Abstract
Although anti-inflammatory effects of American ginseng metabolites have been investigated at systemic and cellular levels, the biological signatures of ginseng microbial metabolite-induced bioactivities are still unknown. To fill this knowledge gap and to support the findings published in the companion research article entitled “American ginseng microbial metabolites attenuated DSS-induced colitis and abdominal pain” (Wang et al., 2018), we are here to provide datasets of enteric microbiome biotransformation and fecal metabolomics. For the microbiome biotransformation study, data were obtained from C57BL6 mice treated with a broad-spectrum antibiotic metronidazole. After oral administration of ginseng extract, we observed that compound K (CK) was undetectable in metronidazole-treated mouse stools but was detected in stools from vehicle-treated mice, suggesting biotransformation of CK is gut microbial dependent. In the fecal metabolomic study, three small molecules which were associated with gut inflammation were identified. In the DSS mice, the levels of lactate, linoleic acid, and malic acid increased significantly in the model group. After ginseng treatment, the expressions of these metabolites reduced significantly. Thus, the selective fecal endogenous metabolites could be used as biological signatures reflecting severity of enteric inflammation and ginseng treatment outcomes. Our results showed the enteric microbiome plays a key role for CK conversion, and the effects of CK on enteric inflammation can be demonstrated by the metabolomics data.
Keywords: American ginseng, Biological signature, Colitis, Enteric microbiome, Gut inflammation, Metabolomics, Microbial metabolites
Specifications table
| Subject area | Gastrointestinal inflammation |
| More specific subject area | Colitis, gut inflammation, intestinal microbiome, biological signature |
| Type of data | Figures |
| How data was acquired | LC/TOF-MS and GC/TOF-MS |
| Data format | Analyzed |
| Experimental factors | Ginsenoside compound K was determined in mouse fecal samples with or without metronidazole treatment. Fecal metabolomic profiles of DSS mice and ginseng treated DSS mice were investigated. |
| Experimental features | Metabolomic fecal profiling from DSS model group and ginseng treated group |
| Data source location | Data was collected at University of Chicago, Chicago, IL, USA |
| Data accessibility | Data is provided within this article |
| Related research article | C.Z. Wang, H. Yao, C.F. Zhang, L. Chen, J.Y. Wan, W.H. Huang, et al., American ginseng microbial metabolites attenuate DSS-induced colitis and abdominal pain, Int. Immunopharmacol. 64 (2018) 246–251.[1](DOI:10.1016/j.intimp.2018.09.005) |
Value of the data
-
•
Broad-spectrum antibiotics-treated mouse is a reliable model for initial ginseng biotransformation observation.
-
•
Enteric microbiome induced compound K possesses different biological activities from its parent compound.
-
•
Fecal endogenous metabolites can be used as biological signatures reflecting enteric inflammation.
-
•
Gut disease severity and treatment outcome can be quantified by metabolomics analysis.
1. Data
1.1. In vivo verification of the requirement of enteric microbiome in ginsenoside compound K (CK) biotransformation
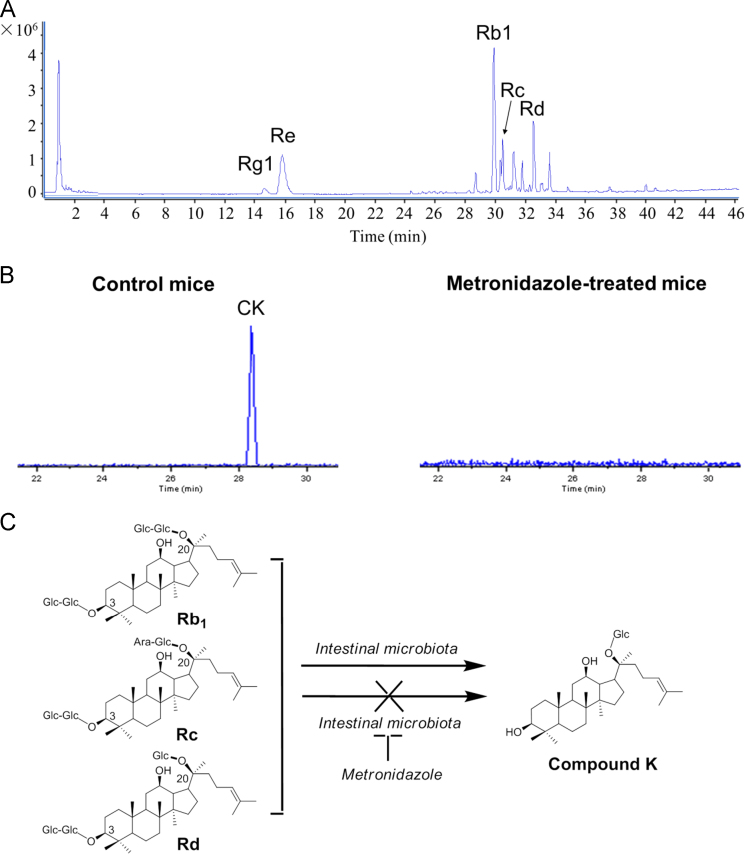
As shown in Fig. 1A, the major ginsenosides in American ginseng extract are Rb1, Rc, Rd, Re, and Rg1, and Rb1 is the primary constituent [2]. After oral ingestion, CK was detected in stools from vehicle control mice, which have normal gut microbiota. In contrast, for the mice pretreated with a broad-spectrum antibiotic metronidazole, CK was not detected in stools (Fig. 1B). Our data suggests that enteric microbiome is required for the biotransformation of CK (Fig. 1C) [3], [4].
Fig. 1.
LC/TOF-MS analysis of ginseng compounds in extract and stool samples. (A) Total ion chromatography of American ginseng extract. Peaks for ginsenosides Rb1, Rc, Rd, Re, and Rg1 in the chromatogram are indicated. Ginsenoside Rb1 is a major constituent in the extract. (B) Extracted ion chromatograms of compound K (CK) with a narrow window of 0.01 Da of mouse stool samples. CK was detected in vehicle control mice, but was not detected in metronidazole-treated mice. (C) Proposed metabolic pathways via gut microbiota from Rb1, Rc, and Rd to CK. Metronidazole eliminated the gut microbiome, and thus inhibited CK biotransformation.
1.2. Metabolomic profiling for biological signatures of ginseng effects on gut inflammation
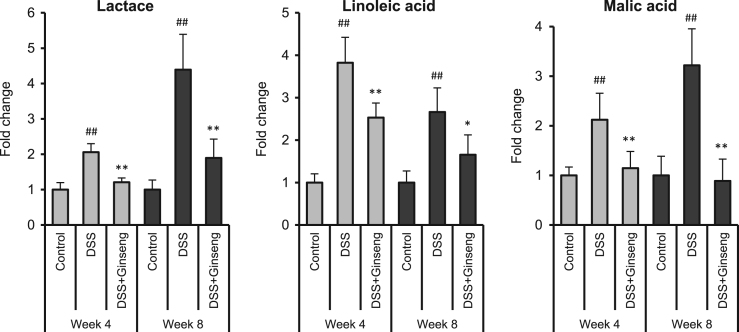
Using the criteria of VIP > 1 and P-value < 0.05, three fecal endogenous metabolites, lactate, linoleic acid, and malic acid, had different expressions in the model and control groups. The metabolite levels were significantly higher in the model group. After ginseng intervention, the levels of these three metabolites decreased significantly (P<0.05 or P<0.01) (Fig. 2). Previous studies have shown that lactate is found in greater abundance in patients with active IBD [5], [6]. Linoleic acid is essential for the synthesis of prostaglandins and their breakdown product PGE2 [7], which is considered as a marker of bowel inflammation [7], [8]. In addition, the malic acid level in plasma and colon tissue significantly increased in DSS mice [9], [10]. Therefore, these three inflammation-related metabolites could be used as biological signatures reflecting gut inflammatory severity and ginseng treatment outcomes.
Fig. 2.
Metabolomic analysis of stool samples in weeks 4 and 8. Three metabolites responsible for the differential expression between the model and control groups were identified. Ginseng treatment restored the expressions of these metabolites. Ordinate values are peak area ratios, that is, relative concentrations (peak area of each groups/peak area of control group) of each metabolite. ##P<0.01 compared with the control group; *P<0.05 and **P<0.01 compared with the model group.
2. Experimental design, materials, and methods
2.1. Chemicals and regents
Ginsenosides Rb1, Rc, Rd, Re, Rg1, and CK were obtained from the Delta Information Center for Natural Organic Compounds (Xuancheng, AH, China). Solvents and other chemical and biological regents were obtained from Fisher Scientific (Pittsburgh, PA, USA) or Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant materials, extract preparation and analysis
The root of American ginseng (Panax quinquefolius L.) was collected from Roland Ginseng, LLC (Wausau, WI, USA). The extraction and LC/MS analysis of American ginseng was carried out as described by Yao et al. [2].
2.3. Microbiome biotransformation of American ginseng extract
This experiment employed 6–8 week-old male C57BL6 mice from Jackson Laboratories (Bar Harbor, ME, USA). All mice received sterile chow diets. Mice were given either un-supplemented drinking water or drinking water supplemented with metronidazole (600 μg/ml) for 7 days (n = 3 per group). Mice then received American ginseng extract in drinking water (30 mg/kg/day) for 3 days. Then mouse stool samples were collected for ginseng metabolite analysis.
Stool samples were extracted using methanol. HPLC/TOF-MS analysis was carried out as described [11]. Extracted ion chromatograms with a 0.01 Da mass window in negative mode (m/z 667.44–667.45) was used for CK detection.
2.4. Fecal metabolomic analysis
Male C57BL6 mice (6–8 week-old) were separated into three groups (n = 5 per group): control, DSS model, and DSS + ginseng groups. The animals in the DSS and DSS + ginseng groups received 2.5% DSS in drinking water for 7 consecutive days [12], [13]. The animals in DSS + ginseng group also consecutively received American ginseng extract in drinking water (30 mg/kg/day) from one week before DSS treatment to 70 days. Stool samples were collected at day 28 (week 4) and day 56 (week 8).
Stool samples were extracted using water and methanol with homogenization [12], [14]. Before analysis, a two-step derivation process was conducted using methoxyamine and BSTFA containing 1% TMCS. GC/TOF-MS analysis and data treatment was carried out as described [12], [15]. The corresponding fold change was calculated, the most changed small molecules were identified and the results were compared to the control.
2.5. Statistical analysis
Data were expressed as mean ± S.D. A student׳s t-test and a one-way ANOVA were used to test the significance of the differences between ginseng treatment and model groups. The statistical significance was set at P<0.05.
Acknowledgements
This study was supported in part by grants from National Institutes of Health (NIH)/National Center for Complementary and Alternative Medicine (NCCAM) AT004418 and AT005362.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.10.131.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Wang C.Z., Yao H., Zhang C.F., Chen L., Wan J.Y., Huang W.H. American ginseng microbial metabolites attenuate DSS-induced colitis and abdominal pain. Int. Immunopharmacol. 2018;64:246–251. doi: 10.1016/j.intimp.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Yao H., Wan J.Y., Zeng J., Huang W.H., Sava-Segal C., Li L. Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncol. Lett. 2018;15:8339–8348. doi: 10.3892/ol.2018.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan J.Y., Liu P., Wang H.Y., Qi L.W., Wang C.Z., Li P. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2013;1286:83–92. doi: 10.1016/j.chroma.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 4.Wan J.Y., Wang C.Z., Liu Z., Zhang Q.H., Musch M.W., Bissonnette M. Determination of American ginseng saponins and their metabolites in human plasma, urine and feces samples by liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1015-1016:62–73. doi: 10.1016/j.jchromb.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjerrum J.T., Wang Y., Hao F., Coskun M., Ludwig C., Gunther U. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn׳s disease and healthy individuals. Metabolomics. 2015;11:122–133. doi: 10.1007/s11306-014-0677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hove H., Nordgaard-Andersen I., Mortensen P.B. Faecal DL-lactate concentration in 100 gastrointestinal patients. Scand. J. Gastroenterol. 1994;29:255–259. doi: 10.3109/00365529409090473. [DOI] [PubMed] [Google Scholar]
- 7.Sheibanie A.F., Yen J.H., Khayrullina T., Emig F., Zhang M., Tuma R. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J. Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 8.Jansson J., Willing B., Lucio M., Fekete A., Dicksved J., Halfvarson J. Metabolomics reveals metabolic biomarkers of Crohn׳s disease. PLoS One. 2009;4:e6386. doi: 10.1371/journal.pone.0006386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong F., Zhang L., Hao F., Tang H., Wang Y. Systemic responses of mice to dextran sulfate sodium-induced acute ulcerative colitis using 1H NMR spectroscopy. J. Proteome Res. 2013;12:2958–2966. doi: 10.1021/pr4002383. [DOI] [PubMed] [Google Scholar]
- 10.Gu X., Song Y., Chai Y., Lu F., Gonzalez F.J., Fan G. GC–MS metabolomics on PPARalpha-dependent exacerbation of colitis. Mol. Biosyst. 2015;11:1329–1337. doi: 10.1039/c5mb00048c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C.Z., Kim K.E., Du G.J., Qi L.W., Wen X.D., Li P. Ultra-performance liquid chromatography and time-of-flight mass spectrometry analysis of ginsenoside metabolites in human plasma. Am. J. Chin. Med. 2011;39:1161–1171. doi: 10.1142/S0192415X11009470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C.Z., Yu C., Wen X.D., Chen L., Zhang C.F., Calway T. American ginseng attenuates colitis-associated colon carcinogenesis in mice: impact on gut microbiota and metabolomics. Cancer Prev. Res. (Phila.) 2016;9:803–811. doi: 10.1158/1940-6207.CAPR-15-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D.S., Kim S.H., Kee J.Y., Han Y.H., Park J., Mun J.G. Eclipta prostrata improves DSS-induced colitis through regulation of inflammatory response in intestinal epithelial cells. Am. J. Chin. Med. 2017;45:1047–1060. doi: 10.1142/S0192415X17500562. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W., Qian S.H., Qian D.W., Li S.L. Screening of intestinal bacterial metabolites of platycodin D using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Am. J. Chin. Med. 2016;44:817–833. doi: 10.1142/S0192415X16500452. [DOI] [PubMed] [Google Scholar]
- 15.Chen X.J., Qiu J.F., Wang Y.T., Wan J.B. Discrimination of three Panax species based on differences in volatile organic compounds using a static headspace GC–MS-based metabolomics approach. Am. J. Chin. Med. 2016;44:663–676. doi: 10.1142/S0192415X16500361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material