Abstract
In this study, we have developed a novel paper based immunoassay for the quantitative detection of immunoreactions using electrochemical impedance spectroscopy. Paper provides an attractive platform for fabrication of simple, low cost, and portable diagnostic devices as it allows passive liquid transport, is biocompatible, and has tunable properties such as hydrophilicity, flexibility, permeability, and reactivity. We have used screen-printing to fabricate interdigitated electrodes (finger width and gap of 200 μm) on the paper substrate, while UV-lithography enables patterning of the paper into hydrophobic/hydrophilic regions. As a proof of concept, we have used this immunosensor to detect the immune response of Human Serum Albumin (HSA) antibody-antigen complex formation. To enable efficient immobilization of HSA antibodies, we have utilized dielectrophoresis to trap microprobes (MPs) on the electrode surface. The microprobes consist of an alumina nanoparticle core with a well-adhered polyaniline outer coating to which the HSA antibodies are conjugated in an oriented manner via covalent chemistry. The efficacy of the impedance-based immunosensor is compared when MPs are immobilized specifically on the electrode surface using dielectrophoresis (DEP) as opposed to being dropped and immobilized via physical absorption on the entire sensing area. Results show that a more reproducible and sensitive response is observed when DEP is utilized to trap the microprobes. Furthermore, the normalized impedance variation during immunosensing shows a linear dependence on the concentration of HSA with an observed limit of detection of 50 μg/ml, which is lower than conventionally used paper based urine dipsticks used for urinary protein detection. Thus, we have developed a low cost paper based immunoassay platform that can be used for the quantitative point of care detection of a wide range of immunoreactions.
I. INTRODUCTION
To enable effective public health monitoring, especially in the developing world, diagnostic tests must fulfill certain criteria which include being affordable, easy to use, portable, and able to operate with little or no external equipment. Paper based biosensors have emerged as an alternative technology for fabricating low cost diagnostic devices, especially for resource limited settings. Besides the fact that paper is inexpensive and widely available in a range of formats, it has certain properties that make it an attractive platform for biosensing applications. These include (i) the ability to wick fluids via capillary action without the need for an external pumping system; (ii) patterning of paper into hydrophobic/hydrophilic regions and fabricating 3-dimensional assays by stacking or folding paper;1–3 (3) the biocompatibility and porosity of paper enables safe storage of reagents; (4) the lightweight and flexibility of paper in addition to the ease of safe disposal via combustion make it a highly suitable platform for single-use tests. The use of paper for fabrication of analytical devices and its application for low cost point of care testing has already been demonstrated. The most common examples of paper-based assays include urinalysis test strips for simultaneous detection of various analytes including protein and glucose and immunochromatographic lateral flow assays (LFAs) such as home pregnancy tests. While the potential of paper for effective calorimetric detection has been demonstrated,4,5 there are still opportunities for fabricating paper based diagnostic devices that utilize other modes of detection, particularly electrical/electrochemical detection, while still retaining low cost and simplicity.
The ability to perform electrochemical detection on paper should enable the fabrication of paper based immunosensors that provide quantitative analysis as opposed to currently used calorimetric assays that are either semi-quantitative or simply give a yes/no response. Immunosensors have been widely employed for several applications including healthcare because they can achieve rapid and sensitive detection of the specific binding of an antigen to its corresponding antibody to form a stable complex.6,7 Immunosensors utilizing electrochemical detection are insensitive to color contamination and can be effectively integrated with the miniaturized hardware, making them arguably the most practical and quantifiable diagnostic technique for detection of proteins.8–10 The fabrication of paper-based immunosensors utilizing electrochemical detection requires electrode integration on the paper substrate that is achieved using several methods among which cost-effective printing protocols like screen11 or inkjet12 printing are most commonly used. Generally, inks made of carbon based materials (graphite or nanomaterials like graphene) and metals like gold, platinum, or silver are good choices for fabricating working and counter electrodes while the reference electrode, if utilized, is generally made of the Ag/AgCl paste.
Among the various electrochemical techniques, impedance based electrical transduction, where the applied electrical signal is alternating as opposed to direct, can be used to analyze both the resistive and the capacitive changes at the electrode surface over a wide frequency range during immunosensing. This analytical approach, known as electrochemical impedance spectroscopy (EIS), is an effective strategy for probing of complex bio-recognition events13 and has been utilized in a wide range of biosensors ranging from bacteria and pathogen detection to immunosensing and DNA characterization.14–16 EIS can be used for real time and direct monitoring of affinity binding events without the need for labeling compounds. To fabricate a sensitive paper based impedimetric immunosensor, it is crucial that the receptor antibody is selectively immobilized on the electrode surface.
The proposed paper based immunosensor utilizes dielectrophoresis (DEP) to trap antibody-conjugated microprobes on interdigitated microelectrodes printed using screen-printing. Furthermore, the efficacy of the immunosensor has been compared when the microprobes are trapped on the electrode surface using DEP as opposed to being dropped on the surface and immobilized via physical absorption. DEP is a versatile technique that has been used for controllable, selective, and accurate manipulation of a wide variety of bioreceptors onto the surface of the electrode sensing platform.17 DEP is defined as the time-averaged force acting on a spherical dielectric particle immersed in a medium and exposed to a spatially non-uniform electric field.18 The magnitude and direction of this force are dependent on the electric field intensity, particle size, and permittivity and on the conductivity of the particle and the suspension medium. In our previous works, we have utilized programmable three step DEP manipulation to effectively distinguish bladder cancer staging using multiple biomarkers on a single glass based lab on a chip device.19 Microelectrodes, in comparison to conventional electrodes, significantly improve sensitivity, especially in low conductivity solutions, and allow rapid reaction kinetics with an improved signal to noise ratio.20 We have chosen an interdigitated electrode design because the fabrication protocol is simpler and the geometry enables improved sensitivity over other microelectrode designs. Furthermore, the conventionally used three electrode design for performing electrochemical measurements has limitations for use in paper as the printed Ag/AgCl reference electrode is unable to maintain a stable potential in the absence of high concentration of chloride ions in solution. To the best of our knowledge, this is the first proof-of-concept paper based immunosensor utilizing DEP for impedance-based detection.
II. EXPERIMENTAL
A. Microprobe synthesis
In this study, we have utilized micrometer-sized probes or microprobes (MPs), which consist of conductive polyaniline (PANI) coated alumina nanoparticles (Al2O3 NPs) conjugated to HSA antibodies, and are referred to as Al2O3/PANI/Ab-HSA MPs. The conjugation of antibodies with nanoparticles has been widely utilized for developing immunosensors as it combines the novel intrinsic properties of nanoparticles and their enhanced surface areas with the selective and specific recognition capabilities of antibodies to antigens. While the dielectric Al2O3 NP core enables effective manipulation using DEP, the polyaniline coating provides a conductive channel to the electrodes and allows for oriented conjugation of antibodies via covalent chemistry.
1. Surface modification of Al2O3 NPs
We have utilized a surface modification technique where dodecyl benzene sulfonic acid (DBSA) was used to obtain a uniform and well-adhered PANI coating on the alumina nanoparticles. Functional protonic acids like DBSA absorb most of the aniline monomers onto their complex network as their long aliphatic chains facilitate efficient mixing while their long hydrocarbon tail allows for easy modification of the polymer-polymer interface.21 To obtain the coating, 3 g of aniline monomer, 8.4115 g of DBSA, 7.35 g of ammonium persulfate (APS), and 2 g of Al2O3 NPs (∼20 nm) were used. First, the Al2O3 NPs were added to the aniline solution and ultra-sonicated for 10 min, followed by the addition of the DBSA solution, and further sonication for 10 min. The APS solution was then added and the system was allowed to react for 2 h. The PANI was polymerized by standard oxidative polymerization of the aniline monomer in the presence of APS as the oxidizing agent. Following polymerization, the dark green Al2O3/PANI microparticle (MP) was filtered, washed multiple times with DI water using centrifugation, and baked in an oven at 60 °C for 30 min to obtain Al2O3/PANI MPs in the dry powdered form.
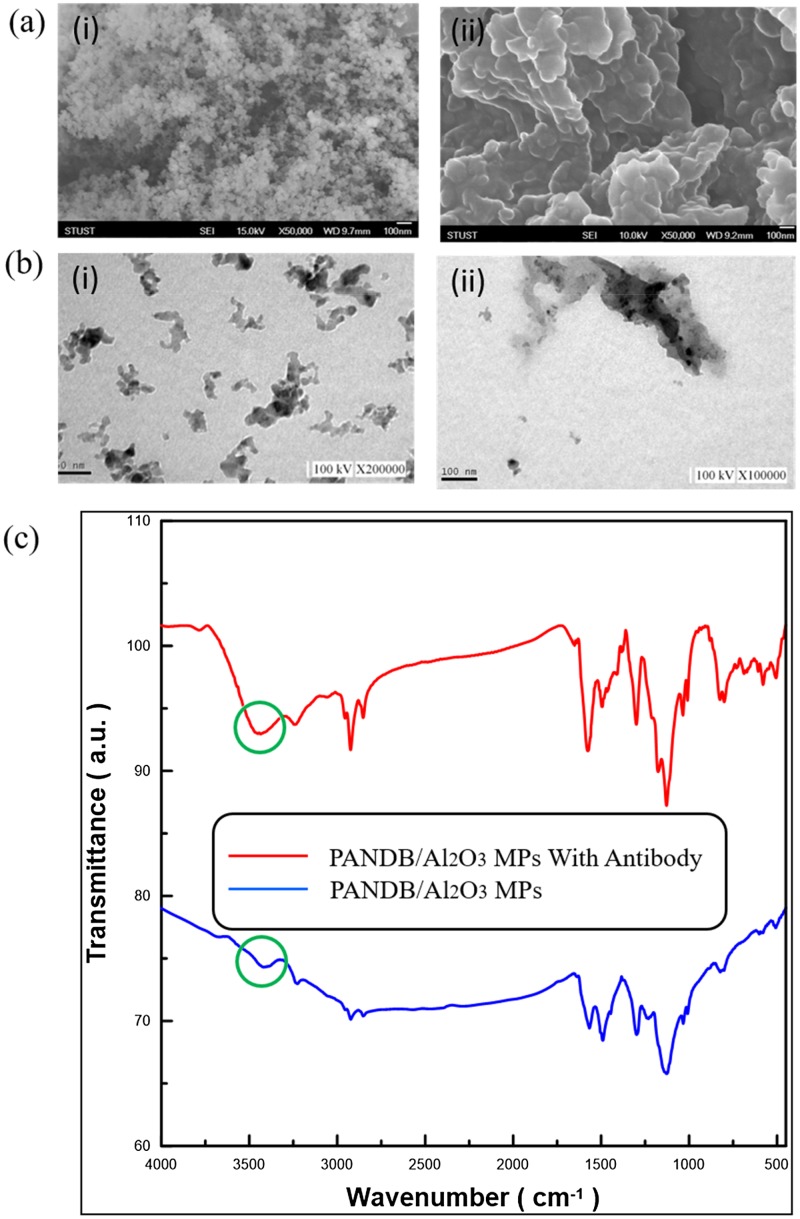
The morphology of the Al2O3 NPs before and after they are surface modified with the PANI coating can be observed in the FE-SEM images shown in Fig. 1(a). It can be seen that the modified Al2O3/PANI microparticles have an irregular and coalesced surface appearance that is markedly different from the powdered Al2O3 NPs. TEM images in Fig. 1(b) show that the PANI surface modification promotes aggregation and cluster formation of the Al2O3 NPs, resulting in sizes in the micrometer range. Furthermore, we have performed a FTIR analysis to confirm the presence of the PANI coating. The peaks appearing at 1562 cm−1 and 1484 cm−1 can be assigned to the C–C stretching modes of quinoid and benzenoid rings, respectively, while the characteristic peak (circled in green) at 3440 cm−1 corresponds to the N–H stretch of amine groups present in PANI as shown in Fig. 1(c). The band at about 1382 cm−1 can be assigned to the C–N stretching vibrations. The strong peak at 1124 cm−1 corresponds to C–H bending vibrations based on the degree of electron delocalization and hence is a characteristic of PANI conductivity. These findings are consistent with previously reported works22,23 and the Al2O3/PANI MPs can thus be used as antibody carriers in our immunosensor.
FIG. 1.
(a) FE-SEM and (b) TEM images of Al2O3 NPs (i) before and (i) after surface modification with PANI (scale bar corresponds to 100 nm). (c) FTIR spectra of the Al2O3/PANI MPs (blue) and after conjugation with HSA (red).
2. Antibody conjugation
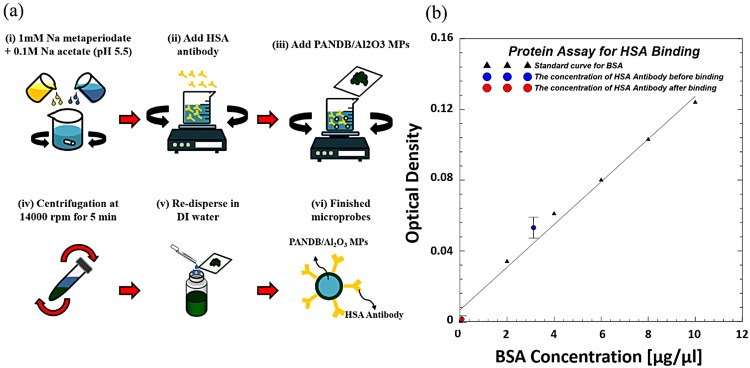
We have used a simple and effective protocol to conjugate the HSA antibodies to the Al2O3/PANI MPs as schematically illustrated in Fig. 2(a). First, the antibodies are oxidized in a solution containing 1 mM of sodium metaperiodate and 0.1M sodium acetate with the pH maintained at 5.5. This step causes the oxidation of the carbohydrate moieties present in the heavy chain of the antibody and results in the formation of aldehyde groups. Next, Al2O3/PANI NPs are added to the oxidized antibody solution and mixed under constant rotational stirring at room temperature for 30 min. During this step, the aldehyde groups of the oxidized antibodies covalently bind with the amino groups of PANI present in the surface modified Al2O3 NPs through the formation of an amide bond. This covalent interaction allows oriented conjugation where the fragment crystallizable region (Fc) of the antibody is bound to the nanoparticle surface while the fragment antigen-binding region (Fab) is available during immunoassay. The synthesized Al2O3/PANI/ab-HSA MPs are collected by centrifugation at 18 000 rpm for 5 min, dispersed in DI water, and stored at 4 °C until further use. We have also performed optical density analysis using a spectrophotometer to study conjugation efficiency of HSA antibodies to the Al2O3/PANI MPs. A 97.8% reduction in optical density of the supernatant before and after conjugation with HSA antibody as shown in Fig. 2(b) confirms that conjugation was successful.
FIG. 2.
(a) The step-by-step fabrication protocol for HSA conjugation to Al2O3/PANI MPs. (b) Optical density analysis confirms successful conjugation.
B. Immunosensor fabrication and operation
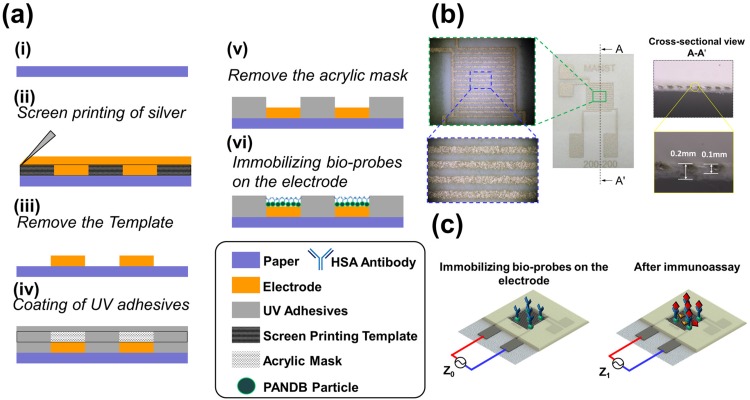
The fabrication of the paper-based immunosensor can be primarily categorized into three stages: (i) Screen printing of interdigitated electrodes (IDEs); (ii) Patterning of paper into hydrophobic/hydrophilic regions using ultraviolet (UV) lithography; (iii) Immobilization of microprobes, which are deposited either directly on the entire sensing area or specifically trapped on the IDEs using positive-dielectrophoresis (p-DEP). The step-by-step fabrication protocol is schematically illustrated in Fig. 3(a). The IDEs are printed on the filter paper (Advantec Grade no: 4a, pore size 1 μm) using a screen printer (SYP-4570, Ag PRO Technology Corporation, Taiwan) operating at a speed of 100 mm/s. Screen printing allows transfer of the microparticulate silver ink onto the paper substrate except in areas that are made impermeable by the use of a blocking stencil. The printed IDE has 8 fingers with each finger having a width and interspacing of 200 μm, respectively, resulting in a total sensing area of 37.2 mm2. The thickness of the filter paper was 0.2 mm and the depth of the ink penetration as seen in the cross sectional image in Fig. 3(b) was about 0.1 mm. After screen printing, the electrodes are thermally cured in an oven at 150° C for 30 min to remove organic solvents present in the ink and sinter the silver microparticles for improved physical and electrical properties. Next, the paper is patterned into hydrophobic/hydrophilic regions using UV-lithography where the sensing areas and the IDE lead regions remain hydrophilic, while the rest of the sensor is hydrophobic. First, UV-curable resin (U54-0, HICO Technology Co., LTD.) is dropped on the filter paper using a plastic dropper and a glass stir rod is then used to evenly spread and penetrate the resin into the pores of the paper. Next, a PMMA mask is fabricated by laser cutting and shaded in black in certain areas to prevent UV light penetration. The mask is then aligned on the paper surface followed by exposure to a 400 W high-pressure mercury lamp (dominant wavelengths of 253 and 365 nm) for curing the resin. Finally, the paper is soaked in ethanol to remove the uncured resin followed by heating on a hotplate at 80 °C for 1 min to evaporate the remaining ethanol. The paper sensor is now ready for immobilization of microprobes, which are deposited on the hydrophilic sensing area using two methods:
FIG. 3.
(a) A schematic of the steps involved in the fabrication of the paper sensor. (b) An image of the completed sensor with enlarged optical microscopy images of the interdigitated microelectrodes. (c) Schematic of the paper sensor before and after immunosensing.
1. Physical absorption
This method involves pipetting the microprobe solution on the hydrophilic sensing areas followed by quick drying using a suction assisted technique. As the Al2O3/PANI/ab-HSA MPs are physically larger than the 1 μm filter paper pore size, they are retained on the sensing area surface and in effect form a thin film that covers the IDEs. 50 μl of Al2O3/PANI MPs in DI water (1 wt. %) is pipetted on the sensing areas and the paper sensor is placed on a micro machined plastic device that is connected to a vacuum pump to enable effective drying within 10 min. After the MPs are trapped, the baseline impedance before immunosensing (Z0) is recorded using an LCR meter (Wayne Kerr Electronics, WK 6420) in a frequency range of 20 Hz to 100 kHz at an applied voltage of 0.1 V. Next, 100 μl of DI water spiked with different concentrations of HSA protein were dropped onto the sensing areas and left for immunosensing for a further 30 min. This is followed by a washing step to remove any unbound proteins and the impedance after immunoassay is recorded (Z1). The impedance measurements for each step (bare electrode, trapping, and immunosensing) were obtained in air. The impedance variation (ΔZ) during immunosensing can be mathematically expressed as Z1–Z0. 10 immunosensors have been used to test each concentration of HSA and the error bars have been added. We have tried to eliminate any differences in initial impedance observed at different electrodes by normalizing the impedance response during immunosensing (ΔZ) with the initial impedance observed after microprobe trapping at that particular electrode (Z0) and this is referred to as the normalized impedance variation (ΔZ/Z0). The normalized impedance variation is calculated at an operating frequency of 10 kHz for which we observe the maximum stable response during immunosensing. While a larger response can be observed at lower frequencies (<1 kHz), it shows lesser reproducibility and a lower signal-to-noise ratio as environmental conditions must be closely monitored and controlled while performing immunosensing.
2. Electrodeposition using p-DEP
This method utilizes DEP to trap the MPs specifically on the electrode surface as opposed to the entire sensing area. The sensitivity of impedimetric biosensors is strongly dependent on the bio-receptor immobilization strategy utilized. One of the aims of this study is to analyze if the immunosensor response is enhanced when MPs are trapped using DEP instead of physical absorption. The paper sensor is immersed in the microprobe solution and an AC signal of 10 Vpp at 1 kHz is applied for 10 min using a function generator (AFG3022, Tektronix) to trap the MPs based on the p-DEP force. We have chosen a frequency of 1 kHz since we observe the largest impedance change after microprobe trapping when using this frequency. The strength of the DEP force strongly depends on the medium and the electrical properties of the particles and their shape and size. Consequently, medium solutions with varying conductivity could be potentially for DEP immobilization, provided optimization of the applied signal frequency is performed. However, since we utilize p-DEP force (the particle attracted toward the region of the strong electrical field) for trapping the microprobes, a low conductivity medium like DI water is preferred so that the Claussius Mosotti factor is dominated by the dielectric properties of the microprobes and not the solution. After DEP trapping, all the steps are the same as that after physical absorption of microprobes as mentioned previously.
Besides DEP, other kinds of AC related electrokinetic forces such as AC electro-osmosis (ACEO) might also be present and effect microprobe manipulation. ACEO results in motion of the liquid when an electrical potential is applied across a porous material or capillary tube like that present in cellulose paper. During application of an AC potential at a frequency of 1 kHz, ACEO may influence fluid movement within the pores of the filter paper between the IDE fingers. However, since the size of the microprobes used in this study is relatively large and they can only be trapped on the surface of the IDEs without being able to penetrate the filter paper, ACEO will not significantly influence microprobe trapping which should ideally be dominated by DEP.
III. RESULTS AND DISCUSSION
A. Physical absorption of MPs
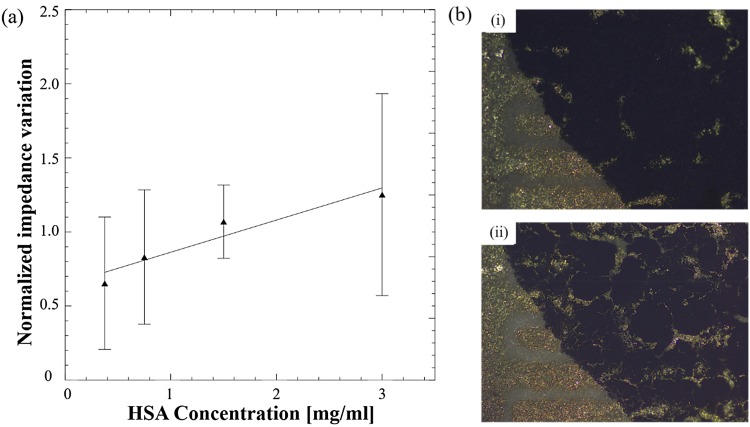
The development of an electrical impedance based immunosensor requires effective immobilization of the antibodies on the electrode surface so that they can selectively capture the antigen, resulting in an impedance change. To enable sensitive and reproducible detection, the electrical pathway between the IDEs through the MPs and the captured antigen should be well defined. When the MPs are dropped onto the sensing area and immobilized via physical absorption, they form in effect a thin film on the surface of the IDEs. The electrical impedance of this film, which consists of semi-conductive polyaniline coated MPs, increases as the HSA protein is captured by the immobilized antibodies. As seen from the results, the normalized impedance variation gradually increases with an increasing concentration of HSA spiked DI water with an impedance variation of 0.65 that increases to 1.25 as the concentration increases from 0.375 mg/ml to 3 mg/ml. However, the repeatability of the observed variations was poor as highlighted by the wide error bars, which overlap for different concentrations. Since the MPs are not specifically immobilized on the IDE surface, the electrical pathway across the IDE fingers is random. Furthermore, physical immobilization results in movement or loss of MPs during the washing steps while cracks develop after drying as shown in Fig. 4(b), resulting in variations between different immunosensors and hence reduced reproducibility.
FIG. 4.
(a) Normalized impedance variations for different concentrations of HSA using the physical absorption method for immobilizing MPs. (b) Optical microscopy image showing a layer of deposited MPs (i) before and (ii) after drying.
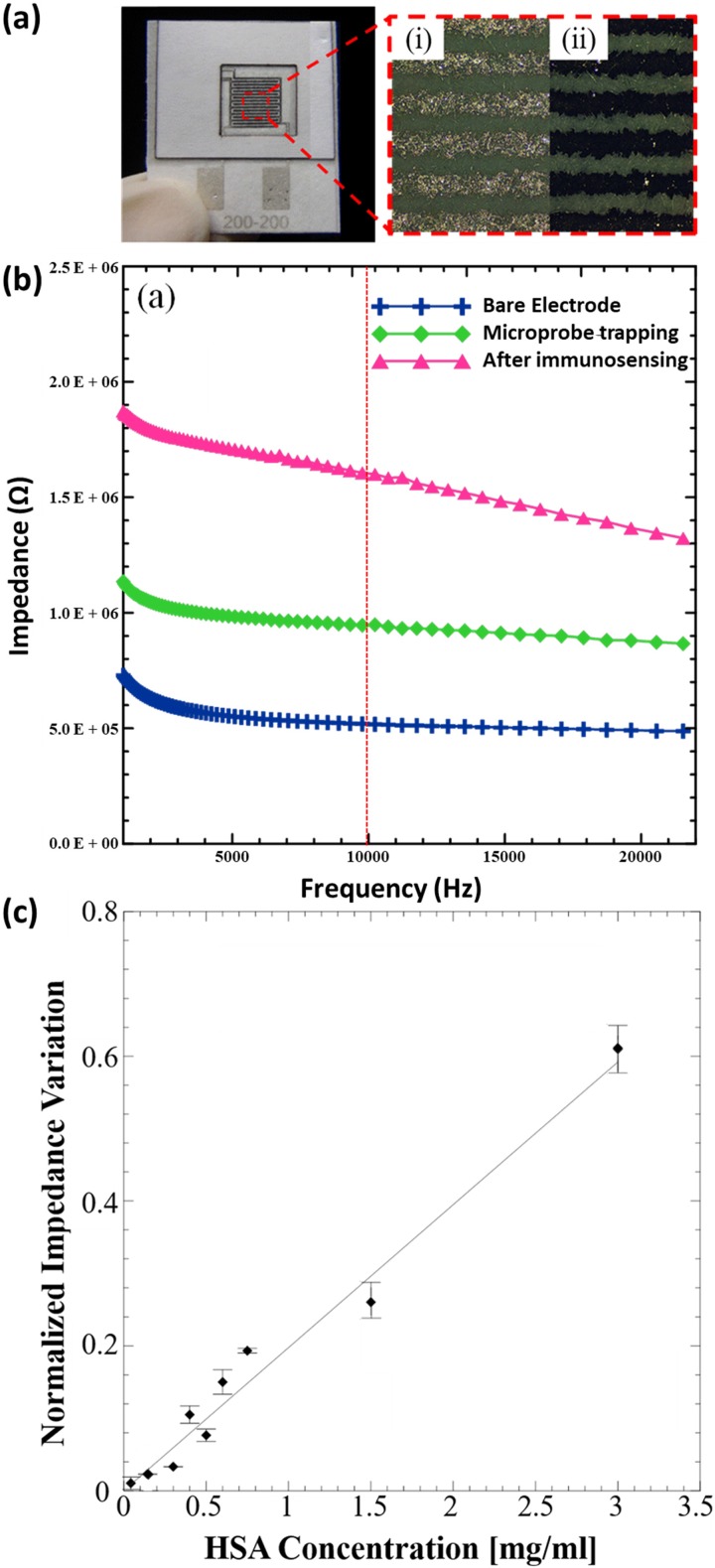
B. Dielectrophoretic trapping of MPs
The physical absorption approach poses challenges when the antibodies need to be immobilized specifically on the electrode surface. For impedimetric immunosensors utilizing IDEs, the bioreceptor is generally immobilized either on the surface of the electrode or the gap in between. This ensures that the changes in electrical properties (resistance and capacitance) at the conductive electrode surface or the non-conductive gap are directly related to the immunoreaction. One way of improving physical immobilization on electrode surfaces is by using AC electrokinetic techniques like DEP to trap the MPs on the electrode surface. The optical microscopy images in Fig. 5(a) show that the MPs are successfully immobilized and uniformly adhere to the IDE surface when a positive dielectrophoretic force is applied. This ensures that the MPs remain firmly bound to the surface of the electrode after the washing step. Owing to the geometry of IDEs, the current flow mainly occurs very close to the electrode surface, and the antibody-antigen complex formation directly impedes this flow when MPs are specifically trapped on the electrode using DEP. The impedance values obtained for each step (bare electrode, microprobe trapping, and after immunosensing) as a function of applied frequency are shown in the bode plot in Fig. 5(b). It can be seen that the impedance almost doubles at 10 kHz after microprobe trapping using p-DEP and further increases after immunosensing with the HSA protein (0.15 mg/ml). Since we measure the impedance change at a frequency of 10 kHz, capacitive effects should ideally dominate the impedance response. Both the microprobe trapping and the antigen binding can be envisioned as increasing the distance between the conducting plates of a capacitor which results in a decrease in the effective capacitance and consequently an increase in the overall impedance. It can be seen from the results in Fig 5(c) that the normalized impedance variation increases linearly over a wide range as the concentration of HSA in DI water increases from 50 μg/ml to 3 mg/ml (linear regression equation is Y = 0.19725 * X + 0.00028 and R2 = 0.962645). The limit of detection or LOD was observed to be 50 μg/ml, which is lower than that achieved using the physical absorption method. In addition, the immunosensor response shows significantly improved reproducibility and relatively lower variability in observed impedance response.
FIG. 5.
(a) Image of the paper based immunosensor with the magnified area showing the silver microelectrode surface observed under an optical microscope (i) before and (ii) after trapping of MPs using p-DEP. (b) EIS spectra (bode plot) for each step during immunosensor fabrication and operation. (c) Normalized impedance variations for different concentrations of HSA.
The proposed paper based immunoassay not only provides quantitative detection but also a lower LOD than the traditional urine dipstick, which displays a negative response under 100 μg/ml. However, in recent years, novel electrochemical biosensors fabricated on hydrophobic substrates and utilizing EIS transduction have been reported with significantly improved sensitivities.24–26 In our previous work, we fabricated a gold interdigitated microelectrode array on glass using a photolithographic protocol, which can detect bladder cancer biomarker Gal-1 with an achievable LOD in the pg/ml range.27 Ding et al. reported an electrochemical immunosensor utilizing interdigitated electrodes fabricated on the silicon wafer and vertically aligned nanotube arrays capable of detecting oral cancer biomarker CIP2A in saliva with a limit of detection of 0.24 pg/ml range.28 While several factors such as the ability to fabricate smaller microelectrode width and gap using photolithography result in lower LOD on hydrophobic surfaces, and there is also a fundamental difference in electrochemical measurements made on paper as compared to those performed in the free fluid on hydrophobic surfaces like glass. This is because the cellulose matrix prevents convection (in stationary fluids), acts as a barrier to diffusion (by increasing the tortuosity of the diffusion path), occupies some of the volume that would otherwise be occupied by fluid, and blocks a portion of the electrode surface (∼30%, depending on the type of paper and fabrication method used).29
Our aim in this research was to analyze the feasibility of fabricating a paper based immunosensing platform utilizing label free detection via EIS that could potentially be further improved to take advantages of paper, primarily its ability to wick fluids, to integrate complexity in assays like sequential fluid delivery. One of the key issues in fabricating label free affinity biosensors on paper is the selective immobilization of the affinity receptor such as antibodies or DNA on/in-between the interdigitated electrode fingers. Herein, we have shown that programmable DEP can be successfully used to trap microprobes selectively on the IDE surface, thus improving sensitivity and repeatability. To further reduce LOD, certain amplification strategies such as a sandwich immunoassay with a secondary antibody conjugated to a label (metal nanoparticles or enzymes) could be utilized. A sequential fluid delivery system can aid to integrate and automate these additional amplification steps, thus increasing suitability for point of care testing. Another challenge in developing paper based label free impedance immunosensors is the issue of specificity due to the non-specific absorption of a multitude of proteins present in clinical samples. While a washing step as performed in this study is helpful, it cannot guarantee the complete removal of physically absorbed proteins. A solution to this issue can be the use of differential analysis by fabricating a test (with antibody) and control (without antibody) site on the same immunosensor as shown below. Differential analysis (the ratio of normalized impedance variation at test and control sites) aids in eliminating non-specific binding effects and ensures that the impedance change after immunosensing is due to the formation of the antibody/antigen complex and not because of the physical immobilization of proteins.
IV. CONCLUSIONS
In summary, we have successfully demonstrated a proof-of-concept paper based electroanalytical platform that uses EIS for quantitative detection of immunoreactions. The combined use of interdigitated microelectrodes and dielectrophoretic trapping of MPs enabled improved sensitivity and reproducibility for label free immunosensing on paper. Future work involves the integration of this low cost, a disposable immunosensing platform with a portable impedance analyzer that can analyze and upload results to the cloud database as developed in our previous study,26 which will enable effective point of care testing and improved public health monitoring. This proposed immunosensing platform could be extended to the development of a range of point of care affinity biosensors.
ACKNOWLEDGMENTS
The authors would like to thank the Ministry of Science and Technology, Taiwan, for financially supporting this research under Contract No. MOST 106-2632-E-218-001.
References
- 1.Martinez A. W. et al. , “Diagnostics for the developing world: Microfluidic paper-based analytical devices,” Anal. Chem. 82, 3–10 (2009). 10.1021/ac9013989 [DOI] [PubMed] [Google Scholar]
- 2.Martinez A. W., Phillips S. T., and Whitesides G. M., “Three-dimensional microfluidic devices fabricated in layered paper and tape,” Proc. Natl. Acad. Sci. 105(50), 19606–19611 (2008). 10.1073/pnas.0810903105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan J. et al. , “A microfluidic origami electrochemiluminescence aptamer-device based on a porous Au-paper electrode and a phenyleneethynylene derivative,” Chem. Commun. 49(14), 1383–1385 (2013). 10.1039/C2CC37402A [DOI] [PubMed] [Google Scholar]
- 4.Fu E. et al. , “Two-dimensional paper network format that enables simple multistep assays for use in low-resource settings in the context of malaria antigen detection,” Anal. Chem. 84(10), 4574–4579 (2012). 10.1021/ac300689s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dungchai W., Chailapakul O., and Henry C. S., “Use of multiple colorimetric indicators for paper-based microfluidic devices,” Anal. Chim. Acta 674(2), 227–233 (2010). 10.1016/j.aca.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 6.Ploeg M., Aben K. K., and Kiemeney L. A., “The present and future burden of urinary bladder cancer in the world,” World J. Urol. 27(3), 289–293 (2009). 10.1007/s00345-009-0383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels J. S. and Pourmand N., “Label-free impedance biosensors: Opportunities and challenges,” Electroanalysis 19(12), 1239–1257 (2007). 10.1002/elan.200603855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S.-J., Andrew Taton T., and Mirkin C. A., “Array-based electrical detection of DNA with nanoparticle probes,” Science 295(5559), 1503–1506 (2002). 10.1126/science.1067003 [DOI] [PubMed] [Google Scholar]
- 9.Zheng G. et al. , “Multiplexed electrical detection of cancer markers with nanowire sensor arrays,” Nat. Biotechnol. 23(10), 1294–1301 (2005). 10.1038/nbt1138 [DOI] [PubMed] [Google Scholar]
- 10.Chikkaveeraiah B. V. et al. , “Electrochemical immunosensors for detection of cancer protein biomarkers,” ACS Nano 6(8), 6546–6561 (2012). 10.1021/nn3023969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominguez R. O., Alonso-Lomillo M. A., and Arcos Martinez M. J., “Recent developments in the field of screen-printed electrodes and their related applications,” Talanta 73(2), 202–219 (2007). 10.1016/j.talanta.2007.03.050 [DOI] [PubMed] [Google Scholar]
- 12.Labroo P. and Cui Y., “Graphene nano-ink biosensor arrays on a microfluidic paper for multiplexed detection of metabolites,” Anal. Chim. Acta 813, 90–96 (2014). 10.1016/j.aca.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 13.Lisdat F. and Schäfer D., “The use of electrochemical impedance spectroscopy for biosensing,” Anal. Bioanal. Chem. 391(5), 1555 (2008). 10.1007/s00216-008-1970-7 [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Afrasiabi R., Fathi F., Wang N., Xiang C., Love R. et al. , “Impedance based detection of pathogenic E. coli O157:H7 using a ferrocene-antimicrobial peptide modified biosensor,” Biosens. Bioelectron. 58, 193–199 (2014). 10.1016/j.bios.2014.02.045 [DOI] [PubMed] [Google Scholar]
- 15.Das L., Das S., and Chatterjee J., “Electrical bioimpedance analysis: A new method in cervical cancer screening,” J. Med. Eng. 2015, 636075 (2015). 10.1155/2015/636075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elshafey R. et al. , “Electrochemical impedance immunosensor based on gold nanoparticles–protein G for the detection of cancer marker epidermal growth factor receptor in human plasma and brain tissue,” Biosens. Bioelectron. 50, 143–149 (2013). 10.1016/j.bios.2013.05.063 [DOI] [PubMed] [Google Scholar]
- 17.Zhang C. et al. , “Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems,” Anal. Bioanal Chem. 396(1), 401–420 (2010). 10.1007/s00216-009-2922-6 [DOI] [PubMed] [Google Scholar]
- 18.Pohl H. A., “The motion and precipitation of suspensoids in divergent electric fields,” J. Appl. Phys. 22(7), 869–871 (1951). 10.1063/1.1700065 [DOI] [Google Scholar]
- 19.Nhan V., “Lab on a chip for multiplexed immunoassays to detect bladder cancer using multifunctional dielectrophoretic manipulations,” Lab Chip 15(14), 3056–3064 (2015). 10.1039/C5LC00352K [DOI] [PubMed] [Google Scholar]
- 20.Harrington M. S. and Anderson L. B., “Analytical strategies using interdigitated filar microelectrodes,” Anal. Chem. 62(6), 546–550 (1990). 10.1021/ac00205a002 [DOI] [Google Scholar]
- 21.Guarino V. et al. , “Electro-Active Polymers (EAPs): A promising route to design bio-organic/bioinspired platforms with on demand functionalities,” Polymers 8(5), 185 (2016). 10.3390/polym8050185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belaabed B. et al. , “Polyaniline-doped benzene sulfonic acid/epoxy resin composites: Structural, morphological, thermal and dielectric behaviors,” Polym. J. 42(7), 546–554 (2010). 10.1038/pj.2010.41 [DOI] [Google Scholar]
- 23.Prabhakar N., Matharu Z., and Malhotra B. D., “Polyaniline Langmuir–Blodgett film based aptasensor for ochratoxin A detection,” Biosens. Bioelectron. 26(10), 4006–4011 (2011). 10.1016/j.bios.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 24.Mahon A. R. et al. , “Molecular detection of invasive species in heterogeneous mixtures using a microfluidic carbon nanotube platform,” PLoS ONE 6(2), e17280 (2011). 10.1371/journal.pone.0017280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basuray S. et al. , “Shear and AC field enhanced carbon nanotube impedance assay for rapid, sensitive, and mismatch-discriminating DNA hybridization,” ACS Nano 3(7), 1823–1830 (2009). 10.1021/nn9004632 [DOI] [PubMed] [Google Scholar]
- 26.Li D. et al. , “A shear-enhanced CNT-assembly nanosensor platform for ultra-sensitive and selective protein detection,” Biosens. Bioelectron. 97, 143–149 (2017). 10.1016/j.bios.2017.05.053 [DOI] [PubMed] [Google Scholar]
- 27.Chuang C.-H. et al. , “Immunosensor for the ultrasensitive and quantitative detection of bladder cancer in point of care testing,” Biosens. Bioelectron. 84, 126–132 (2016). 10.1016/j.bios.2015.12.103 [DOI] [PubMed] [Google Scholar]
- 28.Ding S. et al. , “CIP2A immunosensor comprised of vertically-aligned carbon nanotube interdigitated electrodes towards point-of-care oral cancer screening,” Biosens. Bioelectron. 117, 68–74 (2018). 10.1016/j.bios.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 29.Maxwell E. J., Mazzeo A. D., and Whitesides G. M., “Paper-based electroanalytical devices for accessible diagnostic testing,” MRS Bull. 38(04), 309–314 (2013). 10.1557/mrs.2013.56 [DOI] [Google Scholar]