Abstract
The neurobiology of stress is studied through behavioral neuroscience, endocrinology, neuronal morphology and neurophysiology. There is a shift in focus toward progressive changes throughout stress paradigms and individual susceptibility to stress that requires methods that allow for longitudinal study design and study of individual differences in stress response. Functional magnetic resonance imaging (fMRI), with the advantages of noninvasiveness and a large field of view, can be used for functionally mapping brain-wide regions and circuits critical to the stress response, making it suitable for longitudinal studies and understanding individual variability of short-term and long-term consequences of stress exposure. In addition, fMRI can be applied to both animals and humans, which is highly valuable in translating findings across species and examining whether the physiology and neural circuits involved in the stress response are conserved in mammals. However, compared to human fMRI studies, there are a number of factors that are essential for the success of fMRI studies in animals. This review discussed the use of fMRI in animal studies of stress. It reviewed advantages, challenges and technical considerations of the animal fMRI methodology as well as recent literature of stress studies using fMRI in animals. It also highlighted the development of combining fMRI with other methods and the future potential of fMRI in animal studies of stress. We conclude that animal fMRI studies, with their flexibility, low cost and short time frame compared to human studies, are crucial to advancing our understanding of the neurobiology of stress.
Keywords: Stress, fMRI, Awake, Rodent
1. Introduction
Stress can have a drastic impact on the central nervous system and lead to the development of neurological and psychological pathologies. Better understanding the neurobiology of stress is a crucial step for the treatment of stress-related brain disorders, which are a leading cause of global disease burden (Ferrari et al., 2013; Baxter et al., 2013). However, the effects of stress are difficult to study due to complex variations in the type of stressors, stress perception, as well as previous exposure to stressors (Mitra et al., 2005; Vyas et al., 2002; Rao et al., 2012). In addition, gender, age and timing (e.g. time and/or duration of exposure) are important factors that can affect stress responses (Lupien et al., 2009). To study the neurobiological effects of stress, behavioral, physiological, genetic and cellular/molecular techniques in neuroscience have been extensively utilized. These techniques have developed substantial knowledge of how stress influences the brain, including the role that different brain systems play in stress response, genetic markers of susceptibility to stress, as well as stress-induced specific changes in neuronal morphology and brain region activity (McEwen et al., 2016; McEwen 1999, 2007, 2012; Lupien et al., 2009; Maren et al., 2013; Takahashi et al., 2005; Sachs et al., 2015; Bremner, 2006; Rogers et al., 2013; Rodrigues et al., 2009; Chattarji et al., 2015; Sapolsky et al., 1990; Krishnan and Nestler, 2008; Russo et al., 2012; De Kloet et al., 2005).
Although the approaches used in studies of stress effects on the brain have provided many advances, they frequently focus on measures of specific brain regions and lack a systems-level view of brain changes. Furthermore, these techniques are often difficult to translate into a clinical setting as the invasive nature of these methods make them undesirable or infeasible for use in humans. Meanwhile, the development of functional neuroimaging techniques has begun to act as an alternative to invasive measures and has become an important addition to the repertoire of tools used to assess the impact of stress on the brain.
Functional magnetic resonance imaging (fMRI), in particular, is commonly used to study brain-wide activity based on blood-oxygenation-level dependent (BOLD) signal, which is an indirect indicator of neuronal activity (Logothetis, 2002). This non-invasive neuroimaging technique has been broadly applied in human research including studies of stress effects (Mueller et al., 2010; Matthews et al., 2006; Seeley et al., 2009; Drysdale et al., 2016; Ochsner et al., 2002; Dedovic et al., 2009a, Dedovic et al., 2009b), which have unveiled neural circuits and regions that are involved in stress response. In parallel to human fMRI research, animal fMRI is highly desirable as it allows discoveries made in pre-clinical studies to inform human studies using the same methodology, and vice versa. Such translational utility is likely needed to facilitate more rapid improvement in the clinic (Steimer, 2011). In addition, this systems-level approach allows stress effects to be studied on the scale of the whole brain, and provides guidance for further in-depth research focusing on brain regions critical in stress response that were overlooked or underexplored in the past with other well-established preclinical tools.
Despite the great advantages of fMRI in animal studies, historically it was significantly underutilized due to a number of technical challenges (see below for more discussion). However, recent advances in animal functional neuroimaging techniques has led to an expansion of translational fMRI studies in mapping stress effects on the brain. In addition, techniques complementary to the fMRI methods are starting to be used in multi-modal methodology experiments to validate and add to the information gained using functional neuroimaging approaches. As the field continues to expand, these powerful techniques will provide a set of tools and eventually become common place in studies that aim to understand how stress effects the brain.
This review will discuss the use of fMRI in animal studies of stress. It will explain advantages, challenges and technical considerations of animal fMRI methods, review recent literature using fMRI in studying the neurobiology of stress, as well as highlight the development of combining fMRI with other techniques and the future potential of fMRI in animal studies of stress.
2. fMRI methodology
fMRI indirectly measures neural activity using BOLD signal (Kwong et al., 1992; Bandettini et al., 1992; Ogawa et al. 1990, 1992). This signal originates from the generalized increase in cerebral blood flow supplies that over-compensate oxygen consumption need, resulting in a net change in deoxyhemoglobin concentration at activated brain regions (Bandettini, 2012; Logothetis et al., 2001). Such changes can be reliably detected in T2/T2*-weighted MRI images due to the paramagnetic nature of deoxyhemoglobin (Ogawa et al., 1990).
fMRI experimental designs include task-based experiments, used to explore brain region activation in response to specific discrete events or blocks of stimuli, as well as resting state experiments, used to explore how brain regions are functionally connected and how brain network connectivity patterns differ at separate states (Bandettini et al., 1993; Petersen and Dubis, 2012; Dale and Buckner, 1997; Poldrack, 2012; Biswal et al., 1995; van den Heuvel and Hulshoff Pol, 2010; Bandettini, 2012). The selection of an experimental design is important in determining the interpretation of results. In regards to functional imaging of stress, both designs have been utilized. Task-based designs were used to functionally map brain regions involved in the perception and processing of stress. These experiments can provide insight into regional functional activity during stress exposure and individual variation in response to stressors. Specific to animal fMRI studies, task-related stimuli can be applied to a particular brain region or neural circuit with cell-type specificity when fMRI is combined with techniques like optogenetics (Lee et al., 2010). Resting state fMRI (rsfMRI) can help further decide neural circuit function related to the stress stimulus (Liang et al., 2014). rsfMRI has particular potential in clinics given its simplicity in experimental design and the consistency afforded due to the absence of task (Fox and Greicius, 2010).
3. Advantages of animal fMRI studies of stress
fMRI has become a prominent tool to investigate the effect of stress on the brain. It offers several advantages; i) it is non-invasive; ii) it is highly translatable; iii) it can be utilized with flexible experimental design, making it simple to use in both human and animal studies; and iv) it offers a field of view large enough to image the whole brain while maintaining relatively high spatial resolution, especially compared to other non-invasive measures of brain activity like electroencephalography (EEG). As a result, fMRI can be used to investigate functional networks implicated in the stress response, help discover new regions of interest, is well suited for longitudinal studies crucial to unveiling progressive changes after stress exposure and define endophenotypes of stress-related susceptibility and disorders.
These advantages have made fMRI a powerful tool to study the impact of stress on the brain. A number of fMRI studies have revealed the involvement of amygdala, hippocampus, medial prefrontal cortex and insula in general stress response. In addition, altered activation patterns in these regions were consistently shown during emotional processing in patients with stress-related brain disorders (Rauch et al., 2006; Etkin and Wager, 2007; Milad et al., 2009; Rauch et al., 2000; Lanius et al., 2001; Phelps et al., 2004; Shin and Wright, 2005; Dedovic et al., 2009a, Dedovic et al., 2009b; Dedovic et al., 2005). In this review we will focus on the results of animal fMRI research, and an in-depth review of human fMRI findings can be found elsewhere. However, consistent results from human research are considered to show relevance and translational value of animal fMRI studies (Hughes and Shin, 2011; Bremner, 2007; Wang et al., 2016).
3.1. Experimental flexibility
Both task-based fMRI and rsfMRI can be used in animal studies. This provides the flexibility of studying any task that can be reasonably employed while the animal is in the scanner or studying the functional connectivity of the brain in animals at rest. Furthermore, techniques like pharmaceutical fMRI (phMRI) can be used to analyze the effects of drug administration on brain function (Reese et al., 2000; Littlewood et al., 2006; Schwarz et al., 2009; Pérez et al., 2018).
One area that boasts a great potential is the use of animal fMRI in combination with other methodology that can provide complementary information of brain function. The addition of another modality that can be used in conjunction with fMRI provides more flexibility and interpretability of results from experiments. Animal fMRI is being combined with techniques including electrophysiology (Logothetis et al., 2001; Pan et al., 2010; Shih et al., 2013), calcium signal recording (Duong et al., 2000; Schulz et al., 2012; Liang et al., 2017), direct brain stimulation (Lee et al., 2010; Kahn et al., 2011; Shih et al., 2014; Albaugh et al., 2016; Decot et al., 2017; Van Den Berge et al., 2017), and designer receptors exclusively activated by designer drugs (DREADDs) (Grayson et al., 2016; Giorgi et al., 2017). These multi-modal techniques allow researchers to harness the advantages of separate techniques to extract complementary and multi-dimensional information in the same animal. For instance, fMRI has the advantage of full-brain coverage at high spatial resolution, but is dependent on an indirect measurement of neuronal activity at slow temporal resolution compared to neuronal activity. These disadvantages can be compensated for by simultaneously measuring neuronal spiking based on calcium signal recording using fiber photometry (Liang et al., 2017; Schulz et al., 2012; Schwalm et al., 2017). The utilization of multi-modal techniques also allows for the comparison of data acquired through different modalities that can further help understand neural mechanisms and provide cross-validation of experimental results.
3.2. Well suited for longitudinal studies
Animal fMRI studies are well suited for longitudinal designs that can differentiate brain changes as a consequence of stress exposure from those that result from predisposition. While human studies have highlighted the importance of fMRI techniques to quantitatively assess stress response and identify brain regions/circuits associated with stress disorders, methods are needed that can distinguish preexisting susceptibility markers in brain function from brain function changes that occur as a result of disease development. This distinction will be important for the capacity to help diagnose mental disorder, develop preventative measures and properly inform therapeutic options through the use of fMRI (Markou et al., 2009; Steimer, 2011). For instance, studies in humans have shown a number of brain regions that exhibit distinct functional differences including the anterior cingulate, prefrontal cortex and amygdala in patients with PTSD compared to healthy controls (Rauch et al., 2006; Maron-Katz et al., 2016; Etkin and Wager, 2007; Bremner, 2007; Hughes and Shin, 2011; Lanius et al., 2001; Wang et al., 2016). However, given that most human studies provide only a snapshot of the state of brain function, it can be difficult to unequivocally interpret the differences found, as these differences could be a result of changes that occurred during the development of PTSD, differences preexisting in subjects that have a varying level of susceptibility to developing PTSD, or both. For example, differences in the hippocampal volume, cortisol levels and activation of brain regions like the anterior cingulate cortex have been shown in PTSD patients versus healthy controls (Yehuda et al., 1990; Schmidt et al., 2013; Bremner et al., 1995; Bonne et al., 2008; Liberzon and Sripada, 2007; Sripada et al., 2012). However, twin studies have demonstrated that some of these differences may be a predisposition, rather than a consequence of trauma exposure. For instance, smaller hippocampal volume and increased anterior cingulate activity were also present in twin siblings of PTSD patients who were not exposed to traumatic stress (e.g. warzones) (Yehuda et al., 1998; Gilbertson, 2002; Shin et al., 2011).
This issue can be easily addressed in animal studies with a research design that acquires baseline, disease development, and disease progression measures (but would be difficult, if possible, to test in humans). Particularly for the study of stress effects, such a longitudinal design can be critical as it allows for the comparison of data both before and after stress within the same subject. Given the non-invasive nature, fMRI is well suitable for this longitudinal design (Gorges et al., 2017).
3.3. Non-invasiveness
Neuroscientists have developed numerous tools to study the brain and its role in behavior and cognition. Many techniques involve invasive procedures that damage the brain and are therefore not readily translatable to human studies. For instance, many measures of neural activity used in neuroscience studies require a surgical procedure that can be stressful and time consuming (Fornari et al, 2012). While neural implants have improved biocompatibility, there are still issues with injuries caused by implantation and foreign body responses that can adversely impact the signal quality of implanted devices (Kozai et al., 2015). Therefore, although these invasive methods have led to major advances in understanding brain function, it is important to use non-invasive tools to study the intact brain and make relevant observations of the brain's role in behavior that are more easily translated to humans. In addition, non-invasive methods allow for longitudinal design that can test the reproducibility of results and/or trace how the brain changes over time under manipulation.
The ability to acquire a measure of neural activity in an entirely non-invasive manner using fMRI in animal studies opens many experimental options, such as measuring neural activity in animals without creating any tissue damage (e.g. surgery-induced inflammation). Additionally, the non-invasive nature allows fMRI experiments to be repeatedly conducted in the same group of subjects, and it has been shown that over different scanning days there is reasonable test-retest reliability in both human and animal fMRI data (Cao et al., 2014; Termenon et al., 2016; Liang et al., 2011; Liang et al., 2012b; Liang et al., 2015a). Therefore, fMRI can play a prominent role in studies of stress effects on the brain.
3.4. Translatable nature
Since the method of fMRI is virtually identical in both animal and human studies, the results are directly translatable. This allows for cyclical flow of information between animal and human studies (Pan et al., 2015). Such translational utility can provide insight into understanding the evolutionary development of relevant brain regions and circuits involved in the stress response (Milad and Quirk, 2012).
3.5. Whole-brain field of view
Another advantage fMRI offers when compared to other measures of neural function is its large field of view. While neural implants would allow for local recordings of neuronal activity at a specific brain region, fMRI can be used to image the whole brain with sub-mm resolution (Glover, 2011; Kajikawa and Schoeder, 2012; Zhang et al., 2010a). This gives the opportunity to study many distinct brain regions simultaneously and study circuit-level brain activity (Hoyer et al., 2014; Liang et al., 2015a, Liang et al., 2015b). This capacity makes it possible to conduct data-driven analyses in fMRI studies that can highlight brain regions that are outside of the typical focus in the field, while still providing information on the brain regions that are the main focus (Maldjian et al., 2003; Cole et al., 2010).
With these specific advantages, fMRI has begun to emerge as an important tool in studies that investigate how an organism responds to stress.
3.6. Technical considerations of animal fMRI studies on stress
Before we comprehensively review the findings of animal fMRI studies on stress, several technical factors that affect fMRI results in animals should be discussed including spatial resolution, comparative neuroanatomy between animal and human brains and the conscious state of the animal. In fact, even with the evident advantages of fMRI in animal studies discussed above, this technique is still under-utilized in animal research, including in the investigation of stress, due to the difficulty to address these issues.
3.7. Resolution and signal-to-noise ratio
The spatial resolution of fMRI is determined by the brain size (more precisely, field of view) divided by the sampling matrix size. Typically, the matrix size of fMRI images is similar between animals and humans (64 × 64 – 128 × 128). However, the size of the brain is significantly larger in humans (average size: 1200 cm3) than that in rats (average size: 1200 mm3), and mice (average size: 415 mm3) (Nopoulos et al., 2000; Sahin et al., 2011). This means that a comparable resolution in a rat requires a 1000-fold smaller voxel size. This difference results in not only considerably smaller signal-to-noise ratio (SNR) in animal fMRI data, but also motion artifacts, distortion and physiological noise that differentially impact image quality. While this had been a barrier to expanding fMRI into animal studies in the past, these challenges are successfully addressed with the improvements of imaging techniques. Higher magnetic field strengths, better restraint methods, improved hardware and improved imaging methods have made it possible to acquire high quality images at high resolutions needed for rodent brain imaging (Wald, 2012; Duyn, 2012). For instance, gradient improvements of pre-clinical systems allow for imaging with much larger matrices than a decade ago. In addition, recent progress made by the functional imaging community in humans has made it possible to achieve extremely high resolutions at higher magnetic field strength (Yacoub et al., 2007; Zimmermann et al., 2011) (for review see (Yacoub and Wald, 2018)). Furthermore, the use of superparamagnetic iron oxide (e.g. MION) as a contrast agent can further improve the spatial specificity (Zhang et al., 2010b) and SNR (Decot et al., 2017) of fMRI. At the resolutions available now it is possible to parcellate functionally distinct brain regions in rats with high reproducibility across animals and even across scanners at distinct field strengths (Ma et al., 2016). Even within a subcortical structure like the thalamus, fMRI has revealed distinct functional profiles for separate sub-nuclei that are in agreement with well-known connectivity patterns of thalamocortical networks in rats (Liang et al., 2013).
3.8. Comparative neuroanatomy
Clinical trials often explore mechanisms implicated through studies of animal models and studies in animals often focus on mechanisms translatable to human studies. Objective measures in clinical research of neurological and psychiatric illness that have homologs in animals are increasingly used for the refinement of animal models (Markou et al., 2009; Deslauriers et al., 2018). This is particularly the case in stress studies with the use of behavioral, endocrine and fMRI measures acting to improve translatability as these methods can also be used in clinical trials (Hooijmans and Ritskes-Hoitinga, 2013). This improved similarity of measures and improved information flow between clinical and pre-clinical work help enhance translatability of studies. However, it is important to acknowledge that the jump from any species to another is not trivial. Even within rodent species careful work must be done to relate fMRI data (Jonckers et al., 2011). All studies with a translational goal must consider the comparative neuroanatomy of the species involved and the replication of results between the species. Granted even if the region of the brain is clearly defined in both species there can be distinctions in function or structure that may be important to consider when interpreting the translational value of any study's results. However, the translational benefit of designing studies of stress in animal models, particularly in rodents, is supported by the extensive knowledge of rodent neuroanatomy, general conservation of the stress response and numerous comparative anatomical studies (Paxinos et al., 1980; George Paxinos, 2001; Semple et al., 2013; Clancy et al., 2007; Hooijmans and Ritskes-Hoitinga, 2013; Milad & Quirk 2002, 2012). Many published fMRI studies have shown general conservation of regions and circuits between rodents and humans (Malter Cohen et al., 2013; Gozzi and Schwarz, 2016; Lu et al., 2012). Increasing the use of fMRI in rodent studies will further improve the understanding of translational relevance of rodent models in studying functional networks of the brain (Sakoğlu et al., 2011; Mogil et al., 2010). For instance, characteristic functional neural networks such as the default-mode network and salience networks were demonstrated in multiple animal species and humans (Fox et al., 2005; Lu et al., 2012; Stafford et al., 2014; Vincent et al., 2007), and use of these network definitions are commonly applied in the study of stress (DiGangi et al., 2016; Hermans et al., 2014; Suo et al., 2015). Taken together, the translational nature of rodent studies of stress can potentially be driven by extensive fMRI data from human studies that can be compared to fMRI data from animal studies, ultimately adding to our understanding of the comparative functional neuroanatomy between animals, animal models and humans.
3.9. Awake vs anesthetized
The expansion of fMRI into a preclinical setting was first seen using anesthetized rodents. In order to acquire meaningful functional neuroimaging data, subjects must remain still during imaging. Even in anesthetized animals motion correction was acknowledged as an important step in data preprocessing during the development of animal fMRI techniques and has been an area of consistent improvement (Hajnal et al., 1994; Oakes et al., 2005; Power et al., 2015). In the case of rodent studies movement of only 0.5 mm in any direction can create a shift in the brain map of a full voxel. Initial fMRI experiments were performed on anesthetized animals partially due to this issue of motion control. However, brain function is profoundly altered across different levels of consciousness, which suggests that anesthesia is a significant confounding factor when studying the effects of stress on the brain (Aksenov et al., 2015; Hamilton et al., 2017; Liang et al., 2012a, Liang et al., 2012b; Ma et al., 2017; Pisauro et al., 2013).
Anesthetic agents are well documented to affect neural activity and different aspects of physiology including body temperature, respiration rate and vascular function that can all have an impact in altering the neurovascular response that underlies fMRI signal (Flecknell, 2009). Even more complicatedly, the effects of anesthesia on fMRI measures depend on the type and dose of anesthetic agents (Grandjean et al., 2014; Jonckers et al., 2011). Recent work has highlighted this complexity by showing unique effects for separate anesthetic protocols on region-specific functional connectivity, the default-mode network configuration and brain topological organization (Paasonen et al., 2018). Specific regions that are particularly sensitive to different anesthetic protocols include the medial prefrontal cortex, hippocampus, thalamus and hypothalamus, which are all implicated in studies of stress. Experiments in our lab also showed a graded effect of anesthetic-induced unconsciousness on functional connectivity. It was found that information exchange between brain regions was reduced with decrease in consciousness as shown through a global reduction in functional connectivity, increase in entropy and reduction in mutual information (Hamilton et al., 2017). Furthermore, we showed that the whole-brain network exhibited distinct connectivity patterns at different anesthetized states (Ma et al., 2017), further suggesting that the impact of anesthesia on the brain is a network phenomenon at the whole-brain scale. Similar results have been found in other species (Aksenov et al., 2015). Due to the results described above, it is difficult to interpret a stress response and correlate brain function measured by fMRI in anesthetized animals with behavioral measures typically assessed in the awake state. This obstacle made it important to extend animal imaging to awake imaging (see review on imaging anesthetized and awake animals (Gao et al., 2017)).
Fortunately, procedures that allow the awake imaging of animals to be performed have been developed and utilized in numerous species including rats, mice, rabbits, dogs and monkeys (Berns et al., 2012; Goense and Logothetis, 2008; Schroeder et al., 2016; Desai et al., 2011; Lahti et al., 1998; King et al., 2005; Bergmann et al., 2016; Chang et al., 2016; Yoshida et al., 2016). These procedures often include a training period to acclimate animals to the scanning environment. Acclimation procedures have been utilized by a number of labs and have been shown on multiple occasions to diminish the motion and stress level of animals, measured by stress hormone levels, respiratory rate, heart rate and behavior (Madularu et al., 2017; Schultz-Darken et al., 2004; Duyn, 2011; King et al., 2005; Ferenczi et al., 2016; Liang et al., 2014). These methods make it possible to image fully awake animals and allow for task-based designs to functionally map stress response in the brain, as well as a more accurate determination of resting-state functional networks related to stress processing in animals. Results from initial experiments in this field are reviewed below. Considering the pronounced confounding effects of anesthesia, in this review we only focus on fMRI studies in awake animals. However, it is worth noting that the use of anesthetics in fMRI studies will likely remain an important methodological tool in experimental protocols that prevent the implementation of acclimation procedures, or that utilize the unique effects of anesthetic agents as part of the study design and results interpretations, as suggested by Paasonen et al. (2018).
4. fMRI studies of effects of stress on the brain in awake animals
The brain's acute response to stress has been studied in awake rats using task-based fMRI design. Intruder threat was one of the first stimuli utilized in these studies. In a study designed to image neural circuitry of aggressive motivation, male rats were imaged when presented with their female cage mate and a novel male intruder rat. This intruder threat stimulus activated the amygdala, lateral hypothalamus, hippocampus, anterior cingulate cortex, retrosplenial cortex, and the anterior thalamus (Ferris et al., 2008). Since an intruder stress is a driver of an aggressive (fight) response, the revealed neural circuitry in this study likely includes the brain's response to stress. This notion is supported by the consistent results in a series of follow-up studies, one of which measured the brain activity of dams in response to a male intruder, giving further insight into the emotional response to threat in female rats. Intruder threat activated stress-related regions such as the septum, anterior cingulate cortex, anterior thalamus, basal amygdala, hippocampus, orbital, insular and prelimbic cortices (Nephew et al., 2009). In a similar intruder threat paradigm, rats conditioned to the presentation of a sable ferret with a gustatory cue were imaged once during the initial fear conditioning and again 14 days later with only the conditioned cue as a stimulus. Significant activation in the anterior thalamus, hippocampus, amygdala, mamillary bodies, somatosensory, orbital, retrosplenial, and prelimbic cortices was observed in response to this conditioned fear stimulus (Ferris et al., 2011).
The results from aforementioned studies have been suggested as a generalized activation of Papez's circuit, which includes many of the regions implicated in the response to emotionally relevant and fear invoking stimuli, and the regions in the salience network, which is involved in detecting behaviorally relevant stimuli. These regions have also been shown to be active in humans during acute stress, suggesting a comparative response to stress in rats and humans (van Marle et al., 2010; Hermans et al., 2014). For example, in a human fMRI study the subject was engaged in an active “escape-pain” task by escaping a two-dimensional maze before being caught by a “virtual predator.” During this task, activity of the anterior cingulate and orbital cortices was enhanced during the initial 2 s of threat detection. During the “chase phase”, increased activity was respectively shown in the central nucleus/bed nuclei of the stria terminalis and the basolateral amygdala depending on whether a high predator threat was distal or proximal (Mobbs et al., 2007).
Other studies have looked at the effect of altering stress hormone levels on modulating brain function. Imaging was performed on awake adrenalectomized rats with intravenous delivery of corticosterone (CORT) at two dosages to mimic serum levels present after moderate and severe stress, respectively. High dose administration of CORT led to a significantly larger increase in BOLD signal from regions localized to the hippocampus and cortex. After a minute of CORT administration, the BOLD signal of the prefrontal cortex, cingulate cortex, motor cortex, CA1 and ventral thalamus was significantly higher in the high dose group. This effect expanded after 5 min of CORT injection with the high dose group showing greater BOLD signal in cortical regions, the hippocampus, dorsal striatum and lateral hypothalamus (Ferris and Stolberg, 2010). These results are supported by structural changes seen in previous studies of stress effects on the brain, ranging from dendritic remodeling in the hippocampus and prefrontal cortex, decreases in cingulate volume, to studies where lesion of anterior thalamic nuclei produced decreased anxiety in rats (McEwen, 1999; McEwen et al., 2016; Dupire et al., 2013).
Functional imaging of awake rodents has expanded in recent years, resulting in the use of more in-depth experimental manipulations and testing the role these manipulations play in altering brain function. Brydges et al. (2013) performed fear conditioning outside of the scanning environment. In one group of rats, paired neutral cue and noxious stimulus were presented, whereas in the other group, the neutral cue and noxious stimulus were unpaired. The response of both groups of rats to the neutral cue was subsequently tested inside the scanner. Comparison of the two groups showed greater BOLD response in the lateral amygdala, hypothalamus, granular insular cortex and somatosensory cortex in the paired group (Brydges et al., 2013). These results were also supported with fMRI of fear conditioned mice, which showed significantly increased BOLD response of the left amygdala and a trend toward significantly increased BOLD response of the hypothalamus and granular insular, similar to what was shown in rats (Harris et al., 2015).
These fMRI studies have provided results that are in general consistent with previous work involving the study of stress, stress hormones and fear conditioning, which show that different forms of stress elicit activation of a set of brain regions typically including the amygdala, hippocampus, hypothalamus, agranular insular and medial prefrontal cortex (Martinez et al., 2013; McEwen et al., 2016; Milad and Quirk, 2012; Moscarello and LeDoux, 2013; Schiller et al., 2013). This consistency has offered confidence for further expanding the application of awake animal fMRI methods to studying stress effects and stress-related disorders.
5. fMRI studies of animal models of stress-related brain disorders
The importance of animal models of brain disorders in neuroscience is beyond doubt (Lister, 1990; Van Den Buuse et al., 2005; Mogil, 2009; Nestler and Hyman, 2010). Often times animal models act to provide a basic scientific understanding of changes that occur in disease using methods that are not plausible for use in human studies and are the first step in testing treatment options. In the case of brain disorders, the development and validation of animal models still play a critical role, given the lack of foundational knowledge of mechanisms of most brain disorders. Therefore, studies of animal models that utilize translatable measures like fMRI are particularly valuable, since these modalities allow for similar experimental designs to be taken in both preclinical and clinical settings. The studies reviewed show clear benefit of conducting research using highly translational methods that is particularly relevant for advancing our understanding of stress-related brain disorders.
In a study similar to the ferret exposure experiment described earlier, two different genetic strains, Flinders sensitive line (FSL), a genetic animal model of depression, and Flinders resistant strain (FRS) of rats were exposed to a stressful event while being scanned. Rats were functionally imaged during the presentation of either a neutral scent or trimethylthiazoline (TMT), a synthetic compound isolated from fox feces that elicits innate fear response in rodents. While there were no significant differences between the two strains in a selection of brain regions associated with fear in response to the neutral scent, response to TMT elicited significantly stronger BOLD signal in FSL rats in the cortical nucleus of the amygdala and insular cortex compared to the FRL rats. FSL rats also showed significantly weaker BOLD signal in response to TMT in the prefrontal cortex compared to the FRL rats. These results show the potential for fMRI to elucidate the differences in the neural substrate of different genetic animal models in relation to the stress response (Huang et al., 2011).
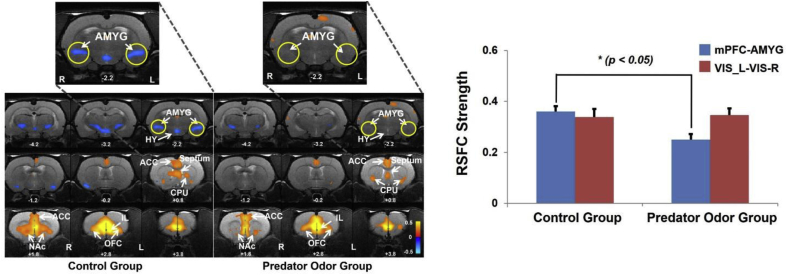
In our lab fMRI of awake rodents has been used to reveal the impact of single exposure of traumatic stress on resting-state functional connectivity of the brain over an extended time frame. We showed that there were impacts on neuroplasticity in rats that were exposed to a single exposure to predator odor scent in an inescapable environment compared to non-exposed rats (Fig. 1). In particular, rsfMRI data measured 7 days post exposure showed that the functional connectivity between the amygdala and the infralimbic (IL) subdivision of medial prefrontal cortex was significantly diminished in the stressed group (Liang et al., 2014). These results provide support for the face validity and construct validity of using predator scent stress as a model of PTSD, given that past work has shown the importance of the infralimbic cortex connectivity with the amygdala in the formation of extinction memories and the inhibition of fear (Milad and Quirk, 2002; Sotres-Bayon and Quirk, 2010). Therefore, our work has demonstrated the potential of awake animal rsfMRI to identify neural circuit plasticity in animal models. Ongoing studies in our lab have begun to employ fMRI in longitudinal studies of disease models, a necessary step in the exploration of disease progression that fMRI is particularly well suited for (Fox and Greicius, 2010).
Fig. 1.
Long-lasting changes in functional connectivity between the medical prefrontal cortex (mPFC) and amygdala (AMYG) in rats exposed a single-episode predator odor in an inescapable environment. rsfMRI measurement was conducted 7 days post exposure. The resting-state functional connectivity (RSFC) strength in the mPFC-AMYG circuit was significantly lower in predator odor rats relative to controls (p < 0.05, n = 8 for each group). In a control circuit, trauma exposure did not induce any changes in the connectivity of the visual system measured by RSFC between the left and right primary visual cortices (p = 0.82, two sample t-test). Bars are SEM. AMYG, amygdala; ACC, anterior cingulate cortex; CPU, caudate Putamen; NAc, nucleus accumbens; OFC, orbital cortex; VIS_L, left visual cortex; VIS_R, right visual cortex. Adapted from (Liang et al., 2014).
Notably, although fMRI has been shown to be a valuable tool to understand stress-related disorders, there are certain caveats that have to be considered when one interprets the fMRI results in disease models. For instance, since the BOLD signal relies on blood flow change to reflect neuronal activity, any alteration in the vascular system and/or neurovascular coupling due to the modeled disorder can lead to misinterpretation of fMRI results in relation to the underlying neuronal activity. In that situation, understanding of disorder-induced changes in vascular response and neurovascular coupling relationship becomes crucial for fMRI studies of the disease.
6. Factors essential for the success of awake animal fMRI experiments
Even though the concept of awake animal imaging has been in use for years, its implementation and applications remain relatively limited. This is attributed to a number of technical factors that are essential for the success of conducting fMRI experiments in awake animals. These technical aspects of imaging are under continuous development for further improvement of imaging data quality.
6.1. Restrainer
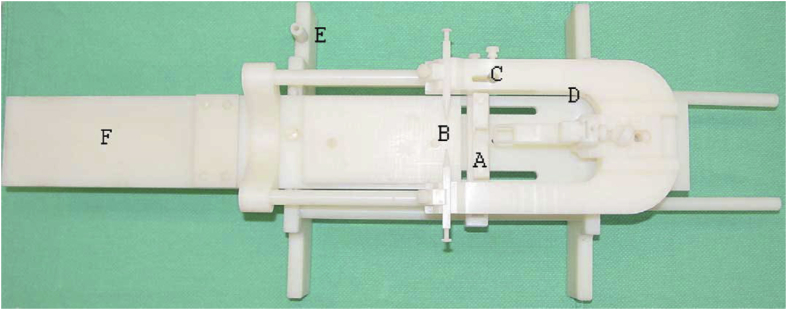
In order to perform fMRI in small animals, a device is needed to hold the animal's head in place to avoid large motion artifacts (Power et al., 2015). A properly-designed device to restrain the animal is important to improve data quality even when imaging anesthetized animals. The setup that has been used in fMRI studies in rodents is relatively straight forward. It often involves some form of body restraint to hold the animal, a stereotaxic-like device that consists of a bite bar and ear bars to hold the animal's head in place, and a form of ventilation or intravenous cannulation for the continuous administration of pharmacological agents (Fig. 2) (Pawela et al., 2008; Hutchison et al., 2010; Kalthoff et al., 2011). These devices can be custom made by the laboratory that performs imaging.
Fig. 2.
The MRI compatible stereotactic frame that incorporated a bite bar (A), ear bars (B), a slot for mounting a radiofrequency (RF) surface coil (C), and a peg (E) to secure the frame against the MRI table. (D) is used to stabilize the animals head and the animal's tail resting on F. Adapted from (Mirsattari et al., 2005).
These devices were effective in reducing motion artifacts in the assessment of BOLD signal (Lahti et al., 1998; Mirsattari et al., 2005). Given the stressful restraint environment during imaging, consideration of stress levels during imaging is necessary. This consideration leads to the development of acclimation procedures to reduce the stress level of animals during imaging.
6.2. Acclimation
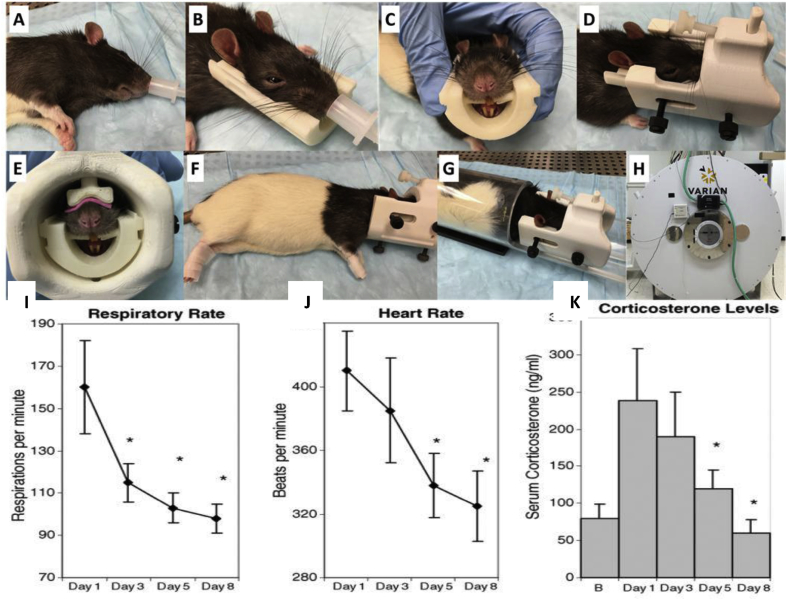
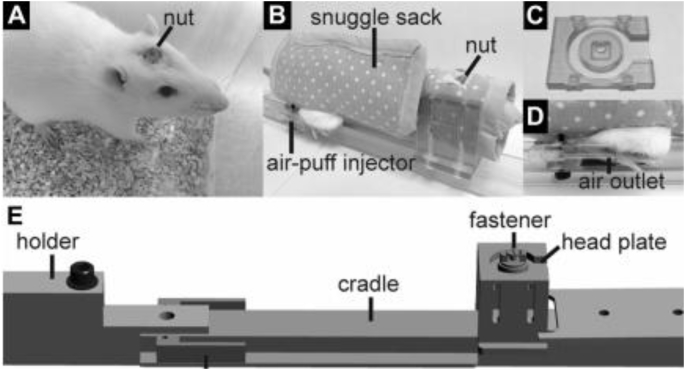
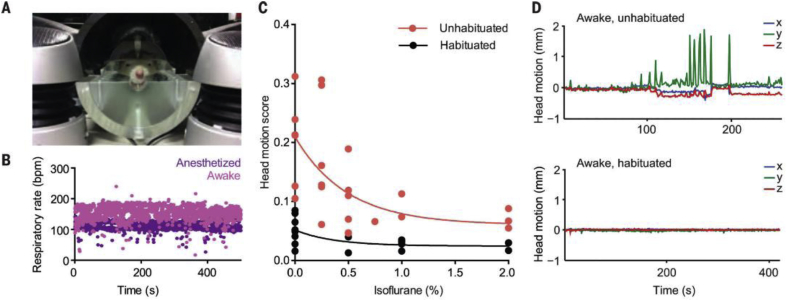
Acclimation is a crucial procedure when performing fMRI in awake animals, particularly for research into stress. Given that neuroimaging can be a stressful event itself, it is important for researchers to minimize imaging-related stress when trying to study the effects of stress on the brain. This is accomplished by acclimating the animals to the scanning environment over a period of days. The procedure for rats involves repeated exposure to a mock imaging environment for 5–7 days. This habituation starts off with a short habituation on day one and increases in length each day, with the last few days lasting the approximate length of an imaging session. On each day of acclimation, the rats are briefly anesthetized and placed into a head restraint and mock coil setup. A topical analgesic (e.g. lidocaine cream) is applied to the rat's head at points of contact with the restraint device to ensure that pressure points do not cause discomfort. This restraint setup is identical to the restrainer used on imaging days, with the mock coil being replaced by a real MR coil. Once restrained the rats are placed in a mock scanner environment that consists of a small enclosed dark space with playback of scanner noise. This procedure is done prior to any experimental procedure to ensure that any changes found in the imaging results are not influenced by the acclimation to the imaging environment. This acclimation procedure and variations of this procedure have been used by a number of labs for imaging of awake rodents and has been shown in multiple experiments to decrease physiological markers of stress during imaging (Fig. 3) (King et al., 2005; Ferris et al., 2014; Ferris et al., 2006a, Ferris et al., 2006b; Zhang et al., 2010; Liang et al., 2011; Liang et al., 2012a, Liang et al., 2012b; Liang et al., 2015a, Liang et al., 2015b). This procedure is adequate to reduce the head motion levels of rats for acquisition of imaging data. Some labs have also utilized surgical procedures to implant a head fixation device onto the skull of the rat to allow for direct fixation of the rat's head to the imaging cradle (Fig. 4, Fig. 5) (Chang et al., 2016; Ferenczi et al., 2016).
Fig. 3.
Acclimation setup and physiological outcomes (A) Animal is briefly anesthetized to allow for setup into an imaging restraint device. (B) Animal is placed into head restraint. (C) Animal's teeth are adjusted around the restraint bite bar. (D) Head restraint is slid into and fastened to a mock coil or real coil. (E) Nose bar is lowered. (F) Hindpaws and forepaws are respectively taped together. (G) Animal is placed into a body tube, the head restraint is fastened in place. (H) Animal is allowed to recover from anesthesia and imaging is performed. (I) The respiratory rate (mean and S.E.M.) as measured on days 1, 3, 5, and 8 of acclimation to the imaging environment. Normal range for respiratory rate ranges from 66 to 114 beats/min. There was a significant decrease in respiratory rate from the third day of acclimation (p < 0.05) as compared to day 1. (J) The heart rate (mean and S.E.M.) as measured on days 1, 3, 5, and 8 of acclimation to the imaging environment. The normal range for heart rate ranges from 330 to 480 beats/min. There was a significant decrease in heart rate by day 5 of acclimation (p < 0.01) as compared to day 1. (K) Plasma corticosterone levels (μg/dl) measured on days 1, 3, 5, and 8 of acclimation (presented are the mean and S.E.M). There was a significant decrease in plasma levels of corticosterone for days 5 and 8 of acclimation as compared to day 1 (p < 0.05). Adapted from (King et al., 2005).
Fig. 4.
Surgical supplemented minimal restraint method. Experimental apparatus. A: photograph of a rat implemented with a nut to allow affixing fastener and head plate to the head. B: a rat in a custom-made snuggle sack with openings that provide access to head nut, hindpaw, and tail. C: head plate with rectangular groove at bottom surface to fix to the head nut and circular groove upon the upper surface for mounting surface coil. D: photograph of a rat with air-puff injector positioned adjacent to its paw. The puff of air comes out of the injector via a small outlet and can be used to induce a response to alerting stimuli during scanning. E: illustration of assembled apparatus with the key components. Adapted from (Chang et al., 2016).
Fig. 5.
Head fixation and habituation in optogenetic fMRI (opto-fMRI) in awake rats. (A) MRI simulation environment for rodent habituation to the scanning procedure. (B) Example of respiratory rate monitoring [bpm, breaths per minute; n = 1 rat; eight sequential scans, four anesthetized (1–2% isoflurane) and four awake (0% isoflurane)]. (C) Effect of habituation on head motion during scanning, as a function of anesthesia depth (n = 2 rats). Head motion score is the root mean square of head translation in three dimensions over the course of a scan. (D) Example head motion plots (head translation in three dimensions, calculated as shifts in the center of mass of all voxels in the image over the course of a single scan) for an unhabituated (top) and a habituated (bottom) subject. The unhabituated scan was aborted early. Adapted from (Ferenczi et al., 2016).
6.3. Imaging-related stress
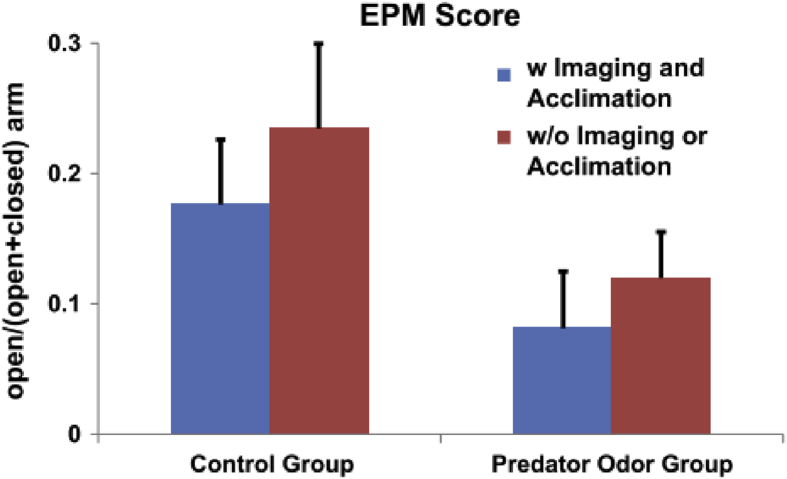
Although acclimation procedures developed have shown to reduce stress during imaging, it would not be appropriate to consider imaging stress free even after the process of acclimation. For this reason undesired behavioral effects resulting from acclimation and imaging are still possible (Low et al., 2016; Ferris et al., 2014). Therefore, the impact of acclimation and imaging-related stress (e.g. repeated handling) on the response to other experimental manipulations and/or imaging results must be considered for each individual experiment. Typically, such potential confounding effects can be ruled out through designs of proper controls. For example, work from our lab on predator odor stress has shown that acclimation stress did not mask the effect of subsequent exposure to a predator odor stressor, and these two processes have a separable impact on rat behavior, shown by negligible interaction between the effect of the two stressors on the measure of elevated plus maze (Fig. 6) (Liang et al., 2014). This suggests that any residual effects from acclimation and imaging can be subtracted out in control animals that were subject to the same acclimation and imaging procedures, but were not exposed to predator odor. This study indicates that running studies that involve imaging of animals, as with any experimental method, requires proper experimental design that considers the effect of the method itself over the timeline of the experiment. As mentioned above, this would involve analyzing the effect of acclimation and imaging on experimental measurements to rule out effects that can possibly influence the analysis of results. Ideally measurements used will have separable effects from acclimation and imaging, as shown in our study, but this must be assessed on an individual experiment basis within each laboratory.
Fig. 6.
Relative behavioral effect induced by a single trauma exposure and acclimation. In all 32 rats, 16 rats underwent the acclimation and imaging procedures (among them 8 rats were exposed to predator odor and the other 8 rats were exposed to air). The other 16 rats were not acclimated or imaged (8 were exposed to predator odor and the other 8 were exposed to air). The EPM score (open/(open + closed) arm) was separately assessed for all four subgroups. Two-way ANOVA with the factors of acclimation and trauma exposure was applied to the four groups. Our results (p_acclimation = 0.34, p_trauma exposure <0.05 and p_interaction = 0.85) confirmed a much smaller effect of acclimation than trauma exposure. They also showed a minimal interaction between the trauma-induced stress and acclimation/imaging-related stress. Adapted from (Liang et al., 2014).
6.4. Data processing
fMRI signal in general has a relatively low SNR. Therefore, data processing is an essential component for cleaning up noise and extracting useful information from raw data. The pipeline commonly used to process animal fMRI data is very similar to that used in human studies. Specifically, brain images of each animal are first registered to a reference template space so that results from different scans and animals can be overlaid, which provides more statistical power for group-level analysis. The template can also provide the anatomical information of any brain voxel based on its coordinates. Motion correction is important for diminishing motion artifact that can occur during the acquisition of imaging data. This is often preceded by brain extraction so that muscle tissue or noise outside of the brain does not affect the performance of motion correction algorithms. Nuisance signals, including signal from the white matter and ventricles, as well as motion parameters are usually regressed out to further remove non-neural-related physiological noise.
Spatial smoothing using a Gaussian kernel is then performed to help increase the SNR. There is often another step of temporal filtering that would be used depending on the study design. In the case of rsfMRI studies, a 0.01 Hz–0.1 Hz band-pass filter is used to separate out the rsfMRI signal from other noise sources. In task-based designs, fMRI time courses can be filtered with a high-pass filter to detrend the drift of BOLD signal. These pre-processing steps maximize the contrast-to-noise ratio of fMRI data, which are optimized for different post-processing strategies and statistical tests that may involve general linear model, independent component analysis, seed-based correlational analysis and graph theoretical analysis.
Based on the paradigm designed, the most commonly used method for analyzing task-based fMRI data is general linear models. On the other hand, rsfMRI data analysis can use hypothesis-driven seed-based correlational analysis or data-driven methods like principal component analysis (PCA), ICA and clustering methods (Soares et al., 2016). These methods have been used to define a number of functional networks in the brain such as the default mode network, salience network and executive control network in both humans and animals (Fox et al., 2005; Greicius et al., 2003; Lu et al., 2012; Seeley et al., 2007; Stafford et al., 2014). Furthermore, graph theoretical analysis has also been widely used to study the topological organization of human and animal brain networks on different scales (Bullmore and Sporns, 2009; De Vico Fallani et al., 2014; Liang et al., 2011; Ma et al., 2016). A comprehensive review on technical considerations of data acquisition and data processing can be referred to more detailed resources (Soares et al., 2016).
7. Future directions
Given its technical advantages, appropriately designed fMRI studies have a large potential to help understand the neurobiology of stress. Although fMRI has been increasingly applied recently, several important areas are still significantly underexplored.
7.1. Longitudinal studies
The non-invasive nature of fMRI allows for longitudinal studies that aren't reliant on endpoint measure. This nature makes them ideal for translationally relevant research projects and can provide insight into how the brain changes over time from hours to months in well controlled animal studies. This is important in studying the individual variability in brain structure and function given the importance of individual difference in neurological and psychological pathologies. Longitudinal measures can allow for the separation of innate susceptibility markers from markers of disease, which has important implications in deciding on therapeutic options. This potential of fMRI in animal studies to non-invasively determine changes that occur over time and across subjects will become a valuable tool in advancing our understanding of mental disorders (Jonckers et al., 2015).
7.2. Multimodal methods
There has been tremendous effort aiming to combine fMRI with other non-invasive techniques in human studies including diffusion tensor imaging (DTI), high density EEG and magnetoencephalography (MEG). These multi-modal methods provide complementary information that can help better understand stress-related disorders in humans.
In animal studies, work in our lab has used simultaneous application of optogenetics and fMRI to further analyze cognitive networks that are otherwise not easily studied in rodents in the scanner (Liang et al., 2015a). This work showed complex connectivity patterns through robust, distributed BOLD signal increases in response to optogenetic stimulation of the infralimbic cortex, which opens up options for opto-fMRI manipulations in awake rats. Similar work that combines different methodologies is important in further understanding how BOLD response relates to neuronal activity, but is also a means to develop fMRI's ability to drive understanding of specific mechanisms mediating behavior in response to stress (Martin, 2014; Ferenczi et al., 2016). An exemplar opto-fMRI paper by Ferenczi et al. (2016) sets a precedent for developing opto-fMRI experiments that can be used to focus on the interaction of particular neural circuits of interest and their behavioral outcomes (in this case reward behavior) (Ferenczi et al., 2016). In addition, quick advancement of simultaneous fMRI-calcium signal recordings (Liang et al., 2017; Schlegel et al., 2018; Schwalm et al., 2017) allows for multi-modal signals that reflects separate aspects of neural activity across different spatiotemporal scales to be concurrently collected. Furthermore, gadolinium probes has been increasingly used for molecular functional imaging. Taken together, multi-modal neuroimaging methods that can bridge wide spatiotemporal scales have become a more and more popular way to study the neurobiology of stress.
7.3. Animal fMRI for pre-clinical pharmaceutical studies
Given the comparable objective measure of neural activity provided by animal and human fMRI, animal fMRI studies have become important for the exploration of therapeutic options. This will likely become more and more common as fMRI in animal studies becomes commonplace (Roses et al., 2014; Crenshaw et al., 2015; Pérez et al., 2018).
8. Conclusion
It is important to note that the studies discussed have validated the awake animal fMRI technique for use in the stress field. These studies have shown an overall convergence of the emerging functional neuroimaging studies in awake animals with human neuroimaging studies and traditional animal studies of stress with other neuroscience methods. The potential of future animal studies utilizing functional neuroimaging techniques has been exemplified and will grow with the expansion of multi-modality studies.
The further expansion of animal studies that utilize fMRI is important in realizing this potential. Animal studies can be used to produce finely controlled experiments that are difficult to achieve in human longitudinal studies, reduce the time needed to acquire meaningful results and allow for more complex neuroscience methods to be used. The methods used in animal models have the potential to provide more specific insight into functional mechanisms of how brain activity can influence behavior and disease development. Importantly, fMRI plays a role in not only providing functional information about brain activity, but also in making these findings translationally relevant and testable in humans. It is foreseeable that the continued increase in fMRI and multi-modal animal studies, with their flexibility, low cost and short time frame, will lead to an improvement in our understanding of the neurobiology of stress and the translatability of findings between animal and human studies.
Conflicts of interest
None.
Acknowledgement
The present study was partially supported by National Institute of Neurological Disorders and Stroke Grant R01NS085200 (PI: Nanyin Zhang, PhD) and National Institute of Mental Health Grant R01MH098003 and RF1MH114224 (PI: Nanyin Zhang, PhD).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.06.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Aksenov D.P. Effects of anesthesia on BOLD signal and neuronal activity in the somatosensory cortex. J. Cerebr. Blood Flow Metabol. 2015;35(11):1819–1826. doi: 10.1038/jcbfm.2015.130. http://journals.sagepub.com/doi/10.1038/jcbfm.2015.130 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaugh D.L. Functional magnetic resonance imaging of electrical and optogenetic deep brain stimulation at the rat nucleus accumbens. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep31613. July. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini P.A. Twenty years of functional MRI: the science and the stories. Neuroimage. 2012;62(2):575–588. doi: 10.1016/j.neuroimage.2012.04.026. Available at: [DOI] [PubMed] [Google Scholar]
- Bandettini P.A. Time course EPI during task activation. Magn. Reson. Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Bandettini P.A. Processing strategies for time-course data sets in functional MRI of the human brain. Magn. Reson. Med.: Official J. Soc. Magn. Reson. Med./Soc. Magn. Reson. Med. 1993;30(2):161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Baxter A.J. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol. Med. 2013;43(5):897–910. doi: 10.1017/S003329171200147X. [DOI] [PubMed] [Google Scholar]
- Bergmann E. The organization of mouse and human cortico-hippocampal networks estimated by intrinsic functional connectivity. Cerebr. Cortex. 2016;26(12):4497–4512. doi: 10.1093/cercor/bhw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns G.S., Brooks A.M., Spivak M. Functional MRI in awake unrestrained dogs. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0038027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med.: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. http://www.ncbi.nlm.nih.gov/pubmed/8524021 Available at: [DOI] [PubMed] [Google Scholar]
- Bonne O. Reduced posterior hippocampal volume in posttraumatic stress disorder. J. Clin. Psychiatr. 2008;69(7):1087–1091. doi: 10.4088/jcp.v69n0707. http://www.ncbi.nlm.nih.gov/pubmed/2684983 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D. Traumatic stress: effects on the brain. Dialogues Clin. Neurosci. 2006;8(4):445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D. Functional neuroimaging in post-traumatic stress disorder. Expert Rev. Neurother. 2007;7(4):393–405. doi: 10.1586/14737175.7.4.393. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D. MRI-based measurement of hippocampal volume in posttraumatic stress disorder. Am. J. Psychiatr. 1995;152(July):973–978. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges N.M. Imaging conditioned fear circuitry using awake rodent fMRI. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0054197. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cao H. Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. Neuroimage. 2014;84:888–900. doi: 10.1016/j.neuroimage.2013.09.013. Available at: [DOI] [PubMed] [Google Scholar]
- Chang P.-C. Novel method for functional brain imaging in awake minimally restrained rats. J. Neurophysiol. 2016;116(1):61–80. doi: 10.1152/jn.01078.2015. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nature Publishing Group. 2015;18(10):1364–1375. doi: 10.1038/nn.4115%5Cnpapers3://publication/doi/10.1038/nn.4115. Available at: [DOI] [PubMed] [Google Scholar]
- Clancy B. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28(5 SPEC. ISS):931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4:1–15. doi: 10.3389/fnsys.2010.00008. http://journal.frontiersin.org/article/10.3389/fnsys.2010.00008/abstract April. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenshaw D.G. Effects of low doses of pioglitazone on resting-state functional connectivity in conscious rat brain. PLoS One. 2015;10(2):1–13. doi: 10.1371/journal.pone.0117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale a M., Buckner R.L. Selective averaging of individual trials using fMRI. Hum. Brain Mapp. 1997;5(5):329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Decot H.K. Coordination of brain-wide activity dynamics by dopaminergic neurons. Neuropsychopharmacology. 2017;42(3):615–627. doi: 10.1038/npp.2016.151. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatr. Neurosci. 2005;30(5):319–325. [PMC free article] [PubMed] [Google Scholar]
- Dedovic K., Rexroth M. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. Available at: [DOI] [PubMed] [Google Scholar]
- Dedovic K., D'Aguiar C., Pruessner J.C. What stress does to your brain: a review of neuroimaging studies. Can. J. Psychiatr. 2009;54(1):6–15. doi: 10.1177/070674370905400104. http://journals.sagepub.com/doi/10.1177/070674370905400104 Available at: [DOI] [PubMed] [Google Scholar]
- Van Den Berge N. Functional circuit mapping of striatal output nuclei using simultaneous deep brain stimulation and fMRI. Neuroimage. 2017;146:1050–1061. doi: 10.1016/j.neuroimage.2016.10.049. October 2016. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Buuse M. Importance of animal models in schizophrenia research. Aust. N. Z. J. Psychiatr. 2005;39(7):550–557. doi: 10.1080/j.1440-1614.2005.01626.x. [DOI] [PubMed] [Google Scholar]
- Desai M., Kahn I., Knoblich U. Mapping brain networks in awake mice using combined optical neural control and fMRI. J. Neurophysiol. 2011;105:1393–1405. doi: 10.1152/jn.00828.2010. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3074423/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers J. Current status of animal models of PTSD: behavioral and biological phenotypes, and future challenges in improving translation. Biol. Psychiatr. 2018;83(10):895–907. doi: 10.1016/j.biopsych.2017.11.019. http://linkinghub.elsevier.com/retrieve/pii/S0006322317322102 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGangi J.A., Tadayyon A., Fitzgerald D.A., Rabinak C.A., Kennedy A., Klumpp H., Rauch S.A., Phan K.L. Reduced default mode network connectivity following combat trauma. Neurosci. Lett. 2016;615:37–43. doi: 10.1016/j.neulet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale A.T. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2016;23(1) doi: 10.1038/nm.4246. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong T.Q. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn. Reson. Med. 2000;43(3):383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Dupire A. A role for anterior thalamic nuclei in affective cognition: interaction with environmental conditions. Hippocampus. 2013;23(5):392–404. doi: 10.1002/hipo.22098. [DOI] [PubMed] [Google Scholar]
- Duyn J.H. In: Magnetic Resonance Neuroimaging. Modo M., Bulte J.W.M., editors. Humana Press; Totowa, NJ: 2011. http://link.springer.com/10.1007/978-1-61737-992-5 Available at: [Google Scholar]
- Duyn J.H. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage. 2012;62(2):1241–1248. doi: 10.1016/j.neuroimage.2011.10.065. http://doi.wiley.com/10.1002/nbm.3066 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi E. a. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351(6268) doi: 10.1126/science.aac9698. http://www.sciencemag.org/cgi/doi/10.1126/science.aac9698 p.aac9698-aac9698. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A.J. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11) doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.F., Stolberg T. Imaging the immediate non-genomic effects of stress hormone on brain activity. Psychoneuroendocrinology. 2010;35(1):5–14. doi: 10.1016/j.psyneuen.2009.09.003. Available at: [DOI] [PubMed] [Google Scholar]
- Ferris C.F. Functional magnetic resonance imaging in conscious animals: a new tool in behavioural neuroscience research. J. Neuroendocrinol. 2006;18(5):307–318. doi: 10.1111/j.1365-2826.2006.01424.x. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.F. VIEWS from the bench functional magnetic resonance imaging in conscious Animals : a new tool in behavioural. Neurosci. Res. 2006;18:307–318. doi: 10.1111/j.1365-2826.2006.01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.F., Stolberg T., Kulkarni P., Murugavel M., Blanchard R., Blanchard D.C., Febo M., Brevard M., Simon N.G. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.F. Functional magnetic resonance imaging in awake animals. Rev. Neurosci. 2011;22(6):665–674. doi: 10.1515/RNS.2011.050. Available at: [DOI] [PubMed] [Google Scholar]
- Ferris C.F. 2014. Functional Magnetic Resonance Imaging in Awake Animals Functional Magnetic Resonance Imaging in Awake animals., (September) [Google Scholar]
- Flecknell P. 2009. Laboratory Animal Anaesthesia. [Google Scholar]
- Fornari R.V. Rodent stereotaxic surgery and animal welfare outcome improvements for behavioral neuroscience. JoVE. 2012;59:1–4. doi: 10.3791/3528. http://www.jove.com/video/3528/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Greicius M. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 2010;4(June) doi: 10.3389/fnsys.2010.00019. http://journal.frontiersin.org/article/10.3389/fnsys.2010.00019/abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.R. Time to wake up: studying neurovascular coupling and brain-wide circuit function in the un-anesthetized animal. Neuroimage. 2017;153:382–398. doi: 10.1016/j.neuroimage.2016.11.069. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- George Paxinos K.F. 2001. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Gilbertson M.W. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi A. Brain-wide mapping of endogenous serotonergic transmission via chemogenetic fMRI. Cell Rep. 2017;21(4):910–918. doi: 10.1016/j.celrep.2017.09.087. Available at: [DOI] [PubMed] [Google Scholar]
- Glover G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. 2011;22(2):133–139. doi: 10.1016/j.nec.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense J.B.M., Logothetis N.K. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr. Biol. 2008;18(9):631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Gorges M. Functional connectivity mapping in the animal model: principles and applications of resting-state fMRI. Front. Neurol. 2017;8(MAY):1–14. doi: 10.3389/fneur.2017.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A., Schwarz A.J. Large-scale functional connectivity networks in the rodent brain. Neuroimage. 2016;127:496–509. doi: 10.1016/j.neuroimage.2015.12.017. Available at: [DOI] [PubMed] [Google Scholar]
- Grandjean J., Schroeter A., Batata I., Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage 102 Pt. 2014;2:838–847. doi: 10.1016/j.neuroimage.2014.08.043. [DOI] [PubMed] [Google Scholar]
- Grayson D.S. The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron. 2016;91(2):453–466. doi: 10.1016/j.neuron.2016.06.005. http://linkinghub.elsevier.com/retrieve/pii/S0896627316302586 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal J.V. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn. Reson. Med. 1994;31(3):283–291. doi: 10.1002/mrm.1910310307. [DOI] [PubMed] [Google Scholar]
- Hamilton C., Ma Y., Zhang N. Global reduction of information exchange during anesthetic-induced unconsciousness. Brain Struct. Funct. 2017;222(7):3205–3216. doi: 10.1007/s00429-017-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.P. Imaging learned fear circuitry in awake mice using fMRI. Eur. J. Neurosci. 2015;42(5):2125–2134. doi: 10.1111/ejn.12939. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.J. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37(6):304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. Available at: [DOI] [PubMed] [Google Scholar]
- Hooijmans C.R., Ritskes-Hoitinga M. Progress in using systematic reviews of animal studies to improve translational research. PLoS Med. 2013;10(7):1–4. doi: 10.1371/journal.pmed.1001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer C. Advantages and challenges of small animal magnetic resonance imaging as a translational tool. Neuropsychobiology. 2014;69:187–201. doi: 10.1159/000360859. Available at: [DOI] [PubMed] [Google Scholar]
- Huang W. Fear induced neuronal alterations in a genetic model of depression: an fMRI study on awake animals. Neurosci. Lett. 2011;489(2):74–78. doi: 10.1016/j.neulet.2010.11.069. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K.C., Shin L.M. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev. Neurother. 2011;11(2):275–285. doi: 10.1586/ern.10.198. http://www.tandfonline.com/doi/full/10.1586/ern.10.198 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M. Functional networks in the anesthetized rat brain revealed by independent component analysis of resting-state fMRI. J. Neurophysiol. 2010;103:3398–3406. doi: 10.1152/jn.00141.2010. Available at: [DOI] [PubMed] [Google Scholar]
- Jonckers E. Functional connectivity fMRI of the rodent brain: comparison of functional connectivity networks in rat and mouse. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonckers E. The power of using functional fMRI on small rodents to study brain pharmacology and disease. Front. Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00231. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I. Characterization of the functional MRI response temporal linearity via optical control of neocortical pyramidal neurons. J. Neurosci. 2011;31(42):15086–15091. doi: 10.1523/JNEUROSCI.0007-11.2011. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0007-11.2011 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa Y., Schoeder E. How local is the local field potential? Neuron. 2012;72(5):847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalthoff D. Functional connectivity in the rat at 11.7T: impact of physiological noise in resting state fMRI. Neuroimage. 2011;54(4):2828–2839. doi: 10.1016/j.neuroimage.2010.10.053. Available at: [DOI] [PubMed] [Google Scholar]
- King J.A. Procedure for minimizing stress for fMRI studies in conscious rats. J. Neurosci. Meth. 2005;148(2):154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Kozai T.D.Y. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015;6(1):48–67. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. The Molecular Neurobiology of Depression. 2008;455(October) doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K.K. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. U.S.A. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=49355&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti K.M. Imaging brain activity in conscious animals using functional MRI. J. Neurosci. Meth. 1998;82:75–83. doi: 10.1016/s0165-0270(98)00037-5. [DOI] [PubMed] [Google Scholar]
- Lanius R.A. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am. J. Psychiatr. 2001;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lee J.H. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465(7299):788–792. doi: 10.1038/nature09108. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., King J., Zhang N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J. Neurosci. 2011;31(10):3776–3783. doi: 10.1523/JNEUROSCI.4557-10.2011. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00005176-200904002-00012 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., King J., Zhang N. Intrinsic organization of the anesthetized brain. J. Neurosci. 2012;32(30):10183–10191. doi: 10.1523/JNEUROSCI.1020-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., King J., Zhang N. Anticorrelated resting-state functional connectivity in awake rat brain. Neuroimage. 2012;59(2):1190–1199. doi: 10.1016/j.neuroimage.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z. Mapping thalamocortical networks in rat brain using resting-state functional connectivity. Neuroimage. 2013;83:237–244. doi: 10.1016/j.neuroimage.2013.06.029. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., King J., Zhang N. Neuroplasticity to a single-episode traumatic stress revealed by resting-state fMRI in awake rats. Neuroimage. 2014;103:485–491. doi: 10.1016/j.neuroimage.2014.08.050. http://linkinghub.elsevier.com/retrieve/pii/S1053811914007241 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Watson G.D.R. Mapping the functional network of medial prefrontal cortex by combining optogenetics and fMRI in awake rats. Neuroimage. 2015;117(May 2015):114–123. doi: 10.1016/j.neuroimage.2015.05.036. http://linkinghub.elsevier.com/retrieve/pii/S1053811915004164 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Liu X., Zhang N. Dynamic resting state functional connectivity in awake and anesthetized rodents. Neuroimage. 2015;104:89–99. doi: 10.1016/j.neuroimage.2014.10.013. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z. Simultaneous GCaMP6-based fiber photometry and fMRI in rats. J. Neurosci. Meth. 2017;289:31–38. doi: 10.1016/j.jneumeth.2017.07.002. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Sripada C.S. The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res. 2007;167(7):151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lister R.G. Ethologically-based animal models of anxiety disorders. Pharmacol. Therapeut. 1990;46(3):321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- Littlewood C.L. Mapping the central effects of ketamine in the rat using pharmacological MRI. Psychopharmacology. 2006;186(1):64–81. doi: 10.1007/s00213-006-0344-0. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K. 2002. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N.K. A neurophysiological investigation of the basis of the BOLD signal in fMRI. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. http://www.nature.com/nature/journal/v412/n6843/full/412150a0.html Available at: [DOI] [PubMed] [Google Scholar]
- Low L.A. Restraint training for awake functional brain scanning of rodents can cause long-lasting changes in pain and stress responses. Pain. 2016;157(8):1. doi: 10.1097/j.pain.0000000000000579. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006396-900000000-99540 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Rat brains also have a default mode network. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(10):3979–3984. doi: 10.1073/pnas.1200506109. http://www.pnas.org/cgi/doi/10.1073/pnas.1200506109 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. http://www.nature.com/doifinder/10.1038/nrn2639 Available at: [DOI] [PubMed] [Google Scholar]
- Ma Z. Functional atlas of the awake rat brain: a neuroimaging study of rat brain specialization and integration. Neuroimage. 2016;170:95–112. doi: 10.1016/j.neuroimage.2016.07.007. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Hamilton C., Zhang N. Dynamic connectivity patterns in conscious and unconscious brain. Brain Connect. 2017;7(1):1–12. doi: 10.1089/brain.2016.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madularu D. A non-invasive restraining system for awake mouse imaging. J. Neurosci. Meth. 2017;287:53–57. doi: 10.1016/j.jneumeth.2017.06.008. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M., Tottenham N., Casey B.J. Translational developmental studies of stress on brain and behavior: implications for adolescent mental health and illness? Neuroscience. 2013;249:53–62. doi: 10.1016/j.neuroscience.2013.01.023. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Phan K.L., Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 2013;14(June):417–428. doi: 10.1038/nrn3492. http://www.nature.com/doifinder/10.1038/nrn3492%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/23635870 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34(1):74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle H.J.F. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53(1):348–354. doi: 10.1016/j.neuroimage.2010.05.070. Available at: [DOI] [PubMed] [Google Scholar]
- Maron-Katz A. A large-scale perspective on stress-induced alterations in resting-state networks. Sci. Rep. 2016;6(July 2015):pp.1–11. doi: 10.1038/srep21503. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. Contributions and complexities from the use of in-vivo animal models to improve understanding of human neuroimaging signals. Front. Neurosci. 2014;8(8 JUL):1–22. doi: 10.3389/fnins.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R.C., Gupta N., Lazaro-Munoz G., Sears R.M., Kim S., Moscarello J.M., LeDoux J.E., Cain C.K. Active vs. reactive threat responding is associated with differential c-Fos expression in specific regions of amygdala and prefrontal cortex. Learn. Mem. 2013;20:446–452. doi: 10.1101/lm.031047.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P.M., Honey G.D., Bullmore E.T. Applications of fMRI in translational medicine and clinical practice. Nat. Rev. Neurosci. 2006;7(9):732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22(1):105–122. doi: 10.1146/annurev.neuro.22.1.105. http://www.annualreviews.org/doi/10.1146/annurev.neuro.22.1.105 Available at: [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Brain on stress: how the social environment gets under the skin. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(Supplement_2):17180–17185. doi: 10.1073/pnas.1121254109. http://www.pnas.org/cgi/doi/10.1073/pnas.1121254109 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R.R., Quirk G.J.J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. http://www.nature.com/nature/journal/v420/n6911/abs/nature01138.html Available at: [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63(1):129–151. doi: 10.1146/annurev.psych.121208.131631. http://www.annualreviews.org/doi/10.1146/annurev.psych.121208.131631 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatr. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. http://linkinghub.elsevier.com/retrieve/pii/S0006322309008968 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsattari S.M. Physiological monitoring of small animals during magnetic resonance imaging. J. Neurosci. Meth. 2005;144(2):207–213. doi: 10.1016/j.jneumeth.2004.11.019. Available at: [DOI] [PubMed] [Google Scholar]
- Mitra R. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. http://www.pnas.org/cgi/content/long/102/26/9371 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J.L., Hassabis D., Weiskopf N., Seymour B., Dolan R.J., Frith C.D. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J.S. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Mogil J.S., Davis K.D., Derbyshire S.W. The necessity of animal models in pain research. Pain. 2010;151(1):12–17. doi: 10.1016/j.pain.2010.07.015. Available at: [DOI] [PubMed] [Google Scholar]
- Moscarello J.M., LeDoux J.E. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J. Neurosci. 2013;33:3815–3823. doi: 10.1523/JNEUROSCI.2596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.C. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew B.C. Blood oxygen level-dependent signal responses in corticolimbic “emotions” circuitry of lactating rats facing intruder threat to pups. Eur. J. Neurosci. 2009;30(5):934–945. doi: 10.1111/j.1460-9568.2009.06875.x. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. Neuroimaging. 2000;98(1):1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Oakes T.R. Comparison of fMRI motion correction software tools. Neuroimage. 2005;28(3):529–543. doi: 10.1016/j.neuroimage.2005.05.058. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of cognitive neuroscience. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ogawa S. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U.S.A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=55275&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. U.S.A. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasonen J., Stenroos P., Salo R.A., Kiviniemi V., Grohn O. Functional connectivity under six anesthesia protocols and the awake condition in rat brain. Neuroimage. 2018;172:9–20. doi: 10.1016/j.neuroimage.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Pan W. Simultaneous fMRI and electrophysiology in the rodent brain. JoVE. 2010;42:4–6. doi: 10.3791/1901. http://www.jove.com/index/Details.stp?ID=1901 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W.J. Considerations for resting state functional MRI and functional connectivity studies in rodents. Front. Neurosci. 2015;9(JUL):1–17. doi: 10.3389/fnins.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela C.P. Resting-state functional connectivity of the rat brain. Magn. Reson. Med. 2008;59(5):1021–1029. doi: 10.1002/mrm.21524. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C.R.R., Emson P.C. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J. Neurosci. Meth. 1980;3(2):129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Pérez P.D. Acute effects of vortioxetine and duloxetine on resting-state functional connectivity in the awake rat. Neuropharmacology. 2018;128:379–387. doi: 10.1016/j.neuropharm.2017.10.038. http://linkinghub.elsevier.com/retrieve/pii/S0028390817305038 Available at: [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Dubis J.W. The mixed block/event-related design. Neuroimage. 2012;62(2):1177–1184. doi: 10.1016/j.neuroimage.2011.09.084. http://doi.wiley.com/10.1002/nbm.3066 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pisauro M.A. Fast hemodynamic responses in the visual cortex of the awake mouse. J. Neurosci. 2013;33(46):18343–18351. doi: 10.1523/JNEUROSCI.2130-13.2013. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.2130-13.2013 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. The future of fMRI in cognitive neuroscience. Neuroimage. 2012;62(2):1216–1220. doi: 10.1016/j.neuroimage.2011.08.007. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.P. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol. Psychiatr. 2012;72(6):466–475. doi: 10.1016/j.biopsych.2012.04.008. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S.L. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study 48. Biol.Psychiatry. 2000;47:0006–3223. doi: 10.1016/s0006-3223(00)00828-3. (Print)), pp.769–776. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Shin L.M., Phelps E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol. Psychiatr. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reese T. Regional brain activation by bicuculline visualized by functional magnetic resonance imaging. Time-resolved assessment of bicuculline-induced changes in local cerebral blood volume using an intravascular contrast agent. NBM (NMR Biomed.) 2000;13(1):43–49. doi: 10.1002/(sici)1099-1492(200002)13:1<43::aid-nbm608>3.0.co;2-s. http://doi.wiley.com/10.1002/mrm.1190 Available at: [DOI] [PubMed] [Google Scholar]
- Rodrigues S.M., LeDoux J.E., Sapolsky R.M. The influence of stress hormones on fear circuitry. Annu. Rev. Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rogers J. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol. Psychiatr. 2013;18(6):700–707. doi: 10.1038/mp.2012.152. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3663915&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses A.D. New applications of disease genetics and pharmacogenetics to drug development. Curr. Opin. Pharmacol. 2014;14(1):81–89. doi: 10.1016/j.coph.2013.12.002. Available at: [DOI] [PubMed] [Google Scholar]
- Russo S.J. Neurobiology of resilience. Nat. Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. http://www.nature.com/doifinder/10.1038/nn.3234 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs B.D., Ni J.R., Caron M.G. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112(8) doi: 10.1073/pnas.1416866112. http://www.pnas.org/content/early/2015/02/03/1416866112 p.201416866. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin B. Brain volumes of the lamb, rat and bird do not show hemispheric asymmetry: a stereological study. Image Anal. Stereol. 2011;20(1):9–13. http://www.ias-iss.org/ojs/IAS/article/view/653%5Cnhttp://www.ias-iss.org/ojs/IAS/article/download/653/556 Available at: [Google Scholar]
- Sakoğlu Ü. Paradigm shift in translational neuroimaging of CNS disorders. Biochem. Pharmacol. 2011;81(12):1374–1387. doi: 10.1016/j.bcp.2010.12.029. http://linkinghub.elsevier.com/retrieve/pii/S0006295211000165 Available at: [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci.: the official journal of the Society for Neuroscience. 1990;10(9):2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D., Kanen J.W., LeDoux J.E., Monfils M.H., Phelps E.A. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20040–20045. doi: 10.1073/pnas.1320322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel F., Sych Y., Schroeter A., Stobart J., Weber B., Helmchen F., Rudin M. Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice. Nat. Protoc. 2018;13:840–855. doi: 10.1038/nprot.2018.003. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Kaltwasser S.F., Wotjak C.T. Review article biomarkers in posttraumatic stress Disorder : overview and implications for future research. 2013;35(1):43–54. doi: 10.1155/2013/835876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder M.P. Intrinsic connectivity of neural networks in the awake rabbit. Neuroimage. 2016;129(5):260–267. doi: 10.1016/j.neuroimage.2016.01.010. http://linkinghub.elsevier.com/retrieve/pii/S1053811916000161 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Darken N.J. Novel restraint system for neuroendocrine studies of socially living common marmoset monkeys. Lab. Anim. (Lond.) 2004;38(4):393–405. doi: 10.1258/0023677041958918. http://www.ncbi.nlm.nih.gov/pubmed/15479554 Available at: [DOI] [PubMed] [Google Scholar]
- Schulz K. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat. Methods. 2012;9(6):597–602. doi: 10.1038/nmeth.2013. [DOI] [PubMed] [Google Scholar]
- Schwalm M., Schmid F., Wachsmuth L., Backhaus H., Kronfeld A., Aedo Jury F., Prouvot P.H., Fois C., Albers F., van Alst T. Cortex-wide BOLD fMRI activity reflects locally-recorded slow oscillation-associated calcium waves. eLife. 2017;6 doi: 10.7554/eLife.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A.J., Gozzi A., Bifone A. Community structure in networks of functional connectivity: resolving functional organization in the rat brain with pharmacological MRI. Neuroimage. 2009;47(1):302–311. doi: 10.1016/j.neuroimage.2009.03.064. Available at: [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B.D. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y.Y.I. Ultra high-resolution fMRI and electrophysiology of the rat primary somatosensory cortex. Neuroimage. 2013;73:113–120. doi: 10.1016/j.neuroimage.2013.01.062. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y.-Y.I. fMRI of deep brain stimulation at the rat ventral posteromedial thalamus. Brain Stimulation. 2014;7(2):190–193. doi: 10.1016/j.brs.2013.11.001. http://linkinghub.elsevier.com/retrieve/pii/S1935861X1300346X Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L., Wright C. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress. Archives. 2005;62(3):1–2. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shin L.M. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am. J. Psychiatr. 2011;168(9):979–985. doi: 10.1176/appi.ajp.2011.09121812. http://www.ncbi.nlm.nih.gov/pubmed/21724666 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J.M. A Hitchhiker's guide to functional magnetic resonance imaging. Front. Neurosci. 2016;10 doi: 10.3389/fnins.2016.00515. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F., Quirk G.J. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;911(35):904–911. doi: 10.1097/PSY.0b013e318273bf33. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3498527&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford J.M., Jarrett B.R., Miranda-Dominguez O., Mills B.D., Cain N., Mihalas S., Lahvis G.P., Lattal K.M., Mitchell S.H., David S.V. Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. U. S. A. 2014;111:18745–18750. doi: 10.1073/pnas.1404346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T. Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues Clin. Neurosci. 2011;13(4):495–506. doi: 10.31887/DCNS.2011.13.4/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo X., Lei D., Li K., Chen F., Li F., Li L., Huang X., Lui S., Li L., Kemp G.J., Gong Q. Disrupted brain network topology in pediatric posttraumatic stress disorder: a resting-state fMRI study. Hum. Brain Mapp. 2015;36:3677–3686. doi: 10.1002/hbm.22871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi L.K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci. Biobehav. Rev. 2005;29(8):1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Termenon M. Reliability of graph analysis of resting state fMRI using test-retest dataset from the Human Connectome Project. Neuroimage. 2016;142:172–187. doi: 10.1016/j.neuroimage.2016.05.062. Available at: [DOI] [PubMed] [Google Scholar]
- De Vico Fallani F., Richiardi J., Chavez M., Achard S. Graph analysis of functional brain networks: practical issues in translational neuroscience. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Patel G.H., Fox M.D., Snyder A.Z., Baker J.T., Van Essen D.C., Zempel J.M., Snyder L.H., Corbetta M., Raichle M.E. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vyas A. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci.: the official journal of the Society for Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald L.L. The future of acquisition speed, coverage, sensitivity, and resolution. Neuroimage. 2012;62(2):1221–1229. doi: 10.1016/j.neuroimage.2012.02.077. http://linkinghub.elsevier.com/retrieve/pii/S1053811912002625 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. Altered resting-state functional activity in posttraumatic stress disorder: a quantitative meta-analysis. Sci. Rep. 2016;6(May):1–14. doi: 10.1038/srep27131. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E., Wald L.L. Pushing the spatio-temporal limits of MRI and fMRI. Neuroimage. 2018;164:1–3. doi: 10.1016/j.neuroimage.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Yacoub E., Shmuel A., Logothetis N., Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage. 2007;37:1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J. Nerv. Ment. Dis. 1990;178(6):366–369. doi: 10.1097/00005053-199006000-00004. http://www.ncbi.nlm.nih.gov/pubmed/2348190 Available at: [DOI] [PubMed] [Google Scholar]
- Yehuda R., McFarlane A.C., Shalev A.Y. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol. Psychiatr. 1998;44(12):1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Yoshida K. Physiological effects of a habituation procedure for functional MRI in awake mice using a cryogenic radiofrequency probe. J. Neurosci. Meth. 2016;274:38–48. doi: 10.1016/j.jneumeth.2016.09.013. Available at: [DOI] [PubMed] [Google Scholar]
- Zhang N. Mapping resting-state brain networks in conscious animals. J. Neurosci. Meth. 2010;189(2):186–196. doi: 10.1016/j.jneumeth.2010.04.001. http://linkinghub.elsevier.com/retrieve/pii/S016502701000186X Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Zhu X.H., Yacoub E., Ugurbil K., Chen W. Functional MRI mapping neuronal inhibition and excitation at columnar level in human visual cortex. Exp. Brain Res. 2010;204:515–524. doi: 10.1007/s00221-010-2318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Zhu X.H., Zhang Y., Park J.K., Chen W. High-resolution fMRI mapping of ocular dominance layers in cat lateral geniculate nucleus. Neuroimage. 2010;50:1456–1463. doi: 10.1016/j.neuroimage.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann J., Goebel R., De Martino F., van de Moortele P.F., Feinberg D., Adriany G., Chaimow D., Shmuel A., Ugurbil K., Yacoub E. Mapping the organization of axis of motion selective features in human area MT using high-field fMRI. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.