Abstract
Background
The risk of hormone positive breast cancer extends beyond 5 years. Extended duration of tamoxifen to 10 years has been shown to improve overall survival (OS) and disease-free survival (DFS). In post-menopausal women aromatase inhibitor (AI) is the gold standard for adjuvant endocrine therapy. Several randomized controlled trials (RCTs) showed benefit with extending the duration of AIs in post-menopausal women. However, the duration and the overall benefit is still controversial.
Methods
Eligible 8 RCTs comprising of 17,190 participants were included in this meta-analysis.
Results
Extending the duration of AI did not show any statistically significant advantage in OS with OR of 1.033 (95% CI: 0.925–1.154, P=0.56), DFS OR of 1.049 (95% CI: 0.930–1.185, P=0.435), recurrence-free survival (RFS) OR of 1.063 (95% CI: 0.952–1.187, P=0.276), and contralateral breast cancer (CBC) OR of 1.094 (95% CI: 0.920–1.301, P=0.311). Higher rates of side-effects of arthralgia, myalgia, hot flushes and bone toxicity was seen among the extended AI group.
Conclusions
Based on this meta-analysis and current literature review, extended use of AI after 5 years of endocrine therapy should be used in selected women with high risk tumour factors. Molecular markers and genomic profiling may assist in identifying the high-risk patients. It is important to consider quality of life and patient satisfaction when considering extending the duration of AI.
Keywords: Adjuvant endocrine therapy, aromatase inhibitor (AI), post-menopausal, extended, breast cancer
Introduction
Management of breast cancer is evolving, aiming to improve survival and decrease recurrence. Effective screening, early diagnosis, improved characterisation of tumour biology and use of neoadjuvant and adjuvant therapies have significantly improved the overall breast cancer outcomes (1-4).
Early breast cancer accounts for majority of all breast cancer. Adjuvant endocrine therapy is the standard of care for oestrogen receptor positive early breast cancer (3-6). It is reported that women with early-stage hormone-positive breast cancer have a prolonged risk for recurrence. This risk extends well beyond 5 years from diagnosis, therefore recurrences can still occur after 5 years of adjuvant endocrine therapy (2-4,7-9). Endocrine therapy includes Tamoxifen, and aromatase inhibitors (AIs). These drugs have been trialled for different period and combinations to reduce the risk of breast cancer recurrence (7,10-12). Published meta-analysis have shown that extended use of Tamoxifen beyond 5 years significantly improved overall survival (OS), recurrence-free survival (RFS), and breast cancer-specific survival (8-10,13-15).
AIs are a gold standard adjuvant endocrine therapy for postmenopausal women with breast cancer (7,11-13). American Society of Clinical Oncology (ASCO) guideline regarding adjuvant endocrine therapy recommends extended tamoxifen treatment for premenopausal women with hormone receptor-positive early breast cancer (5). For postmenopausal patients, a choice remains between four different treatment regimens. They include: AI only for 5 years, sequenced treatment with tamoxifen and AIs for 5 years, extended tamoxifen only for 10 years, or tamoxifen followed by extended AIs for 10 years (12,15-17).
Early clinical trials assessing the duration and efficacy of the AIs published promising results (11,12,15-22). However recent studies did not demonstrate significant reduction in recurrence or breast cancer related mortality. Due to these discrepancies the optimal duration of AIs in post-menopausal women is still debatable. This meta-analysis aims to examine the randomized controlled trials (RCTs) for the potential benefits of extended use of AIs after 5 years of endocrine therapy in the post-menopausal women with early breast cancer. The effect on OS, disease-free survival (DFS), RFS and contralateral breast cancer (CBC) were analysed.
Methods
A systematic literature review of the published RCTs was performed and the meta-analysis was conducted in accordance with the Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This meta-analysis did not require any review of protocols or registration.
Data sources and search strategy
A detailed search strategy was used to search the PubMed, Medline, Ebsco, Embase, Cinahl and Cochrane Library databases were searched for all RCTs until December 2017. Database search used the keywords “breast cancer”, “cancer or carcinoma or malignant or neoplasm”, “hormone or endocrine or anti-hormone”, “early or locally advanced”, “therapy or treatment”, “anastrozole”, “letrozole”, “exemestane”, “duration or period”, “randomised trial or RCT or clinical trial”, “trial or study” and “extended or continued or prolonged”. Abstracts of the yearly conferences of the San Antonio Breast Cancer Symposium (SABCS) and the ASCO were searched for relevant trials (and substituted by full papers if published before December 2017).
Inclusion criteria
We included all the RCTs which met the following criteria: were published in English, patients of any age with hormone receptor positive early or locally advanced breast cancer, RCT’s which investigated the outcomes of extended adjuvant endocrine or hormone therapy in post-menopausal women, assessed the local recurrence, DFS, OS and CBC, and there was no time restriction on publication dates.
Exclusion criteria
Studies, which were not RCTs, case series, reviews, letters, editorials, non-peer reviewed studies, and duplicates were excluded from the analysis.
Data collection and analysis
Study selection and data extraction
Three authors assessed the eligible studies. Disagreements were resolved by discussion and consensus. The following information was extracted from each trial: number of participants, details on the study design, authors, year of publication, type of study, menopausal status, sample size of each arm and subgroups, medial follow-up and outcome measures. The quality of each study was assessed by two authors.
Quality assessment and risk of bias
Cochrane risk of bias tool was used to assess the risk of bias in the included studies. Each RCT was evaluated for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Potential conflicts of interest were determined by considering funding sources. Each domain was assigned a “high,” “low,” or “unclear” risk of bias independently by two reviewers with disagreements adjudicated by a third reviewer (23). Funnel plot was used to assess the risk of publication bias.
Qualitative summary measures of the included studies
The odds ratios and forest plots for OS, DFS, RFS and CBC are summarised in Table 1 and Figures 1-4.
Table 1. OR of outcome measures from the RCT’s.
| Trial | OS | DFS | RFS | CBC |
|---|---|---|---|---|
| MA 17 | 1.03 (0.77–1.37) | 1.07 (0.79–1.44) | 1.02 (0.76–1.36) | 1.02 (0.76–1.36) |
| ABCSG 6a | 1.3 (0.93–1.81) | – | 1.29 (0.93–1.78) | 1.27 (0.92–1.74) |
| NSABP-33 | 0.979 (0.72–1.31) | 1.01 (0.74–1.38) | 1.01 (0.74–1.37) | – |
| NSABP-42 | 0.99 (0.74–1.34) | 1.05 (0.77–1.38) | 1.04 (0.77–1.4) | 1.03 (0.76–1.38) |
| DATA | 1.01 (0.74–1.37) | 1.06 (0.76–1.46) | 1.07 (0.78–1.47) | – |
| LATER | 1.02 (0.67–1.55) | 1.11 (0.73–1.67) | 1.17 (0.77–1.78) | – |
| IDEAL | 1.005 (0.74–1.36) | 1.01 (0.73–1.39) | 0.98 (0.73–1.32) | – |
| MA 17R | 0.98 (0.73–1.33) | 1.04 (0.77–1.41) | 1.01 (0.75–1.37) | – |
Data are presented as OR (95% CI). OS, overall survival; DFS, disease-free survival; RFS, recurrence-free survival; CBC, contralateral breast cancer; OR, odds ratio; RCT, randomized controlled trial.
Figure 1.
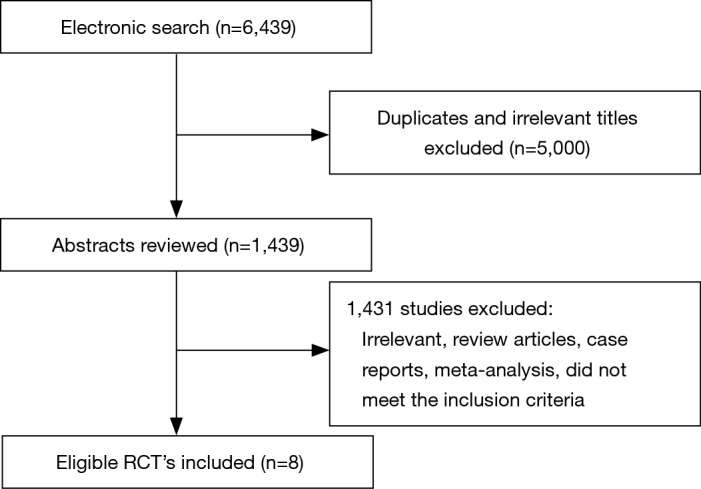
Flowchart of literature search. RCT, randomized controlled trial.
Figure 2.
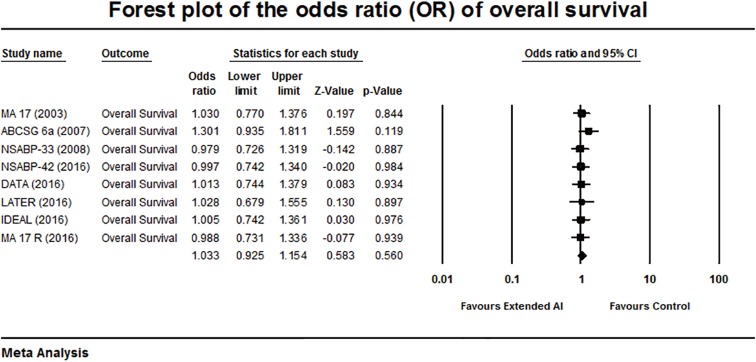
Forest plot of the odds ratio of overall survival.
Figure 3.
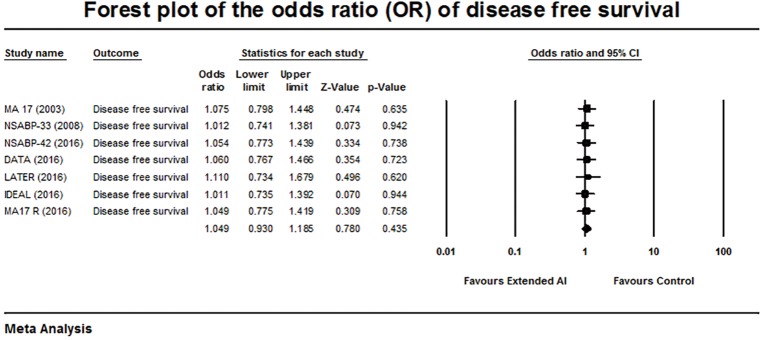
Forest plot of the odds ratio of disease-free survival.
Figure 4.
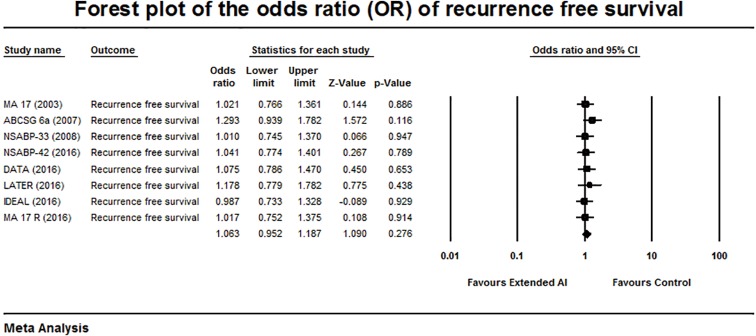
Forest plot of the odds ratio of recurrence-free survival.
Statistical analysis
All the statistical analyses were performed using Comprehensive Meta-analysis (Biostat, version 3.3.070, Englewood, New Jersey, USA) and the SPSS (IBM SPSS Statistics for Windows, version 23.0., New York, USA). The Chi-squared (χ2) test and I2 statistic of inconsistency was used to detect heterogeneity among the studies. The homogeneity among the trials were evaluated by P (or I2). If P≥0.1 (I2≤50%), the trials were classified to be homogeneous, then a fixed-effects model was used. If P<0.1 (I2>50%), the trials were classified as heterogeneous, then a random effects model was used. The combined odds ratio (OR) (outcome measure) with 95% confidence intervals (CI) were calculated using fixed and random-effects models, and the publication bias was assessed with the funnel plot analysis. All statistical tests were two-sided with a statistical significance was set at P<0.05.
Results
Baseline characteristics
The flow diagram for the search results and study selection is shown in Figure 1. A total of 6,439 potentially relevant articles were identified. After excluding duplicates and irrelevant titles a total of 8 RCTs, which met the inclusion criteria were included for analysis (7,13,15,18,19,21,22,24-27). These trials had a total of 17,190 participants (8,553 extended AI arm and 8,637 control arm). All women were post-menopausal with a median follow-up of 5.1 years. Seven out of 8 studies reported OS. Summary baseline characteristics of the included trials are shown in Table 2.
Table 2. Baseline characteristics of the included studies.
| Trial | Year | Description of trial arms | Trial population | Outcome measures | Median follow-up (years) |
|---|---|---|---|---|---|
| MA 17 | 2003 | Phase-III placebo controlled; 5 y Letrozole vs. placebo after 5 y Tamoxifen | 5,157 | DFS, OS, DDFS, CBC | 5.3 |
| ABCSG 6a | 2007 | Phase-III open-label; 3 y Anastrozole vs. observation after 5 y Tamoxifen | 856 | OS, RFS, CBC | 5.2 |
| NSABP-33 | 2008 | Phase-III placebo controlled; 5 y Exemestane vs. placebo after 5 y Tamoxifen | 1,598 | DFS, RFS | 2.5 |
| NSABP-42 | 2016 | Phase-III placebo controlled; 5 y Letrozole vs. placebo after 5 y Tamoxifen ± AI | 3,923 | OS, DFS, DRFS, CBC | 6.9 |
| DATA | 2016 | Phase-III open-label; 6 y vs. 3 y Anastrozole after 5 years Tamoxifen ± AI | 1,660 | DFS, OS, RFS | 4.1 |
| LATER | 2016 | Phase-III open-label; 5 y Letrozole vs. observation after 4≥ Tamoxifen ± AI | 360 | RFS, OS | 3.9 |
| IDEAL | 2016 | Phase-III open-label; 5 y vs. 2.5 y Letrozole after initial 5 y Tamoxifen ± AI | 1,824 | DFS, OS, RFS | 6.6 |
| MA 17R | 2016 | Phase-III placebo controlled; 5 y Letrozole vs. placebo after initial 5 y Tamoxifen ± AI | 1,918 | OS, DFS, RFS | 6.3 |
OS, overall survival; DFS, disease-free survival; RFS, recurrence-free survival; DRFS, distant recurrence-free survival; CBC, contralateral breast cancer; DDFS, distant disease-free survival.
The meta-analysis of the reported OS showed a pooled OR of 1.033, P=0.560 and no heterogeneity among these studies were (Q-statistic showing a Q =2.18, P=0.94, I2=0%). There was no significant advantage in the OS was achieved with extended AI (Figure 2).
Seven out of 8 trials reported DFS with an OR of 1.049, 95% CI: 0.930–1.185, P=0.435 with heterogeneity among the studies (Q-statistic showing a Q =0.2, P=1, and I2=0%) (Figure 3). There was no significant benefit in the DFS was demonstrated with extended AI.
Analysis of the RFS among the trials showed an OR of 1.063, 95% CI: 0.952–1.187, P=0.276 and no heterogeneity (Q =2.2, P=0.94, and I2= 0%) (Figure 4). There was no overall benefit in the RFS was demonstrated with extended AI.
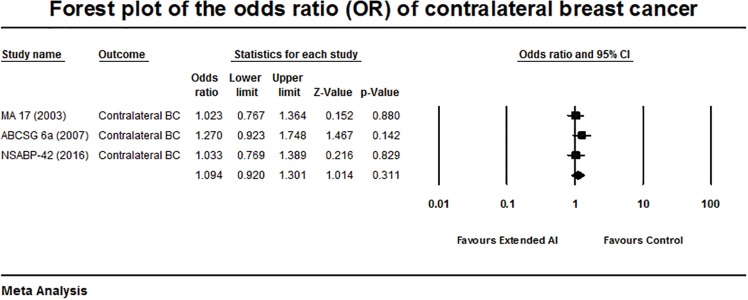
Analysis of the risk of CBC was assessed from 3 out of 8 included trials. The meta-analysis showed no significant difference within the groups with an OR of 1.094, 95% CI: 0.920–1.301, P=0.311 (Q statistics showing Q =1.19, P=0.55, and I2=0% (Figure 5). There was no heterogeneity among the included studies.
Figure 5.
Forest plot of the odds ratio of contralateral breast cancer.
Based on these data there was no significant difference in the odds ratio between the groups to demonstrate a significant benefit with the use of extended AIs.
Publication bias
Cochrane risk assessment tool showed an overall low degree of bias (Table 3). Funnel plot was also performed to investigate the presence of publication bias of the selected studies. The shapes of the funnel plots did not show any evidence of significant asymmetry within the trials and quantitatively there was no publication bias.
Table 3. Cochrane risk of bias tool of the included trials.
| Trial | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting | Other potential sources of bias |
|---|---|---|---|---|---|---|
| MA 17 | Low | High | Unclear | Low | Low | Low |
| ABCSG 6a | Low | High | Low | Low | Low | Unclear |
| NSABP-33 | Low | High | Low | Low | Low | Low |
| NSABP-42 | Low | Unclear | Low | Low | Low | Low |
| DATA | Low | Low | Low | Low | Low | Low |
| LATER | Low | High | Unclear | High | Low | High |
| IDEAL | Low | High | Low | Low | Low | Unclear |
| MA 17R | Low | High | Unclear | Low | Low | Low |
Toxicity associated with extended AIs
Six out of 8 trials reported toxicity and adverse events. Table 4 summarises the common adverse events reported in the included trials. It is evident that osteoporosis, hot flushes, arthralgia and myalgia were higher in the extended AI group.
Table 4. Toxicity related major adverse events (extended aromatase inhibitor vs. placebo).
| Trial | Osteoporosis, % | Hot flushes, % | Arthralgia, % | Myalgia, % |
|---|---|---|---|---|
| MA 17 | 3.6 vs. 2.9 | 47.2 vs. 40.5 | 21.3 vs. 16.6 | 11.8 vs. 9.5 |
| ABCSG 6a | 0.8 vs. 1.1 | 39 vs. 22.4 | – | – |
| NSABP-33 | – | – | – | – |
| NSABP-42 | – | – | – | – |
| DATA | 21 vs. 16 | – | 58 vs. 53 | – |
| LATER | 73.2 vs. 64.1 | – | 74.5 vs. 63.5 | 33.5 vs. 19.9 |
| IDEAL | 10.2 vs. 7.5 | 11.8 vs. 10.5 | 14 vs. 13.2 | – |
| MA 17R | 11 vs. 6 | 38 vs. 37 | 53 vs. 50 | 28 vs. 25 |
Discussion
This meta-analysis included 8 published RCTs, which included a total of 17,190 participants to assess the use of extended adjuvant AI after 5 years of endocrine therapy in post-menopausal women with early hormone positive breast cancer. There were 8,553 participants in the extended AI arm and 8,637 within the control arm. Early hormone-receptor-positive breast cancer is a chronic relapsing disease that can remain clinically dormant for many years. It is well known that in post-menopausal women with hormone positive early breast cancer, the use of AI is superior to Tamoxifen (3-6). Although the RCTs demonstrated minimal benefits, this meta-analysis did not demonstrate a statistically significant overall benefit with the use of extended AIs in the OS, DFS, RFS, or CBC.
The main limitation among the included trials was the duration of the follow-up. This meta-analysis showed a mean follow-up of 5.1 years. Long-term follow-up studies are needed to further assess the role of extended AI and the optimal duration. Most trials showed a decrease with the recurrence rate with the use of extended AI in node positive patients. Current literature shows that the patients with high risk tumour factors such as node positivity and large size should be considered for extended AI (11,12,14,26,28-31). Women with low risk tumours may not receive significant benefits from the use of extended AI. Therefore, their role in low risk patients may be limited. The recent advances in molecular markers and genomic profiling can also assist in identifying the high-risk patients who might benefit from the use of extended AI (32).
It is also well documented that there is an increased risk of bone related side effects such as bone loss and fracture rates with the use of AIs. The use of AI can suppress the conversion of androgens to oestrogens, which leads to osteopenia and osteoporosis. This can lead to increased rate of fragility fractures among those patients taking AI (7,11,12,16,24,27,33). The side-effects and toxicity of AI can also lead to non-compliance and increased levels of unwanted toxicity with its extended use after 5 years. The problem of non-adherence to endocrine therapy is estimated to be between 30% and 60% and may increase with the extended duration of AI. Side effects and absence of conviction are the main factors contributing to non-compliance. Non-compliance can increase the rate of recurrence and mortality. Regular follow-up can improve adherence to AI. Bisphosphonates, cholecalciferol and calcium supplementation is crucial in protecting the patient’s bone health. The use of bisphosphonates can not only reduce bone loss, it is also shown to improve DFS (33-35).
Therefore, based on our meta-analysis and current literature review, extended use of AI after 5 years of endocrine therapy should be used in selected women with high risk tumour factors. Bisphosphonates, calcium and cholecalciferol supplementation should be used in those women on the extended regime to minimise bone loss. Regular follow-up can also improve patient compliance. Newly emerging biomarkers, molecular targets and genomic profiling may also help in identifying high risk patient subgroups. It is also vital to consider quality of life and patient satisfaction when considering extending the duration of AI. Further trials with long-term follow-up is needed to assess the overall benefits of extending the duration of AIs.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. 10.1016/S0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 2.Cianfrocca M. Overcoming recurrence risk: extended adjuvant endocrine therapy. Clin Breast Cancer 2008;8:493-500. 10.3816/CBC.2008.n.059 [DOI] [PubMed] [Google Scholar]
- 3.Yu KD, Wu J, Shen ZZ, et al. Hazard of breast cancer-specific mortality among women with estrogen receptor-positive breast cancer after five years from diagnosis: implication for extended endocrine therapy. J Clin Endocrinol Metab 2012;97:E2201-9. 10.1210/jc.2012-2423 [DOI] [PubMed] [Google Scholar]
- 4.Petrelli F, Coinu A, Cabiddu M, et al. Five or more years of adjuvant endocrine therapy in breast cancer: a meta-analysis of published randomised trials. Breast Cancer Res Treat 2013;140:233-40. 10.1007/s10549-013-2629-4 [DOI] [PubMed] [Google Scholar]
- 5.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J Clin Oncol 2014;32:2255-69. 10.1200/JCO.2013.54.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennecke HF, Olivotto IA, Speers C, et al. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Ann Oncol 2007;18:45-51. 10.1093/annonc/mdl334 [DOI] [PubMed] [Google Scholar]
- 7.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349:1793-802. 10.1056/NEJMoa032312 [DOI] [PubMed] [Google Scholar]
- 8.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomized trial. Lancet 2013;381:805-16. 10.1016/S0140-6736(12)61963-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013;31:abstr 5.
- 10.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) , Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771-84. 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol 2007;25:2664-70. 10.1200/JCO.2006.08.8054 [DOI] [PubMed] [Google Scholar]
- 12.Goldvaser H, AlGorashi I, Ribnikar D, et al. Efficacy of extended adjuvant therapy with aromatase inhibitors in early breast cancer among common clinicopathologically-defined subgroups: A systematic review and meta-analysis. Cancer Treat Rev 2017;60:53-9. 10.1016/j.ctrv.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 13.Higgins MJ, Liedke PE, Goss PE. Extended adjuvant endocrine therapy in hormone dependent breast cancer: the paradigm of the NCIC-CTG MA.17/BIG 1-97 trial. Crit Rev Oncol Hematol 2013;86:23-32. 10.1016/j.critrevonc.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 14.Gadde KM, Allison DB, Ryan DH, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomized trials. Lancet 2015;386:1341-52. 10.1016/S0140-6736(15)61074-1 [DOI] [PubMed] [Google Scholar]
- 15.Goss PE, Ingle JN, Martino S, et al. Efficacy of letrozole extended adjuvant therapy according to estrogen receptor and progesterone receptor status of the primary tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J Clin Oncol 2007;25:2006-11. 10.1200/JCO.2006.09.4482 [DOI] [PubMed] [Google Scholar]
- 16.Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. New Engl J Med 2016;375:209-19. 10.1056/NEJMoa1604700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H, Tu D, Zhao N, et al. Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol 2012;30:718-21. 10.1200/JCO.2010.34.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 2008;26:1965-71. 10.1200/JCO.2007.14.0228 [DOI] [PubMed] [Google Scholar]
- 19.Mamounas EP, Bandos H, Lembersky BC, et al. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy (tx) with letrozole (L) in postmenopausal women with hormone-receptor (+) breast cancer (BC) who have completed previous adjuvant tx with an aromatase inhibitor (AI): Results from NRG Oncology/NSABP B-42. Cancer Res 2017;77:Abstract nr S1-5.
- 20.Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 2007;99:1845-53. 10.1093/jnci/djm246 [DOI] [PubMed] [Google Scholar]
- 21.Zdenkowski N, Forbes JF, Boyle FM, et al. , Australia New Zealand, Breast Cancer Trials G. Observation versus late reintroduction of letrozole as adjuvant endocrine therapy for hormone receptor-positive breast cancer (ANZ0501 LATER): an open-label randomised, controlled trial. Ann Oncol 2016;27:806-12. 10.1093/annonc/mdw055 [DOI] [PubMed] [Google Scholar]
- 22.Goss PE, Ingle JN, Martino S, et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann Oncol 2013;24:355-61. 10.1093/annonc/mds330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Moher D, et al. The Cochrane collaborations tool for assessing risk of bias in randomized controlled trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontein DBY, Nortier JWR, Liefers GJ, et al. High non-compliance in the use of letrozole after 2.5 years of extended adjuvant endocrine therapy. Results from the IDEAL randomized trial. Eur J Surg Oncol 2012;38:110-7. 10.1016/j.ejso.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 25.Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy; results of the randomized phase III IDEAL trial (BOOG 2006-05). J Natl Cancer Inst 2018;110(1). 10.1093/jnci/djx134 [DOI] [PubMed] [Google Scholar]
- 26.Tjan-Heijnen VC, van Hellemond IEG, Peer PG, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol 2017;18:1502-11. 10.1016/S1470-2045(17)30600-9 [DOI] [PubMed] [Google Scholar]
- 27.Gnant M, Mlineritsch B, Stoeger H, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol 2015;26:313-20. 10.1093/annonc/mdu544 [DOI] [PubMed] [Google Scholar]
- 28.Zhang XH-F, Giuliano M, Trivedi MV, et al. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res 2013;19:6389-97. 10.1158/1078-0432.CCR-13-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohlmann PR, Isaacs C. Extended adjuvant endocrine therapy for postmenopausal women: Treating many to benefit a few. J Natl Cancer Inst 2018;110(1). 10.1093/jnci/djx142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldvaser H, Barnes TA, Seruga B, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: A systematic review and meta-analysis. J Natl Cancer Inst 2018;110:31-9. 10.1093/jnci/djx141 [DOI] [PubMed] [Google Scholar]
- 31.van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 2011;377:321-31. 10.1016/S0140-6736(10)62312-4 [DOI] [PubMed] [Google Scholar]
- 32.Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298-305. 10.1158/1078-0432.CCR-13-1845 [DOI] [PubMed] [Google Scholar]
- 33.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 2008;26:556-62. 10.1200/JCO.2007.11.5451 [DOI] [PubMed] [Google Scholar]
- 34.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 2012;134:459-78. 10.1007/s10549-012-2114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the Breast International Group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol 2016;34:2452-9. 10.1200/JCO.2015.63.8619 [DOI] [PMC free article] [PubMed] [Google Scholar]