SUMMARY
Lymphoma incidence and survival in adolescent and young adult (AYA, defined as 15 to 39 years of age) and adult patients (> 39 years) were assessed using Surveillance, Epidemiology, and End Results (SEER) data. From 2000 to 2014, 431,721 lymphoma cases were reported to SEER, 9% in AYA patients. In the AYA group, the highest age-adjusted incidence rate was for classical Hodgkin lymphoma (HL; 3.4 per 100,000 person-years; 95% confidence interval [CI], 3.38–3.49) followed by diffuse large B cell lymphoma (DLBCL; 1.56, 95% CI,1.53–1.60) and for adults, it was plasma cell neoplasms (14.17, 95% CI, 14.07–14.27), DLBCL (13.86, 95% CI, 13.76–13.96) and chronic lymphocytic leukaemia (CLL; 13.19, 95% CI, 13.09–13.29). HL comprised 42% of lymphoma cases for AYAs, but only 4% in adults. The occurrence of DLBCL among AYAs and adults was similar, 18% and 20%, respectively. Twenty-eight percent of AYAs compared with 9% of adults presented with stage II disease, and 21% of AYAs compared with 10% of adults had B-symptoms. Extranodal disease was less common (33%) in AYAs than adults (59%). Overall, AYA patients with lymphoma have better 2- and 5-year relative survival rates (RSRs) to adults. When restricted to HL and DLBCL, RSR of AYA patients exceeds adult RSR.
Keywords: Adolescent and young adult (AYA), Hodgkin lymphoma, non-Hodgkin lymphoma, Surveillance Epidemiology and End Results (SEER), epidemiology, incidence, survival
INTRODUCTION
According to a recent Surveillance, Epidemiology, and End Results (SEER) monograph (Bleyer et al, 2006), the most common malignancies in adolescent and young adult (AYA) patients include lymphomas (20%), melanoma (11%), testicular cancer (11%), thyroid (11%), female genital tract (10%), sarcoma (8%), leukaemia (6%), central nervous system tumours (6%), and breast cancer (5%). With respect to lymphomas in AYA patients, Hodgkin lymphoma (HL) is the most common lymphoid malignancy, accounting for 12% of lymphoma cases and non-Hodgkin lymphoma (NHL) comprises about 7% of cases. However, a comprehensive description of these lymphoid malignancies by World Health Organization (WHO) subtype (Swerdlow et al, 2008; Swerdlow et al, 2016) in the AYA population with a comparison to the adult population has not been conducted. Ultimately, an understanding of the differences in incidence and survival of specific HL and NHL subtypes in the AYA population will help to direct future studies designed to examine differences in the biology, treatment and long-term outcomes of AYA and adult patients with lymphoma.
An understanding of the scope and incidence of specific WHO lymphoma subtypes in the AYA patient population is critical as several studies suggest that this patient group may have inferior outcomes compared to adult patients; or at least slower rates of survival improvements with treatment advances (Henderson et al, 2018; Kahn et al, 2016; Keegan et al, 2016). Data specifically evaluating treatment approaches in AYA lymphomas is currently limited to two studies (Henderson et al, 2018; Giulino-Roth et al, 2017) A recent retrospective study compared AYA HL patients aged 17–21 years treated on a Children’s Oncology Group (COG) protocol with patients in the same age group treated on an adult Eastern Cooperative Oncology Group (ECOG) HL trial, demonstrating an improved 3-year failure-free survival (FFS) of 83% with the COG approach versus 70% on the adult protocol (Henderson et al, 2018). In the NHL category, one multi-centre retrospective study reported results on 156 AYA and adult patients treated with dose adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab (DA-EPOCH-R) for primary mediastinal B-cell lymphoma (Giulino-Roth et al, 2017). Currently, no comparative data on outcomes of AYA patients with other common subtypes of NHL, including diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma, follicular lymphoma (FL) and anaplastic large T-cell lymphoma (ALCL) treated with paediatric versus adult type therapy exists. Furthermore, little is known on the biology, pathology and distribution of specific HL and NHL subtypes in the AYA patient group.
Therefore, this analysis sought to determine the incidence rates, sex and race distribution, and disease characteristics of specific lymphoid neoplasms, as defined by the WHO classification (Swerdlow et al 2008), in AYA patients aged 15–39 years in comparison to adult patients aged 40 years and older diagnosed in the United States (US). In addition, this study reports survival statistics for these specific lymphoma subtypes by age group (AYA vs. adult).
METHODS
Data Source
This project uses SEER data with incidence case data from 11 states (California, Connecticut, Georgia, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, Utah and Hawaii), six metropolitan areas (Detroit, Seattle/Puget Sound, San-Francisco/Oakland, Los Angeles, San Jose/Monterey and Atlanta), and the Native American population in Arizona. The data collected by SEER from these areas include patient demographics and characteristics, such as age, sex, race, anatomic site, and histology of the cancer. There are a total of 18 registries, accounting for approximately 28% of the US population (National Cancer Institute, 2017 a). For this analysis, we examined cases identified between 2000 and 2014 in 18 SEER registries (National Cancer Institute, 2017 b)
Study Cohort
Lymphoma cases were identified using the 2008 World Health Organization (WHO) classification (Swerdlow et al, 2008) and the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes reported by the 18 SEER registries. The updated WHO manual, released in 2016 (Swerdlow et al, 2016), was not utilized for this project because the surveillance data available in SEER are up to the end of 2014 and do not reflect the 2016 updates. The ability to re-classify cases according to 2016 WHO criteria is limited due to requirements for additional molecular or genetic testing; however, the 2016 changes to the WHO lymphoid classification should not greatly impact the overall findings and conclusions on the incidence and survival of AYA HL and NHL in the US observed in this analysis. The lymphoma subtypes and the selection of lymphoma cases for analysis of incidence and survival outcomes are depicted in Table I.
TABLE I.
Age-Adjusted Incidence Rates of Lymphoma in the AYA (age 15–39 years) and adult (age > 39 years) patient populations
| AYA | Adult | ||||
|---|---|---|---|---|---|
| Lymphoma Subtypes | ICD-0–3 codes | N | Age-Adjusted Incidence Rate per 100,000 person-years (95% CI) |
N | Age-Adjusted Incidence Rate per 100,000 person- years (95% CI) |
| Hodgkin lymphoma | |||||
| Classical Hodgkin lymphoma | 9650–9655, 9663– 9667, 9661,9662 |
15451 | 3.44 (3.38–3.49) | 15249 | 2.82 (2.77–2.86) |
| Nodular lymphocyte predominant Hodgkin lymphoma |
9659 | 638 | 0.14 (0.13–0.16) | 949 | 0.17 (0.16–0.18) |
| Non-Hodgkin lymphoma, B-cell | |||||
| Precursor B-cell lymphoblastic leukaemia/lymphoma, NOS |
9727 (B), 9728, 9835(B), 9836, 9811 |
3176 | 0.71 (0.69–0.74) | 4336 | 0.79 (0.76–0.81) |
| Precursor B-cell lymphoblastic leukaemia/lymphoma, with recurrent genetic abnormalities |
9812–9818 | 110 | 0.025 (0.02–0.03) | 212 | 0.038 (0.03–0.04) |
| Chronic lymphocytic leukaemia/small lymphocytic lymphoma |
9670, 9823 | 617 | 0.15 (0.14–0.16) | 70785 | 13.19 (13.09–13.29) |
| Prolymphocytic leukaemia, B-cell | 9832 (B), 9833 | NA | 0.002 (0.001–0.004) | 296 | 0.056 (0.05–0.06) |
| Mantle cell lymphoma | 9673 | 86 | 0.021 (0.017–0.026) | 9453 | 1.752 (1.12–1.79) |
| Lymphoplasmacytic lymphoma, including Waldenstrom macroglobulinaemia |
9671, 9761 | 75 | 0.018 (0.014–0.022) | 7817 | 1.47(1.44–1.50) |
| DLBCL, NOS | 9684 (B), 9680 (excluding C44.0– 44.9, C49.9, C71.0– 71.9) |
6289 | 1.45 (1.41–1.49) | 73346 | 13.7 (13.6–13.8) |
| Primary DLBCL of the CNS | 9680 (C71.0–71.9) | 246 | 0.058 (0.051–0.065) | 3109 | 0.578 (0.56–0.60) |
| Primary cutaneous DLBCL, leg type | 9680 (C44.0–44.9) | 56 | 0.013 (0.010–0.017) | 1317 | 0.25 (0.23–0.26) |
| T-cell/histiocycte rich large B-cell lymphoma | 9688 | 54 | 0.012 (0.009–0.016) | 208 | 0.038 (0.033– 0.044) |
| Intravascular large B-cell lymphoma | 9712, 9680 (C49.9) | NA | 45 | 0.008 (0.006– 0.011) |
|
| ALK positive large B-cell lymphoma | 9737 | NA | 0.002 (0.001–0.003) | NA | 0.002 (0.001– 0.004) |
| Plasmablastic lymphoma | 9735 | 58 | 0.013 (0.010–0.017) | 267 | 0.048 (0.043–0.055) |
| Large B-cell lymphoma HHV8-associated multicentric Castleman disease |
9738 | NA | 0.002 (0.001–0.004) | 27 | 0.005 (0.003– 0.007) |
| Primary effusion lymphoma | 9678 | 25 | 0.006 (0.004–0.008) | 107 | 0.02 (0.016–0.024) |
| Primary mediastinal (thymic) large B-cell lymphoma |
9679 | 349 | 0.079 (0.071–0.88) | 220 | 0.041 (0.036– 0.047) |
| Burkitt lymphoma/leukaemia | 9687, 9826 | 1157 | 0.26 (0.25–0.28) | 2654 | 0.49 (0.47–0.51) |
| Marginal Zone lymphoma | 9689, 9699, 9764 | 1023 | 0.24 (0.22–0.25) | 22128 | 4.10 (4.05–4.16) |
| Follicular lymphoma | 9690, 9691, 9695, 9688 |
2095 | 0.5 (0.49–0.52) | 42497 | 7.78 (7.70–7.85) |
| Hairy cell leukaemia | 9940 | 307 | 0.075 (0.067–0.084) | 3402 | 0.62 (0.60–0.64) |
| Hairy cell leukaemia variant | 9591 (site 42.1–32.2) | 18 | 0.004 (0.002–0.007) | 1651 | 0.31 (0.30–0.33) |
| Plasma cell neoplasms | 9731, 9734, 9732, 9733 |
1129 | 0.27 (0.26–0.29) | 76200 | 14.17 (14.07–14.27) |
| B-cell lymphoid neoplasms, NOS | 9590 (B), 9591 (B), 9675 (B), 9820 (B), 9970 (B) |
880 | 0.20 (0.19–0.22) | 18328 | 3.73 (3.39–3.49) |
| Non-Hodgkin lymphoma, T/NK-cell | |||||
| Precursor T/NK-cell lymphoblastic leukaemia/lymphoma, NOS |
9727 (T), 9729, 9835(T), 9837 |
1277 | 0.28 (0.27–0.30) | 817 | 0.15 (0.14–0.16) |
| Mycosis Fungoides/Sezary syndrome | 9700, 9701 | 742 | 0.17 (0.16–0.18) | 4718 | 0.86 (0.84–0.89) |
| Peripheral T/NK-cell lymphoma | 9702, 9705, 9716, 9717, 9714, 9724, 9726, 9708, 9709 |
1595 | 0.37 (0.35–0.39) | 11019 | 2.04 (2.00–2.08) |
| Adult T-cell leukaemia/lymphoma | 9827 | 66 | 0.015 (0.012–0.019) | 385 | 0.071 (0.064– 0.079) |
| Extranodal NK/T-cell lymphoma, nasal type | 9719 | 206 | 0.047 (0.04–0.05) | 591 | 0.107 (0.099– 0.116) |
| T-cell large granular lymphocytic leukaemia | 9831 | NA | 0.003 (0.002–0.005) | 193 | 0.036 (0.031– 0.042) |
| T-cell prolymphocytic leukaemia | 9834, 9832(T) | NA | 0.003 (0.001–0.005) | 402 | 0.075 (0.067– 0.082) |
| Aggressive NK-cell leukaemia | 9948 | NA | 0.003 (0.002–0.006) | 88 | 0.016 (0.013–0.020) |
| Primary cutaneous CD30 + lymphoproliferative disorders |
9718 | 157 | 0.036 (0.31–0.42) | 950 | 0.17 (0.16–0.19) |
| T/NK-cell lymphoid neoplasms, NOS | 9591(T), 9590(T), 9684(T), 9820(T), 9970(T) |
63 | 0.014 (0.011–0.018) | 546 | 0.102 (0.093–0.110) |
AYA: adolescent and young adult; CI: confidence interval; DLBCL: diffuse large B cell lymphoma; HHV8: human herpes virus 8; ICD-0–3: International Classification of Diseases for Oncology, third edition; NA: not available, too few cases to be displayed; NK: Natural Killer; NOS: not otherwise specified
Incidence Calculations
The analysis was restricted to lymphoma cases that were microscopically confirmed with the histology code ending in “/3”. Patients with lymphoma with unknown age (n=0), unknown race (n= 5,830), age <15 years (n=13,033), human immunodeficiency virus (HIV) positive (n=449), and diagnosis based on death certificate or autopsy only (n=0) were excluded from the incidence analysis (Figure 1). However, it is important to recognize that reporting of HIV status varies by SEER registry and lymphoid malignancy and there is no reporting requirement for follow-up or to record a later HIV diagnosis following the cancer diagnosis (Howlader et al, 2016). Cases with known race were classified as white, black or other (Asian, Pacific Islander, and American Indian or Alaska Native). Examination of Hispanic ethnicity was beyond the scope of this study, because ethnicity was not recorded reliably during the time period. Cases were classified into two groups: AYA patients, who were 15–39 years of age per National Cancer Institute guidelines (National Institutes of Health et al, 2006) (n=38,013), and adult patients, defined as 40 years and older (n=374,396). Data on patient age at diagnosis, gender, race, Ann Arbor stage, presence of extranodal disease and occurrence of B-symptoms were collected. Data were missing regarding stage in 178,671 cases, B-symptoms in 262,919 cases and extranodal disease in 651 cases.
Figure 1. Incidence Analysis and Survival Analysis.
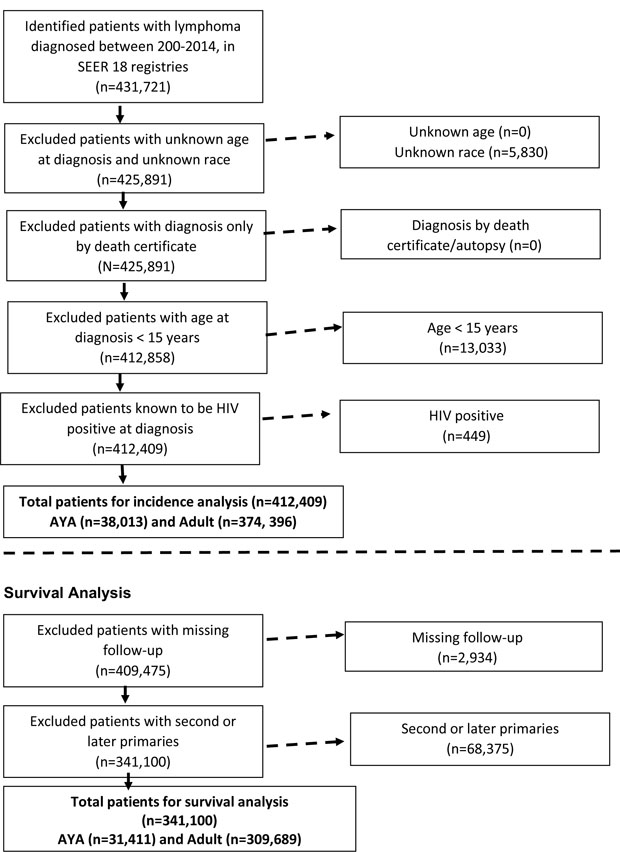
AYA: adolescent and young adult; HIV: human immunodeficiency syndrome; SEER: Surveillance, Epidemiology, and End Results.
The age-adjusted incidence rates for the lymphoma subtypes were expressed as new cases per 100,000 person-years and adjusted using the 2000 US standard population. Two-sided T-tests and Chi-squared tests were calculated to compare baseline characteristics at diagnosis across age groups. Trends in incidence rates of selected subtypes of lymphoma (HL, B-NHL, and T-NHL) in AYA and adult patients from 2000–2014 were assessed.
Survival calculations
For the survival analyses, the survival time was calculated using the date of diagnosis and one of the following as the end point: date of death, date last known to be alive, or date of study cut-off (31 December 2014). Patients who were not actively followed (n=2,934) or with second or later malignancies (n=68,375) were excluded for the survival analysis (Figure 1). The final cohort for the survival analyses was 341,100 cases, including 31,411 AYA patients.
Two- and five-year relative survival rates (RSRs) were calculated by actuarial methods and Z test was used to test the equivalence. Relative survival represents cancer survival in the absence of other causes of death and is defined as the proportion of observed survivors in a cohort of cancer patients compared to the proportion of expected survivors in a comparable set of cancer-free individuals. The expected survival rate at each point of follow-up was calculated so that the matched individuals were considered to be at risk until the corresponding cancer patient died or was censored. Kaplan-Meier survival curves were constructed and compared using the log-rank test. A level of significance (α) of 0.05 was considered statistically significant. All statistics were computed using the National Cancer Institute SEER*Stat software, version 8.3.4 (www.seer.cancer.gov/seerstat), and SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Incidence of lymphoma in AYA and adult patients
A total of 38,013 and 374,396 cases of lymphoma were identified in the AYA and adult patient cohorts, respectively. Classical HL was most common AYA lymphoma with 15,451 cases and an age-adjusted incidence rate of 3.44 per 100,000 person-years (Table I). DLBCL not otherwise specified (NOS) was the second most commonly encountered lymphoid neoplasm in this age group, with 6,291 cases (1.45 age-adjusted incidence rate). In the adult population, plasma cell neoplasms occurred most frequently (76,200 cases, age-adjusted incidence rate of 14.17), followed by DLBCL NOS (73,419 cases, age-adjusted incidence rate of 13.7) and chronic lymphocytic leukaemia (CLL, 70,785 cases, age-adjusted incidence rate of 13.19). Interestingly, the total number of classical HL cases in the adult population was 15,249, very similar to the AYA group, with an age-adjusted incidence rate of 2.82. Distribution of lymphoma cases by age group is depicted in Figure 2, with HL and DLBCL representing 42% and 18% of lymphoma cases for AYA patients, and DLBCL, plasma cell neoplasms and CLL each representing about 20% of lymphoma cases in adults. The incidence of HL, B-NHL, and T-NHL has remained relatively stable for both AYA and adult patients from 2000–2014 and these trends are provided in Figure 3.
Figure 2. Distribution of lymphoma subtypes among AYA (15–39 years) and adult patients (> 39 years).
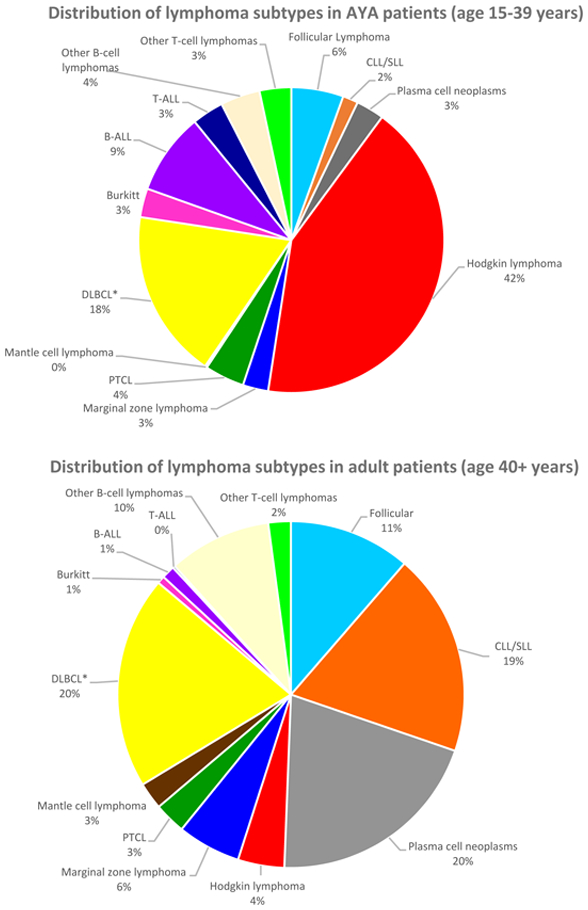
*DLBCL includes DLBCL not otherwise specified (NOS), T-cell/histiocyte rich B-NHL, intravascular B-NHL and ALK+ B-NHL. DLBCL excludes primary DLBCL of central nervous system and primary cutaneous DLBCL, leg type.
ALL: acute lymphoblastic leukaemia; CLL: chronic lymphocytic leukaemia; DLBCL: diffuse large b cell lymphoma; NHL: Non-Hodgkin lymphoma; PTCL: peripheral T-cell lymphoma; SLL: small lymphocytic lymphoma.
Figure 3. Incidence rate trends for Hodgkin lymphoma, B-NHL, and T-NHL in AYA (15–39 years) and adult patients (> 39 years) from 2000–2014.
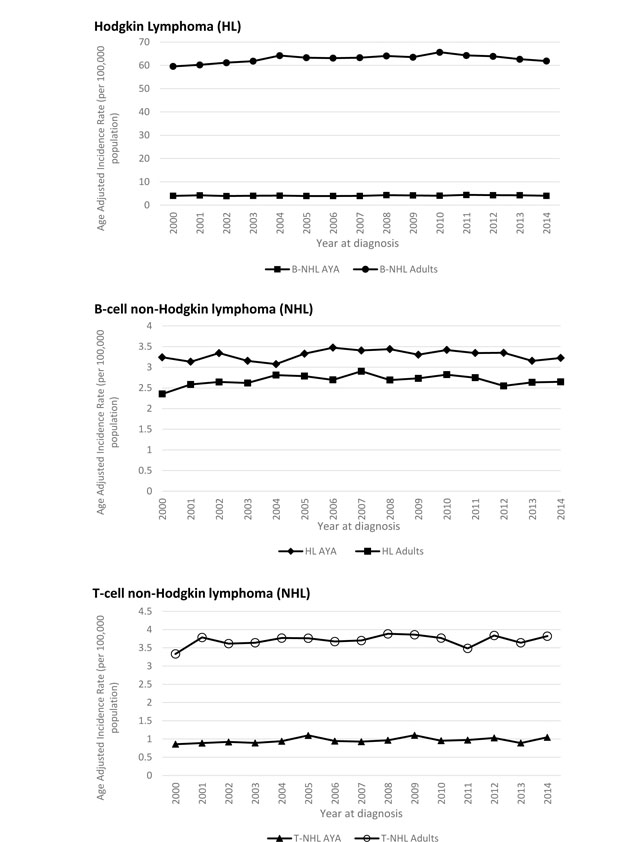
AYA: adolescent and young adult; NHL: Non-Hodgkin lymphoma.
Patient characteristics
For the AYA patient group, the mean age at diagnosis was 28 years and in adults, the mean age was 68 years (Table II). Fifty-seven and 55% of patients were male in the AYA and adult cohorts, and 78% and 85% of patients were white, respectively. More patients in the AYA group had stage II disease at diagnosis, 28% compared to 9% of adults. Extranodal disease was more common in the adult population, but B-symptoms were more frequently encountered in patients 15–39 years. Patient characteristics for AYA patients with HL, DLBCL, Burkitt lymphoma, and FL are shown in Table III.
Table II.
Adolescent and young adult (AYA) and adult lymphoma patient characteristics
| AYA (15–39 years) | Adult (40 years and older) | |||
|---|---|---|---|---|
| Disease Characteristics |
N | % | N | % |
| Number | 38,013 | 9 | 374,396 | 91 |
| Age at diagnosis (years) | ||||
| Mean | 28 | 68 | ||
| Median | 29 | 68 | ||
| Male | 21,719 | 57.0 | 207,573 | 55.0 |
| Race | ||||
| White | 29,840 | 78.0 | 317,847 | 85.0 |
| Black | 5,241 | 14.0 | 35,794 | 10.0 |
| Other | 2,932 | 8.0 | 20,755 | 5.0 |
| Stage | ||||
| I | 6,971 | 18.3 | 58,079 | 15.5 |
| I | 10,745 | 28.3 | 34,779 | 9.3 |
| III | 4,804 | 12.6 | 35,954 | 9.6 |
| IV | 7,319 | 19.3 | 75,087 | 20.1 |
| Missing | 8,174 | 21.5 | 170,497 | 45.5 |
| Extranodal | ||||
| Yes | 12,545 | 33.0 | 222,164 | 59.3 |
| No | 25,441 | 66.9 | 151,608 | 40.5 |
| Missing | 27 | 0.1 | 624 | 0.2 |
| B-symptoms | ||||
| Yes | 8,141 | 21.4 | 36,182 | 9.7 |
| No | 11,639 | 30.6 | 93,528 | 25.0 |
| Missing | 18,233 | 48.0 | 244,686 | 65.4 |
*
Table III.
AYA patient and disease characteristics by lymphoma subtype (think about deleting this vs. adding other most common subtypes – precursor B-ALL, MZL, PCN, T-ALL, Peripheral T cell NHL)
| HL | DLBCL | BL | FL | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | N | % | N | % | N | % | N | % |
| N | 16,089 | 7,096 | 1,157 | 2,095 | ||||
| Male | 8,247 | 51% | 4,281 | 60% | 909 | 79% | 1,124 | 54% |
| Stage | ||||||||
| I | 2,348 | 15% | 2,154 | 30% | 254 | 22% | 564 | 27% |
| II | 7,731 | 48% | 1,753 | 25% | 191 | 17% | 314 | 15% |
| III | 2,891 | 18% | 826 | 12% | 129 | 11% | 442 | 21% |
| IV | 2,427 | 15% | 2,102 | 30% | 543 | 47% | 649 | 31% |
| Missing | 692 | 4% | 261 | 4% | 40 | 3% | 126 | 6% |
| Extranodal | ||||||||
| Yes | 380 | 2% | 2,701 | 38% | 313 | 27% | 295 | 14% |
| No | 15,709 | 98% | 4,383 | 62% | 839 | 73% | 1,800 | 86% |
| Missing | 0 | 0% | 12 | 0% | 5 | 0% | 0 | 0% |
| B symptoms | ||||||||
| Yes | 4,748 | 30% | 1,723 | 24% | 334 | 29% | 288 | 14% |
| No | 5,611 | 35% | 2,728 | 38% | 454 | 39% | 887 | 42% |
| Missing | 5730 | 35% | 2645 | 37% | 369 | 32% | 920 | 44% |
ALL: acute lymphoblastic leukaemia; AYA: adolescent and young adult; BL: Burkitt lymphoma; DLBCL: diffuse large B cell lymphoma; FL: follicular lymphoma; HL: Hodgkin lymphoma; MZL: marginal zone lymphoma; NHL: Non-Hodgkin lymphoma; PCN: plasma cell neoplasm
Incidence rates by age, sex and race
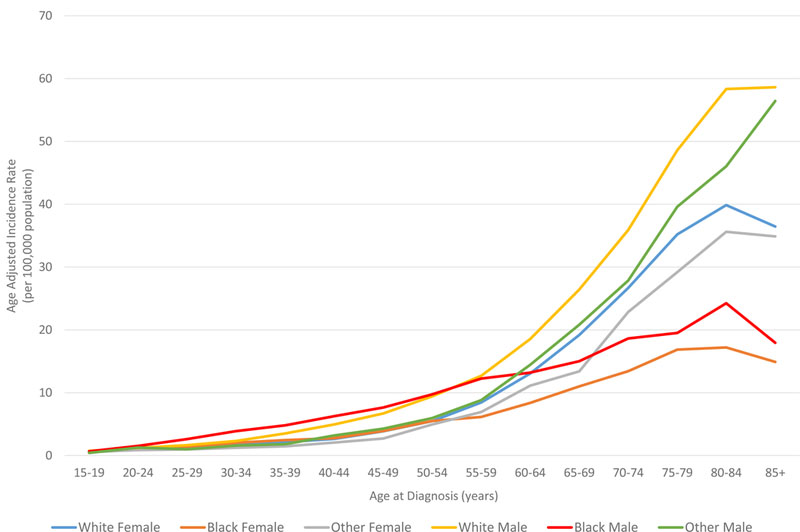
In all races, the incidence of lymphoma increased from age 15 to 70 years (Figures 4A and5A). Men had higher rates of lymphoma across all age groups (Figure 4A), with the highest incidences in black men from age 25–65 years (Figures 4A and 5A). After 65 years of age, the number of cases of lymphoma declined in black patients while continuing to rise in whites (Figure 5A). In AYA patients, HL was more commonly observed in white males and females aged 15–25 years, with similar incidences in whites and blacks aged 25–39 years (Figure 4C, 5C, and 6E). In contrast, the distribution of NHL cases for patients aged 15–35 years was similar across the races, although men were more commonly affected than women (Figures 4B and 5B). For DLBCL, after the age of 20 years, the age-adjusted incidence was higher for black males until 59 years of age, at which point the incidence of DLBCL in white males quickly exceeded all other groups and continued to rise until 80 years of age (Figure 6A and6B). In FL, however, white males and females were most affected after the age of 30 years, but the incidence was relatively similar across the races for patients aged 15–30 years (Figure 6C and 6D).
Figure 4. A. Race and sex-specific age-adjusted incidence rates of lymphoma (HL and NHL) by age at diagnosis. B. Race and sex-specific age-adjusted incidence rates of NHL for the AYA cohort. C. Race and sex-specific age adjusted incidence rates of HL for the AYA cohort.
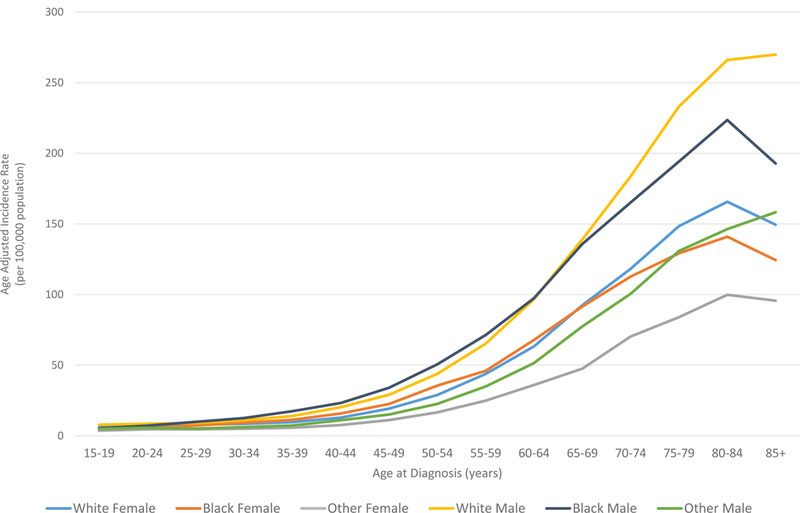
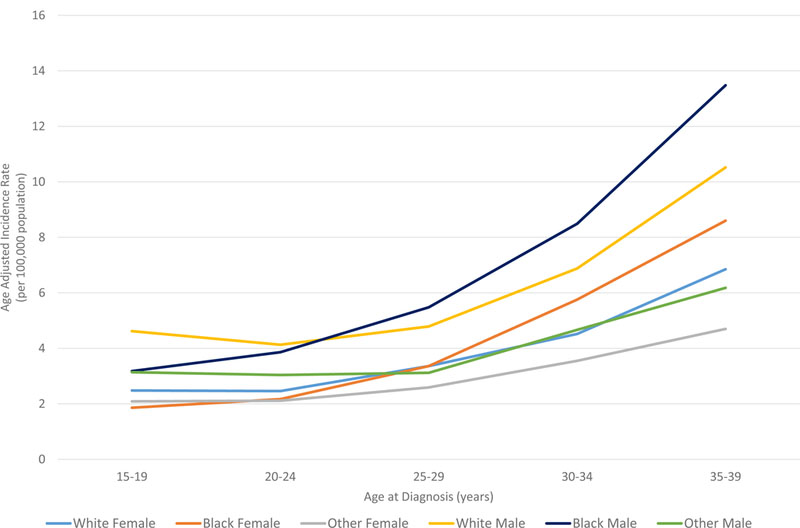
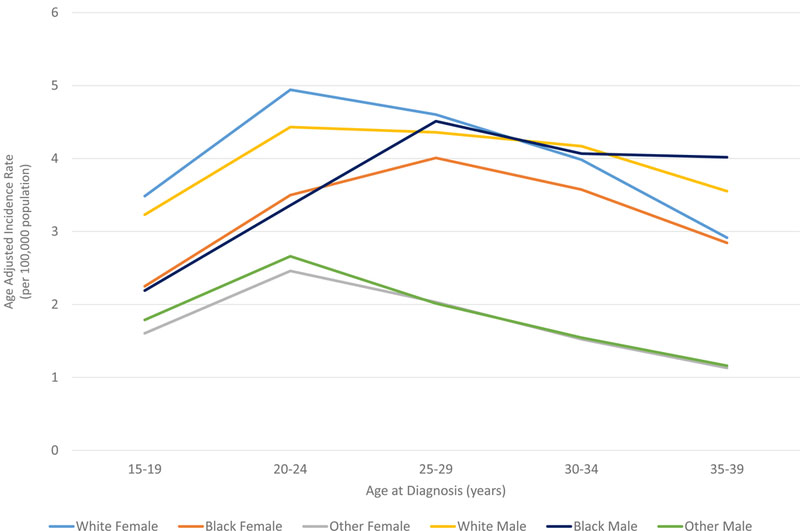
AYA: adolescent and young adult; HL: Hodgkin lymphoma; NHL: Non-Hodgkin lymphoma.
Figure 5. A. Age distribution of lymphoma (HL and NHL) cases shown by race. B. Age distribution of NHL cases for the AYA cohort. C. Age distribution of HL cases for the AYA cohort.
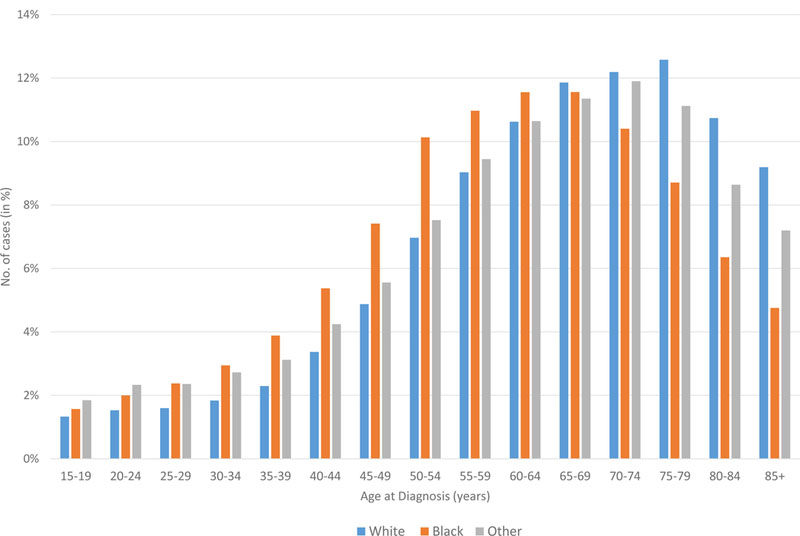
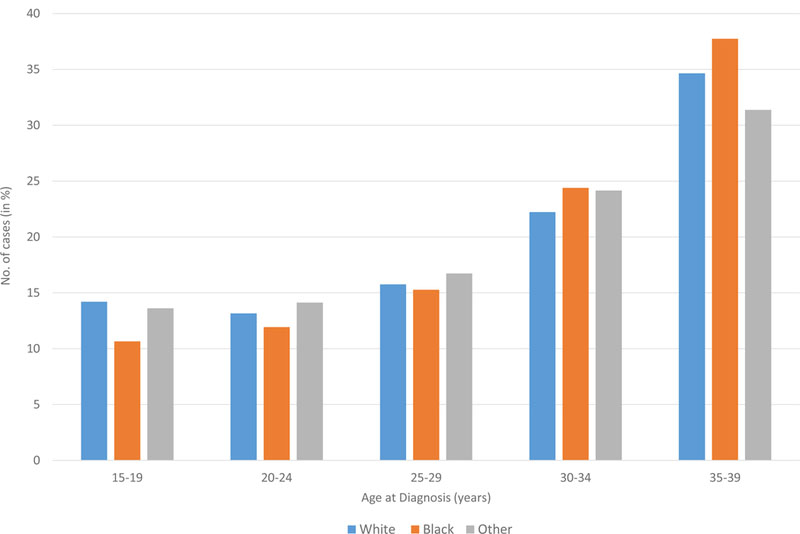
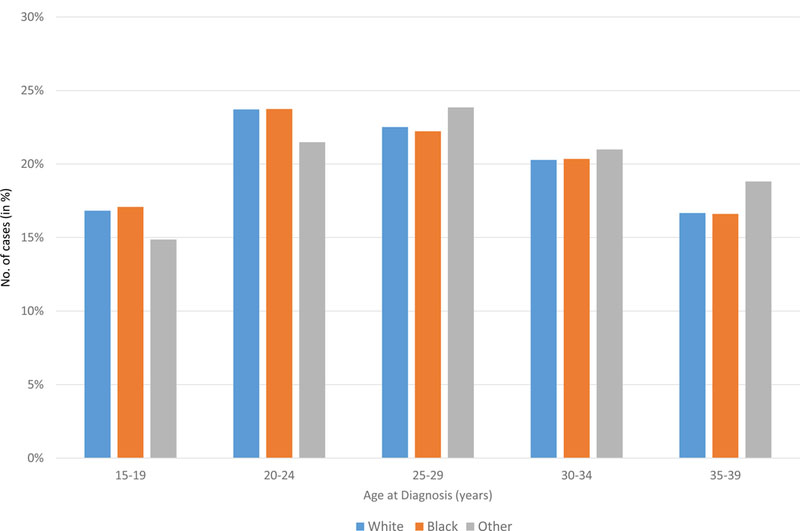
AYA: adolescent and young adult; HL: Hodgkin lymphoma; NHL: Non-Hodgkin lymphoma.
Figure 6. A. Race and sex-specific age-adjusted incidence rates for DLBCL at age of diagnosis . B. Race and sex-specific age-adjusted incidence rates for DLBCL in AYA cohort. C. Race and sex-specific age-adjusted incidence rates for FL at age of diagnosis. D. Race and sex-specific age-adjusted incidence rates for FL in AYA cohort. E. Race and sex-specific age-adjusted incidence rates for HL at age of diagnosis.
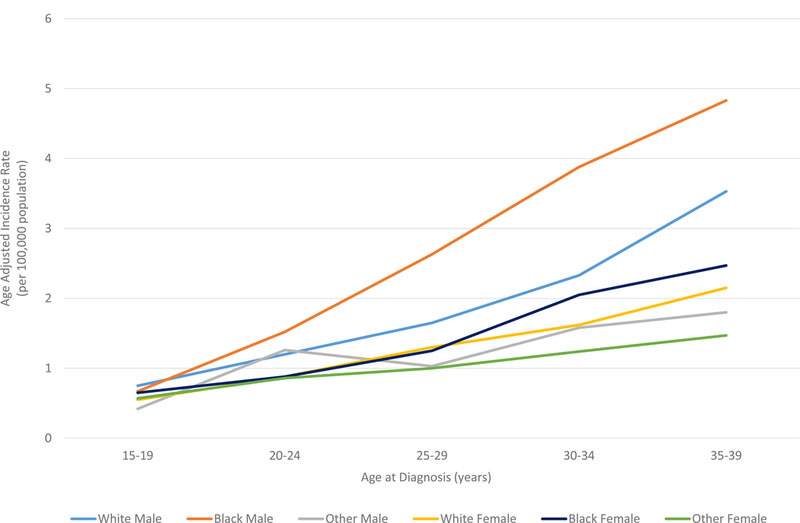
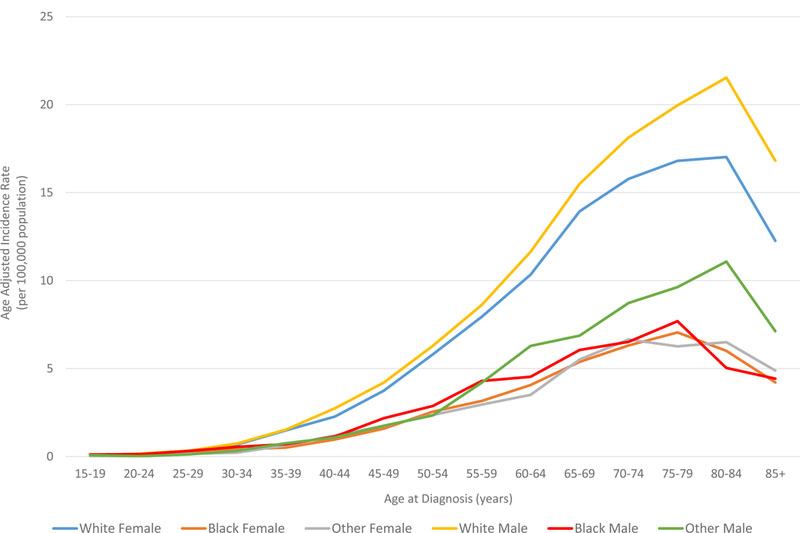
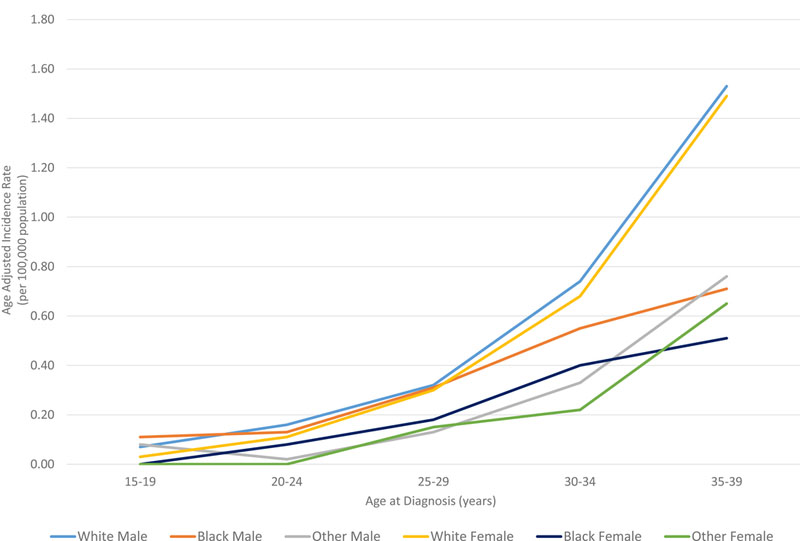
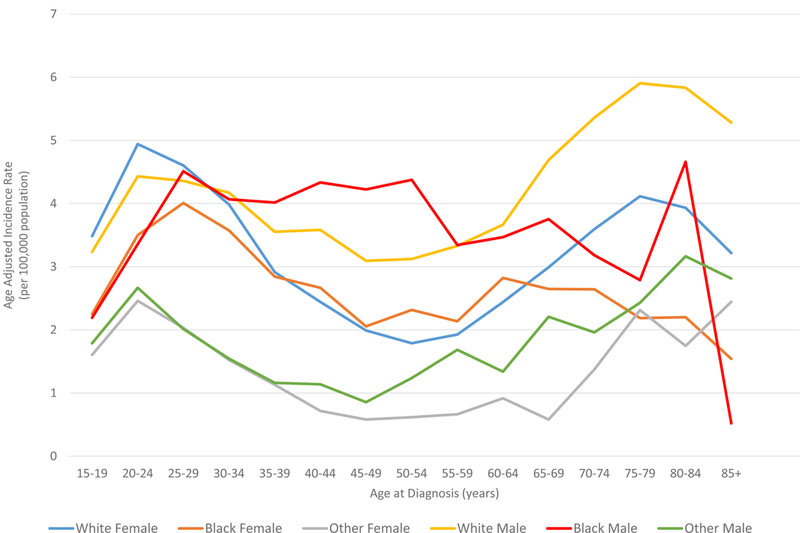
AYA: adolescent and young adult; DLBCL: diffuse large B cell lymphoma; FL: follicular lymphoma; HL: Hodgkin lymphoma.
Survival analysis
The overall 2-year and 5-year RSRs were 87.9% and 83.1% for AYA patients and 75.8% and 65.4% for adults, respectively (Table IV). The 2- and 5- year RSRs for AYA patients with classical HL were 96.7% and 94%, and for adults with classical HL they were 79.1% and 72.7%. In addition, the 2- and 5-year RSRs for AYAs with DLBCL were 82.7% and 79%, and 65% and 59% for adults with DLBCL.
Table IV.
Two and 5-year relative survival rates with confidence intervals by lymphoma subtype among AYA (15–39 years) and adult (> 39 years) cohorts, 18 SEER registries, 2000–2014
| AYA | Adult | |||||||
|---|---|---|---|---|---|---|---|---|
| 2-year | 5-year | 2-year | 5-year | |||||
| RSR | CI | RSR | CI | RSR | CI | RSR | CI | |
| Overall | 87.90% | 87.6–88.3 | 83.10% | 82.7–83.5 | 75.80% | 75.6–76 | 65.40% | 65.2–65.6 |
| Hodgkin lymphoma | 96.80% | 96.5–97.1 | 93.60% | 93.2–94.1 | 80.00% | 79.1–80.6 | 73.90% | 72.9–74.7 |
| Classical Hodgkin lymphoma | 96.70% | 96.4 – 97 | 94% | 93 – 93.9 | 79.10% | 78.3 – 79.8 | 72.70% | 71.8 – 73.7 |
| Nodular lymphocyte predominant Hodgkin lymphoma |
99.00% | 97.4 – 99.6 | 97% | 94.7 – 98.3 | 95% | 92.7 – 96.5 | 93% | 90.1 – 95.3 |
| Non-Hodgkin lymphoma, B-cell | 82.60% | 82–83.2 | 76.40% | 75.7–77.1 | 76.20% | 76–76.3 | 65.40% | 65.2–65.7 |
| Precursor B-cell lymphoblastic leukaemia/lymphoma, NOS |
68.80% | 67 – 70.5 | 54% | 51.9 – 55.8 | 37% | 35.7 – 39.1 | 25% | 23.2 – 26.6 |
| Precursor B-cell lymphoblastic leukaemia/lymphoma, with recurrent genetic abnormalities” |
74.60% | 62.4 – 83.4 | NA | NA | 50% | NA | NA | NA |
| Chronic lymphocytic leukaemia/small lymphocytic lymphoma |
96.30% | 94.2 – 97.7 | 87% | 83.2 – 89.7 | 90% | 89.5 – 90.2 | 80% | 79.3 – 80.4 |
| Prolymphocytic leukaemia, B-cell | 87.50% | 38.6 – 98.2 | 66% | 15.6 – 91 | 62% | 54.2 – 68.5 | 46% | 37.6 – 53.7 |
| Mantle cell lymphoma | 89.00% | 78.8 – 94.5 | 84% | 72.8 – 91.3 | 72% | 71 – 73.3 | 55% | 53 – 56 |
| Lymphoplasmacytic lymphoma, including Waldenstrom macroglobulinemia |
87.90% | 77.1 – 93.8 | 83% | 71 – 90.3 | 88% | 87.2 – 89.4 | 79% | 77.6 – 80.8 |
| DLBCL, NOS | 82.70% | 81.7 – 83.7 | 79% | 78.1 – 80.3 | 65% | 64.3 – 65.2 | 59% | 58.6 – 59.6 |
| Primary DLBCL of the CNS | 52.00% | 45.2 – 58.4 | 45% | 38.1 – 51.8 | 40% | 38.4 – 42.5 | 26% | 23.7 – 27.8 |
| Primary cutaneous DLBCL, leg type | 90.10% | 77.4 – 95.9 | 88% | 73.9 – 94.6 | 82% | 78.4 – 84.4 | 68% | 63.8 – 71.9 |
| T-cell/histiocycte rich large B-cell lymphoma |
87.70% | 72.6 – 94.8 | NA | NA | 74% | 65.3 – 80.8 | NA | NA |
| Intravascular large B-cell lymphoma | NA | NA | NA | NA | 42% | 24 – 59.2 | NA | NA |
| ALK positive large B-cell lymphoma | 66.90% | 19.4 – 90.6 | NA | NA | 100% | NA | NA | NA |
| Plasmablastic lymphoma | 50.90% | 34.1 – 65.4 | NA | NA | 45% | 36.5 – 52.6 | NA | NA |
| Large B-cell lymphoma HHV8- associated multicentric Castleman disease |
65.90% | 26.9 – 87.6 | NA | NA | 90% | 63 – 97.5 | NA | NA |
| Primary effusion lymphoma | 25.10% | 7.8 – 47.3 | 17% | 3.2 – 39.6 | 35% | 24.1 – 46.9 | 29% | 17.5 – 41.3 |
| Primary mediastinal (thymic) large B- cell lymphoma |
91.90% | 88 – 94.6 | 89% | 83.9 – 92.2 | 83% | 76.3 – 87.9 | 80% | 72.2 – 85.9 |
| Burkitt lymphoma/leukaemia | 70.90% | 68 – 73.5 | 68% | 65.3 – 71 | 48% | 46 – 50.3 | 45% | 42.4 – 47 |
| Marginal Zone lymphoma | 98.30% | 97.1 – 99 | 97% | 95.3 – 98 | 93% | 92.7 – 93.8 | 88% | 87.3 – 89 |
| Follicular lymphoma | 96.50% | 95.6 – 97.3 | 93% | 91.7 – 94.2 | 91% | 90.6 – 91.3 | 85% | 84.4 – 85.5 |
| Hairy cell leukaemia | 98.70% | 96.5 – 99.5 | 99% | 95.9 – 99.5 | 95% | 93.9 – 96.1 | 93% | 91.5 – 94.8 |
| Hairy cell leukaemia variant | 75.80% | 47.6 – 90.1 | 76% | 47.6 – 90.1 | 84% | 81 – 86.1 | 77% | 72.3 – 81.1 |
| Plasma cell neoplasms | 86.10% | 83.7 – 88.1 | 72% | 69.2 – 75.3 | 66% | 65.2 – 66.1 | 44% | 43.5 – 44.5 |
| B-cell lymphoid neoplasms, NOS | 81.80% | 78.9 – 84.4 | 78% | 74.2 – 80.4 | 71% | 70.4 – 72.1 | 65% | 62.5 – 64.7 |
| Non-Hodgkin lymphoma, T/NK-cell | 75.10% | 73.7–76.5 | 69.20% | 67.6–70.7 | 66.10% | 65.3–66.9 | 57.50% | 56.6–58.5 |
| Precursor T/NK-cell lymphoblastic leukaemia/lymphoma, NOS |
70.20% | 67.4 – 72.8 | 59% | 56.1 – 62.2 | 43% | 39.3 – 47.2 | 32% | 27.7 – 35.6 |
| Mycosis Fungoides/Sezary syndrome | 98.40% | 96.9 – 99.1 | 96.20% | 94.1 – 97.6 | 94.4% | 93.3 – 95.3 | 88.2% | 86.5 – 89.7 |
| Peripheral T/NK-cell lymphoma | 72.20% | 69.8 – 74.5 | 68% | 65.6 – 70.7 | 57.6% | 56.5 – 58.7 | 48.3% | 47 – 49.5 |
| Adult T-cell leukaemia/lymphoma | 45.70% | 32 – 58.4 | 29% | 15.6 – 42.7 | 34.2% | 28.4 – 39.9 | 23.5% | 18.1 – 29.3 |
| Extranodal NK/T-cell lymphoma, nasal type |
48.40% | 40.8 – 55.7 | 43.30% | 35.5 – 51 | 46.1% | 41.4 – 50.6 | 37.7% | 32.7 – 42.7 |
| T-cell large granular lymphocytic leukaemia |
78.60% | 47.2 – 92.6 | 78.60% | 47.2 – 92.6 | 80.5% | 72.5 – 86.5 | 69.7% | 60.2 – 77.4 |
| T-cell prolymphocytic leukaemia | 49.50% | 17.5 – 75.3 | 49.50% | 17.5 – 75.3 | 38.0% | 32 – 44 | 15.70% | 10.8 – 21.3 |
| Aggressive NK-cell leukaemia | 21.70% | 5.3 – 45.3 | 21.70% | 5.3 – 45.3 | 47.1% | 34.3 – 58.8 | 41.9% | 28.9 – 54.3 |
| Primary cutaneous CD30 + lymphoproliferative disorders |
95.50% | 90.6 – 97.9 | 94% | 88.4 – 97.2 | 91.5% | 88.5 – 93.8 | 87.3% | 83 – 90.7 |
| T/NK-cell lymphoid neoplasms, NOS | 53.80% | 39.9 – 65.8 | 47.90% | 34.1 – 60.4 | 45.0% | 40 – 49.9 | 35.8% | 30.7 – 40.9 |
AYA: adolescent and young adult; CI: confidence interval; DLBCL: diffuse large B cell lymphoma; HHV8: human herpes virus 8; NA: Not available, unable to calculate due to small numbers of patients; NK: Natural Killer; NOS: not otherwise specified; RSR: relative survival rate; SEER: Surveillance, Epidemiology, and End Results
Five-year survivals exceeded 90% in AYA patients with classical HL, nodular lymphocyte predominant (LP) HL, marginal zone lymphoma, FL, hairy cell leukaemia, CD30+ cutaneous lymphoma and mycosis fungoides. In adults, 5-year survivals exceeded 90% only for patients with nodular LP HL and hairy cell leukaemia. Five-year survival rates below 50% were observed in AYA patients with primary DLBCL of the central nervous system (CNS), primary effusion lymphoma, T-cell prolymphocytic leukaemia, extranodal T/Natural Killer (NK) cell lymphoma nasal type, and adult T/NK cell leukaemia/lymphoma. In adults, 5-year RSRs below 50% were observed in patients with precursor B-cell lymphoblastic leukaemia, B-cell prolymphocytic leukaemia, primary DLBCL of the CNS, primary effusion lymphoma, Burkitt lymphoma, plasma cell neoplasms, precursor T/NK cell leukaemia/lymphoma, T-cell prolymphocytic leukaemia, peripheral T-cell lymphoma, extranodal NK/T-cell lymphoma nasal type and T/NK cell leukaemia/lymphoma.
The only subgroups with superior survivals in adults when compared with AYA patients were aggressive NK cell lymphomas, primary effusion lymphoma, Castleman disease, and ALK+ large B-cell lymphoma, but the number of cases was very small for these subgroups, particularly in the AYA cohort. Notably, survival rates were similar for AYA and adult patients (≤ 5% difference) with nodular LP HL, lymphoplasmacytic lymphoma/Waldenstrom macroglobulinaemia, and hairy cell leukaemia/variant, all of which are typically indolent lymphomas associated with late relapses.
Overall survival by the Kaplan-Meier method was superior for AYA patients with lymphoma, compared to the other age groups (40–59 years, 60–79 years, ≥ 80 years) as demonstrated in Figure 7A (log rank test, P< 0.0001). In addition, a similar trend was seen for disease-specific Kaplan-Meier survival curves (log-rank test, P<0.0001, Figures 7B-7C), comparing AYAs with classical HL and DLBCL to the other age groups.
Figure 7. A. Kaplan-Meier estimate of overall survival for all patients by age group (n=341,100). B. Kaplan-Meier estimate of Overall Survival for patients with DLBCL by age group. C. Kaplan-Meier estimate of Overall Survival for patients with HL by age group.
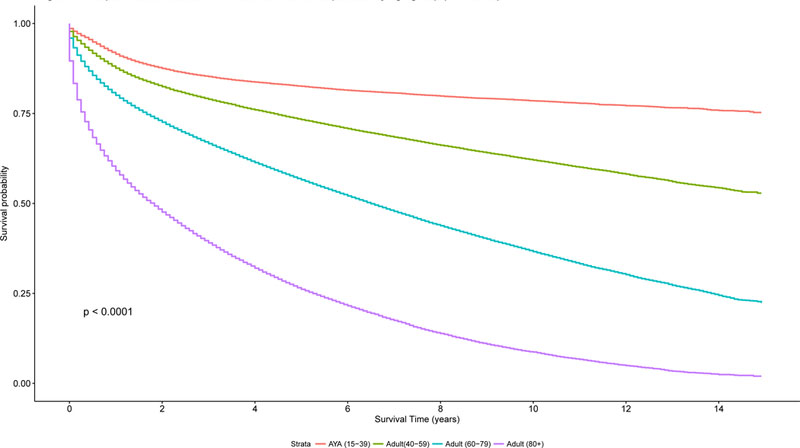
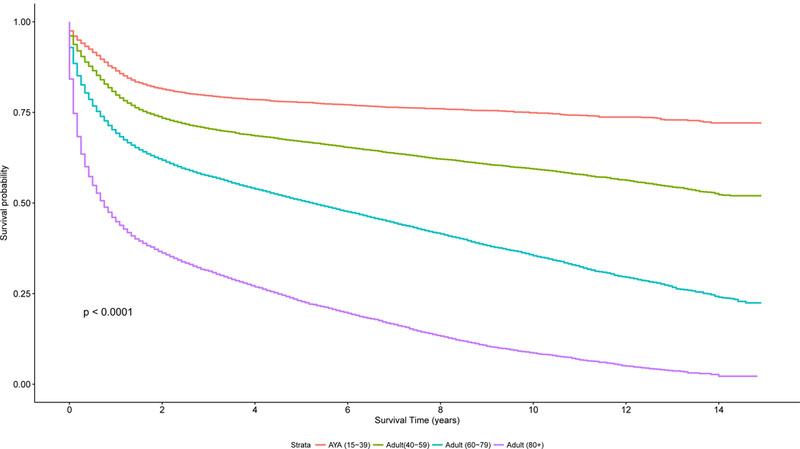
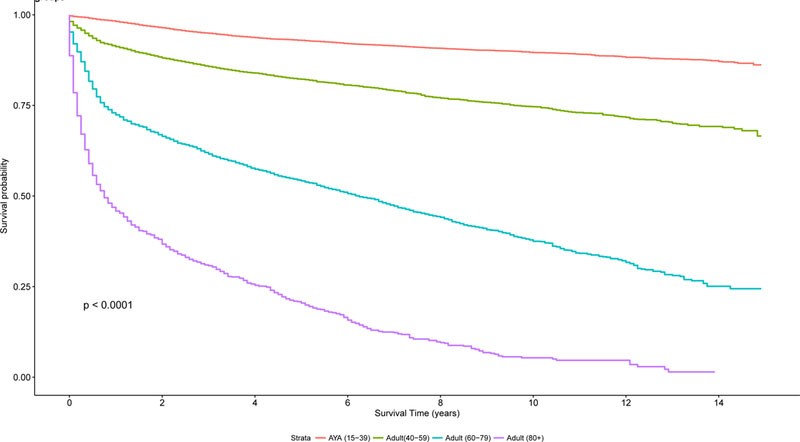
AYA: adolescent and young adult; DLBCL: diffuse large B cell lymphoma; HL: Hodgkin lymphoma.
DISCUSSION
This report provides data on the incidence, disease-specific characteristics and survival of AYA patients (15–39 years) with lymphoma, in comparison to adult patients (> 39 years). Utilizing data available from 18 SEER registries collected between 2000 and 2014, 38,013 AYA patients with lymphoma were identified, about 9% of the total number of lymphoma cases in the US. While 42% of the lymphomas in AYA patients consist of HL, HL only represents 4% of the lymphoma cases in adults. DLBCL is observed at a similar frequency in AYA and adult groups at 18% and 20%, respectively. For adults, DLBCL, plasma cell neoplasms, and CLL are the most commonly diagnosed lymphoid neoplasms, each representing about 20% of adult lymphoma cases. AYA patients with lymphoma commonly present with stage II disease and B-symptoms, unlike adults where advanced stage disease occurs more frequently and only about 10% have B-symptoms. Additionally, extranodal disease is less frequently encountered in AYA patients. Males are most typically affected in both AYA and adult cohorts. The incidence of lymphoma in patients aged 15–25 years is similar across white and black populations, is highest in black males aged 25–65 years, and is highest in white males after 65 years of age. These variations differ according to histological subtype for AYA patients; for example, the age-adjusted incidence rate of DLBCL is highest in black males; however, FL occurs more frequently in white males and females than blacks. Although, this study excluded HIV positivity when it was available, it is difficult to accurately assess the impact of HIV and immunosuppression on the distribution of these lymphomas across the age and race groups as the reporting of HIV status varies by SEER registry (Howlader et al, 2016).
In addition to the differences between incidence, race and gender distribution and disease features at diagnosis among the AYA and adult cohorts, survivals for specific HL and NHL subtypes are typically higher in AYA patients than adults. Two-year and 5-year RSRs in the AYA group at 87.9% are 83.1%, compared to 75.8% and 65.4% for adults, respectively. However, there are subgroups with very favourable outcomes in both adults and AYA patients, particularly LP HL and hairy cell leukaemia. Interestingly, both of these diseases are indolent lymphomas with frequent late relapses. Despite these relapses, survivals exceed 90% in all age groups and it is therefore critical to minimize therapeutic toxicity with a goal of long-term survival in this group. Furthermore, primary CNS lymphoma, primary effusion lymphoma, extranodal NK/T-cell lymphoma nasal type, T/NK cell leukaemia/neoplasms and prolymphocytic leukaemia have very poor outcomes in both AYA and adult patients and new treatment paradigms are desperately needed for these diseases.
Limitations of this study include lack of central pathology review, which may lead to misclassification of lymphoma cases in the AYA and adult cohorts. However, for the most commonly encountered lymphomas in both AYA and adults including classical HL, DLBCL, CLL, plasma cell neoplasms and FL, pathological concordance is typically 85% or higher (The Non-Hodgkin’s Lymphoma Classification Project 1997; Morton et al, 2006). Furthermore, cases from 2000–2001 originally classified according to ICD-0–2 before the introduction of the WHO classification were re-classified to ICD-0–3 codes, and there is 77–82% agreement between computer converted ICD-0–2 codes to ICD-03 codes in SEER (Clarke et al, 2006).
Although the SEER registries incorporate rigorous evaluation for data quality and completeness with extensive training of SEER reviewers, resulting in ascertainment of ≥ 98% of data across all registries (National Cancer Institute 2017 a, b), the large numbers of missing data regarding histological subtype, stage, B-symptoms, extranodal disease, treatment, follow-up and survival in the SEER registries also affected this analysis. The small numbers of cases for uncommon lymphomas in both the AYA and adult populations; for example, Castleman disease, primary effusion lymphoma, plasmablastic lymphoma, prolymphocytic leukaemia, and T/NK-cell leukaemia, limits any conclusions and survival comparisons. Additionally, the number of cases for some of these lymphoma subtypes may be restricted due to the exclusion of patients with known HIV, as some of these diseases (including primary effusion lymphomas) are more commonly encountered patients with HIV. Missing data in 22% of AYA patients and 46% of adult patients regarding stage and in 48% of AYA patients and 65% of adult patients regarding B-symptoms may make our conclusions on the increased frequency of early stage disease and B-symptoms in the AYA group incorrect. Lastly, comparisons of paediatric patients aged less than 15 years with lymphoma to AYA and adult patients with lymphoma will be critical for future research and publications. However, due to the limited numbers of paediatric cases available in the SEER database (13,033 cases) comparisons with the AYA and adult populations were challenging and we elected to focus on AYA and adult cases for this initial publication. Future publications should attempt to examine this paediatric cohort and compare it to the AYA and adult groups.
Data are emerging on the differences in biology, presentation, genetic features and treatment outcomes for AYA patients with lymphoblastic leukaemia/lymphoma compared to adults (Stock et al, 2008; Kuchinskaya et al, 2005; Sallan 2006; DeAngelo et al, 2015). However, although lymphomas represent 20% of the AYA cancers compared to only 6% for leukaemia, much less is known about differences in biology, genetics and treatment outcomes for this group. This limited information may be partly due to the complexity and multiple subtypes of HL and NHL as well as the limited understanding of the genetics of these diseases when compared to leukaemia. Despite the overall excellent outcomes for most AYA patients with lymphoma, differences in histological subtype, disease characteristics and race at presentation from their adult counterparts confirm the need for selective research in this population, identifying opportunities for therapeutic advancements that may differ from treatments available for adult patients.
In a recent analysis of survival trends in AYA patients using available SEER data from 1992–2006, Keegan et al (2016) identified 2651 cases of AYA lymphoma, with 5-year survival of 94.6%, similar to the 5-year survivals of 94% and 97% in the classical HL and LP HL reported in this study. The survivals of the AYA group were inferior to that seen in paediatric patients with HL, whose survivals reached 96.2% and were superior to the 5-year OS of 74.9% in adults. For NHL, the 5-year survival for 2857 AYA patients was 77.6%, again inferior to paediatric cases where 5-year OS reached 85% but improved over adult OS of 68.2% (Keegan et al, 2016). Encouragingly, these survival figures mirror the 5-year RSR in AYA patients with HL and NHL in the present study. In the study reported by Keegan et al (2016), survival improvements over time were less for AYA HL patients than adult HL patients, suggesting that treatment advances in the field have not yet been utilized in the AYA population or do not lead to the same improvements as in adults. Additionally, a recent study has shown that while white AYA patients with HL had improvements in OS, black patients did not, with 5-year survivals of whites with HL at 96.4% compared to 92% for blacks (Kahn et al, 2016). These findings highlight the need to examine the biology and treatment options for AYA patients independently from the paediatric and adult populations and to ensure these trials are equally available to all races. Prospective cohort studies that include the collection of clinical, epidemiological, treatment and outcomes data along with biological samples from the patient and tumour are needed to perform comprehensive analyses comparing paediatric, AYA and adult patients.
It is unclear if the differences in outcomes in AYA and adult patients result from variable access to diagnostic and treatment advances, differing biology or distinct treatment approaches. Traditionally, and perhaps an over-simplification, it has been assumed that paediatric malignancies result from 1–2 key mutations whereas as adult malignancies are more complex due to the accumulation of defects and mutations over time. However, limited data exist with lymphomas to confirm or refute this, and there are few identified essential genetic abnormalities in most subtypes of lymphoma, unlike in acute lymphoid or myeloid leukaemias. With the expanding use of sequencing techniques, comparisons between distinct NHL and HL subtypes in AYA and adult patients can now occur. One small series of 75 AYA NHL cases and 120 adult NHL cases demonstrated an average of 4.5 genomic alterations in AYA patients compared to 5.1 in adults (Johnson et al, 2017). IGH/IGK/IGL and TP53 rearrangements occurred at similar rates in AYAs and adults, but more frequent alterations in B2M, CD58, CIITA, and ALK were observed in AYA cases. In this same series, adult cases more often had CDKN2B, KMT2D, CREBBP, CD79B, and MYD88 mutations. This study included multiple subtypes of NHL and, hopefully, future sequencing studies in the AYA population will focus on distinct NHL and HL subtypes, providing us further insight into the genetic differences, if any, in AYA and adult HL and NHL.
The inferior outcomes of AYA patients with lymphoma compared to their paediatric counterparts suggest that perhaps inferior treatment is a potential cause. A recent retrospective study compared HL patients aged 17–21 years treated on a COG protocol (n=391) with patients enrolled on the adult ECOG 2496 trial (n=114), demonstrating a 3-year FFS of 83% with the COG approach versus 70% with the adult protocol (Henderson et al, 2018). Treatment consisted of ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide) with and without radiation on the COG protocol vs. ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) or Stanford V with and without radiation for the adult protocol. This analysis also demonstrated that the AYA population of patients aged 17–21 years enrolled on the ECOG 2496 study had an inferior 3-year FFS of 70% compared to 79% in patients aged 22–44 years on the same study. However, in addition to the small sample size, this retrospective study was also limited by differences in the patient populations, including varying stage, bulk and B-symptoms as well as differing rates of radiation use for patients enrolled on the two studies. In the NHL category, one multi-centre retrospective study has reported results on 156 AYA and adult patients treated with DA-EPOCH-R for primary mediastinal B-cell lymphoma (Giulino-Roth et al, 2017). Patients treated by paediatric oncologists (aged 9–21 years) had similar outcomes when compared to patients treated by adult oncologists (aged 18–70 years), with 3-year event-free survivals of 81.0% vs 87.4%, respectively. As has been the case in AYA ALL (Stock et al, 2008; Kuchinskaya et al 2005; DeAngelo et al, 2015) the findings from these two studies are provocative and support future prospective examination of paediatric vs. adult regimens in AYA HL and NHL patients. Such trials are in active development through collaborations with the COG and the US Intergroup in HL and primary mediastinal DLBCL. Hopefully, the results of this SEER survey will provide better insights into what lymphomas most frequently affect AYA patients, expected patient disease characteristics, race and gender variations, and expected survival by subtype utilizing current treatment paradigms. These data should guide the design of future prospective studies assessing the biology and evaluating novel treatment approaches for the AYA population. This study identifies research in AYA HL and DLBCL as areas of greatest need based on patient volumes; however, there are a number of other lymphoma subtypes in the AYA population where outcomes are quite poor and new treatments are needed. In these less common lymphoma subtypes, trial design may benefit from combined enrolment and analysis of paediatric, AYA and adult patients. Additionally, for AYA lymphomas subtypes associated with excellent long-term outcomes, focusing on survivorship, minimizing long-term toxicity and examining differences in biology from less favourable adult cases is also critical.
ACKNOWLEDGEMENTS
KAB, AP, and CRF conceived and designed the study, collected the data, and analysed the data. KAB wrote the manuscript. FGK, SC, and CRF reviewed the data and reviewed and edited the manuscript.
REFERENCES
- Bleyer A, O’Leary M, Barr R, Ries LAG (2006) Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 years of age, including SEER Incidence and Survival: 1995–2000. National Cancer Institute, NIH Publication 06–5767. Bethesda, MD. [Google Scholar]
- Clarke AC, Undurraga DM, Harasty PJ,. Glaser SL., Morton LM., Holly EA. (2006) Changes in cancer registry coding for lymphoma subtypes: reliability over time and relevance for surveillance and study. Cancer Epidemiol Biomarkers Prev 15:630–638. [DOI] [PubMed] [Google Scholar]
- DeAngelo DJ, Stevenson KE, Dahlberg SE, Silverman LB, Couban S, Supko JG, Amrein PC, Ballen KK, Seftel MD, Turner AR, Leber B, Howson-Jan K, Kelly K, Cohen S, Matthews JH, Savoie L, Wadleigh M, Sirulinik LA, Galinsky I, Neuberg DS, Sallan SE, Stone RM (2015) Long term outcome of a pediatric inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia 39:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulino-Roth L, O’Donohue T, Chen Z, Bartlett NL, LaCasce A, Martin-Doyle W, Barth MJ, Davies K, Blum KA, Christian B, Casulo C, Smith SM, Godfrey J, Termuhlen A, Oberley MJ, Alexancer S, Weitzman S, Appel B, Mizukawa B, Svoboda J, Afify Z, Pauly M, Dave H, Gardner R, Stephens DM, Zeitler WA, Forlenza C, Levine J, Williams ME, Sima JL, Bollard CM, and Leonard JP (2017) Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Hematol 179:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TO, Parsons SK, Wroblewski KE, Chen L, Hong F, Smith SM, McNeer JL, Advani RH, Gascoyne RD, Constine LS, Horning S, Bartlett NL, Shah B, Connors JM, Leonard JL, Kahl BS, Kelly KM, Schwartz CL, Li H, Friedberg JW, Friedman DL, Gordon LI, Evens AM (2018) Outcomes in Adolescents and Young Adults with Hodgkin Lymphoma treated on US Cooperative Group Protocols: An Adult Intergroup (E2496) and Children’s Oncology Group (COG AHOD0031) Comparative Analysis. Cancer 124:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Shiels MS, Mariotto AB, Engels EA (2016) Contributions of HIV to non-Hodgkin lymphoma mortality trends in the United States. Cancer Epidemiol Biomarkers Prev 25:1289–1296. [DOI] [PubMed] [Google Scholar]
- Johnson A, Morosini D, Vergilio JA, Yelensky R, Rosenzweig M Khaira D, Ali SM, Palma N, Lipson D, Juhn F, Erlich R, Stephens PJ, Ross JS, Miller VA, Wang K (2017) Unique genomic features in adolescent and young adult, as compared to older adult, non-Hodgkin lymphoma and potential therapeutic targets. Br. J Hematol 178:640–642. [DOI] [PubMed] [Google Scholar]
- Kahn JM, Keegan THM, Tao L, Abrahao R, Bleyer A, Viny AD (2016) Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer 122:2723–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan TH, Reis LAG, Barr RD, Geiger AM, Dahlke DV, Pollock BH, Bleyer WA (2016) Comparison of Cancer Survival Trends in the United States of Adolescents and Young Adults with those in Children and Older Adults. Cancer 122:1009–1016. [DOI] [PubMed] [Google Scholar]
- Kuchinskaya E, Heyman M, Grander D, Linderholm M, Soderhall S, Zaritskey A, Nordgren A, Porwit-Macdonald A, Zueva E, Pawitan Y, Corcoran M, Nordenskjold M, Blennow E (2005) Children and adults with acute lymphoblastic leukemia have similar gene expression profiles. Eur J Hematol 74:466–480. [DOI] [PubMed] [Google Scholar]
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS (2006) Lymphoma Incidence patterns by WHO subtype in the United States, 1992–2001. Blood 107:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. (2017 a) Surveillance, Epidemiology, and End Results (SEER) Program. “Overview of the SEER Program” https://seer.cancer.gov/about/overview.html. Accessed 9 November 2017. [Google Scholar]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. (2017 b) “About the SEER Registries”. https://seer.cancer.gov/registries. Accessed 9 November 2017. [Google Scholar]
- National Institutes of Health. (2006) Adolescent and Young Adult Oncology Progress Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adult Oncology Progress Review Group Bethesda MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, and the LIVESTRONG Young Adult Alliance, 2006. Available at: http://planning.cancer.gov/library/AYAO_PRG_Report_2006_FINAL.pdf [Google Scholar]
- The Non-Hodgkin’s Lymphoma Classification Project. (1997) A clinical evaluation of the International lymphoma study group classification of non-Hodgkin’s lymphoma. Blood 89:3909–3918. [PubMed] [Google Scholar]
- Sallan SE (2006) Myths and lessons in the adult/pediatric interface in acute lymphoblastic leukemia. Hematology Am Soc Hematology Education Program 2006,128–132. [DOI] [PubMed] [Google Scholar]
- Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, Larson RA, and Nachman J (2008) What determines outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood 112:1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Narris HL (2008). WHO classification of tumors of hematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumors Lyon, France: IARC. [Google Scholar]
- Swerdlow SH, Campo E, Pilera SA, Harris NL, Stein H, Siebert R, Advani R, Ghilemini M, Salles GA, Zelenetz AD, and Jaffe ES (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]


