Abstract
BACKGROUND:
Men with locally (LAPCa) or regionally advanced (RAPCa) prostate cancer are at high risk of death from their disease. Clinical guidelines support multi-modal approaches, which include radical prostatectomy (RP) followed by radiotherapy (XRT) or radiotherapy plus androgen deprivation therapy (ADT). However, limited data exists comparing these substantially different treatment approaches. Using SEER-Medicare data, we compare survival outcomes and adverse effects associated with RP+XRT vs XRT+ADT in these men.
METHODS:
SEER-Medicare data was queried for men with cT3-T4, N0, M0 (LAPCa) or cT3-T4, N1, M0 (RAPCa) prostate cancer. Propensity score methods were used to balance cohort characteristics between treatment arms. Survival analyses were analyzed using the Kaplan-Meier method and Cox proportional hazards models.
RESULTS:
From 1992 to 2009, 13,856 men (≥65 years) were diagnosed with LAPCa or RAPCa, of which 6.1% received RP+XRT vs 23.6% who received XRT+ADT. At a median follow-up of 14.6 years, there were 2189 deaths in the cohort, of which 702 were secondary to prostate cancer. Irrespective of tumor stage and Gleason score, adjusted 10-year prostate cancer-specific survival and 10-year overall survival favored men who underwent RP+XRT when compared to XRT+ADT. However, RP+XRT vs. XRT+ADT was associated with higher rates of erectile dysfunction (28% vs. 20%, p=0.0212, respectively) and urinary incontinence (49% vs. 19%, p<0.001, respectively).
CONCLUSIONS:
Men with LAPCa or RAPCa treated initially with RP+XRT had a lower risk of prostate cancer-specific death and improved overall survival when compared to those men treated with XRT+ADT, but experienced higher rates of erectile dysfunction and urinary incontinence.
Keywords: Prostate Cancer, Combined Modality Therapy, Population and Observational Studies, Survival, Outcomes, Comparative Effectiveness, Surgery, Radiation, Hormone Therapy
Precis:
Clinical practice guidelines support multimodal therapeutic approaches for men with locally advanced or regionally advanced prostate cancer. This comparative effectiveness study using population-based data demonstrates more favorable survival outcomes in these men who received radical prostatectomy with adjuvant radiotherapy when compared to those who received radiotherapy plus androgen deprivation therapy.
INTRODUCTION
Despite widespread prostate-specific antigen (PSA)-based screening, at least 10% of men will have locally advanced (LAPCa) or regionally advanced (RAPCa) prostate cancer at the time of diagnosis.1–3 Unlike localized prostate cancer, where 5-year relative survival approaches 100%,3 men with LAPCa (T3-T4, N0, M0) or RAPCa (T3-T4, N1, M0) are at an increased risk of PSA failure, need for secondary therapy, metastatic progression, and death from prostate cancer.4
Treatment approaches vary within this high-risk group of men and the optimal initial treatment for these patients remains undetermined and widely debated. Locally and regionally advanced prostate cancers represent a heterogeneous population of tumors with varying risks of PSA failure and distant metastatic potentials after treatment. Though monotherapy alone may adequately control a subset of these cancers, clinical practice guidelines generally support multi-modal treatment approaches, which include radical prostatectomy (RP) followed by adjuvant radiotherapy (XRT) or primary XRT with adjunctive androgen deprivation therapy (ADT).4, 5 To our knowledge, little comparative data in the form of well-controlled or randomized trials exists that compares these two substantially different multimodal approaches for men with locally and/or regionally advanced prostate cancers.
We present a population-based comparative effectiveness study of RP+XRT versus XRT+ADT in men with LAPCa and RAPCa to examine differences in prostate-cancer specific survival and overall survival. As a secondary endpoint, the prevalence of treatment-associated adverse events in the RP+XRT versus XRT+ADT groups was evaluated.
MATERIALS AND METHODS
Data Sources
The study cohort comprised patients from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, which links patient demographic and tumor-specific data collected by SEER cancer registries to health care claims for Medicare enrollees. Information on incident cancer cases was available from 17 affiliated cancer registries from January 1, 1992 through December 31, 2009, covering 28% of the US population, and linked to Medicare claims through 2010. Greater California, Kentucky, Louisiana, and New Jersey case contributions began in 2000.6 SEER registries collect data on each patient’s cancer site, extent of disease, histology, date of diagnosis, and initial treatment. We staged patients according to the American Joint Committee on Cancer (AJCC) criteria.7
The Medicare program provides health care benefits to 97% of the US population 65 years or older. Hospitalization information for those eligible for Medicare Part A is available from the Medicare Provider Analysis and Review files. Outpatient and physician/supplier Medicare files for services rendered in physicians’ offices and hospital outpatient departments are available for the 96% of Medicare beneficiaries who elect Part B coverage. Approximately 94% of SEER patients ≥ 65 years have been successfully linked with their Medicare claims.8 This study was deemed exempt from review by the institutional review board at Rutgers Cancer Institute of New Jersey.
Study Population
From 1992 to 2009, 648,042 patients were diagnosed with prostate cancer. Men were excluded if they had a history of previous malignancy (n=60,770), clinical stage T1, T2, in situ, or M1 (n=561,093), distant lymph node involvement (n=1,284), age at diagnosis <65 years (n=6,646), those with HMO coverage during 6 months subsequent to diagnosis (n=315), and those without Part A or B Medicare coverage during 6 months subsequent to diagnosis (n=426). After further excluding men whose treatments could not be classified and those who received primary chemotherapy (n=3,652), 13,856 men comprised the final analytic cohort.
Definition of Variables
Socio-demographic, clinical, and treatment-related variables were derived from their respective data sources from the SEER-Medicare database. Patient comorbidity was categorized according to the modification by Romano et al9 of the index developed by Charlson et al,10 and is analogous to the methods described and used in prior studies.11
Primary treatment was categorized according to the following 2 treatment groups: RP with adjuvant XRT, or XRT with adjunctive ADT. Men who underwent surgical therapies not considered curative, (i.e. cryotherapy, subtotal prostatectomy, transurethral resection of the prostate, etc…) were excluded from the RP group. To differentiate between neoadjuvant and adjuvant therapies, both SEER codes noting radiation sequence with surgery and Medicare claims detailing date therapies were delivered were used. Adjuvant XRT was defined as XRT received within 6 months after date of RP. XRT+ADT was defined as XRT plus any ADT delivered 2 months before XRT until any time 3 years after XRT.
Complications associated with therapy were identified using diagnostic (ICD) and current procedural terminology (CPT, HCPCS) codes from Medicare claims which are detailed in the Appendix. Complications were evaluated as adverse effects occurring within 12 months following primary treatment delivery.
As the quality of PSA data captured in SEER has come into question,12 we performed sensitivity analyses to determine the impact of its inclusion or exclusion in our study. The inclusion or exclusion of PSA in our analyses did not significantly alter our overall findings. Therefore, all data shown includes analyses excluding the PSA variable.
Statistical Analysis
The primary study end points were prostate cancer-specific survival and overall survival, according to cancer stage, Gleason score, and treatment received. Secondary study end points assessed the prevalence of treatment-related complications, according to treatment.
Propensity score methods were used to balance patient characteristics between groups and to control for observed confounding factors. Propensity score was estimated from a logistic regression model of the probability of RP+XRT relative to XRT+ADT as a function of clinical and demographic characteristics. The covariates included age, comorbidity status, primary tumor stage, nodal stage, tumor grade, year of diagnosis, marital status, ethnicity, race, SEER region, population of residence quartile, income quartile, urban residence and educational attainment quartile. The standardized mortality ratio weighting (SMRW) were used and SMRW assigns weights of 1 for of RT+XRT and a weight of [ps/(1-ps)] for XRT+ADT. Propensity score adjusted (SMRW) hazard ratios and 95% CIs were estimated. To further reduce residual confounding by controlling for proxies of unmeasured confounders, high-dimensional propensity score adjustment was applied13 by empirically identifying candidate dichotomous covariates from ICD 9 diagnosis code, HCPCS procedure code and ICD 9 surgery code within 1 year prior to cancer diagnosis. Propensity score was estimated from a logistic regression model of the probability of RP+XRT relative to XRT+ADT as a function of clinical and demographic characteristics and the empirical covariates.
The Kaplan-Meier method was used to determine 10-year prostate cancer-specific and 10-year overall survival. Adverse effects from treatment were ascertained from Medicare claims and χ2 testing was used to examine the association between each adverse event and treatment.
All analyses were two-tailed and were conducted using SAS version 9.1 (SAS Institute Inc, Cary, NC). A P value <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics and Relation to Treatment
Of 13,856 men diagnosed as having LAPCa or RAPCa, approximately 50% of men received a single intervention, with 20.1% (n=2884), 18.3% (n=2541), and 11.2% (n=1545) undergoing treatment with RP alone, XRT alone, and ADT alone, respectively. Almost 30% of men received multimodal treatment, with 6.1% (n=848) and 23.6% (n=3272) receiving RP+XRT and XRT+ADT, respectively. Among men who received adjuvant XRT following RP, 29.8% (n=253) received concurrent ADT. Finally, 20% of men (n=2766) received no treatment within 6 months of diagnosis.
Older men, those with more comorbid conditions, unmarried men, and those diagnosed in more contemporary eras were more likely to receive XRT+ADT versus RP+XRT (Table 1). For example, among men who received RP+XRT, when compared to XRT+ADT, >55.7% versus 26.7% were aged 65–69, 9.6% versus 26.1% were aged 75–79, and <1.3% versus 13.5% were over 80 years old, respectively (p<0.0001); 90.1% versus 79.2%, 7.8% versus 13.7%, and 2.1% versus 7.1% with comorbidity index score 0, 1, 2+, respectively, received RP+XRT versus XRT+ADT (p<0.0001). The proportion of men undergoing RP+XRT decreased significantly over time, from 9.4% in 1992–1997 to 4.0% in 2004–2009, p<0.001. Conversely, the proportion of men receiving XRT+ADT increased more than 3-fold from 11.6% in 1992–1997 to 37.8% in 2004–2009, p<0.001.
Table 1.
Demographic and Tumor Characteristics of Men undergoing Radical Prostatectomy and Adjuvant Radiotherapy vs Men undergoing Radiotherapy plus Androgen Deprivation Therapy
| Before Propensity Weighting |
P value |
After Propensity Weighting(ITPW) |
P value |
After Propensity Weighting(SMRW) |
P value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | RP+XRT N= 848 No. (%) |
XRT+ADT N= 3272 No. (%) |
RP+XRT N= 3811 No. (%) |
XRT+ADT N= 4135 No. (%) |
RP+XRT N= 848 No. (%) |
XRT+ADT N= 864 No. (%) |
||||
| Age at diagnosisβ | ||||||||||
| 65–69 | >473 (>55.7%) | 875 (26.7) |
<.0001 |
1380 (36.2) | 1365 (33) |
0.7139 |
>473 (>55.7) | >488 (>56.4) |
0.9888 |
|
| 70–74 | 283 (33.4) | 1100 (33.6) | 1418 (37.2) | 1385 (33.5) | 283 (33.4) | 285 (33.0) | ||||
| 75–79 | 81 (9.6) | 854 (26.1) | 766 (20.1) | 935 (22.6) | 81 (9.6) | 80 (9.3) | ||||
| 80+ | <11 (<1.3) | 443 (13.5) | 247 (6.5) | 450 (10.9) | <11 (<1.3) | <11 (<1.3) | ||||
| Race | ||||||||||
| White | 711 (83.8) | 2686 (82.1) |
0.2175 |
3087 (81.0) | 3403 (82.3) |
0.9008 |
711 (83.8) | 719 (83.2) |
0.6483 |
|
| Black | 60 (7.1) | 295 (9.0) | 396 (10.4) | 356 (8.6) | 60 (7.1) | 60 (7.0) | ||||
| Other/Unknown | 77 (9.0) | 291 (8.9) | 328 (8.6) | 376 (9.1) | 77 (9.0) | 85 (9.8) | ||||
| Hispanic ethnicity | ||||||||||
| Not Hispanic | >803 (>94.7) | 3030 (92.6) |
0.0072 |
>3609 (>94.7) | 3846 (93.0) |
0.4566 |
>803 (>94.7) | >810 (>93.8) |
0.6313 |
|
| Hispanic | 34 (4.0) | 219 (6.7) | 191 (5.0) | 261 (6.3) | 34 (4.0) | 43 (5.0) | ||||
| Unknown | <11 (<1.3) | 23 (0.7) | <11 (<0.3) | 28 (0.7) | <11 (<1.3) | <11 (<1.3) | ||||
| Marital Status | ||||||||||
| Married | >721 (>85.0) | 2434 (74.4) |
<.0001 |
2976 (78.1) | 3163 (76.5) |
0.6207 |
>721 (>85.0) | 730 (84.5) |
0.9586 |
|
| Not Married | 116 (13.7) | 691 (21.1) | 747 (19.6) | 815 (19.7) | 116 (13.7) | 122 (14.1) | ||||
| Unknown | <11 (<1.3) | 147 (4.5) | 88 (2.3) | 157 (3.8) | <11 (<1.3) | 12 (1.4) | ||||
|
Tumor Stage |
||||||||||
| T3 | ||||||||||
| T3N0M0 | 666 (78.5) | 2048 (62.6) | 2275 (59.7) | 2688 (65.0) | 666 (78.5) | 644 (74.5) | ||||
| T3N1M0 | 93 (11.0) | 115 (3.5) | 301 (7.9) | 248 (6.0) | 93 (11.0) | 133 (15.4) | ||||
| T3NXM0 | 20 (2.4) | 717 (21.9) | 758 (19.9) | 736 (17.8) | 20 (2.4) | 20 (2.3) | ||||
| T4 | ||||||||||
| T4N0M0 | 52 (6.1) | 232 (7.1) | <.0001 | 377 (9.9) | 283 (6.8) | 0.6512 | 52 (6.1) | 51 (5.9) | 0.2599 | |
| T4N1M0/T4NXM0 | 17 (2.0) | 160 (4.9) | 100 (2.6) | 180 (4.3) | 17 (2.0) | 16 (1.9) | ||||
| Gleason’s sum | ||||||||||
| 8–10 | 436 (51.4) | 1767 (54.0) |
0.0117 |
1978 (51.9) | 2237 (54.1) |
0.0598 |
436 (51.4) | 473 (54.7) |
0.5450 |
|
| 5–7 | 381 (44.9) | 1371 (41.9) | 1486 (39.0) | 1733 (41.9) | 381 (44.9) | 361 (41.8) | ||||
| 2–4 | 17 (2.0) | 36 (1.1) | 42 (1.1) | 50 (1.2) | 17 (2.0) | 13 (1.5) | ||||
| Unknown | 14 (1.7) | 98 (3.0) | 305 (8.1) | 115 (2.8) | 14 (1.7) | 17 (2.0) | ||||
| Comorbidity index | ||||||||||
| 0 | 764 (90.1) | 2591 (79.2) |
<.0001 |
3235 (84.9) | 3378 (81.7) |
0.3908 |
764 (90.1) | 788 (91.3) |
0.6441 |
|
| 1 | 66 (7.8) | 449 (13.7) | 473 (12.4) | 509 (12.3) | 66 (7.8) | 60 (6.9) | ||||
| 2+ | 18 (2.1) | 232 (7.1) | 103 (2.7) | 248 (6.0) | 18 (2.1) | 16 (1.8) | ||||
| Diagnosis Year | ||||||||||
| 1992–1997 | 655 (77.2) | 811 (24.8) |
<.0001 |
1582 (41.5) | 1485 (35.9) |
0.5842 |
655 (77.2) | 672 (77.8) |
0.9512 |
|
| 1998–2003 | 61 (7.2) | 1227 (37.5) | 1060 (27.8) | 1286 (31.1) | 61 (7.2) | 62 (7.1) | ||||
| 2004–2009 | 132 (15.6) | 1234 (37.7) | 1169 (30.7) | 1364 (33.0) | 132 (15.6) | 130 (15.1) | ||||
| SEER Region | ||||||||||
| Northeast | 90 (10.6) | 521 (15.9) |
0.0002 |
734 (20.3) | 620 (15.0) |
0.4403 |
90 (10.6) | 103 (11.9) |
0.9665 |
|
| North Central | 149 (17.6) | 605 (18.5) | 534 (14.0) | 758 (18.3) | 149 (17.6) | 149 (17.2) | ||||
| West | 513 (60.5) | 1724 (52.7) | 1997 (52.4) | 2241 (54.2) | 513 (60.5) | 516 (59.7) | ||||
| South | 71 (8.4) | 304 (9.3) | 381 (10.0) | 376 (9.1) | 71 (8.4) | 72 (8.4) | ||||
| Unknown | 25 (2.9) | 118 (3.6) | 126 (3.3) | 140 (3.4) | 25 (2.9) | 24 (2.8) | ||||
| Population of county Residencea |
||||||||||
| Bottom quartile | 207 (24.4) | 824 (25.2) |
0.6860 |
990 (26.0) | 1036 (25.1) |
0.9765 |
207 (24.4) | 213 (24.7) |
0.8893 |
|
| Second quartile | 205 (24.2) | 824 (25.2) | 860 (22.6) | 1015 (24.6) | 205 (24.2) | 192 (22.2) | ||||
| Third quartile | 226 (26.6) | 805 (24.6) | 982 (25.8) | 1042 (25.2) | 225 (26.6) | 239 (27.6) | ||||
| Top quartile | 210 (24.8) | 819 (25.1) | 979 (25.7) | 1042 (25.2) | 210 (24.8) | 220 (25.5) | ||||
|
Percentage of men with less than a high school education in census tract of residencea |
||||||||||
| Bottom quartile | 222 (26.2) | 808 (24.7) |
0.0360 |
877 (23.0) | 1053 (25.5) |
0.6540 |
222 (26.2) | 245 (28.4) |
0.8714 |
|
| Second quartile | 233 (27.5) | 798 (24.4) | 1159 (30.4) | 1024 (24.8) | 233 (27.5) | 229 (26.5) | ||||
| Third quartile | 211 (24.8) | 820 (25.1) | 922 (24.2) | 1034 (25.0) | 210 (24.8) | 213 (24.6) | ||||
| Top quartile | 182 (21.5) | 846 (25.9) | 853 (22.4) | 1024 (24.8) | 183 (21.5) | 177 (20.5) | ||||
|
Percentage household income (U.S. dollars) in census tract of residencea |
||||||||||
| Bottom quartile | 176 (20.8) | 854 (26.1) |
0.0063 |
1064 (27.9) | 1034 (25.0) |
0.5104 |
176 (20.8) | 181 (21.0) |
0.9531 |
|
| Second quartile | 218 (25.7) | 811 (24.8) | 729 (19.1) | 1021 (24.7) | 217 (25.7) | 211 (24.3) | ||||
| Third quartile | 239 (28.2) | 792 (24.2) | 1083 (28.4) | 1038 (25.1) | 239 (28.2) | 246 (28.5) | ||||
| Top quartile | 215 (25.3) | 815 (24.9) | 935 (24.5) | 1042 (25.2) | 216 (25.3) | 226 (26.2) | ||||
| Residence | ||||||||||
| Rural | 108 (12.7) | 537 (16.4) |
0.0092 |
701 (18.4) | 641 (15.5) |
0.5319 |
108 (12.7) | 106 (12.3) |
0.8033 |
|
| Urban | 740 (87.3) | 2735 (83.6) | 3110 (81.6) | 3494 (84.5) | 740 (87.3) | 758 (87.7) | ||||
Population of county residence, educational levels and median income based on census tract data (year 2000) if available. Otherwise, data from zip code of residence (year 2000) was used, followed by census tract data (year 1990, with adjustment for year 2010) if zip code data was unavailable.
Tumor stage and Gleason score were associated with treatment received. Men whose cancers extended beyond the prostatic capsule (T3a N0 M0) or invaded the seminal vesicles (T3b N0 M0) but did not involve regional lymph nodes were more likely to undergo RP+XRT. The observed treatment pattern was independent of regional lymph node involvement, as men with regional nodal metastasis and extracapsular extension (T3a N1 M0) or seminal vesicle invasion (T3b N1 M0) were also more likely to have undergone RP+XRT than XRT+ADT [5.1% versus 2.0% with T3a N1 M0 disease undergoing RP+XRT versus XRT+ADT, respectively; 5.9% versus 1.5% with T3b N1 M0 disease undergoing RP+XRT versus XRT+ADT, respectively (p<0.001)]. Patients with tumor fixation to adjacent structures (T4 N0 M0) were more likely to undergo XRT+ADT versus RP+XRT (7.1% versus 6.1%), p<0.001.
Survival Analysis
At a median follow-up of 14.6 years, there were 2189 deaths in the cohort, of which 702 deaths were secondary to prostate cancer. Without adjustments for other variables, men who received RP+XRT were less likely to die from prostate cancer and less likely to die from any cause, when compared to those who received XRT+ADT. These findings were independent of primary tumor stage, nodal stage, and Gleason score (Tables 2, 3). For example, men who received XRT+ADT, when compared to RP+XRT, experienced more than a two-fold increase in death from prostate cancer if their cancers had evidence of extracapsular extension without nodal involvement, (hazard ratio [HR], 2.90; 95% CI, 1.92–4.36) or if their cancers involved adjacent structures without involving regional lymph nodes, (HR, 2.59, 95% CI 1.26–5.33), respectively. Patients with regional lymph node involvement who received XRT+ADT versus RP+XRT were also found to be at increased risk of dying from prostate cancer and of dying from other causes; however, the 95% CIs in the subgroup of men with disease involving regional lymph nodes was wide (Table 2a).
Table 2.
Risk of Mortality According to Tumor Stage, Gleason Score, and Treatment (RP+XRT vs XRT+ADT) using Cox Multivariate Analysis
| Cox Proportional Hazards Regression Model Analysis | ||||||
|---|---|---|---|---|---|---|
| Radical Prostatectomy with Adjuvant Radiotherapy |
Radiotherapy and Androgen Deprivation Therapy |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Adjusted HR (95% CI)a |
Adjusted HR (95% CI)b |
|
| Tumor Stage and Gleason Score |
Event Rate per 1000 | Event Rate per 1000 | ||||
| Prostate Cancer-Specific Mortality | ||||||
| T3aN0M0 | 0.7 | 1.6 | 2.9(1.92,4.36) | 3.82(2.44,5.99) | 4.22(2.83,6.28) | 1.93(1.37,2.71) |
| T3bN0M0 | 2.0 | 2.3 | 1.4(1.03,1.92) | 2.11(1.42,3.12) | 1.84(1.34,2.53) | 1.49(1.02,2.17) |
| T3aN1M0 | 1.5 | 3.2 | 2.46(0.89,6.77) | 14.94(1.96,113.93) | 2.12(0.83,5.41) | NA |
| T3bN1M0 | 3.0 | 4.0 | 1.27(0.58,2.76) | 1.2(0.33,4.29) | 0.98(0.51,1.87) | NA |
| T3aNXM0 | 2.4 | 2.5 | 1.01(0.32,3.23) | 2.07(0.6,7.2) | 1.36(0.32,5.81) | NA |
| T3bNXM0 | 1.8 | 3.0 | 1.9(0.46,7.91) | 2.9(0.62,13.49) | 3.23(0.52,20.22) | NA |
| T4N0M0 | 1.6 | 4.2 | 2.59(1.26,5.33) | 1.48(0.57,3.86) | 2.31(1.05,5.12) | NA |
| T4N1M0 | 2.7 | 7.3 | 2.42(0.68,8.59) | 12.2(1.59,92.49)* | 2.68(0.66,10.9) | NA |
| T4NXM0 | 5.5 | 5.6 | 2.53(0.55,11.75) | 3(0.53,17.1) | 2.13(0.34,13.37) | NA |
| Gleason 5–7 | 1.0 | 1.7 | 2.31(1.65,3.23) | 2.73(1.85,4.01) | 2.62(1.83,3.73) | 2.34(1.20,4.54) |
| Gleason 8–10 | 2.0 | 3.2 | 1.69(1.34,2.14) | 1.99(1.52,2.61) | 1.96(1.53,2.53) | 1.78(1.17,2.71) |
| Overall Mortality | ||||||
| T3aN0M0 | 4.2 | 5.3 | 1.87(1.57,2.22) | 1.69(1.37,2.07) | 1.75(1.45,2.11) | 2.15(1.78,2.61) |
| T3bN0M0 | 5.0 | 5.6 | 1.5(1.24,1.81) | 1.42(1.12,1.79) | 1.49(1.22,1.84) | 1.36(1.08,1.71) |
| T3aN1M0 | 6.8 | 6.6 | 1.11(0.67,1.86) | 1.61(0.73,3.54) | 0.88(0.54,1.44) | NA |
| T3bN1M0 | 6.6 | 7.0 | 1.17(0.69,1.98) | 0.93(0.43,2.05) | 0.74(0.47,1.17) | NA |
| T3aNXM0 | 7.8 | 6.7 | 0.99(0.52,1.88) | 1.26(0.62,2.56) | 0.97(0.40,2.37) | NA |
| T3bNXM0 | 4.1 | 6.8 | 2.03(0.82,5.01) | 2.47(0.92,6.59) | 2.46(0.75,8.05) | NA |
| T4N0M0 | 4.5 | 8.7 | 2.12(1.4,3.22) | 1.59(0.9,2.79) | 1.62(1.00,2.64) | NA |
| T4N1M0 | 7.6 | 8.2 | 0.97(0.43,2.17) | 0.62(0.06,6.21) | 1.06(0.39,2.86) | NA |
| T4NXM0 | 8.1 | 10.0 | 2.97(0.91,9.65) | 2.86(0.75,10.86) | 2.66(0.62,11.45) | NA |
| Gleason 5–7 | 4.2 | 5.1 | 1.77(1.51,2.09) | 1.68(1.38,2.04) | 1.62(1.35,1.96) | 1.34(0.99,1.80) |
| Gleason 8–10 | 5.5 | 7.2 | 1.59(1.38,1.83) | 1.49(1.26,1.76) | 1.33(1.13,1.56) | 1.39(1.08,1.78) |
Abbreviations: ADT, androgen deprivation therapy; CI, confidence interval; HR, hazard ratio; NA, not available; RP, radical prostatectomy; XRT, radiotherapy.
Propensity score weighting method(SMRW) was applied.
High dimensional propensity score method was applied. Only men with Part A/Part B coverage 1 year prior cancer diagnosis were included in the analysis. For small samples propensity score can’t be obtained from the logistic regression model.
Table 3.
Adjusted 10-year Overall and Prostate Cancer-Specific Survival according to Tumor Stage in Men Undergoing Radical Prostatectomy with Adjuvant Radiotherapy versus Radiotherapy plus Androgen Deprivation Therapy
| Tumor Stage | Radical Prostatectomy with Adjuvant Radiotherapy |
Radiotherapy and Androgen Deprivation Therapy | Difference (95% CI) | ||
|---|---|---|---|---|---|
| Event rate per thousand |
Adjusted Survival(%) |
Event rate per thousand |
Adjusted Survival(%) | ||
| 10-year Prostate Cancer-Specific Survival | |||||
| T3a-bN0-XM0 | 1.3 | 88.9 | 2.0 | 74.2 | 14.7(11.4,17.2) |
| T3a-bN1M0 | 2.2 | 75.7 | 3.7 | 58.6 | 17.1(−0.8,34.2) |
| T4N0-XM0 | 2.0 | 72.0 | 4.6 | 60.5 | 11.6(0.8,16.9) |
| T4N1M0 | 3.5 | NA | 8.0 | NA | NA |
| 10-year Overall Survival | |||||
| T3a-bN0-XM0 | 3.4 | 64.2 | 5.3 | 48.3 | 15.8(11.3,20.2) |
| T3a-bN1M0 | 5.6 | 44.3 | 6.5 | 40.5 | 3.8(−10.8,22.5) |
| T4N0-XM0 | 4.0 | 49.6 | 8.8 | 34.9 | 14.7(−0.1,30.5) |
| T4N1M0 | 7.8 | NA | 9.0 | NA | NA |
Note: Covariates included age, race, year of diagnosis, marital status, comorbidity status, income quartiles, urban residence, SEER region, education quartiles, population quartiles. 95% CIS are bootstrapped. 10-year survival was not available in stage T4N1M0 men.
Using a conventional Cox proportional hazards model without propensity score adjustment, RP+XRT, when compared to XRT+ADT, was associated with a lower risk of death from prostate cancer or death from other causes, after adjusting for all other covariates. Using this model, patients irrespective of primary tumor stage, nodal status, or Gleason score, experienced improved prostate cancer-specific survival and improved overall survival if they underwent RP+XRT versus XRT+ADT (Table 2). Propensity score adjustment did not materially alter the risk estimates from the conventional model. Patients with T3aN0M0, T3bN0M0, T4N0M0 disease who received RP+XRT versus XRT+ADT were less likely to die from prostate cancer [(HR, 4.22, 95% CI 2.83–6.28), (HR, 1.84, 95% CI 1.34–2.53), and (HR, 2.31, 95% CI 1.05–5.12), respectively] and less likely to die from any cause [(HR, 1.75, 95% CI 1.45–2.11), (HR, 1.49, 95% CI 1.22–1.84), and (HR, 1.62, 95% CI 1.00–2.64), respectively] (Table 2).
Adjusted Kaplan-Meier prostate cancer-specific survival and overall survival curves for the study cohort, according to tumor stage are shown in Figures 1a and 1b, respectively. Adjusted 10-year prostate cancer specific survival and 10-year overall survival probabilities for the entire cohort, according to tumor stage and treatment, is shown in Table 3. The 10-year actuarial disease-specific survival in men undergoing RP+XRT versus XRT+ADT was 88.9% versus 74.2% (Survival difference, 14.7%, 95% CI 11.4–17.2) for men with T3a/b N0-NX M0 disease, 75.7% versus 58.6% (Survival difference, 17.1%, 95% CI −0.8 – 34.2) for men with T3a/b N1 M0 disease, and 72.0% versus 60.5% (Survival difference, 11.6%, 95% CI 0.8 – 16.9) for men with T4 N0-NX M0 disease, respectively. The 10-year actuarial overall survival in men undergoing RP+XRT versus XRT+ADT was 64.2% versus 48.3% (Survival difference, 15.8%, 95% CI 11.3–20.2) for men with T3a/b N0-NX M0 disease, 44.3% versus 40.5% (Survival difference, 3.8%, 95% CI −10.8 – 22.5) for men with T3a/b N1 M0 disease, and 49.6% versus 34.9% (Survival difference, 14.7%, 95% CI −0.1 – 30.5) for men with T4 N0-NX M0 disease, respectively.
Figure 1a.
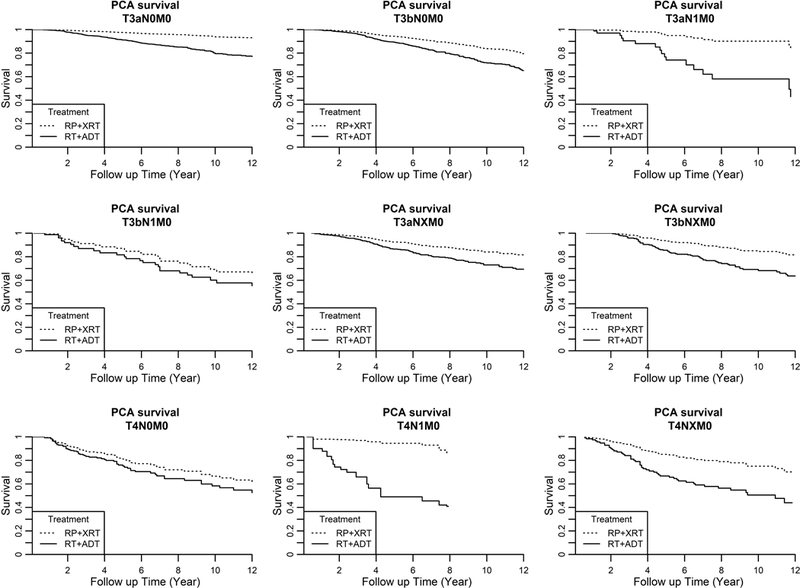
Adjusted Prostate Cancer-Specific Survival in Men undergoing Radical Prostatectomy with Adjuvant Radiotherapy versus Radiotherapy plus Androgen Deprivation Therapy, according to Tumor Stage
Adverse Outcomes by Treatment
In propensity-adjusted analysis (Table 4), men undergoing RP+XRT versus XRT+ADT were more often diagnosed as having urinary incontinence (49.1% versus 19.4%, p<0.0001) and erectile dysfunction (28.3% versus 20.4%, p=0.0212) and more oftentimes underwent procedures to address urinary incontinence (12.4% versus 1.6%, p=0.0007) or erectile dysfunction (8.4% versus 3.7%, p=0.0186). Also, men who received RP+XRT were more often diagnosed with bladder neck contractures (37.6% versus 18.3%, p<0.0001) and more oftentimes required procedures to address bladder neck contracture (34.3% versus 12.8%, p<0.0001), when compared to those undergoing XRT+ADT. The need to undergo procedures for urinary retention was higher in the XRT+ADT group.
Table 4.
Propensity Model-Adjusted Adverse Outcomes by Treatment
| Adverse Outcomes | Treatment | ||
|---|---|---|---|
| Radical Prostatectomy and Adjuvant Radiotherapy (N=451) % |
Radiotherapy and Androgen Deprivation Therapy (N=2590) % |
P-value | |
| Urinary Incontinence | |||
| Diagnosis | 49.1 | 19.4 | <.0001 |
| Procedures | 12.4 | 1.6 | 0.0007 |
| Erectile Dysfunction | |||
| Diagnosis | 28.3 | 20.4 | 0.0212 |
| Procedures | 8.4 | 3.7 | 0.0186 |
| Other Genitourinary | |||
| Radiation cystitis | 8.6 | 6.0 | 0.1635 |
| Hematuria | 40.7 | 34.0 | 0.0963 |
| Bladder neck contracture/ Urethral stricture |
|||
| Diagnosis | 37.6 | 18.3 | <.0001 |
| Procedure | 34.3 | 12.8 | <.0001 |
| Urinary fistula | 2.9 | 1.8 | 0.2872 |
| Gastrointestinal | |||
| Radiation proctitis | 5.6 | 7.7 | 0.2345 |
| Diarrhea | 24.7 | 23.5 | 0.7229 |
| Rectal bleeding | 47.1 | 46.9 | 0.9575 |
| Cardiac | |||
| Acute myocardial infarction | 19.2 | 18.3 | 0.7648 |
| Ventricular arrhythmias | 18.7 | 12.1 | 0.0152 |
| Coronary artery disease | 55.4 | 44.0 | 0.0083 |
| Pulmonary | |||
| Pulmonary embolus | 7.2 | 8.8 | 0.4455 |
| Vascular | |||
| Venous embolism/thrombosis | 25.4 | 24.0 | 0.6714 |
| Transient cerebral ischemia/stroke |
47.1 | 42.0 | 0.1997 |
| Peripheral arterial disease | 39.8 | 37.4 | 0.5227 |
| Endocrine | |||
| Diabetes Mellitus | 42.4 | 32.5 | 0.0157 |
| Musculoskeletal | |||
| Fracture | 33.6 | 30.7 | 0.4003 |
| Osteoporosis | 18.9 | 19.8 | 0.8027 |
No statistically significant differences were observed between the two treatment groups in rates of hematuria, radiation cystitis, rectal bleeding, urinary or GI fistulas, or radiation proctitis.
In patients who received XRT+ADT, we did not observe higher rates of cardiovascular, metabolic or musculoskeletal morbidity, when compared to men who received RP+XRT. More specifically, rates of acute myocardial infarction, sudden cardiac death, coronary artery disease, thromboembolic events, skeletal fractures, or osteoporosis did not differ significantly between the two groups. Results of adjusted analyses comparing adverse outcomes were generally consistent with unadjusted analyses (data not shown).
DISCUSSION
Though several contemporary studies in various settings show that men with high-risk disease characteristics may have a lower risk of metastatic progression and prostate cancer-specific death after RP compared with other treatment modalities,14, 15 the surgical treatment of patients with high-risk prostate cancer, defined in our study as locally advanced (cT3-T4, N0, M0) or regionally advanced (cT3-T4, N1, M0) disease, has traditionally been discouraged, in part because patients are at increased risk of positive surgical margins or distant relapse, and the procedure can be technically challenging.16, 17 As a result, primary XRT with ADT has traditionally been offered to these men, despite the National Comprehensive Cancer Network (NCCN) and European Association of Urology (EAU)-European Society for Radiotherapy & Oncology (ESTRO)-guidelines on prostate cancer supporting both XRT+ADT and RP+XRT as appropriate modalities.4, 5
More recently, there has been renewed interest in surgery for this group of men, as emerging retrospective series have shown durable intermediate- to long-term cancer-specific survival and overall survival.18–21 A recent population-based study comparing outcomes of men with locally advanced prostate cancer undergoing single-modality primary treatment with either radical prostatectomy vs external beam radiation found significant differences in toxicity and survival, with survival outcomes more favorable in the radical prostatectomy group.22 The theoretical benefits of RP as first-line treatment are to achieve tumor volume reduction and optimal local control. Furthermore, evaluation of prostatectomy and nodal specimens and postoperative PSA levels allow for more accurate staging and risk stratification that will better select for men who may benefit from adjuvant treatment.23 Cancer-specific survival rates for men with clinical stage T3, N0, M0 prostate cancer at 10- and 15-years ranging between 85–92% and 62–84%, respectively, have been reported.18–21, 24, 25 A few cohort studies provide survival data for surgery of clinical stage T3b-T4, N0, M0 prostate cancer, and report 10-year cancer-specific and overall survival of 87–92% and 65–71%, respectively.16, 26, 27 Finally, limited data exists evaluating the role of RP in clinically node positive (cN+) patients. In a recent study comparing the outcomes of 50 men with cN+ disease to 252 men with pN1 disease, a subset of men with cN+ experienced similar clinical outcomes to those with normal preoperative imaging (cN0) in the setting of lymph node metastasis.28
Our observational analysis aimed to determine which initial multimodal approach for high-risk prostate cancer was associated with improved survival outcomes. Several key findings were observed. First, non-adherence to practice guidelines was seen. Though NCCN and EAU/ESTRO guidelines recommend multimodal treatment for men with high-risk prostate cancer,4, 5 approximately half the entire study cohort of 13,856 men received single intervention and almost 20% (n=2766) did not receive treatment. For those receiving multimodal treatment, XRT+ADT was most commonly delivered (23.6%), followed by RP+XRT (6.1%). Not surprisingly, patient age, comorbid conditions, and cancer stage were associated with treatment received. For instance, men who were younger, had less comorbid conditions, and whose cancers exhibited extracapsular extension (T3a, N0, M0) or seminal vesicle invasion (T3b, N0, M0) without regional lymph node involvement were more likely to receive RP+XRT. Men with regional lymph node involvement and extracapsular extension or seminal vesicle invasion were also more likely to have undergone RP+XRT than XRT+ADT. Older men, those with more significant comorbid conditions, and those with tumor fixation to adjacent structures (cT4) were more likely to receive XRT+ADT. These findings may be attributable to biases in choice of treatment based on a patient’s clinical stage or also in part to pathologic stage information being more accurately captured for surgically treated patients when compared to those treated by primary XRT+ADT.
Second, men who received primary radical prostatectomy followed by post-surgery radiotherapy were less likely to die from prostate cancer and experienced improved overall survival when compared to those who received XRT+ADT. These findings were independent of primary tumor stage, nodal stage, and Gleason score, though the survival advantage benefited those men without lymph node metastasis most. This data suggests that even men with high-risk disease that is not clinically localized can achieve durable long-term cancer-specific survival and overall survival with multimodal treatment, with adjusted 10-year prostate cancer–specific survival rates for men with T3a/b, N0, M0 disease, T3a/b N1, M0 disease, T4, N0, M0 disease of 88.9%, 75.7%, and 72%, respectively, for those who received RP+XRT and 74.2%, 58.6%, 60.5%, respectively, for those who received XRT+ADT. Most men with T4, N1, M0 disease, irrespective of treatment received, died from disease by 10 years. Though we found RP as a primary intervention to be associated with more favorable survival outcomes than XRT in this high-risk population, the questions, as other investigators have suggested, should focus not on which modality is best, but instead, on optimizing treatment sequences and timing, optimizing intensities of treatment, and integrating more effective systemic therapies with optimal local treatments.29
Finally, men who underwent RP+XRT had higher rates of erectile dysfunction (28% versus 20%), higher rates of urinary incontinence (49% versus 19%), and higher rates of bladder neck contractures (BNC) and urethral strictures (38% vs 18%) when compared to those who underwent XRT+ ADT. Anastamotic strictures are the most commonly reported complication following radical prostatectomy.30 BNC and urethral stricture rates in men undergoing RP+XRT appear high in our study. There are several reasons why this may be. First, published rates of BNC and/or urethral strictures following radical prostatectomy alone for men with clinically localized prostate cancer range widely in the literature, occurring in 2.7% to 26% of cases, depending on how and where the data was collected, the definition of what constitutes a stricture, and differences in surgical volume.30–33 For example, the lowest published rates of BNC (2.7% to 5%) have been reported using physician-assessed definitions in academic practices.33 Data from CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor), which enrolls patients from 43 community urology practices, academic medical centers, and VA hospitals throughout the United States, reports BNC rates of 8.4%.31 Data from population-based sources (SEER-Medicare data) reports BNC rates in the literature ranging from 17% to 26%.32, 34 Even among surgeons with high radical prostatectomy volumes (>60 per year), BNC rates of 22% were reported using claims data from Medicare beneficiaries.30 Our study reveals BNC and/or urethral stricture rates following RP+XRT to be 37.6%, with 34.3% requiring a procedure to manage the BNC and/or urethral stricture. The use of Medicare claims to identify the incidence of treating urethral strictures that occur after RP have been found to be reliable, demonstrating excellent sensitivity, specificity, and positive predictive value.34 The diagnostic codes and procedure codes used in our study are analogous to what has been previously published in the literature (Appendix).32 Since men in our RP+XRT cohort had high risk factors for BNC/stricture formation when compared to men with clinically localized prostate cancer, namely more advanced disease, receipt of adjuvant XRT, older age >65 years, all risk factors for BNC and stricture formation, it is not surprising that BNC/strictures rates are higher in our study. Finally, whereas several published series focus on BNC only, we defined and evaluated stricture in any location that required treatment as an event.
No significant differences were observed in rates of hematuria, radiation cystitis, or proctitis. Though several studies have reported an association between ADT and an increased risk of myocardial infarction and cardiovascular events, metabolic syndrome, and risk of fractures,35–37 we did not observe higher rates of acute myocardial infarction, sudden cardiac death, osteoporosis, or fracture in men receiving XRT+ADT versus RP+XRT. However, our study was not designed to assess adverse effects of ADT when compared to other treatment modalities.
Several limitations of our study warrant mention. First, our findings should be interpreted within the limitations of an observational study design. Since our patients were not randomized, the two treatment groups may differ in measured and unmeasured ways that are associated with differences in survival, despite our best efforts to rigorously adjust for confounders. Our propensity score analyses utilized information from all measured covariates to balance observed factors between treatment groups. Though propensity score analysis can also reduce the bias associated with unobserved factors, so long as relationships between unobservable and measured factors exist, there is likely to be unmeasured confounding and selection biases that we are not able to control for that may have accounted for a portion of the survival differences observed in our study, as has been reported in other studies using cancer registry data.38, 39 To further reduce residual confounding by controlling for proxies of unmeasured confounders, high-dimensional propensity score adjustment was also applied.13 In an attempt to further balance observed and unobserved characteristics between treatment groups over what is possible with propensity score adjustments, we explored the possibility of utilizing instrumental variable analysis.40 However, an acceptable instrument could not be identified, as there was not sufficient variation in the use of radiotherapy plus androgen deprivation therapy in our study cohort.
Second, administrative claims are designed for billing purposes. Thus, key information that may influence outcome, such as radiation dosage, the field included within the radiation portal, or whether or not nerve-sparing was performed during surgery, is not precisely captured. A contemporary study of men with high-risk prostate cancer using the National Cancer Database found that among 14,817 patients undergoing primary radiation, 51.3% received whole pelvic radiotherapy versus 48.7% who received prostate-only radiotherapy.41 Based on this study, and on prospective randomized studies evaluating the radiation portal (i.e. whole pelvic vs prostate-only) in men with high-risk prostate cancer, whole pelvic radiotherapy does not appear to be associated with an overall survival advantage when compared to prostate-only radiotherapy.41–43 Also, whether radiotherapy was delivered for adjuvant or salvage intent cannot be determined by Medicare claims. To minimize inclusion of men receiving salvage radiotherapy, we defined adjuvant radiotherapy strictly as radiotherapy being delivered within 6 months after surgery..44
Third, measuring complications of cancer treatment using Medicare claims may result in a relatively crude estimate of treatment-related adverse effects. These codes may lack clinical specificity and may not always be adequate to identify diagnoses or procedures related to complications. As physician reimbursement is dictated by procedures rather than by diagnoses, complications that are not procedurally based may risk being underreported. Also, cancer patients can experience complications that impact negatively on quality of life to which they do not seek treatment for.34
Finally, controlling the impact that concurrent technological advances in surgery and radiation has had on treatment outcomes over time is not possible with Medicare claims.
CONCLUSIONS
To summarize, though our results are limited by the usual biases of an observational study design, men with locally advanced prostate cancer or regionally advanced prostate cancer who received primary radical prostatectomy with post-surgery radiotherapy had a lower risk of death from prostate cancer and had an improved overall survival when compared to those treated with primary radiotherapy plus ADT. Men who received RP+XRT had higher rates of erectile dysfunction (28% vs 20%, p=0.0212, respectively) and higher rates of urinary incontinence (49% vs 19%, p<0.001, respectively) when compared to those who received XRT+ADT. These findings should be verified with prospective trial data and suggest the need to include a surgical arm in future trials for men with high-risk prostate cancer.
Supplementary Material
Figure 1b.

Adjusted Overall Survival in Men undergoing Radical Prostatectomy with Adjuvant Radiotherapy versus Radiotherapy plus Androgen Deprivation Therapy, according to Tumor Stage
Acknowledgements:
We acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute, the Office of Information Services, and the Office of Strategic Planning, Center for Medicare & Medicaid Services; Information Management Services Inc, and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Funding Support:
1. New Jersey Health Foundation (TLJ)
2. National Cancer Institute P30 CA-072720 (Rutgers Cancer Institute of New Jersey Biometrics Shared Resource)
3. National Cancer Institute CA-116399 (GLY)
Footnotes
Conflict of Interest Disclosures:
Dr. Jang reports grants from National Cancer Institute (P30 CA-072720), grants from New Jersey Health Foundation, during the conduct of the study.
Dr. Scardino reports other from OPKO, outside the submitted work (Clinical Advisory Board); In addition, Dr. Scardino has a patent OPKO issued.
Dr. Stein reports personal fees from Merck, outside the submitted work;
All other authors declare no competing interests.
Contributor Information
Thomas L. Jang, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Neal Patel, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Izak Faiena, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Kushan Radadia, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Dirk F. Moore, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Sammy E. Elsamra, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Eric A. Singer, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Mark N. Stein, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Yong Lin, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
Isaac Y. Kim, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ.
James A. Eastham, Memorial Sloan-Kettering Cancer Center, Department of Surgery, Urology Service, Weill Cornell Medical College, New York, NY.
Peter T. Scardino, Memorial Sloan-Kettering Cancer Center, Department of Surgery, Urology Service, Weill Cornell Medical College, New York, NY.
Grace L. Lu-Yao, Sidney Kimmel Cancer Center, Thomas Jefferson University, Department of Medical Oncology, Sidney Kimmel Medical College, Jefferson College of Population Health, Philadelphia, PA (GLY).
REFERENCES
- 1.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012;366: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst 2009;101: 1280–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67: 7–30. [DOI] [PubMed] [Google Scholar]
- 4.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71: 618–629. [DOI] [PubMed] [Google Scholar]
- 5.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw 2016;14: 19–30. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Surveillance, Epidemiology, and End Results Available from URL: http://seer.cancer.gov [accessed July 2, 2017.
- 7.AJCC Cancer Staging Manual, 7th Edition New York, NY: Springer -Verlag, 2010. [Google Scholar]
- 8.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care 1993;31: 732–748. [PubMed] [Google Scholar]
- 9.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46: 1075–1079; discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis . 1987;40: 373–383. [DOI] [PubMed] [Google Scholar]
- 11.Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med 2010;170: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furlow B US National Cancer Institute investigates PSA coding errors. Lancet Oncol 2015;16: 614. [DOI] [PubMed] [Google Scholar]
- 13.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer 2010;116: 5226–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 2010;28: 1508–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joniau S, Hsu CY, Gontero P, Spahn M, Van Poppel H. Radical prostatectomy in very high-risk localized prostate cancer: long-term outcomes and outcome predictors. Scand J Urol Nephrol 2012;46: 164–171. [DOI] [PubMed] [Google Scholar]
- 17.Hodgson D, Warde P, Gospodarowicz M. The management of locally advanced prostate cancer. Urol Oncol 1998;4: 3–12. [DOI] [PubMed] [Google Scholar]
- 18.Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int 2005;95: 751–756. [DOI] [PubMed] [Google Scholar]
- 19.Carver BS, Bianco FJ Jr., Scardino PT, Eastham JA. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol 2006;176: 564–568. [DOI] [PubMed] [Google Scholar]
- 20.Gerber GS, Thisted RA, Chodak GW, et al. Results of radical prostatectomy in men with locally advanced prostate cancer: multi-institutional pooled analysis. Eur Urol 1997;32: 385–390. [PubMed] [Google Scholar]
- 21.Hsu CY, Joniau S, Oyen R, Roskams T, Van Poppel H. Outcome of surgery for clinical unilateral T3a prostate cancer: a single-institution experience. Eur Urol 2007;51: 121–128; discussion 128–129. [DOI] [PubMed] [Google Scholar]
- 22.Feldman AS, Meyer CP, Sanchez A, et al. Morbidity and Mortality of Locally Advanced Prostate Cancer: A Population Based Analysis Comparing Radical Prostatectomy versus External Beam Radiation. J Urol 2017;198: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 23.Joniau S, Tosco L, Briganti A, et al. Results of surgery for high-risk prostate cancer. Curr Opin Urol 2013;23: 342–348. [DOI] [PubMed] [Google Scholar]
- 24.Loeb S, Smith ND, Roehl KA, Catalona WJ. Intermediate-term potency, continence, and survival outcomes of radical prostatectomy for clinically high-risk or locally advanced prostate cancer. Urology 2007;69: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 25.Freedland SJ, Partin AW, Humphreys EB, Mangold LA, Walsh PC. Radical prostatectomy for clinical stage T3a disease. Cancer 2007;109: 1273–1278. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone PA, Ward KC, Goodman M, Assikis V, Petros JA. Radical prostatectomy for clinical T4 prostate cancer. Cancer 2006;106: 2603–2609. [DOI] [PubMed] [Google Scholar]
- 27.Moltzahn F, Karnes J, Gontero P, et al. Predicting prostate cancer-specific outcome after radical prostatectomy among men with very high-risk cT3b/4 PCa: a multi-institutional outcome study of 266 patients. Prostate Cancer Prostatic Dis 2015;18: 31–37. [DOI] [PubMed] [Google Scholar]
- 28.Moschini M, Briganti A, Murphy CR, et al. Outcomes for Patients with Clinical Lymphadenopathy Treated with Radical Prostatectomy. Eur Urol 2016;69: 193–196. [DOI] [PubMed] [Google Scholar]
- 29.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA 2015;314: 80–82. [DOI] [PubMed] [Google Scholar]
- 30.Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol 2003;21: 401–405. [DOI] [PubMed] [Google Scholar]
- 31.Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol 2007;178: 529–534; discussion 534. [DOI] [PubMed] [Google Scholar]
- 32.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med 2002;346: 1138–1144. [DOI] [PubMed] [Google Scholar]
- 33.Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol 2004;172: 2227–2231. [DOI] [PubMed] [Google Scholar]
- 34.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care 2002;40: IV-62–68. [DOI] [PubMed] [Google Scholar]
- 35.Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin 2010;60: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol 2007;25: 2420–2425. [DOI] [PubMed] [Google Scholar]
- 37.Shao YH, Moore DF, Shih W, Lin Y, Jang TL, Lu-Yao GL. Fracture after androgen deprivation therapy among men with a high baseline risk of skeletal complications. BJU Int 2013;111: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross ME, Kreider AR, Huang YS, Matone M, Rubin DM, Localio AR. Propensity Score Methods for Analyzing Observational Data Like Randomized Experiments: Challenges and Solutions for Rare Outcomes and Exposures. Am J Epidemiol 2015;181: 989–995. [DOI] [PubMed] [Google Scholar]
- 39.Williams SB, Huo J, Chamie K, et al. Discerning the survival advantage among patients with prostate cancer who undergo radical prostatectomy or radiotherapy: The limitations of cancer registry data. Cancer 2017;123: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst 2010;102: 1780–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amini A, Jones BL, Yeh N, Rusthoven CG, Armstrong H, Kavanagh BD. Survival Outcomes of Whole-Pelvic Versus Prostate-Only Radiation Therapy for High-Risk Prostate Cancer Patients With Use of the National Cancer Data Base. Int J Radiat Oncol Biol Phys 2015;93: 1052–1063. [DOI] [PubMed] [Google Scholar]
- 42.Lawton CA, DeSilvio M, Roach M 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94–13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys 2007;69: 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol 2007;25: 5366–5373. [DOI] [PubMed] [Google Scholar]
- 44.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 2009;302: 1557–1564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


