Abstract
Vitamin K antagonist therapy is associated with an increased bleeding risk, and clinicians often reverse anticoagulation in patients who require emergency surgical procedures. Current guidelines for rapid anticoagulation reversal for emergency surgery recommend 4-factor prothrombin complex concentrate and vitamin K co-administration. We reviewed the current evidence on prothrombin complex concentrate treatment for vitamin K antagonist reversal in the perioperative setting, focusing on comparative studies and in the context of intracranial hemorrhage and cardiac surgery. Cochrane and PubMed were searched between January 2008 and December 2017 and retrieved 423 English language papers, which were then screened for relevance to the perioperative setting, and 36 papers were identified and included in this review. Prothrombin complex concentrate therapy was consistently shown to reduce international normalized ratio rapidly and control bleeding effectively. In comparative studies with plasma, prothrombin complex concentrate use was associated with a greater proportion of patients achieving target international normalized ratios rapidly, with improved hemostasis. No differences in thromboembolic event rates were seen between prothrombin complex concentrate and plasma, with prothrombin complex concentrate also demonstrating a lower risk of fluid overload events. Overall, the studies reviewed support current recommendations favoring prothrombin complex concentrate therapy in patients requiring vitamin K antagonist reversal prior to emergency surgery.
Keywords: anticoagulants, bleeding, hemorrhage, plasma, vitamin K antagonists, warfarin
Summary Statement:
Patients anticoagulated with warfarin often require emergency surgery. Although fresh frozen plasma is still frequently used, guidelines for rapid reversal recommend 4-factor prothrombin complex concentrates. We review the current evidence supporting these recommendations.
Introduction
Despite the increasing use of non-vitamin K antagonist oral anticoagulants, vitamin K antagonists, such as warfarin, are still widely used in patients with atrial fibrillation, venous thromboembolism and mechanical heart valves. In 2015, approximately 3 million patients were prescribed warfarin in the USA alone.1 As with all anticoagulants, the main risk associated with vitamin K antagonist therapy is an increased risk for bleeding. Thus, annual rates of major hemorrhagic events ranged from 1.0–7.4% in a systematic review of patients with atrial fibrillation receiving vitamin K antagonist therapy for stroke prevention, while rates of intracranial hemorrhage (ICH) in the same population ranged from 0.1–2.5%.2
Patients receiving vitamin K antagonist therapy who require a surgery or invasive procedure present a specific challenge to clinicians, with an estimated 250,000–400,000 patients affected per year in North America alone.3 Data from the Randomized Evaluation of Long-Term Anticoagulation Therapy trial demonstrated that major bleeding (defined as ≥2 g/dL reduction in hemoglobin, transfusion of ≥2 units of red blood cells, or a critical area/organ bleed) occurred in 3.3% of warfarin-treated patients undergoing elective surgery, increasing to 21.6% in patients who required emergency surgery.4 Consequently, effective perioperative management is a key consideration in this population. In patients undergoing elective surgery, current guidelines recommend discontinuing vitamin K antagonist therapy 5 days before the procedure to restore the patient’s international normalized ratio to a normal range and minimize the risk of perioperative bleeding.3 However, in patients who require an emergency surgical procedure, rapid vitamin K antagonist reversal is recommended by replacing the vitamin K-dependent coagulation factors II, VII, IX and X.5
Intravenous vitamin K monotherapy is recommended only for vitamin K antagonist reversal in patients in whom surgery can be delayed6 because it can take >48 hours to normalize functional factor levels and restore them to the normal range.5 Therefore, in situations requiring rapid vitamin K antagonist reversal, treatment with prothrombin complex concentrates, concomitantly with vitamin K, is more commonly administered. Although fresh frozen plasma (plasma frozen within 8 hours following collection) or plasma (frozen within 24 hours following collection) was traditionally used for rapid reversal of anticoagulation with vitamin K antagonists, there are multiple limitations to its use, including the need for blood-type matching prior to administration; time required to thaw the product; and risks of fluid overload, pathogen transmission, and transfusion-related acute lung injury (TRALI).5 Further, only minimal benefits of plasma have been shown when reducing the international normalized ratio below 1.7 in adults, as well as minimal efficacy for anticoagulation reversal.7,8
Prothrombin complex concentrates, which are classed as either 4-factor prothrombin complex concentrates; (containing coagulation factors II, VII, IX and X) or 3-factor prothrombin complex concentrate ; (containing factors II, IX and X, but only minimal levels of factor VII) (Table 1), are stored at room temperature, administered in a smaller volume and shorter infusion time than plasma and are virally inactivated to minimize the risk of pathogen transmission. Current treatment guidelines recommend prothrombin complex concentrates, specifically 4F-PCCs, with concomitant intravenous vitamin K, as the preferred therapy for urgent vitamin K antagonist reversal (Table 2).5,6,9,10
Table 1.
Composition of available prothrombin complex concentrates
| Product (Manufacturer) |
Coagulation factor content (IU) | Antithrombotic content (IU) | |||||
|---|---|---|---|---|---|---|---|
| II | VII | IX | X | Protein C | Protein S | ATIII | |
| Beriplex P/N (CSL Behring) |
400–960 | 200–500 | 400–620 | 440–1200 | 300–900 | 240–760 | 4–30 |
| Octaplex (Octapharma) |
280–760 | 180–480 | 500 | 360–600 | 260–620 | 240–640 | 0 |
| Prothromplex Total (Shire/Baxalta) |
480–900 | 500 | 600 | 600 | 400 | Not declared | Not declared |
| Cofact/PPSB SD/Kanokad (Sanquin/CAF) |
280–700 | 140–400 | 500 | 280–700 | 222–780 | 20–160 | ≤0.6 |
| Uman Complex (Kedrion) |
500 | Not declared | 500 | 400 | Not declared | Not declared | 2.5 |
| Profilnine (Grifols) |
150 | 35 | 100 | 100 | Not declared | Not declared | Not declared |
| Bebulin (Shire/Baxalta) |
Not declared |
Not declared (low) | Not declared | Not declared | Not declared | Not declared | Not declared |
| FEIBA (Shire/Baxalta) |
Present* mainly non- activated |
Present* activated |
Present* mainly non- activated |
Present,* mainly non- activated |
Not declared |
Not declared | Not declared |
ATIII, antithrombin III. Data are based on the prescribing information of each product, as of January 2017.
Indicates that values are not provided in the prescribing information, just the presence or absence of the coagulation factor
Table 2.
Current guideline recommendations for reversal of vitamin K antagonist anticoagulation in patients with bleeding events or requiring surgery
| Condition | Guidance |
|
|---|---|---|
| US guidelines3,9,55,107 | European guidelines6,10 | |
| Elective surgery |
• Cessation of VKAs approximately 5 days before surgery |
• VKAs should not be taken for 5 days prior to surgery |
| • PCC should not be used to enable elective surgery |
||
| Emergency surgery |
• Intravenous vitamin K should be administered in patients whose surgery can be delayed for 6–12 hours |
|
| • In patients with life-threatening bleeding and an INR >1.5, 4F-PCC 20–40 IU/kg and intravenous vitamin K 10 mg should be administered |
||
| Non-major bleeding |
• Intravenous vitamin K 1–3 mg should be administered |
|
| Major/life- threatening bleeding |
• 4F-PCC 25–50 IU/kg concomitant with intravenous vitamin K 5–10 mg should be administered |
• 4F-PCC 25–50 IU/kg concomitant with intravenous vitamin K 5–10 mg should be administered |
| • In patients with VKA-associated ICH |
• rFVIIa is not recommended for anticoagulation in this setting |
|
| ○ PCCs might be considered over FFP |
• 4F-PCC is preferred over plasma | |
| ○ If INR ≥ 1.4: intravenous vitamin K 10 mg plus 3F- or 4F-PCC should be administered |
||
3F, 3-factor; 4F, 4-factor; FFP, fresh frozen plasma; ICH, intracranial hemorrhage; PCC, prothrombin complex concentrate; rFVIIa, activated recombinant factor VII; VKA, vitamin K antagonist
The perioperative management of hemostasis in patients receiving vitamin K antagonist s was previously reviewed in this Journal in 2008.11 Since then, multiple new studies have investigated vitamin K antagonist reversal in perioperative and periprocedural settings, and prothrombin complex concentrates have become more widely available in the United States and are recommended in guidance documents. Despite the fact that prothrombin complex concentrate is recommended in all guidelines, plasma is still frequently administered for vitamin K antagonist reversal.12 This article provides an update on the latest evidence for the use of prothrombin complex concentrates in patients requiring urgent vitamin K antagonist reversal for emergency surgery, but will also review current use for non vitamin K antagonist oral anticoagulant reversal.
Methods
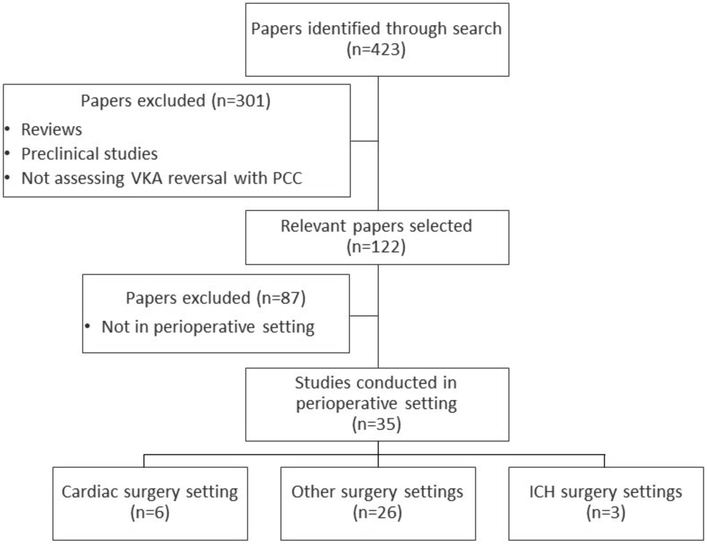
A Cochrane and PubMed search for publications between January 2008 and December 2017 was conducted using the following search terms: prothrombin complex concentrate* AND (warfarin OR [vitamin K antagonist*]). The search retrieved 423 English language papers, which were then screened for relevance to the perioperative setting (Figure 1). We excluded preclinical studies and reviews but included all other studies, including case studies.
Figure 1. Literature search process.
ICH, intracranial hemorrhage; PCC, prothrombin complex concentrate; VKA, vitamin K antagonist.
In total, 35 papers investigating the use of prothrombin complex concentrate for vitamin K antagonist reversal in perioperative settings were identified and included in this review. A further paper investigating prothrombin complex concentrate use in cardiac surgery was identified through a recent meta-analysis of warfarin reversal with prothrombin complex concentrate or fresh frozen plasma,13 bringing the total of papers included to 36. Of these papers, six studies in cardiac surgery and three in neurosurgical settings were identified.
Results
Non-comparative studies of prothrombin complex concentrates
The majority of studies identified in the search were of a retrospective observational design, with limited numbers of patients and lacking a comparator treatment arm. In general, perioperative bleeding episodes were well controlled with prothrombin complex concentrate therapy. The proportion of patients achieving effective hemostasis (no reports of excessive bleeding or bleeding controlled with no requirement for additional products) ranged from 90% to 100%,14–19 while in a study of 20 patients treated with a 4 factor-prothrombin complex concentrate, blood loss decreased significantly, from an average 829 mL in the 6 hours preceding 4 factor-prothrombin complex concentrate administration to 283 mL 6 hours after administration.20
Reversing vitamin K antagonist anticoagulation, as reflected by a normalized international normalized ratio is often required for most surgeries/procedures. In the studies identified, prothrombin complex concentrate therapy consistently reduced patients’ international normalized ratio to 1.1–1.9 from baseline values of 1.6–4.2.15,16,20–35 Critically, these reduced international normalized ratios are in line with the target international normalized ratio for patients undergoing surgery of <1.5.3
As well as reducing the risk for bleeding during surgery, rapid vitamin K antagonist reversal with prothrombin complex concentrates may also reduce time to surgery. For minor procedures such as a lumbar puncture, the time between administration of prothrombin complex concentrate and the start of the procedure was as short as 15–30 minutes.16,24 In patients requiring more extensive surgery (e.g., heart transplantation, neurosurgery), this time period ranged from 2.5–5.2 hours.22,23,36
All-cause mortality rates were generally between 10% and 25%,14,22,23,30,37 although one study in patients requiring neurosurgery due to a life-threatening intracranial hemorrhage reported a mortality rate of 43.5%.21 It should be noted that this study included high-risk patients with serious head trauma; the authors also highlighted that delays in therapy administration, subtherapeutic doses of prothrombin complex concentrate and incorrect vitamin K use may also have been factors contributing to this high mortality rate.21
Studies comparing prothrombin complex concentrate and fresh frozen plasma/plasma
Given that fresh frozen plasma/plasma is still often used by clinicians for the urgent reversal of vitamin K antagonist anticoagulation, it is pertinent to look at studies that specifically compared this treatment option with prothrombin complex concentrate in patients undergoing emergency surgical procedures. Overall, three randomized trials38–40 and one retrospective study41 comparing these treatment options in this setting were identified. Outside of the literature search, a further study was identified that investigated the administration of prothrombin complex concentrate versus fresh frozen plasma in patients who experienced coagulopathy while undergoing elective pulmonary endarterectomy (Table 3).42 All studies used either fresh frozen plasma or plasma frozen within 24 hours of collection (frozen plasma). Compared with fresh frozen plasma, only levels of factors V and VIII are slightly reduced in frozen plasma; therefore, for the purposes of this review, the terms fresh frozen plasma or plasma can be used interchangeably.
Table 3.
Comparative studies of PCC versus plasma for urgent VKA reversal in perioperative settings – 2008–2017
| Citation & location of study |
Study design | Surgical indication |
Patients (n) | PCC used- manufacturer |
Comparator | Key efficacy results | Key safety results |
|---|---|---|---|---|---|---|---|
| Agarwal et al. Neurosurger 2017; doi: 10.1093/neurs/nyx327(USA) |
Retrospective cohort analysis |
Neurosurgery | PCC: 28 FFP: 35 |
Not specified | FFP | • INR decreased from 3.36 to 1.36 with PCC and 2.92 to 1.33 with FFP • No significant difference between post-treatment INR for the PCC and FFP groups |
• 1 and 0 TEEs were reported in the PCC and FFP groups within 72 h after infusion • No significant difference in in hospital mortality rates were observed (PCC, 17.9%; FFP, 14.3%) |
| Demeyereet al. Vox Sang. 2010; 99: 251– 60 (Belgium) |
Prospective, randomized, two-arm, open label |
Cardiac surgery |
PCC: 18 FFP: 20 |
Cofact (Sanquin) |
FFP | • 15 min post-CPB, INR ≤1.5 was reached by 7 and 0 patients receiving PCC and FFP, respectively • Median INR decrease was greater with PCC (from 2.7 to 1.6) than with FFP (2.6 to 2.3) 15 min post-CPB • 6 and 20 patients required an additional dose to reach INR target in the PCC and FFP groups, respectively |
• 7 and 9 AEs were reported in the PCC and FFP groups, respectively • 2 patients in the FFP group reported excessive oozing |
| Fariboz Farsad et al. Iranian J Pharm Res 2015; 14: 877– 85 (Iran) |
Randomized Study comparing PCC with FFP |
Cardiac procedure |
PCC: 25 FFP: 25 |
Uman Complex (Kedrion) |
FFP | • 30 min post infusion, mean INR decreased from 4.02 to 2.34 for the PCC group and from 4.88 to 3.1 for FFP • 76% and 20% of patients achieved INR <2.5 in the PCC and FFP groups, respectively • 20% and 68% of patients needed additional doses to achieve target INR in the PCC and FFP groups, respectively |
• No cases of hemorrhage were reported |
| Goldstein et al. Lancet 2015; 385: 2077–87 (USA, Belarus, Bulgaria, Lebanon, Romania, Russia) |
Phase 3b, prospective randomized, open-label, active- control, multicentre study |
Urgent surgery |
PCC: 89 FFP: 90 |
Beriplex/Kcent a (CSL Behring) |
FFP | • Effective hemostasis was achieved in 90% and 75% of patients in the PCC and plasma groups, respectively • INR ≤1.3 at 30 min post administration was achieved in 55% and 10% of patients in the PCC and plasma groups, respectively • Median time from start of infusion to start of surgery was significantly shorter in the PCC group (p=0.0098) |
• AEs were seen in 56% and 60% of patients in the PCC and plasma groups, respectively • TEEs occurred in 7% and 8%, fluid overload developed in 3% and 13%, and late bleeding occurred in 3% and 5% of patients in the PCC and plasma groups, respectively • By Day 45, 3 and 8 deaths were reported in the PCC and plasma groups, respectively. Only 1 death (plasma group) was deemed related to treatment |
| Ortmann et al. Anesth Analg. 2015; 121: 26– 33 (UK) |
Exploratory cohort study |
Cardiac surgery |
PCC: 45 FFP: 55 |
Beriplex/Kcent a (CSL Behring) and Octaplex (Octapharma) |
FFP | • Cumulative blood loss was lower in the PCC group 1 and 12 hours following surgery compared with FFP • Similar numbers of units of red blood cells were transfused in both groups |
• No DVT, pulmonary embolisms or MIs were seen in either group • Rates of cerebral infarction, hemorrhage and 30-day mortality were similar between the two groups |
| Refaai et al. Emerg Med Int. 2017; 2017: 8024356 |
Post hoc analysis |
GI bleeding | PCC: 22 FFP: 20 |
Beriplex/Kcent a (CSL Behring) |
FFP | • INR ≤1.3 30 min after infusion was achieved in 65% of patients with PCC vs 0% in patients with FFP • Median time between start of treatment and first procedure was 17.5 h with PCC vs 23.9 h with FFP |
• TEEs occurred in 1 and 2 patients in the PCC and FFP groups, respectively • 1 and 4 fluid overload events occurred in the PCC and FFP groups, respectively |
AE, adverse event; CPB, cardiopulmonary bypass; DVT, deep vein thrombosis; FFP, fresh frozen plasma; INR, international normalized ratio; MI, myocardial infarction; PCC, prothrombin complex concentrate; TEE, thromboembolic event
Effect of prothrombin complex concentrate vs. plasma on international normalized ratio
In the randomized trials, prothrombin complex concentrate was consistently shown to reduce the international normalized ratio more rapidly than plasma. One study, by Goldstein et al., in various surgical indications demonstrated superiority of 4F-prothrombin complex concentrate over plasma for rapid international normalized ratio reduction, with 55% of patients treated with a 4-factor prothrombin complex concentrate achieving a target international normalized ratio of ≤1.3 versus 10% of patients in the plasma group at 30 minutes after the end of infusion (treatment difference: 45.3%; 95% confidence interval [CI]: 31.9–56.4%; p<0.0001).40 Patients undergoing cardiac surgery with cardiopulmonary bypass demonstrated a significant treatment difference 15 minutes after infusion, with 17.5% of patients receiving 4 factor-prothrombin complex concentrate achieving a target international normalized ratio of ≤1.5 compared with no patients who received fresh frozen plasma (p=0.0068).38 These quicker international normalized ratio reduction times seen with prothrombin complex concentrate compared with plasma should also be considered in the context of the smaller volume that needs to be administered (40–100 mL with prothrombin complex concentrate compared with 520–1200 mL with plasma), which leads to a shorter infusion time.38–40 Thus, in the Goldstein et al. study, mean infusion times were 21 minutes for 4 factor- prothrombin complex concentrate and 141 minutes for plasma. Therefore, despite plasma having almost 2 additional hours to start exerting a treatment effect, international normalized ratio reduction 30 minutes after end of infusion was still superior with 4 factor- prothrombin complex concentrate.40
An important advantage of a more rapid and predictable international normalized ratio reduction is the ability to proceed to surgery quickly in emergency situations. The length of time from start of infusion to start of surgery was reported in one study: patients who received 4 factor- prothrombin complex concentrate had a significantly shorter median time to surgery than patients who received plasma (3.6 hours vs 8.5 hours, respectively; p=0.0098).40 In a post-hoc analysis of patients with GI bleeding requiring procedures in the Goldstein et al. study and another study investigating 4 factor- prothrombin complex concentrate for warfarin reversal in patients with acute bleeding,43 the mean time between the start of treatment and the first procedure was significantly shorter in patients given 4 factor- prothrombin complex concentrate compared with plasma (p=0.037).44
Unlike the randomized trials, the retrospective analysis comparing prothrombin complex concentrate versus fresh frozen plasma in patients undergoing emergency neurosurgery did not investigate the time taken to achieve international normalized ratio reversal. However, both prothrombin complex concentrate and fresh frozen plasma were shown to significantly decrease the international normalized ratio from baseline (p<0.001), with no significant difference between either group for post-treatment values.41
Effect of Prothrombin complex concentrate vs. Plasma on Clinical Outcomes
As well as demonstrating more rapid international normalized ratio reduction, 4 factor- prothrombin complex concentrates have also been associated with greater clinical efficacy compared with plasma. In a study by Goldstein et al. in patients undergoing various surgical/invasive procedures, effective hemostasis (defined as intraoperative blood loss not exceeding predicted loss by 50 mL or 30%, normal hemostasis, and no requirement for additional coagulation products) was achieved in 90% of patients who received 4 factor- prothrombin complex concentrate compared with 75% of patients who received plasma. This treatment difference was significant (p=0.0142) and demonstrated superiority of 4 factor- prothrombin complex concentrate over plasma.40 In another study involving patients who received 4 factor-prothrombin complex concentrate or plasma for vitamin K antagonist reversal while undergoing elective pulmonary endarterectomy, cumulative blood loss was significantly lower up to 12 hours postoperatively in the 4 factor- prothrombin complex concentrate group compared with the plasma group (277 mL and 650 mL, respectively; p=0.0078).42
In general, similar numbers of patients receiving prothrombin complex concentrate and plasma required transfusions of additional blood products (i.e., platelets, red blood cells, cryoprecipitate).40,42 However, one study in patients undergoing cardiopulmonary bypass reported a significantly greater proportion of patients who received plasma requiring additional doses of plasma or 4 factor- prothrombin complex concentrate versus patients who originally received 4 factor- prothrombin complex concentrate (100% vs 30% of patients receiving plasma or 4 factor- prothrombin complex concentrate, respectively; p<0.001).38
Mortality rates were reported in two studies. Although fewer deaths occurred among patients who received 4 factor- prothrombin complex concentrate compared with plasma (3.4% vs. 9.1%40 and 6.7% vs. 7.3%42), this difference did not reach statistical significance.40,42 A recent systematic review and meta-analysis of 13 studies comparing prothrombin complex concentrate versus plasma in patients with warfarin-related bleeding also demonstrated a nonsignificant reduction in mortality outcomes in a subgroup analysis of studies evaluating patients who underwent urgent surgical procedures.13 By contrast, when all warfarin-related bleeding events were included, and not just those in the perioperative setting, this meta-analysis demonstrated that prothrombin complex concentrate therapy was associated with a significant reduction in all-cause mortality compared with plasma (p=0.006).13
Studies comparing 3 factor prothrombin complex concentrates and 4 factor prothrombin complex concentrates
Both 3 and 4 factor prothrombin complex concentrates were used in the studies identified in our search, although no studies directly compared these different formulations in a surgical setting. However, current guidelines recommend the use of 4 factor- prothrombin complex concentrates for patients who require rapid vitamin K antagonist reversal.6,9 These recommendations are aligned with the findings of retrospective studies conducted in patients experiencing major bleeding, which demonstrated that a greater proportion of patients achieved vitamin K antagonist reversal (as measured by achievement of target international normalized ratios ranging from ≤1.3 to 1.5) with 4 factor- prothrombin complex concentrate than 3 factor- prothrombin complex concentrate,45–48 reaching statistical significance in two studies.45,47 One study also reported a significantly higher mortality rate (p=0.001) in patients who received 3 factor compared with 4 factor prothrombin complex concentrate.48
Studies comparing prothrombin complex concentrate and recombinant FVIIa
Despite being off-label, use of recombinant FVIIa has been reported for vitamin K antagonist reversal. Although recombinant FVIIa completely normalizes the international normalized ratio, it does not correct the coagulation defect based on peak thrombin levels and endogenous thrombin potential.49,50 Two retrospective studies investigated the use of recombinant FVIIa in comparison with a 3 factor- prothrombin complex concentrate. In one analysis, recombinantFVIIa was shown to reduce the international normalized ratio more rapidly than prothrombin complex concentrates, although this difference did not result in clinical benefit, with a greater proportion of patients receiving recombinant FVIIa experiencing hematoma expansion.51 The second study also reported more rapid international normalized ratio reduction with recombinant FVIIa versus prothrombin complex concentrate; however, there were no significant differences in thromboembolic events or mortality rates.52 In another retrospective review, a significantly greater proportion of patients achieved a target international normalized ratio of <1.3 when receiving a combination of 3 factor- prothrombin complex concentrate and recombinantFVIIa (79.4%) compared with patients who received either recombinant FVIIa (45.7%) or 4 factor- prothrombin complex concentrate (50.0%) alone; however, this combination therapy was associated with a significantly higher proportion of deep vein thromboses (18.7%) compared with either recombinant FVIIa (4.2%) or 4 factor- prothrombin complex concentrate (6.1%).53 The high number of patients achieving international normalized ratio <1.3 and experiencing deep vein thromboses with the combination therapy might be indicative of “double dosing” of coagulation factors and the fact that 3 factor- prothrombin complex concentrates lack small amounts of anticoagulant factors (protein C and S) present in 4 factor- prothrombin complex concentrates.53
Current guidelines do not recommend recombinant FVIIa for urgent vitamin K antagonist anticoagulation reversal5,6; further investigative studies would be beneficial to compare the efficacy and safety of prothrombin complex concentrates and rFVIIa to help inform future practice.
Studies conducted in specific surgical indications
The majority of surgeries carry an inherent risk of bleeding; however, certain surgical indications are associated with an increased bleeding risk in patients receiving vitamin K antagonists.3 Furthermore, uncontrolled bleeding in patients undergoing cardiac, intracranial or spinal surgery can result in serious clinical consequences.3
Intracranial hemorrhage
Intracranial hemorrhage is a particular concern in patients treated with vitamin K antagonists. A report from a large U.S. cohort of over 13,500 patients with atrial fibrillation demonstrated that almost 88% of deaths due to warfarin-associated bleeding were intracranial hemorrhage events, and over 40% in patients who developed an intracranial hemorrhage died.54 Although surgical intervention in cases of intracranial hemorrhage remains controversial, it can be considered in patients who are deteriorating neurologically, have brainstem compression or hydrocephalus owing to ventricular obstruction,55 or those with supratentorial intracranial hemorrhage and a Glasgow coma score of 9–12.56
Few studies have investigated vitamin K antagonist reversal in patients with intracranial hemorrhage in the perioperative setting, and a comprehensive examination of prothrombin complex concentrate use in patients presenting with intracranial hemorrhage, not just those requiring surgical intervention, is outside the scope of this review. A retrospective analysis by Agarwal et al. investigated prothrombin complex concentrate use versus plasma in warfarin-treated patients undergoing emergency surgery for treatment of intracranial hemorrhage. As highlighted earlier, both prothrombin complex concentrate and plasma significantly reduced international normalized ratio from baseline (p<0.001); however, no difference between the post-treatment international normalized ratio values were seen between prothrombin complex concentrate and plasma. In-hospital mortality rates were similar between the two treatments with a rate of 17.9% and 14.3% in the prothrombin complex concentrate and plasma groups, respectively.
In studies investigating plasma and prothrombin complex concentrate treatment in warfarin treated patients presenting with intracranial hemorrhage and not just patients undergoing neurosurgery, prothrombin complex concentrate use versus plasma resulted in more rapid international normalized ratio reversal,57–60 and a greater proportion of patients achieved the target international normalized ratio.58,59,61,62 Mortality rates were not significantly different between treatments,41,57,59,63,64 while fewer patients experienced neurological deterioration63 or required neurosurgical intervention following prothrombin complex concentrate treatment.57 In a study comparing plasma, 3 factor- prothrombin complex concentrate and recombinant FVIIa in patients with intracranial hemorrhage, time to anticoagulation reversal was almost twice as long with plasma compared with 3 factor- prothrombin complex concentrate and recombinant FVIIa; international normalized ratio rebound was seen more frequently in patients who received recombinant FVIIa compared with either plasma or prothrombin complex concentrate, and the mortality rate was lowest in patients who received 3 factor- prothrombin complex concentrate (although it should be noted that the population size for these groups was small).65 In another retrospective study conducted in the intracranial hemorrhage setting, recombinant FVIIa was shown to reduce international normalized ratio to ≤1.3 in 83% of patients, compared with just 20% of patients treated with 3 factor- prothrombin complex concentrate. However, this improved international normalized ratio reversal did not translate into clinical efficacy, with hematoma expansion occurring in a greater proportion of patients receiving recombinant FVIIa.51
Cardiac surgery
Major bleeding events in patients undergoing cardiac surgery have been shown to significantly increase the risk of operative mortality, and are also a precursor to reoperation and increased red blood cell transfusions, both of which are associated with increased morbidity and mortality.66 As such, rapid vitamin K antagonist reversal is essential for patients requiring emergency cardiac surgery. In a study in patients undergoing cardiopulmonary bypass, international normalized ratio reversal to ≤1.5 within 15 minutes was achieved in 35.0% of patients administered 4 factor- prothrombin complex concentrate compared with 0% of plasma recipients,38 whereas another study of prothrombin complex concentrate in patients undergoing heart transplantation showed that 12% and 75% of patients achieved an international normalized ratio<1.5, and <1.7, respectively, before transplantation.67 International normalized ratio reduction to <1.5 was achieved in all four patients in a small case series of patients undergoing heart transplantation; however, the average time to achieve this was 2.45 hours (still within the recommended 2–3-hour window between dosing and incision).36
In comparison with plasma, prothrombin complex concentrate treatment was associated with a more rapid international normalized ratio decrease,38 a greater proportion of patients achieving the target international normalized ratio prior to cardiac surgery39 and less cumulative postoperative blood loss.38,42 Nonsignificant decreases in blood product use (e.g., red blood cells, plasma, platelets and cryoprecipitate), patients requiring reoperation for bleeding, and in-hospital mortality were also seen in patients undergoing heart transplantation treated with 4 factor- prothrombin complex concentrate compared with a nonfactor concentrate historical control group.67
In a retrospective analysis of patients undergoing orthotopic heart transplantation, significantly fewer units of cryoprecipitate and packed red blood cells (RBCs) were transfused in patients who received 4 factor- prothrombin complex concentrate compared with those who did not (p<0.001).68 Furthermore, the median time to chest closure was significantly shorter in patients receiving 4 factor- prothrombin complex concentrate (547.9 min) versus those who did not (618.8 min; p=0.008).68 No significant difference in in-hospital mortality was observed.68
Reversing vitamin K antagonist therapy in trauma patients
Patients who are receiving vitamin K antagonist therapy and present with trauma represent a challenging medical emergency. In the U.S., the proportion of trauma patients who are taking warfarin has been shown to be approximately 4%, which increases to almost 13% when considering patients over 65 years of age.69 Furthermore, anticoagulant use prior to trauma has been associated with an increased risk of mortality, even when adjusting for confounders such as age and pre-existing medical conditions.69,70
While rapid reversal of the anticoagulant effect is essential in any vitamin K antagonist -treated patient suffering a traumatic injury, simply replenishing the vitamin K-dependent coagulation factors does not provide volume replacement, which is often required in patients who are in hypovolemic shock following major blood loss. For patients with a suspected massive bleed, current European guidelines recommend transfusion of fresh frozen plasma(or pathogen-inactivated plasma) in conjunction with packed RBCs in a plasma–RBC ratio of at least 1:2.71 The recent Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial investigated the effectiveness and safety of a 1:1:1 plasma, platelet, RBC transfusion ratio compared with a 1:1:2 ratio.72 No significant differences were seen in overall mortality after 24 hours or 30 days, however, the 1:1:1 transfusion ratio was associated with a significantly greater proportion of patients achieving hemostasis (86.1% vs. 78.1%; p=0.006) and significantly fewer patients dying due to exsanguination (9.2% vs. 14.6%; p=0.03).72
However, the administration of large amounts of fresh frozen plasma and RBCs to restore blood volume can lead to a dilution of the coagulation factors, leading to a delay in coagulopathy reversal.71 As such, the administration of prothrombin complex concentrate in conjunction with fresh frozen plasma has been proposed as an alternative therapeutic option for the rapid correction of traumatic coagulopathy while also restoring volume, and should be considered in a vitamin K antagonist treated patient. While studies have also investigated 3 factor- prothrombin complex concentrate for the treatment of traumatic coagulopathy, 4 factor- prothrombin complex concentrate is recommended by current guidelines for the reversal of vitamin K antagonist -related anticoagulation5,6,9,10 and a number of studies have investigated the use of 4 factor- prothrombin complex concentrate to reverse the anticoagulant effect in vitamin K antagonist -treated trauma patients.47,60,75–77 In a retrospective study of 4 factor- prothrombin complex concentrate administered to 26 trauma patients on warfarin, mean international normalized ratio was shown to significantly decrease from 5.7 to 1.5 (p<0.001) and this was sustained for over 2 days.75 No patients developed venous thromboembolic events and no in-hospital mortality was reported.75 A prospective study investigated 4 factor- prothrombin complex concentrate treatment for warfarin-associated coagulopathy following traumatic brain injury.60 Of 5 patients treated with 4 factor- prothrombin complex concentrate, international normalized ratio was corrected to ≤1.2 from a baseline >2.0 in all patients, and for patients requiring surgery, the time to anesthesia induction was 159 minutes, which compared favorably with patients who received fresh frozen plasma (307 minutes).60 Administration of 4 factor- prothrombin complex concentrate has also been shown to result in significantly lower transfusion requirements of RBC and platelet concentrate units (p<0.001), as well as fewer trauma patients requiring transfusion when compared with patients receiving fresh frozen plasma.76
When comparing with prothrombin complex concentrates, 4 factor- prothrombin complex concentrate has been shown to result in a significantly lower international normalized ratio (1.3 vs 1.6; p<0.001) and a significantly greater proportion of trauma patients achieving successful reversal of anticoagulation (83% vs 50%; p=0.022).47 3 factor- prothrombin complex concentrate was also associated with a greater number of venous thromboembolic events in patients compared with 4 factor- prothrombin complex concentrate (15% vs 0%), although this difference did not reach statistical significance.47 In a retrospective analysis of warfarin-treated trauma patients comparing 4 factor- prothrombin complex concentrate with 3 factor- prothrombin complex concentrate plus recombinantFVIIa, the combination therapy of a 3 factor- prothrombin complex concentrate and recombinant FVIIa achieved a significantly lower international normalized ratio compared with 4 factor- prothrombin complex concentrate (0.75 vs 1.28; p<0.001); however, no difference was seen between treatments for patients achieving a target international normalized ratio <1.5.77 Furthermore, the combination therapy was associated with a significantly increased risk of deep vein thrombosis development occurring in 22.6% of patients compared with 2.9% in the 4 factor- prothrombin complex concentrate group (p=0.01).
While these studies demonstrate that prothrombin complex concentrate results in a rapid reversal of the coagulopathy, as measured by international normalized ratio, as stated earlier, it is important to remember that owing to its concentrated nature, prothrombin complex concentrate does not provide the volume support that can be required to correct hypoperfusion associated with major blood loss, and therefore, administration of plasma is still recommended in this patient population.71
Safety
Thromboembolic and bleeding events
Historically, the use of prothrombin complex concentrates has been associated with a potential increase in venous thromboembolic events, possibly because activated coagulation factors were included in the earlier formulations of prothrombin complex concentrates,78 but also because patients on anticoagulants are treated for hypercoagulable disorders. Current formulations use nonactivated clotting factors and include antithrombotic components (protein C and S), which may mitigate the risk of developing venous thromboembolic events.79 In a meta-analysis of 27 studies investigating prothrombin complex concentrate therapy for vitamin K antagonist-treated patients in various settings, the overall risk of venous or arterial venous thromboembolic events was only 1.4%, which decreased to 0.8% in the subset of patients undergoing a surgical procedure.80
In the studies identified in our search, rates of venous thromboembolic events in patients receiving prothrombin complex concentrates varied considerably from 0% to 26.3%.19,22,23,25,31,37,41,64,81–83 We also identified two case studies which each reported a patient undergoing surgery who developed a venous thromboembolic event within 1 hour following 3 factor- prothrombin complex concentrate administration.35,84 It should be noted that many of these studies included few patients and the patient populations investigated often had a number of comorbidities; moreover, once vitamin K antagonist therapy is reversed, the underlying risk that first necessitated anticoagulation is restored, and as a result, caution should be taken when interpreting these findings.
In a comparative study of patients requiring vitamin K antagonist reversal prior to heart transplantation, venous thromboembolic events were reported more frequently in patients receiving 3 factor- prothrombin complex concentrate compared with a historical cohort who received vitamin K and plasma (18.7% vs 10%, respectively), although this difference was not significant.67 In another comparative study of 4 factor- prothrombin complex concentrate and plasma in patients undergoing emergency surgery, no significant difference was noted in the proportion of patients with venous thromboembolic events, with 7% and 8% of patients who received prothrombin complex concentrate and plasma, respectively, experiencing a venous thromboembolic event,40 which is in line with a recent meta-analysis demonstrating no increase in risk of venous thromboembolic events with prothrombin complex concentrates compared with plasma.13 However, none of the studies were designed to compare the incidence of thromboembolic events between prothrombin complex concentrates and plasma.
Across four studies, no significant differences in overall adverse event rates were seen in patients who received prothrombin complex concentrate compared with plasma.38–40,42 One study reported similar rates of late bleeding events in 4 factor- prothrombin complex concentrate - and plasma-treated patients,40 whereas another study reported abnormal bleeding in two patients who received plasma but none in those treated with 4 factor- prothrombin complex concentrate.38 These abnormal bleeding events were likely linked to plasma’s lower effectiveness at reducing patients’ international normalized ratio.38
Because rapid infusion of prothrombin complex concentrates have potential safety concerns, a multinational trial evaluated 43 patients given prothrombin complex concentrates for emergency warfarin reversal to evaluate the effect of infusion rate on international normalized ratio correction and thrombogenicity.85 The Infusion speed ranged from 2.0 to 40.0 mL min(−1) (median of 7.5 mL min(−1). The investigators noted the speed of infusion did not affect international normalized ratio measured at 30 min following prothrombin complex concentrate completion and measured thrombogenicity parameters were not affected by infusion speed.85 Currently, recommendations for 4 component- prothrombin complex concentrate administration is reconstitution in 20 ml, and the solution should be administered intravenously (not more than 3 IU/kg/min, max. 210 IU/min, approximately 8 ml/min).
Fluid overload
Owing to the increased volumes administered with plasma compared with prothrombin complex concentrate, there is a greater risk of fluid overload in patients treated with plasma.86 In the study by Goldstein et al., fluid overload or similar cardiac events were reported in 3% of patients who received 4 factor- prothrombin complex concentrate compared with 13% of patients who received plasma.40 In another study, one patient who received plasma experienced a significant increase in pulmonary and/or atrial pressure following plasma administration, which is indicative of fluid overload; no patients treated with 4 factor- prothrombin complex concentrate demonstrated fluid overload events.38 Taken together, these safety findings are in line with those reported in a recent meta-analysis of warfarin-treated patients who required urgent reversal owing to major bleeding or urgent surgical intervention: no significant difference between prothrombin complex concentrate and plasma was seen in relation to thromboembolic risk, and fluid overload was less likely in patients treated with prothrombin complex concentrate compared with plasma.13 In summary, large volumes of plasma are required to reverse vitamin K antagonists, however they ineffectively increase the concentration of coagulation factors, expose patients to allogeneic blood products with all the inherent risks, and should not be recommended/used for vitamin K antagonist reversal as also recommended in guidelines.
Future directions: role of prothrombin complex concentrates for reversal of oral factor Xa anticoagulants
In contrast with vitamin K antagonists, the nonvitamin K oral anticoagulants specifically inhibit either coagulation factors IIa or Xa, and unlike vitamin K antagonists, have few drug-drug interactions.87 However, as with vitamin K antagonists, increased bleeding risk remains a concern with non-vitamin K oral anticoagulants.88 In cases of emergency surgical intervention, there are currently no approved specific-reversal agents for factor Xa inhibitors, although andexanet alfa, a recombinant factor Xa decoy receptor protein was approved in May 2018 for patients treated with rivaroxaban and apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. The use of specific reversal strategies for non-vitamin K oral anticoagulants, also called antidotes, is an evolving strategy for treating bleeding with these agents.89 However, andexanet has not been studied in surgical patients, will be available initially in a limited number of medical centers, and its role for perioperative use remains to be determined.
Based on preclinical evidence and recent reports, current guidelines suggest that prothrombin complex concentrates could be used as part of a multimodal approach in patients requiring urgent surgery or experiencing life-threatening bleeding.10,90–93 Infusion of 4 factor- prothrombin complex concentrate has been shown to reduce prothrombin time and/or increase endogenous thrombin potential in studies of healthy volunteers or patients who received apixaban, edoxaban or rivaroxaban.94–98 Furthermore, infusion of 4 factor- prothrombin complex concentrate following edoxaban administration demonstrated a dose-dependent effect on reducing bleeding duration and volume within 30 minutes, with a dose of 50 IU/kg decreasing bleeding duration and volume below baseline levels in patients receiving therapeutic doses.98
An increasing amount of clinical data on prothrombin complex concentrate use for treatment of acute major bleeding associated with factor Xa anticoagulation is emerging from large patient registries and observational studies. Data from a large prospective registry of patients receiving nonvitamin K oral anticoagulants, the Dresden registry,99 demonstrated the rates, management and outcome of rivaroxaban-related bleeding. Of 1776 patients, 66 patients experienced a major bleeding event and 6 patients received prothrombin complex concentrates (dose range: 18–47 IU/kg). Only one patient had a significant improvement in coagulation parameters (international normalized ratio, prothrombin time ratio and activated partial thromboplastin time), however, five of the six patients demonstrated hemorrhage stabilization.99 In a retrospective review of patients developing hemorrhage secondary to dabigatran or rivaroxaban therapy, a median dose of PCC 40 IU/kg was administered in 3 out of 25 patients.100 All three patients had rivaroxaban-associated bleeds (one major, two life-threatening) and administration of prothrombin complex concentrate successfully resolved the bleeding in all cases.100 With regards to the perioperative setting, a retrospective, multicentre study investigated patients who received 4 factor- prothrombin complex concentrate for treatment of the anticoagulation effects of factor Xa inhibitors when developing a pericardial effusion during or after atrial fibrillation ablation.101 In total, 11 patients were administered 4 factor- prothrombin complex concentrate. Two patients required further surgery for treatment of the pericardial effusion, while the other nine patients were hemodynamically stable and there was no recurrence of the pericardial effusion, demonstrating that 4 factor- prothrombin complex concentrate is an effective management option in this patient population.101
There have also been a few case reports of patients on factor Xa inhibitors (either apixaban or rivaroxaban) being treated with prothrombin complex concentrate prior to undergoing a surgical procedure.102–104 Overall, administration of prothrombin complex concentrate was associated with successful completion of surgery and no bleeding complications were reported.102–104
A recent prospective evaluation reported 84 patients receiving rivaroxaban or apixaban who were treated with prothrombin complex concentrates for major bleeding, and evaluated for thromboembolic events and all-cause mortality within 30 days.105 Prothrombin complex concentrates were administered at a median dose of 2000 IU dose (1500–2000 IU) for patients with an intracranial hemorrhage (n = 59; 70.2%) or gastrointestinal bleeding (n=13;15.5%). Treatment to stop bleeding was considered effective in 58 (69.1%) and ineffective in 26 (30.9%) of treated patients. The majority of the patients with ineffective hemostasis has intracranial hemorrhage (n = 16; 61.5%), and two patients developed an ischemic stroke 5 and 10 days after prothrombin complex concentrate administration. A total of 27 (32%) patients died within 30 days, however there was no control group in the report.105
An additional report from Canada evaluated major bleeding in 66 apixaban or rivaroxaban treated patient treated with 2,000 units of prothrombin complex concentrates and evaluated thromboembolism or mortality 30-days later.106 Using a specific evaluation scale, the investigators reported cessation of bleeding was as good in 65%, moderate in 20%, and poor/none in 15% of patients and included patients with intracranial hemorrhage or gastrointestinal bleeding. Overall reversal was considered to be effective in 68% of patients and ineffective in 32%, and mortality was14% in 30 days, with an 8% risk of thromboembolic events.106
Conclusions
Overall, the studies identified in this review support current guideline recommendations that 4 factor- prothrombin complex concentrate is a preferred treatment option for urgent reversal of vitamin K antagonist anticoagulation in patients requiring urgent surgical or invasive procedures. Prothrombin complex concentrates consistently and rapidly reduced patients’ international normalized ratio. Comparative studies with plasma demonstrated greater clinical efficacy with prothrombin complex concentrates in patients requiring emergency surgery. Furthermore, prothrombin complex concentrate treatment was associated with lower rates of fluid overload owing to its lower infusion volume compared with plasma, and no instances of viral transmission. prothrombin complex concentrates are recommended in guidelines for rapid reversal of anticoagulation in vitamin K antagonist -treated patients and represent an important therapeutic option for emergency surgical interventions.
Acknowledgments
JHL developed the first draft, and revised and updated with the authors subsequent versions of the manuscript. All the current authors made additional contributions, editing, and further input for the manuscript. Writing assistance was also provided with a grant from CSL Behring. The authors had full editorial control and no additional financial support was received.
Conflicts of interest: JHL serves on research and advisory committees for Boehringer-Ingelheim, CSL Behring, Grifols, Instrumentation Laboratories, Janssen, Merck, Octapharma, Portola. JD has no conflicts of interest to disclose. TS has received a research grant form Octapharma, consultancy fees from Bayer, BMS-Pfizer, Boehringer-Ingelheim, and Daiichi Sankyo, and speakers’ honoraria from Bayer, BMS-Pfizer, Boehringer-Ingelheim, and Daiichi Sankyo, and holds shares from Novo Nordisk. JNG has received research funding from Boehringer Ingelheim, Pfizer, and Portola. TJM serves on research steering committees for PHRI, Portola, Boehringer-Ingelheim, and CSL Behring, and a speakers’ bureau for Janssen. He has also received funding from the Seton Dell Medical School Stroke Institute as a Principal Investigator; NHLBI K23 1K23HL127227–01A1.
References
- 1.IMS data Global Prescription Audit; MAT (moving annual total) 2014/15 to 2015/16.
- 2.Lip GY, Andreotti F, Fauchier L, Huber K, Hylek E, Knight E, Lane D, Levi M, Marin F, Palareti G, Kirchhof P, European Heart Rhythm A: Bleeding risk assessment and management in atrial fibrillation patients. Executive Summary of a Position Document from the European Heart Rhythm Association [EHRA], endorsed by the European Society of Cardiology [ESC] Working Group on Thrombosis. Thromb Haemost 2011; 106: 997–1011 [DOI] [PubMed] [Google Scholar]
- 3.Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R, American College of Chest P: Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest 2012; 141: e326S–50S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S, Themeles E, Heidbuchel H, Avezum A, Reilly P, Connolly SJ, Yusuf S, Ezekowitz M, Investigators R-L: Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126: 343–8 [DOI] [PubMed] [Google Scholar]
- 5.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, American College of Chest P: Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest 2012; 141: e44S–88S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, Kitchen S, Makris M, British Committee for Standards in H: Guidelines on oral anticoagulation with warfarin - fourth edition Br J Haematol 2011; 154: 311–24 [DOI] [PubMed] [Google Scholar]
- 7.Holland LL, Brooks JP: Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results. Am J Clin Pathol 2006; 126: 133–9 [DOI] [PubMed] [Google Scholar]
- 8.Dzik WH: The James Blundell Award Lecture 2006: transfusion and the treatment of haemorrhage: past, present and future. Transfus Med 2007; 17: 367–74 [DOI] [PubMed] [Google Scholar]
- 9.Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH, American College of Chest P: Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest 2012; 141: e152S–84S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, De Robertis E, Faraoni D, Filipescu DC, Fries D, Haas T, Jacob M, Lancé MD, Pitarch JVL, Mallett S, Meier J, Molnar ZL, Rahe-Meyer N, Samama CM, Stensballe J, Van der Linden PJF, Wikkelsø AJ, Wouters P, Wyffels P, Zacharowski K. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol. 2017. June;34(6):332–395. [DOI] [PubMed] [Google Scholar]
- 11.Levy JH, Tanaka KA, Dietrich W: Perioperative hemostatic management of patients treated with vitamin K antagonists. Anesthesiology 2008; 109: 918–26 [DOI] [PubMed] [Google Scholar]
- 12.Halbritter K, Beyer-Westendorf J, Nowotny J, Pannach S, Kuhlisch E, Schellong SM: Hospitalization for vitamin-K-antagonist-related bleeding: treatment patterns and outcome. J Thromb Haemost 2013; 11: 651–9 [DOI] [PubMed] [Google Scholar]
- 13.Chai-Adisaksopha C, Hillis C, Siegal DM, Movilla R, Heddle N, Iorio A, Crowther M: Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal. A systematic review and meta-analysis. Thromb Haemost 2016; 116: 879–890 [DOI] [PubMed] [Google Scholar]
- 14.Altorjay A, Szabo E, Boda Z, Kramer L, Ngo LY, Engl W, Firth CL, Ahlstrom ER, Gelmont DM, Pabinger I: An international, multicenter, prospective study of a prothrombin complex concentrate, Prothromplex Total(R), in anticoagulant reversal. Thromb Res 2015; 135: 485–91 [DOI] [PubMed] [Google Scholar]
- 15.Majeed A, Eelde A, Agren A, Schulman S, Holmstrom M: Thromboembolic safety and efficacy of prothrombin complex concentrates in the emergency reversal of warfarin coagulopathy. Thromb Res 2012; 129: 146–51 [DOI] [PubMed] [Google Scholar]
- 16.Morimoto Y, Niwa H, Nakatani T: On the use of prothrombin complex concentrate in patients with coagulopathy requiring tooth extraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110: e7–10 [DOI] [PubMed] [Google Scholar]
- 17.Varga C, Al-Touri S, Papadoukakis S, Caplan S, Kahn S, Blostein M: The effectiveness and safety of fixed low-dose prothrombin complex concentrates in patients requiring urgent reversal of warfarin. Transfusion 2013; 53: 1451–8; quiz 1450 [DOI] [PubMed] [Google Scholar]
- 18.Wozniak M, Kruit A, Padmore R, Giulivi A, Bormanis J: Prothrombin complex concentrate for the urgent reversal of warfarin. Assessment of a standard dosing protocol. Transfus Apher Sci 2012; 46: 309–14 [DOI] [PubMed] [Google Scholar]
- 19.Kushimoto S, Fukuoka T, Kimura A, Toyoda K, Brainsky A, Harman A, Chung T, Yasaka M: Efficacy and safety of a 4-factor prothrombin complex concentrate for rapid vitamin K antagonist reversal in Japanese patients presenting with major bleeding or requiring urgent surgical or invasive procedures: a prospective, open-label, single-arm phase 3b study. Int J Hematol 2017; 106: 777–786 [DOI] [PubMed] [Google Scholar]
- 20.Leal-Noval SR, Lopez-Irizo R, Bautista-Paloma J, Casado M, Arellano-Orden V, Leal-Romero M, Fernandez-Hinojosa E, Puppo-Moreno A, Munoz M: Efficacy of the prothrombin complex concentrate prothromplex in patients requiring urgent reversal of vitamin K antagonists or presenting with uncontrolled bleeding: a retrospective, single center study. Blood Coagul Fibrinolysis 2013; 24: 862–8 [DOI] [PubMed] [Google Scholar]
- 21.Barillari G, Pasca S, Barillari A, De Angelis V: Emergency reversal of anticoagulation: from theory to real use of prothrombin complex concentrates. A retrospective Italian experience. Blood Transfus 2012; 10: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beynon C, Potzy A, Jungk C, Unterberg AW, Sakowitz OW: Rapid Anticoagulation Reversal With Prothrombin Complex Concentrate Before Emergency Brain Tumor Surgery. J Neurosurg Anesthesiol 2015; 27: 246–51 [DOI] [PubMed] [Google Scholar]
- 23.Beynon C, Potzy A, Unterberg AW, Sakowitz OW: Prothrombin complex concentrate facilitates emergency spinal surgery in anticoagulated patients. Acta Neurochir (Wien) 2014; 156: 741–7 [DOI] [PubMed] [Google Scholar]
- 24.Butler JJ: Use of low-dose prothrombin complex concentrate before lumbar puncture. Am J Health Syst Pharm 2015; 72: 203–5 [DOI] [PubMed] [Google Scholar]
- 25.Carvalho MC, Rodrigues AG, Conceicao LM, Galvao ML, Ribeiro LC: Prothrombin complex concentrate (Octaplex): a Portuguese experience in 1152 patients. Blood Coagul Fibrinolysis 2012; 23: 222–8 [DOI] [PubMed] [Google Scholar]
- 26.Hurlburt L, Roscoe A, van Rensburg A: The use of prothrombin complex concentrates in two patients with non-pulsatile left ventricular assist devices. J Cardiothorac Vasc Anesth 2014; 28: 345–6 [DOI] [PubMed] [Google Scholar]
- 27.Kar R, Abel E, Burcham P, Firstenberg MS: Prothrombin complex concentrate for warfarin-induced bleeding in a patient with a mechanical aortic valve. Interact Cardiovasc Thorac Surg 2013; 17: 421–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pabinger I, Brenner B, Kalina U, Knaub S, Nagy A, Ostermann H, Beriplex PNARSG: Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost 2008; 6: 622–31 [DOI] [PubMed] [Google Scholar]
- 29.Skerritt C, Mannion S: Prothrombin complex concentrate for rapid reversal of warfarin anticoagulation to allow neuraxial blockade. Case Rep Anesthesiol 2014; 2014: 126864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sridharan M, Wysokinski WE, Pruthi R, Oyen L, Freeman WD, Rabinstein AA, McBane RD: Periprocedural warfarin reversal with prothrombin complex concentrate. Thromb Res 2016; 139: 160–5 [DOI] [PubMed] [Google Scholar]
- 31.Laible M, Beynon C, Sander P, Purrucker J, Muller OJ, Mohlenbruch M, Ringleb PA, Rizos T: Treatment With Prothrombin Complex Concentrate to Enable Emergency Lumbar Puncture in Patients Receiving Vitamin K Antagonists. Ann Emerg Med 2016; 68: 340–4 [DOI] [PubMed] [Google Scholar]
- 32.Burk DR, Smith JL, Wild JR: Prothrombin Complex Concentrates: An Alternative to Fresh Frozen Plasma. Orthopedics 2017; 40: e367–e369 [DOI] [PubMed] [Google Scholar]
- 33.Chaudhuri K, Phillips CW, Chaudhuri S, Wasnick J: Use of Prothrombin Complex Concentrate for Warfarin Reversal Before the Performance of an Epidural Blood Patch in a Patient With Cortical Vein Thrombosis and Subdural Hematoma: A Case Report. A A Case Rep 2017; 8: 36–38 [DOI] [PubMed] [Google Scholar]
- 34.Farley TM, Andreas EM: Reversal of anticoagulation with four-factor prothrombin complex concentrate without concurrent vitamin K (phytonadione) for urgent surgery in a patient at moderate-to-high risk for thromboembolism. BMJ Case Rep 2016; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keung CYY, Metz AJ, Sood S: Rapid large pulmonary embolism after prothrombin complex concentrate for warfarin reversal during colonoscopy. Intern Med J 2017; 47: 710–711 [DOI] [PubMed] [Google Scholar]
- 36.Nuckles KB, Pratt JH, Cameron CM, Ingemi AI: Case series of four-factor prothrombin complex concentrate for warfarin reversal before heart transplantation. Transplant Proc 2015; 47: 841–3 [DOI] [PubMed] [Google Scholar]
- 37.Joseph R, Burner J, Yates S, Strickland A, Tharpe W, Sarode R: Thromboembolic outcomes after use of a four-factor prothrombin complex concentrate for vitamin K antagonist reversal in a real-world setting. Transfusion 2016; 56: 799–807 [DOI] [PubMed] [Google Scholar]
- 38.Demeyere R, Gillardin S, Arnout J, Strengers PF: Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sang 2010; 99: 251–60 [DOI] [PubMed] [Google Scholar]
- 39.Fariborz Farsad B, Golpira R, Najafi H, Totonchi Z, Salajegheh S, Bakhshandeh H, Hashemian F: Comparison between Prothrombin Complex Concentrate (PCC) and Fresh Frozen Plasma (FFP) for the Urgent Reversal of Warfarin in Patients with Mechanical Heart Valves in a Tertiary Care Cardiac Center. Iran J Pharm Res 2015; 14: 877–85 [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein JN, Refaai MA, Milling TJ Jr., Lewis B, Goldberg-Alberts R, Hug BA, Sarode R: Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet 2015; 385: 2077–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal P, Abdullah KG, Ramayya AG, Nayak NR, Lucas TH: A Retrospective Propensity Score-Matched Early Thromboembolic Event Analysis of Prothrombin Complex Concentrate vs Fresh Frozen Plasma for Warfarin Reversal Prior to Emergency Neurosurgical Procedures. Neurosurgery 2017 [DOI] [PubMed] [Google Scholar]
- 42.Ortmann E, Besser MW, Sharples LD, Gerrard C, Berman M, Jenkins DP, Klein AA: An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anesth Analg 2015; 121: 26–33 [DOI] [PubMed] [Google Scholar]
- 43.Sarode R, Milling TJ Jr., Refaai MA, Mangione A, Schneider A, Durn BL, Goldstein JN: Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128: 1234–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Refaai MA, Kothari TH, Straub S, Falcon J, Sarode R, Goldstein JN, Brainsky A, Omert L, Lee ML, Milling TJ: Four-Factor Prothrombin Complex Concentrate Reduces Time to Procedure in Vitamin K Antagonist-Treated Patients Experiencing Gastrointestinal Bleeding: A Post Hoc Analysis of Two Randomized Controlled Trials. Emerg Med Int 2017; 2017: 8024356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Majzoub O, Rybak E, Reardon DP, Krause P, Connors JM: Evaluation of Warfarin Reversal with 4-Factor Prothrombin Complex Concentrate Compared to 3-Factor Prothrombin Complex Concentrate at a Tertiary Academic Medical Center. J Emerg Med 2016; 50: 7–13 [DOI] [PubMed] [Google Scholar]
- 46.Jones GM, Erdman MJ, Smetana KS, Mohrien KM, Vandigo JE, Elijovich L: 3-Factor Versus 4-Factor Prothrombin Complex Concentrate for Warfarin Reversal in Severe Bleeding: A Multicenter, Retrospective, Propensity-Matched Pilot Study. J Thromb Thrombolysis 2016; 42: 19–26 [DOI] [PubMed] [Google Scholar]
- 47.Mangram A, Oguntodu OF, Dzandu JK, Hollingworth AK, Hall S, Cung C, Rodriguez J, Yusupov I, Barletta JF: Is there a difference in efficacy, safety, and cost-effectiveness between 3-factor and 4-factor prothrombin complex concentrates among trauma patients on oral anticoagulants? J Crit Care 2016; 33: 252–6 [DOI] [PubMed] [Google Scholar]
- 48.Voils SA, Holder MC, Premraj S, Catlin JR, Allen BR: Comparative effectiveness of 3- versus 4-factor prothrombin complex concentrate for emergent warfarin reversal. Thromb Res 2015; 136: 595–8 [DOI] [PubMed] [Google Scholar]
- 49.Skolnick BE, Mathews DR, Khutoryansky NM, Pusateri AE, Carr ME: Exploratory study on the reversal of warfarin with rFVIIa in healthy subjects. Blood 2010; 116: 693–701 [DOI] [PubMed] [Google Scholar]
- 50.Tanaka KA, Szlam F, Dickneite G, Levy JH: Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb Res 2008; 122: 117–23 [DOI] [PubMed] [Google Scholar]
- 51.Pinner NA, Hurdle AC, Oliphant C, Reaves A, Lobo B, Sills A: Treatment of warfarin-related intracranial hemorrhage: a comparison of prothrombin complex concentrate and recombinant activated factor VII. World Neurosurg 2010; 74: 631–5 [DOI] [PubMed] [Google Scholar]
- 52.Chapman SA, Irwin ED, Abou-Karam NM, Rupnow NM, Hutson KE, Vespa J, Roach RM: Comparison of 3-Factor Prothrombin Complex Concentrate and Low-Dose Recombinant Factor VIIa for Warfarin Reversal. World J Emerg Surg 2014; 9: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeLoughery E, Avery B, DeLoughery TG: Retrospective study of rFVIIa, 4-factor PCC, and a rFVIIa and 3-factor PCC combination in improving bleeding outcomes in the warfarin and non-warfarin patient. Am J Hematol 2016; 91: 705–8 [DOI] [PubMed] [Google Scholar]
- 54.Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, Singer DE: Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120: 700–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D, American Heart Association Stroke C, Council on C, Stroke N, Council on Clinical C: Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015; 46: 2032–60 [DOI] [PubMed] [Google Scholar]
- 56.Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, Thomas BM, Toni D, Unterberg A, Wagner M, European Stroke O: European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014; 9: 840–55 [DOI] [PubMed] [Google Scholar]
- 57.Edavettal M, Rogers A, Rogers F, Horst M, Leng W: Prothrombin complex concentrate accelerates international normalized ratio reversal and diminishes the extension of intracranial hemorrhage in geriatric trauma patients. Am Surg 2014; 80: 372–6 [PubMed] [Google Scholar]
- 58.Siddiq F, Jalil A, McDaniel C, Brock DG, Pineda CC, Bell RD, Lee K: Effectiveness of Factor IX complex concentrate in reversing warfarin associated coagulopathy for intracerebral hemorrhage. Neurocrit Care 2008; 8: 36–41 [DOI] [PubMed] [Google Scholar]
- 59.Steiner T, Poli S, Griebe M, Husing J, Hajda J, Freiberger A, Bendszus M, Bosel J, Christensen H, Dohmen C, Hennerici M, Kollmer J, Stetefeld H, Wartenberg KE, Weimar C, Hacke W, Veltkamp R: Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol 2016; 15: 566–73 [DOI] [PubMed] [Google Scholar]
- 60.Yanamadala V, Walcott BP, Fecci PE, Rozman P, Kumar JI, Nahed BV, Swearingen B: Reversal of warfarin associated coagulopathy with 4-factor prothrombin complex concentrate in traumatic brain injury and intracranial hemorrhage. J Clin Neurosci 2014; 21: 1881–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alonso de Lecinana M, Huertas N, Egido JA, Muriel A, Garcia A, Ruiz-Ares G, Diez-Tejedor E, Fuentes B: Questionable reversal of anticoagulation in the therapeutic management of cerebral haemorrhage associated with vitamin K antagonists. Thromb Haemost 2013; 110: 1145–51 [DOI] [PubMed] [Google Scholar]
- 62.Frontera JA, Gordon E, Zach V, Jovine M, Uchino K, Hussain MS, Aledort L: Reversal of coagulopathy using prothrombin complex concentrates is associated with improved outcome compared to fresh frozen plasma in warfarin-associated intracranial hemorrhage. Neurocrit Care 2014; 21: 397–406 [DOI] [PubMed] [Google Scholar]
- 63.Fong WC, Lo WT, Ng YW, Cheung YF, Wong GC, Ho HF, Chan JH, Li PC: The benefit of prothrombin complex concentrate in decreasing neurological deterioration in patients with warfarin-associated intracerebral haemorrhage. Hong Kong Med J 2014; 20: 486–94 [DOI] [PubMed] [Google Scholar]
- 64.Majeed A, Meijer K, Larrazabal R, Arnberg F, Luijckx GJ, Roberts RS, Schulman S: Mortality in vitamin K antagonist-related intracerebral bleeding treated with plasma or 4-factor prothrombin complex concentrate. Thromb Haemost 2014; 111: 233–9 [DOI] [PubMed] [Google Scholar]
- 65.Woo CH, Patel N, Conell C, Rao VA, Faigeles BS, Patel MC, Pombra J, Akins PT, Axelrod YK, Ge IY, Sheridan WF, Flint AC: Rapid Warfarin reversal in the setting of intracranial hemorrhage: a comparison of plasma, recombinant activated factor VII, and prothrombin complex concentrate. World Neurosurg 2014; 81: 110–5 [DOI] [PubMed] [Google Scholar]
- 66.Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G, Surgical, Clinical Outcome Research G: Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg 2013; 96: 478–85 [DOI] [PubMed] [Google Scholar]
- 67.Kantorovich A, Fink JM, Militello MA, Wanek MR, Smedira NG, Soltesz EG, Moazami N: Low-dose 3-factor prothrombin complex concentrate for warfarin reversal prior to heart transplant. Ann Pharmacother 2015; 49: 876–82 [DOI] [PubMed] [Google Scholar]
- 68.Sun GH, Patel V, Moreno-Duarte I, Zahedi F, Ursprung E, Couper G, Chen FY, Welsby IJ, Comenzo R, Kao G, Cobey FC: Intraoperative Administration of 4-Factor Prothrombin Complex Concentrate Reduces Blood Requirements in Cardiac Transplantation. J Cardiothorac Vasc Anesth 2017 [DOI] [PubMed] [Google Scholar]
- 69.Dossett LA, Riesel JN, Griffin MR, Cotton BA: Prevalence and implications of preinjury warfarin use: an analysis of the National Trauma Databank. Arch Surg 2011; 146: 565–70 [DOI] [PubMed] [Google Scholar]
- 70.Lecky FE, Omar M, Bouamra O, Jenks T, Edwards A, Battle CE, Evans PA: The effect of preinjury warfarin use on mortality rates in trauma patients: a European multicentre study. Emerg Med J 2015; 32: 916–20 [DOI] [PubMed] [Google Scholar]
- 71.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, Neugebauer EA, Ozier Y, Riddez L, Schultz A, Vincent JL, Spahn DR: The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition Crit Care 2016; 20: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O’Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G, Group PS: Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015; 313: 471–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joseph B, Aziz H, Pandit V, Hays D, Kulvatunyou N, Yousuf Z, Tang A, O’Keeffe T, Green D, Friese RS, Rhee P: Prothrombin complex concentrate versus fresh-frozen plasma for reversal of coagulopathy of trauma: is there a difference? World J Surg 2014; 38: 1875–81 [DOI] [PubMed] [Google Scholar]
- 74.Joseph B, Khalil M, Harrison C, Swartz T, Kulvatunyou N, Haider AA, Jokar TO, Burk D, Mahmoud A, Latifi R, Rhee P: Assessing the Efficacy of Prothrombin Complex Concentrate in Multiply Injured Patients With High-Energy Pelvic and Extremity Fractures. J Orthop Trauma 2016; 30: 653–658 [DOI] [PubMed] [Google Scholar]
- 75.Berndtson AE, Huang WT, Box K, Kobayashi L, Godat LN, Smith AM, Weingarten D, Coimbra R: A new kid on the block: Outcomes with Kcentra 1 year after approval. J Trauma Acute Care Surg 2015; 79: 1004–8 [DOI] [PubMed] [Google Scholar]
- 76.Innerhofer P, Westermann I, Tauber H, Breitkopf R, Fries D, Kastenberger T, El Attal R, Strasak A, Mittermayr M: The exclusive use of coagulation factor concentrates enables reversal of coagulopathy and decreases transfusion rates in patients with major blunt trauma. Injury 2013; 44: 209–16 [DOI] [PubMed] [Google Scholar]
- 77.Martin DT, Barton CA, Dodgion C, Schreiber M: Emergent reversal of vitamin K antagonists: addressing all the factors. Am J Surg 2016; 211: 919–25 [DOI] [PubMed] [Google Scholar]
- 78.Kohler M: Thrombogenicity of prothrombin complex concentrates. Thromb Res 1999; 95: S13–7 [DOI] [PubMed] [Google Scholar]
- 79.Kalina U, Bickhard H, Schulte S: Biochemical comparison of seven commercially available prothrombin complex concentrates. Int J Clin Pract 2008; 62: 1614–22 [DOI] [PubMed] [Google Scholar]
- 80.Dentali F, Marchesi C, Giorgi Pierfranceschi M, Crowther M, Garcia D, Hylek E, Witt DM, Clark NP, Squizzato A, Imberti D, Ageno W: Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta-analysis. Thromb Haemost 2011; 106: 429–38 [DOI] [PubMed] [Google Scholar]
- 81.Desmettre T, Dubart AE, Capellier G, Fanara B, Puyraveau M, Kepka S, Coquart J, Sheppard F, Tazarourte K: Emergency reversal of anticoagulation: the real use of prothrombin complex concentrates: a prospective multicenter two year French study from 2006 to 2008. Thromb Res 2012; 130: e178–83 [DOI] [PubMed] [Google Scholar]
- 82.Sin JH, Berger K, Lesch CA: Four-factor prothrombin complex concentrate for life-threatening bleeds or emergent surgery: A retrospective evaluation. J Crit Care 2016; 36: 166–172 [DOI] [PubMed] [Google Scholar]
- 83.Toth P, van Veen JJ, Robinson K, Maclean RM, Hampton KK, Laidlaw S, Makris M: Real world usage of PCC to “rapidly” correct warfarin induced coagulopathy. Blood Transfus 2013; 11: 500–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldhammer JE, Bakowitz MJ, Milas BL, Patel PA: Intracardiac Thrombosis after Emergent Prothrombin Complex Concentrate Administration for Warfarin Reversal. Anesthesiology 2015; 123: 458. [DOI] [PubMed] [Google Scholar]
- 85.Pabinger I, Tiede A, Kalina U, Knaub S, Germann R, Ostermann H, Beriplex PNARSG: Impact of infusion speed on the safety and effectiveness of prothrombin complex concentrate: a prospective clinical trial of emergency anticoagulation reversal. Ann Hematol 2010; 89: 309–16 [DOI] [PubMed] [Google Scholar]
- 86.Refaai MA, Goldstein JN, Lee ML, Durn BL, Milling TJ Jr., Sarode R: Increased risk of volume overload with plasma compared with four-factor prothrombin complex concentrate for urgent vitamin K antagonist reversal. Transfusion 2015; 55: 2722–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levy JH, Key NS, Azran MS. Novel oral anticoagulants: implications in the perioperative setting. Anesthesiology. 2010;113:726–45. [DOI] [PubMed] [Google Scholar]
- 88.Sarich TC, Seltzer JH, Berkowitz SD, Costin J, Curnutte JT, Gibson CM, Hoffman M, Kaminskas E, Krucoff MW, Levy JH, Mintz PD, Reilly PA, Sager PT, Singer DE, Stockbridge N, Weitz JI, Kowey PR: Novel oral anticoagulants and reversal agents: Considerations for clinical development. Am Heart J 2015; 169: 751–7 [DOI] [PubMed] [Google Scholar]
- 89.Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI; Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:623–7. [DOI] [PubMed] [Google Scholar]
- 90.Faraoni D, Levy JH, Albaladejo P, Samama CM, Groupe d’Interet en Hemostase P: Updates in the perioperative and emergency management of non-vitamin K antagonist oral anticoagulants. Crit Care 2015; 19: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, Florido R, Hucker W, Mehran R, Messé SR, Pollack CV Jr., Rodriguez F, Sarode R, Siegal D, Wiggins BS. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:3042–3067. [DOI] [PubMed] [Google Scholar]
- 92.Levy JH, Douketis J, Weitz JI. Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol. 2018;15:273–281. [DOI] [PubMed] [Google Scholar]
- 93.Raval AN, Cigarroa JE, Chung MK, Diaz-Sandoval LJ, Diercks D, Piccini JP, Jung HS, Washam JB, Welch BG, Zazulia AR, Collins SP, American Heart Association Clinical Pharmacology Subcommittee of the Acute Cardiac C, General Cardiology Committee of the Council on Clinical C, Council on Cardiovascular Disease in the Y, Council on Quality of C, Outcomes R: Management of Patients on Non-Vitamin K Antagonist Oral Anticoagulants in the Acute Care and Periprocedural Setting: A Scientific Statement From the American Heart Association. Circulation 2017; 135: e604–e633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M: Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124: 1573–9 [DOI] [PubMed] [Google Scholar]
- 95.Levi M, Moore KT, Castillejos CF, Kubitza D, Berkowitz SD, Goldhaber SZ, Raghoebar M, Patel MR, Weitz JI, Levy JH: Comparison of three-factor and four-factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost 2014; 12: 1428–36 [DOI] [PubMed] [Google Scholar]
- 96.Nagalla S, Thomson L, Oppong Y, Bachman B, Chervoneva I, Kraft WK: Reversibility of Apixaban Anticoagulation with a Four-Factor Prothrombin Complex Concentrate in Healthy Volunteers. Clin Transl Sci 2016; 9: 176–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schultz NH, Tran HTT, Bjornsen S, Henriksson CE, Sandset PM, Holme PA: The reversal effect of prothrombin complex concentrate (PCC), activated PCC and recombinant activated factor VII against anticoagulation of Xa inhibitor. Thromb J 2017; 15: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zahir H, Brown KS, Vandell AG, Desai M, Maa JF, Dishy V, Lomeli B, Feussner A, Feng W, He L, Grosso MA, Lanz HJ, Antman EM: Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation 2015; 131: 82–90 [DOI] [PubMed] [Google Scholar]
- 99.Beyer-Westendorf J, Forster K, Pannach S, Ebertz F, Gelbricht V, Thieme C, Michalski F, Kohler C, Werth S, Sahin K, Tittl L, Hansel U, Weiss N: Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood 2014; 124: 955–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pahs L, Beavers C, Schuler P: The Real-World Treatment of Hemorrhages Associated With Dabigatran and Rivaroxaban: A Multicenter Evaluation. Crit Pathw Cardiol 2015; 14: 53–61 [DOI] [PubMed] [Google Scholar]
- 101.Gianni C, DIB L, Mohanty S, Trivedi C, Bai R, Al-Ahmad A, Burkhardt JD, Gallinghouse GJ, Horton RP, Sanchez JE, Hranitzky PM, Lakkireddy D, Mansour MC, Santangeli P, Zado ES, Marchlinski FE, Beheiry S, Hao SC, Couts L, Gibson D, Natale A: Management of Periprocedural and Early Pericardial Effusions With Tamponade Following Ablation of Atrial Fibrillation With Uninterrupted Factor Xa Inhibitors: A Case Series. J Cardiovasc Electrophysiol 2016; 27: 399–403 [DOI] [PubMed] [Google Scholar]
- 102.Beynon C, Potzy A, Unterberg AW, Sakowitz OW: Emergency neurosurgical care in patients treated with apixaban: report of 2 cases. Am J Emerg Med 2015; 33: 858 e5–7 [DOI] [PubMed] [Google Scholar]
- 103.Chic Acevedo C, Velasco F, Herrera C: Reversal of rivaroxaban anticoagulation by nonactivated prothrombin complex concentrate in urgent surgery. Future Cardiol 2015; 11: 525–9 [DOI] [PubMed] [Google Scholar]
- 104.Liu M, Aguele C, Darr U: Use of four-factor prothrombin complex concentrate for the mitigation of rivaroxaban-induced bleeding in an emergent coronary artery bypass graft. Clin Case Rep 2017; 5: 636–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Majeed A, Agren A, Holmstrom M, Bruzelius M, Chaireti R, Odeberg J, Hempel EL, Magnusson M, Frisk T, Schulman S: Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood 2017; 130: 1706–1712 [DOI] [PubMed] [Google Scholar]
- 106.Schulman S, Gross PL, Ritchie B, Nahirniak S, Lin Y, Lieberman L, Carrier M, Crowther MA, Ghosh I, Lazo-Langner A, Zondag M, Study I: Prothrombin Complex Concentrate for Major Bleeding on Factor Xa Inhibitors: A Prospective Cohort Study. Thromb Haemost 2018 [DOI] [PubMed] [Google Scholar]
- 107.Frontera JA, Lewin JJ 3rd, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, del Zoppo GJ, Kumar MA, Peerschke EI, Stiefel MF, Teitelbaum JS, Wartenberg KE, Zerfoss CL Guideline for reversal of antithrombotics in intracranial hemorrhage: A statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care 2016; 24:6–46 [DOI] [PubMed] [Google Scholar]