid tumors predominated at NCH, particularly carcinomas.
Table 1:
Clinical Characteristics of Adolescents/Young Adult Cohort (n=152)
| CHLA1 n (%) |
NCH2 n (%) |
|
|---|---|---|
| Total | 68 (43.9) | 84 (56.1) |
| Age Distribution (years) | ||
| 15-17 | 50 (73.5) | 0 (0) |
| 18-21 | 18 (26.5) | 4 (4.8) |
| 22-25 | 0 (0) | 14 (16.7) |
| 26-30 | 0 (0) | 19 (22.6) |
| 31-35 | 0 (0) | 23 (27.4) |
| 36-39 | 0 (0) | 24 (28.6) |
| Sex | ||
| Male | 43 (63.2) | 53 (63.1) |
| Female | 25 (36.8) | 31 (36.9) |
| Race/Ethnicity | ||
| Hispanic | 40 (58.8) | 37 (44.0) |
| White/Non-Hispanic | 12 (17.6) | 20 (23.8) |
| Asian | 4 (5.9) | 13 (15.5) |
| Black | 4 (5.9) | 7 (8.3) |
| Other | 8 (11.8) | 7 (8.3) |
| Diagnosis | ||
| Leukemia/lymphoma | ||
| Acute Lymphoblastic Leukemia | 16 (23.5) | 5 (6.0) |
| Acute Myeloid Leukemia | 7 (10.3) | 4 (4.8) |
| Hodgkin Lymphoma | 10 (14.7) | 3 (3.6) |
| Non-Hodgkin Lymphoma | 7 (10.3) | 11 (13.1) |
| Solid Tumor | ||
| Brain Tumors | 5 (7.4) | 8 (9.5) |
| Extra-Cranial Germ Cell Tumor | 12 (17.6) | 15 (17.9) |
| Carcinoma | 3 (4.4)3 | 29 (34.5)4 |
| Sarcoma | 7 (10.3) | 7 (8.3) |
| Other Solid Tumor | 1 (1.5)5 | 2 (2.4)6 |
Children’s Hospital Los Angeles
Norris Cancer Hospital
Thyroid (3)
Breast (8), Colorectal (7), Cervical (4) Esophageal (2), Hepatocellular (1), Lung (1), Nasopharyngeal (1), Renal (4), and Thyroid (1)
Melanoma (1)
Desmoplastic Small Round Cell Tumor (1), Gastrointestinal Stromal Tumor (1)
CCT Existence, Availability, and Enrollment Proportions
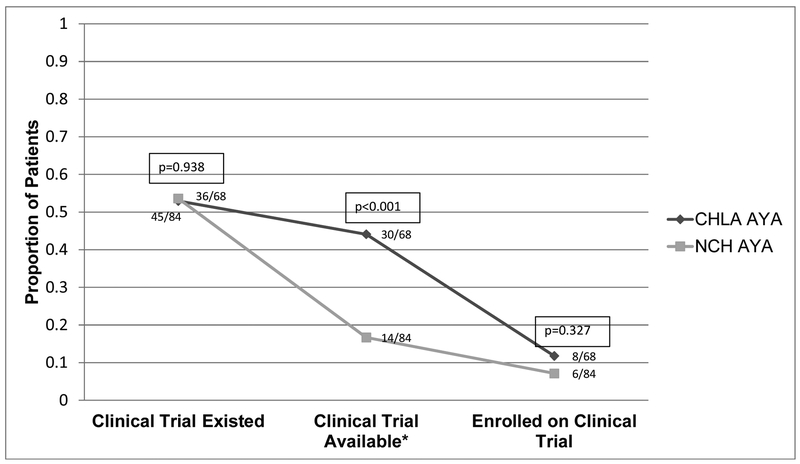
The proportions of CCT existence, availability, and enrollment were compared for AYAs treated at CHLA and NCH (Figure 2). For having an existing CCT, the proportion of AYAs at CHLA and NCH were similar (36/68 [52.9%] versus 45/84 [53.6%], respectively; p=0.938). However, the proportion of AYAs having an available CCT was significantly lower at NCH than CHLA (14/84 [16.7%] versus 30/68 [44.1%], respectively; p<0.001). Despite the greater availability of CCTs at CHLA, enrollment proportions at CHLA and NCH were similarly low (8/68 [11.8%] versus 6/84 [7.1%], respectively; p=0.327).
Figure 2:
Comparison of cancer clinical trial existence, availability, and enrollment of adolescents and young adults at Children’s Hospital Los Angeles (CHLA, n=68) and the Norris Cancer Hospital (NCH, n=84). See Methods for operational definitions of cancer clinical trial existence, availability and enrollment.
The characteristics of patients who were evaluated for CCTs are displayed in Supplemental Table 1. Although CCTs existed in all four diagnostic categories at both sites, at NCH trials were available only to AYAs with non-brain solid tumors. In this study, five AYAs had available CCTs only at the other site: at NCH, four AYAs had available CCTs at CHLA (acute lymphoblastic leukemia (ALL) = 3, Ewing sarcoma = 1), while one AYA at CHLA had a CCT available at NCH (intermediate risk testicular cancer).
A total of 30 AYAs had a trial available to them but did not enroll (CHLA=22, NCH=8). At both sites, patient age and ethnicity were similar between those enrolled and not enrolled but at CHLA males were more likely to have not enrolled compared with NCH (14/22 [63.6%] and 6/8 [75.0%]). Non-enrolled AYAs at CHLA had the following diagnoses: ALL (9), Hodgkin lymphoma (5), acute myeloid leukemia (4), acute promyelocytic leukemia (2), Ewing sarcoma (1), and synovial sarcoma (1). This differed at NCH where non-enrolled AYAs had the following diagnoses: testicular carcinoma (5), cervical carcinoma (2), and malignant peripheral nerve sheath tumor (1).
CCT Characteristics
The characteristics of CCTs that existed, were available, and were utilized for enrollment at the two sites are displayed in Table 2. At the national level, thirty-five unique trials existed for AYAs in this study. Of these, eight existed for AYAs only at CHLA, twenty-one for those only at NCH, and six for AYAs across sites. Of the trials that existed nationally, at CHLA 8/14 (57.1%) were available while at NCH only 4/27 (14.8%) were available. Of the six trials that existed nationally for AYAs across sites, three were available only at CHLA, one was available only at NCH, one was available at both sites, and one was available at neither site. The single CCT available at both sites was a COG study for non-rhabdomyosarcomatous soft tissue sarcoma opened at NCH via the NCI Cancer Trials Support Unit (CTSU) mechanism (www.ctsu.org). The eight CCTs available at CHLA included the all four diagnostic categories (leukemia, lymphoma, brain tumors, and non-brain solid tumors), while the four at NCH were for only non-brain solid tumors. At CHLA, all available CCTs were phase 3 and sponsored by the COG. In contrast, of the four available CCTs at NCH, one each was phase 1, 2, 2/3, and 3; three of these were sponsored by adult-focused NCTN groups, and one was a multi-institutional collaboration. Notably, none of the available CCTs at either site were industry-sponsored.
Table 2:
Number and Types of Cancer Clinical Trials by Treatment Site (n=35)
| Cancer Clinical Trials at CHLA1 |
Cancer Clinical Trials at NCH2 |
|||||
|---|---|---|---|---|---|---|
| Existed3
|
Available3
|
Utilized for Enrollment3 |
Existed |
Available |
Utilized for Enrollment |
|
| Total | 14 | 8 | 4 | 27 | 4 | 3 |
| Target Cancer | ||||||
| Leukemia | 4 | 4 | 2 | 3 | -- | -- |
| Lymphoma | 6 | 1 | 1 | 8 | -- | -- |
| Non-brain Solid Tumor |
3 | 2 | -- | 13 | 4 | 3 |
| Brain Tumor | 1 | 1 | 1 | 3 | -- | -- |
| Phase | ||||||
| Phase 1 | -- | -- | -- | 4 | 1 | 1 |
| Phase 1/2 | -- | -- | -- | 1 | -- | -- |
| Phase 2 | 6 | -- | -- | 14 | 1 | 1 |
| Phase 2/3 | 1 | 1 | -- | 2 | 1 | -- |
| Phase 3 | 7 | 7 | 4 | 6 | 1 | 1 |
| Sponsor | ||||||
| NCTN4 | 10 | 8 | 4 | 12 | 3 | 2 |
| Multi-site | 2 | -- | -- | 7 | 1 | 1 |
| Investigator Initiated | 2 | -- | -- | 4 | -- | -- |
| Industry | -- | -- | -- | 4 | -- | -- |
Children’s Hospital Los Angeles
Norris Cancer Hospital
See Methods for operational definitions of cancer clinical trial existence, availability and enrollment.
NCI National Clinical Trials Network
DISCUSSION
The objective of this study was to determine the existence, availability, and utilization of CCTs for enrollment of AYAs treated at either an academic children’s hospital (CHLA) or an affiliated adult cancer hospital (NCH). Our findings shed much-needed light on the CCT enrollment fate of AYAs having a first diagnosis of cancer. Although CCTs existed nationally for more than half of our AYAs at both sites, CCTs were available for only one third as many AYAs at the adult cancer hospital. Although the overall low CCT enrollment of about 10% at both sites was not unexpected and is consistent with previous reports,1,8,13,17 our findings of national-level similarity in CCT existence, but site-level disparity in CCT availability, are novel. They are significant because they demonstrate the need to account for treatment setting when considering solutions for the difficult problem of low CCT enrollment of AYAs. To our knowledge, this is the first prospective study to compare CCT existence and availability in explaining low CCT enrollment among AYAs treated at a pediatric or adult cancer hospital.
This study adds to the scant published research concerning causes for low AYA enrollment onto CCTs. In addition to our recent prospective study of CCT availability within a children’s hospital,14 we are aware of only three other reports of CCT availability for AYAs, two from one cancer center using internal data13,15 and one from the NCI using population-based estimates.18 All three of these studies were retrospective, detected relatively small effects, and yielded mixed results. Here, we have more definitively compared CCT existence and availability for AYAs treated concurrently at either a pediatric hospital (CHLA) or an adult cancer specialty hospital (NCH). The CCT enrollment pathway can be viewed as a linear process that first requires having CCTs existing at the national level, which are then made available at the institutional level, offered by the provider, and finally accepted by the patient/family.5 As potential “upstream” enrollment barriers, CCT existence and availability are particularly crucial because without available CCTs, “downstream” barriers at the provider- and patient/family level become irrelevant. In our recent study comparing CCT availability and enrollment for children versus early AYAs (15–21 years old) treated in an academic children’s hospital, we found similar CCT availability but significantly lower enrollment of early AYAs.14 Whereas those results suggested that patient/family- and provider-level barriers were more important for AYAs in the pediatric setting, our current study demonstrates unequivocally that low CCT availability was the major barrier for AYAs in our adult cancer hospital, irrespective of target cancer, phase, or sponsor of existing CCTs. However, it is important to note that the proportion of AYAs who enrolled onto a CCT when one was available was actually higher at our adult cancer hospital than at the children’s hospital. Although our study was not designed to assess physician motivation or other “downstream” barriers and facilitators of enrollment on available CCTs, it is clear from our data that distinctly different explanations may account for low CCT enrollment of AYAs, depending on their treatment setting.
Appreciating alternative causes for low CCT enrollment is important for both informing future research and developing potential solutions.5 In our study, CCT existence clearly was not the primary barrier, where, in fact, twice as many CCTs existed for AYAs at the adult cancer hospital than at the children’s hospital. The striking disparity in CCT availability in our adult site appears to have reflected a focus on solid tumors and early-phase trials (Table 2). This diagnostic specificity may not be altogether inappropriate given that three quarters of those AYAs had non-hematological cancers (Table 1). However, this emphasis on new-agent studies may not serve well the population of AYAs with newly diagnosed cancer, for whom only one phase 3 CCT was available. In this respect, the five AYAs diagnosed with ALL at our adult site would have benefited from having access to the appropriate CCT available at our children’s hospital. Now that these important gaps have been identified, further research has been initiated to elucidate the factors influencing institutional prioritization of CCTs, and development of effective mechanisms for enrolling AYAs across affiliated sites like ours.
The setting and design of our study afforded several strengths and some limitations. A significant strength was prospective, concurrent case ascertainment at two affiliated sites combined with real-time, case-linked evaluation of CCT existence and availability. This provided a level of accuracy difficult to achieve retrospectively because CCTs open and close to enrollment over time, and detailed clinical information is needed to assess trial-specific eligibility fully for individual patients. The volume and heterogeneity of patients made it feasible to conduct our study at two sites over a relatively short period of time. In order to identify patients in real-time, different methods of case ascertainment were needed at the two sites, which could have introduced different selection biases in the two cohorts. However, there were a sizeable number of relapsed/refractory patients at NCH, who were not included. Therefore, our focus on patients with first cancers prevents commenting on CCT availability for relapsed cancer, which could be important for AYAs who often have high-risk disease. Additionally, our results may not be generalizable to community-based hospitals where the majority of AYAs are treated, or to academic hospitals serving the full AYA age spectrum at a single site. Research investigating CCT availability is needed in both these populations. As our study was designed to assess only CCT availability and enrollment, reasons for non-enrollment onto available CCTs cannot be assessed from these data.
Nonetheless, several insights about the relative importance of CCT availability for AYAs can be gained from our data. First, in view of CCT existence being similar at both sites simply opening more trials nationally, as has been suggested,7,12,13 is unlikely to alter overall AYA enrollment. Low institutional CCT availability appears to be the more promising target. However, solutions for this may require a mix of site-specific and general measures. For example, while our adult site emphasized solid tumors and new agents, centers prioritizing phase 3 trials for newly-diagnosed patients with more prevalent AYA diagnoses including leukemia and lymphoma would offer greater CCT availability and would theoretically have the greatest impact on improving outcomes. This should be done in a site-specific manner, such that existing CCTs are opened for the most common AYA cancers referred to that institution. On the other hand, certain barriers to opening CCTs may apply across adult centers, such as the financial burden of conducting NCTN studies compared with industry trials (although it is notable that existing industry CCTs were equally unavailable for AYAs in our study).19 Thus, strategies that reduce burden for institutions in opening AYA-relevant phase 3 trials are likely to be impactful. In this regard, the NCI Central IRB approval process has lessened the burden for scientific review at the local sites. Finally, institutions having a relatively seamless clinical research operation are at a distinct advantage when it comes to enrolling the full age spectrum of AYAs onto CCTs. On-site partnerships across pediatric and adult oncology coupled with sharing one electronic health record and IRB, for studies that cannot utilize the NCI Central IRB, would likely facilitate CCT enrollment. Such partnering could include joint tumor boards that identify AYAs eligible for CCTs across institutions, and development of mechanisms to facilitate their enrollment, such as reciprocal cross-group affiliation of medical and pediatric oncologists and clinical cooperation in the delivery of protocol therapy. The NCI CTSU provides a “just in time” mechanism for accomplishing cross-group enrollment, which is rapid activation of CCTs for patients presenting with rare diagnoses.20 However, not all CCTs are available through the CTSU. Further, treatment sites must still have the resources and motivation to open those CCTs that are. In theory, development of an AYA program could heighten institutional awareness of these issues, garner additional resources, provide educational opportunities about the importance of trial enrollment in this population, and foster collaborations to overcome these barriers.21 Even then, as our experience illustrates, the challenges remain daunting and highlight the need for having accurate CCT availability and enrollment data in order to address the most salient barriers.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Ms. Gwendolyn Lynch for identifying newly diagnosed AYAs at NCH, and Drs. Joel Milam and Richard Sposto for reviewing an early draft of the manuscript.
Funding: This work was supported in part by the David Stroud Adolescent and Young Adult Oncology Fellowship Fund (SMT) and by grant UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health (SMT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bleyer WA, Tejeda H, Murphy SB, et al. National cancer clinical trials: children have equal access; adolescents do not. J Adolesc Health. 1997;21(6):366–373. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute LAF. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer: Report of the Adolescent and Young Adult Oncology Progress Review Group. 2006. [Google Scholar]
- 3.Smith AW, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleyer A, Montello M, Budd T, Saxman S. National survival trends of young adults with sarcoma: lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103(9):1891–1897. [DOI] [PubMed] [Google Scholar]
- 5.Freyer DR, Seibel NL. The Clinical Trials Gap for Adolescents and Young Adults with Cancer: Recent Progress and Conceptual Framework for Continued Research. Current Pediatrics Reports. 2015;3(2):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krailo MD, Bernstein L, Sullivan-Halley J, Hammond GD. Patterns of enrollment on cooperative group studies. An analysis of trends from the Los Angeles County Cancer Surveillance Program. Cancer. 1993;71(10 Suppl):3325–3330. [DOI] [PubMed] [Google Scholar]
- 7.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol 2011;29(30):4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins CL, Malvar J, Hamilton AS, Deapen DM, Freyer DR. Case-linked analysis of clinical trial enrollment among adolescents and young adults at a National Cancer Institute-designated comprehensive cancer center. Cancer. 2015;121(24):4398–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw PH, Ritchey AK. Different rates of clinical trial enrollment between adolescents and young adults aged 15 to 22 years old and children under 15 years old with cancer at a children’s hospital. J Pediatr Hematol Oncol 2007;29(12):811–814. [DOI] [PubMed] [Google Scholar]
- 10.Felgenhauer J, Hooke MC. Regulatory barriers to clinical trial enrollment of adolescent and young adult oncology patients. Pediatrics. 2014;133 Suppl 3:S119–122. [DOI] [PubMed] [Google Scholar]
- 11.Parsons HM, Harlan LC, Schmidt S, et al. Who Treats Adolescents and Young Adults with Cancer? A Report from the AYA HOPE Study. J Adolesc Young Adult Oncol 2015;4(3):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuk MK, Mulugeta Y, Roth-Cline M, Mehrotra N, Reaman GH. Enrolling Adolescents in Disease/Target-Appropriate Adult Oncology Clinical Trials of Investigational Agents. Clin Cancer Res 2017;23(1):9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob SA, Shaw PH. No improvement in clinical trial enrollment for adolescents and young adults with cancer at a children’s hospital. Pediatr Blood Cancer. 2017. [DOI] [PubMed] [Google Scholar]
- 14.Thomas SM, Malvar J, Tran H, Shows J, Freyer DR. A prospective, observational cohort study comparing cancer clinical trial availability and enrollment between early adolescents/young adults and children. Cancer. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs-Canner S, Shaw PH. A comparison of clinical trial enrollment between adolescent and young adult (AYA) oncology patients treated at affiliated adult and pediatric oncology centers. J Pediatr Hematol Oncol 2009;31(12):927–929. [DOI] [PubMed] [Google Scholar]
- 16.IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. In. [Google Scholar]
- 17.Sanford SD, Beaumont JL, Snyder MA, Reichek J, Salsman JM. Clinical research participation among adolescent and young adults at an NCI-designated Comprehensive Cancer Center and affiliated pediatric hospital. Support Care Cancer. 2017;25(5):1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seibel N, Hunsberger S, O’Mara AM, et al. Adolescent and young adult oncology (AYAO) patient enrollments onto National Cancer Institute (NCI)-supported trials from 2000 to 2010. Journal of Clinical Oncology. 2014;32(15_suppl):10058–10058. [Google Scholar]
- 19.Seow HY, Whelan P, Levine MN, et al. Funding oncology clinical trials: are cooperative group trials sustainable? J Clin Oncol 2012;30(13):1456–1461. [DOI] [PubMed] [Google Scholar]
- 20.Weiss AR, Nichols CR, Freyer DR. Enhancing Adolescent and Young Adult Oncology Research Within the National Clinical Trials Network: Rationale, Progress, and Emerging Strategies. Semin Oncol 2015;42(5):740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw PH, Boyiadzis M, Tawbi H, et al. Improved clinical trial enrollment in adolescent and young adult (AYA) oncology patients after the establishment of an AYA oncology program uniting pediatric and medical oncology divisions. Cancer. 2012;118(14):3614–3617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.