Abstract
Background:
Long-term studies characterizing disease course of cutaneous lupus erythematosus (CLE) patients on standard-of-care treatments are lacking.
Objective:
We characterized and compared disease course of CLE patients using Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI).
Methods:
In total, 83 CLE patients with CLASI scores collected from ≥3 study visits within 2 years had disease activity and damage trends calculated by average change scores (ACS). Trends were classified as improved (ACS ≤−3), worsened (ACS ≥3), or stable (−3 < ACS < 3). Linear regression models compared CLASI trends between groups.
Results:
Most patients (72.73%) with initial CLASI activity (CLASI-A) scores >9 (N = 33) had improved disease activity versus 14.00% of those with initial CLASI-A scores ≤9 (N = 50). Linear regression analyses showed significant improvement in CLASI-A scores in patients of minority races (P < .05), with baseline CLASI-A scores >9 (P <.0001), baseline CLASI damage (CLASI-D) scores ≥10 (P = .0001), and CLE disease duration ≤1 year (P = .01). Of 28 patients with baseline CLASI-D scores ≥10, 35.71% had improvements in damage, while 5.26% of patients with initial CLASI-D scores of 5–9 (N = 19) and 0% with initial CLASI-D scores <5 (N = 36) (P = .0005) had improvements.
Limitations:
Limitations include small sample size.
Conclusion:
Baseline CLASI-A score >9, minority race, and short disease duration predict CLE disease activity improvement. A baseline CLASI-D score ≥10 is associated with disease damage improvement.
Keywords: Cutaneous Lupus Disease Area and Severity Index, cutaneous lupus erythematosus, disease activity, disease damage, longitudinal
Cutaneous lupus erythematosus (CLE) is a photosensitive skin disease with an array of manifestations that can make diagnosis challenging. Two CLE subtypes are discoid lupus erythematosus (DLE) and subacute cutaneous lupus erythematosus (SCLE). Their clinical presentations vary, with a variety of disease activities (eg, erythema, scale) and disease damage (eg, dyspigmentation, scarring). Because there is no curative treatment, CLE can persist in patients, with varying disease courses. Moreover, it is unknown how often and what type of patients with CLE improve, worsen, or are stable over time.
The Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) provides a simple, quantitative clinical tool that standardizes the description of disease activity and damage in CLE and provides guidelines for identifying clinical change.1 The content validity, interrater and intrarater validity, and practical applicability of CLASI has been demonstrated in previous studies. The CLASI score has also been shown to be sensitive to clinically significant improvement in most subsets of CLE.2–6 However, several studies have been limited to 2–5 individual time points over a few months.3–5,7,8
There have not yet been any long-term studies elucidating the disease course of CLE with the CLASI. In general, prospective long-term studies in CLE have been rare due to lack of follow-up data. Thus, given the chronic nature of the disease course in CLE, it is important to determine how CLE patients fare over a longer period of time. A better understanding of the natural history of disease activity and damage in CLE could then be used as a basis for further clinical studies to evaluate the effectiveness of various treatment regimens.
Therefore, in this study, we aim to characterize the disease course of patients with CLE on standard-of-care treatments and identify clinical features associated with different disease activity and damage trends. On the basis of our clinical experiences, we hypothesized that patients with high disease activity at baseline will have improved disease activity over time.
METHODS
Patient recruitment
This was a prospective longitudinal cohort study of patients with a clinicopathologic diagnosis of CLE. Patients were at least 18 years of age at the time of study enrollment and had CLASI scores collected from ≥3 study visits within at least a 2-year period. Patients were recruited from outpatient dermatology clinics at University of Texas Southwestern Medical Center, Parkland Health and Hospital System, and University of Pennsylvania School of Medicine during January 2007–August 2016. This study was approved by the institutional review boards at University of Texas Southwestern and University of Pennsylvania School of Medicine.
Clinical and demographic measures
Each CLE patient had clinical evaluations at each visit by physical examination and medical chart review. Patients were assigned a predominant CLE subtype at baseline visit (ie, generalized or localized DLE, annular or papulosquamous SCLE). Skin disease activity and damage were measured at each visit by using the CLASI.1 Lesions are scored by body site, with increased emphasis on head and neck areas. CLASI activity (CLASI-A) score comprises lesion scores for erythema and scale. CLASI damage (CLASI-D) score accounts for dyspigmentation and scarring, with additional points given for the presence of scarring alopecia. Higher CLASI scores correspond to greater disease activity or damage. Other clinical and demographic characteristics obtained at baseline visit included sex, race, smoking status, age at baseline visit, age at CLE diagnosis, CLE disease duration, and individual American College of Rheumatology diagnostic criteria for systemic lupus erythematosus (SLE).9
Outcome and predictor variables
The primary outcome variables were average change scores (ACSs) for both disease activity and damage. The ACS represented the mean of the differences in the CLASI-A or CLASI-D scores taken at baseline visit, and the CLASI-A or CLASI-D scores at each follow-up visit. Disease course for either activity or damage was classified as improved if the ACS was ≥−3, worsened if the ACS was ≥3, and stable if the ACS was between these 2 values (−3 < ACS < 3). The cut-off of the absolute value of 3 was chosen to match the physician’s estimate of predicted progression frequency in the disease population and paralleled results from prior studies.10 Secondary outcomes included variability of disease activity, defined as the number of disease flares per year for each patient. Disease flare was defined as a 4-point or a 20% increase in CLASI-A score. Predictor variables included baseline CLASI-A and CLASI-D scores, which were categorized by disease severity, and demographic and clinical characteristics, including age at baseline visit, age at disease onset, disease duration at baseline visit, sex, race, CLE subtype, and presence of SLE based on American College of Rheumatology criteria.
Statistical analysis
Different activity and damage trends were compared between patient subgroups separated by the aforementioned predictor variables by using Mann-Whitney or Kruskal-Wallis tests (continuous variables) and chi-squared tests (categorical variables). For each predictor, a linear mixed model using the predictor, time, and predictor-time interaction, was fitted to assess its time-varying effect on disease activity.
RESULTS
Baseline patient characteristics
There were 83 patients who met inclusion criteria. Table I shows the baseline patient characteristics of the cohort. Most patients were female (81.9%) and white (59.0%). About two-thirds of the population had DLE (68.7%) and one-third SCLE (31.3%). Patients with SLE involvement (27.7%, n = 23) had DLE (n = 20) as their predominant CLE subtype. At the first visit, patients had a mean age of 49 years and disease duration of 9.81 years, with a mean follow-up of 3.61 years per patient. Most patients were current or past smokers (60.2%).
Table I.
Baseline patient characteristics (N = 83)
| Characteristic | Value |
|---|---|
| Sex, n (%) | |
| Female | 68 (81.93) |
| Male | 15 (18.07) |
| Race, n (%) | |
| White | 49 (59.04) |
| Black | 29 (34.94) |
| Hispanic/Latino | 4 (4.82) |
| Asian | 1 (1.20) |
| Age at diagnosis, years, mean (SD) | 39.12 (14.93) |
| Age at first visit, years, mean (SD) | 49.0 (12.42) |
| Predominant CLE subtype, n (%) | |
| Discoid LE | 57 (68.67) |
| Localized | 31 (37.35) |
| Generalized | 26 (31.33) |
| Subacute LE | 26 (31.33) |
| Annular | 8 (9.64) |
| Papulosquamous | 22 (26.51) |
| Systemic LE involvement, n (%) | 23 (27.71) |
| Discoid LE | 20 (24.10) |
| Localized | 7 (8.43) |
| Generalized | 13 (15.66) |
| Subacute LE | 3 (3.61) |
| Annular | 0 (0) |
| Papulosquamous | 3 (3.61) |
| Disease duration at baseline visit, years, mean (SD) | 9.81 (10.88) |
| Duration of follow-up per patient, years, mean (SD) | 3.61 (2.18) |
| No. follow-up visits per patient, mean (SD) | 7.53 (3.75) |
| Smoking status, n (%) | |
| Nonsmoker | 38 (45.78) |
| Current smoker | 33 (39.76) |
| Past smoker | 12 (14.46) |
CLE, Cutaneous lupus erythematosus; LE, lupus erythematosus; SD, standard deviation.
The characteristics of the patient cohort by CLASI-A and CLASI-D severity are displayed in Supplemental Table I (available at http://www.jaad.org). Most patients had mild disease activity (ie, CLASI-A ≤9) at baseline (60.2%, n = 50) with a mean CLASI-A of 3.70 ± 2.68, and 39.8% (n = 33) had moderate-severe disease activity (ie, CLASI-A >9) at baseline, with a mean CLASI-A of 20.97 ± 8.86. Of note, patients with moderate-severe disease activity at baseline also had greater disease damage, with a mean CLASI-D of 13.79 ± 12.54. Those who had mild activity at baseline had a mean CLASI-D score of 5.16 ± 4.95. In total, 36 (43.4%) patients, 19 (22.9%) patients, and 28 (33.7%) patients had baseline CLASI-D scores of 0–4, 5–9, and ≥10, respectively. Patients with a CLASI-D score ≥10 at baseline had both a high baseline CLASI-D score of 19.50 ± 9.13 and CLASI-A score of 15.07 ± 10.69.
Characteristics of patients with CLE disease activity trends
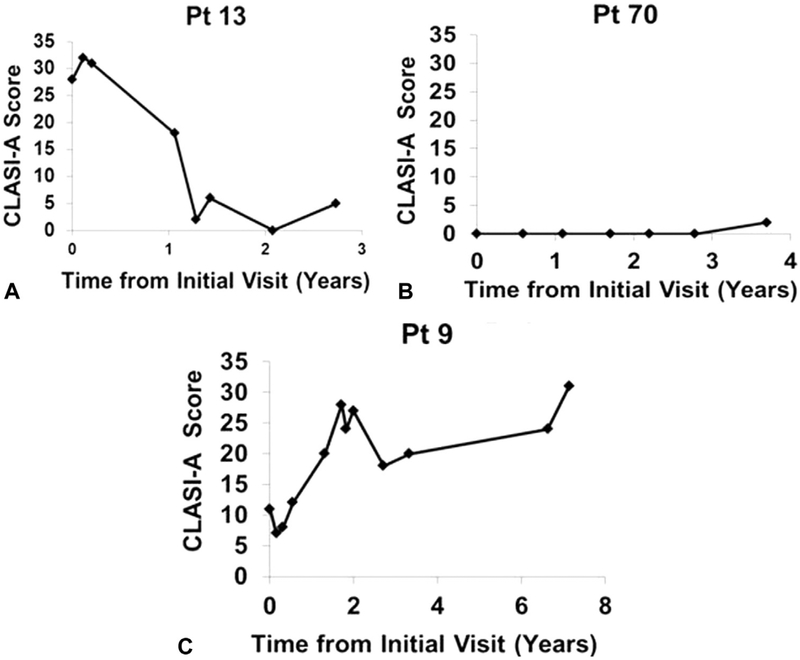
Over time, most CLE patients showed improved (37.3%, n = 31) or stable (45.8%, n = 38) disease activity trends. A minority (16.9%, n = 14) demonstrated worsened disease activity. Individual patient examples of each trend are shown in Fig 1. A large proportion of patients (72.7%, n = 24) with moderate-severe disease activity at baseline had improved disease activity. A majority of patients with baseline mild activity (62.0%, n = 31) showed stable disease activity (P < .0001). Patients with baseline CLASI-A scores >9 had greater improvements in disease activity, with an ACS of e6.71 ± 7.93, compared with patients with baseline CLASI-A scores ≤9, with an ACS of 0.93 ± 4.20 (P < .0001) (Supplemental Table II; available at http://www.jaad.org).
Fig 1.
CLASI-A scores over time for cutaneous lupus erythematosus patients with improving, stable, or worsening disease activity. The following are examples of the disease course of a patient each with improved (A), stable (B), and worsened (C) disease activity over time. CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index activity; Pt, patient.
Among the various CLE subtypes, most patients with localized (61.3%, n = 19) and generalized DLE (50.0%, n = 13) had stable disease, whereas few patients with SCLE had stable disease (23.1%, n = 6). A greater percentage of patients with SCLE (46.2%, n = 12) than generalized (34.6%, n = 9) or localized(32.3%, n = 10) DLE had improved disease. Likewise, a greater percentage of patients with SCLE (30.8%, n = 8) than generalized (15.4%, n = 4) or localized (6.5%, n = 2) DLE had worsened disease (P = .03). There were no significant differences in disease activity outcome variables on the basis of patient sex, race, age, SLE involvement, disease duration, smoking status, or initial CLASI-D score (Supplemental Table II).
Linear regression analyses performed to show predictors of progression of disease activity over time are displayed in Table II. There was significant improvement in CLASI-A scores over time in patients who were black (P = .049) or Hispanic or Asian (P = .02), had a disease duration of 1 year or less (P = .01), had moderate-severe disease activity at baseline (P < .0001), or had high baseline disease damage (P = .0001). The effects of age, sex, CLE subtype, SLE involvement, and smoking status did not play a significant role in predicting progression of disease activity.
Table II.
Predictors of progression of disease activity over time
| Patient characteristic | N | Estimated baseline CLASI-A, mean (SD) | P value for baseline mean | Estimated CLASI-A change per year, mean (SD) | P value for change per year |
|---|---|---|---|---|---|
| Baseline CLASI-A score | |||||
| ≤9 | 50 | 4.11 (0.89) | Referent | 0.22 (0.18) | Referent |
| >9 | 33 | 16.87 (1.08) | <1 × 10−16 | −1.00 (0.24) | 4 × 10−5 |
| Baseline CLASI-D score | |||||
| 0–4 | 36 | 7.24 (1.32) | Referent | 0.09 (0.25) | Referent |
| 5–9 | 19 | 4.89 (1.80) | .29 | 0.67 (0.27) | .11 |
| ≥10 | 28 | 14.42 (1.46) | .0003 | −1.19 (0.23) | .0001 |
| Sex | |||||
| Female | 68 | 9.70 (1.01) | Referent | −0.33 (0.17) | Referent |
| Male | 15 | 7.36 (2.13) | .32 | −0.03 (0.31) | .39 |
| Race | |||||
| White | 49 | 8.48 (1.20) | Referent | 0.07 (0.20) | Referent |
| Black | 29 | 9.60 (1.55) | .57 | −0.54 (0.24) | .049 |
| Other* | 5 | 13.86 (3.72) | .17 | −1.29 (0.53) | .02 |
| Age at first visit, years | |||||
| ≤50 | 46 | 11.03 (1.19) | Referent | −0.33 (0.18) | Referent |
| >50 | 37 | 7.04 (1.34) | .026 | −0.16 (0.24) | .58 |
| CLE subtype | |||||
| Localized DLE | 31 | 5.16 (1.40) | Referent | −0.09 (0.26) | Referent |
| Generalized DLE | 26 | 13.33 (1.51) | 7 × 10−5 | −0.72 (0.22) | .07 |
| SCLE | 26 | 9.71 (1.54) | .03 | −0.23 (0.28) | .40 |
| Systemic LE involvement | |||||
| No | 60 | 8.18 (1.06) | Referent | −0.11 (0.18) | Referent |
| Yes | 23 | 11.94 (1.70) | .06 | −0.57 (0.25) | .14 |
| Disease duration at baseline visit, years | |||||
| ≤1 | 18 | 11.37 (1.98) | Referent | −1.10 (0.37) | Referent |
| >1 | 65 | 8.76 (1.03) | .24 | −0.11 (0.16) | .01 |
| Smoking status | |||||
| Past or never smoker | 50 | 7.25 (1.13) | Referent | −0.25 (0.19) | Referent |
| Current smoker | 33 | 12.32 (1.38) | .004 | −0.28 (0.22) | .91 |
Bold values are statistically significant.
CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index activity; CLASI-D, Cutaneous Lupus Erythematosus Disease Area and Severity Index damage; CLE, cutaneous lupus erythematosus; DLE, discoid lupus erythematosus; LE, lupus erythematosus; SD, standard deviation.
Includes 4 Hispanic/Latino patients and 1 Asian patient.
Characteristics of patients with CLE disease damage trends
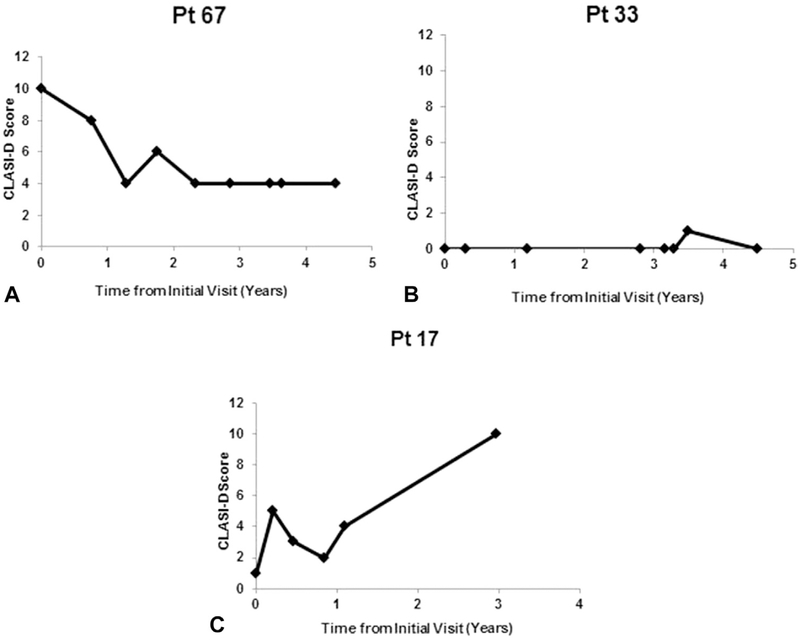
Over time, most CLE patients showed stable (65.1%, n = 54) disease damage trends. Fewer patients demonstrated improved (13.3%, n = 11) or worsened (21.7%, n = 18) disease damage patterns. Individual patient examples of each trend are shown in Fig 2. Of note, improved disease was more often seen in patients with CLASI-D scores ≥10 (35.7%, n = 10) at baseline compared with those with CLASI-D scores of 5–9 (5.3%, n = 1) or 0–4 (0%, n = 0) (P = .0005). Patients with baseline CLASI-D scores of 0–4 and 5–9 tended to have stable skin damage over time compared with those with CLASI-D scores ≥10 (80.6%, n = 29; 68.4%, n = 13; 42.9%, n = 12, respectively; P = .0005). Patients with baseline CLASI-D scores ≥10 (n = 28) had a lower ACS (e1.12 ± 5.02) t scores of 0–4 (1.81 ± 2.35, n = 36) or 5–9 (1.52 ± 3.82, n = 19) (P = .004). Patients with CLE disease duration of 1 year or less at the baseline visit (n = 18; 2.57 ± 3.15) had a higher ACS than those with disease duration [1 year (n = 65; 0.35 ± 4.04; P = .02). There were no significant differences in disease damage outcome variables on the basis of sex, race, age, CLE subtype, SLE involvement, disease duration, smoking status, or initial CLASI-A score (Supplemental Table III; available at http://www.jaad.org).
Fig 2.
CLASI-D scores over time for cutaneous lupus erythematosus patients with improving, stable, and worsening disease damage. The following are examples of the disease course of a patient each with improved (A), stable (B), and worsened (C) skin damage over time. CLASI-D, Cutaneous Lupus Erythematosus Disease Area and Severity Index damage; Pt, patient.
DISCUSSION
Because of the paucity of longitudinal studies that delineate the disease course of CLE over time, this study sought to characterize changes in disease activity and damage of CLE patients on standard-of-care therapies by using the CLASI. Most CLE patients demonstrated improved or stable disease activity and stable disease damage trends. Most patients with moderate-severe disease activity at baseline showed improvement, and those with initially mild disease activity tended to show stable disease activity. Patients with DLE also tended to show more stable disease activity over time than those with SCLE, suggesting that SCLE might have a more variable disease course. Interestingly, significantly higher numbers of CLE patients with initial CLASI-D score ≥10 showed improved disease damage, and those with milder damage tended to have stable skin damage.
Most patients in this study had mild disease activity at baseline (CLASI-A ≤9) and lower CLASI-D scores. Similar results were seen in those with moderate-severe disease activity at baseline, who also had greater disease damage at the initial visit. Patients with high baseline damage (CLASI-D ≥10) tended to be black and current smokers with generalized DLE and SLE involvement. A previous study showed that black patients tended to have DLE and experienced damage early in their disease course, frequently in conjunction with disease activity.11 In another study, it was found that among the different CLE subtypes, patients with DLE were noted to have greater degrees of disease damage due to dyspigmentation and scarring.12 Smoking is associated with CLE in general, especially DLE,13 and smokers with CLE tend to have greater disease activity than nonsmokers.14 In a recent study by Kuhn et al,15 the total CLASI activity and damage scores of patients with CLE was higher in those who had ever smoked than in nonsmokers. This is also reflected in our study, where smokers showed greater disease damage early in the disease course.
In comparison with patients with mild disease activity, more patients with moderate-severe disease activity (with CLASI-A scores >9) showed improved disease activity over time. In contrast, most of those with baseline mild activity (CLASI-A ≤9) showed stable disease activity over time. Any significant change in activity might be better appreciated in those with moderate-severe disease. Those with mild disease have a floor effect where significant changes in disease activity can be more difficult to detect when starting with low disease activity scores. To further account for these differences, we hypothesize that patients with higher disease activity at baseline were more likely to receive aggressive immunosuppressant treatments.
Patients with DLE (localized or generalized) had a stable disease activity course over time. Although this might be acceptable for those with minimal disease activity, this highlights the refractory nature of DLE in patients with more severe disease. This also underscores the need for newer therapies that can effectively treat this refractory group. We also found that SCLE patients had a more variable disease course, with most (46.2%) demonstrating improved disease, and others (30.8%) showing worsened disease over time. The relatively higher percentage of SCLE patients with progressing disease activity also emphasizes a gap in existing therapies in effectively treating SCLE. Furthermore, our patients with CLE are initially treated with a combination of antimalarials (eg, hydroxychloroquine or chloroquine ± quinacrine). For those who show lack of significant improvement, varying doses of prednisone; other steroid-sparing immunosuppressants (most commonly, methotrexate, mycophenolate mofetil, and thalidomide); or both are added. Despite following these treatment regimens, we found a sizeable portion of our CLE patients had worsening disease activity trends and required additional treatments.
About 13% of our CLE patients showed improving trends in their skin damage. A prior study showed that overall median disease damage in CLE did not improve over time.11 However, in the current study, when grouping by CLASI damage severity, significantly more patients with high CLASI damage scores (CLASI-D ≥10) showed improved disease damage trends and negative ACS of CLASI-D scores. While this might be partially attributed to the floor effect associated with those with lower baseline CLASI-D scores, our data provides some hope to patients with significant disease damage that their chronic skin lesions can improve with current existing therapies.
We found significant improvement in disease activity over time in patients of racial minorities, patients with high disease activity and damage at baseline, and patients with short disease duration of 1 year or less at baseline. Patients seeking treatment early in the disease course likely received timely appropriate treatment, resulting in improved disease activity. Because patients of minority races started with higher scores at baseline compared with white patients, they had greater room for improvement, which likely contributed to their significant reduction in disease activity. In contrast to our findings, studies on minorities with SLE have demonstrated worse prognosis, particularly in those with lupus nephritis.16,17 Whether or not the skin behaves differently than other organs in minorities needs to be further explored with larger scale studies.
Limitations of this study include referral bias; our tertiary care centers receive outside referrals for refractory patients and, thus, have higher numbers of patients with moderate-severe disease activity at baseline. The sample size in our study was small due to the required follow-up times of at least 2 years. Some patients had large gaps between visits, resulting in missing information about disease course. Information on therapy in terms of the types and doses of treatment, duration, and compliance were not included in this study. This might have resulted in a potential for confounding of unmeasured variables, which in turn affected disease course.
In conclusion, over a long-term period, our CLE patient cohort mostly demonstrated improving or stable disease activity and stable disease damage trends. High baseline disease activity, minority race, and short duration of disease predict CLE disease activity improvement. High baseline disease damage is associated with CLE damage improvement. This natural disease course data may be used as historical controls for future clinical trials.
Supplementary Material
CAPSULE SUMMARY.
There are few longitudinal studies characterizing disease activity and damage of cutaneous lupus erythematosus (CLE) patients.
In this study, the Cutaneous Lupus Erythematosus Disease Area and Severity Index was used to delineate the disease course of CLE patients on standard-of-care treatments.
Having high baseline disease activity, minority race, or CLE disease duration <1 year predict CLE activity improvement.
Acknowledgments
Funding sources: Supported by an internal grant awarded by Biogen Incorporated, the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (award no. K23AR061441 to Dr Chong and K24AR02207 to Dr Werth), the Rheumatology Research Foundation Career Development Bridge Funding Award (to Dr Chong) and the United States Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development) (to Dr Werth).
Abbreviations used:
- ACS
average change score
- CLASI
Cutaneous Lupus Erythematosus Disease Area and Severity Index
- CLASI-A
Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity
- CLASI-D
Cutaneous Lupus Erythematosus Disease Area and Severity Index Damage
- CLE
cutaneous lupus erythematosus
- DLE
discoid lupus erythematosus
- SCLE
subacute lupus erythematosus
- SLE
systemic lupus erythematosus
Footnotes
Conflicts of interest: Dr Chong has received research grants (paid to his institution) from Biogen Incorporated and Daavlin Corporation. Dr Chong is an investigator for Pfizer Incorporated and has also received an honorarium from Celgene Corporation. Dr Werth has received research grants from Celgene Corporation, Janssen, Pfizer, Biogen, Corbus Pharmaceuticals, LuCIN, Genentech, Syntimmune, and AstraZeneca and honoraria from Celgene, Medimmune, Resolve, Neovacs, ACI, Immune Pharmaceuticals, Genetech, Idera, Octapharma, BSL Behring, Janssen, Lilly, Pfizer, Biogen, BMS, Biostrategies, Gilead, Amgen, Medscape, Principia, Nektar, Syntimmune, Incyte, and EMD Sorona. The University of Pennsylvania owns the copyright for the Cutaneous Lupus Erythematosus Disease Area and Severity Index. The remaining authors have no conflicts of interest to disclose.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of The University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, University of Pennsylvania School of Medicine, the National Center for Research Resources, the National Institutes of Health, and the Rheumatology Research Foundation.
REFERENCES
- 1.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilla-Martinez ZL, Albrecht J, Troxel AB, et al. The Cutaneous Lupus Erythematosus Disease Area and Severity Index: a responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol. 2008;144:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626–632. [DOI] [PubMed] [Google Scholar]
- 4.Kreuter A, Gaifullina R, Tigges C, et al. Lupus erythematosus tumidus: response to antimalarial treatment in 36 patients with emphasis on smoking. Arch Dermatol. 2009;145:244–248. [DOI] [PubMed] [Google Scholar]
- 5.Kreuter A, Tomi NS, Weiner SM, et al. Mycophenolate sodium for subacute cutaneous lupus erythematosus resistant to standard therapy. Br J Dermatol. 2007;156:1321–1327. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbach MOJ, Krathen M, Braunstein I, et al. Clinical and histopathological analysis of subjects treated with lenalidomide for refractory chronic cutaneous lupus erythematosus [abstract]. J Invest Dermatol. 2009;129:S22. [Google Scholar]
- 7.Chang AY, Piette EW, Foering KP, et al. Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis. Arch Dermatol. 2011;147:1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokogawa N, Kato Y, Sugii S, et al. Response to hydroxychloroquine in Japanese patients with systemic lupus erythematosus using the cutaneous lupus erythematosus disease area and severity index (CLASI). Mod Rheumatol. 2012;22:249–255. [DOI] [PubMed] [Google Scholar]
- 9.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. [DOI] [PubMed] [Google Scholar]
- 10.Chansky PB, Olazagasti JM, Feng R, et al. Cutaneous dermatomyositis disease course followed over time using the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI). J Am Acad Dermatol. October 21, 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma SM, Okawa J, Propert KJ, et al. The impact of skin damage due to cutaneous lupus on quality of life. Br J Dermatol. 2014;170:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santiago-Casas Y, Vilá LM, McGwin G Jr, et al. Association of discoid lupus erythematosus with clinical manifestations and damage accrual in a multiethnic lupus cohort. Arthritis Care Res (Hoboken). 2012;64:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miot HA, Bartoli Miot LD, Haddad GR. Association between discoid lupus erythematosus and cigarette smoking. Dermatology. 2005;211:118–122. [DOI] [PubMed] [Google Scholar]
- 14.Piette EW, Foering KP, Chang AY, et al. Impact of smoking in cutaneous lupus erythematosus. Arch Dermatol. 2012;148: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn A, Sigges J, Biazar C, et al. Influence of smoking on disease severity and antimalarial therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. Br J Dermatol. 2014;171:571–579. [DOI] [PubMed] [Google Scholar]
- 16.Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006; 69:1846–1851. [DOI] [PubMed] [Google Scholar]
- 17.Alarcón GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus. 1999;8:197–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.