Abstract
Eating disorders (ED), including Anorexia Nervosa (AN), Bulimia Nervosa (BN), and Binge Eating Disorder (BED), are medically dangerous psychiatric disorders of unknown etiology. Accumulating evidence supports a biopsychosocial model that includes genetic heritability, neurobiological vulnerability, and psychosocial factors, such as stress, in the development and maintenance of ED. Notably, stress hormones influence appetite and eating, and dysfunction of the physiological stress response has been implicated in ED pathophysiology. Stress signals also appear associated with food reward neurocircuitry response in ED, providing a possible mechanism for the role of stress in appetite dysregulation. This paper provides a review of some of the interacting psychological, behavioral, physiological, and neurobiological mechanisms involved in the stress response among individuals with ED, and discusses novel neuroimaging techniques to address potential physiological confounds of studying neural correlates of stress in ED, such as calibrated fMRI.
Keywords: Eating disorders, Stress, Anorexia nervosa, Bulimia nervosa, Functional neuroimaging, HPA-Axis
1. Introduction
Eating disorders (EDs) affect 1–3% of individuals (American Psychiatric Association, 2000; Hudson et al., 2007; Keski-Rahkonen et al., 2009) and are associated with substantial and costly medical morbidity (McKenzie and Joyce, 1992), high rates of comorbid psychopathology (e.g., substance abuse, anxiety, depression), significant psychosocial impairment (American Psychiatric Association, 2000; Wonderlich and Mitchell, 1997), and the highest mortality rate of any psychiatric disorder (Birmingham et al., 2005; Papadopoulos et al., 2009; Sullivan, 1995). Empirically-supported psychotherapies achieve symptom abstinence in only 30–60% of ED treatment-completers (Iacovino et al., 2012; Lock et al., 2010; Loeb and Le Grange, 2009; Mitchell et al., 2007; Wilson et al., 2007); the rest tend to have a chronic, relapsing course (Herzog et al., 1992; Keel et al., 1999; Klein and Walsh, 2003). ED diagnoses have traditionally been differentiated based on physical symptoms, though overlap is common. For example, Anorexia Nervosa (AN) is characterized by severe food restriction, leading to significantly low body weight (American Psychiatric Association, 2013). Bulimia Nervosa (BN) is characterized by recurrent episodes of binge eating, defined as consuming an objectively large amount of food combined with a sense of “loss of control” over eating (Mond et al., 2010; Shoemaker et al., 2010; Wolfe et al., 2009), and compensatory behaviors to avoid weight gain, like self-induced vomiting and highly restricted intake (American Psychiatric Association, 2013). Both AN and BN share an undue influence of body weight or shape on self-evaluation. In contrast, Binge Eating Disorder (BED) is characterized by recurrent episodes of binge eating in the absence of compensatory behaviors, and body weight/shape concerns are not central to the disorder (American Psychiatric Association, 2013). Although the etiology of ED is unknown, there is growing evidence for a biopsychosocial model that includes psychosocial factors, genetic heritability, and neurobiological vulnerabilities (Bulik et al., 2006; Kaye et al., 2009; Kaye et al., 2008; Kaye et al., 2013; Kendler et al., 1991; Lilenfeld et al., 1998; Strober et al., 2000).
2. Stress as a potential risk factor for the development and maintenance of ED
The role of stress in relation to eating behavior has been of empirical interest for more than half a century (Stunkard, 1959; Pare, 1965; Morley et al., 1983). The experience of stress, activated in the face of a current or anticipated physiological or psychological stressor, is commonly described as a state in which an individual is faced with demands that are perceived as being greater than the available resources to cope with them (Lazarus and Folkman, 1984; McEwen, 2008). Stress is thought to play a role in the development and maintenance of EDs (Schmidt et al., 1997; Soukop et al., 1990; Troop et al., 1998). Notably, patients often report experiencing stressful life events or trauma prior to the onset of the ED (Chapman et al., 2007; Dube et al., 2003; Jacobi et al., 2004; Kessler et al., 2010; Monteleone et al., 2016a, Monteleone et al., 2016b; Monteleone et al., 2017; Rojo et al., 2006), and demonstrate maladaptive coping strategies when ill (Burkert et al., 2015), consistent with models proposing that chronic stress or an inadequate stress response facilitates emotional disorders (Chida and Hamer, 2008; de Kloet, 2008; McEwen, 2003).
Importantly, individuals with an ED tend to share certain temperament and personality traits, which often first present in childhood before illness onset, do not change over the course of treatment (Harrison et al., 2016), and persist after recovery, suggesting they are susceptibility traits (Wagner et al., 2006) contributing to the pathogenesis of EDs. These temperament and personality factors include anxiety (Kaye et al., 2004), perfectionism (Friederich and Herzog, 2011), obsessionality (Anderluh et al., 2003; van den Heuvel et al., 2005), punishment sensitivity (Harrison et al., 2010; Jappe et al., 2011), interoceptive deficits (Lilenfeld et al., 2006), and harm avoidance (Fassino et al., 2002) — a temperament trait (Cloninger et al., 1994) containing elements of anxiety, inhibition, and inflexibility. However, temperament findings do not clearly respect diagnostic boundaries: while all ED subtypes share high punishment sensitivity in the ill and remitted states (Harrison et al., 2010; Jappe et al., 2011; Matton et al., 2013), findings of reward sensitivity are mixed. For example, while some suggest it is elevated in both AN and BN (Glashouwer et al., 2014; Jappe et al., 2011), others show subtle subtype differences that might be symptom-driven (Harrison et al., 2010; Matton et al., 2013). Similarly, elevated impulsivity is more characteristic of disorders with a binge and/or purge component than AN restricting subtype (Farstad et al., 2016; Favaro et al., 2005; Waxman, 2009), suggesting that these temperament traits are transdiagnostic and may relate more closely to neurobiology than diagnostic classification. Importantly, together these traits are thought to increase vulnerability to stress, which might maintain ED symptoms through maladaptive coping strategies.
Individuals with AN and BN report global difficulties with effectively regulating their emotional experiences (Brockmeyer et al., 2014), and ED behaviors tend to be used for coping with or modulating emotion. For instance, women with ED tend to be less effective in their coping, with evidence suggesting they are more likely to use negative (e.g., cognitive avoidance or cognitive rumination) vs positive coping strategies in response to severe events or difficulties (Burkert et al., 2015; Troop et al., 1998). In AN, dietary restraint and reduced daily caloric intake appears to serve as a means of anxiety reduction (Kaye et al., 2003a, Kaye et al., 2003b; Steinglass et al., 2010; Vitousek and Manke, 1994), whereas eating increases dysphoric mood (Frank and Kaye, 2012). For BN, negative affect, mood lability, and stress may trigger binge eating episodes. Although the binge and purge cycle appears to reduce dysphoria and/or anxiety in the short term (Abraham and Beaumont, 1982; Crosby et al., 2009; Haedt-Matt and Keel, 2011; Johnson and Larson, 1982; Kaye et al., 1986; Smyth et al., 2007), supporting the notion that bulimic behaviors are negatively reinforcing, recent naturalistic data suggest binge-purge behavior may ultimately lead to greater overall affective dysregulation (Berner et al., 2017). However, the biological stress response in ED, and how it might interact with behavior to elicit or maintain ED symptoms is not well understood.
3. The stress response and its role in eating behavior
The stress response is characterized by activation of two core physiological systems. The more immediate response is mediated by the sympathetic nervous system (SNS), which facilitates rapid adaptation, traditionally referred to as the fight or flight response, through the release of epinephrine and norepinephrine (Het et al., 2015; Romeo, 2013; Ulrich-Lai and Herman, 2009). Complementing the rapid actions of the SNS are more protracted features of the stress response that are mediated by the hypothalamic-pituitary-adrenal (HPA) axis. A hormonal cascade begins with release of corticotrophin releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus, which functions to stimulate release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland, which in turn initiates release of glucocorticoids from the adrenal cortex (GCs; e.g., cortisol in humans) (Adam and Epel, 2007; Herman et al., 2003; Ulrich-Lai and Herman, 2009). GCs bind to two receptor types in the brain: the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR) (ter Heegde et al., 2015). Ultimately this process serves to mobilize energy reserves to facilitate either reactive or anticipatory adaption to the stressor that initiated the response (Herman et al., 2016). Under normal circumstances, following cessation of the stressor, GCs exert inhibition through negative feedback on the HPA axis, with additional inhibitory influences on cortical regions external to the HPA axis including limbic areas and the prefrontal cortex (Herman et al., 2003, 2016; Oitzl et al., 2010).
With regard to influences on appetite and eating, the primary hormones involved in the HPA axis-mediated stress response have complex and in some cases opposing effects on appetitive behavior. Typically, in the context of acute stress, CRH is anorexigenic in nature and promotes a similarly anorexigenic effect of cortisol in the short-term. However, in the context of prolonged stress, chronically elevated GCs promote inhibition of CRH and ultimately result in an overall orexigenic effect (Cavagnini et al., 2000; Crespo et al., 2014). This shift from acute to chronic effects is commonly noted as a potentially important mechanism underlying the apparent association between stress and weight gain (Gluck, 2006; Torres and Nowson, 2007). Empirically, evidence suggests that increased food intake is a common reaction to stress, although a minority of individuals experience stress-induced reductions in food intake (Adam and Epel, 2007; Greeno and Wing, 1994). In particular, studies point to a particularly potent role of elevated cortisol in relation to food choice, with higher levels of stress and cortisol promoting selection and intake of more palatable and calorically dense foods (i.e., those with greater fat and sugar content) (Born et al., 2010; Epel et al., 2001; Newman et al., 2007; Oliver et al., 2000). Furthermore, acute stress has been shown to promote non-homeostatic eating (i.e., eating in the absence of hunger) in normal and overweight individuals (Rutters et al., 2009). Notably, although a detailed review is beyond the scope of this paper, peripheral hormones (e.g., leptin, ghrelin) can exert modulatory effects on HPA axis activity and thus also have influences on appetite behavior (Ross et al., 2016). For example, leptin can function to suppress cortisol through negative feedback at the adrenal cortex or through inhibition of hypothalamic CRH release (Bornstein et al., 1997; Heiman et al., 1997), whereas ghrelin can stimulate release of ACTH (van der Lely et al., 2004).
4. Functioning of the stress response system in ED
As described above, a substantial theoretical and empirical literature focused on behavioral and psychological factors suggests the salience of stress in relation to the development and maintenance of ED, as well as in the treatment of these disorders. For instance, stress-related experiences have been highlighted in terms of the potential risk of ED onset (e.g., history of childhood trauma) (Carr et al., 2013; Caslini et al., 2016), the occurrence and maintenance of ED symptoms (e.g., severity of momentary stress preceding and following ED behaviors) (Mason et al., 2017; Smyth et al., 2007), and the potential utility of targeting stress, coping, and related factors (e.g., emotion regulation, distress tolerance) in ED treatment (Wonderlich et al., 2015; Safer et al., 2009). Although the role of the glucocorticoid receptor (GR) in stress reactivity is well-established, recent work suggests that the mineralocorticoid (MR) receptor, which is predominantly expressed in the limbic system, is also a critically important modulator of stress and influences basal as well as stress-induced HPA-axis activity (ter Heegde et al., 2015). Notably, variation in MR expression is thought to influence the vulnerability to develop a psychiatric disorder after exposure to traumatic stress, with a loss of MR expression in limbic areas associated with psychiatric disorders. Thus, a decrease in MR functionality is posited to lead to a maladaptive stress response that consequently increases one's vulnerability to the effects of chronic stress. Although no studies have examined mineralocorticoid receptors in ED, the correspondence between early life stress and psychiatric disorders has led investigators to posit that the impact of early life stress on later stress responsivity is mediated by changes in gene expression of GR and MR (Struber et al., 2014), resulting in either a glucocorticoid hyperfunction or hypofunction, with the direction of change in glucocorticoid functionality specifically associated with the development of different psychiatric disorders.
Findings from an increasing number of psychophysiological and neurobiological studies of stress in ED samples further support the importance of the stress response in ED pathophysiology. For example, findings have demonstrated that individuals with ED exhibit a pattern of altered SNS activity across a number of indices including heart rate variability, blood pressure, and salivary alpha-amylase, among others (Het et al., 2015; Koo-Loeb et al., 1998; Mazurak et al., 2011; Messerli-Bürgy et al., 2010; Peschel et al., 2016). With regard to the more protracted mechanisms of the stress response mediated by the HPA axis, evidence also points to dysregulation. For instance, HPA axis hyperactivity has been found in individuals with AN and BN, most notably characterized by elevated serum and salivary cortisol (Culbert et al., 2016). Furthermore, findings support the presence of an elevated cortisol awakening response (CAR) in women with current AN versus controls, although such differences are not evident in BN (Culbert et al., 2016; Monteleone et al., 2014, Monteleone et al., 2016a, Monteleone et al., 2016b). Additional evidence suggests that in AN, the binge-purge subtype is characterized by the highest CAR levels (Monteleone et al., 2017), a finding which is notable as it suggests potentially additive relationships of chronic starvation and binge-purge behaviors with regard to HPA axis dysfunction in AN. Compared to studies focused on average or basal HPA axis functioning, an arguably greater degree of heterogeneity has emerged from studies of reactivity to acute, laboratory-based physical and/or psychosocial stress exposure (Culbert et al., 2016; Het et al., 2015; Vannucci et al., 2015). Overall, however, contrasting with the evidence of elevated basal cortisol in those with AN and BN, findings regarding stress reactivity are suggestive of a blunted cortisol response in individuals with EDs, potentially reflecting HPA axis downregulation due to chronic and pervasive stress exposure (Culbert et al., 2016; Ginty et al., 2012; Het et al., 2015; Vannucci et al., 2015; Zonnevylle-Bender et al., 2005).
Recent research has also sought to more directly examine the associations between HPA axis activity and core symptoms characterizing ED. For example, in a study of women across the weight spectrum from underweight to obese, average nocturnal cortisol levels were found to be positively associated with multiple cognitive measures of ED psychopathology, controlling for body mass index (Lawson et al., 2011a, Lawson et al., 2011b). Additionally, in a study of obese participants with and without BED, cortisol response to a stress task was positively associated with change in the desire to binge eat in the BED group (Rosenberg et al., 2013). However, in a study of AN that examined associations between cortisol and appetite, Lawson et al. (2013) found that cortisol levels were inversely related to both homeostatic and hedonic appetite in currently ill and weight restored AN. Interestingly, Zonnevylle-Bender et al. (2005) found that adolescent AN patients self-reported higher levels of stress-related anxiety following a stress exposure task, but this was not reflected in the cortisol response. In contrast, increases in both self-reported anxiety and cortisol were found in controls, suggesting that the blunted cortisol response that seems to characterize AN may not translate into a blunted perception of stress. Such findings highlight the complexity of understanding the interacting psychological, behavioral, physiological, and neurobiological mechanisms involved in the stress response among individuals with ED.
Taken together, the existing literature thus supports the presence of dysfunction in the stress response system in ED, including altered SNS activity and disturbance of HPA axis functioning with regard to both acute (blunted) and chronic (elevated) stressors (Lo Sauro et al., 2008; Mazurak et al., 2011; Peschel et al., 2016). As noted by Herman et al. (2016), this is particularly concerning given that dysfunctional and/or protracted activation of the HPA axis is energetically costly and may function as a potent maintaining factor for psychiatric illness. However, a number of issues related to the stress response in ED are in need of further study. For instance, findings have been mixed with regard to persistence of HPA axis dysfunction following ED recovery, and the extent to which such dysfunction represents a predisposing physiological risk factor remains unclear (Culbert et al., 2016). Furthermore, the distinctive features of each ED (e.g., differences in body mass index, nutritional status, etc.) suggest the need to clarify how these underlying mechanisms may vary across the disorders. Finally, the unique mechanisms underlying HPA axis dysfunction in ED, and the interacting effects of ED symptoms (including bulimic behaviors, malnutrition, and starvation), psychosocial stressors, and the physiological components of the stress response system, requires further elaboration. As such, more work is needed to determine the degree to which HPA dysfunction in ED is secondary to physical effects of ED behaviors (i.e., malnourishment, chemical and hormonal imbalances) or is attributable to psychological stress. It is also possible that some of the conflicting findings across basal and acute measures of stress could be resolved by mapping these cortisol patterns onto particular constructs that are transdiagnostic across EDs (e.g., harm avoidance).
5. Association of stress signals and food reward neurocircuitry in ED
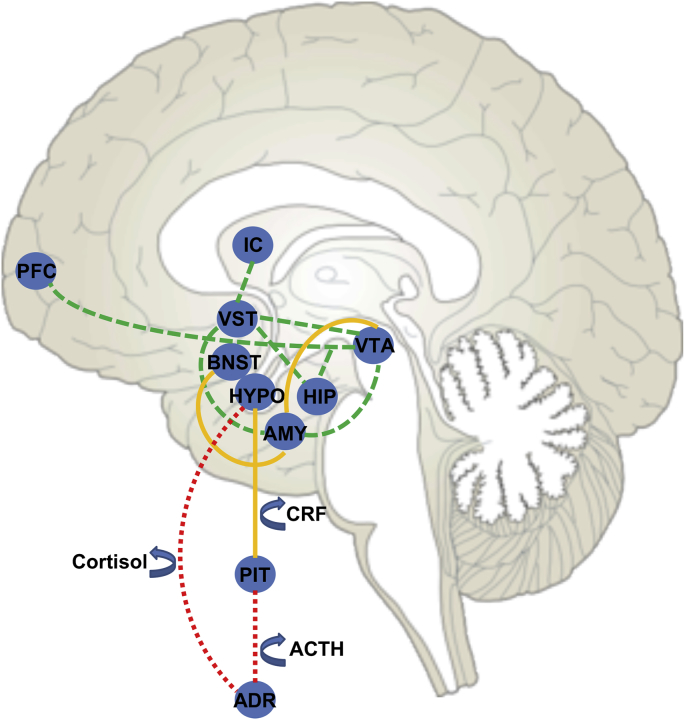
There is considerable overlap in the limbic and paralimbic neural circuitry regulating stress and food reward, including the hypothalamus, amygdala, hippocampus, ventral striatum and insula (Cleck and Blendy, 2008; Monteleone et al., 2018) (see Fig. 1). These regions contain dense expression of receptors (CRF1, CRF2, GR, and MR) of HPA hormones to modulate both the stress response and appetite (Chalmers et al., 1995; Chalmers et al., 1996; Lawson et al., 2013; McEwen, 1988; McEwen et al., 1986; McEwen et al., 1969; Struber et al., 2014). The hypothalamus is central to integrating peripheral feeding signals with cortical and subcortical food motivation circuits. The hippocampus regulates food intake by processing hunger and satiety signals and detecting learned signals which are paired with eating and the consequences of eating (i.e., Pavlovian conditioning, operant conditioning, negative occasion setting) (Benoit et al., 2010; Davidson and Jarrard, 1993; Davidson et al., 2007; Hargrave et al., 2016). The amygdala is implicated in non-homeostatic feeding and is influenced by acute and chronic stress (Rudenga et al., 2013). The ventral striatum, including the nucleus accumbens and putamen (Braun et al., 1982), plays a role in assessing the reward value of food, learning satiety cues, and regulating consumption via connections to the insula (Fudge et al., 2005). The insula, which includes the primary gustatory cortex, assesses sensory taste experience (Fudge et al., 2005; Small, 2010) and integrates this information with interoceptive (e.g., visceral, homeostatic, emotional) signaling from the ventral striatum and hypothalamus to guide motivated behavior. Together, this circuitry integrates autonomic function, learning, reward value, and motor control (Devinsky et al., 1995; Freedman et al., 2000; Paus, 2001; Price and Drevets, 2012; Williams et al., 2004) in the context of metabolic states to motivate feeding (Appelhans, 2009; Rolls, 2008; see review Kaye et al., 2009; Rolls, 2016; Volkow et al., 2011).
Fig. 1.
Schematic representation of overlapping stress and food reward circuitry (Cleck & Blendy, 2008; Monteleone et al., 2018). The reward pathway (green) is composed of dopamine cell bodies in the ventral tegmental area (VTA) that project to the ventral striatum (VST). The VTA also projects to parts of the prefrontal cortex (PFC), hippocampus (HIP), insular cortex (IC), and the amygdala (AMY). Central corticosterone-releasing factor (CRF) circuitry (yellow) consists of CRF-containing cell bodies located in the central nucleus of the amygdala, which projects to the bed nucleus of the stria terminalis (BNST). CRF projections from the amygdala also innervate the VTA, thus completing the circuit. The hypothalamic CRF projections are directed to the pituitary gland (PIT) located outside the blood-brain barrier. CRF in the PIT stimulates the endocrine output of the HPA axis (red) including the release of adrenocorticotropin hormone (ACTH), which acts on the adrenal gland (ADR) to stimulate the secretion of cortisol. A negative feedback system allows for cortisolmediated regulation of continuous CRF production in the HPA axis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Neuroimaging studies employing stress induction tasks have revealed an association between stress and the neural substrates of appetite in individuals across the weight spectrum. In healthy weight individuals, the experience of acute stress dampens brain response to food cues in limbic reward circuitry (Born et al., 2010). In patients with AN, higher reported stress was associated (trend level) with smaller hippocampal volume, which may explain deficits in coping and emotional regulation (McEwen and Gianaros, 2010). This is consistent with prior studies showing that stressful life events influence the neuroplasticity of the hippocampus and associated regions, and alterations in neuroplasticity of the hippocampus can affect stress reactivity and coping (McEwen and Gianaros, 2010). In patients with BN, activation in the precuneus, anterior cingulate cortex, amygdala, and ventral medial prefrontal cortex while viewing food cues was reduced following acute stress (Fischer et al., 2017), and this decreased response was associated with self-reported increased perceived stress prior to binges (Fischer et al., 2017). Findings of decreased precuneus activation in BN to food cues following acute stress, thought to reflect decreased self-referential thought, were subsequently replicated (Collins et al., 2017) and interpreted to support escape theories of emotion regulation in BN (i.e., the notion that binge eating is triggered by acute stress and serves to distract from self-awareness and negative self-referential thoughts) (Heatherton and Baumeister, 1991; Collins et al., 2017). In contrast, in overweight or obese individuals, acute stress elicits greater amygdala response to taste of milkshake, with a significant association between basal cortisol and amygdala response (Rudenga et al., 2013), which may drive appetitive “stress” eating.
Studies that have examined associations between brain activation and cortisol levels provide more direct evidence that HPA activation may be involved in the pathogenesis and maintenance of AN via the suppression of appetitive drive through directly affecting appetite-regulating regions of the brain (Lawson et al., 2013). For example, Lawson and colleagues (Lawson et al., 2013) reported elevated serum cortisol levels in individuals with active AN or who were weight restored, and associations between increased cortisol and lower fasting homeostatic and hedonic measures of subjective appetite. Moreover, associations between elevated cortisol levels and between-group differences in brain activation (e.g., decreased activation in food-motivation brain regions including the hypothalamus, amygdala, hippocampus, OFC, and insula) were evident in the patient groups compared to healthy controls, independent of BMI, when viewing palatable food images. Together, these results suggest the HPA dysregulation in AN is associated with altered perception of appetite and hypoactive food motivation circuitry. However, despite these findings that suggest stress may impact structural and functional brain response in ED, most studies do not account for stress when examining the neurobiological substrates of ED.
6. Physical outcomes of eating disorder behaviors as a unique stressor
There are several unique challenges to using neuroimaging to study ED. Malnutrition and starvation reflect physical stressors of the illness, and the impact of pathological eating and malnutrition on neural processes in ED remains a major methodological question. Malnutrition in AN is associated with changes in brain structure (e.g., reduction in gray matter, altered white matter integrity) and as mentioned above, profound metabolic, electrolyte, and endocrine disturbances (Blasel et al., 2012; Castro-Fornieles et al., 2007; Friederich et al., 2012; Van den Eynde et al., 2012). Studies in animals suggest that diet and weight can influence dopamine (Avena et al., 2012; Johnson and Kenny, 2010) and serotonin metabolism (Kaye et al., 2003a, Kaye et al., 2003b). The studies reviewed above raise the possibility that stress signals may also confound the fMRI blood oxygen level dependent (BOLD) response. To date, the most common strategy to avoid the confounding effects of abnormal nutritional status on brain structure and function has been to study individuals who are weight-restored or have recovered from an ED, although the definition of recovery varies across studies and it remains conjectural whether abnormal findings reflect traits or scars. An alternative strategy that may have potential utility in reducing these physiological confounds and isolating the effects of stress on brain function is to use calibrated fMRI.
7. The potential utility of calibrated fMRI to reduce these physiological confounds in ED and better understand stress
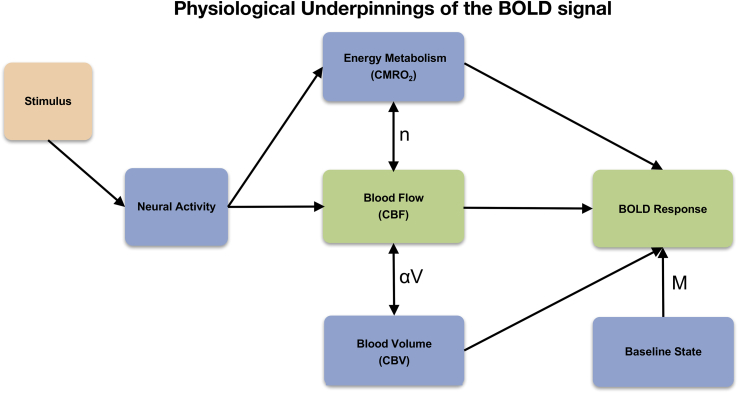
Although BOLD fMRI is the most widely used technique to assess human brain function, it does not directly measure neural activity, but depends on a cascade of physiological events that link neural activity to the BOLD signal. Stress may alter the physiological underpinnings of the BOLD signal and influence the relationship between BOLD response and neural activity. Previous patient fMRI studies (including our own) have not explicitly addressed these potential confounds in the interpretation of BOLD results. Specifically, the BOLD signal reflects local changes in deoxyhemoglobin content, which in turn exhibits a complex dependence on changes in cerebral blood flow (CBF), cerebral blood volume (CBV) and the cerebral metabolic rate of oxygen consumption (CMRO2) (Buxton, 2009; Buxton et al., 2004) (see Fig. 2). CBF is tightly coupled with brain metabolism underlying cognition by increasing local delivery of oxygen and glucose to support neural function and remove metabolic bi-products (Brown et al., 2007). This hemodynamic neurovascular coupling ensures a strong increase of CBF and neuronal glucose uptake with enhanced neural activity (Buxton, 2009). Thus, CBF is an indirect marker of neuronal activity and metabolism, and is most commonly measured with positron emission tomography (PET) 15O-labeled water, single-photon emission tomography (SPECT), and more recently, arterial spin labeling (ASL) functional magnetic resonance imaging (fMRI). Factors that affect CBF or the coupling between CBF and CMRO2—as is possible with stress—may therefore alter the BOLD response even when neural activity is unchanged (Buxton et al., 2004; Wierenga and Bondi, 2007) (see Fig. 3 for a conceptual framework).
Fig. 2.
The BOLD signal is modulated by the baseline state (M: baseline oxygen extraction fraction; arterial, capillary, venous CBV; hematocrit) and the combination of venous CBV change (αV) and CBF/CMRO2 coupling ratio (n) (Buxton, 2009; Buxton et al., 2004).
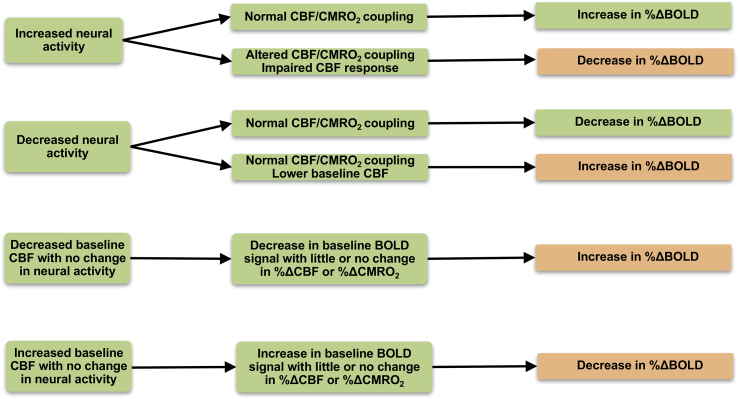
Fig. 3.
Possible neural and perfusion mechanisms resulting in either an increase or decrease in the BOLD response. BOLD = blood oxygen level dependent; CBF = cerebral blood flow; CMRO2 = cerebral metabolic rate of oxygen consumption (Buxton et al., 2004; Wierenga & Bondi, 2007).
Altered CBF has been reported in ED (Frank et al., 2000), with relatively consistent findings of hypoperfusion at rest in regions of the gustatory/homeostatic circuit in ill AN (Kojima et al., 2005; Lask et al., 2005; Naruo et al., 2001; Takano et al., 2001). Findings (Frank et al., 2000) in remitted AN and BN are more discrepant, with some studies reporting hypoperfusion in remitted AN (Rastam et al., 2001; Wierenga et al., 2017) and other studies (Frank et al., 2007; Sheng et al., 2015) reporting no regional differences in CBF. The heterogeneity in methods used to quantify CBF may contribute to these discrepant findings, with higher perfusion reported in ASL than SPECT (Iwanga et al., 2014; Takahashi et al., 2014) images. The meaning of these alterations in CBF is not well understood, and could reflect the influence of stress.
HPA axis hyperactivity may compromise arterial function. GCs like cortisol can facilitate sympathetic actions, such that during stress, their overall physiological effects are to permissively prolong cardiovascular reactivity (i.e., the process by which the cerebral vasculature adjusts CBF). Moreover, arterial tone, which contributes to cardiovascular reactivity by impacting vascular mechanics, is regulated in part by cortisol. Inhibition of cortisol production before mental stress has been shown to prevent endothelial dysfunction (which largely regulates arterial stiffness), and lower levels of circulating cortisol following a 30-min laughter intervention improved arterial stiffness, suggesting inhibition of the HPA axis has beneficial effects on arterial function (Vlachopoulos et al., 2009), or alternatively, that excitation of the HPA axis has detrimental effects on arterial function that could impact CBF and compromise the fMRI BOLD signal in patient populations characterized by high stress.
Changes in CBF may also reflect the function of astrocytes, given their critical role in mediating the coupling of neuronal activity with metabolic and vascular responses (Magistretti and Allaman, 2015). Astrocytic end feet wrap around the endothelium of capillaries and via this contact, they can influence CBF and control the transport of nutrients, such as glucose, in and out of the brain to ensure proper brain homeostasis and support neurons metabolically (Magistretti and Allaman, 2015; Zhang et al., 2012). Recent evidence suggests astrocytes, similar to neurons, respond directly to multiple nutrient and endocrine signals and, in turn, contribute to adjusting central nervous system control of systemic metabolism according to nutrient availability (García-Cáceres et al., 2016). This raises the question of whether findings of altered CBF reflect an aberrant neural-astrocyte response to peptides, enzymes, or metabolic or stress hormones that disrupt appetite signaling.
Supporting this notion is evidence of CBF alteration in other disorders (in addition to EDs) associated with HPA axis activity. For example, PTSD, OCD, and other anxiety disorder patients have demonstrated widespread regions of hyperperfusion in frontal, temporal, and parietal cortices, subcortical structures (caudate, putamen, thalamus), and the cerebellum (Bonne et al., 2003; Kim et al., 2007; Lacerda et al., 2003; Sachinvala et al., 2000), and hypoperfusion in orbitofrontal, anterior cingulate, and thalamic regions, when compared to healthy controls (Busatto et al., 2000; Kim et al., 2007). Symptom severity among these patients also appears to correlate (both positively and negatively) with CBF levels (Busatto et al., 2000; Lucey et al., 1997; Osuch et al., 2000). Moreover, functional CBF shows differential change among those with social anxiety, with an observed pattern of subcortical CBF increase during stress induction tasks, compared to increased cortical (rather than subcortical) CBF among normal controls under the same acute stress conditions (Tillfors et al., 2001). There is also evidence that everyday stress in the absence of psychopathology leads to functional CBF alteration, with experimentally induced psychological stress among healthy normal controls being associated with increased CBF within the prefrontal cortex, the putamen, and the cerebellum (Ito et al., 2003; Wang et al., 2005, 2007), and reduced CBF within the orbitofrontal cortex (Pruessner et al., 2008; Tillfors et al., 2001; Wang et al., 2005, 2007).
Cortisol levels are also linked to both increased and decreased CBF among those with stress-related disorders (i.e., PTSD, social anxiety) most notably in frontal, medial temporal, and hypothalamic regions (Ahs et al., 2006; Bonne et al., 2003). Similar associations between cortisol and CBF are evident among normal controls during acute stress (Wang et al., 2005, 2007). These results are consistent with animal studies showing rich cortisol receptor in the prefrontal cortex, hippocampus, and amygdala (Carrasco and Van De Kar, 2003; Charney, 2004). Corticotropin-releasing factor also appears to be associated with CBF alteration, with animal evidence suggesting its capacity to provoke major increases in CBF (De Michele et al., 2005). Overall, these findings support the notion that HPA axis function is associated with resting and functional CBF alteration, which could serve to confound the BOLD signal.
Taken together, this suggests that populations characterized by high stress, such as EDs, are likely to show CBF and BOLD signal alteration that reflects stress response, rather than directly reflecting neural activation. Since neurons necessarily expend energy to accomplish their work (Hyder, 2004), CMRO2 is thought to be a more direct measure of neuronal activity than is CBF or BOLD. Although measures of CBF may be particularly useful when investigating the direct impact of stress on clinical populations, CMRO2 is likely to be a more accurate measure when interested in investigating neural activity controlling for stress. Future work is needed to examine the differential relationships between physiological measures of stress (i.e., cortisol, GR and MR expression) and CBF, the BOLD signal, and CMRO2, to better elucidate the impact of stress on brain function and its relationship to clinical symptoms. Revealing potential associations between stress, brain response, and symptom severity is of particular clinical relevance.
The positive BOLD response observed in most fMRI experiments reflects the fact that CBF increases relatively more than CMRO2, so that local capillary and venous blood are more oxygenated during increased brain activity. In general, the actual amplitude of the BOLD response reflects a delicate balance between the relative increases in CBF and CMRO2 (Brown et al., 2007; Buxton et al., 2004). For example, decreased BOLD response to food cues in reward circuitry in AN, thought to reflect reduced reward sensitivity and motivation to eat, may thus reflect impaired CBF response and/or altered CBF/CMRO2 coupling, or a true decrease in neural activity with normal CBF/CMRO2 coupling. However, without a quantitative estimate of functional changes in CMRO2, the impact of physiological effects (e.g., stress hormones, malnutrition) on CBF and the BOLD response is unclear. To improve the interpretability of future BOLD FMRI studies, Iannetti & Wise (Iannetti and Wise, 2007) have argued that calculation of CMRO2 is the best method to extract meaningful information of neural activity from fMRI BOLD studies in patients by disentangling potential vascular confounds.
Calculation of CMRO2 has traditionally been accomplished through the use of calibrated fMRI. This innovative functional imaging techniques estimates functional changes in CMRO2 by simultaneously acquiring BOLD and CBF data during a functional task in combination with a calibration experiment and analyzing these data within the context of a mathematical model of the BOLD response (Davis et al., 1998; Liu and Wong, 2005). The calibration constant M, corresponding to the maximum possible BOLD signal increase (i.e. the signal increase that could be achieved upon total elimination of all dHb from tissues), is estimated using blood gas manipulation techniques, most often involving CO2 or O2 gas mixture breathing. Assuming that these hypercapnic techniques do not induce changes in CMRO2, M can then be quantified from the measurement of BOLD and CBF changes relative to baseline, allowing for subsequent quantification of fractional changes in CMRO2 (Davis et al., 1998). Regionally specific changes in CMRO2 in response to a functional task can then be used as outcome measures in case-control comparisons of neural function differences.
Although the calibrated fMRI method offers one of the best noninvasive techniques currently available for measuring oxygen metabolism in the brain with good spatial and temporal resolution (Griffeth and Buxton, 2011), some limitations of this technique should be noted. The David model has become the standard model for the calibrated fMRI experiment, but this model makes several assumptions, that if incorrect, could lead to errors in calculating CMRO2. For example, the Davis model assumes 1) CBV changes are uniformly distributed across vascular compartments, 2) CBF/CBF coupling is always the same, and 3) hypercapnia does not affect CMRO2. The Davis model also neglects intravascular signal changes (despite evidence that intravascular signal changes and CBV exchange effects can bias estimated CMRO2). Moreover, although directly measuring CMRO2 using a hypercapnic challenge to calibrate the BOLD signal across participants allows for the most accurate estimate of CMRO2, it may also be cumbersome to acquire in some populations (e.g., older adults) (Griffeth and Buxton, 2011; Hoge, 2012). As such, newer scan sequences are being developed to assess CMRO2 without requiring hypercapnia (Blockley et al., 2015; Yücel et al., 2012). Furthermore, because calibrated fMRI relies on measurement of CBF, limitations of arterial spin labeling methods, including magnetization transfer effects, reduced tagging efficiency, insensitivity to transit time effects, and low spatial resolution may also apply.
8. Conclusion
Stress and altered HPA axis activity have been implicated in the pathogenesis and maintenance of ED, and the impact of stress on the neurobiological substrates of appetite regulation and food-related reward processing is beginning to be revealed. With the increasing application of fMRI techniques to the study of stress in ED, there is a growing need for quantitative measures that can more accurately reflect neural activity without confounding physiological effects of stress and malnutrition. One promising area for future efforts entails the use ASL/BOLD calibrated fMRI in order to advance our understanding of the unique features associated with the pathophysiology of ED. One clear benefit of this approach is to control for physiological effects of stress and ED behaviors on traditional measures of neural activation to avoid potentially confounding effects of stress with differences in neural function. Moreover, the ability to differentiate between CBF, BOLD and CMRO2 also allows for model comparisons to directly assess the differential influence of physiological measures of stress on each measured brain response. The potential utility of calibrated fMRI lies in its ability to help resolve some of the discrepancies in the literature and depends on carefully designed studies aimed at directly mapping complex neurobiological stress profiles onto specific symptom constructs and functional brain response.
Declaration of interest
None.
Acknowledgements
Support provided by the National Institute of Mental Health [R01MH113588 to C.E.W; K23MH101342 to J.L.] and the National Science Foundation Graduate Research Fellowship Program [2015207525 C.C.H.].
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.08.006.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abraham S., Beaumont P. How patients describe bulimia or binge eating. Psychol. Med. 1982;12(3):625–635. doi: 10.1017/s0033291700055732. [DOI] [PubMed] [Google Scholar]
- Adam T.C., Epel E.S. Stress, eating and the reward system. Physiol. Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Ahs F., Furmark T., Michelgard A., Langstrom B., Appel L., Wolf O. Hypothalamic blood flow correlates positively with stress-induced cortisol levels in subjects with social anxiety disorder. Psychosom. Med. 2006;68(6):859–862. doi: 10.1097/01.psy.0000242120.91030.d8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . In: Diagnostic & Statistical Manual of Mental Disorders. fourth ed. Association A.P., editor. DSM:VI-TR; Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association . fifth ed. DSM-V; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anderluh M.B., Tchanturia K., Rabe-Hesketh S., Treasure J. Childhood obsessive-compulsive personality traits in adult women with eating disorders: defining a broader eating disorder phenotype. Am. J. Psychiatr. 2003;160(2):242–247. doi: 10.1176/appi.ajp.160.2.242. 12562569. [DOI] [PubMed] [Google Scholar]
- Appelhans B. Neurobehavioral inhibition of reward‐driven feeding: implications for dieting and obesity. Obesity (Silver Springs) 2009;17(4):640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- Avena N., Borcarsly M., Hoebel B. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Meth. Mol. Biol. 2012;829:351–365. doi: 10.1007/978-1-61779-458-2_23. 22231826. [DOI] [PubMed] [Google Scholar]
- Benoit S., Davis J., Davidson T. Learned and cognitive controls of food intake. Brain Res. 2010;1350:71–76. doi: 10.1016/j.brainres.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner L.C., RD, Cao L., Engel S., Lavender J., Mitchell J., Wonderlich S. Temporal associations between affective instability and dysregulated eating behavior in bulimia nervosa. J. Psychiatr. Res. 2017;92:183–190. doi: 10.1016/j.jpsychires.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham C., Su J., Hlynsky J., Goldner E., Gao M. The mortality rate from anorexia nervosa. Int. J. Eat. Disord. 2005;38:143–146. doi: 10.1002/eat.20164. 16134111. [DOI] [PubMed] [Google Scholar]
- Blasel S., Pilatus U., Magerkurth J., von Stauffenberg M., Vronski D., Mueller M. Metabolic gray matter changes of adolescents with anorexia nervosa in combined MR proton and phosphorus spectroscopy. Neuroradiology. 2012;54(7):753–764. doi: 10.1007/s00234-011-1001-9. 22201349. [DOI] [PubMed] [Google Scholar]
- Blockley N.P., Griffeth V.E.M., Simon A.B., Dubowitz D.J., Buxton R.B. Calibrating the BOLD response without administering gases: comparison of hypercapnia calibration with calibration using an asymmetric spin echo. Neuroimage. 2015;104:423–429. doi: 10.1016/j.neuroimage.2014.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O., Gilboa A., Louzoun Y., Brandes D., Yona I., Lester H. Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biol. Psychiatr. 2003;54(10):1077–1086. doi: 10.1016/s0006-3223(03)00525-0. 14625150. [DOI] [PubMed] [Google Scholar]
- Born J., Lemmens S., Rutters F., Nieuwenhuizen A., Formisano E., Goebel L. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int. J. Obes. 2010;34(1):172–181. doi: 10.1038/ijo.2009.221. [DOI] [PubMed] [Google Scholar]
- Bornstein S.R., Uhlmann K., Haidan A., Ehrhart-Bornstein M., Scherbaum W.A. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland. Leptin inhibits cortisol release directly. Diabetes. 1997;46:1235–1238. doi: 10.2337/diab.46.7.1235. [DOI] [PubMed] [Google Scholar]
- Braun J., Lasiter P., Kiefer S. The gustatory neocortex of the rat. Physiol. Psychol. 1982;10:13–45. [Google Scholar]
- Brockmeyer T., Skunde M., Wu M., Bresslein E., Rudofsky G., Herzog W. Difficulties in emotion regulation across the spectrum of eating disorders. Compr. Psychiatr. 2014;55(3):565–571. doi: 10.1016/j.comppsych.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Brown G., Clark C., Liu T. Measurement of cerebral perfusion with arterial spin labeling. Part 2. Applications. J. Int. Neuropsychol. Soc. 2007;13:526–538. doi: 10.1017/S1355617707070634. 17445302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik C., Sullivan P.F., Tozzi F., Furberg H., Lichtenstein P., Pedersen N.L. Prevalence, heritability and prospective risk factors for anorexia nervosa. Arch. Gen. Psychiatr. 2006;63(3):305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- Burkert N., Koschutnig K., Ebner F., Frieidl W. Structural hippocampal alterations, perceived stress, and coping deficiencies in patients with anorexia nervosa. Int. J. Eat. Disord. 2015;48(6):670–676. doi: 10.1002/eat.22397. [DOI] [PubMed] [Google Scholar]
- Busatto G., Zamignani D., Buchpiguel C., Garrido G., Glabus M., Rocha E. A voxel-based investigation of regional cerebral blood flow abnormalities in obsessive-compulsive disorder using single photon emission computed tomography (SPECT) Psychiatr. Res. 2000;99(1):15–27. doi: 10.1016/s0925-4927(00)00050-0. 10891646. [DOI] [PubMed] [Google Scholar]
- Buxton R. Second. Cambridge University Press; New York: 2009. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. [Google Scholar]
- Buxton R., Uludağ K., Dubowitz D., Liu T. Modeling the hemodynamic response to brain activation. Neuroimage. 2004 doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Carr C.P., Martins C.M., Stingel A.M., Lemgruber V.B., Juruena M.F. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J. Nerv. Ment. Dis. 2013;201(12):1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Carrasco G., Van De Kar L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003;463(1–3):235–272. doi: 10.1016/s0014-2999(03)01285-8. 12600714. [DOI] [PubMed] [Google Scholar]
- Caslini M., Bartoli F., Crocamo C., Dakanalis A., Clerici M., Carrà G. Disentangling the association between child abuse and eating disorders: a systematic review and meta-analysis. Psychosom. Med. 2016;78(1):79–90. doi: 10.1097/PSY.0000000000000233. [DOI] [PubMed] [Google Scholar]
- Castro-Fornieles J., Bargallo N., Lazaro L., Andres S., Falcon C., Plana M. Adolescent anorexia nervosa: cross-sectional and follow-up frontal gray matter disturbances detected with proton magnetic resonance spectroscopy. J. Psychatr. Res. 2007;41(11):952–958. doi: 10.1016/j.jpsychires.2006.09.013. 17112540. [DOI] [PubMed] [Google Scholar]
- Cavagnini F., Croci M., Putignano P., Petroni M.L., Invitti C. Glucocorticoids and neuroendocrine function. Int. J. Obes. Relat. Metab. Disord. 2000;24(Suppl. 2):S77–S79. doi: 10.1038/sj.ijo.0801284. [DOI] [PubMed] [Google Scholar]
- Chalmers D., Lovenberg T., De Souza E. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J. Neurosci. 1995;15(10):6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. 7472399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers D.L., TWGrigoriadis D.E., Behan D., De Souza E. Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol. Sci. 1996;17(4):166–172. doi: 10.1016/0165-6147(96)81594-x. 8984745. [DOI] [PubMed] [Google Scholar]
- Chapman D., Dube S.A., Anda R.F. Adverse childhood events as risk factors for negative mental health outcomes. Psychiatr. Ann. 2007;37:359–364. [Google Scholar]
- Charney D. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am. J. Psychiatr. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. 14754765. [DOI] [PubMed] [Google Scholar]
- Chida Y., Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol. Bull. 2008;134(6):829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Cleck J.N., Blendy J.A. Making a bad thing worse: adverse effects of stress on drug addiction. J. Clin. Investig. 2008;118(2):454–461. doi: 10.1172/JCI33946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger C., Przybeck T., Svrakic D., Wetzel R. vol. 2. Center for Psychobiology of Personality, Washington University; St. Louis MO: 1994. pp. 19–28. (The Temperament and Character Inventory (TCI): a Guide to its Development and Use). Chapter 4. ISBN 0-9642917-1-1. [Google Scholar]
- Collins B., Breithaupt L., McDowell J., Miller L., Thompson J., Fischer S. The impact of acute stress on the neural processing of food cues in bulimia nervosa: replication in two samples. J. Abnorm. Psychol. 2017;126(5):540–551. doi: 10.1037/abn0000242. [DOI] [PubMed] [Google Scholar]
- Crespo C.S., Cachero A.P., Jiménez L.P., Barrios V., Ferreiro E.A. Peptides and food intake. Front. Endocrinol. 2014;5:58. doi: 10.3389/fendo.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby R., Wonderlich S., Engel S., Simonich H., Smyth J., Mitchell J. Daily mood patterns and bulimic behaviors in the natural environment. Behav. Res. Ther. 2009;47(3):181–188. doi: 10.1016/j.brat.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert K.M., Racine S.E., Klump K.L. Hormonal factors and disturbances in eating disorders. Curr. Psychiatr. Rep. 2016;18(7):65. doi: 10.1007/s11920-016-0701-6. [DOI] [PubMed] [Google Scholar]
- Davidson T., Jarrard L. A role for hippocampus in the utilization of hunger signals. Behav. Neural. Biol. 1993;59(2):167–171. doi: 10.1016/0163-1047(93)90925-8. 8476385. [DOI] [PubMed] [Google Scholar]
- Davidson T., Kanoski S., Schier L., Clegg D., Benoit S. A potential role for the hippocampus in energy intake and body weight regulation. Curr. Opin. Pharmacol. 2007;7(6):613–616. doi: 10.1016/j.coph.2007.10.008. 18032108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T., Kwong K., Weisskoff R., Rosen B. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. 9465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E. About stress hormones and resilience to psychopathology. J. Neuroendocrinol. 2008;206:885–892. doi: 10.1111/j.1365-2826.2008.01707.x. [DOI] [PubMed] [Google Scholar]
- De Michele M., Touzani O., Foster A.F.C., Sette G., McCulloch J. Corticotropin-releasing factor: effect on cerebral blood flow in physiologic and ischaemic conditions. Exp. Brain Res. 2005;165(3):375–382. doi: 10.1007/s00221-005-2303-0. 15864562. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. 7895011. [DOI] [PubMed] [Google Scholar]
- Dube S., Felitti V., Dong M., Giles W., Anda R. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev. Med. 2003;37(3):268–277. doi: 10.1016/s0091-7435(03)00123-3. 12914833. [DOI] [PubMed] [Google Scholar]
- Epel E., Lapidus R., McEwen B., Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoendocrin. 2001;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Farstad S.M., McGeown L.M., von Ranson K.M. Eating disorders and personality, 2004-2016: a systematic review and meta-analysis. Clin. Psychol. Rev. 2016;46:91–105. [Google Scholar]
- Fassino S., Abbate-Daga G., Amianto F., Leombruni P., Boggio S., Rovera G.G. Temperament and character profile of eating disorders: a controlled study with the temperament and character inventory. Int. J. Eat. Disord. 2002;32:412–425. doi: 10.1002/eat.10099. [DOI] [PubMed] [Google Scholar]
- Favaro A., Zanetti T., Tenconi E., Degortes D., Ronzan A., Veronese A., Santonastaso P. The relationship between temperament and impulsive behaviors in eating disordered subjects. Eat. Disord. 2005;13(1):61–70. doi: 10.1080/10640260590893647. [DOI] [PubMed] [Google Scholar]
- Fischer S., Breithaupt L., Wonderlich J., Westwater M.L., Crosby R.D., Engel S.G., Thompson J., Lavender J., Wonderlich S. Impact of the neural correlates of stress and cue reactivity on stress related binge eating in the natural environment. J. Psychiatr. Res., Sep. 2017;92:15–23. doi: 10.1016/j.jpsychires.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Frank G., Bailer U.F., Meltzer C.C., Price J., Mathis C., Wagner A. Regional cerebral blood flow after recovery from anorexia and bulimia nervosa. Int. J. Eat. Disord. 2007;40(6):488–492. doi: 10.1002/eat.20395. [DOI] [PubMed] [Google Scholar]
- Frank G., Kaye W. Current status of functional imaging in eating disorders. Int. J. Eat. Disord. 2012;45(6):723–736. doi: 10.1002/eat.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G.K., Kaye W.H., Greer P., Meltzer C.C., Price J.C. Regional cerebral blood flow after recovery from bulimia nervosa. Psychiatr. Res. 2000;100(1):31–39. doi: 10.1016/s0925-4927(00)00069-x. 11090723. [DOI] [PubMed] [Google Scholar]
- Freedman L.J., Insel T.R., Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J. Comp. Neurol. 2000;421:172–188. 10813780. [PubMed] [Google Scholar]
- Friederich H., Herzog W. Cognitive-behavioral flexibility in anorexia nervosa. Curr. Top Behav. Neurosci. 2011;6:111–123. doi: 10.1007/7854_2010_83. 21243473. [DOI] [PubMed] [Google Scholar]
- Friederich H., Walther S., Bendszus M., Biller A., Thomann P., Zeigermann S. Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage. 2012;59(2):1106–1113. doi: 10.1016/j.neuroimage.2011.09.042. 21967727. [DOI] [PubMed] [Google Scholar]
- Fudge J., Breibart M., Danish M., Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J. Comp. Neurol. 2005;490(2):101–118. doi: 10.1002/cne.20660. 16052493 PMCID: PMC2474655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cáceres C., Quarta C.V.L., Gao Y., Gruber T., Legutko B., Jastroch M. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell. 2016;166(4):867–880. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty A.T., Phillips A.C., Higgs S., Heaney J.L., Carroll D. Disordered eating behaviour is associated with blunted cortisol and cardiovascular reactions to acute psychological stress. Psychoneuroendocrinology. 2012;37(5):715–724. doi: 10.1016/j.psyneuen.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Glashouwer K., Bloot L., Veensra E., Franken I., de Jong P. Heightened sensitivity to punishment and reward in anorexia nervosa. Appetite. 2014;75:97–102. doi: 10.1016/j.appet.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Gluck M.E. Stress response and binge eating disorder. Appetite. 2006;46(1):26–30. doi: 10.1016/j.appet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Greeno C.G., Wing R.R. Stress-induced eating. Psychol. Bull. 1994;115(3):444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- Griffeth V.E., Buxton R.B. A theoretical framework for estimating cerebral oxygen metabolism changes using the calibrated-BOLD method: modeling the effects of blood volume distribution, hematocrit, oxygen extraction fraction, and tissue signal properties on the BOLD signal. Neuroimage. 2011 Sep 1;58(1):198–212. doi: 10.1016/j.neuroimage.2011.05.077. Epub 2011 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt A., Keel P. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychol. Bull. 2011;137(4):660–681. doi: 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave S., Jones S., Davidson T. The outward spiral: a vicious cycle model of obesity and cognitive dysfunction. Curr. Opin. Behav. Sci. 2016;9:40–46. doi: 10.1016/j.cobeha.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A., O'Brien N., Lopez C., Treasure J. Sensitivity to reward and punishment in eating disorders. Psychol. Res. 2010;177(1–2):1–11. doi: 10.1016/j.psychres.2009.06.010. 20381877. [DOI] [PubMed] [Google Scholar]
- Harrison A., Steinheim L., O'Hara C., Oldershaw A., Schmidt U. Do reward and punishment sensitivity change after treatment for anorexia nervosa? Pers. Indiv. Differ. 2016;96(40–6) [Google Scholar]
- Heatherton T.F., Baumeister R.F. Binge eating as escape from self-awareness. Psychol. Bull. 1991;110(1):86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Heiman M.L., Ahima R.S., Craft L.S., Schoner B., Stephens T.W., Flier J.S. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138(9):3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Figueiredo H., Mueller N.K., Ulrich-Lai Y., Ostrander M.M., Choi D.C., Cullinan W.E. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comp. Physiol. 2016;6(2):603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog D.B., Keller M.B., Lavori P.W., Kenny G.M., Sacks N.R. The prevalence of personality disorders in 210 women with eating disorders. J. Clin. Psychiatr. 1992;53(5):147–152. 1592839. [PubMed] [Google Scholar]
- Het S., Vocks S., Wolf J.M., Hammelstein P., Herpertz S., Wolf O.T. Blunted neuroendocrine stress reactivity in young women with eating disorders. J. Psychosom. Res. 2015;78(3):260–267. doi: 10.1016/j.jpsychores.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Hoge R.D. Calibrated FMRI. Neuroimage. 2012 Aug 15;62(2):930–937. doi: 10.1016/j.neuroimage.2012.02.022. Epub 2012 Feb 17. Review. [DOI] [PubMed] [Google Scholar]
- Hudson J., Hiripi E., Pope H., Kessler R. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychol. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. 16815322 PMCID: PMC1892232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F. Neuroimaging with calibrated FMRI. Stroke. 2004;35(11 Suppl. 1):2635–2641. doi: 10.1161/01.STR.0000143324.31408.db. 15388903. [DOI] [PubMed] [Google Scholar]
- Iacovino J., Gredysa D., Altman M.W., DE Psychological treatments for binge eating disorder. Curr. Psychiatr. Rep. 2012;14(4):432–446. doi: 10.1007/s11920-012-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti G., Wise R. BOLD functional MRI in disease and pharmacological studies: room for improvement? Mag. Reson. Imaging. 2007;25(6):978–988. doi: 10.1016/j.mri.2007.03.018. 17499469. [DOI] [PubMed] [Google Scholar]
- Ito H., Kanno I., Hatazawa J., Miura S. Changes in human cerebral blood flow and myocardial blood flow during mental stress measured by dual positron emission tomography. Ann. Nucl. Med. 2003;17(5):381–386. doi: 10.1007/BF03006605. 12971636. [DOI] [PubMed] [Google Scholar]
- Iwanga T., Harada M., Kubo H., Funakoshi Y., Kunikane Y., Matsuda T. Operator-bias-free comparison of quantitative perfusion maps acquired with pulsed-continuous arterial spin labeling and single-photon-emission computed tomography. Magn. Reson. Med. 2014;13(4):239–249. doi: 10.2463/mrms.2013-0117. 25167874. [DOI] [PubMed] [Google Scholar]
- Jacobi C., Hayward C., de Zwaan M., Kraemer H., Agras W. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol. Bull. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. 14717649. [DOI] [PubMed] [Google Scholar]
- Jappe L., Frank G., Shott M., Rollin M., Pryor T., Hagman J. Heightened sensitivity to reward and punishment in anorexia nervosa. Int. J. Eat. Disord. 2011;44(4):317–324. doi: 10.1002/eat.20815. 21472750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Larson R. Bulimia: an analysis of mood and behavior. Psychosom. Med. 1982;44(4):341–351. doi: 10.1097/00006842-198209000-00003. [DOI] [PubMed] [Google Scholar]
- Johnson P., Kenny P. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. 20348917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W., Bulik C., Thornton L., Barbarich N., Masters K., Fichter M., Halmi K., Kaplan A., Strober M., Woodside D.B., Bergen A., Crow S., Mitchell J., Rotondo A., Mauri M., Cassano G., Keel P.K., Plotnicov K., Pollice C., Klump K., Lilenfeld L.R., Devlin B., Quadflieg R., Berrettini W.H. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am. J. Psychiatr. 2004;161(12):2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kaye W., Fudge J., Paulus M. New insight into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009;10(8):573–584. doi: 10.1038/nrn2682. 19603056. [DOI] [PubMed] [Google Scholar]
- Kaye W., Strober M., Jimerson D. The neurobiology of eating disorders. In: Charney D., Nestler E., editors. The Neurobiology of Mental Illness. third ed. Oxford Press; New York: 2008. [Google Scholar]
- Kaye W., Strober M., Klump K.L. Neurobiology of eating disorders. In: Martin A., Scahill L., Charney D.S., Leckman J.F., editors. Pediatric Psychopharmacology, Principles & Practice. Oxford University Press; New York: 2003. pp. 224–237. [Google Scholar]
- Kaye W., Wierenga C., Bailer U., Simmons A., Wagner A., Bischoff-Grethe A. Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biol. Psychiatr. 2013;73(9):836–842. doi: 10.1016/j.biopsych.2013.01.002. 23380716 PMCID:PMC3755487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W.H., Barbarich N.C., Putnam K., Gendall K.A., Fernstrom J., Fernstrom M. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int. J. Eat. Disord. 2003;33(3):257–267. doi: 10.1002/eat.10135. 12655621. [DOI] [PubMed] [Google Scholar]
- Kaye W.H., Gwirtsman H.E., George D.T., Weiss S.R., Jimerson D.C. Relationship of mood alterations to bingeing behaviour in bulimia. Br. J. Psychiatry. 1986;149:479–485. doi: 10.1192/bjp.149.4.479. [DOI] [PubMed] [Google Scholar]
- Keel P.K., Mitchell J.E., Miller K.B., Davis T.L., Crow S.J. Long-term outcome of bulimia nervosa. Arch. Gen. Psychiatr. 1999;56(1):63–69. doi: 10.1001/archpsyc.56.1.63. 9892257. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., MacLean C., Neale M., Kessler R., Heath A., Eaves L. The genetic epidemiology of bulimia nervosa. Am. J. Psychiatr. 1991;148(12):1627–1637. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A., Hoek H., Linna M., Raevuori A., Sihvola E., Bulik C. Incidence and outcomes of bulimia nervosa: a nationwide population-based study. Psychol. Med. 2009;39(5):823–831. doi: 10.1017/S0033291708003942. [DOI] [PubMed] [Google Scholar]
- Kessler R.M., KA, Green J., Gruber M., Sampson N.Z., AM, Aguilar-Gaxiola S., Alhamzawi A. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br. J. Psychiatry. 2010;197(5):378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lyoo I., Lee Y., Kim J., Sim M., Bae S. Decreased cerebral blood flow of thalamus in PTSD patients as a strategy to reduce re-experience symptoms. Acta Psychiatr. Scand. 2007;116(2):145–153. doi: 10.1111/j.1600-0447.2006.00952.x. 17650277. [DOI] [PubMed] [Google Scholar]
- Klein D., Walsh B. Eating disorders. Int. Rev. Psychiatr. 2003;15:205–216. doi: 10.1080/0954026031000136839. [DOI] [PubMed] [Google Scholar]
- Kojima S., Nagai N., Nakabeppu Y. Comparison of regional cerebral blood flow in patients with anorexia nervosa before and after weight gain. Psychiatr. Res. 2005;140:251–258. doi: 10.1016/j.pscychresns.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Koo-Loeb J.H., Pedersen C., Girdler S.S. Blunted cardiovascular and catecholamine stress reactivity in women with bulimia nervosa. Psychiatr. Res. 1998;80(1):13–27. doi: 10.1016/s0165-1781(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Lacerda A., Dalgalarrondo P., Caetano D., Camargo E.E., EC, Soares J. Elevated thalamic and prefrontal regional cerebral blood flow in obsessive-compulsive disorder: a SPECT study. Psychiatr. Res. 2003;123(2):125–134. doi: 10.1016/s0925-4927(03)00061-1. 12850251. [DOI] [PubMed] [Google Scholar]
- Lask B., Gordon I., Christie D., Frampton I., Chowdhury U., Watkins B. Functional neuroimaging in early-onset anorexia nervosa. Int. J. Eat. Disord. 2005;37(Suppl. S49–51) doi: 10.1002/eat.20117. Discussion S87-89. [DOI] [PubMed] [Google Scholar]
- Lawson E.A., Eddy K.T., Donoho D., Misra M., Miller K.K., Meenaghan E., Lydecker J., Herzog D., Klibanski A. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur. J. Endocrinol. 2011;164(2):253–261. doi: 10.1530/EJE-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson E., Holsen L., Desanti R., Santin M., Meenaghan E., Herzog D. Increased hypothalamic-pituitary-adrenal drive is associated with decreased appetite and hypoactivation of food-motivation neurocircuitry in anorexia nervosa. Eur. J. Endocrinol. 2013;169(5):639–647. doi: 10.1530/EJE-13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson E.E., KT, Donoho D., Misra M., Miller K., Meenaghan E., Lydecker J. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur. J. Endocrinol. 2011;164(2):253–261. doi: 10.1530/EJE-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R.S., Folkman S. Springer Publishing Company; New York: 1984. Stress, Appraisal, and Coping. [Google Scholar]
- Lilenfeld L., Wonderlich S., Riso L.P., Crosby R., Mitchell J. Eating disorders and personality: a methodological and empirical review. Clin. Psychol. Rev. 2006;26(3):299–320. doi: 10.1016/j.cpr.2005.10.003. 16330138. [DOI] [PubMed] [Google Scholar]
- Lilenfeld L.R., Kaye W.H., Greeno C.G., Merikangas K.R., Plotnicov K., Pollice C. A controlled family study of anorexia nervosa and bulimia nervosa: psychiatric disorders in first-degree relatives and effects of proband comorbidity. Arch. Gen. Psychiatr. 1998;55(7):603–610. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- Liu T.T., Wong E.C. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005 Jan 1;24(1):207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Lo Sauro C., Ravaldi C., Cabras P.L., Faravelli C., Ricca V. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology. 2008;57(3):95–115. doi: 10.1159/000138912. [DOI] [PubMed] [Google Scholar]
- Lock J., Le Grange D., Agras W., Moye A., Bryson S., Jo B. Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch. Gen. Psychiatr. 2010;67:1025–1032. doi: 10.1001/archgenpsychiatry.2010.128. 20921118 PMCID:PMC3038846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb K., Le Grange D. Family-based treatment for adolescent eating disorders: current status, new applications and future directions. Int. J. Child Adolesc. Health. 2009;2(2):243–254. 20191109 PMCID:PMC2828763. [PMC free article] [PubMed] [Google Scholar]
- Lucey J.C., DC, Adshead G., Deahl M., Busatto G., Gacinovic S., Travis M. Brain blood flow in anxiety disorders. OCD, panic disorder with agoraphobia, and post-traumatic stress disorder on 99mTcHMPAO single photon emission tomography (SPET) Br. J. Psychiatry. 1997;171 doi: 10.1192/bjp.171.4.346. 346-250. [DOI] [PubMed] [Google Scholar]
- Magistretti P., Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86(4):883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Matton A., Goossens L., Braet C., Vervaet M. Punishment and reward sensitivity: are naturally occurring clusters in these traits related to eating and weight problems in adolescents? Eur. Eat Disord. Rev. 2013;21:184–194. doi: 10.1002/erv.2226. [DOI] [PubMed] [Google Scholar]
- Mason T.B., Lavender J.M., Wonderlich S.A., Crosby R.D., Engel S.G., Mitchell J.E., Crow S.J., Le Grange D., Peterson C.B. Examining a momentary mediation model of appearance-related stress, anxiety, and eating disorder behaviors in adult anorexia nervosa. Eat. Weight Disord. 2017 doi: 10.1007/s40519-017-0404-y. Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurak N., Enck P., Muth E., Teufel M., Zipfel S. Heart rate variability as a measure of cardiac autonomic function in anorexia nervosa: a review of the literature. Eur. Eat Disord. Rev. 2011;19(2):87–99. doi: 10.1002/erv.1081. [DOI] [PubMed] [Google Scholar]
- McEwen B. Glucocorticoid receptors in the brain. Hosp. Pract. 1988;23(8):107–111. doi: 10.1080/21548331.1988.11703523. 119-121.2841347. [DOI] [PubMed] [Google Scholar]
- McEwen B. Mood disorders and allostatic load. Biol. Psychiatr. 2003;54(3):200–207. doi: 10.1016/s0006-3223(03)00177-x. 12893096. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B., De Kloet E., Rosetene W. Adrenal steroid receptors and actions in the nervous system. Physiol. Rev. 1986;66(41121–1188):3532143. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen B., Gianaros P. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B., K.W, Schwartz L. Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 1969;16(1):227–241. doi: 10.1016/0006-8993(69)90096-1. 5348850. [DOI] [PubMed] [Google Scholar]
- McKenzie J.M., Joyce P.R. Hospitalization for anorexia nervosa. Int. J. Eat. Disord. 1992;11(3):235–241. [Google Scholar]
- Messerli-Bürgy N., Engesser C., Lemmenmeier E., Steptoe A., Laederach-Hofmann K. Cardiovascular stress reactivity and recovery in bulimia nervosa and binge eating disorder. Int. J. Psychophysiol. 2010;78(2):163–168. doi: 10.1016/j.ijpsycho.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Mitchell J., Agras S., Wonderlich S. Treatment of bulimia nervosa: where are we and where are we going? Int. J. Eat. Disord. 2007;40(2):95–101. doi: 10.1002/eat.20343. 17080448. [DOI] [PubMed] [Google Scholar]
- Mond J.L., JD, Hay P., Owen C., Rodgers B. Objective and subjective bulimic episodes in the classification of bulimic-type eating disorders: another nail in the coffin of a problematic distinction. Behav. Brain Res. Ther. 2010;48(7):661–669. doi: 10.1016/j.brat.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Monteleone A., Castellini G., Volpe U., Ricca V., Lelli L., Monteleone P., Maj M. Neuroendocrinology and brain imaging of reward in eating disorders: a possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2018;80:132–142. doi: 10.1016/j.pnpbp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Monteleone A., Monteleone P., Marciello F., Pellegrino F., Castellini G., Maj M. Differences in cortisol awakening response between binge-purging and restrictive patients with anorexia nervosa. Eur. Eat Disord. Rev. 2016;25(1):13–18. doi: 10.1002/erv.2485. [DOI] [PubMed] [Google Scholar]
- Monteleone A., Monteleone P., Serino I., Amodio R., Monaco F., Maj M. Underweight subjects with anorexia nervosa have an enhanced salivary cortisol response not seen in weight restored subjects with anorexia nervosa. Psychoneuroendocrinology. 2016;70:118–121. doi: 10.1016/j.psyneuen.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Monteleone A., Monteleone P., Volpe U., De Riso F., Fico G., Giuliano R. Impaired cortisol awakening response in eating disorder women with childhood trauma exposure: evidence for a dose-dependent effect of the traumatic load. Psychol. Med. 2017 doi: 10.1017/S0033291717002409. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Monteleone P., Scognamiglio P., Monteleone A.M., Perillo D., Maj M. Cortisol awakening response in patients with anorexia nervosa or bulimia nervosa: relationships to sensitivity to reward and sensitivity to punishment. Psychol. Med. 2014;44(12):2653–2660. doi: 10.1017/S0033291714000270. [DOI] [PubMed] [Google Scholar]
- Morley J.E., Levine A.S., Rowland N.E. Stress induced eating. Life Sci. 1983;32(19):2169–2182. doi: 10.1016/0024-3205(83)90415-0. [DOI] [PubMed] [Google Scholar]
- Naruo T., Nakabeppu Y., Deguchi D., Nagai N., Tsutsui J., Nakajo M. Decreases in blood perfusion of the anterior cingulate gyri in anorexia nervosa restricters assessed by SPECT image analysis. BMC Psychiatr. 2001;2:1. doi: 10.1186/1471-244X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E., O’Connor D.B., Conner M. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32:125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Oitzl M.S., Champagne D.L., van der Veen R., de Kloet E.R. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci. Biobehav. Rev. 2010;34(6):853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Oliver G., Wardle J., Gibson E.L. Stress and food choice: a laboratory study. Psychosom Med. 2000;62:853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Osuch E.A., Ketter T.A., Kimbrell T.A., George M.S., Benson B.E., Willis M.W. Regional cerebral metabolism associated with anxiety symptoms in affective disorder patients. Biol. Psychiatr. 2000;48(10):1020–1023. doi: 10.1016/s0006-3223(00)00920-3. 11082477. [DOI] [PubMed] [Google Scholar]
- Papadopoulos F., Ekbom A., Brandt L., Ekselius L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br. J. Psychiatry. 2009;194(1):10–17. doi: 10.1192/bjp.bp.108.054742. 19118319. [DOI] [PubMed] [Google Scholar]
- Pare W.P. Stress and consummatory behavior in the albino rat. Psychol. Rep. 1965;16:399–405. doi: 10.2466/pr0.1965.16.2.399. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Peschel S.K., Feeling N.R., Vögele C., Kaess M., Thayer J.F., Koenig J. A systematic review on heart rate variability in Bulimia Nervosa. Neurosci. Biobehav. Rev. 2016;63:78–97. doi: 10.1016/j.neubiorev.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Price J., Drevets W. Neural circuits underlying the pathophysiology of mood disorders. Trends Cognit. Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Pruessner J., Dedovic K., Khalili-Mahani N., Engert V., Pruessner M., Buss C. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. Psychiatr. 2008;63(2):234–240. doi: 10.1016/j.biopsych.2007.04.041. 17686466. [DOI] [PubMed] [Google Scholar]
- Rastam M., Bjure J., Vestergren E., Uvebrant P., Gillberg I.C., Wentz E. Regional cerebral blood flow in weight-restored anorexia nervosa: a preliminary study. Dev. Med. Child Neurol. 2001;43(4):239–242. doi: 10.1017/s0012162201000457. 21199960; PMC11305400. [DOI] [PubMed] [Google Scholar]
- Rojo L., Conesa L., bermudez O., Livianos L. Influence of stress in the onset of eating disorders: data from a two-stage epidemiologic controlled study. Psychosom. Med. 2006;68(4):628–635. doi: 10.1097/01.psy.0000227749.58726.41. 16868274. [DOI] [PubMed] [Google Scholar]
- Rolls E. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiol. Hung. 2008;95(2):131–164. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- Rolls E. Reward systems in the brain and nutrition. Annu. Rev. Nutr. 2016 doi: 10.1146/annurev-nutr-071715-050725. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Romeo R.D. The teenage brain: the stress response and the adolescent brain. Curr. Dir. Psychol. Sci. 2013;22(2):140–145. doi: 10.1177/0963721413475445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Bloch M., Ben Avi I., Rouach V., Schreiber S., Stern N., Greenman Y. Cortisol response and desire to binge following psychological stress: comparison between obese subjects with and without binge eating disorder. Psychiatr. Res. 2013;208(2):156–161. doi: 10.1016/j.psychres.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Ross R.A., Mandelblat-Cerf Y., Verstegen A.M. Interacting neural processes of feeding, hyperactivity, stress, reward, and the utility of the activity-based anorexia model of anorexia nervosa. Harv. Rev. Psychiatr. 2016;24(6):416–436. doi: 10.1097/HRP.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenga K., Sinha R., Small D. Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. Int. J. Obes. 2013;37(2):309–316. doi: 10.1038/ijo.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutters F., Nieuwenhuizen A.G., Lemmens S.G., Born J.M., Westerterp-Plantenga M.S. Acute stress-related changes in eating in the absence of hunger. Obesity. 2009;17(1):72–77. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- Sachinvala N., Kling A., Suffin S., Lake R., Cohen M. Increased regional cerebral perfusion by 99mTc hexamethyl propylene amine oxime single photon emission computed tomography in post-traumatic stress disorder. Mil. Med. 2000;165(6):473–479. 10870367. [PubMed] [Google Scholar]
- Safer D.L., Telch C.G., Chen E.Y. Guilford Press; New York, NY: 2009. Dialectical Behavior Therapy for Binge Eating and Bulimia. [Google Scholar]
- Schmidt U., Tiller J., Blanchard M., Andrews B., Treasure J. Is there a specific trauma precipitating anorexia nervosa? Psychol. Med. 1997;27(3):523–530. doi: 10.1017/s0033291796004369. 9153673. [DOI] [PubMed] [Google Scholar]
- Sheng M., Lu H., Liu P., Thomas B., McAdams C. Cerebral perfusion differences in women currently with and recovered from anorexia nervosa. Psychol. Res. 2015;23(2):175–183. doi: 10.1016/j.pscychresns.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker L., Tanofsky-Kraff M.E.,C., Wolkoff L., Columbo K., Ranzenhofer L., Roza C. Salience of loss of control for pediatric binge episodes: does size really matter? 2010;43(8):707–716. doi: 10.1002/eat.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. Taste representation in the human insula. Brain Struct. Funct. 2010;214(5–6):551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Smyth J., Wonderlich S., Heron K., Sliwinski M., Crosby R., Mitchell J. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. J. Consult. Clin. Psychol. 2007;75(4):629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- Soukop V., Beiler M., Terrell F. Stress, coping style, and problem solving ability among eating-disordered inpatients. J. Clin. Psychol. 1990;46(5):592–599. doi: 10.1002/1097-4679(199009)46:5<592::aid-jclp2270460508>3.0.co;2-y. 2246365. [DOI] [PubMed] [Google Scholar]
- Steinglass J., Sysko R., Mayer L., Berner L., Schebendach J., Wang Y. Pre-meal anxiety and food intake in anorexia nervosa. Appetite. 2010;55(2):214–218. doi: 10.1016/j.appet.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober M., Freeman R., Lampert C., Diamond J., Kaye W. Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am. J. Psychiatr. 2000;157(3):393–401. doi: 10.1176/appi.ajp.157.3.393. [DOI] [PubMed] [Google Scholar]
- Struber N., Struber D., Roth G. Impact of early adversity on glucocorticoid regulation and later mental disorders. Neurosci. Biobehav. Rev. 2014;38:17–37. doi: 10.1016/j.neubiorev.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Stunkard A.J. Eating patterns and obesity. Psychiatr. Q. 1959;33(2):284–295. doi: 10.1007/BF01575455. [DOI] [PubMed] [Google Scholar]
- Sullivan P.F. Mortality in anorexia nervosa. Am. J. Psychiatr. 1995;152(7):1073–1074. doi: 10.1176/ajp.152.7.1073. 7793446. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Ishii K., Hosokaw C., Hyodo T., Kashiwagi N., Matsuki M. Clinical application of 3D arterial spin-labelled brain perfusion imagine for Alzheimer Disease: comparison with brain perfusion spect. Am. J. Neuroradiol. 2014;35(5):906–911. doi: 10.3174/ajnr.A3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A., Shiga T., Kitagawa N., Koyama T., Katoh C., Tsukamoto E. Abnormal neuronal network in anorexia nervosa studied with I-123-IMP SPECT. Psychiatr. Res. Neuroimaging. 2001;107(1):45–50. doi: 10.1016/s0925-4927(01)00093-2. Jul 2001. [DOI] [PubMed] [Google Scholar]
- ter Heegde F., De Rijk R.H., Vinkers C.H. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology. 2015;52:92–110. doi: 10.1016/j.psyneuen.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Tillfors M., Furmark T., Marteinsdottir I., Fischer H., Pissiota A., Langstrom B. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am. J. Psychiatr. 2001;158(8):1220–1226. doi: 10.1176/appi.ajp.158.8.1220. 11481154. [DOI] [PubMed] [Google Scholar]
- Torres S.J., Nowson C.A. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Troop N., Holbrey A., Treasure J. Stress, coping, and crisis support in eating disorders. Int. J. Eat. Disord. 1998;24(2):157–166. doi: 10.1002/(sici)1098-108x(199809)24:2<157::aid-eat5>3.0.co;2-d. 9697014. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynde F., Suda M., Broadbent H., Guillaume S., Van den Eynde M., Steiger H. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur. Eat Disord. Rev. 2012;20(2):94–105. doi: 10.1002/erv.1163. 22052722. [DOI] [PubMed] [Google Scholar]
- van den Heuvel O., Veltman D., Groenewegen H., Witter M., Merkelbach J., Cath D. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch. Gen. Psychiatr. 2005;62(8):922–933. doi: 10.1001/archpsyc.62.8.922. 16061770. [DOI] [PubMed] [Google Scholar]
- van der Lely A.J., Tschöp M., Heiman M.L., Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr. Rev. 2004;25(3):426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- Vannucci A., Nelson E.E., Bongiorno D.M., Pine D.S., Yanovski J.A., Tanofsky-Kraff M. Behavioral and neurodevelopmental precursors to binge-type eating disorders: support for the role of negative valence systems. Psychol. Med. 2015;45(14):2921–2936. doi: 10.1017/S003329171500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek K., Manke F. Personality variables and disorders in anorexia nervosa and bulimia nervosa. J. Abnorm. Psychol. 1994;103(1):137–147. doi: 10.1037//0021-843x.103.1.137. 8040475. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Xaplanteris P., lexopoulos N., Aznaouridas K., Vasiliadou C., Baou K. Divergent effects of laughter and mental stress on arterial stiffness and central hemodynamics. Psychosom. Med. 2009;71(4):446–453. doi: 10.1097/PSY.0b013e318198dcd4. [DOI] [PubMed] [Google Scholar]
- Volkow N., Wang G., Baler R. Reward, dopamine and the control of food intake: implications for obesity. Trends Cognit. Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A., Barbarich N., Frank G., Bailer U., Weissfeld L., Henry S. Personality traits after recovery from eating disorders: do subtypes differ? Int. J. Eat. Disord. 2006;39(4):276–284. doi: 10.1002/eat.20251. 16528697. [DOI] [PubMed] [Google Scholar]
- Wang J., Korczykowski M., Rao H., Fan Y., Pluta J., Gur R. Gender difference in neural response to psychological stress. Soc. Cognit. Affect Neurosci. 2007;2(3):227–239. doi: 10.1093/scan/nsm018. 17873968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Rao H., Wetmore G., Furlan P., Korczykowski M., Dinges D. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc. Natl. Acad. Sci. U.S.A. 2005;102(49):17804–17809. doi: 10.1073/pnas.0503082102. 16306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. A systematic review of impulsivity in eating disorders. Eur. Eat Disord. Rev. 2009;17:408–425. doi: 10.1002/erv.952. [DOI] [PubMed] [Google Scholar]
- Wierenga C., Bischoff-Grethe A., Rasmussen G., Bailer U., Berner L., Liu T. Aberrant cerebral blood flow in response to hunger and satiety in women remitted from anorexia nervosa. Front. Nutr. 2017;4:32. doi: 10.3389/fnut.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga C.E., Bondi M.W. Use of functional magnetic resonance imaging in the early identification of Alzheimer's disease. Neuropsychol. Rev. 2007;17(2):127–143. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Z., Biush G., Rauch S., Cosgrove G., Eskandar E. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat. Neurosci. 2004;7(12):1370–1375. doi: 10.1038/nn1354. 15558064. [DOI] [PubMed] [Google Scholar]
- Wilson G., Grilo C., Vitousek K. Psychological treatment of eating disorders. Am. Psychol. 2007;62(3):199–216. doi: 10.1037/0003-066X.62.3.199. 17469898. [DOI] [PubMed] [Google Scholar]
- Wolfe B., Baker C., Smith A., Kelly-Weeder S. Validity and utility of the current definition of binge eating. Int. J. Eat. Disord. 2009;42(8):674–686. doi: 10.1002/eat.20728. [DOI] [PubMed] [Google Scholar]
- Wonderlich S.A., Mitchell J.E. Eating disorders and comorbidity: empirical, conceptual, and clinical implications. Psychopharmacol. Bull. 1997;33(3):381–390. 9550882. [PubMed] [Google Scholar]
- Wonderlich S.A., Peterson C.B., Smith T.L., Mitchell J.E., Crow S.J. Guilford; New York: 2015. Integrative Cognitive-affective Therapy for Bulimia Nervosa: a Treatment Manual. [Google Scholar]
- Yücel M.A., Huppert T.J., Boas D.A., Gagnon L. Calibrating the BOLD signal during a motor task using an extended fusion model incorporating DOT, BOLD and ASL data. Neuroimage. 2012;61(4):1268–1276. doi: 10.1016/j.neuroimage.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Badaut J., Tang J., Obenaus A., Hartman R., Pearce W. The vascular neural network—a new paradigm in stroke pathophysiology. Nat. Rev. Neurosci. 2012;8(12):711–716. doi: 10.1038/nrneurol.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonnevylle-Bender M.J., van Goozen S.H., Cohen-Kettenis P.T., Jansen L.M., van Elburg A., van Engeland H. Adolescent anorexia nervosa patients have a discrepancy between neurophysiological responses and self-reported emotional arousal to psychosocial stress. Psychiatr. Res. 2005;135(1):45–52. doi: 10.1016/j.psychres.2004.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.