Abstract
Traditional models of neural reorganization of language skills in patients with chronic stroke-induced aphasia (PWA) propose activation of reperfused or spared left hemisphere tissue results in the most favorable language outcomes. However, these models do not fully explain variable behavioral recovery patterns observed in chronic patients. Instead, investigation of connectivity patterns of critical network nodes may elucidate better-informed recovery models. In the present study, we combined fMRI and dynamic causal modeling (DCM) to examine effective connectivity of a simple three-node left hemisphere network during a semantic feature decision task in 25 PWA and 18 age-matched neurologically intact healthy controls. The DCM model space utilized in Meier, Kapse, & Kiran (2016), which was organized according to exogenous input to one of three regions (i.e., left inferior frontal gyrus, pars triangularis [LIFGtri], left posterior middle temporal gyrus [LpMTG], or left middle frontal gyrus [LMFG]) implicated in various levels of lexical-semantic processing, was interrogated. This model space included all possible combinations of uni- and bidirectional task-modulated connections between LIFGtri, LMFG and LpMTG, resulting in 72 individual models that were partitioned into three separate families (i.e., Family #1: Input to LIFGtri, Family #2: Input to LMFG, Family #3: Input to LpMTG). Family-wise Bayesian model selection revealed Family #2: Input to LMFG best fit both patient and control data at a group level. Both groups relied heavily on LMFG’s modulation of the other two model regions. By contrast, between-group differences in task-modulated coupling of LIFGtri and LpMTG were observed. Within the patient group, the strength of activity in LIFGtri and connectivity of LpMTG→LIFGtri were positively associated with lexical-semantic abilities inside and outside of the scanner, whereas greater recruitment of LpMTG was associated with poorer lexical-semantic skills.
Keywords: connectivity, aphasia, lexical-semantics, dynamic causal modeling
1. Introduction
Approximately one-third of stroke survivors present with aphasia, a neurogenic communication disorder characterized by a constellation of deficits in multiple domains of language usually following infarct in the left middle cerebral artery (MCA) territory (Engelter et al., 2006; Flowers et al., 2016; Gialanella, Bertolinelli, Lissi, & Prometti, 2011; Laska, Hellblom, Murray, Kahan, & Von Arbin, 2001; Maas et al., 2012; Pedersen, Jørgensen, Nakayama, Raaschou, & Olsen, 1995; Wade, Hewer, David, & Enderby, 1986). Aphasia that persists into the chronic stage of recovery has devastating effects on patients’ communication, participation in everyday activities and quality of life (Flowers et al., 2016; Gialanella et al., 2011; Hilari, Wiggins, Roy, Byng, & Smith, 2003; Lam & Wodchis, 2010). Research that elucidates patterns of co-occurring neural and behavioral recovery in persons with aphasia (PWA) is an essential resource for maximizing language recovery in these individuals as improved behavioral, neuromodulatory, and/or pharmaceutical treatments will naturally follow a better understanding of brain-behavior relationships in stroke aphasia. However, predictive computational models of aphasia recovery based on neural and demographic variables are only now emerging (e.g., Hope, Leff, & Price, 2018; Hope, Seghier, Leff, & Price, 2013; Price, Seghier, & Leff, 2010), and thus, predictions regarding treatment-related and spontaneous recovery potential for individual patients are currently unreliable (Charidimou et al., 2014; Heiss, 2017; Lazar, Speizer, Festa, Krakauer, & Marshall, 2008; Lazar & Antoniello, 2008; Lazar & Boehme, 2017; Price, Hope, & Seghier, 2017).
Traditional theories of neural reorganization in chronic aphasia (Anglade, Thiel, & Ansaldo, 2014; Heiss & Thiel, 2006) provide a general framework for understanding recovery by juxtaposing left versus right hemisphere recruitment for language. For example, Heiss and Thiel (2006) proposed a three-tiered framework of chronic aphasia in which optimal, satisfactory, and poor language recovery were associated with, respectively, preservation or reactivation of primary language areas; damage to primary language cortex but spared and functional secondary left hemisphere language areas; and extensive left hemisphere damage with reliance on the contralesional (but possibly ill-suited) right hemisphere to mediate language. However, the past 25 years of collective fMRI and PET research in aphasia has resulted in conflicting evidence for these patterns, particularly the contested role of the right hemisphere in aphasia recovery (see Cappa, 2011; Cocquyt, De Ley, Santens, Van Borsel, & De Letter, 2017; Crosson et al., 2007; Kiran, 2012; Meinzer, Harnish, Conway, & Crosson, 2011; Price & Crinion, 2005; Saur & Hartwigsen, 2012; Thompson & den Ouden, 2008; Zahn, Schwarz, & Huber, 2006 for reviews). Imaging findings accord with the general assertion that left hemisphere activation—and perilesional activity in particular—results in better language recovery in PWA (Fridriksson, Richardson, Fillmore, & Cai, 2012; Heiss, Kessler, Thiel, Ghaemi, & Karbe, 1999; Léger et al., 2002; Marcotte & Ansaldo, 2010; Meinzer et al., 2008; Meinzer & Breitenstein, 2008; Menke et al., 2009; Rosen et al., 2000; Szaflarski, Allendorfer, Banks, Vannest, & Holland, 2013; van Hees, McMahon, Angwin, de Zubicaray, & Copland, 2014; Vitali et al., 2007; Warburton, Price, Swinburn, & Wise, 1999; Winhuisen et al., 2007).
However, definitive evidence from the patient activation literature supporting Heiss and Thiel’s (2006) distinction between optimal and satisfactory recovery patterns (i.e., activation of canonical language cortex versus activation of ipsilesional regions, respectively) has not been demonstrated. For example, certain studies (Abel, Weiller, Huber, Willmes, & Specht, 2015; Fridriksson, Bonilha, Baker, Moser, & Rorden, 2010; Sims et al., 2016) have shown that patients with the most recovered language abilities recruit traditional perisylvian language regions (e.g., inferior frontal, superior and middle temporal, and angular and supramarginal gyri) as well as left hemisphere nodes outside of the canonical language network (e.g., superior and middle frontal gyri, superior parietal lobule). In a meta-analysis of 12 fMRI studies in PWA, Turkeltaub, Messing, Norise, and Hamilton (2011) discovered that across a variety of language tasks, PWA activated crucial language nodes homotopic to activation peaks observed in left inferior frontal gyrus pars opercularis (LIFGop), LIFG pars triangularis (LIFGtri), and left middle temporal gyrus (LMTG) in healthy controls. When the authors accounted for LIFG lesions, LH peaks were still found within canonical language regions but were shifted to functionally-homologous intact perilesional regions (i.e., LIFG pars orbitalis [LIFGorb]) or were recruited for a presumed different function (i.e., anterior insula). Critically, patients with and without lesions in LIFG showed activation in neighboring dorsolateral prefrontal cortex (i.e., LMFG), leading the authors to conclude that LMFG may be critical for supporting linguistic processes in some manner for PWA, regardless of lesion in LIFG. Within Heiss and Thiel’s (2006) hierarchy of chronic aphasia recovery, LIFG and LMTG likely fall into the category of “classic” language regions whereas LMFG would probably be considered secondary left hemisphere language cortex. Nevertheless, the necessity of perisylvian and extrasylvian left hemisphere recruitment for optimal language recovery in PWA is reasonable given both the nature of linguistic deficits in PWA and the potential functional roles of these regions, as described below.
1.1. Aphasic deficits and the functional role of frontotemporal cortex for language
It has been proposed that deficits in PWA are a consequence of impaired controlled access to, rather than destruction of, linguistic representations (Corbett, Jefferies, & Lambon Ralph, 2009; Corbett, Jefferies, Ehsan, & Lambon Ralph, 2009; Crutch & Warrington, 2005; Gotts & Plaut, 2002; Hoffman, Jefferies, & Lambon Ralph, 2011; Jefferies, Baker, Doran, & Lambon Ralph, 2007; Jefferies, Patterson, & Lambon Ralph, 2008; Jefferies & Lambon Ralph, 2006; McCarthy & Warrington, 2016; Mirman & Britt, 2013; Rogers, Patterson, Jefferies, & Lambon Ralph, 2015; Thompson & Jefferies, 2013; Warrington, 1975; Warrington & Cipolotti, 1996; Warrington & Mccarthy, 1983, 1987). In the neuropsychological literature, the difference between representation and access deficits has been studied most often within the semantic system. Error analysis supports the existence of intact semantic knowledge in patients with stroke aphasia as PWA with semantic control deficits retrieve related but incorrect semantic information during tasks such as picture naming. For example, PWA often produce category coordinate errors (e.g., ‘shorts’ for ‘coat’) or responses that fall outside of the intended category due to thematic associations (e.g., ‘snow’ for a cold weather clothing). In addition, phonological cues improve word retrieval (e.g., correct naming of ‘coat’ with a phonological cue of /k/) for these individuals while miscues result in paraphasias (e.g., incorrect naming of ‘shorts’ for ‘coat’ with phonological cue of /ʃ/) (Jefferies et al., 2008; Schwartz, 2013).
In alignment with the access deficit hypothesis, it stands to reason that beneficial neural reorganization of language in post-stroke aphasia involves the recruitment of and communication between regions implicated in access to and/or executive control of linguistic information. Neurocognitive models of healthy semantic processing (e.g., Binder & Desai, 2011) confer an executive role to certain cortical regions, most often frontal cortex. Findings from seminal fMRI studies from the late 1990s and early 2000s support LIFG—and LIFGorb and LIFGtri in particular—in semantic access or control in cognitively demanding tasks (e.g., resolution of semantic ambiguity, selection of a target among semantic competitors, suppression of strong semantic associations with activation of weaker associations) (Badre & D’Esposito, 2007; Badre, Poldrack, Paré-Blagoev, Insler, & Wagner, 2005; Devlin, Matthews, & Rushworth, 2003; Gold & Buckner, 2002; Poldrack et al., 1999; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997; Wagner, Paré-Blagoev, Clark, & Poldrack, 2001). Lambon Ralph, Jefferies, Patterson, and Rogers (2016) proposed a larger semantic control network which includes not only LIFG but also the posterior LMTG (LpMTG), intraparietal sulcus (IPS), pre-supplementary motor area (pre-SMA), and medial prefrontal/anterior cingulate cortex (mPFC/ACC). Other work has suggested that a multiple demand (MD) network (including portions of the middle frontal gyrus [MFG], IFG pars opercularis [IFGop], the frontal operculum, anterior insula, dorsal ACC, SMA, and IPS) becomes active across a variety of tasks (e.g., verbal working memory, sentence comprehension, visual working memory, etc.) particularly when task difficulty increases (Duncan, 2010; Fedorenko, Behr, & Kanwisher, 2011; Fedorenko, Duncan, & Kanwisher, 2012; Fedorenko & Thompson-Schill, 2014).
Collectively, these papers highlight that regions implicated in domain-specific (i.e., semantic) and/or domain-general regions are activated in healthy individuals with increased executive processing demands. Given that PWA struggle with language, it is not inconceivable that regions that mediate executive processes would be crucial in patient networks. Alternatively, patients’ reliance on regions typically associated with domain-specific (e.g., LIFGtri, LpMTG) or domain-general (e.g., LMFG) executive processing could reflect some undetermined—but possibly critical—consequence of stroke (e.g., reorganization of language to redundant systems, heightened cognitive control load due to concomitant cognitive deficits).
1.2. Domain specificity versus generality in aphasia
One critical question is if post-stroke language recovery—and lexical-semantic recovery in particular—must be mediated through reactivation of spared tissue in critical hubs associated with semantic representation or control or if redundant systems allow for domain-general MD regions to compensate for lost domain-specific systems. Recent work has illustrated that PWA rely on domain-general networks and/or connectivity between language and domain-general regions to a greater extent for language tasks compared to age-matched healthy controls (Geranmayeh, Chau, Wise, Leech, & Hampshire, 2017; Geranmayeh, Leech, & Wise, 2016; Sharp, Turkheimer, Bose, Scott, & Wise, 2010). Moreover, changes in activation in PWA following language therapy have been found not only in perisylvian language areas but also in other left hemisphere regions outside the canonical language network (e.g., Fridriksson, 2010; Fridriksson, Bonilha, Baker, Moser, & Rorden, 2010; Kiran, Meier, Kapse, & Glynn, 2015; Marcotte et al., 2012; Menke et al., 2009; Sandberg, Bohland, & Kiran, 2015). Unlike perisylvian language cortex—including the two regions most often implicated in semantic control (i.e., anterior LIFG and LpMTG)—MD regions such as LMFG fall on the outskirts of the MCA territory and may represent the only remaining ipsilesional spared tissue available for bootstrapping linguistic processing in PWA. Therefore, informative conclusions regarding the neural reorganization of language in PWA would likely result from quantifying the extent of damage to canonical language regions, measuring the degree of activation or connectivity of regions within language and MD networks, and relating these neural metrics to specific linguistic skills.
To date, few researchers have made direct associations between lesion characteristics and PWA’s activation or connectivity patterns during lexical-semantic processing. Of the limited existing studies, Griffis and colleagues (Griffis, Nenert, Allendorfer, & Szaflarski, 2017b; Griffis, Nenert, Allendorfer, Vannest, et al., 2017) found that patients with a greater amount of spared tissue in left temporoparietal cortex demonstrated greater activity in a canonical left frontoparietal semantic network and better performance on language tasks inside and outside of the scanner. In the same vein, Hallam et al. (2018) discovered that patients with semantic aphasia with damage to LIFG demonstrated greater activity in LpMTG relative to healthy individuals for a semantic control task. By contrast, Sims et al. (2016) found that greater damage to canonical anterior (i.e., LIFGorb) and posterior (i.e., angular/supramarginal gyri [LAG/LSMG] and LMTG) language cortex was associated with greater percent signal change for semantic decisions in bilateral dorsolateral and dorsomedial prefrontal cortex, regions most often implicated in domain-general cognitive control.
These collective findings suggest LIFGtri and LpMTG are integral for semantic processing and potentially play a critical role in domain-specific (i.e., semantic control) processing for individuals with aphasia. Likewise, a reliance on regions implicated in domain-general processes (e.g., LMFG) may be essential for the recovery of lexical-semantics in PWA. Nevertheless, the relative importance of canonical perisylvian regions versus traditional domain-general areas for lexical-semantic processing in PWA remains unknown.
1.3. Study aims
The present study aimed to extend previous work by interrogating functional integration (i.e., connectivity) of residual left hemisphere tissue in regions potentially most primed to mediate recovery of lexical-semantic skills in PWA. Specifically, we used a systematic approach for identifying regional activity during a semantic feature decision task in three left hemisphere regions of interest (ROIs): LIFGtri for its presumed role in controlled semantic access, LMFG for its presumed role in domain-general cognitive processes, and LpMTG for its assumed role in either semantic access and/or convergence of semantic information from modality-specific regions during semantic tasks. We used dynamic causal modeling (DCM; Friston, Harrison, & Penny, 2003) to investigate differences between PWA and age-matched healthy controls in effective connectivity of the LIFGtri-LMFG-LpMTG subnetwork and to determine relationships between connectivity parameters and lexical-semantic skills in PWA. To address these goals, we explored the DCM model space from Meier, Kapse, & Kiran (2016), in which exogenous task input was modeled to either LIFGtri, LMFG, or LpMTG and all possible combinations of task-modulated connections were specified to examine connectivity during lexical retrieval in PWA.
In Meier et al. (2016), we found that healthy controls demonstrated a preference for best-fit DCM models with input to LIFGtri whereas PWA preferred models with input to LMFG. Furthermore, we found that the strength of several connections including LMFG was related to the amount of spared tissue in network nodes and naming abilities in PWA. As such, we hypothesized healthy controls in the present study would demonstrate a preference for DCM models with input to and the strongest connections from LIFGtri. Alternatively, if LIFGtri and LpMTG play a similar role in semantic access (Noonan et al., 2013; Lambon Ralph et al., 2016), we predicted no clear preference for models that emphasize input to either one of these two regions. For PWA, we hypothesized a high likelihood of local damage to and greater functional disconnection of LIFGtri and LpMTG. Similar to findings from Meier et al. (2016), we predicted that best-fit DCM models for PWA would include input to LMFG and that modulatory connections from LMFG to LIFGtri and/or LpMTG would be most predictive of lexical-semantic skills in the patient group.
2. Materials and methods
This study was conducted as part of a large, multi-site investigation of the neural bases of language recovery in individuals with chronic aphasia secondary to stroke (NIH/NIDCD grant 1P50DC012283) within the Center for the Neurobiology of Language Recovery (CNLR; http://cnlr.northwestern.edu/).
2.1. Participants
Thirty-two individuals with chronic aphasia (22M, mean age = 61.75 ± 11.26 years) following left middle cerebral artery MCA infarct and 21 neurologically intact healthy controls (10M, mean age = 59.61 ± 13.45 years) were recruited for the present study. Case history information including neurological history, age, handedness, race, and ethnicity was collected for all participants via questionnaire. None of the participants had major psychiatric disorders, neurological disease (besides stroke in PWA) or active medical conditions that would preclude participation. All participants primarily spoke English and had normal or corrected-to-normal vision and adequate hearing (i.e., bilateral threshold ≤ 40 dB at 500, 1000 and 4000 Hz). Enrolled patients presented with aphasia due to a single left hemisphere CVA2 and were in the chronic phase of stroke recovery (mean post-stroke onset = 49.56 ± 48.93 months).
Final inclusion in the present study was determined based on fMRI data quality. Data were unusable for seven PWA and three controls due to artifacts from implanted material (n = 1 patient), low signal-to-noise from excessive motion (n = 6 PWA, 2 controls), and participant removal from the scanner due to claustrophobia prior to scan completion (n = 1 control). As such, behavioral and neuroimaging data were analyzed for 25 PWA (17M, mean age = 62.00 ± 11.77 years) and 18 healthy controls (10M; mean age = 60.35 ± 10.93 years). Of note, a subset of the participants in the current study3 were included in Meier et al. (2016).
Prior to scanning, PWA completed a behavioral testing battery that included the Western Aphasia Battery-Revised (WAB; Kertesz, 2007) to quantify overall aphasia severity per the Aphasia Quotient (AQ); the Pyramids and Palm Trees Test (PPT; Howard & Patterson, 1992) to assess nonverbal semantic association abilities, and subtest 51: Word Semantic Association from the Psycholinguistic Assessment of Language Processing in Aphasia (PALPA; Kay, Coltheart, & Lesser, 1992) to assess lexical-semantic association skills. Accuracy on PALPA 51 served as an outside-of-scanner proxy for lexical-semantic abilities. Lexical-semantic skills in general (and semantic feature processing in particular) were investigated due to the importance of semantic processes in single word production (Dell, Schwartz, Martin, Saffran, & Gagnon, 1997; Levelt, Roelofs, & Meyer, 1999; Schwartz, Dell, Martin, Gahl, & Sobel, 2006). Moreover, as part of the CNLR, many patients in the present study completed a 12-week language therapy that targeted word-retrieval deficits by training patients on semantic features of target items. While a detailed summary of the therapy is outside the scope of this paper, the results of the treatment portion of the study (see Gilmore, Meier, Johnson, & Kiran, 2018) additionally illustrate that semantic feature knowledge is critical for successful word retrieval in PWA. See Table 1 for stroke, demographic and testing information in the patient group.
Table 1.
Demographic, stroke, and behavioral data for PWA
| ID | Gender | Age | Handedness | MPO | Lesion Volume* |
WAB- R AQ |
PPT | PALPA51 |
|---|---|---|---|---|---|---|---|---|
| P1 | M | 55 | R | 12 | 74508 | 87.20 | 96.00 | 76.67 |
| P2 | M | 79 | R | 13 | 92057 | 74.10 | 94.00 | 60.00 |
| P3 | M | 67 | R | 8 | 172344 | 30.80 | 92.00 | 30.00 |
| P4 | M | 49 | R | 113 | 324719 | 66.60 | 92.00 | 73.00 |
| P5 | M | 55 | R | 137 | 210628 | 48.00 | 88.00 | 40.00 |
| P6 | F | 71 | R | 37 | 11279 | 95.20 | 96.00 | 86.67 |
| P7 | F | 53 | R | 12 | 68088 | 80.40 | 94.00 | 80.00 |
| P8 | M | 68 | R | 104 | 210383 | 40.00 | 88.00 | 40.00 |
| P9 | M | 42 | L | 18 | 8097 | 92.70 | 94.00 | 70.00 |
| P10 | F | 64 | R | 24 | 59140 | 64.40 | 94.00 | 53.33 |
| P11 | F | 70 | R | 62 | 130489 | 87.20 | 85.00 | 53.33 |
| P12 | M | 50 | R | 71 | 321907 | 33.60 | 79.00 | 10.00 |
| P13 | M | 61 | R | 152 | 159060 | 74.30 | 98.00 | 70.00 |
| P14 | F | 70 | R | 152 | 154879 | 78.00 | 96.15 | 50.00 |
| P15 | M | 80 | R | 22 | 87744 | 28.90 | 82.69 | 26.67 |
| P16 | F | 48 | R | 20 | 257144 | 13.00 | 94.23 | 33.33 |
| P17 | M | 69 | R | 164 | 235770 | 40.40 | 94.00 | 26.67 |
| P18 | F | 76 | R | 33 | 136854 | 37.50 | 65.00 | 63.33 |
| P19 | F | 64 | R | 115 | 279144 | 58.00 | 69.00 | 40.00 |
| P20 | M | 63 | R | 22 | 111102 | 56.00 | 98.08 | 50.00 |
| P21 | M | 49 | R | 49 | 79770 | 85.50 | 94.00 | 66.67 |
| P22 | M | 82 | R | 12 | 57440 | 73.80 | 94.23 | 73.33 |
| P23 | M | 39 | R | 18 | 13867 | 71.30 | 100.00 | 46.67 |
| P24 | M | 64 | L | 13 | 56449 | 79.60 | 96.15 | 56.67 |
| P25 | M | 62 | L | 21 | 5256 | 92.00 | 94.00 | 70.00 |
| MEAN | 62.00 | 56.16 | 132724.72 | 63.54 | 90.70 | 53.85 | ||
| STDEV | 11.77 | 53.01 | 97283.45 | 23.49 | 8.65 | 19.53 | ||
MPO = months post-onset; WAB-R AQ = Western Aphasia Battery-Revised Aphasia Quotient; PPT = Pyramids and Palm Trees Test; PALPA 51 = Psycholinguistic Assessment of Linguistic Processing in Aphasia, subtest 51;
lesion volume in number of 1×1×1 mm voxels
Behavioral procedures for PWA were executed according to IRB protocols at Boston University. Neuroimaging procedures for all participants were performed according to IRB protocols at Massachusetts General Hospital.
2.2. MR data acquisition
Imaging data were collected at the Athinoula A. Martinos Center in Charlestown, MA, on a 3T Siemens Trio Tim scanner with a 20 channel head+neck coil. For all participants, a high resolution, T1-weighted 3D sagittal volume (parameters: TR/TE = 2300/2.91ms, T1 = 900ms, flip angle = 9°, matrix = 256×256mm, FOV = 256mm, slice thickness = 1mm3, 176 sagittal slices) and functional scans via a gradient echo T2*-weighted EPI sequence (parameters: TR/TE = 2570/30ms, flip angle = 90°, matrix = 80×78mm, FOV = 220×220mm, 40 axial, 3mm slices with 2×2×3mm voxels, parallel imaging with acceleration factor or 2) were acquired.
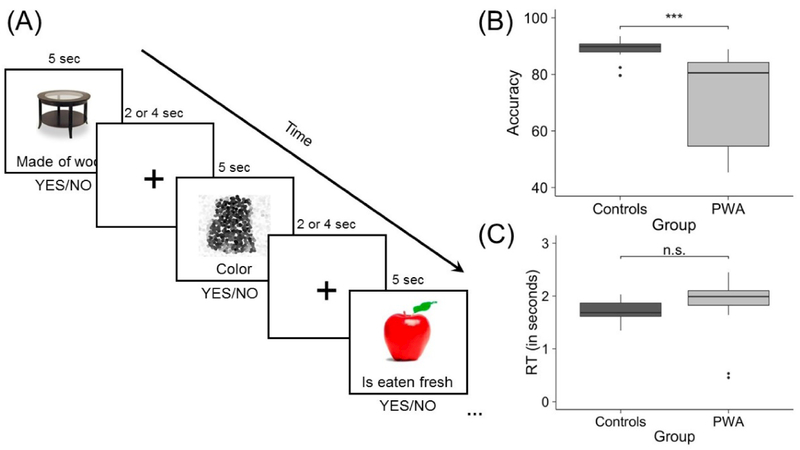
To measure the neural correlates of semantic processing, an event-related semantic feature verification task with a jittered inter-stimulus interval was used. Experimental stimuli included 108 real pictured items from three of five semantic categories (i.e., birds, vegetables, furniture, clothing, and fruit) that were balanced for familiarity, length, lexical frequency, and concreteness using the CELEX (Van der Wouden, 1990) and MRC Psycholinguistic (Coltheart, 1981) databases. Category assignment was pseudo-randomly counterbalanced across participants. Control stimuli included 36 scrambled, pixelated images in black and white or color. Trials were randomized and presented in two separate runs (i.e., 54 experimental and 18 control stimuli per run). During the task, an image appeared on the screen followed one second later by a written feature below the image. Features for the experimental items were based on results of a MTurk pilot study (https://www.mturk.com/mturk) in which participants decided whether a certain feature applied to a given item. Features were classified as being either related (i.e., contextual, characteristic, physical, and functional features) or unrelated to the item. During the fMRI experiment, participants decided whether the written feature applied to the image and responded via a yes/no button press (see Figure 1A). Accuracy and reaction time (RT) data were collected and compared between groups using unequal variance Welch’s t-tests. PWA were significantly less accurate than controls were in making semantic judgments during the fMRI task (t(26.23) = −4.89, p < 0.001; mean PWA accuracy: 72.38 ± 15.81%, mean control accuracy: 88.72 ± 3.47%) (Figure 1B). However, no significant differences in reaction times were noted between groups (t(33.68) = 1.62, p = 0.11; mean PWA RT: 1.89 ± 0.47 seconds, mean control RT: 1.72 ± 0.21 seconds) (Figure 1C).
Figure 1.
fMRI task. (A) Example experimental and control trials and comparison of (B) fMRI task accuracy and (C) reaction times between participant groups
2.3. MR data analysis
MR data were analyzed using SPM12 (Wellcome Trust Centre for Neuroimaging).
2.3.1. Preprocessing
A standard pipeline with additional steps for patient data was used to preprocess the MR data. First, slice timing correction with reference to the middle slice was performed to account for differences in the timing of slice acquisition. Next, realignment of functional scans to the mean image via 4th degree B-spline interpolation was performed, and functional and structural images were coregistered. Images were then segmented into gray matter, white matter and cerebrospinal fluid based on SPM12’s tissue probability maps, and segmented functional and structural images were normalized to the MNI template. Last, data were smoothed using a relatively small smoothing kernel of 4mm. A smoothing kernel approximately twice the functional voxel dimensions has been recommended for patient data to increase the credibility of activation peaks obtained from 1st-level activation maps and to reduce the likelihood of activation shifting into lesioned areas (Meinzer et al., 2013). In addition, lesion masks were manually drawn in MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/) using each patient’s T1-weighted structural image. Binarized lesion masks (in which lesioned voxels were deleted) and lesion maps (in which lesioned voxels were preserved) were included during preprocessing to improve coregistration and normalization of the patient data (Brett, Leff, Rorden, & Ashburner, 2001). Finally, the ArtRepair toolbox was used to account for large volume-to-volume motion; specifically, repaired functional files were used when repair reduced the standard deviation of the estimation error for the contrast of interest in the 1st-level general linear model (GLM) (Mazaika, Hoeft, Glover, & Reiss, 2009).
2.3.2. Localization of regional activity
The following procedures were performed to identify task activation in the three left hemisphere regions of interest (ROIs) for the effective connectivity analysis.
2.3.2.1. 1st-level analysis
For all participants, a 1st-level GLM was specified to obtain activation associated with semantic processing during the feature verification task. The GLM included the conditions pictures (i.e., experimental stimuli), scrambled pictures (i.e., control stimuli), and fixation. Stimulus onsets and durations for each condition were modeled and convolved with the canonical hemodynamic response function and its temporal derivative. The contrast of interest pictures – scrambled pictures was used to capture core processes involved in making semantic judgments (i.e., lexical-semantics, semantic control) while controlling for visual and motor processes. Activation maps were obtained at an uncorrected threshold (p < 0.001) to identify peaks within each ROI for each participant.
2.3.2.2. 2nd-level analysis
As a critical data validation step prior to effective connectivity analysis, 2nd-level analyses were first performed for the contrast pictures – scrambled pictures using the Statistical nonparametric Mapping (SnPM) toolbox in SPM (http://warwick.ac.uk/snpm) to check whole-brain activation in patients and controls. Within each group, multi-subject one-sample t-tests with a cluster-defining threshold of p < 0.01 and F.W.E. cluster-corrected threshold of p < 0.05 over 10,000 permutations were conducted. The results of these analyses are shown in Supplemental Figure 1.
2.3.2.3. Definition of anatomically-constrained bounding masks
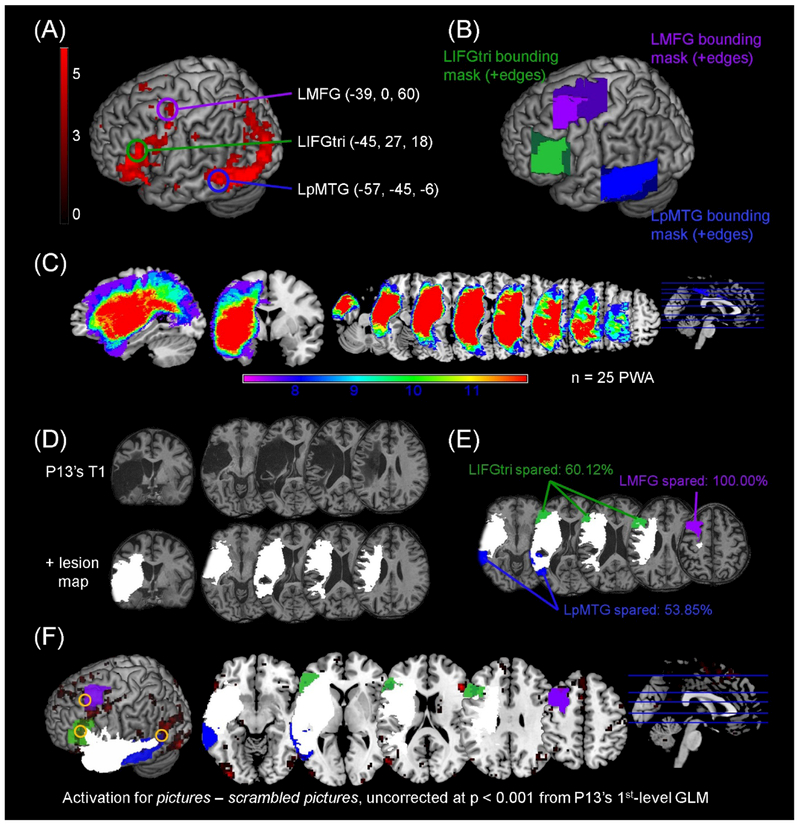
Masks within the anatomical boundaries of LIFGtri, LMFG and LpMTG were created to ensure that the signal extracted for the effective connectivity analysis was in a similar anatomical location across participants. First, one-sample t-test results in the control group at an uncorrected threshold (p < 0.001) were used to localize unique peak maxima within each region of interest (Figure 2A). The MNI coordinates corresponding to each peak were entered as the center input of rectangular bounding masks created in the MarsBaR toolbox (Brett, Anton, Valabregue, & Poline, 2002) for LIFGtri (mask dimensions: 35×35×35mm), LMFG (mask dimensions: 35×50×35mm) and LpMTG (mask dimensions: 35×50×35mm). Next, the rectangular masks were trimmed to fit the anatomical boundaries of each region per the SPM Anatomy toolbox (Eickhoff et al., 2005) (Figure 2B). To account for PWA’s lesions (see Figure 2C for patient group lesion overlay), a manually-drawn lesion map for each patient (e.g., Figure 2D) was overlaid onto the bounding masks and lesioned voxels were subtracted from each mask. The amount of spared tissue within each anatomically constrained bounding mask was determined for each patient by subtracting their normalized lesion volume from the volume of the bounding mask and dividing that amount by the volume of the bounding mask (Figure 2E). Thus, bounding masks were tailored for each patient so that the area from which signal was extracted reflected tissue without frank damage from stroke (Figure 2F).
Figure 2.
Definition of anatomically-constrained bounding masks. (A) Rendered results of the control group 2nd-level analysis for pictures – scrambled pictures, uncorrected p < 0.001 are shown and activation peaks within each ROI that were used as the center input in the anatomically-constrained bounding masks are circled. (B) Rectangular bounding boxes at a search depth of 12mm are shown as dark edges. The edges were trimmed to constrain the masks to the anatomical boundaries of the ROIs, resulting in the three lightly-shaded masks used in other analyses. (C) Overlay of lesion maps from the patient group included in the DCM analysis are shown in sagittal, coronal and axial slices. (D) Lesion masks (in which lesioned voxels were deleted) were manually drawn slice-by-slice on each patient’s T1 structural image in native space to create lesion maps (in which lesioned voxels were preserved). The normalized lesion map overlaid on the normalized T1 structural image for one participant (i.e., P13) is shown to illustrate this process. (E) Each patient’s lesion map was overlaid onto the anatomical bounding masks shown in (B) in order to create individually-tailored bounding masks reflecting the spared tissue within each mask. The percentage of residual tissue within each mask was determined by subtracting the patient’s lesion volume from the volume of the anatomically-constrained bounding mask, divided by the volume of the mask. The lesion map (in white) and individually-tailored bounding masks are shown for P13. (F) Visual inspection of overlaid t maps for pictures – scrambled pictures, lesion maps, and individually-constrained bounding masks for each patient ensured that the extracted VOIs fell outside the lesion and within (or approximate to) the bounding mask borders. The location of P13’s VOIs extracted for the connectivity analysis are denoted by the yellow circles.
2.3.2.4. Extraction of contrast estimates
To assess potential between-group differences in activation strength within the three ROIs and the potential effects such differences might have on connectivity, contrast estimates for pictures – scrambled pictures were extracted from the 2nd-level one-sample t-tests from each anatomically constrained bounding mask with MarsBaR (Brett et al., 2002). Raw data with the grand mean scaled to zero were estimated for each participant. Differences in contrast estimates between groups were compared via a one-way MANOVA with participant group as the independent variable and regional contrast estimates as the dependent variables.
2.4. Effective connectivity analysis
Dynamic causal modeling (DCM) is a hypothesis-driven method of effective connectivity used to make inferences about how the coupling between modeled brain regions is influenced by an experimental task (Friston et al., 2003; Kahan & Foltynie, 2013; Seghier, Zeidman, Neufeld, Leff, & Price, 2010; Stephan et al., 2010; Stephan, Weiskopf, Drysdale, Robinson, & Friston, 2007). More specifically, DCM utilizes Bayesian estimations on parameters of a dynamic multiple input-state-output model. The basic DCM implementation includes three main components: (1) inputs that are stimulus functions that correspond to external experimental manipulations (i.e., experimental task or conditions), (2) state variables that include neuronal and neurophysiological variables required to form outputs, and (3) outputs that correspond to hemodynamic responses in fMRI studies. DCM models five state variables for each region of interest. Neuronal activity within each region is fed forward into a hemodynamic balloon model that models four state variables (i.e., signal increases from vasodilation, blood inflow, changes in blood volume, and changes in deoxyhemoglobin). Observed changes in hemodynamic response are then linked to “hidden” neuronal states in the form of synaptic activity; as such, DCM allows for inferences about direct neural activity via the fifth and final state variable (Friston et al., 2003). Critically, three parameters within the neuronal state equation are estimated; specifically, differential equations within the DCM-A matrix model the intrinsic coupling between regions in the absence of task input; the DCM-B matrix models the change in the rate of coupling between regions induced by an external task; and the DCM-C matrix models exogenous perturbation of task input on a given region in the model. The remainder of this section describes the DCM implementation for the present study.
2.4.1. Model specification
The DCM model space from Meier et al. (2016) was utilized in the present study to test our hypotheses regarding dynamic connectivity during semantic judgments. As alluded to in the Introduction, prior fMRI studies have highlighted the importance of the three left hemisphere regions included in the model space (i.e., LIFGtri, LMFG, and LpMTG) for different cognitive functions during lexical-semantic processing in both healthy individuals and PWA. For this reason, Meier et al. (2016) constructed the model space to examine effective connectivity during overt picture naming, a task that has different (e.g., increased executive control during semantic selection) but overlapping (e.g., lexical-semantic processing) task demands as the semantic feature verification task. As such, comparing the results from these two investigations can provide additional insight into left hemisphere connectivity dynamics in chronic stroke patients.
Specification of the structure of the DCM-A, -B, and -C matrices followed Meier et al. (2016). In brief, fully inter-connected intrinsic connections were modeled in the DCM-A matrix given the robust network of white matter structures connecting these regions (Bajada et al., 2017; Catani & Mesulam, 2008; Catani, Howard, Pajevic, & Jones, 2002; Catani, Jones, & ffytche, 2005; Cloutman & Lambon Ralph, 2012; Dick, Bernal, & Tremblay, 2014; Frey, Campbell, Pike, & Petrides, 2008; Glasser & Rilling, 2008; Jung, Cloutman, Binney, & Lambon Ralph, 2017; Sarubbo, De Benedictis, Maldonado, Basso, & Duffau, 2013). The condition effect of pictures was systematically modeled onto connections between regions in the DCM-B matrix, such that all possible combinations of task modulation on uni- and bidirectional connections were specified. Last, direct task-induced perturbation from the pictures condition was modeled in the DCM-C matrix to one of the three regions within each model. Not only have other studies using DCM (Allen et al., 2008; den Ouden et al., 2012; Eickhoff, Heim, Zilles, & Amunts, 2009; Hartwigsen, Saur, Price, Baumgaertner, et al., 2013; Hartwigsen, Saur, Price, Ulmer, et al., 2013; Heim, Eickhoff, & Amunts, 2009; Seghier, Josse, Leff, & Price, 2011; Volz, Eickhoff, Pool, Fink, & Grefkes, 2015) similarly modeled input to higher-level cortex, but also this implementation was particularly useful in the present sample of PWA as these individuals exhibited varying degrees of regional damage that may have influenced connectivity patterns. The final model space included 72 individual models and was partitioned into three families, with exogenous input modeled to LIFGtri for the first 24 models (i.e., Family #1), LMFG for the next 24 models (i.e., Family #2) and LpMTG for the final 24 models (i.e., Family #3) (see Supplemental Figure 2).
2.4.2. Volume of interest (VOI) extraction and model estimation
For each participant, peak maxima within the LIFGtri, LMFG and LpMTG bounding masks were identified from the 1st-level GLM at an uncorrected threshold (p < 0.001). If the local maxima within a given anatomical region fell outside the regional bounding mask, the MNI coordinates of the second or third most-active peaks at p < 0.001 or the strongest peak at a reduced threshold (p < 0.01) were identified. Volumes of interest (VOIs) were extracted as 8mm eigenvariate spheres surrounding the MNI coordinates of selected peaks. For PWA with less than approximately 50% spared tissue within a regional bounding mask who also did not exhibit perilesional activation within the mask, a noisy signal (i.e., at a threshold of uncorrected, p = 1.0) was extracted from the damaged region at the coordinates corresponding to the controls’ regional peak. This method, which has been used in previous studies of network reorganization in stroke patients (Seghier, Bagdasaryan, Jung, & Price, 2014; Seghier et al., 2012), constitutes a good approximation of damage within the language system (Seghier et al., 2010) and allows for the inclusion of PWA who would otherwise be excluded from the DCM analysis. Following VOI extraction, models according to the model space were estimated for all participants. To minimize the complexity of the models while still capturing potential excitatory and inhibitory inter-regional modulation, a bilinear, deterministic two-state implementation was utilized.
2.4.3. DCM model and parameter inference
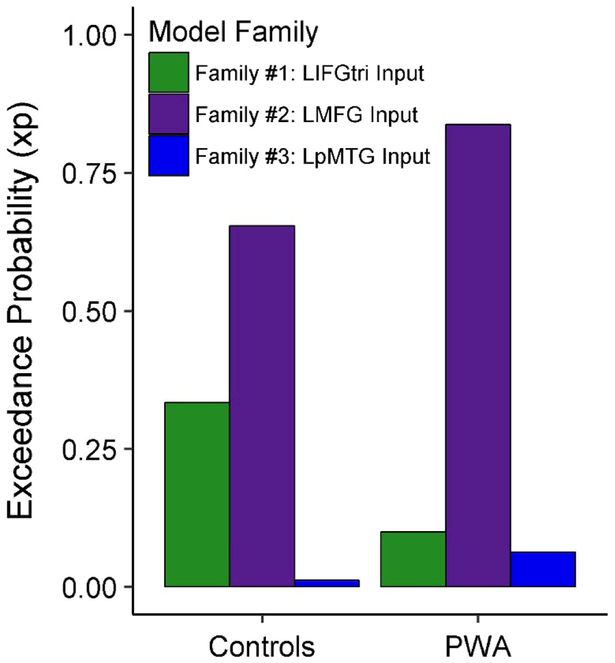
Inferences at the model and parameter levels were made to evaluate the DCM results. First, fixed effects Bayesian parameter averaging (BPA) was applied for each participant across outputs that corresponded to each run of the task. In the BPA analysis, the individual posterior densities from the two runs were combined by treating the posterior of one run as the prior for the other run (Stephan et al., 2010). This method was useful for obtaining one representative set of models for each participant. Next, to account for potential model structure uncertainty, a random-effects family-wise Bayesian model selection (BMS) procedure was used to establish which family of models best fit the data at both the individual and group levels (Penny et al., 2010). In this analysis, model inference is made on the posterior probability estimates of model family frequency, and as such, a model family’s exceedance probability (xp) value reflects the certainty that a given family of models is more likely than other model families to fit the data (Stephan, Penny, Daunizeau, Moran, & Friston, 2009).
Because we expected best-fit families to differ between participant groups, we additionally applied Bayesian model averaging (BMA) across model families for parameter extraction. BMA resulted in one set of parameters that reflected weighted average connectivity of the six connections (i.e., LIFGtri→LMFG, LIFGtri→LpMTG, LMFG→LIFGtri, LMFG→LpMTG, LpMTG→LIFGtri, LpMTG→LMFG) wherein models with high evidence for a participant contributed more greatly to the strength of task-modulated connections (measured by Ep.B values) than models with low evidence. One-sample t-tests were used to determine significant connections within each participant group. A one-way MANOVA was conducted to determine between-group differences in connection strength. In order to mitigate potential effects of regional activity to input regions influencing between-group differences, two-way MANOVAs with independent factors of group and regional activity within bounding regions (captured via contrast estimates described in section 2.3.2.4) were also conducted. Finally, backward stepwise regression was used to determine neural metrics (i.e., DCM parameters, contrast estimates) that were associated with lexical-semantic skills inside and outside of the scanner in the patient group.
3. Results
3.1. ROI analyses
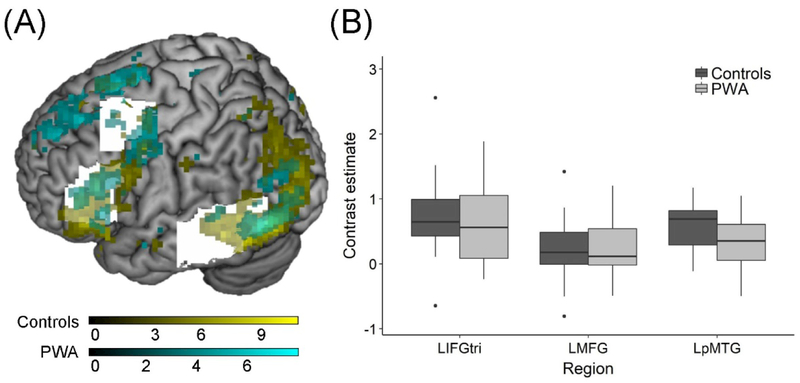
The location of peak maxima was most similar between groups in the two frontal ROIs. Specifically, both groups showed peak activity in the dorsal portion of LIFGtri (MNI coordinates for controls: −45, 27, 18 and PWA: −51, 27, 21) and in LMFG bordering the precentral gyrus (MNI coordinates for controls: −39, 0, 60 and PWA: −36, −3, 60). While the left middle temporal peak was located in LpMTG in both groups, the patient group peak was superior to the controls’ peak (MNI coordinates for controls: −57, −45, −6 and PWA: −57, −42, 6). The spatial extent of activity within the ROIs was smaller in LIFGtri and LpMTG and greater in LMFG in PWA compared to controls (Figure 3A). The overall MANOVA model testing for between-group differences in the strength of regional activity in the three anatomical bounding masks was not significant (F(3,38) = 2.364, Pillai’s trace = 0.157, p = 0.086) (Figure 3B). The fact that the extent—but not strength—of activation differed between groups accords with the relative damage to LIFGtri and LpMTG and sparing of LMFG in the individuals with aphasia, as shown in Table 2.
Figure 3.
Activity within bounding masks. (A) Rendered t-maps from the one-sample t-tests (at p < 0.001, uncorrected) in each group show activated clusters within the anatomical bounding masks (in white). (B) The comparison of contrast estimates extracted from the anatomical bounding masks for each participant revealed no significant differences.
Table 2.
Percentage of spared tissue per bounding mask
| ID | LIFGtri | LMFG | LpMTG |
|---|---|---|---|
| P1 | 99.91 | 100.00 | 84.99 |
| P2 | 100.00 | 100.00 | 36.30 |
| P3 | 84.82 | 100.00 | 14.91 |
| P4 | 15.88 | 68.24 | 11.44 |
| P5 | 96.98 | 98.16 | 81.00 |
| P6 | 100.00 | 100.00 | 100.00 |
| P7 | 100.00 | 100.00 | 99.10 |
| P8 | 34.49 | 100.00 | 85.78 |
| P9 | 100.00 | 100.00 | 95.21 |
| P10 | 77.86 | 95.61 | 87.10 |
| P11 | 78.04 | 77.02 | 99.97 |
| P12 | 36.03 | 28.01 | 10.99 |
| P13 | 60.12 | 100.00 | 53.85 |
| P14 | 84.26 | 100.00 | 52.53 |
| P15 | 66.57 | 100.00 | 100.00 |
| P16 | 2.60 | 98.48 | 42.82 |
| P17 | 64.72 | 98.56 | 35.33 |
| P18 | 95.17 | 100.00 | 65.60 |
| P19 | 30.69 | 51.72 | 90.22 |
| P20 | 71.36 | 100.00 | 25.66 |
| P21 | 98.51 | 100.00 | 91.12 |
| P22 | 100.00 | 100.00 | 41.57 |
| P23 | 100.00 | 100.00 | 100.00 |
| P24 | 98.24 | 100.00 | 100.00 |
| P25 | 100.00 | 100.00 | 100.00 |
| MEAN | 77.95 | 94.56 | 67.77 |
| STDEV | 28.38 | 15.82 | 31.98 |
Across the patient group, LpMTG was the most damaged anatomical ROI whereas LMFG was the most spared. Eleven PWA had approximately 50% or less spared tissue within at least one regional mask. Three individuals (i.e., P4, P12 and P16) had less than 50% spared tissue in two or all three ROI masks. Of the patients with highly damaged ROIs, noisy VOIs were extracted in LIFGtri for three PWA and in LpMTG for five PWA. Perilesional activity noted outside the boundaries of the masks was extracted for some PWA in order to maximize the number of functional peaks and connections. Ultimately, at least two functional peaks were extracted for all PWA excluding P12. This patient exhibited a high degree of damage to all three regions, and only one perilesional functional peak was identified; thus, this participant was excluded from the remaining analyses.
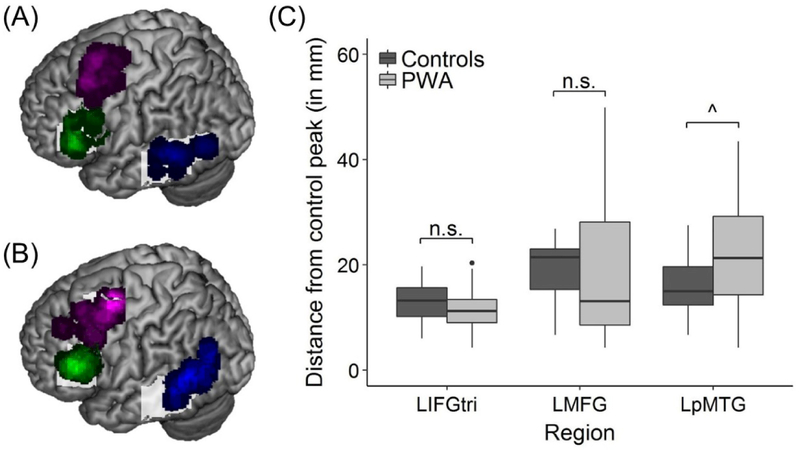
To ensure that all participants’ regional peaks were proximal to the center of each regional mask, the distance between the control group’s peaks (i.e., LIFGtri: −45, 27, 18; LMFG: −39, 0, 60; and LpMTG: −57, −45, −6) and each subject’s corresponding regional peak was calculated for both PWA (Figure 4A) and controls (Figure 4B) using the distance formula:
There were no significant differences between groups in the distance between individual peaks and the control group’s peak for LIFGtri (t(36.55) = −1.261, p = 0.215; controls’ mean distance = 12.87 ± 3.88mm; PWA’s mean distance = 11.27 ± 4.07mm) or LMFG (t(35.841) = −0.022, p = 0.983; controls’ mean distance = 18.83 ± 6.55mm; PWA’s mean distance = 18.76 ± 12.87mm). By contrast, the distance between the controls’ LpMTG peak and individual LpMTG peaks approached significance wherein a greater mean distance was found for PWA compared to controls (t(29.241) = 2.025, p = 0.052; controls’ mean distance = 16.03 ± 6.00mm; PWA’s mean distance = 21.97 ± 11.50mm) (Figure 4C). The results are expected given the degree of damage to the LpMTG bounding mask across the patient group and the necessity of extracting perilesional peaks outside the mask for some PWA. Nevertheless, these collective results provide some certainty that potential between-group differences in connectivity were not solely due to between-group differences in the location of regional activity.
Figure 4.
VOI location. Overlays shown of all regional peaks for all (A) controls and (B) patients. (C) The results of the MANOVA revealed the distance between each subject’s regional peak and the corresponding bounding mask peak did not differ between groups for LIFGtri and LMFG but approached significance for LpMTG. Note: for overlay of patients’ VOIs, only functional spheres are shown (i.e., n = 21 LIFGtri VOIs, 24 LMFG VOIs, and 20 LpMTG VOIs)
3.3. DCM model inference
In line with our hypotheses, the best-fit model family in the patient group was Family #2: Input to LMFG (xp = 0.837). Contrary to our hypotheses, Family #2 was also the model family that best characterized the control group’s data (xp = 0.654). However, Family #2 was not uniformly the best family to model all controls’ data, as evidenced by the exceedance probability value for Family #1: Input to LIFGtri at the group level (Figure 5). Three of the 72 individual models best characterized controls’ data, including one fully-connected bidirectional model from Family #1: Input to LIFGtri (i.e., model #24 [xp = 0.297]) and two highly-connected bidirectional models from Family #2: Input to LMFG (i.e., models #42 [xp = 0.252] and #48 [xp = 0.265]). (See Supplemental Figure 2 for visualization of model #24 and Supplemental Figure 3 for visualization of models #42 and #48). While the exceedance probability for Family #2 was higher for the patient group, six PWA demonstrated a preference for Family #1: Input to LIFGtri and six PWA showed a preference for Family #3: Input to LpMTG at the single-subject level. Given the heterogeneity of model fit, averaged parameters weighed according to model evidence across all models (obtained via the BMA procedure) were extracted for further analysis.
Figure 5.
Family-wise Bayesian model selection within each group
3.4. DCM parameter inference
First, to validate the use of the noisy VOI methodology in a larger sample of stroke patients than has been explored previously, we performed an analysis with the subgroup of PWA for whom noisy VOIs were extracted. For this analysis, connections were coded as noisy if the noisy VOI was either the modulatory or the target region and as potentially functional if the noisy VOI was neither the modulatory nor the target region. For example, if a noisy VOI was extracted for LpMTG for a patient, connections involving LpMTG (i.e., LIFGtri→LpMTG, LMFG→LpMTG, LpMTG→LIFGtri, and LpMTG→LMFG) were coded as noisy, and connections without LpMTG (i.e., LIFGtri→LMFG and LMFG→LIFGtri) were considered potentially functional. A one-way ANOVA with connection type (i.e., noisy/functional) as the independent variable and the strength of task-modulated connections (i.e., Ep.B values) as the dependent variable revealed that potentially functional connections had significantly higher Ep.B values than noisy connections (F(1,40) = 9.501, p = 0.004), indicating that noisy signals did not artificially induce higher Ep.B values. Furthermore, across the entire patient group, the amount of spared tissue within the anatomical bounding masks of driving regions was not associated with the strength of task-modulated connections (range: r = −0.034 – 0.368, p = 0.386 – 0.873 after FDR correction for multiple comparisons), indicating that patient connectivity was not merely a reflection of the integrity of network regions.
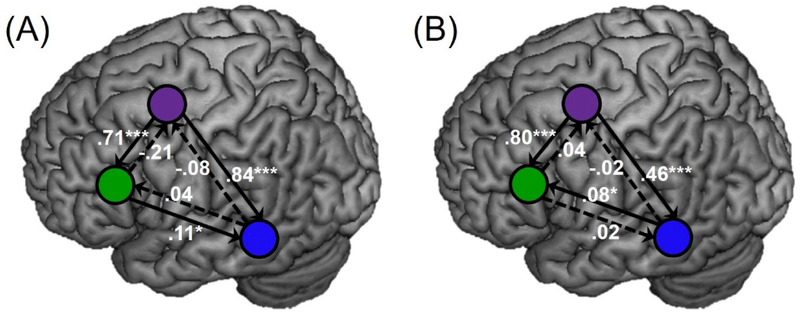
Next, modulatory connections within and between groups were further interrogated. In controls, significant connections per one-sample t-tests included LIFGtri→LpMTG (t(17) = 2.928, p = 0.019), LMFG→LIFGtri (t(17) = 5.118, p < 0.001) and LMFG→LpMTG (t(17) = 4.800, p < 0.001) after FDR correction for multiple tests (Figure 6A). After FDR correction, significant task-modulated connections for PWA also included LMFG→LIFGtri (t(23) = 6.219, p < 0.001) and LMFG→LpMTG (t(23) = 4.725, p < 0.001), as well as LpMTG→LIFGtri (t(23) = 2.819, p = 0.020) (Figure 6B). It should be noted that positive Ep.B values are typically interpreted as denoting an excitatory influence of one region on another, whereas negative values reflect inhibitory effects. Given that all significant Ep.B values were positive across groups, these results indicate that driving regions had an excitatory effect on target regions, where the greater the value, the stronger the effect.
Figure 6.
Modulatory connections within the (A) controls and (B) PWA, with statistically significant and non-significant connections per one-sample t-tests denoted by solid and dashed lines, respectively. Purple = LMFG, blue = LpMTG, green = LIFGtri
Comparisons between groups in modulatory connections did not reach significance (F(6,35) = 1.826, p = 0.123). Given that the model space was structured according to exogenous input to ROIs, it stands to reason that regional activity may have influenced comparisons in connection strength between groups. In other words, we wanted to ensure that the null between-group result was not merely the consequence of the magnitude of activity in a given region, given that DCM connectivity parameters reflect how the rate of activity change in one region influences the rate of change in another region. Therefore, the previous analysis was re-run as three two-way MANOVAs with independent variables of group and contrast estimates from the LIFGtri, LMFG, or LpMTG bounding masks and dependent variables of Ep.B values per connection. In the models controlling for regional activation, the main effect of group still was not significant when contrast estimates from the LIFGtri (F(6,34) = 1.903, p = 0.109), LMFG (F(6,34) = 1.803, p = 0.128), and LpMTG (F(6,34) = 1.804, p = 0.128) masks were included in the models. The effect of activation in the bounding masks also was not significant in any model (LIFGtri beta weights: F(6,34) = 1.573, p = 0.185; LMFG beta weights: F(6,34) = 0.821, p = 0.562; LpMTG beta weights: F(6,34) = 0.580, p = 0.743). Despite the suprising lack of statistically-significant between-group differences in strength of connections, these results indicate that similarities in control and patient connectivity were not primarily driven by activation within the anatomical bounding masks.
3.5. Relationship between activation, connectivity, and behavior in PWA
Finally, the relationship between neural metrics and lexical-semantic skills was examined in the patient group. While interrogating within-scanner performance (via accuracy on the fMRI task) allowed for the tightest inferences regarding brain and behavior relationships, examining lexical-semantic skills outside the scanner (via accuracy on PALPA 51) avoided the potential confounds of practice effects (as PWA were trained extensively on the fMRI task before scanning) and reductions in patient accuracy due to time constraints imposed by the fMRI protocol. To ensure that accuracy on the fMRI task and PALPA 51 reflected a similar (but not entirely overlapping) skill, we first conducted a Spearman correlation between these two behavioral metrics. A strong, positive correlation between patients’ accuracy on the scanner task and their performance on PALPA 51 (r = 0.770, p < 0.001) was found. Therefore, each behavioral metric was entered into separate backward stepwise regression models as the dependent variable. The independent variables in each regression included Ep.B values for each connection and contrast estimates in the three anatomical bounding masks.
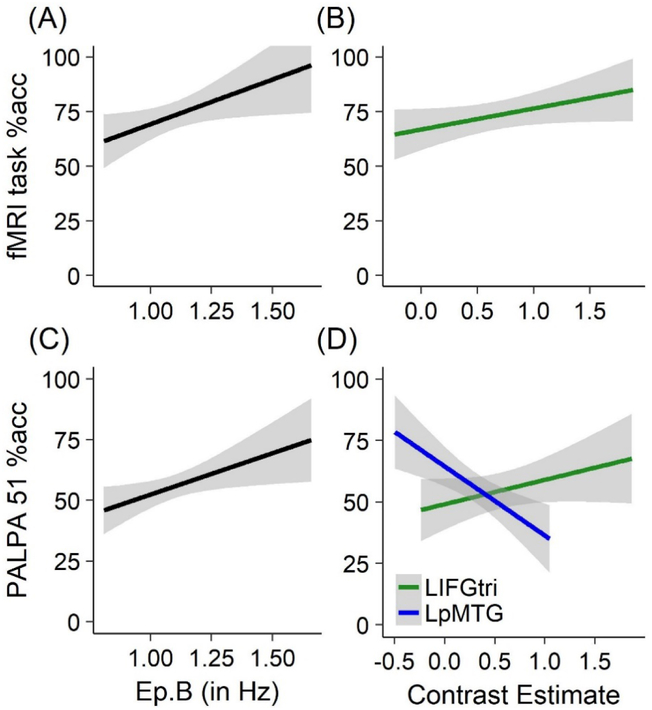
The final model predicting fMRI task accuracy from functional metrics was significant (F(4,18) = 3.910, p = 0.019, adjusted R2 = 0.346) and included two of the six connections (i.e., LMFG→LpMTG and LpMTG→LIFGtri) and contrast estimates from the LIFGtri and LMFG masks as factors. Significant independent positive predictors of behavioral accuracy included the LpMTG→LIFGtri connection (β = 0.415, SE = 0.157, t = 2.637, p = 0.017) and LIFGtri beta weights (β = 0.135, SE = 0.056, t = 2.416, p = 0.027). In other words, greater excitatory coupling (indicated by higher Ep.B values) from LpMTG to LIFGtri and stronger activity within the LIFGtri mask were associated with better performance on the fMRI task (Figure 7A/B).
Figure 7.
Neural metrics predicting lexical-semantics showing relationships between (A) fMRI task accuracy and the influence of task-modulation on the strength of LpMTG→LIFGtri per Ep.B values (B) between fMRI task accuracy and contrast estimates within the LIFGtri bounding mask (C) between accuracy on PALPA 51 and the strength of LpMTG→LIFGtri (D) and between accuracy on PALPA 51 and contrast estimates extracted from the LIFGtri and LpMTG bounding masks.
Similarly, the final model predicting PALPA 51 accuracy was significant (F(4,19) = 7.613, p < 0.001, adjusted R2 = 0.535) and included two of the six connections (i.e., LMFG→LIFGtri and LpMTG→LIFGtri) as well as constrast estimates from the LIFGtri and LpMTG masks. As with fMRI task accuracy, significant positive predictors of semantic association skills per PALPA 51 were LpMTG→ LIFGtri (β = 0.497, SE = 0.176, t = 2.830, p = 0.011) and LIFGtri contrast estimates (β = 0.125, SE = 0.047, t = 2.649, p = 0.016). Contrast estimates extracted from the LpMTG bounding mask also significantly predicted PALPA 51 accuracy, but an inverse relationship was found (β = −0.262, SE = 0.068, t = −3.867, p = 0.001). In other words, greater activity within the LpMTG bounding mask was linked to lower accuracy on PALPA 51 (Figure 7C/D). In all, these results indicate that similar patterns of excitatory connectivity and stronger LIFGtri activity were linked to optimal patient performance on lexical-semantic tasks inside and outside of the scanner, whereas a dissociation in LpMTG activation and connectivity during lexical-semantic processing in PWA was observed.
4. Discussion
In this study, we investigated differences between healthy controls and a heterogeneous group of PWA in left hemisphere effective connectivity during a semantic feature judgment task. We also determined relationships between neural metrics and lexical-semantic processing abilities in the patient group. Using a systematic approach to localize activation and account for lesioned tissue in PWA, we found no differences between patients and controls in the strength of regional activity within anatomical bounding masks in LIFGtri, LMFG or LpMTG for semantic feature decisions. The DCM analysis revealed that the best-fit model family for both PWA and control group data was Family #2: Input to LMFG, although heterogeneity in model fit across groups was noted. At the connection level, modulation of LpMTG and LIFGtri by LMFG characterized the strongest connections in both participant groups. By contrast, the LIFGtri→LpMTG connection was significant within the control network whereas PWA relied on the reverse connection. Last, within the patient group, we discovered that better performance on lexical-semantic tasks inside and outside the scanner was associated with greater coupling of LpMTG→LIFGtri as well as stronger activity within the LIFGtri. Each of the aforementioned results is considered below in greater detail in the context of findings from previous fMRI studies.
First, activation analyses that preceded DCM revealed similar patterns of left hemisphere activity in patients and controls, yet the spatial extent of activated clusters differed between groups (i.e., greater spatial extent in LMFG and domain-general cortex for PWA and in LIFGtri and LpMTG for controls) (see Supplemental Figure 1). Despite several PWA demonstrating a fair degree of damage to LIFGtri and LpMTG, the strength of activity within the anatomical bounding masks did not significantly differ between groups. It is possible that the surprisingly high activation in the patient group was driven by PWA with mostly spared ROIs. Alternatively, because contrast estimates in PWA were extracted from anatomical masks that accounted for lesion, the activation within the masks may be perilesional activity in PWA with damaged ROIs. If so, such perilesional activity may reflect some mechanism of earlier neural recovery that allowed for reinstatement of premorbid left hemisphere activation patterns by the chronic post-stroke stage (Saur et al., 2006). Critically, these results also lend credence to our activation localization approach in PWA and provide a basis for interpreting between-group comparisons of the connectivity results.
Turning to the DCM findings, the fact that the fit of Family #2: Input to LMFG models was so high in the PWA group indicates that the majority of patients, despite heterogeneous behavioral and lesion profiles, preferred this family of models. This finding aligns with our hypotheses and previous studies (Geranmayeh et al., 2017; Meier et al., 2016; Turkeltaub et al., 2011) that have demonstrated the importance of dorsal prefrontal cortex—and LMFG specifically—within the language network in individuals with chronic aphasia. By itself, this result also provides support for the theory that patients rely on MD regions for language processing when cognitive demands are high (Fedorenko, Duncan, & Kanwisher, 2013; Fedorenko et al., 2012; Fedorenko & Thompson-Schill, 2014). However, contrary to our hypotheses, Family #2: Input to LMFG was also the winning model family for the control group. Notably, model fit in controls was split evenly between three individual models, including one model from Family #1: Input to LIFGtri and two models from Family #2: Input to LMFG. Full, bidirectional task-modulated connections (excluding LIFGtri→LpMTG in one model) comprised each of these models. As such, it may be that the primary driving force behind model fit in the control group was the degree of connectivity between regions while the particular input region exerted a smaller—although still critical—impact. In general, the control results align with literature that reports a heightened reliance in older adults on prefrontal cortex for tasks requiring retrieval of lexical-semantic information (Baciu et al., 2016; Davis, Zhuang, Wright, & Tyler, 2014; Manenti, Brambilla, Petesi, Miniussi, & Cotelli, 2013; Meinzer et al., 2009; Obler et al., 2010). However, this is a tentative explanation given that the current results cannot be compared to connectivity findings from another, less cognitive-demanding task in this older adult sample nor did we enroll a group of younger healthy controls for comparison to our healthy control group.
At the connection level, both groups demonstrated significant task-modulated connectivity of LMFG→LpMTG and LMFG→LIFGtri. One possible interpretation of these findings is that modulation of LpMTG by LMFG assisted participants in rapidly activating candidate semantic features whereas modulation of LIFGtri by LMFG assisted in the selection of the correct features of the target item. The directionality of these connections suggests cognitive control mechanisms were at play during semantic decisions (Fedorenko, Duncan, & Kanwisher, 2013; Fedorenko et al., 2012). For example, the location of the LMFG bounding mask aligns with a dorsal anterior premotor region posited as a potential hub for domain-general cognitive control and implicated in contextual control across a variety of tasks (Badre & Nee, 2018). Along the same vein, Xu, Lin, Han, He, and Bi (2016) found that the same region of LMFG was one of two key hubs that connected three dissociable modules of the semantic network (i.e., perisylvian network, fronto-parietal network and DMN). Binder and Desai (2011) posited that the role of this portion of the prefrontal cortex is in the translation of internal states into “a plan for top-down activation of semantic fields relevant to the problem at hand” (p. 532). Although the exact role of LMFG for participants in either group cannot be ascertained for certain, these connection results further cement the central role of LMFG within this small network in both PWA and healthy controls.
Surprisingly, the test comparing the strength of all connections between patients and controls did not reach significance. It should be noted that trends of stronger connectivity of LMFG→LpMTG in controls (Ep.B in controls = 0.84 and patients = 0.46) and marginally stronger connectivity of LMFG→LIFGtri in patients (Ep.B in controls = 0.71 and patients = 0.80) were observed, and thus, the lack of significant between-group results could be an issue of statistical power. Given that the area of greatest brain damage in the patients spanned tissue between LMFG and LpMTG (see Figure 2C), the weaker LMFG→LpMTG connection in PWA may reflect the consequence of functional disconnect for some individuals. On the other hand, the slightly stronger LMFG→LIFGtri patient connection value may indicate a need for heightened semantic control for some PWA in making semantic judgments or may be a consequence of functional reorganization of the semantic system following prior language treatment that targeted the semantic system (e.g., Kiran et al., 2015).
By contrast, striking differences between PWA and controls were also observed in the coupling of LIFGtri and LpMTG. Specifically, the one-sample t-test in controls revealed LpMTG→LIFGtri connection was not signicantly involved in their task-based semantic network. However, for PWA, greater connectivity of LpMTG→LIFGtri was associated with better accuracy on lexical-semantic tasks inside and outside the scanner and was a significant network connection per the one-sample test. Interestingly, the reverse connection (i.e., LIFGtri→LpMTG) was not significantly modulated by the task in PWA—unlike in controls—and was not related to lexical-semantic abilities per either behavioral measure in the patient group. It is possible that the directionality difference in LpMTG-LIFGtri coupling reflects neural reorganization in the patients that occurred in the months after stroke. Alternatively, the between-group difference could purely be a consequence of structural damage and/or disconnect in some PWA.
For PWA, better lexical-semantic abilities (per PALPA 51) were also associated with stronger activation within the LIFGtri bounding mask but weaker activity in the LpMTG mask. The former result is unsurprising since spared tissue and activation within LIFG has been linked with greater spontaneous and treatment-induced recovery of language skills in numerous patient studies (Abel, Weiller, Huber, & Willmes, 2014; Abel et al., 2015; Fridriksson et al., 2010; Kiran et al., 2015; Marcotte et al., 2012; Rochon et al., 2010; Rosen et al., 2000; Sims et al., 2016; van Hees, McMahon, Angwin, de Zubicaray, Read, et al., 2014; van Oers et al., 2010). On the other hand, the latter finding is unexpected given that temporoparietal cortex is considered a convergence zone of structural and functional networks that are crucial to semantic processing (Buckner et al., 2009; Davey et al., 2016; Griffis, Nenert, Allendorfer, & Szaflarski, 2017a, 2017b; Turken & Dronkers, 2011; Wei et al., 2012; Xu et al., 2016). One potential explanation is that strong local activity within LpMTG without communication to other regions (e.g., LIFGtri in this subnetwork) may be the consequence of maladaptive activation patterns or structural disconnection that results in poorer semantic performance (Abel et al., 2015; Griffis, Nenert, Allendorfer, & Szaflarski, 2017b). Furthermore, it is reasonable to assume heavy reliance on LpMTG without recruitment of frontal regions might be detrimental to performance on PALPA 51 as this semantic association task requires subjects to select a target item in the presence of multiple—including semantic—distractors. However, this conclusion is tentative as explicit investigation of LIFGtri-LpMTG activation and connectivity patterns during a semantic association fMRI task was not undertaken in the present investigation.
Overall, these collective results suggest that the activation and connectivity of regions implicated in domain-general processing (e.g., LMFG) and domain-specific semantic mechanisms (e.g., LIFGtri) are critical for patients with chronic aphasia during semantic feature decision-making. While the particular cognitive mechanisms mediated by these regions cannot be definitively determined from this experiment, these results loan credence to the notion that the connectivity of left hemisphere regions that potentially mediate domain-general processing (e.g., LMFG) constitutes ideal neural organization patterns in chronic stroke-induced aphasia. Certainly, the positive relationships between patients’ lexical-semantic abilities and LIFGtri activity and LpMTG→LIFGtri connectivity aligns with Heiss and Thiel’s (2006) proposal that regions within the canonical language network must be recruited for optimal language task performance in chronic aphasia.
A final contribution of the present work is that, to our knowledge, no other study has incorporated a similar multipronged approach to account for individual lesion variability and also provided conclusions regarding task-based connectivity at the group level in patients with chronic aphasia. Several steps were taken to ensure reliability of the signal extracted for the effective connectivity analysis, including the creation of individualized anatomical bounding masks for each patient. As a result, we were able to successfully account for a diverse group of PWA with different lesion profiles, consistent with the greater population of PWA. One of the greatest challenges in investigating neural reorganization in post-stroke aphasia is the inherent heterogeneity of PWA. As such, despite our best efforts, P12 was still excluded from the connectivity analysis given the size of his lesion and damage to the ROIs. One way to accommodate such heterogeneity—including between-group differences in certain demographic variables (i.e., handness, gender)—would be to recruit a larger sample of PWA so that participants could be split into subgroups according to behavioral, demographic and/or stroke profiles.
Future work could also mitigate such limitations of the present study by interrogating right hemisphere and interhemispheric connectivity in addition to left hemisphere interactions. Furthermore, it is possible that the inclusion of other regions implicated in semantic processing (e.g., posterior mPFC, ITG) would alter the present results. As such, follow-up investigations that pair exploratory functional connectivity and hypothesis-driven effective connectivity methods would be beneficial for providing further information regarding the most critical regions and connections for semantic processing in PWA. Last, while we accounted for the structural integrity of local regions, we did not incorporate metrics reflecting the integrity of white matter pathways connecting the three left hemisphere cortical nodes. As shown in emerging work (e.g., Pustina et al., 2017), multimodal investigations that measure both structural and functional connectivity may provide the best explanatory power of language skills in PWA and should be the focus of future work regarding the neural bases of lexical-semantic processing in PWA.
5. Conclusions
In sum, the present investigation revealed that at a high level, individuals with chronic aphasia with diverse behavioral and stroke profiles demonstrate some similarities to age-matched healthy controls in activation and connectivity patterns during lexical-semantic deicisions. Specifically, similar to controls, PWA recruited canonical left hemisphere language regions (i.e., LIFGtri, LpMTG) for the fMRI task although the extent of activation in such classic language regions was less for PWA compared to their healthy counterparts. By contrast, the extent of activity in regions associated with domain-general processing (e.g., LSFG, LMFG, left posterior mPFC) was greater in PWA relative to controls. Nonetheless, both groups relied on LMFG-driven connections during the fMRI task, a finding which aligns with work highlighting the importance of dorsolateral prefrontal cortex in cognitively-demanding tasks in healthy and disordered populations. Fine-grained differences in LIFGtri and LpMTG coupling and recruitment within each participant group further revealed neural reorganization patterns associated with healthy aging versus chronic aphasia. In particular, optimal lexical-semantic abilities in PWA were linked to greater excitatory modulation of LIFGtri by LpMTG, presumably resulting in beneficial heightened recruitment of LIFGtri for the task. Future work should endeavor to further disentangle beneficial LIFG-LMTG effective connectivity patterns for related tasks, determine the structural pathways most critical for mediating such connections, and determine the role of right hemisphere homologues of these regions from a network perspective.
Supplementary Material
Highlights.
Both patients with aphasia and age-matched healthy adults demonstrated left-lateralized frontotemporal activation during the semantic feature judgment task, including activity in the three regions (i.e., LIFGtri, LMFG, and LpMTG) selected a priori for effective connectivity analysis.
Task-based effective connectivity was characterized by a similar reliance on LMFG-driven connections in patients and controls.
Within the patient group, greater modulation of LIFGtri by LpMTG and greater local recruitment of LIFGtri for the semantic feature task was positively associated with lexical-semantic abilities inside and outside of the scanner.
Acknowledgments
First and foremost, we would like to thank the individuals with and without aphasia who participated in this study. We also acknowledge past and present members of the Boston University Aphasia Research Laboratory and our collaborators who assisted with data collection and analysis, in particular Kushal Kapse, Yansong Geng, Yue Pan, Jen Michaud, Kelly Martin, Natalie Gilmore, and Maria Dekhtyar.
Funding: This work was supported by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (1P50DC012283 and 1F31DC015940).
Abbreviations
- AG
angular gyrus
- ACC
anterior cingulate cortex
- aSTG
anterior superior temporal gyrus
- BMA
Bayesian model averaging
- BPA
Bayesian parameter averaging
- BMS
Bayesian model selection
- CSC
controlled semantic cognition
- DCM
dynamic causal modeling
- DMN
default mode network
- IFG
inferior frontal gyrus
- op
pars opercularis of IFG
- orb
pars orbitalis of IFG
- tri
pars triangularis of IFG
- ITG
inferior temporal gyrus
- iOCC
inferior occipital cortex
- GLM
general linear model
- IPS
intraparietal sulcus
- L prefix
denotes left hemisphere lateralization
- mPFC
medial prefrontal cortex
- MCA
middle cerebral artery
- MCC
mid-cingulate cortex
- MFG
middle frontal gyrus
- MPO
months post-onset
- MTG
middle temporal gyrus
- pMTG
posterior portion of MTG
- MD
multiple demand
- PWA
persons with aphasia
- R prefix
denotes right hemisphere lateralization
- ROI(s)
region(s) of interest
- RT
reaction time
- SFG
superior frontal gyrus
- SMA
supplementary motor area
- SMG
supramarginal gyrus
- VOI(s)
volume(s) of interest
- xp
exceedance probability value
Footnotes
Visual inspection of the T1-weighted images revealed three patients (i.e., P11, P18, and P19) exhibited right hemisphere ischemic changes of unknown etiology of the deep white matter abutting the lateral ventricles. As none of these participants had a known history of right hemisphere cortical infarct, their data were included in analyses within the present investigation.
Behavioral and neuroimaging data pertaining to oral naming abilities and connectivity from 12 PWA (i.e., P1-P3, P5-P7, P9, P11-P13, P15 and P21) and 10 controls included in the present study were analyzed in the previous investigation.
References
- Abel S, Weiller C, Huber W, & Willmes K (2014). Neural underpinnings for model-oriented therapy of aphasic word production. Neuropsychologia, 57, 154–165. 10.1016/j.neuropsychologia.2014.03.010 [DOI] [PubMed] [Google Scholar]
- Abel S, Weiller C, Huber W, Willmes K, & Specht K (2015). Therapy-induced brain reorganization patterns in aphasia. Brain, 138(4), 1097–1112. 10.1093/brain/awv022 [DOI] [PubMed] [Google Scholar]
- Allen P, Mechelli A, Stephan KE, Day F, Dalton J, Williams S, & McGuire PK (2008). Fronto-temporal interactions during overt verbal initiation and suppression. Journal of Cognitive Neuroscience, 20(9), 1656–1669. 10.1162/jocn.2008.20107 [DOI] [PubMed] [Google Scholar]
- Anglade C, Thiel A, & Ansaldo AI (2014). The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: A critical review of literature. Brain Injury, 28(2), 138–145. 10.3109/02699052.2013.859734 [DOI] [PubMed] [Google Scholar]
- Baciu M, Boudiaf N, Cousin E, Perrone-Bertolotti M, Pichat C, Fournet N, … Krainik A (2016). Functional MRI evidence for the decline of word retrieval and generation during normal aging. AGE, 38(1). 10.1007/s11357-015-9857-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, & D’Esposito M (2007). Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience, 19(12), 2082–2099. [DOI] [PubMed] [Google Scholar]
- Badre D, & Nee DE (2018). Frontal Cortex and the Hierarchical Control of Behavior. Trends in Cognitive Sciences, 22(2), 170–188. 10.1016/j.tics.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, & Wagner AD (2005). Dissociable Controlled Retrieval and Generalized Selection Mechanisms in Ventrolateral Prefrontal Cortex. Neuron, 47(6), 907–918. 10.1016/j.neuron.2005.07.023 [DOI] [PubMed] [Google Scholar]
- Bajada CJ, Haroon HA, Azadbakht H, Parker GJM, Lambon Ralph MA, & Cloutman LL (2017). The tract terminations in the temporal lobe: Their location and associated functions. Cortex, 97, 277–290. 10.1016/j.cortex.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, & Desai RH (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536. 10.1016/j.tics.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, & Poline JB (2002). Region of interest analysis using the MarsBar toolbox for SPM 99., 16(2), S497. [Google Scholar]
- Brett M, Leff AP, Rorden C, & Ashburner J (2001). Spatial Normalization of Brain Images with Focal Lesions Using Cost Function Masking. NeuroImage, 14(2), 486–500. 10.1006/nimg.2001.0845 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, … Johnson KA (2009). Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. Journal of Neuroscience, 29(6), 1860–1873. 10.1523/JNEUROSCI.5062-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappa SF (2011). The neural basis of aphasia rehabilitation: Evidence from neuroimaging and neurostimulation. Neuropsychological Rehabilitation, 21(5), 742–754. 10.1080/09602011.2011.614724 [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, & Jones DK (2002). Virtual in Vivo Interactive Dissection of White Matter Fasciculi in the Human Brain. NeuroImage, 17(1), 77–94. 10.1006/nimg.2002.1136 [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, & ffytche DH (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57(1), 8–16. 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- Catani M, & Mesulam M (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex, 44(8), 953–961. 10.1016/j.cortex.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charidimou A, Kasselimis D, Varkanitsa M, Selai C, Potagas C, & Evdokimidis I (2014). Why Is It Difficult to Predict Language Impairment and Outcome in Patients with Aphasia after Stroke? Journal of Clinical Neurology, 10(2), 75 10.3988/jcn.2014.10.2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, & Lambon Ralph MA (2012). Connectivity-based structural and functional parcellation of the human cortex using diffusion imaging and tractography. Frontiers in Neuroanatomy, 6 10.3389/fnana.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquyt E-M, De Ley L, Santens P, Van Borsel J, & De Letter M (2017). The role of the right hemisphere in the recovery of stroke-related aphasia: A systematic review. Journal of Neurolinguistics, 44, 68–90. 10.1016/j.jneuroling.2017.03.004 [DOI] [Google Scholar]
- Coltheart M (1981). The MRC Psycholinguistic Database. The Quarterly Journal of Experimental Psychology Section A, 33(4), 497–505. 10.1080/14640748108400805 [DOI] [Google Scholar]
- Corbett F, Jefferies E, Ehsan S, & Lambon Ralph MA (2009). Different impairments of semantic cognition in semantic dementia and semantic aphasia: evidence from the non-verbal domain. Brain, 132(9), 2593–2608. 10.1093/brain/awp146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, & Lambon Ralph MA (2009). Exploring multimodal semantic control impairments in semantic aphasia: Evidence from naturalistic object use. Neuropsychologia, 47(13), 2721–2731. 10.1016/j.neuropsychologia.2009.05.020 [DOI] [PubMed] [Google Scholar]
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang Y-L, … White KD (2007). Functional MRI of Language in Aphasia: A Review of the Literature and the Methodological Challenges. Neuropsychology Review, 17(2), 157–177. 10.1007/s11065-007-9024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch SJ, & Warrington EK (2005). Gradients of semantic relatedness and their contrasting explanations in refractory access and storage semantic impairments. Cognitive Neuropsychology, 22(7), 851–876. 10.1080/02643290442000374 [DOI] [PubMed] [Google Scholar]
- Davey J, Thompson HE, Hallam G, Karapanagiotidis T, Murphy C, De Caso I, … Jefferies E (2016). Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. NeuroImage, 137, 165–177. 10.1016/j.neuroimage.2016.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Zhuang J, Wright P, & Tyler LK (2014). Age-related sensitivity to task-related modulation of language-processing networks. Neuropsychologia, 63, 107–115. 10.1016/j.neuropsychologia.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, & Gagnon DA (1997). Lexical access in aphasic and nonaphasic speakers. Psychological Review, 104(4), 801–838. [DOI] [PubMed] [Google Scholar]
- den Ouden D-B, Saur D, Mader W, Schelter B, Lukic S, Wali E, … Thompson CK (2012). Network modulation during complex syntactic processing. NeuroImage, 59(1), 815–823. 10.1016/j.neuroimage.2011.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, & Rushworth MF (2003). Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience, 15(1), 71–84. [DOI] [PubMed] [Google Scholar]
- Dick AS, Bernal B, & Tremblay P (2014). The language connectome: new pathways, new concepts. The Neuroscientist, 20(5), 453–467. [DOI] [PubMed] [Google Scholar]
- Duncan J (2010). The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14(4), 172–179. 10.1016/j.tics.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, & Amunts K (2009). A systems perspective on the effective connectivity of overt speech production. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 367(1896), 2399–2421. 10.1098/rsta.2008.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, & Zilles K (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25(4), 1325–1335. 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Engelter ST, Gostynski M, Papa S, Frei M, Born C, Ajdacic-Gross V, … Lyrer PA (2006). Epidemiology of Aphasia Attributable to First Ischemic Stroke: Incidence, Severity, Fluency, Etiology, and Thrombolysis. Stroke, 37(6), 1379–1384. 10.1161/01.STR.0000221815.64093.8c [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Behr MK, & Kanwisher N (2011). Functional specificity for high-level linguistic processing in the human brain. Proceedings of the National Academy of Sciences, 108(39), 16428–16433. 10.1073/pnas.1112937108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2012). Language-Selective and Domain-General Regions Lie Side by Side within Broca’s Area. Current Biology, 22(21), 2059–2062. 10.1016/j.cub.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences, 110(41), 16616–16621. 10.1073/pnas.1315235110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, & Thompson-Schill SL (2014). Reworking the language network. Trends in Cognitive Sciences, 18(3), 120–126. 10.1016/j.tics.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers HL, Skoretz SA, Silver FL, Rochon E, Fang J, Flamand-Roze C, & Martino R (2016). Poststroke Aphasia Frequency, Recovery, and Outcomes: A Systematic Review and Meta-Analysis. Archives of Physical Medicine and Rehabilitation, 97(12), 2188–2201.e8. 10.1016/j.apmr.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JSW, Pike GB, & Petrides M (2008). Dissociating the Human Language Pathways with High Angular Resolution Diffusion Fiber Tractography. Journal of Neuroscience, 28(45), 11435–11444. 10.1523/JNEUROSCI.2388-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J (2010). Preservation and Modulation of Specific Left Hemisphere Regions is Vital for Treated Recovery from Anomia in Stroke. Journal of Neuroscience, 30(35), 11558–11564. 10.1523/JNEUROSCI.2227-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Bonilha L, Baker JM, Moser D, & Rorden C (2010). Activity in Preserved Left Hemisphere Regions Predicts Anomia Severity in Aphasia. Cerebral Cortex, 20(5), 1013–1019. 10.1093/cercor/bhp160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, & Cai B (2012). Left hemisphere plasticity and aphasia recovery. NeuroImage, 60(2), 854–863. 10.1016/j.neuroimage.2011.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, & Penny W (2003). Dynamic causal modelling. NeuroImage, 19(4), 1273–1302. 10.1016/S1053-8119(03)00202-7 [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Chau TW, Wise RJS, Leech R, & Hampshire A (2017). Domain-general subregions of the medial prefrontal cortex contribute to recovery of language after stroke. Brain, 140(7), 1947–1958. 10.1093/brain/awx134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Leech R, & Wise RJ (2016). Network dysfunction predicts speech production after left hemisphere stroke. Neurology, 86(14), 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialanella B, Bertolinelli M, Lissi M, & Prometti P (2011). Predicting outcome after stroke: the role of aphasia. Disability and Rehabilitation, 33(2), 122–129. [DOI] [PubMed] [Google Scholar]
- Gilmore N, Meier EL, Johnson JP, & Kiran S (in press). Typicality-based semantic treatment for anomia results in multiple levels of generalization Neuropsychological Rehabilitation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore N, Meier EL, Johnson JP, & Kiran S (2018). Typicality-based semantic treatment for anomia results in multiple levels of generalisation. Neuropsychological Rehabilitation, 1–27. 10.1080/09602011.2018.1499533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, & Rilling JK (2008). DTI Tractography of the Human Brain’s Language Pathways. Cerebral Cortex, 18(11), 2471–2482. 10.1093/cercor/bhn011 [DOI] [PubMed] [Google Scholar]
- Gold BT, & Buckner RL (2002). Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron, 35(4), 803–812. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, & Plaut DC (2002). The impact of synaptic depression following brain damage: a connectionist account of “access/refractory” and “degraded-store” semantic impairments. Cognitive, Affective & Behavioral Neuroscience, 2(3), 187–213. [DOI] [PubMed] [Google Scholar]
- Griffis JC, Nenert R, Allendorfer JB, & Szaflarski JP (2017a). Damage to white matter bottlenecks contributes to language impairments after left hemispheric stroke. NeuroImage: Clinical, 14, 552–565. 10.1016/j.nicl.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis JC, Nenert R, Allendorfer JB, & Szaflarski JP (2017b). Linking left hemispheric tissue preservation to fMRI language task activation in chronic stroke patients. Cortex, 96, 1–18. 10.1016/j.cortex.2017.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis JC, Nenert R, Allendorfer JB, Vannest J, Holland S, Dietz A, & Szaflarski JP (2017). The canonical semantic network supports residual language function in chronic post-stroke aphasia: Canonical Networks Support Aphasia Recovery. Human Brain Mapping, 38(3), 1636–1658. 10.1002/hbm.23476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam GP, Thompson HE, Hymers M, Millman RE, Rodd JM, Lambon Ralph MA, Jefferies E (2018). Task-based and resting-state fMRI reveal compensatory network changes following damage to left inferior frontal gyrus. Cortex, 99, 150–165. 10.1016/j.cortex.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Saur D, Price CJ, Baumgaertner A, Ulmer S, & Siebner HR (2013). Increased facilitatory connectivity from the pre-SMA to the left dorsal premotor cortex during pseudoword repetition. Journal of Cognitive Neuroscience, 25(4), 580–594. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Saur D, Price CJ, Ulmer S, Baumgaertner A, & Siebner HR (2013). Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proceedings of the National Academy of Sciences, 110(41), 16402–16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, & Amunts K (2009). Different roles of cytoarchitectonic BA 44 and BA 45 in phonological and semantic verbal fluency as revealed by dynamic causal modelling. NeuroImage, 48(3), 616–624. 10.1016/j.neuroimage.2009.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, & Karbe H (1999). Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Annals of Neurology, 45(4), 430–438. [DOI] [PubMed] [Google Scholar]
- Heiss W-D (2017). Contribution of Neuro-Imaging for Prediction of Functional Recovery after Ischemic Stroke. Cerebrovascular Diseases, 44(5–6), 266–276. 10.1159/000479594 [DOI] [PubMed] [Google Scholar]
- Heiss W-D, & Thiel A (2006). A proposed regional hierarchy in recovery of post-stroke aphasia. Brain and Language, 98(1), 118–123. 10.1016/j.bandl.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Hilari K, Wiggins R, Roy P, Byng S, & Smith S (2003). Predictors of health-related quality of life (HRQL) in people with chronic aphasia. Aphasiology, 17(4), 365–381. 10.1080/02687030244000725 [DOI] [Google Scholar]
- Hoffman P, Jefferies E, & Lambon Ralph MA (2011). Explaining semantic short-term memory deficits: Evidence for the critical role of semantic control. Neuropsychologia, 49(3), 368–381. 10.1016/j.neuropsychologia.2010.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope TMH, Leff AP, & Price CJ (2018). Predicting language outcomes after stroke: Is structural disconnection a useful predictor? NeuroImage: Clinical, 19, 22–29. 10.1016/j.nicl.2018.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope TMH, Seghier ML, Leff AP, & Price CJ (2013). Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage: Clinical, 2, 424–433. 10.1016/j.nicl.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, & Patterson KE (1992). The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company. [Google Scholar]
- Jefferies E (2013). The neural basis of semantic cognition: Converging evidence from neuropsychology, neuroimaging and TMS. Cortex, 49(3), 611–625. 10.1016/j.cortex.2012.10.008 [DOI] [PubMed] [Google Scholar]
- Jefferies E, Baker SS, Doran M, & Ralph MAL (2007). Refractory effects in stroke aphasia: A consequence of poor semantic control. Neuropsychologia, 45(5), 1065–1079. 10.1016/j.neuropsychologia.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Jefferies E, & Lambon Ralph MA (2006). Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain, 129(8), 2132–2147. 10.1093/brain/awl153 [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, & Ralph MAL (2008). Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia, 46(2), 649–658. 10.1016/j.neuropsychologia.2007.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Cloutman LL, Binney RJ, & Lambon Ralph MA (2017). The structural connectivity of higher order association cortices reflects human functional brain networks. Cortex, 97, 221–239. 10.1016/j.cortex.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan J, & Foltynie T (2013). Understanding DCM: Ten simple rules for the clinician. NeuroImage, 83, 542–549. 10.1016/j.neuroimage.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Kay J, Coltheart M, & Lesser R (1992). Psycholinguistic Assessments of Language Processing in Aphasia, (PALPA): Auditory Processing. Lawrence Erlbaum Associates. [Google Scholar]
- Kertesz A (2007). Western Aphasia Battery (Revised) PsychCorp. San Antonio, Tx, (Journal Article). [Google Scholar]
- Kiran S (2012). What Is the Nature of Poststroke Language Recovery and Reorganization? ISRN Neurology, 2012, 1–13. 10.5402/2012/786872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, Meier EL, Kapse KJ, & Glynn PA (2015). Changes in task-based effective connectivity in language networks following rehabilitation in post-stroke patients with aphasia. Frontiers in Human Neuroscience, 9 10.3389/fnhum.2015.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JM, & Wodchis WP (2010). The relationship of 60 disease diagnoses and 15 conditions to preference-based health-related quality of life in Ontario hospital-based long-term care residents. Medical Care, 48(4), 380–387. [DOI] [PubMed] [Google Scholar]
- Laska AC, Hellblom A, Murray V, Kahan T, & Von Arbin M (2001). Aphasia in acute stroke and relation to outcome. Journal of Internal Medicine, 249(5), 413–422. [DOI] [PubMed] [Google Scholar]
- Lazar RM, & Antoniello D (2008). Variability in recovery from aphasia. Current Neurology and Neuroscience Reports, 8(6), 497–502. [DOI] [PubMed] [Google Scholar]
- Lazar RM, & Boehme AK (2017). Aphasia As a Predictor of Stroke Outcome. Current Neurology and Neuroscience Reports, 17(11). 10.1007/s11910-017-0797-z [DOI] [PubMed] [Google Scholar]
- Lazar RM, Speizer AE, Festa JR, Krakauer JW, & Marshall RS (2008). Variability in language recovery after first-time stroke. Journal of Neurology, Neurosurgery & Psychiatry, 79(5), 530–534. 10.1136/jnnp.2007.122457 [DOI] [PubMed] [Google Scholar]
- Léger A, Démonet J-F, Ruff S, Aithamon B, Touyeras B, Puel M, … Cardebat D (2002). Neural Substrates of Spoken Language Rehabilitation in an Aphasic Patient: An fMRI Study. NeuroImage, 17(1), 174–183. 10.1006/nimg.2002.1238 [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22(1), 1–38. [DOI] [PubMed] [Google Scholar]
- Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, … Furie KL (2012). The Prognosis for Aphasia in Stroke. Journal of Stroke and Cerebrovascular Diseases, 21(5), 350–357. 10.1016/j.jstrokecerebrovasdis.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti R, Brambilla M, Petesi M, Miniussi C, & Cotelli M (2013). Compensatory networks to counteract the effects of ageing on language. Behavioural Brain Research, 249, 22–27. 10.1016/j.bbr.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Marcotte K, Adrover-Roig D, Damien B, de Préaumont M, Généreux S, Hubert M, & Ansaldo AI (2012). Therapy-induced neuroplasticity in chronic aphasia. Neuropsychologia, 50(8), 1776–1786. 10.1016/j.neuropsychologia.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Marcotte K, & Ansaldo A (2010). The Neural Correlates of Semantic Feature Analysis in Chronic Aphasia: Discordant Patterns According to the Etiology. Seminars in Speech and Language, 31(01), 052–063. 10.1055/s-0029-1244953 [DOI] [PubMed] [Google Scholar]
- Mazaika P, Hoeft F, Glover GH, & Reiss AL (2009). A. L. (2009). Methods and software for fMRI analysis for clinical subjects (poster presented at) Hum. Brain Mapp, 1–1. Poster Presentation presented at the Organization for Human Brain Mapping, San Francisco, CA. [Google Scholar]
- McCarthy RA, & Warrington EK (2016). Past, present, and prospects: Reflections 40 years on from the selective impairment of semantic memory (Warrington, 1975). Quarterly Journal of Experimental Psychology (2006), 69(10), 1941–1968. 10.1080/17470218.2014.980280 [DOI] [PubMed] [Google Scholar]
- Meier EL, Kapse KJ, & Kiran S (2016). The Relationship between Frontotemporal Effective Connectivity during Picture Naming, Behavior, and Preserved Cortical Tissue in Chronic Aphasia. Frontiers in Human Neuroscience, 10 10.3389/fnhum.2016.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Beeson PM, Cappa S, Crinion J, Kiran S, Saur D, Thompson CK (2013). Neuroimaging in aphasia treatment research: Consensus and practical guidelines for data analysis. NeuroImage, 73, 215–224. 10.1016/j.neuroimage.2012.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, & Breitenstein C (2008). Functional imaging studies of treatment?induced recovery in chronic aphasia. Aphasiology, 22(12), 1251–1268. 10.1080/02687030802367998 [DOI] [Google Scholar]
- Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, & Rockstroh B (2008). Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. NeuroImage, 39(4), 2038–2046. 10.1016/j.neuroimage.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, Crosson B (2009). Neural Signatures of Semantic and Phonemic Fluency in Young and Old Adults. Journal of Cognitive Neuroscience, 21(10), 2007–2018. 10.1162/jocn.2009.21219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Harnish S, Conway T, & Crosson B (2011). Recent developments in functional and structural imaging of aphasia recovery after stroke. Aphasiology, 25(3), 271–290. 10.1080/02687038.2010.530672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke R, Meinzer M, Kugel H, Deppe M, Baumgärtner A, Schiffbauer H, Breitenstein C (2009). Imaging short- and long-term training success in chronic aphasia. BMC Neuroscience, 10(1), 118 10.1186/1471-2202-10-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, & Britt AE (2013). What we talk about when we talk about access deficits. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1634), 20120388–20120388. 10.1098/rstb.2012.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, & Lambon Ralph MA (2013). Going beyond Inferior Prefrontal Involvement in Semantic Control: Evidence for the Additional Contribution of Dorsal Angular Gyrus and Posterior Middle Temporal Cortex. Journal of Cognitive Neuroscience, 25(11), 1824–1850. 10.1162/jocn_a_00442 [DOI] [PubMed] [Google Scholar]
- Obler LK, Rykhlevskaia E, Schnyer D, Clark-Cotton MR, Spiro III A, Hyun J, Albert ML (2010). Bilateral brain regions associated with naming in older adults. Brain and Language, 113(3), 113–123. 10.1016/j.bandl.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PM, Stig Jørgensen H, Nakayama H, Raaschou HO, & Olsen TS (1995). Aphasia in acute stroke: Incidence, determinants, and recovery: Aphasia in Stroke. Annals of Neurology, 38(4), 659–666. 10.1002/ana.410380416 [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, Schofield TM, & Leff AP (2010). Comparing Families of Dynamic Causal Models. PLoS Computational Biology, 6(3), e1000709 10.1371/journal.pcbi.1000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, & Gabrieli JD (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage, 10(1), 15–35. 10.1006/nimg.1999.0441 [DOI] [PubMed] [Google Scholar]
- Price CJ, & Crinion J (2005). The latest on functional imaging studies of aphasic stroke. Current Opinion in Neurology, 18(4), 429–434. [DOI] [PubMed] [Google Scholar]
- Price CJ, Hope TM, & Seghier ML (2017). Ten problems and solutions when predicting individual outcome from lesion site after stroke. NeuroImage, 145, 200–208. 10.1016/j.neuroimage.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Seghier ML, & Leff AP (2010). Predicting language outcome and recovery after stroke: the PLORAS system. Nature Reviews. Neurology, 6(4), 202–210. 10.1038/nrneurol.2010.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustina D, Coslett HB, Ungar L, Faseyitan OK, Medaglia JD, Avants B, & Schwartz MF (2017). Enhanced estimations of post-stroke aphasia severity using stacked multimodal predictions: Enhanced Predictions of Aphasia Severity. Human Brain Mapping, 38(11), 5603–5615. 10.1002/hbm.23752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MAL, Jefferies E, Patterson K, & Rogers TT (2016). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18(1), 42–55. 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- Rochon E, Leonard C, Burianova H, Laird L, Soros P, Graham S, & Grady C (2010). Neural changes after phonological treatment for anomia: An fMRI study. Brain and Language, 114(3), 164–179. 10.1016/j.bandl.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Patterson K, Jefferies E, & Lambon Ralph MA (2015). Disorders of representation and control in semantic cognition: Effects of familiarity, typicality, and specificity. Neuropsychologia, 76, 220–239. 10.1016/j.neuropsychologia.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Corbetta M (2000). Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology, 55(12), 1883–1894. [DOI] [PubMed] [Google Scholar]
- Sandberg CW, Bohland JW, & Kiran S (2015). Changes in functional connectivity related to direct training and generalization effects of a word finding treatment in chronic aphasia. Brain and Language, 150, 103–116. 10.1016/j.bandl.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, & Duffau H (2013). Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Structure and Function, 218(1), 21–37. 10.1007/s00429-011-0372-3 [DOI] [PubMed] [Google Scholar]
- Saur D, & Hartwigsen G (2012). Neurobiology of Language Recovery After Stroke: Lessons From Neuroimaging Studies. Archives of Physical Medicine and Rehabilitation, 93(1), S15–S25. 10.1016/j.apmr.2011.03.036 [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, & Weiller C (2006). Dynamics of language reorganization after stroke. Brain, 129(6), 1371–1384. 10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- Schwartz M, Dell G, Martin N, Gahl S, & Sobel P (2006). A case-series test of the interactive two-step model of lexical access: Evidence from picture naming☆. Journal of Memory and Language, 54(2), 228–264. 10.1016/j.jml.2005.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF (2013). Theoretical analysis of word production deficits in adult aphasia. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1634), 20120390–20120390. 10.1098/rstb.2012.0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier. (2010). Identifying abnormal connectivity in patients using Dynamic Causal Modelling of fMRI responses. Frontiers in Systems Neuroscience. 10.3389/fnsys.2010.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Bagdasaryan J, Jung DE, & Price CJ (2014). The Importance of Premotor Cortex for Supporting Speech Production after Left Capsular-Putaminal Damage. Journal of Neuroscience, 34(43), 14338–14348. 10.1523/JNEUROSCI.1954-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Josse G, Leff AP, & Price CJ (2011). Lateralization is Predicted by Reduced Coupling from the Left to Right Prefrontal Cortex during Semantic Decisions on Written Words. Cerebral Cortex, 21(7), 1519–1531. 10.1093/cercor/bhq203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Neufeld NH, Zeidman P, Leff AP, Mechelli A, Nagendran A, Price CJ (2012). Reading without the left ventral occipito-temporal cortex. Neuropsychologia, 50(14), 3621–3635. 10.1016/j.neuropsychologia.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Turkheimer FE, Bose SK, Scott SK, & Wise RJ (2010). Increased frontoparietal integration after stroke and cognitive recovery. Annals of Neurology, 68(5), 753–756. [DOI] [PubMed] [Google Scholar]
- Sims JA, Kapse K, Glynn P, Sandberg C, Tripodis Y, & Kiran S (2016). The relationships between the amount of spared tissue, percent signal change, and accuracy in semantic processing in aphasia. Neuropsychologia, 84, 113–126. 10.1016/j.neuropsychologia.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, & Friston KJ (2009). Bayesian model selection for group studies. NeuroImage, 46(4), 1004–1017. 10.1016/j.neuroimage.2009.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HEM, Daunizeau J, & Friston KJ (2010). Ten simple rules for dynamic causal modeling. NeuroImage, 49(4), 3099–3109. 10.1016/j.neuroimage.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Weiskopf N, Drysdale PM, Robinson PA, & Friston KJ (2007). Comparing hemodynamic models with DCM. NeuroImage, 38(3), 387–401. 10.1016/j.neuroimage.2007.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Allendorfer JB, Banks C, Vannest J, & Holland SK (2013). Recovered vs. not-recovered from post-stroke aphasia: the contributions from the dominant and non-dominant hemispheres. Restorative Neurology and Neuroscience, 31(4), 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, & den Ouden D-B (2008). Neuroimaging and recovery of language in aphasia. Current Neurology and Neuroscience Reports, 8(6), 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HE, & Jefferies E (2013). Semantic control and modality: An input processing deficit in aphasia leading to deregulated semantic cognition in a single modality. Neuropsychologia, 51(10), 1998–2015. 10.1016/j.neuropsychologia.2013.06.030 [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, & Farah MJ (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences, 94(26), 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Messing S, Norise C, & Hamilton RH (2011). Are networks for residual language function and recovery consistent across aphasic patients? Neurology, 76(20), 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, & Dronkers NF (2011). The Neural Architecture of the Language Comprehension Network: Converging Evidence from Lesion and Connectivity Analyses. Frontiers in System Neuroscience, 5 10.3389/fnsys.2011.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wouden T (1990). Celex: Building a multifunctional polytheoretical lexical data base. Proceedings of BudaLex, 88, 363–373. [Google Scholar]
- van Hees S, McMahon K, Angwin A, de Zubicaray G, & Copland DA (2014). Neural activity associated with semantic versus phonological anomia treatments in aphasia. Brain and Language, 129, 47–57. 10.1016/j.bandl.2013.12.004 [DOI] [PubMed] [Google Scholar]
- van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, & Copland DA (2014). A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post-stroke aphasia: Resting-State fMRI and Aphasia Treatment. Human Brain Mapping, 35(8), 3919–3931. 10.1002/hbm.22448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers CAMM, Vink M, van Zandvoort MJE, van der Worp HB, de Haan EHF, Kappelle LJ, Dijkhuizen RM (2010). Contribution of the left and right inferior frontal gyrus in recovery from aphasia. A functional MRI study in stroke patients with preserved hemodynamic responsiveness. NeuroImage, 49(1), 885–893. 10.1016/j.neuroimage.2009.08.057 [DOI] [PubMed] [Google Scholar]
- Vitali P, Abutalebi J, Tettamanti M, Danna M, Ansaldo A-I, Perani D, Cappa SF (2007). Training-Induced Brain Remapping in Chronic Aphasia: A Pilot Study. Neurorehabilitation and Neural Repair, 21(2), 152–160. 10.1177/1545968306294735 [DOI] [PubMed] [Google Scholar]
- Volz LJ, Eickhoff SB, Pool E-M, Fink GR, & Grefkes C (2015). Differential modulation of motor network connectivity during movements of the upper and lower limbs. NeuroImage, 119, 44–53. 10.1016/j.neuroimage.2015.05.101 [DOI] [PubMed] [Google Scholar]
- Wade DT, Hewer RL, David RM, & Enderby PM (1986). Aphasia after stroke: natural history and associated deficits. Journal of Neurology, Neurosurgery, and Psychiatry, 49(1), 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, & Poldrack RA (2001). Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron, 31(2), 329–338. [DOI] [PubMed] [Google Scholar]
- Warburton E, Price CJ, Swinburn K, & Wise RJ (1999). Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. Journal of Neurology, Neurosurgery & Psychiatry, 66(2), 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK (1975). The selective impairment of semantic memory. The Quarterly Journal of Experimental Psychology, 27(4), 635–657. 10.1080/14640747508400525 [DOI] [PubMed] [Google Scholar]
- Warrington EK, & Cipolotti L (1996). Word comprehension: The distinction between refractory and storage impairments. Brain, 119(2), 611–625. 10.1093/brain/119.2.611 [DOI] [PubMed] [Google Scholar]
- Warrington EK, & Mccarthy R (1983). CATEGORY SPECIFIC ACCESS DYSPHASIA. Brain, 106(4), 859–878. 10.1093/brain/106.4.859 [DOI] [PubMed] [Google Scholar]
- Warrington EK, & Mccarthy RA (1987). CATEGORIES OF KNOWLEDGE: FURTHER FRACTIONATIONS AND AN ATTEMPTED INTEGRATION. Brain, 110(5), 1273–1296. 10.1093/brain/110.5.1273 [DOI] [PubMed] [Google Scholar]
- Wei T, Liang X, He Y, Zang Y, Han Z, Caramazza A, & Bi Y (2012). Predicting Conceptual Processing Capacity from Spontaneous Neuronal Activity of the Left Middle Temporal Gyrus. Journal of Neuroscience, 32(2), 481–489. 10.1523/JNEUROSCI.1953-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, & Heiss WD (2007). The Right Inferior Frontal Gyrus and Poststroke Aphasia: A Follow-Up Investigation. Stroke, 38(4), 1286–1292. 10.1161/01.STR.0000259632.04324.6c [DOI] [PubMed] [Google Scholar]
- Xu Y, Lin Q, Han Z, He Y, & Bi Y (2016). Intrinsic functional network architecture of human semantic processing: Modules and hubs. NeuroImage, 132, 542–555. 10.1016/j.neuroimage.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Zahn R, Schwarz M, & Huber W (2006). Functional activation studies of word processing in the recovery from aphasia. Journal of Physiology-Paris, 99(4–6), 370–385. 10.1016/j.jphysparis.2006.03.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.